Abstract
Background
Low skeletal muscle area or density, such as myosteatosis, identified on computed tomography (CT) is associated with poor prognosis in patients with cardiovascular diseases. However, there is a lack of evidence regarding the clinical process of skeletal muscle decline as a short‐term change during acute care settings. This study focused on the use of routine CT imaging for aortic disease management and investigated the changes in skeletal muscle before and after acute care.
Methods
This prospective study included 123 patients who underwent abdominal CT before and after acute care. The all‐abdominal and each abdominal muscle areas were divided into eight parts (e.g. rectus abdominis, psoas, and erector spine), and their areas and densities were measured at the third lumbar vertebra level after the patients were discharged and de‐identified with blinding to avoid measurement bias. Short physical performance battery (SPPB) was measured at the start and end of in‐hospital cardiac rehabilitation. A generalized linear model with patients as random effects was made to investigate skeletal muscle loss during acute care. Multivariate linear regression analysis was also used to assess the relationship between the change in skeletal muscle during acute care and SPPB during in‐hospital cardiac rehabilitation.
Results
The median age of the patients was 70 (interquartile: 58–77) years, and 69.9% (86/123) were men. The median day between acute care from the day of surgery or hospital admission and follow‐up CT was 7 (interquartile: 3–8) days. Overall muscle density declined after acute care (estimate value: −3.640, 95% confidence interval [CI]: −4.538 to −2.741), and each abdominal muscle density consistently declined (interaction: F value = 0.099, P = 0.998). In contrast, there was no significant change in the overall muscle area (estimate value: −0.863, 95% CI: −2.925 to 1.200). Changes in the muscle area were different for each skeletal muscle (interaction: F value = 2.142, P = 0.037), and only the erector spine muscle significantly declined (estimate value: −1.836, 95% CI: −2.507 to −1.165). After adjusting for confounding factors, a greater decline in muscle density was associated with lower recovery score on SPPB (β = 0.296, 95% CI: 0.066 to 0.400).
Conclusions
Muscle density consistently declined after acute care, especially the erector spine muscles, which also significantly decreased in size. A higher decline in muscle density was associated with a slower recovery of physical function during in‐hospital cardiac rehabilitation in patients with aortic diseases.
Keywords: Skeletal muscle wasting, Acute care, Physical function, Aortic disease
Introduction
As the population of ageing people is increasing worldwide, diseases associated with ageing, such as sarcopenia, are becoming increasingly important. 1 , 2 A decline in the quantity and quality of skeletal muscles is one of the major physical changes associated with ageing, 3 and it is an important factor for patients with diseases. 4 , 5 , 6 Furthermore, low skeletal muscle quality combined with low physical function leads to poor prognosis. 7 Therefore, the clinical course of skeletal muscle mass and quality should be properly monitored for appropriate disease management and intervention, in conjunction with a decline in physical function.
The evaluation of skeletal muscle using medical imaging modalities, such as computed tomography (CT) imaging, is considered the gold standard. 2 In recent years, there has been an emphasis on in‐hospital treatment or bed rest acquired weakness, such as loss of muscle mass and physical dysfunction, in acute care settings. 8 Particularly, several studies have reported on acute skeletal muscle loss identified by CT imaging in cancer patients who underwent surgery, 9 , 10 patients who were admitted to the intensive care unit (ICU), or patients that are critically ill. 11 , 12 , 13 , 14 , 15 However, limited evidence is available to detail the changes in skeletal muscle area and density, especially in patients with cardiovascular disease. Changes in muscle area and density are expected to vary from muscle to muscle, 3 , 16 although most have measured these changes in all muscles or in only using a single muscle. 17 Moreover, there is little evidence regarding the relationship between change in skeletal muscle identified by CT during acute care and recovery in physical function during in‐hospital rehabilitation.
In Japan, routine CT imaging of patients with aortic diseases is recommended for disease management. 18 We thought that focusing on CT images routinely taken for the management of aortic disease would be a possible solution. Therefore, the present study sought to grasp the features of skeletal muscle wasting identified by CT during acute care and its relationship with short‐term changes in the physical function of patients with aortic diseases.
Methods
Study population
This study was a part of a single‐centre prospective study on cardiac rehabilitation. We included patients admitted to Kitasato University Hospital between September 2018 and August 2020 for the treatment of aortic disease. The criteria for enrolment in this study were as follows: (i) patients for whom CT imaging before and after acute treatment were recommended in the guidelines; (ii) patients with adequate transverse abdominal CT images taken before and after acute treatment; and (iii) patients who underwent in‐hospital cardiac rehabilitation at least once.
This study was conducted in accordance with the Declaration of Helsinki and was performed as a part of a prospective observational study approved by the Ethics Committee of Kitasato University Hospital (B18‐083). An overview of the comprehensive prospective study was published in a publicly available University Hospital Information Network (UMIN‐CTR, unique identifier: UMIN000038373), and information about the research was made public by opt‐out. The participants were informed of the option to drop out.
Clinical data collection and measurement of physical function
From the electronic medical records, data on patient characteristics, including age, sex, height, body weight, body mass index (BMI), type of treatment (medical or surgical), length of ICU stay, haemoglobin, albumin, creatinine, and C‐reactive protein, were collected, and the patient's background and confounding factors were used. Change in body weight (ΔBW) between acute care from the day of surgery or hospital admission and follow‐up CT was calculated as a confounding factor in this study. In addition, baseline left ventricular ejection fraction, co‐morbidities, previous history of myocardial infarction, and heart failure were collected as background factors.
A short physical performance battery (SPPB), which is simple and measures the bedside score, was used as a physical function parameter. 1 , 2 SPPB comprises three items: gait speed, balance, and time of 5‐sit to stand. Gait speed was assessed using the patient's comfortable speed from the start of the gait at 4 m away from the goal. The use of a walking aid was not defined. The balance test consisted of three components: closed‐foot stance, semi‐tandem standing, and tandem standing. The time of 5‐sit to stand was measured from the sitting chair until the fifth repeated motion of standing up. Each item was scored from 0 to 4, with the best score at 12 points. A score of 0 point was given when patients could not assess anything due to impaired function or severe conditions. Patients were assessed at the beginning of cardiac rehabilitation near bedside and at discharge with a rehabilitation room or outside the hospital room. The time difference between the start of cardiac rehabilitation and hospital discharge was calculated (ΔSPPB).
