Abstract
Background
Interventions to preserve functional capacities at advanced age are becoming increasingly important. So far, exercise provides the only means to counteract age‐related decrements in physical performance and muscle function. Unfortunately, the effectiveness of exercise interventions in elderly populations is hampered by reduced acceptance and compliance as well as disuse complications. We therefore studied whether application of interleukin‐6 (IL‐6), a pleiotropic myokine that is induced by skeletal muscle activity and exerts broad systemic effects in response to exercise, affects physical performance and muscle function alone or in combination with training in aged mice.
Methods
Sedentary old male mice (Sed+Saline, n = 15) were compared with animals that received recombinant IL‐6 (rIL‐6) in an exercise‐mimicking pulsatile manner (Sed+IL‐6, n = 16), were trained with a moderate‐intensity, low‐volume endurance exercise regimen (Ex+Saline, n = 13), or were exposed to a combination of these two interventions (Ex+IL‐6, n = 16) for 12 weeks. Before and at the end of the intervention, mice underwent a battery of tests to quantify endurance performance, muscle contractility in situ, motor coordination, and gait and metabolic parameters.
Results
Mice exposed to enhanced levels of IL‐6 during endurance exercise bouts showed superior improvements in endurance performance (33% more work and 12% greater peak power compared with baseline), fatigue resistance in situ (P = 0.0014 vs. Sed+Saline; P = 0.0199 vs. Sed+IL‐6; and P = 0.0342 vs. Ex+Saline), motor coordination (rotarod performance, P = 0.0428), and gait (gait speed, P = 0.0053) following training. Pulsatile rIL‐6 treatment in sedentary mice had only marginal effects on glucose tolerance and some gait parameters. No increase in adverse events or mortality related to rIL‐6 treatment was observed.
Conclusions
Administration of rIL‐6 paired with treadmill running bouts potentiates the adaptive response to a moderate‐intensity low‐volume endurance exercise regimen in old mice, while being safe and well tolerated.
Keywords: Ageing, Muscle, Exercise, Myokine, Interleukin‐6, Frailty, Fatigue
Introduction
Besides neurodegenerative events, functional deterioration of skeletal muscle is one of the major causes for loss of independence, admission to nursing homes, morbidity, and mortality in the elderly. Because of the high costs associated with care of these patients, sarcopenia and frailty put an escalating burden on healthcare systems. 1 In light of an expanding global geriatric population, 2 effective interventions to preserve or improve physical performance and functional capacities at advanced ages are thus becoming increasingly important, on both the individual and societal levels.
Endurance exercise improves cardiorespiratory, neuromuscular, and metabolic function, as it requires successful integration of these systems. 3 Endurance training could thus provide a powerful countermeasure for muscular decline, loss of motor coordination and mobility, and metabolic deteriorations with ageing. 4 , 5 Unfortunately, however, several factors hamper the effectiveness of exercise‐based interventions, in particular in the elderly. First, at older age, muscle may show an attenuated immediate 6 and/or an abbreviated 7 response to mechanical loading and thus require more intense and/or more frequent workouts to significantly adapt. 8 Second, aged muscle may be more vulnerable to exercise‐induced injury compared with young muscle 9 , 10 and display decreased regenerative capacity, 11 which can limit exercise intensity, the amount of training, or the potential for improvement. Third, many elderly people have accumulated disuse complications and suffer from morbidity, frailty, and coordinative deficits, 12 which may not allow to manage or tolerate the strenuous or sustained training required to induce significant adaptations. Taken together, novel and effective strategies to leverage the beneficial effects of exercise training on physical performance and neuromuscular function in ageing are highly desirable as effective and safe pharmacological therapies are still lacking. 13
Myokines, signalling molecules produced and secreted by skeletal muscle, may play a pivotal role in meditating the positive muscular and systemic effects of exercise training, by their autocrine, paracrine, and/or endocrine action. 14 Interleukin‐6 (IL‐6), the founder member of the myokine family, increases up to 100‐fold in the circulation in response to exercise, leading to a systemic spike towards the end of or shortly after a single bout of exercise, while returning back to baseline quickly thereafter. 15 During exercise, IL‐6 acts as metabolic coordinator in the inter‐organ crosstalk, by promoting hepatic glucose production 16 and lipolysis in adipose tissue. 17 , 18 , 19 IL‐6 further facilitates the uptake and catabolism of energy substrates (i.e. glucose and fatty acids) in muscle fibres and thereby improves muscle function during single endurance exercise bouts. 20
Even though IL‐6 is a strong pro‐inflammatory cytokine important for an adequate immune response to infection, for example, by activating immune cells, mounting the acute response in the liver, or initiating the fever response in the hypothalamus, 21 potent systemic anti‐inflammatory effects have been attributed to IL‐6 when released as a myokine. 22 This dichotomy in IL‐6 function results in differential therapeutic avenues for pathological contexts. Anti‐IL‐6 agents are tested or used in the treatment of diseases characterized by a persistent, sterile inflammation, such as type 2 diabetes, multiple sclerosis, atherosclerosis, or rheumatoid arthritis. In comparison, the application of recombinant IL‐6 (rIL‐6) to mirror the anti‐inflammatory and hence potentially beneficial effects of IL‐6 as a myokine is still in its infancy for therapeutic use. For example, rIL‐6‐based therapy has been proposed to mitigate neuropathies associated with cancer chemotherapy 23 and diabetes. 24 , 25 Surprisingly, the use of rIL‐6 treatment mimicking the myokine function on age‐associated decrements in physical performance and muscle function, and the combination with exercise to facilitate training and achieve synergistic effects, has not been studied so far.
Given the impaired exercise training capacity and/or blunted adaptive response of aged muscle to exercise stimuli, coupled with the proposed role of IL‐6 in exercise adaptation, the aims of the current study were to investigate whether (i) a moderate‐intensity, low‐volume 12 week endurance exercise regimen is sufficient to improve functional capacities in old mice, (ii) the effect of the same intervention can be potentiated by exposing the animals to elevated IL‐6 levels during training sessions, and (iii) long‐term, pulsatile rIL‐6 treatment can elicit endurance training‐like effects in old sedentary mice in a safe and tolerable manner.
Methods
Animals
Aged and young male C57BL/6JRj mice were obtained from Janvier Labs (Le Genest‐Saint‐Isle, France) at an age of 19 and 5 months, respectively, and then kept under a 12 h light‐to‐dark cycle with light onset at 6 a.m. (Zeitgeber Time 0) at 23°C in the animal facility of the Biozentrum (Basel, Switzerland) until the end of the study. Mice were housed single caged with enrichment and received ad libitum access to regular chow and water. All experiments were approved by the veterinary office of the canton Basel‐Stadt (Switzerland) and performed in accordance with the Swiss federal guidelines for animal experimentation under consideration of the well‐being of the animals and the 3R (replace, reduce, and refine) principle.
Please see the Supporting Information for detailed experimental procedures.
Results
To study the adaptive response to endurance training in old mice and to test whether rIL‐6 can act as a therapeutic agent to induce exercise‐like effects or to potentiate training adaptation when combined with exercise bouts, we used aged male C57BL/6JRj mice as an experimental model. After a baseline characterization, mice were divided into four experimental groups at the age of 22 months (Figure 1A). Two groups were kept under sedentary conditions, one of which was subcutaneously injected with rIL‐6 (10 μg/kg) three times per week for 12 weeks to mimic the transient increase of IL‐6 plasma levels observed in response to single endurance exercise bouts (Sed+Saline, n = 15; Sed+IL‐6, n = 16). Two additional groups were engaged in a moderate‐intensity low‐volume treadmill training regimen, in one group as a single intervention and in the other group combined with rIL‐6 administration to enhance systemic IL‐6 levels during and shortly after each exercise bout (Ex+Saline, n = 13; Ex+IL‐6, n = 16). Maximum speed was 12 m/min in the first training session, which then was gradually increased every following session until 19 m/min (reached in the fifth week). Each training session lasted 45–50 min until all mice covered the same total distance.
Figure 1.
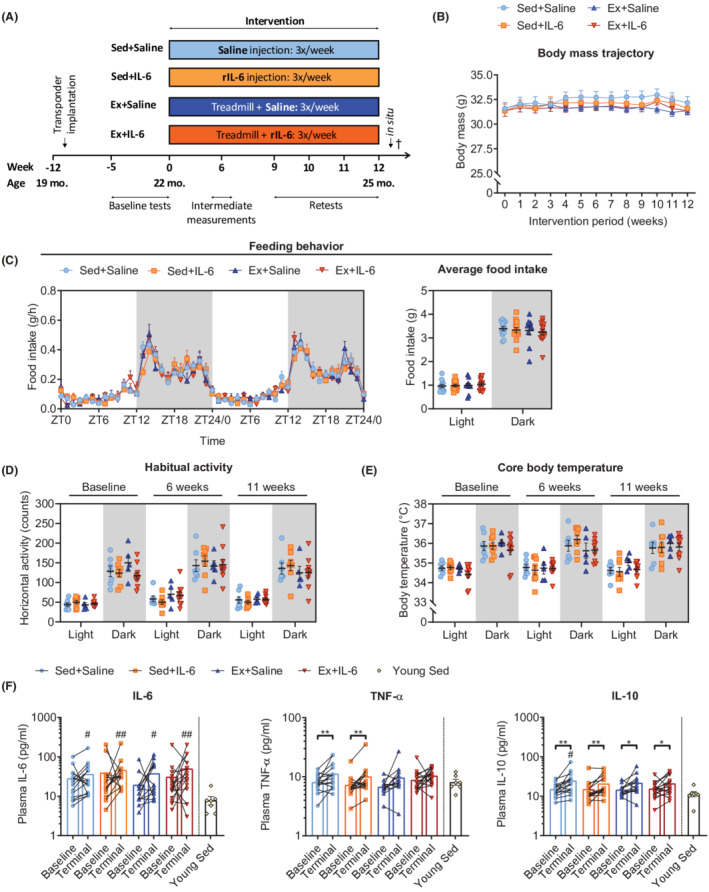
Long‐term rIL‐6 treatment is safe and well tolerated. (A) Graphical illustration of the experimental approach of treadmill training and/or rIL‐6 treatment. (B) Body mass trajectory for all four groups from start (0 weeks) to end (12 weeks) of the intervention (Sed+Saline, n = 15; Sed+IL‐6, n = 16; Ex+Saline, n = 13; and Ex+IL‐6, n = 16). (C) Feeding behaviour over 48 h (left panel; ZT, Zeitgeber time) and average food intake during light and dark phases (right panel) for this period assessed during Week 11 of the intervention (Sed+Saline, n = 13; Sed+IL‐6, n = 13; Ex+Saline, n = 10; and Ex+IL‐6, n = 15). (D) Habitual activity and (E) core body temperature during light and dark phases at baseline, in the 6th week and 11th week (Sed+Saline, n = 7; Sed+ IL‐6, n = 8; Ex+Saline, n = 5; and Ex+IL‐6, n = 10). (F) Plasma cytokine levels before (Baseline) and at the end (Terminal) of the study of interleukin‐6 (IL‐6), tumour necrosis factor‐α (TNF‐α), and interleukin‐10 (IL‐10) (Sed+Saline, n = 15; Sed+IL‐6, n = 15; Ex+Saline, n = 13; Ex+IL‐6, n = 16; and young Sed, n = 7). (F) Y‐axis has a logarithmic scale (log10). Data are presented as mean ± SEM (B, C) including individual values (right panel in C–E), mean, and individual paired values connected with a black line (F). (F) Paired Student's t‐test and one‐way ANOVA followed by Sidak's multiple comparisons of terminal measures of old groups and the young Sed group. *P < 0.05 and **P < 0.01. In (F): # P < 0.05 and ## P < 0.01 old groups vs. young Sed.
Long‐term recombinant interleukin‐6 treatment alone or in combination with exercise training is safe and well tolerated
Epidemiological evidence suggests a link between IL‐6 and age‐related diseases associated with chronic systemic low‐grade inflammation, 26 while there is a general lack of data regarding the safety of long‐term recurrent rIL‐6 administration. We therefore monitored general behaviour and well‐being and assessed metabolic and inflammatory profiles under habitual non‐exercise conditions. All four groups showed similar body mass trajectories throughout the study (Figure 1B). Comprehensive analysis of feeding behaviour (Figure 1C), habitual activity (Figure 1D), and several metabolic parameters [rate of oxygen consumption (V̇O2), carbon dioxide production (V̇CO2), respiratory exchange ratio (calculated as V̇CO2/V̇O2), and heat production; Figure S1A–S1D] revealed no significant differences between groups. In line with these observations, terminal plasma analyses of a suite of markers of blood biochemistry revealed no obvious metabolic changes or adverse effects of long‐term rIL‐6 treatment (Figure S2A). ALT and AST, two common markers to assess liver toxicity, were either unchanged or, in the case of ALT, even reduced in the Ex+IL‐6 group. Moreover, LDH was lowered by rIL‐6 and/or training, indicating reduced cellular damage in these mice.
Because IL‐6 has been implicated in fever generation upon peripheral immune challenge and inflammation, 27 we implanted small transponders into the abdominal cavity of mice to tightly monitor core body temperature throughout the study. Animals treated with rIL‐6 did not display higher body temperatures, suggesting that repeated rIL‐6 injections did not elicit persistent pyrogenic effects (Figure 1E). To gain further insights into whether rIL‐6 treatment and/or exercise affected the inflammatory status of the animals, we measured cytokines in the plasma at baseline and after 12 weeks of treatment. Resting IL‐6 levels remained constant in all four groups during the intervention and thus were not affected by regular rIL‐6 administration (Figure 1F). While interferon‐γ and IL‐1β levels also remained unchanged (Figure S2B), TNF‐α plasma concentrations increased significantly during the intervention, but only in the sedentary groups, whereas IL‐10 increased in all groups (Figure 1F). Comparison of terminal measures of the old groups with the values of an independent group of young sedentary mice revealed elevated levels of IL‐6 and IL‐1β in all old mice and IL‐10 in Sed+Saline mice (Figures 1F and S2B). Finally, we did not observe an increase in adverse events or mortality related to rIL‐6 treatment (Table S1). On the contrary, only one mouse in the rIL‐6‐treated groups (sedentary and exercised) died during the intervention, while seven animals died in the saline‐treated groups (two in the sedentary and five in the exercise group).
Elevated IL‐6 concentrations in response to exercise, as well as acute and short‐term rIL‐6 treatment, increased insulin secretion and glucose tolerance in young mice. 28 In the current study, exercise training resulted in significantly lower retest (in the ninth week of treatment) glucose tolerance test (GTT) curves compared with the Sed+Saline group, while rIL‐6 treatment alone failed to do so (Figure 2A). The corresponding area under the curves (AUCs) were not different between the two exercise groups; however, only the AUC of IL‐6 treated mice was significantly smaller compared with Sed+Saline mice (Figure 2B). Moreover, change from baseline AUC was negative only in the Ex+IL‐6 group (Figure S3A). Figure 2C–2F shows the GTT curve and corresponding AUC comparisons between the baseline measurements (−2 weeks) and the retests for the individual groups, in which only the Sed+Saline group showed a significant decrease in glucose tolerance.
Figure 2.
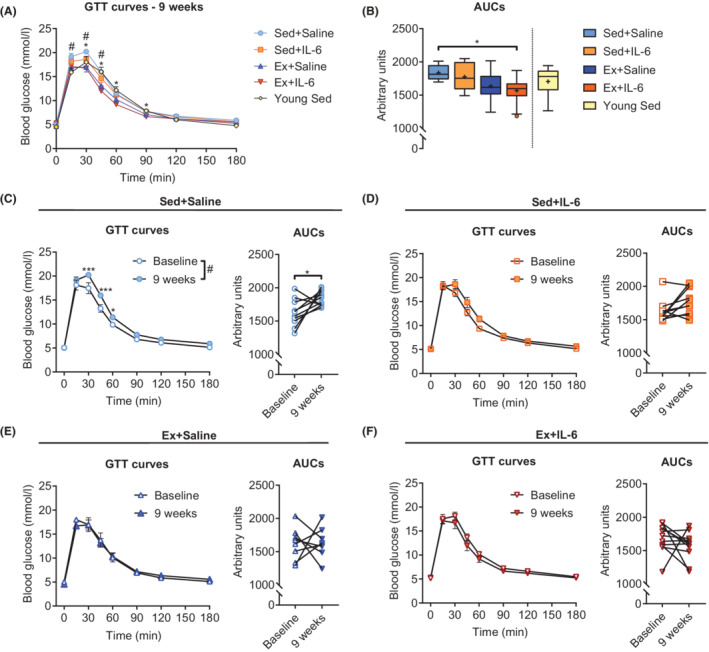
Effects of moderate‐intensity low‐volume endurance training on glucose tolerance. (A) Glucose tolerance test (GTT) curves and (B) corresponding area under the curves (AUCs) measured in the ninth week of treatment in the old groups and in young sedentary mice (Sed+Saline, n = 10; Sed+IL‐6, n = 10; Ex+Saline, n = 8; Ex+IL‐6, n = 11; and young Sed, n = 12). GTT curves and corresponding AUCs measured at baseline (−2 weeks) and after 9 weeks of treatment: (C) Sed+Saline group (n = 10), (D) Sed+IL‐6 group (n = 10), (E) Ex+Saline group (n = 8), and (F) Ex+IL‐6 group (n = 11). Data are presented as mean ± SEM (GTT curves), individual paired values connected with a black line (AUCs of C–F). Two‐way ANOVA (repeated measures) followed by Sidak's multiple comparisons for GTT curves (A, C–F), one‐way ANOVA followed by Sidak's multiple comparisons (B), and paired Student's t‐test for AUCs (C–F). In (A): *P < 0.05 Sed+Saline vs. Ex+IL‐6; # P < 0.05 Sed+Saline vs. Ex+Saline. In (C): # P < 0.05 result of the two‐way ANOVA for the column factor. *P < 0.05 and ***P < 0.001.
Body composition analysis revealed significantly reduced fat mass at 12 weeks compared with baseline in the two exercise groups (Figure S4A), whereas lean mass increased in all groups (Figure S4B), except for the Ex+IL‐6 group. This resulted in a significant overall reduction of body fat percentage after 12 weeks in the two exercise groups as well as in the Sed+IL‐6, but not the Sed+Saline group (Figure S4C). Interestingly, the Ex+IL‐6 group had significantly less epididymal white adipose tissue (eWAT, a visceral fat depot in mice) compared with the Sed+Saline group, while no difference in subcutaneous white adipose tissue (sWAT) was detected at the end of the study (Figure S4D). In accordance with the lean body mass measurements, no differences in individual limb muscle masses were observed at the end of the study (Figure S4E).