Skeletal muscle composition measurement
Skeletal muscle area and density were assessed using abdominal CT imaging with two consecutive slices at the third lumbar vertebra level, including the transverse processes. Baseline CT was determined as either the most recent preoperative image or the most recent pre‐acute care image. Follow‐up CT images were identified between 2 and 14 days, based on the date of surgery or hospital admission as a post‐acute care imaging. 11 , 12 The tube potential was set between 100–120 kV with a 512 × 512 matrix, and the electrical current (mA) was set automatically for each slice. Slice thickness and contrast enhancement were not standardized. The observers (M. Y., S. U., T. N., and N. Y.) measured skeletal muscle area and density after being sufficiently trained until their measurement results exceeded 0.95 in intraclass correlation coefficients using 20 randomly selected slices. 4
An example of these measurements is shown in the Supporting information Figure S1. The area and density of the all‐abdominal skeletal muscles were measured in this study. In addition, as a sensitivity analysis, muscle measurements were separated according to a previous study, 16 including the rectus abdominis, internal oblique, external oblique, psoas, quadratus lumborum, latissimus dorsi, transverse spinal, erector spine, and other muscles. The area and density of all‐abdominal muscles and each muscle were measured using the Slice‐O‐Matic ver. 5.0 (Tomovision, Canada). Measurements were conducted in a blinded fashion, wherein participants could not be identified to avoid measurement bias. Furthermore, these were performed at a well‐timed interval following patient discharge. The range of Hounsfield units (HUs) for the skeletal muscle was defined from −29 to 150. The area (cm2) and density (HU) were calculated as the average of the two slices after the measurement was obtained.
Inpatient cardiac rehabilitation
According to the Guidelines of the Japanese Circulation Society, 19 a multi‐domain inpatient cardiac rehabilitation intervention involving supervised rehabilitation therapy was implemented. Two exercise stages were applied by a multi‐disciplinary team consisting of doctors, nurses, and physical therapists to improve the strength, balance, mobility, and endurance of patients by utilizing reproducible and targeted exercises. The first stage of the supervised rehabilitation therapy programme at the bedside consisted of gradual mobilization, which included basic activity training, such as sitting up in bed, sit‐to‐stand motion, and walking within the hospital ward. Once the patients were shown to be clinically stable, they proceeded to the second stage, which consisted of a gym‐based exercise training programme at the rehabilitation room with 5–10 min of stretching, balance training, and resistance training with the patient's own body weight, and 20–40 min of aerobic training using a cycling ergometer or treadmill, including warm‐up and cool‐down periods. The exercise intensity in both types of training was prescribed at a rating of perceived exertion (RPE) of 11–13 on the Borg RPE scale (range: 6–20) while managing blood pressure, heart rate, and arrhythmia. Exercise intensity was increased progressively in each session. Patients participated in the rehabilitation programme for 1 h daily, 5 days per week, during the period of hospitalization for as long as there were no adverse symptoms or events.
Statistical analysis
Continuous variables were expressed as median and interquartile range (IQR), and categorical variables were expressed as n (%). All analyses were performed for patients with complete data.
Decline in muscle area and density were assessed using the generalized linear mixed model (GLMM), which were compared with pre‐acute and post‐acute treatment. The random effect was defined as patients, and covariates were defined as follows: Model 1 with baseline patient characteristics (age, sex, and baseline BMI), Model 2 with Model 1 plus treatment‐related factors (medical or surgical, ICU length, and time to follow‐up CT scan) and imaging conditions (tube voltage, tube current, thickness, and using contrast enhancement), and Model 3 with Model 2 plus severity (haemoglobin, albumin, creatinine, and C‐reactive protein) and change in body weight during the acute care (ΔBW). Results for each model showed an estimated value and 95% confidence interval (95% CI). Furthermore, the same analyses were performed using each muscle after adjustment by Model 3, as a sensitivity analysis and to check for interaction. The same analysis was also performed for participants who were divided into elective or emergency.
In order to determine the cut‐off value for muscle wasting, minimal detectable changes (MDC) in the all‐abdominal skeletal muscle area and skeletal muscle density were calculated based on the standard error of measurement (SEM) agreement, which was determined from two consecutive CT images taken before acute care. The MDC at the individual level was calculated as ‘1.96 × √2 × SEM agreement’, making it 95% confident that the observed changes could not be attributed to measurement errors and could be regarded as actual changes.
Univariate and multivariate linear regression analyses were used to assess the relationship between changes in muscle composition during acute care (Δmuscle area, Δmuscle density) and the change in physical function (ΔSPPB) during in‐hospital cardiac rehabilitation. Confounding factors were identified as follows: Model 1 with baseline characteristics, Model 2 with Model 1 plus treatment‐related factors, and Model 3 with Model 2 plus severity and ΔBW. Similarly, results showed a standardized regression coefficient (β) and 95% CI.
All analyses were performed using JMP (version 15.1; SAS Institute Inc., Cary, NC, USA), R version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria), and Stata version 16.0 (Stata Corp., College Station, TX). Two‐tailed P values <0.05 were indicative of statistical significance.
Results
Patient flow in the present study is shown in Figure 1. Around 254 patients were hospitalized for the treatment of aortic disease and underwent in‐hospital cardiac rehabilitation at least once over 2 years. Patients who underwent thoracic endovascular aortic repair or endovascular aortic repair were excluded due to difference in pathology and since post‐acute CT images were not taken routinely in Japan. Exactly 29 patients, who did not undergo baseline or follow‐up CT imaging for any reason, had a lack of whole abdominal imaging, and had increased intensity on CT imaging due to the presence of metal, were also excluded. Therefore, a total of 123 patients were enrolled in this study.
Figure 1.
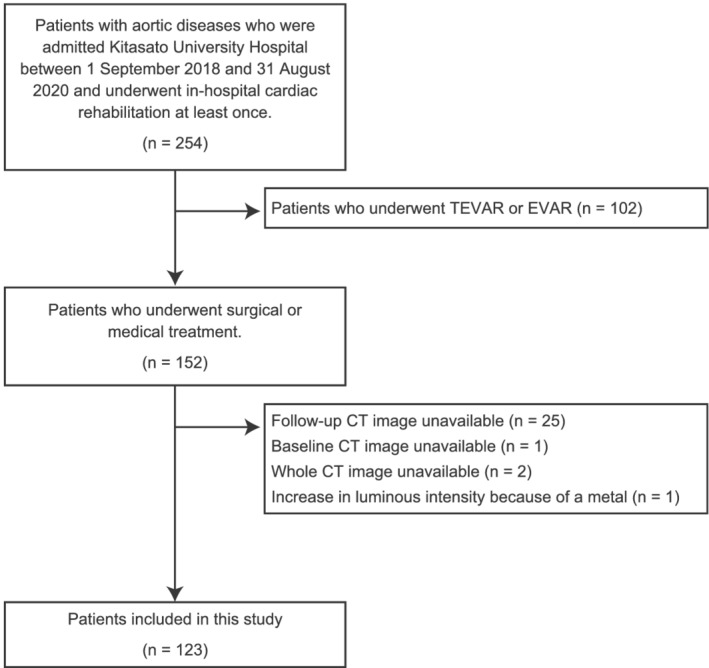
Patient flow in this study. CT, computed tomography; EVAR, endovascular aortic repair; TEVAR, thoracic endovascular aortic repair.
Baseline patient characteristics are shown in Table 1. The median age of the patients was 70 years, and 69.9% (86/123) were men. Two‐thirds of the patients (82/123) received surgical treatment, and one‐third of the patients (41/123) received medical treatment. During the period between acute treatment and post‐treatment CT scan (median: 7, IQR: 3–8 days), body weight changed by −1.4 (IQR: −3.8 to 0.6) kg. The change in SPPB score was 3 (IQR: 0–5) points during in‐hospital rehabilitation. Supporting Information Table S1 shows the imaging conditions of the baseline and follow‐up CT. Compared with pre‐acute care, a higher percentage of patients underwent contrast‐enhanced CT after acute care. Contrarily, CT with thicker slices was higher before acute care than that after acute care. Tube voltage and tube current were not significant.
Table 1.
Patients' characteristics
| Factor | All case (n = 123) | |
|---|---|---|
| Median [IQR] or n (%) | ||
| Age, year | 70 | [58, 77] |
| Male, n (%) | 86 | (69.9) |
| Baseline BMI, kg/m2 | 24.0 | [22.0, 27.0] |
| Baseline LVEF, % | 66.3 | [62.0, 70.4] |
| Co‐morbidities, n (%) | ||
| Hypertension | 73 | (59.3) |
| Diabetes mellitus | 11 | (8.9) |
| Dyslipidaemia | 24 | (19.5) |
| Arterial fibrillation | 10 | (8.1) |
| Chronic kidney disease | 80 | (65.0) |
| Prior myocardial infarction | 2 | (1.6) |
| Prior heart failure | 4 | (3.3) |
| Baseline albumin, mg/dL | 3.9 | [3.5, 4.2] |
| Baseline creatinine, mg/dL | 1.01 | [0.80, 1.21] |
| Baseline CRP, mg/dL | 0.16 | [0.05, 1.04] |
| Baseline haemoglobin, mg/dL | 12.8 | [11.10, 14.30] |
| Treatment, n (%) | ||
| Surgical treatment | 82 | (66.7) |
| Medical treatment | 41 | (33.3) |
| Emergency, n (%) | 78 | (63.4) |
| ICU length, day | 3 | [2.0,6.5] |
| SPPB at start CR, points | 7 | [4, 10] |
| SPPB at discharge, points | 11 | [8, 12] |
| ΔSPPB, points | 3 | [0, 5] |
| ΔBody weight, kg | −1.4 | [−3.8, 0.6] |
BMI, body mass index; IQR, interquartile range; LVEF, left ventricular ejection fraction; CRP, C‐reactive protein; ICU, intensive care unit; SPPB, short physical performance battery; CR, cardiac rehabilitation.
Table 2 shows changes in skeletal muscle area and density during acute care using GLMM. All skeletal muscle areas showed a significant difference when adjusted to Model 2 (baseline patients' characteristics, treatment‐related factors, and imaging conditions). However, the difference was not maintained after adjustment for severity and ΔBW (estimated value: −0.863, 95% CI: −2.925 to 1.200, P = 0.412). Notably, the area of the erector spine muscles consistently decreased during the acute care phase (estimated value: −1.836, 95% CI: −2.507 to −1.165, P < 0.001). In contrast, all skeletal muscle density was decreased, even after adjusting for confounding factors (estimated value: −3.640, 95% CI: −4.538 to −2.741, P < 0.001). Furthermore, a similar trend of decrease in skeletal muscle density was observed in each skeletal muscle (all: P < 0.001). The same trend was observed in the analysis stratified by elective and emergency (Supporting information Tables S2 and S3). The SEM agreement of the all‐abdominal muscle area was 2.894, and that in muscle density was 0.737. The MDC value for muscle area and muscle density was 8.016 and 2.042, respectively.
Table 2.