Elevated levels of interleukin‐6 during endurance training bouts improve treadmill running capacity and muscle fatigue resistance in situ
Endurance exercise capacity and its physiological determinants, such as peak oxygen uptake (V̇O2peak), are strong independent predictors of mortality 29 and decrease with age. 30 We therefore subjected mice to a short and intense ramp‐sprint protocol (Figure S5A) to determine V̇O2peak at baseline (−3 weeks) and in the 10th week of the intervention. Blood lactate levels of all four groups rose above 12 mmol/L when V̇O2peak plateaued, indicating that the animals reached their peak performance (Figure S5B). Old endurance‐trained mice showed higher retest V̇O2peak values (Figure 3A) resulting from a pronounced decline in the two sedentary groups (Figure S5E). However, V̇O2peak of the trained groups remained below the levels of young sedentary mice (Figure 3A), despite the absence of significant differences in speed reached and distance covered (Figure S5C and S5D). Of note, compared with young sedentary mice, delta lactate was higher in all groups except for the Ex+IL‐6 group (Figure S5F). While V̇O2peak is largely determined by the ability of the cardiorespiratory system to deliver oxygen to exercising muscles, 31 muscle intrinsic aspects, such as diffusive oxygen transport and metabolic processes, strongly determine submaximal endurance performance and fatigue resistance. We therefore challenged the same mice with a long duration incremental step protocol (Figure S5G) at baseline and at the end of the intervention (12 weeks). In this test, the two sedentary groups showed no change from baseline (Figures 3B–3D and S5H), indicating that despite the marked reduction in V̇O2peak, fatigue resistance at submaximal performance may be unaffected. Surprisingly, performance of the two exercise groups was strongly diverging. Mice that were exposed to higher levels of rIL‐6 during exercise training sessions clearly improved their running capacity, as running distance covered (Figure 3B) and time to exhaustion (Figure 3C) were both significantly longer compared with baseline. Moreover, these mice performed 33% more work (Figure 3D) and reached 12% greater peak power (Figure S5H) in the retest. In stark contrast, the Ex+Saline group did not improve in any of the assessed parameters and thus was more similar to the two sedentary control groups. In addition, measurements of blood lactate concentrations before (basal) and at the end (exhausted) of each test indicated a ‘lactate threshold’ decrease during the intervention, which was less pronounced when treated with rIL‐6 or endurance trained, and even absent when rIL‐6 and training were combined (Figures 3E, 3F, and S5I). Moreover, even though they performed 19% more work and had a 10% higher power output at exhaustion, Ex+IL‐6 mice accumulated significantly less lactate compared with Sed+Saline mice in the retest, and their delta lactate was not higher compared with young sedentary mice (Figures 3E and S5I). Western blot analysis for the lactate dehydrogenase heart subunit (LDH‐H, transcribed from the Ldhb gene) that drives the conversion of lactate to pyruvate revealed a higher abundance of this form in Ex+IL‐6 mice compared with the other old groups (Figure 3F), suggesting enhanced capacity for lactate clearance in these animals.
Figure 3.
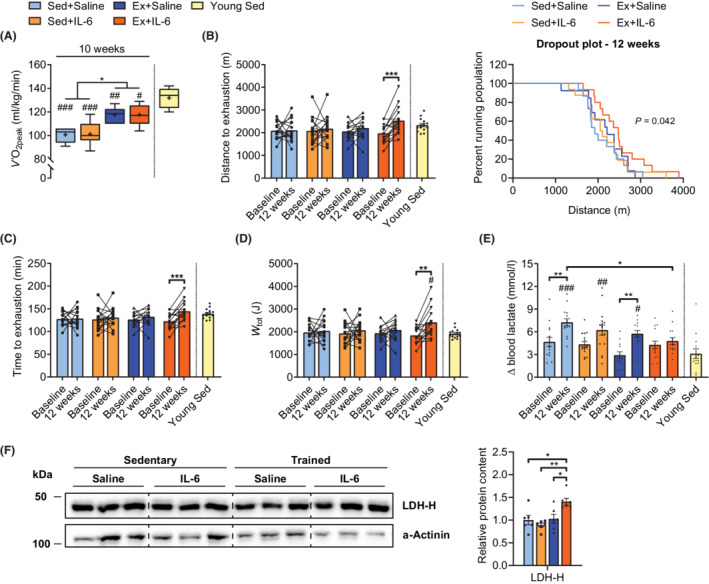
rIL‐6 treatment combined with moderate‐intensity low‐volume endurance training improves treadmill running capacity. (A) Peak oxygen uptake (V̇O2peak) in old groups in the 10th week and in an independent cohort of young sedentary mice reached during a short ramp‐sprint protocol (Sed+Saline, n = 6; Sed+IL‐6, n = 7; Ex+Saline, n = 6; Ex+IL‐6, n = 5; and young Sed, n = 14). (B–E) Parameters assessed with a long duration incremental step protocol: (B) distance covered until exhaustion at baseline and after 12 weeks (retest) in old groups and young sedentary mice (left panel) and Kaplan–Meier plot for the retest showing the percentage of mice running at indicated distances (right panel). (C) Time to exhaustion and (D) total work (W tot) performed at baseline and after 12 weeks in the older groups and in young sedentary mice. (E) Blood lactate levels expressed as the difference exhausted‐basal (Δ) at baseline and after 12 weeks in older groups and in young sedentary mice. (B–E) Sed+Saline, n = 15; Sed+IL‐6, n = 16; Ex+Saline, n = 13; Ex+IL‐6, n = 15; and young Sed, n = 14. (F) Representative western blot of M. quadriceps femoris lactate dehydrogenase heart subunit (LDH‐H) and corresponding band quantification. LDH‐H values are normalized to α‐actinin and expressed relative to Sed+Saline (n = 6 per group). Data are presented as box and whiskers in (A), as mean and individual paired values connected with a black line (B–D), or mean ± SEM including individual values (Young Sed group in B–F). One‐way ANOVA followed by Sidak's multiple comparisons (A, E and F), paired Student's t‐test (B–D), and log‐rank test for trend (B). *P < 0.05, **P < 0.01, and ***P < 0.001 as indicated. # P < 0.05, ## P < 0.01, and ### P < 0.001 old groups vs. Young Sed group.
To further characterize muscle intrinsic aspects, we assessed in situ fatigability and contractile properties of the M. tibialis anterior by sciatic nerve stimulation at the end of the study. In response to a 4‐min fatigue protocol, the Ex+IL‐6 group showed the slowest drop in force production, leading to a clear separation of its fatigue curve from the curves of the other old groups, very reminiscent of young sedentary mice (Figure 4A). Intriguingly, the resulting AUC (Figure 4B) of the Ex+IL‐6 group was not only significantly larger compared with the two sedentary groups but also compared with the Ex+Saline group, which suggests that additional IL‐6 during training sessions was required to increase fatigue resistance in response to the 12 week endurance training programme in the M. tibialis anterior. There was no difference in area of the curve of the recovery period between the older groups (Figures 4A and S6A). Of note, the observed gain in muscular endurance was not at the expense of muscle force, as grip strength measures in vivo (Figure S6B) as well as single twitch and tetanic forces in situ (Figure S6C–S6F) were similar in all groups. Furthermore, contraction and relaxation velocities did not differ between groups (Figure S6G and S6H).
Figure 4.
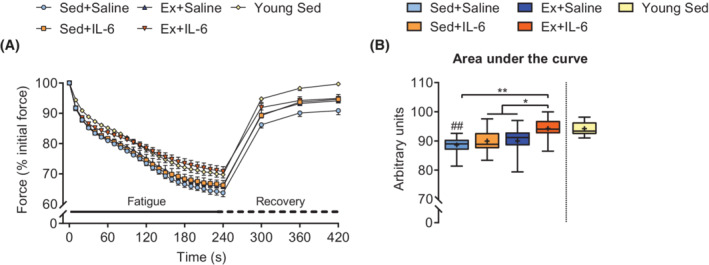
rIL‐6 treatment combined with moderate‐intensity low‐volume endurance training improves muscle fatigue resistance in situ. (A) Average curves showing the force decline during a 4‐min in situ muscle fatigue protocol on the M. tibialis anterior and of muscle force recovery up to 3 min after fatigue with (B) area under the curves corresponding to the force decline during muscle fatigue (Sed+Saline, n = 14; Sed+IL‐6, n = 15; Ex+Saline, n = 12; Ex+IL‐6, n = 14; and young Sed, n = 9). One‐way ANOVA followed by Sidak's multiple comparisons (B). *P < 0.05 and **P < 0.01 as indicated. ## P < 0.01 old groups vs. Young Sed group.
Administration of recombinant interleukin‐6 during endurance training bouts improves gait and motor coordination in old mice
Walking speed is a strong independent predictor of life expectancy, 32 and unsteady gait and deficits in motor coordination largely contribute to the increased risk of falls and the ensuing devastating health consequences in elderly adults. We thus used the CatWalk XT voluntary gait analysis system and a rotarod‐based test to examine gait and motor coordination. Compared with baseline, gait speed remained constant in both sedentary groups after 12 weeks (Figure 5A), but the number of steps per second (cadence) appeared to decrease significantly in the Sed+Saline group (Figure 5B). Moreover, compared with young sedentary mice, gait speed and cadence were reduced in Sed+Saline mice (Figure 5A and 5B). The decrease in cadence was mainly due to an increased duration of step cycle (sum of swing and stand phase, Figure S7A), which was driven by a longer duration of swing phase of both hindlimbs and forelimbs (Figure S7C and S7F). The slower limb movement in old sedentary mice at the end of the study was not observed in rIL‐6‐treated and/or endurance‐trained mice. Moreover, rIL‐6 administration during exercise bouts increased gait speed significantly after 12 weeks of training, whereas exercise alone did not (Figure 5A). While cadence remained unchanged, overall (i.e. hindlimbs and forelimbs combined) stride length increased significantly in both exercise groups (Figure 5B and 5C), suggesting that exercise induced an increase in step size, which was, however, not sufficient to improve gait speed. The additional increase in swing speed of forelimbs (Figure S7B) and decrease in stand phase of hindlimbs (Figure S7G) achieved by Ex+IL‐6 mice, together with the increase in hindlimb swing phase in the Ex+Saline group (Figure S7F), might explain the observed discrepancy in gait speed. In addition, Ex+IL‐6 mice reduced the base of support of their forepaws (Figure 5D), which is indicative of a more confident and secure gait. In accordance with the observed changes in voluntary gait, the Ex+IL‐6 group was the only group that improved rotarod performance within the treatment period and even reached the level of young sedentary mice (Figures 5E and S7I).
Figure 5.
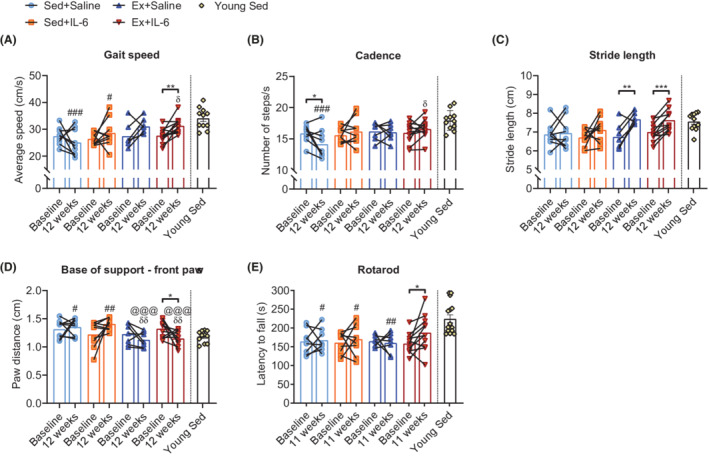
rIL‐6 treatment improves gait and motor coordination in endurance‐trained mice. (A–E) Parameters of quantitative gait analysis at baseline and after 12 weeks of the intervention in old groups and in an independent cohort of young sedentary mice: (A) average body speed (cm/s), (B) cadence (number of steps/s), (C) average stride length (in cm) of hindlimbs and forelimbs, and (D) base of support, that is, distance (cm) between paws of forelimbs (Sed+Saline, n = 9; Sed+IL‐6, n = 9; Ex+Saline, n = 7; Ex+IL‐6, n = 11; and young Sed, n = 11). (E) Motor coordination assessed with an accelerated rotarod test at baseline and in the 11th week of the intervention in old groups and in young sedentary mice (Sed+Saline, n = 8; Sed+IL‐6, n = 9; Ex+Saline, n = 7; Ex+IL‐6, n = 11; and young Sed, n = 14). Data are presented as mean and individual paired values connected with a black line and mean ± SEM including individual values (Young Sed group in A–E). Paired Student's t‐test and one‐way ANOVA followed by Sidak's multiple comparisons between retests of old groups and young sedentary group. *P < 0.05, **P < 0.01, and ***P < 0.001 as indicated. # P < 0.05, ## P < 0.01, and ### P < 0.001 old groups vs. young Sed group. @@@ P < 0.001 Sed+Saline vs. Ex+Saline or Ex+IL‐6; δδ P < 0.01 Sed+IL‐6 vs. Ex+Saline or Ex+IL‐6.
Exercised mice treated with recombinant interleukin‐6 show increased expression of mitochondrial complex I components and pyruvate dehydrogenase kinase 4
The fact that only Ex+IL‐6 mice improved fatigue resistance in situ together with the observation that rIL‐6 increased running performance in vivo without affecting V̇O2peak suggested that IL‐6 potentiates skeletal muscle intrinsic adaptations to training. To get more insights on molecular changes, we assessed mRNA expression of metabolic genes in the M. quadriceps femoris, a muscle heavily used during treadmill running and displaying mixed fibre‐type composition. This analysis revealed no strong transcriptional differences between groups in key components of the electron transport chain except for complex I (Figure 6A). Moreover, genes encoding for proteins involved in fatty acid transport, synthesis, and oxidation, as well as components involved in glucose metabolism and storage, remained unaffected by either intervention (Figure S8A and S8B). In contrast, pyruvate dehydrogenase kinase 4 (Pdk4), a gene encoding for a kinase that inactivates pyruvate dehydrogenase (PDH), which in turn is a positive regulator of carbohydrate‐derived energy substrate utilization in mitochondria, 33 was up‐regulated in muscles of Ex+IL‐6 mice (Figure 6B). Both the PDH component subunit E1 alpha (Pdha1) and pyruvate dehydrogenase phosphatase 1 (Pdp1, encoding a PDH activating phosphatase) showed similar expression in all groups.
Figure 6.
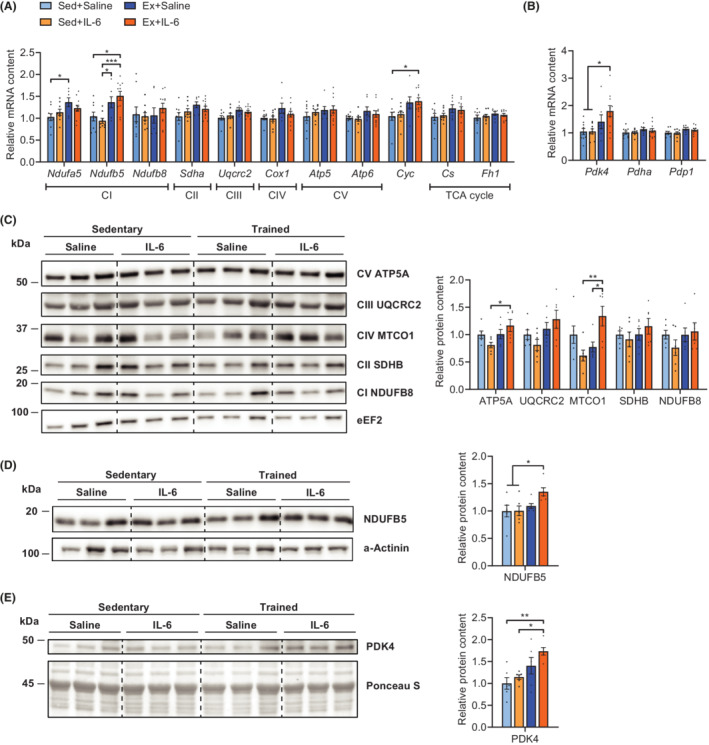
rIL‐6‐treated endurance‐trained mice show increased expression of mitochondrial complex I components and PDK4. Relative M. quadriceps femoris mRNA levels of (A) mitochondrial genes of the electron transport chain [complex (C) I–V, Cyc] and tricarboxylic acid (TCA) cycle and (B) of pyruvate dehydrogenase kinase 4 (Pdk4), pyruvate dehydrogenase E1 component subunit alpha (Pdha1), and pyruvate dehydrogenase phosphatase 1 (Pdp1). Sed+Saline, n = 9; Sed+IL‐6, n = 9; Ex+Saline, n = 7; and Ex+IL‐6, n = 11. Expression values were determined by qPCR and normalized to Tbp. Data are shown as the mean fold change ± SEM including individual values relative to the expression of the control (Sed+Saline) set to 1. Relative M. quadriceps femoris protein levels of OXPHOS (C), NDUFB5 (D), and PDK4 (E) assessed by western blot (n = 6 per group). Target band intensities were normalized to the loading control (eEF2, α‐actinin, or Ponceau S stain), and data are shown as the mean fold change ± SEM including individual values relative to the control (Sed+Saline) set to 1. One‐way ANOVA followed by Sidak's multiple comparisons (A–E). *P < 0.05, **P < 0.01, and ***P < 0.001.
To determine whether the transcriptional status is reflecting protein levels at the time of analysis, we performed western blots for OXPHOS proteins and PDK4 with tissue from the same muscles. Ex+IL‐6 mice showed higher complex IV and V protein abundance in the OXPHOS blot (Figure 6C). An additional western blot for the complex I component NDUFB5, which showed clearest difference in gene expression, revealed a slight up‐regulation in the Ex+IL‐6 group compared with the two sedentary groups (Figure 6D). Also, in accordance with gene expression, PDK4 protein levels were highest in IL‐6‐treated exercised mice (Figure 6E). The increase in OXPHOS proteins and enhanced levels of PDK4 in skeletal muscle, together with elevated LDH‐H, is indicative of a boosted oxidative capacity and lactate as well as fatty acid‐derived substrate flux, which may provide performance advantage during prolonged exercise at the submaximal level. Of note, total resting levels of the downstream effector protein of IL‐6 signalling, signal transducer and activator of transcription 3 (STAT3), were increased in skeletal muscle by both rIL‐6 treatment and endurance training (Figure S8C).