The change in skeletal muscle before and after acute care
| Unadjusted | Model 1 | Model 2 | Model 3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate value | 95% CI | P value | Estimate value | 95% CI | P value | Estimate value | 95% CI | P value | Estimate value | 95% CI | P value | |
| [Muscle area] | ||||||||||||
| All‐abdominal muscle | −1.602 | −3.388 to 0.184 | 0.079 | −1.584 | −3.384 to 0.216 | 0.085 | −2.201 | −4.217 to −0.185 | 0.032 | −0.863 | −2.925 to 1.200 | 0.412 |
| Recutus abdominis | 0.032 | −0.199 to 0.262 | 0.788 | 0.029 | −0.203 to 0.262 | 0.806 | 0.100 | −0.167 to 0.367 | 0.461 | 0.155 | −0.130 to 0.440 | 0.286 |
| Internal oblique | 0.046 | −0.633 to 0.726 | 0.893 | 0.057 | −0.628 to 0.741 | 0.871 | −0.140 | −0.910 to 0.631 | 0.723 | 0.236 | −0.553 to 1.025 | 0.558 |
| External oblique | 0.658 | 0.154 to 1.161 | 0.010 | 0.664 | 0.157 to 1.172 | 0.010 | 0.419 | −0.160 to 0.997 | 0.156 | 0.613 | 0.011 to 1.214 | 0.046 |
| Psoas | −0.119 | −0.510 to 0.272 | 0.551 | −0.106 | −0.499 to 0.288 | 0.599 | 0.144 | −0.308 to 0.596 | 0.533 | 0.453 | 0.016 to 0.890 | 0.042 |
| Quadratus lumborum | −0.245 | −0.469 to −0.020 | 0.033 | −0.240 | −0.466 to −0.014 | 0.038 | −0.154 | −0.412 to 0.104 | 0.241 | −0.035 | −0.295 to 0.225 | 0.790 |
| Latissimus dorsi | −0.018 | −0.222 to 0.186 | 0.862 | −0.017 | −0.223 to 0.189 | 0.873 | 0.053 | −0.184 to 0.291 | 0.660 | 0.052 | −0.200 to 0.304 | 0.685 |
| Transverse spinal | −0.341 | −0.618 to −0.065 | 0.015 | −0.348 | −0.626 to −0.070 | 0.014 | −0.095 | −0.406 to 0.215 | 0.547 | 0.046 | −0.274 to 0.366 | 0.779 |
| Erector spine | −1.898 | −2.451 to −1.345 | <0.001 | −1.896 | −2.454 to −1.338 | <0.001 | −2.144 | −2.793 to −1.494 | <0.001 | −1.836 | −2.507 to −1.165 | <0.001 |
| [Muscle density] | ||||||||||||
| All‐abdominal muscle | −2.932 | −3.703 to −2.161 | <0.001 | −2.933 | −3.711 to −2.156 | <0.001 | −3.520 | −4.378 to −2.662 | <0.001 | −3.640 | −4.538 to −2.741 | <0.001 |
| Recutus abdominis | −2.346 | −3.538 to −1.155 | <0.001 | −2.372 | −3.572 to −1.172 | <0.001 | −2.717 | −4.038 to −1.395 | <0.001 | −3.063 | −4.398 to −1.727 | <0.001 |
| Internal oblique | −3.026 | −4.045 to −2.008 | <0.001 | −3.029 | −4.056 to −2.002 | <0.001 | −3.619 | −4.739 to −2.498 | <0.001 | −3.883 | −5.040 to −2.726 | <0.001 |
| External oblique | −2.426 | −3.393 to −1.459 | <0.001 | −2.439 | −3.414 to −1.464 | <0.001 | −2.761 | −3.790 to −1.732 | <0.001 | −2.760 | −3.838 to −1.682 | <0.001 |
| Psoas | −2.885 | −3.925 to −1.846 | <0.001 | −2.858 | −3.905 to −1.812 | <0.001 | −3.924 | −5.046 to −2.802 | <0.001 | −4.118 | −5.271 to −2.965 | <0.001 |
| Quadratus lumborum | −3.104 | −4.475 to −1.733 | <0.001 | −3.122 | −4.505 to −1.740 | <0.001 | −4.759 | −6.206 to −3.312 | <0.001 | −4.959 | −6.454 to −3.463 | <0.001 |
| Latissimus dorsi | −3.261 | −4.387 to −2.135 | <0.001 | −3.280 | −4.415 to −2.145 | <0.001 | −3.376 | −4.645 to −2.108 | <0.001 | −3.406 | −4.731 to −2.080 | <0.001 |
| Transverse spinal | −2.888 | −4.134 to −1.642 | <0.001 | −2.838 | −4.090 to −1.586 | <0.001 | −3.747 | −5.146 to −2.347 | <0.001 | −3.906 | −5.371 to −2.442 | <0.001 |
| Erector spine | −2.887 | −3.971 to −1.803 | <0.001 | −2.898 | −3.991 to −1.805 | <0.001 | −3.751 | −4.953 to −2.550 | <0.001 | −3.876 | −5.129 to −2.622 | <0.001 |
The random effect is defined as patients. Model 1: age, sex, and baseline body mass index. Model 2: Model 1 plus medical or surgical treatment, intensive care unit length, time to follow‐up computed tomography scan, tube voltage, tube current, slice thickness, and contrast enhancement. Model 3: Model 2 plus baseline haemoglobin, baseline albumin, baseline creatine, baseline C‐reactive protein, and change in body weight.
CI, confidence interval.
Figure 2 shows the change in the area and density of each skeletal muscle after adjustment with Model 3. An interaction between the type of skeletal muscle area and time was confirmed (F value: 2.142, P = 0.037), but no significant main effect was identified on time (F value: 0.003, P = 0.953). Although a significant main effect of muscle type was observed (F value: 1349.350, P < 0.001). There was also no significant interaction effect (F value: 0.099, P = 0.998); however, there was a significant main effect on the density of each skeletal muscle (F value: 299.726, P < 0.001) and time (F value: 84.654, P < 0.001), indicating a consistent decrease in density in all skeletal muscles.
Figure 2.
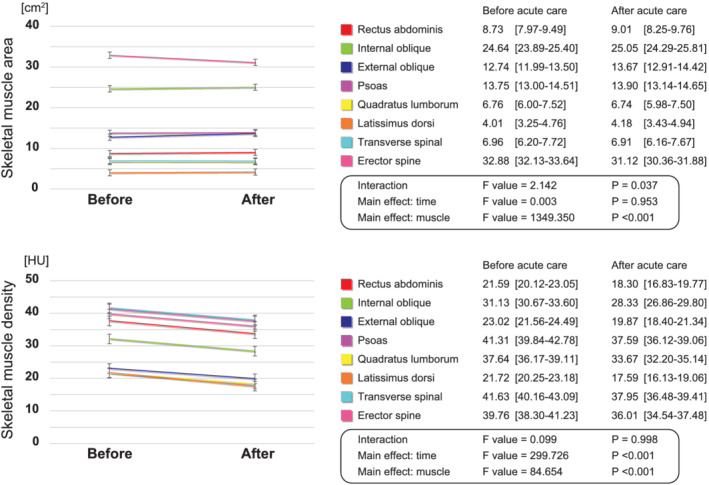
The change in area and density of each skeletal muscle before and after acute care. The red line is the rectus abdominis, green line is the internal oblique, blue line is the external oblique, purple line is the psoas, yellow line is the quadratus lumborum, orange line is the latissimus dorsi, light blue line is the transverse spinal, and the pink line is the erector spine. HU, Hounsfield unit.
Table 3 shows the multivariate linear regression analysis of the relationship between the change in skeletal muscle (Δmuscle area, Δmuscle density) and the change in physical function during the in‐hospital cardiac rehabilitation (ΔSPPB). A positive association between Δmuscle density and ΔSPPB was observed even after adjustment for multiple variables (β = 0.296, 95% CI: 0.067 to 0.400, P = 0.007). In other words, a smaller decrease in skeletal muscle density during the acute care period (larger Δmuscle density values) was associated with better SPPB recovery. On the other hand, Δmuscle area and ΔSPPB were not associated (β: 0.103, 95% CI: −0.040 to 0.108, P = 0.364).
Table 3.
The relationship between change in skeletal muscle and physical function
| Unadjusted | Model 1 | Model 2 | Model 3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | 95% CI | P value | β | 95% CI | P value | β | 95% CI | P value | β | 95% CI | P value | |
| All‐abdominal muscle area | 0.089 | −0.044 to 0.104 | 0.416 | 0.100 | −0.045 to 0.113 | 0.392 | 0.109 | −0.042 to 0.116 | 0.352 | 0.103 | −0.040 to 0.108 | 0.364 |
| All‐abdominal muscle density | 0.241 | 0.020 to 0.371 | 0.030 | 0.268 | 0.036 to 0.398 | 0.020 | 0.284 | 0.048 to 0.412 | 0.014 | 0.296 | 0.066 to 0.400 | 0.007 |
Model 1: age, sex, and baseline body mass index. Model 2: Model 1 plus medical or surgical treatment, intensive care unit length, and time to follow‐up computed tomography scan. Model 3: Model 2 plus baseline haemoglobin, baseline albumin, baseline creatine, baseline C‐reactive protein, and change in body weight.
CI, confidence interval.
Discussion
To the best of our knowledge, this is the first report to examine in detail the deterioration of skeletal muscle before and after acute care using CT imaging, as well as examine its relationship with the improvement of physical function before and after comprehensive in‐hospital rehabilitation. Skeletal muscle density consistently decreased in all muscles, and the decrease in muscle density affected the recovery of physical function during in‐hospital rehabilitation. These observations suggest that skeletal muscle density reflects acute treatment‐related weaknesses, such as ICU‐acquired weakness (ICU‐AW). Additionally, these findings suggest that loss of muscle density during acute care may trigger post‐acute care dysfunction, including post‐intensive care syndrome.
Focusing on skeletal muscle density, systemic skeletal muscle changes could be observed in a short period. Moreover, a decrease in skeletal muscle density was shown to be involved in the subsequent recovery process. A previous study showed that a decrease in skeletal muscle density of more than 10% during hospitalisation was associated with higher in‐hospital mortality. 20 Therefore, it is necessary to appropriately manage whole skeletal muscle density during hospitalisation to reduce loss. In fact, Dusseaux et al. reported that the decrease in skeletal muscle density during acute care was more likely to occur with infection, resulting in a more severe systemic inflammatory state among critically ill patients. 21 Yeh et al. also investigated the relationship between the percentage of skeletal muscle density loss and protein deficits, suggesting that early nutritional deficits correlated with muscle quality deterioration. 13 These findings suggest that loss of skeletal muscle density during acute care critically reflects the consequences of acute treatment, such as acquired weakness, especially systemic weakness of the skeletal muscles.
However, it is interesting to note a difference in the decrease in the skeletal muscle area. Some previous studies in patients with cancer reported that similar to our findings, skeletal muscle area did not change in the short term and was not associated with poor prognosis. 22 , 23 In addition, Baggerman reported that oedema formation was significantly associated with increased skeletal muscle area and a higher sequential organ failure assessment (SOFA) score. 24 Therefore, when using abdominal CT scans to assess changes in muscle mass and quality in critically ill patients, researchers must be aware and careful with the interpretation of the results. Generally, when oedema occurs, skeletal muscle area and mass increase, whereas skeletal muscle density decreases. Nevertheless, only the erector spine muscle area was significantly reduced after acute treatment in the present study. Lambell et al. showed that skeletal muscle area is markedly reduced in the first week after admission to the ICU, and this trend gradually slows down. 12 Similarly, Haines et al. reported that the psoas and all‐skeletal muscle area decreased daily. 11 These findings differ from our results, although they are important for understanding the clinical course of skeletal muscles in acute care. It should be noted that the erector spine muscle is the largest of the muscles identified by abdominal cross‐sectional CT imaging and is one of the major antigravity muscles that mainly act to maintain posture in sitting and standing positions. Therefore, it may have been easier to identify the atrophy of antigravity muscles caused by resting and lying in bed.