Discussion
The age‐associated decline in skeletal muscle function is one of the main drivers of loss of independence, admission to nursing homes, increased risk for chronic diseases, morbidity, and mortality in the elderly. The only efficacious method to prevent and mitigate sarcopenia, frailty, and other pathologies associated with this functional decline is exercise based, using both resistance training to address loss in muscle mass and strength, and endurance training to improve cardiovascular function, fatigue, and frailty. Unfortunately, training interventions are notoriously difficult to implement and adhere to, in the general population, but due to pre‐existing frailty, impaired gait as well as reduced balance and motor coordination, co‐morbidities, and other events, even more so in older individuals. 34 We therefore assessed how a low‐volume, moderate‐intensity endurance training could be combined with the application of rIL‐6 to achieve synergy in improving the functional capacity of skeletal muscle in old mice.
Notably, even when only performed at old age, and despite the low volume, endurance training conferred potent beneficial effects on various parameters, most of which could not be recapitulated by the pulsatile administration of rIL‐6 as performed in this study. Importantly, however, when combined with training, rIL‐6 powerfully enhanced a broad spectrum of biological programmes that are impaired in ageing. Indeed, following 12 weeks of training, Ex+IL‐6 mice displayed higher endurance performance and prolonged contractions at higher relative force in situ, which excelled the effects of training. The combined treatment thereby most efficiently improved running capacity and fatigability in old mice. Second, the Ex+IL‐6 group showed ameliorated gait and motor coordination, none of which could be improved to a similar extent in mice trained without rIL‐6 administration. Even though increased muscular endurance is important for motor coordination, neuronal changes (e.g. vestibular and proprioceptive input, nerve conduction velocity, or neuromuscular junction functionality) and motor planning are additional crucial components that could potentially be affected by IL‐6. Intriguingly, rIL‐6 treatment has been shown to be protective in chemotherapy‐induced and diabetes‐induced neuropathies, 23 , 24 , 25 alluding to a direct effect of rIL‐6 on neuronal integrity and potentially function. This potential therapeutic effect of rIL‐6 in combination with exercise on skeletal muscle‐autonomous as well as systemic functions is underlined by the third outcome, the moderate beneficial effect on glucose tolerance and body composition. The sedentary group showed a decrease in glucose tolerance during the intervention, which was not observed when treated with rIL‐6 or endurance trained. Even though there was no difference in retest GTT AUCs between the two sedentary or exercise groups, the combination of rIL‐6 and training significantly lowered GTT AUC compared with the Sed+Saline group. Glucose tolerance is indicative of metabolic flexibility, and its impairments are a hallmark of the age‐related metabolic syndrome. Often, insulin sensitivity and glucose tolerance are closely linked to body composition. In our cohorts, both training groups exhibited a reduction in fat mass, but only in the combination group (Ex+IL‐6) a significant decrease in the mass of eWAT was observed. In contrast to sWAT, excessive eWAT has been linked to pro‐inflammatory events and the development of insulin resistance and type 2 diabetes. 35
Despite the equal values of V̇O2peak, submaximal treadmill running capacity increased remarkably in animals that were exposed to higher levels of IL‐6 during single training bouts, whereas endurance training alone was not sufficient to elevate any of the measured parameters. Therefore, the two exercise groups may differ in the proportion of V̇O2peak (i.e. the rate of ‘fractional utilization’) sustained during prolonged exercise. The fact that Ex+IL‐6 mice had less lactate accumulation at exhaustion despite the higher power output supports the notion that adaptations within skeletal muscle are responsible for the improved performance, as higher lactate thresholds are often associated with training‐induced increases in skeletal muscle oxidative capacity and optimized production, disposal, and clearance of lactate. 36
In skeletal muscle, rIL‐6 treatment elevated basal levels of its downstream target STAT3, similar to what was observed in endurance‐trained mice. Endurance exercise‐induced STAT3 activation is necessary for the beneficial effects of training on exercise performance and glucose homeostasis. 37 While STAT3 exerts many effects in different cells, a direct regulatory link between this transcription factor and metabolic genes might exist. For example, low Stat3 expression was associated with low Pdk4 expression in human prostate tumours, and subsequent analyses of several chromatin immunoprecipitation DNA‐sequencing (ChIP‐Seq) data sets showed binding of STAT3 to the promoter region of Pdk4. 38 In addition, ChIP assays on control and shSTAT3 cells with or without prior IL‐6 stimulation showed highest STAT3 levels and Pdk4 promoter region binding with IL‐6 stimulation and a reduction with Stat3 knockdown. 38 In line with these reports, exercise combined with rIL‐6 up‐regulated resting levels of PDK4 gene and protein expression in skeletal muscle in our study. PDK4 is an inhibitor of PDH, which in turn is a positive regulator of carbohydrate‐derived energy substrate utilization in mitochondria. Higher PDK4 levels may thus allow a rapid or more efficient up‐regulation of fatty acid oxidation during exercise and thereby providing performance advantage by sparing glycogen. It has previously been shown that a single injection of rIL‐6 decreases skeletal muscle PDH activity in mice in the fed state 39 and that skeletal muscle PDH activity is higher in muscle‐specific IL‐6 knockouts compared with controls at rest and at 60 min of exercise. 40 Concomitant with elevated PDK4, the combination of training and rIL‐6 increased the transcript and protein levels of OXPHOS components and those of LDH‐H in our study. Collectively, this metabolic remodelling could favour more efficient oxidation of lipids and lactate and thereby contribute to the higher endurance and fatigue resistance in this group.
Many therapeutic approaches aim at neutralizing the pro‐inflammatory effects of IL‐6, and hence, application of exogenous rIL‐6 could theoretically be regarded as an unsafe approach. To mitigate potential deleterious effects of persistently elevated IL‐6 as observed in many chronic diseases, we therefore aimed at a pulsatile, low‐dose application replicating the regulation of systemic IL‐6 as a myokine during and after acute exercise bouts. Because IL‐6 levels return to baseline within ~2 h after injection, 28 IL‐6 was indeed not chronically elevated in terminal plasma measurements in our mice. Second, the absence of any specific change in other pro‐inflammatory cytokines, for example, TNF‐α, IL‐1β, or interferon‐γ, in the rIL‐6‐treated groups compared with the levels in the control groups indicates that the application of rIL‐6 did not induce a pro‐inflammatory environment. This conclusion is supported by the absence of any pyrogenic activity, modulation of spontaneous locomotion, body mass, or food intake by rIL‐6. Furthermore, inconspicuous ALT and AST levels in the rIL‐6 groups indicate normal liver health, and the reduction in plasma LDH by rIL‐6 and/or training could imply an even reduced cellular damage in these mice. Collectively, these data demonstrate that a pulsatile, low‐dose administration of rIL‐6 over 12 weeks, with or without concomitant endurance training, is safe and well tolerated and lacks any discernable adverse effects in old mice.
Conclusions
Our results demonstrate that the application of pulsatile, low‐dose rIL‐6 can potentiate the beneficial effects of a low‐volume endurance training on muscle intrinsic and systemic parameters in old mice. Of note, no detectable adverse effects of this treatment were observed, implying that such an intervention is safe and well tolerated. Even though future studies will aim at a careful investigation of sarcopenia, healthspan, and lifespan, the present data demonstrate functional improvements in endurance performance, fatigue resistance, gait and motor coordination, and potential benefits on glucose tolerance. Thus, if this intervention can be translated to elderly individuals, rIL‐6 could not only facilitate training interventions, and hence increase adherence and compliance, but also directly result in a massive improvement of quality of life by affecting gait and motor coordination. Importantly, fatigability, a key hallmark of human frailty, 41 could likewise be significantly mitigated. Collectively, these improvements could alleviate insecurity, avoidance of physical activity and exercise, falls and the ensuing fractures and hospitalizations, and the loss of independence as well as admission to nursing homes delayed. Clinical trials and safety studies with rIL‐6 have already been performed in non‐geriatric individuals. Therefore, following a careful evaluation of safety, tolerability, and adverse effects in the elderly, the use of combined rIL‐6 training interventions in the prevention and treatment of age‐associated functional decline could be initiated in a relatively short amount of time and help to overcome the reduced training response or even exercise intolerance often observed in this population. 41
Conflict of interest
Y.S. is a consultant for Sonnet Biotherapeutics CH SA, a company promoting the development of low‐dose IL‐6 treatments for peripheral neuropathies. The other authors declare no competing interests.
Ethics statement
All procedures involving animals were approved by the veterinary office of the canton Basel‐Stadt (Switzerland) and performed in accordance with the Swiss federal guidelines for animal experimentation under consideration of the well‐being of the animals and the 3R (replace, reduce, and refine) principle. The manuscript does not contain clinical studies or patient data.
Funding
This research was supported by the Innosuisse ‐ Schweizerische Agentur für Innovationsförderung Grant 44112.1 IP‐LS, Swiss National Science Foundation (Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung), the European Research Council (ERC) Consolidator Grant 616830‐MUSCLE_NET, Swiss Cancer Research (Krebsforschung Schweiz) Grant KFS‐3733‐08‐2015, the Swiss Society for Research on Muscle Diseases (SSEM), SystemsX.ch, the Novartis Stiftung für Medizinisch‐Biologische Forschung, and the University of Basel (Universität Basel).