Atrophy of skeletal muscle tissues associated with a few days of bed rest is more likely to occur in type I fibres, 25 predominantly in antigravity muscles. 26 In a validation experiment using dry immersion in young participants, muscle fibre atrophy after 3 days of bed rest, increased inflammatory cytokines associated with increased catabolism of skeletal muscles, and a partial process of intramuscular fat deposition were all observed. 27 Another previous study showed that insulin resistance increases after 1 week of resting and lying in bed, and changes in the antigravity skeletal muscle area were clearly captured on CT. 28 This is possible because skeletal muscle density reflects the size and amount of muscle fibres and intramuscular fat 29 and is related to insulin sensitivity, inflammable cytokines, and lipid and protein metabolism. 30 , 31 Furthermore, it is considered to be synergistic with the pathogenesis of cardiovascular diseases. 32 Therefore, protein catabolism, such as insulin resistance and increased inflammation, may be a mechanism for the decline in overall skeletal muscle quality, as found in the present validation, throughout acute care. 33
In this study, we performed multivariate analysis that is adjusted for basic patient background factors, acute treatment factors that induce skeletal muscle atrophy, certain laboratory data associated with skeletal muscle metabolism, and body weight changes as a surrogate marker of body water content, considering the previously mentioned mechanisms. In particular, even though the CT imaging conditions before and after acute care were not constant, the same results were obtained after adjustment for some CT settings. Most skeletal muscle studies using CT images did not specify the CT imaging conditions, which was a major limiting factor. 17 This is important as the imaging conditions of CT images affect the penetration of radioactivity, resulting in errors in the evaluation of tissue density. Therefore, it will be necessary to verify the results by standardizing the imaging conditions in future studies. Nevertheless, this study will help clinical practice, at least in understanding short‐term muscle atrophy in skeletal muscles.
Correct identification of skeletal muscle is important in the evaluation of skeletal muscle disorders during ICU stay. However, it is still controversial whether muscle mass loss is a present symptom of ICU‐AW. 34 One reason for this controversy is the uncertainty of a valid method for measuring muscle mass in the ICU, such as overestimation due to oedema. 24 From the results of the present study, muscle mass loss in trunk muscle groups varies based on muscle type, muscle quality is consistently decreased, and these longitudinal changes predict improvement in physical function with in‐hospital rehabilitation. In recent years, early rehabilitation in the ICU has become popular. 35 , 36 However, there are very few reports on longitudinal and quantitative evaluation of changes in the quantity and quality of skeletal muscle, except for reports on electrical muscle stimulation for lower limb muscles 37 or parenteral nutrition. 15 Thus, the imaging evaluation of skeletal muscle quantity and quality showed that this study might be a useful index for assessing the response to treatment and understanding the pathophysiology and risk stratification of ICU‐AW in clinical practice.
Although this was a novel study with some strengths, it also had several limitations that should be considered when interpreting the findings. First, although this study was performed prospectively, only Japanese patients with aortic disease, who underwent in‐hospital cardiac rehabilitation at a single centre and had their CT images taken twice, were recruited. CT imaging should not be performed solely for muscle measurement due to radiation exposure. On the other hand, magnetic resonance imaging may be an alternative due to better image resolution and less invasiveness. In the future, it will be necessary to establish a less invasive method to evaluate skeletal muscle quality. Nevertheless, more than 80% of the inpatient cardiac rehabilitation patients were included in this study. Therefore, we believe that the present study reflects the patient population in actual clinical practice to some extent, making it one of the strengths of this study. Second, treatment‐related factors, such as surgical‐related factors, muscle relaxants, catecholamines, or steroid use, were not considered in this study. In particular, it should be emphasized that the effects of treatment‐related systemic oedema were not fully accounted for, although weight change was used as a surrogate marker. As such, the actual change in muscle might have been masked. Whether treatment‐related factors are associated with skeletal muscle loss will need to be tested in the future. Third, we investigated skeletal muscle loss after acute care, although we could not verify recovery in skeletal muscle after implementation of pre‐admission, in‐hospital, and outpatient rehabilitation. Several randomized controlled trials have focused on changes in the skeletal muscle area and density in healthy older men 38 and medium‐term to long‐term interventions. 29 , 39 However, there have only been a few reports on hospitalized patients undergoing in‐hospital and outpatient rehabilitation, and reports on skeletal muscle changes involving the improvement process of physical functions are still limited. We believe that a comprehensive approach by medical doctors, nurses, physiotherapists, dietitians, and pharmacists, which includes early nutritional intervention and careful but aggressive early rehabilitation, based on each patient's condition is key to preventing skeletal muscle loss. Therefore, an early assessment of muscle wasting or malnutrition using the imaging evaluation in addition to clinical screening in each patient is important for identifying the best strategy for exercise and nutrition. Further research is needed on how the area and density of skeletal muscles change with comprehensive rehabilitation.
Conclusion
Skeletal muscle density, especially that and muscle size in the erector spine muscles, consistently decreased during acute care. In addition, when the degree of loss of skeletal muscle density was higher, physical function during in‐hospital cardiac rehabilitation worsened recovery. Further research is needed to study the effects of early weaning and nutritional interventions on preventing muscle wasting, mainly in antigravity muscles, and on the improvement of skeletal muscle quality and physical function through comprehensive rehabilitation.
Conflict of interest
All authors have no conflicts of interest to declare.
Supporting information
Data S1. Supporting information
Figure S1. An example of skeletal muscle measurement using computed tomography. These measurements belong to a 73‐year‐old woman undergoing medical treatment 1) at the time of admission (left side: before acute treatment), and 2) two days after admission to the intensive care unit (right side: after acute treatment). The top row shows plain images before measurement, the middle row shows images of all skeletal muscles measured, and the bottom row shows images coloured according to each type of skeletal muscle.
Table S1. Comparison of before and after computed tomography setting and body weight
Table S2. Changes in skeletal muscle before and after acute care in elective patients
Table S3. Changes in skeletal muscle before and after acute care in patients at emergency
Acknowledgements
The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia, and Muscle. 40 We would also like to thank Mr. Suzuki and Mr. Ueno for their advice on statistical analysis. This study was supported by a grant from the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Numbers 20J10290 and 21H03309.
Yamashita M., Kamiya K., Matsunaga A., Kitamura T., Hamazaki N., Ichikawa T., Uchida S., Noda T., Yanagi N., Maekawa E., Yamaoka‐Tojo M., Ako J., and Miyaji K. (2022) Features of trunk muscle wasting during acute care and physical function recovery with aortic disease, Journal of Cachexia, Sarcopenia and Muscle, 13, 1054–1063, 10.1002/jcsm.12935
References
- 1. Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc 2020;21:300–7.e2. [DOI] [PubMed] [Google Scholar]
- 2. Cruz‐Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Johannesdottir F, Allaire B, Anderson DE, Samelson EJ, Kiel DP, Bouxsein ML. Population‐based study of age‐ and sex‐related differences in muscle density and size in thoracic and lumbar spine: the Framingham study. Osteoporos Int 2018;29:1569–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yamashita M, Kamiya K, Matsunaga A, Kitamura T, Hamazaki N, Matsuzawa R, et al. Prognostic value of psoas muscle area and density in patients who undergo cardiovascular surgery. Can J Cardiol 2017;33:1652–1659. [DOI] [PubMed] [Google Scholar]
- 5. Aleixo GFP, Shachar SS, Nyrop KA, Muss HB, Malpica L, Williams GR. Myosteatosis and prognosis in cancer: systematic review and meta‐analysis. Crit Rev Oncol Hematol 2020;145:102839, 10.1016/j.critrevonc.2019.102839 [DOI] [PubMed] [Google Scholar]
- 6. Ahn H, Kim DW, Ko Y, Ha J, Shin YB, Lee J, et al. Updated systematic review and meta‐analysis on diagnostic issues and the prognostic impact of myosteatosis: a new paradigm beyond sarcopenia. Ageing Res Rev 2021;70:101398, 10.1016/j.arr.2021.101398 [DOI] [PubMed] [Google Scholar]
- 7. Yamashita M, Kamiya K, Matsunaga A, Kitamura T, Hamazaki N, Nozaki K, et al. Low skeletal muscle density combined with muscle dysfunction predicts adverse events after adult cardiovascular surgery. Nutr Metab Cardiovasc Dis 2021;31:1782–1790. [DOI] [PubMed] [Google Scholar]
- 8. Fan E, Cheek F, Chlan L, Gosselink R, Hart N, Herridge MS, et al. An official American Thoracic Society Clinical Practice guideline: the diagnosis of intensive care unit‐acquired weakness in adults. Am J Respir Crit Care Med 2014;190:1437–1446. [DOI] [PubMed] [Google Scholar]
- 9. Huang DD, Ji YB, Zhou DL, Li B, Wang SL, Chen XL, et al. Effect of surgery‐induced acute muscle wasting on postoperative outcomes and quality of life. J Surg Res 2017;218:58–66. [DOI] [PubMed] [Google Scholar]
- 10. Kobayashi A, Kaido T, Hamaguchi Y, Okumura S, Taura K, Hatano E, et al. Impact of postoperative changes in sarcopenic factors on outcomes after hepatectomy for hepatocellular carcinoma. J Hepatobiliary Pancreat Sci 2016;23:57–64. [DOI] [PubMed] [Google Scholar]
- 11. Haines RW, Zolfaghari P, Wan Y, Pearse RM, Puthucheary Z, Prowle JR. Elevated urea‐to‐creatinine ratio provides a biochemical signature of muscle catabolism and persistent critical illness after major trauma. Intensive Care Med 2019;45:1718–1731. [DOI] [PubMed] [Google Scholar]
- 12. Lambell KJ, Goh GS, Tierney AC, Forsyth A, Nanjayya V, Nyulasi I, et al. Marked losses of computed tomography‐derived skeletal muscle area and density over the first month of a critical illness are not associated with energy and protein delivery. Nutrition 2021;82:111061, 10.1016/j.nut.2020.111061 [DOI] [PubMed] [Google Scholar]
- 13. Yeh DD, Ortiz‐Reyes LA, Quraishi SA, Chokengarmwong N, Avery L, Kaafarani HMA, et al. Early nutritional inadequacy is associated with psoas muscle deterioration and worse clinical outcomes in critically ill surgical patients. J Crit Care 2018;45:7–13. [DOI] [PubMed] [Google Scholar]
- 14. Nakamura K, Kihata A, Naraba H, Kanda N, Takahashi Y, Sonoo T, et al. Efficacy of belt electrode skeletal muscle electrical stimulation on reducing the rate of muscle volume loss in critically ill patients:a randomized controlled trial. J Rehabil Med 2019;51:705–711. [DOI] [PubMed] [Google Scholar]
- 15. Casaer MP, Langouche L, Coudyzer W, Vanbeckevoort D, De Dobbelaer B, Güiza FG, et al. Impact of early parenteral nutrition on muscle and adipose tissue compartments during critical illness. Crit Care Med 2013;41:2298–2309. [DOI] [PubMed] [Google Scholar]
- 16. Anderson DE, D'Agostino JM, Bruno AG, Demissie S, Kiel DP, Bouxsein ML. Variations of CT‐based trunk muscle attenuation by age, sex, and specific muscle. J Gerontol A Biol Sci Med Sci 2013;68:317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Amini B, Boyle SP, Boutin RD, Lenchik L. Approaches to assessment of muscle mass and myosteatosis on computed tomography (CT): a systematic review. J Gerontol A Biol Sci Med Sci 2019;74:1671–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Okita Y, Okada Y, Otsuji Y, Komeda M, Nakatani S, Matsuzaki M, et al. Guidelines for surgical and interventional treatment of valvular heart disease (JCS 2012). Jpn Circ J 2012;1–75. [Google Scholar]
- 19. JCS Joint Working Group . Guidelines for rehabilitation in patients with cardiovascular disease (JCS 2012). Circ J 2014;78:2022–2093. [DOI] [PubMed] [Google Scholar]
- 20. van Grinsven J, van Vugt JLA, Gharbharan A, Bollen TL, Besselink MG, van Santvoort HC, et al. The association of computed tomography‐assessed body composition with mortality in patients with necrotizing pancreatitis. J Gastrointest Surg 2017;21:1000–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dusseaux MM, Antoun S, Grigioni S, Béduneau G, Carpentier D, Girault C, et al. Skeletal muscle mass and adipose tissue alteration in critically ill patients. PLoS ONE 2019;14:e0216991, 10.1371/journal.pone.0216991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brewster DJ, Strauss BJ, Crozier TM. Measuring visceral fat, subcutaneous fat and skeletal muscle area changes by computed tomography in acute pancreatitis: a retrospective, single‐centre study. Crit Care Resusc 2014;16:42–47. [PubMed] [Google Scholar]