Supporting information
Data S1. Supporting Information
Figure S1. Equal metabolic parameters under non‐exercise conditions. (A) Oxygen uptake (V̇O2), (B) carbon dioxide uptake (V̇CO2), (C) respiratory exchange ratio (RER; V̇CO2/V̇O2) and (D) heat production over 48 h (left panels; ZT: Zeitgeber time) and averaged for light and dark phase of this period (right panels) during week 11 of the intervention (Sed+Saline, n = 13; Sed+IL‐6, n = 13; Ex+Saline, n = 10; Ex+IL‐6, n = 15). Data are presented as mean ± SEM (left panels A‐D) including individual values (right panels in A‐D).
Figure S2. Normal blood biochemistry in rIL‐6 treated mice. (A) Blood plasma levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin, lactate dehydrogenase (LDH), total cholesterol, low‐density lipoprotein (LDL), high‐density lipoprotein (HDL), triglycerides and lipase for all four groups after 12 weeks of treatment. (B) Plasma levels of interferon‐gamma (INF‐γ) and interleukin‐1 beta (IL‐1β) before (Baseline) and at the end (Terminal) of the study and of an independent cohort of young sedentary mice (Sed+Saline, n = 15; Sed+IL‐6, n = 15; Ex+Saline, n = 13; Ex+IL‐6, n = 16; Young Sed, n = 7). (B) Y‐axis has a logarithmic scale (log 10). Data are presented as box and whiskers (A) or individual paired values connected with a black line (B). One‐way ANOVA followed by Sidak's multiple comparisons (A), paired Student's t‐test and one‐way ANOVA followed by Sidak's multiple comparisons of Terminal measures of old groups and the Young Sed group (B). *P < 0.05. In (J and K): ## P < 0.01, ### P < 0.001 old groups vs. Young Sed group.
Figure S3. Moderate‐intensity low‐volume endurance training preserves glucose tolerance with aging. Relative change from baseline area under the curve (Sed+Saline, n = 10; Sed+IL‐6, n = 10; Ex+Saline, n = 8; Ex+IL‐6, n = 11; Young Sed, n = 12).
Figure S4. Effects of moderate‐intensity low‐volume endurance training and/or rIL‐6 treatment on body composition. (A) Absolute fat mass at baseline and after 12 weeks of the intervention (left) and relative fat mass change from baseline to 12 weeks in percent (right). (B) Absolute lean mass at baseline and after 12 weeks (left) and relative lean mass change from baseline to 12 weeks in percent (right). (C) Percent body fat at baseline and after 12 weeks. (A‐C) Sed+Saline, n = 6; Sed+IL‐6, n = 7; Ex+Saline, n = 6; Ex+IL‐6, n = 6. (D) Fat depot masses upon dissection of epididymal white adipose tissue (eWAT) and anterior subcutaneous white adipose tissue (sWAT). (E) Individual muscle masses of M. quadriceps femoris (QUAD), M. gastrocnemius (GAS), M. triceps brachii (TRI), M. extensor digitorum longus (EDL), M. soleus (SOL) and M. plantaris (PLAN) upon dissection (Sed+Saline, n = 15; Sed+IL‐6, n = 16; Ex+Saline, 11 n = 13; Ex+IL‐6, n = 16). Data are presented as mean and individual paired values connected with a black line (left panel in A and B and in C), box and whiskers (right panel in A and B), or individual values and mean ± SEM (F and G). Paired Student's t‐test (left panel in A and B and in C) and one‐way ANOVA followed by Sidak's multiple comparisons (individual tissues in D). *P < 0.05, **P < 0.01, ***P < 0.001 as indicated.
Figure S5. Treadmill protocols and additional corresponding measurements. (A) Graphical representation of the ramp‐sprint protocol used to assess peak oxygen uptake (V̇O2peak). (B) Blood lactate levels before (Basal) and after (Exhausted) the ramp‐sprint test at baseline and in the 10th week (retest) in the old groups. (C) Peak speed reached and (D) distance covered until exhaustion during the retest (10 weeks) in old groups and in an independent young sedentary group. (E) Relative V̇O2peak change from baseline (–3 weeks) to 10 weeks in percent. (F) Increase in blood lactate expressed as the difference exhausted‐basal (Δ) during the retest (10 weeks) and in young sedentary mice. (B‐F) Sed+Saline, n = 6; Sed+IL‐6, n = 7; Ex+Saline, n = 6; Ex+IL‐6, n = 5; Young Sed, n = 14. (G) Graphical representation of the long duration incremental step protocol used to evaluate running capacity. (H) Peak power (Ppeak) achieved at baseline and after 12 weeks in old groups and in young sedentary mice. (I) Blood lactate levels in basal and exhausted state. (H and I) Sed+Saline, n = 15; Sed+IL‐6, n = 16; Ex+Saline, n = 13; Ex+IL‐6, n = 15; Young Sed, n = 14. Data are presented as mean and paired individual values connected with a black line (A and B), mean ± SEM (E) including individual values (H), and box and whiskers (C, D, E, F). One‐way ANOVA followed by Sidak's multiple comparisons (B‐F, H and I) and paired Student's t‐test (H). In (B and I) ***P < 0.001 basal vs. exhausted within group; # P < 0.05, ### P < 0.001 Baseline exhausted vs. 12 weeks exhausted within group; In (C, D, F and H) # P < 0.05 ## P < 0.01 ### P < 0.001, Young Sed vs. older groups. In (E) * or # P < 0.05, Ex‐IL‐6 vs. Sed + Saline or Sed+IL‐6; ** or ## P < 0.01 Ex+Saline vs. Sed+Saline or Sed+IL‐6. In (E) δP < 0.05, δδδP < 0.001 Sed + Saline 12 weeks exhausted vs. Ex+Saline or Ex+IL‐6 12 weeks exhausted.
Figure S6. No effect of moderate‐intensity low‐volume endurance training and/or rIL‐6 treatment on contractile properties in situ and grip strength in vivo. (A) Area of the curves (AOCs) of the recovery period following the fatigue protocol. (B) Normalized in vivo muscle force estimated by measuring peak force of whole limb grip (kgf, kilogram‐force; one kgf is equal to 9.806650 N). Electrical sciatic nerve stimulation evoked (C) absolute muscle force‐frequency relationship, (D) specific twitch force and (E) specific tetanic force. (F) Masses and lengths of the M. tibialis anterior used to calculate specific forces. (G) Single twitch time‐to‐peak tension and (H) single twitch half‐relaxation time. Sed+Saline, n = 14; Sed+IL‐6, n = 15; Ex+Saline, n = 12; Ex+IL‐6, n = 14, Young Sed, n = 9. Data are presented as box and whiskers (A, B, D, E, G and H), mean ± SEM (C) including 12 individual values (F). One‐way ANOVA followed by Sidak's multiple comparisons (C). # P < 0.05 Young Sed vs. old groups.
Figure S7. Additional parameters obtained with the CatWalk voluntary gait analysis system and Rotarod test. (A‐H) Additional parameters of quantitative voluntary gait analysis at baseline and after 12 weeks of the intervention and an independent cohort of young sedentary mice: (A‐F) Swing speed (cm/s), time (s) of swing phase and time (s) of stand phase of hind limbs (A‐C) and fore limbs (D‐F). (G) Average time (s) of a step cycle and (H) base of support i.e., distance (cm) between paws of hind limbs. (A‐H) Sed+Saline, n = 9; Sed+IL‐6, n = 9; Ex+Saline, n = 7; Ex+IL‐6, n = 11; Young Sed, n = 11. (I) Motor coordination assessed by challenging mice with an accelerated Rotarod test at baseline and in the 11th week of the intervention (Sed+Saline, n = 8; Sed+IL‐6, n = 9; Ex+Saline, n = 7; Ex+IL‐6, n = 11; Young Sed, n = 14). Data are presented as mean and individual paired values connected with a black line. Paired Student's t‐test and one‐way ANOVA followed by Sidak's multiple comparisons of retest of old groups and young sedentary group. *P < 0.05, **P < 0.01; # P < 0.05, ## P < 0.01, ### P < 0.001 Young Sed vs. old groups.
Figure S8. Expression of fatty acid and glucose metabolism related genes. Relative M. quadriceps femoris mRNA levels of genes involved in (A) fatty acid transport, synthesis and oxidation and (B) glucose metabolism (Sed+Saline, n = 9; Sed+IL‐6, n = 9; Ex+Saline, n = 7; Ex+IL‐6, n = 11). Expression values were determined by qPCR and normalized to Tbp. Data are presented as the mean fold‐change ± SEM relative to the expression of the control (Sed+Saline) set to 1. (C) Relative M. quadriceps femoris total STAT3 protein levels assed by western blot (n = 6 per group). Target band intensities were normalized to the loading control (Ponceau S stain) and data are shown as the mean fold‐change ± SEM including individual values relative to the control (Sed+Saline) set to 1. One‐way ANOVA followed by Sidak's multiple comparisons (C). **P < 0.01, ***P < 0.001.
Table S1. Dying and excluded animals
Table S2. Primer sequences used for qPCR
Acknowledgements
We thank M. Donath, M. Böni‐Schnetzler, and the rest of their group for providing equipment and assistance with the cytokine measurements. We also thank J. Delezie for the help with the telemetric data acquisition and interpretation. The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. 42
Leuchtmann A. B., Furrer R., Steurer S. A., Schneider‐Heieck K., Karrer‐Cardel B., Sagot Y., and Handschin C. (2022) Interleukin‐6 potentiates endurance training adaptation and improves functional capacity in old mice, Journal of Cachexia, Sarcopenia and Muscle, 13, 1164–1176, 10.1002/jcsm.12949
References
- 1. Pinedo‐Villanueva R, Westbury LD, Syddall HE, Sanchez‐Santos MT, Dennison EM, Robinson SM, et al. Health care costs associated with muscle weakness: a UK population‐based estimate. Calcif Tissue Int 2019;104:137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Harper S. Economic and social implications of aging societies. Science 2014;346:587–591. [DOI] [PubMed] [Google Scholar]
- 3. Harridge SD, Lazarus NR. Physical activity, aging, and physiological function. Physiology (Bethesda) 2017;32:152–161. [DOI] [PubMed] [Google Scholar]
- 4. Pedersen BK, Saltin B. Exercise as medicine—evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sports 2015;25:1–72. [DOI] [PubMed] [Google Scholar]
- 5. Garatachea N, Pareja‐Galeano H, Sanchis‐Gomar F, Santos‐Lozano A, Fiuza‐Luces C, Moran M, et al. Exercise attenuates the major hallmarks of aging. Rejuvenation Res 2015;18:57–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kumar V, Selby A, Rankin D, Patel R, Atherton P, Hildebrandt W, et al. Age‐related differences in the dose–response relationship of muscle protein synthesis to resistance exercise in young and old men. J Physiol 2009;587:211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. West DWD, Marcotte GR, Chason CM, Juo N, Baehr LM, Bodine SC, et al. Normal ribosomal biogenesis but shortened protein synthetic response to acute eccentric resistance exercise in old skeletal muscle. Front Physiol 2018;9:1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bickel CS, Cross JM, Bamman MM. Exercise dosing to retain resistance training adaptations in young and older adults. Med Sci Sports Exerc 2011;43:1177–1187. [DOI] [PubMed] [Google Scholar]
- 9. Hughes DC, Marcotte GR, Baehr LM, West DWD, Marshall AG, Ebert SM, et al. Alterations in the muscle force transfer apparatus in aged rats during unloading and reloading: impact of microRNA‐31. J Physiol 2018;596:2883–2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brooks SV, Faulkner JA. The magnitude of the initial injury induced by stretches of maximally activated muscle fibres of mice and rats increases in old age. J Physiol 1996;497:573–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Joanisse S, Nederveen JP, Snijders T, McKay BR, Parise G. Skeletal muscle regeneration, repair and remodelling in aging: the importance of muscle stem cells and vascularization. Gerontology 2017;63:91–100. [DOI] [PubMed] [Google Scholar]
- 12. Hung WW, Ross JS, Boockvar KS, Siu AL. Recent trends in chronic disease, impairment and disability among older adults in the United States. BMC Geriatr 2011;11:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Leuchtmann AB, Handschin C. Pharmacological targeting of age‐related changes in skeletal muscle tissue. Pharmacol Res 2019;154:104191. 10.1016/j.phrs.2019.02.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Leuchtmann AB, Adak V, Dilbaz S, Handschin C. The role of the skeletal muscle secretome in mediating endurance and resistance training adaptations. Front Physiol 2021;12:709807. 10.3389/fphys.2021.709807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle‐derived interleukin‐6. Physiol Rev 2008;88:1379–1406. [DOI] [PubMed] [Google Scholar]
- 16. Febbraio MA, Hiscock N, Sacchetti M, Fischer CP, Pedersen BK. Interleukin‐6 is a novel factor mediating glucose homeostasis during skeletal muscle contraction. Diabetes 2004;53:1643–1648. [DOI] [PubMed] [Google Scholar]
- 17. van Hall G, Steensberg A, Sacchetti M, Fischer C, Keller C, Schjerling P, et al. Interleukin‐6 stimulates lipolysis and fat oxidation in humans. J Clin Endocrinol Metab 2003;88:3005–3010. [DOI] [PubMed] [Google Scholar]
- 18. Petersen EW, Carey AL, Sacchetti M, Steinberg GR, Macaulay SL, Febbraio MA, et al. Acute IL‐6 treatment increases fatty acid turnover in elderly humans in vivo and in tissue culture in vitro. Am J Physiol Endocrinol Metab 2005;288:E155–E162. [DOI] [PubMed] [Google Scholar]
- 19. Wedell‐Neergaard AS, Lang Lehrskov L, Christensen RH, Legaard GE, Dorph E, Larsen MK, et al. Exercise‐induced changes in visceral adipose tissue mass are regulated by IL‐6 signaling: a randomized controlled trial. Cell Metab 2019;29:844, e3–855. [DOI] [PubMed] [Google Scholar]
- 20. Chowdhury S, Schulz L, Palmisano B, Singh P, Berger JM, Yadav VK, et al. Muscle‐derived interleukin 6 increases exercise capacity by signaling in osteoblasts. J Clin Invest 2020;130:2888–2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Munoz‐Canoves P, Scheele C, Pedersen BK, Serrano AL. Interleukin‐6 myokine signaling in skeletal muscle: a double‐edged sword? FEBS J 2013;280:4131–4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Petersen AM, Pedersen BK. The anti‐inflammatory effect of exercise. J Appl Physiol (1985) 2005;98:1154–1162. [DOI] [PubMed] [Google Scholar]
- 23. Callizot N, Andriambeloson E, Glass J, Revel M, Ferro P, Cirillo R, et al. Interleukin‐6 protects against paclitaxel, cisplatin and vincristine‐induced neuropathies without impairing chemotherapeutic activity. Cancer Chemother Pharmacol 2008;62:995–1007. [DOI] [PubMed] [Google Scholar]
- 24. Andriambeloson E, Baillet C, Vitte PA, Garotta G, Dreano M, Callizot N. Interleukin‐6 attenuates the development of experimental diabetes‐related neuropathy. Neuropathology 2006;26:32–42. [DOI] [PubMed] [Google Scholar]
- 25. Cameron NE, Cotter MA. The neurocytokine, interleukin‐6, corrects nerve dysfunction in experimental diabetes. Exp Neurol 2007;207:23–29. [DOI] [PubMed] [Google Scholar]
- 26. Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age‐associated diseases. J Gerontol A Biol Sci Med Sci 2014;69:S4–S9. [DOI] [PubMed] [Google Scholar]
- 27. Nilsberth C, Elander L, Hamzic N, Norell M, Lonn J, Engstrom L, et al. The role of interleukin‐6 in lipopolysaccharide‐induced fever by mechanisms independent of prostaglandin E2 . Endocrinology 2009;150:1850–1860. [DOI] [PubMed] [Google Scholar]
- 28. Ellingsgaard H, Hauselmann I, Schuler B, Habib AM, Baggio LL, Meier DT, et al. Interleukin‐6 enhances insulin secretion by increasing glucagon‐like peptide‐1 secretion from L cells and alpha cells. Nat Med 2011;17:1481–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med 2002;346:793–801. [DOI] [PubMed] [Google Scholar]
- 30. Wilson TM, Tanaka H. Meta‐analysis of the age‐associated decline in maximal aerobic capacity in men: relation to training status. Am J Physiol Heart Circ Physiol 2000;278:H829–H834. [DOI] [PubMed] [Google Scholar]
- 31. Bassett DR Jr, Howley ET. Limiting factors for maximum oxygen uptake and determinants of endurance performance. Med Sci Sports Exerc 2000;32:70–84. [DOI] [PubMed] [Google Scholar]
- 32. Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, et al. Gait speed and survival in older adults. JAMA 2011;305:50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Harris RA, Bowker‐Kinley MM, Huang B, Wu P. Regulation of the activity of the pyruvate dehydrogenase complex. Adv Enzyme Regul 2002;42:249–259. [DOI] [PubMed] [Google Scholar]
- 34. Billot M, Calvani R, Urtamo A, Sanchez‐Sanchez JL, Ciccolari‐Micaldi C, Chang M, et al. Preserving mobility in older adults with physical frailty and sarcopenia: opportunities, challenges, and recommendations for physical activity interventions. Clin Interv Aging 2020;15:1675–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yudkin JS, Eringa E, Stehouwer CD. “Vasocrine” signalling from perivascular fat: a mechanism linking insulin resistance to vascular disease. Lancet 2005;365:1817–1820. [DOI] [PubMed] [Google Scholar]
- 36. San‐Millan I, Brooks GA. Assessment of metabolic flexibility by means of measuring blood lactate, fat, and carbohydrate oxidation responses to exercise in professional endurance athletes and less‐fit individuals. Sports Med 2018;48:467–479. [DOI] [PubMed] [Google Scholar]
- 37. Knudsen NH, Stanya KJ, Hyde AL, Chalom MM, Alexander RK, Liou YH, et al. Interleukin‐13 drives metabolic conditioning of muscle to endurance exercise. Science 2020;368. 10.1126/science.aat3987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Oberhuber M, Pecoraro M, Rusz M, Oberhuber G, Wieselberg M, Haslinger P, et al. STAT3‐dependent analysis reveals PDK4 as independent predictor of recurrence in prostate cancer. Mol Syst Biol 2020;16:e9247. 10.15252/msb.20199247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Biensø RS, Knudsen JG, Brandt N, Pedersen PA, Pilegaard H. Effects of IL‐6 on pyruvate dehydrogenase regulation in mouse skeletal muscle. Pflugers Arch 2014;466:1647–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gudiksen A, Schwartz CL, Bertholdt L, Joensen E, Knudsen JG, Pilegaard H. Lack of skeletal muscle IL‐6 affects pyruvate dehydrogenase activity at rest and during prolonged exercise. PLoS One 2016;11:e0156460. 10.1371/journal.pone.0156460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lewsey SC, Weiss K, Schar M, Zhang Y, Bottomley PA, Samuel TJ, et al. Exercise intolerance and rapid skeletal muscle energetic decline in human age‐associated frailty. JCI Insight 2020;5. 10.1172/jci.insight.141246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle update 2021. J Cachexia Sarcopenia Muscle 2021;12:2259–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting Information
Figure S1. Equal metabolic parameters under non‐exercise conditions. (A) Oxygen uptake (V̇O2), (B) carbon dioxide uptake (V̇CO2), (C) respiratory exchange ratio (RER; V̇CO2/V̇O2) and (D) heat production over 48 h (left panels; ZT: Zeitgeber time) and averaged for light and dark phase of this period (right panels) during week 11 of the intervention (Sed+Saline, n = 13; Sed+IL‐6, n = 13; Ex+Saline, n = 10; Ex+IL‐6, n = 15). Data are presented as mean ± SEM (left panels A‐D) including individual values (right panels in A‐D).