- 23. Reisinger KW, Bosmans JW, Uittenbogaart M, Alsoumali A, Poeze M, Sosef MN, et al. Loss of skeletal muscle mass during neoadjuvant chemoradiotherapy predicts postoperative mortality in esophageal cancer surgery. Ann Surg Oncol 2015;22:4445–4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Baggerman MR, van Dijk DPJ, Winkens B, Schnabel RM, van Gassel RJJ, Bol ME, et al. Edema in critically ill patients leads to overestimation of skeletal muscle mass measurements using computed tomography scans. Nutrition 2021;89:111238, 10.1016/j.nut.2021.111238 [DOI] [PubMed] [Google Scholar]
- 25. Demangel R, Treffel L, Py G, Brioche T, Pagano AF, Bareille MP, et al. Early structural and functional signature of 3‐day human skeletal muscle disuse using the dry immersion model. J Physiol 2017;595:4301–4315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Johnson MA, Polgar J, Weightman D, Appleton D. Data on the distribution of fibre types in thirty‐six human muscles. An autopsy study J Neurol Sci 1973;18:111–129. [DOI] [PubMed] [Google Scholar]
- 27. Pagano AF, Brioche T, Arc‐Chagnaud C, Demangel R, Chopard A, Py G. Short‐term disuse promotes fatty acid infiltration into skeletal muscle. J Cachexia Sarcopenia Muscle 2018;9:335–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dirks ML, Wall BT, van de Valk B, Holloway TM, Holloway GP, Chabowski A, et al. One week of bed rest leads to substantial muscle atrophy and induces whole‐body insulin resistance in the absence of skeletal muscle lipid accumulation. Diabetes 2016;65:2862–2875. [DOI] [PubMed] [Google Scholar]
- 29. Aas SN, Breit M, Karsrud S, Aase OJ, Rognlien SH, Cumming KT, et al. Musculoskeletal adaptations to strength training in frail elderly: a matter of quantity or quality? J Cachexia Sarcopenia Muscle 2020;11:663–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zoico E, Rossi A, Di Francesco V, Sepe A, Olioso D, Pizzini F, et al. Adipose tissue infiltration in skeletal muscle of healthy elderly men: relationships with body composition, insulin resistance, and inflammation at the systemic and tissue level. J Gerontol A Biol Sci Med Sci 2010;65:295–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vella CA, Nelson MC, Unkart JT, Miljkovic I, Allison MA. Skeletal muscle area and density are associated with lipid and lipoprotein cholesterol levels: the multi‐ethnic study of atherosclerosis. J Clin Lipidol 2020;14:143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Strassburg S, Springer J, Anker SD. Muscle wasting in cardiac cachexia. Int J Biochem Cell Biol 2005;37:1938–1947. [DOI] [PubMed] [Google Scholar]
- 33. Jolley SE, Bunnell AE, Hough CL. ICU‐acquired weakness. Chest 2016;150:1129–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zorowitz RD. ICU‐acquired weakness: a rehabilitation perspective of diagnosis, treatment, and functional management. Chest 2016;150:966–971. [DOI] [PubMed] [Google Scholar]
- 35. Anekwe DE, Biswas S, Bussières A, Spahija J. Early rehabilitation reduces the likelihood of developing intensive care unit‐acquired weakness: a systematic review and meta‐analysis. Physiotherapy 2020;107:1–10. [DOI] [PubMed] [Google Scholar]
- 36. Fuke R, Hifumi T, Kondo Y, Hatakeyama J, Takei T, Yamakawa K, et al. Early rehabilitation to prevent postintensive care syndrome in patients with critical illness: a systematic review and meta‐analysis. BMJ Open 2018;8:e019998, 10.1136/bmjopen-2017-019998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Reidy PT, McKenzie AI, Brunker P, Nelson DS, Barrows KM, Supiano M, et al. Neuromuscular electrical stimulation combined with protein ingestion preserves thigh muscle mass but not muscle function in healthy older adults during 5 days of bed rest. Rejuvenation Res 2017;20:449–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Smeuninx B, Elhassan YS, Manolopoulos KN, Sapey E, Rushton AB, Edwards SJ, et al. The effect of short‐term exercise prehabilitation on skeletal muscle protein synthesis and atrophy during bed rest in older men. J Cachexia Sarcopenia Muscle 2021;12:52–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. de Almeida AC, Aily JB, Pedroso MG, Gonçalves GH, de Carvalho Felinto J, Ferrari RJ, et al. A periodized training attenuates thigh intermuscular fat and improves muscle quality in patients with knee osteoarthritis: results from a randomized controlled trial. Clin Rheumatol 2020;39:1265–1275. [DOI] [PubMed] [Google Scholar]
- 40. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2021. J Cachexia Sarcopenia Muscle 2021;12:2259–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting information
Figure S1. An example of skeletal muscle measurement using computed tomography. These measurements belong to a 73‐year‐old woman undergoing medical treatment 1) at the time of admission (left side: before acute treatment), and 2) two days after admission to the intensive care unit (right side: after acute treatment). The top row shows plain images before measurement, the middle row shows images of all skeletal muscles measured, and the bottom row shows images coloured according to each type of skeletal muscle.
Table S1. Comparison of before and after computed tomography setting and body weight
Table S2. Changes in skeletal muscle before and after acute care in elective patients
Table S3. Changes in skeletal muscle before and after acute care in patients at emergency