Figure S2. Normal blood biochemistry in rIL‐6 treated mice. (A) Blood plasma levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin, lactate dehydrogenase (LDH), total cholesterol, low‐density lipoprotein (LDL), high‐density lipoprotein (HDL), triglycerides and lipase for all four groups after 12 weeks of treatment. (B) Plasma levels of interferon‐gamma (INF‐γ) and interleukin‐1 beta (IL‐1β) before (Baseline) and at the end (Terminal) of the study and of an independent cohort of young sedentary mice (Sed+Saline, n = 15; Sed+IL‐6, n = 15; Ex+Saline, n = 13; Ex+IL‐6, n = 16; Young Sed, n = 7). (B) Y‐axis has a logarithmic scale (log 10). Data are presented as box and whiskers (A) or individual paired values connected with a black line (B). One‐way ANOVA followed by Sidak's multiple comparisons (A), paired Student's t‐test and one‐way ANOVA followed by Sidak's multiple comparisons of Terminal measures of old groups and the Young Sed group (B). *P < 0.05. In (J and K): ## P < 0.01, ### P < 0.001 old groups vs. Young Sed group.
Figure S3. Moderate‐intensity low‐volume endurance training preserves glucose tolerance with aging. Relative change from baseline area under the curve (Sed+Saline, n = 10; Sed+IL‐6, n = 10; Ex+Saline, n = 8; Ex+IL‐6, n = 11; Young Sed, n = 12).
Figure S4. Effects of moderate‐intensity low‐volume endurance training and/or rIL‐6 treatment on body composition. (A) Absolute fat mass at baseline and after 12 weeks of the intervention (left) and relative fat mass change from baseline to 12 weeks in percent (right). (B) Absolute lean mass at baseline and after 12 weeks (left) and relative lean mass change from baseline to 12 weeks in percent (right). (C) Percent body fat at baseline and after 12 weeks. (A‐C) Sed+Saline, n = 6; Sed+IL‐6, n = 7; Ex+Saline, n = 6; Ex+IL‐6, n = 6. (D) Fat depot masses upon dissection of epididymal white adipose tissue (eWAT) and anterior subcutaneous white adipose tissue (sWAT). (E) Individual muscle masses of M. quadriceps femoris (QUAD), M. gastrocnemius (GAS), M. triceps brachii (TRI), M. extensor digitorum longus (EDL), M. soleus (SOL) and M. plantaris (PLAN) upon dissection (Sed+Saline, n = 15; Sed+IL‐6, n = 16; Ex+Saline, 11 n = 13; Ex+IL‐6, n = 16). Data are presented as mean and individual paired values connected with a black line (left panel in A and B and in C), box and whiskers (right panel in A and B), or individual values and mean ± SEM (F and G). Paired Student's t‐test (left panel in A and B and in C) and one‐way ANOVA followed by Sidak's multiple comparisons (individual tissues in D). *P < 0.05, **P < 0.01, ***P < 0.001 as indicated.
Figure S5. Treadmill protocols and additional corresponding measurements. (A) Graphical representation of the ramp‐sprint protocol used to assess peak oxygen uptake (V̇O2peak). (B) Blood lactate levels before (Basal) and after (Exhausted) the ramp‐sprint test at baseline and in the 10th week (retest) in the old groups. (C) Peak speed reached and (D) distance covered until exhaustion during the retest (10 weeks) in old groups and in an independent young sedentary group. (E) Relative V̇O2peak change from baseline (–3 weeks) to 10 weeks in percent. (F) Increase in blood lactate expressed as the difference exhausted‐basal (Δ) during the retest (10 weeks) and in young sedentary mice. (B‐F) Sed+Saline, n = 6; Sed+IL‐6, n = 7; Ex+Saline, n = 6; Ex+IL‐6, n = 5; Young Sed, n = 14. (G) Graphical representation of the long duration incremental step protocol used to evaluate running capacity. (H) Peak power (Ppeak) achieved at baseline and after 12 weeks in old groups and in young sedentary mice. (I) Blood lactate levels in basal and exhausted state. (H and I) Sed+Saline, n = 15; Sed+IL‐6, n = 16; Ex+Saline, n = 13; Ex+IL‐6, n = 15; Young Sed, n = 14. Data are presented as mean and paired individual values connected with a black line (A and B), mean ± SEM (E) including individual values (H), and box and whiskers (C, D, E, F). One‐way ANOVA followed by Sidak's multiple comparisons (B‐F, H and I) and paired Student's t‐test (H). In (B and I) ***P < 0.001 basal vs. exhausted within group; # P < 0.05, ### P < 0.001 Baseline exhausted vs. 12 weeks exhausted within group; In (C, D, F and H) # P < 0.05 ## P < 0.01 ### P < 0.001, Young Sed vs. older groups. In (E) * or # P < 0.05, Ex‐IL‐6 vs. Sed + Saline or Sed+IL‐6; ** or ## P < 0.01 Ex+Saline vs. Sed+Saline or Sed+IL‐6. In (E) δP < 0.05, δδδP < 0.001 Sed + Saline 12 weeks exhausted vs. Ex+Saline or Ex+IL‐6 12 weeks exhausted.
Figure S6. No effect of moderate‐intensity low‐volume endurance training and/or rIL‐6 treatment on contractile properties in situ and grip strength in vivo. (A) Area of the curves (AOCs) of the recovery period following the fatigue protocol. (B) Normalized in vivo muscle force estimated by measuring peak force of whole limb grip (kgf, kilogram‐force; one kgf is equal to 9.806650 N). Electrical sciatic nerve stimulation evoked (C) absolute muscle force‐frequency relationship, (D) specific twitch force and (E) specific tetanic force. (F) Masses and lengths of the M. tibialis anterior used to calculate specific forces. (G) Single twitch time‐to‐peak tension and (H) single twitch half‐relaxation time. Sed+Saline, n = 14; Sed+IL‐6, n = 15; Ex+Saline, n = 12; Ex+IL‐6, n = 14, Young Sed, n = 9. Data are presented as box and whiskers (A, B, D, E, G and H), mean ± SEM (C) including 12 individual values (F). One‐way ANOVA followed by Sidak's multiple comparisons (C). # P < 0.05 Young Sed vs. old groups.
Figure S7. Additional parameters obtained with the CatWalk voluntary gait analysis system and Rotarod test. (A‐H) Additional parameters of quantitative voluntary gait analysis at baseline and after 12 weeks of the intervention and an independent cohort of young sedentary mice: (A‐F) Swing speed (cm/s), time (s) of swing phase and time (s) of stand phase of hind limbs (A‐C) and fore limbs (D‐F). (G) Average time (s) of a step cycle and (H) base of support i.e., distance (cm) between paws of hind limbs. (A‐H) Sed+Saline, n = 9; Sed+IL‐6, n = 9; Ex+Saline, n = 7; Ex+IL‐6, n = 11; Young Sed, n = 11. (I) Motor coordination assessed by challenging mice with an accelerated Rotarod test at baseline and in the 11th week of the intervention (Sed+Saline, n = 8; Sed+IL‐6, n = 9; Ex+Saline, n = 7; Ex+IL‐6, n = 11; Young Sed, n = 14). Data are presented as mean and individual paired values connected with a black line. Paired Student's t‐test and one‐way ANOVA followed by Sidak's multiple comparisons of retest of old groups and young sedentary group. *P < 0.05, **P < 0.01; # P < 0.05, ## P < 0.01, ### P < 0.001 Young Sed vs. old groups.
Figure S8. Expression of fatty acid and glucose metabolism related genes. Relative M. quadriceps femoris mRNA levels of genes involved in (A) fatty acid transport, synthesis and oxidation and (B) glucose metabolism (Sed+Saline, n = 9; Sed+IL‐6, n = 9; Ex+Saline, n = 7; Ex+IL‐6, n = 11). Expression values were determined by qPCR and normalized to Tbp. Data are presented as the mean fold‐change ± SEM relative to the expression of the control (Sed+Saline) set to 1. (C) Relative M. quadriceps femoris total STAT3 protein levels assed by western blot (n = 6 per group). Target band intensities were normalized to the loading control (Ponceau S stain) and data are shown as the mean fold‐change ± SEM including individual values relative to the control (Sed+Saline) set to 1. One‐way ANOVA followed by Sidak's multiple comparisons (C). **P < 0.01, ***P < 0.001.
Table S1. Dying and excluded animals
Table S2. Primer sequences used for qPCR


