Abstract
Background
Routinely used cardiac medications, based on pharmacokinetics, are hypothesized to increase drug levels of direct oral anticoagulants (DOACs), with the potential to increase the risk of hemorrhage. We set out to compare the risk for hemorrhage following initiation of amiodarone, verapamil, or diltiazem (moderate cytochrome P450 3A4 and/or P-glycoprotein activity) vs metoprolol or amlodipine (weak or no activity), among older adults prescribed DOACs.
Methods
We conducted a population-based, retrospective cohort study of all adults (aged ≥ 66 years) on a DOAC (dabigatran, apixaban, rivaroxaban; n = 295,038) who were newly prescribed amiodarone (n = 4872), verapamil (n = 1284), or diltiazem (n = 14,638), compared with metoprolol or amlodipine, from Ontario, Canada (2009-2016). The outcome was hospital admission or emergency room visit with a major hemorrhage (upper or lower gastrointestinal tract, intracranial), examined using weighted models.
Results
A total of 1737 hemorrhage events occurred (amiodarone, 80 [1.6%] vs metoprolol 503 [2.3%]; verapamil, 32 [2.5%] vs amlodipine, 406 [1.6%]; diltiazem, 312 [2.1%] vs amlodipine, 404 [1.5%]). The weighted risk of major hemorrhage was not elevated with amiodarone, verapamil, or diltiazem initiation in DOAC users, compared to metoprolol or amlodipine, during the full follow-up period (hazard ratio [HR; 95% confidence interval]: amiodarone HR 0.77 [0.61-0.97]; verapamil HR 1.32 [0.88-1.98]; diltiazem HR 0.99 [0.85-1.15]). This finding was consistent with a broader definition of bleeding, adjusting for kidney function, by DOAC type or dosage.
Conclusions
Hemorrhage risk with amiodarone, verapamil, and diltiazem was similar to that with comparators, among DOAC users aged > 66 years.
Résumé
Contexte
Les médicaments cardiaques couramment utilisés, selon la pharmacocinétique, devraient théoriquement augmenter les taux d’anticoagulants oraux directs (AOD), ce qui s'accompagne d'un risque accru d’hémorragie. Nous avons entrepris de comparer le risque d’hémorragie après l’instauration de l’amiodarone, du vérapamil ou du diltiazem (activité modérée du cytochrome P450 3A4 ou de la P-glycoprotéine) par rapport au métoprolol ou à l’amlodipine (activité faible ou nulle), chez des personnes âgées à qui l’on avait prescrit des AOD.
Méthodologie
Nous avons mené une étude de cohortes rétrospective en population auprès de tous les adultes (âgés de 66 ans et plus) prenant un AOD (dabigatran, apixaban, rivaroxaban; n = 295 038) à qui l’on venait de prescrire de l’amiodarone (n = 4872), du vérapamil (n = 1284) ou du diltiazem (n = 14 638), comparativement au métoprolol ou à l’amlodipine, en Ontario, au Canada (2009-2016). Le critère d’évaluation était une admission à l’hôpital ou une consultation à l’urgence pour une hémorragie grave (voie gastro-intestinale supérieure ou inférieure, intracrânienne), examiné à l’aide de mo-dèles pondérés.
Résultats
Au total, 1 737 événements hémorragiques sont survenus (amiodarone, 80 [1,6 %] contre métoprolol, 503 [2,3 %]; vérapamil, 32 [2,5 %] contre amlodipine, 406 [1,6 %]; diltiazem, 312 [2,1 %] contre amlodipine, 404 [1,5 %]). Le risque pondéré d’hémorragie grave ne s’est pas accru avec l’instauration de l’amiodarone, du vérapamil ou du diltiazem chez les utilisateurs d’AOD, comparativement au métoprolol ou à l’amlodipine, pendant toute la période de suivi (rapport des risques instantanés [RRI; intervalle de confiance à 95 %] : amiodarone : RRI 0,77 [0,61-0,97]; vérapamil : RRI 1,32 [0,88-1,98]; diltiazem : RRI 0,99 [0,85-1,15]). Ce résultat concorde avec une définition plus large du saignement, après ajustement pour la fonction rénale, par type ou posologie d’AOD.
Conclusions
Le risque d’hémorragie associé à l’amiodarone, au vérapamil et au diltiazem était semblable à celui des médicaments de comparaison chez les utilisateurs d’AOD âgés de plus de 66 ans.
Direct oral anticoagulants (DOACs) are a class of commonly prescribed anticoagulants used in the prevention of stroke in atrial fibrillation and in the treatment and prevention of venous thrombosis.1, 2, 3, 4 Many patients who require anticoagulation for atrial fibrillation or venous thrombosis are concurrently treated with medications to stabilize their heart rate and rhythm.5, 6, 7, 8, 9, 10, 11, 12, 13 Although DOACs have fewer drug–drug interactions than vitamin-K antagonists (VKAs), interactions still exist that can alter drug concentrations, efficacy, and safety and result in increased risks of thrombosis and bleeding.8,14, 15, 16, 17, 18 Both events result in significant morbidity and mortality.
Several studies have investigated the pharmacokinetics/pharmacodynamics of DOACs when exposed to inhibitors/inducers of their metabolism and excretion.17,19, 20, 21, 22, 23, 24 Rivaroxaban, apixaban, and dabigatran are excreted by permeability glycoprotein (P-gp), with rivaroxaban and apixaban additionally metabolized in the liver by the cytochrome P450 3A4 (CYP3A4) pathway.8,25, 26, 27, 28 Commonly utilized cardiovascular anti-arrhythmic agents, such as amiodarone, verapamil, and diltiazem, are proposed to interfere with similar metabolic pathways.11,12,29, 30, 31 Verapamil, diltiazem, and amiodarone are all moderate inhibitors of both CYP3A4 and P-gp activity, and the latter has been shown to increase the area under the concentration–time curve (AUC) for DOACs by 36% to > 100%, as well as their peak serum concentrations (Cmax) by 40% to 61%.5,7,10,11,29,30,32 Verapamil and diltiazem are reported to increase AUC and Cmax for DOACS by 196% and 40%, and 250% and 31%, respectively.17,21,22,33,34 Despite the literature demonstrating increases in anticoagulant serum concentration levels, the reported clinical implications of these interactions are inconsistent.6,12 As a result, product monographs and published guidelines provide differing, and in some cases conflicting, recommendations on the management of patients who are taking these medications concomitantly.1,2,26, 27, 28,35,36
Given the limited information regarding the clinical significance of the interactions between DOACs and amiodarone, verapamil, and diltiazem, we conducted a retrospective observational study to determine the relative risk of bleeding in patients exposed to a DOAC concurrently with one of these medications. We selected 2 similar, commonly prescribed medications (metoprolol and amlodipine) to act as our active comparators to amiodarone and our calcium-channel blockers (CCBs), respectively.10 These medications were selected because they demonstrate no or minimal influence on P-gp/CYP3A4 activity.10 We hypothesized that DOAC users concurrently prescribed amiodarone, verapamil, or diltiazem would experience a higher risk of clinically significant bleeding compared with DOAC users concurrently prescribed metoprolol or amlodipine.
Methods
Data sources
We used encoded, linked databases housed at the ICES (see Supplemental Table S1 for a description of databases used in this study). Demographics and vital status information were obtained from the Ontario Registered Persons Database. Medication information was obtained from the Ontario Drug Benefit (ODB) Program claims database. Ontario is Canada’s largest province, with over 14 million residents.37 All citizens have access to universal public healthcare with drug coverage for individuals over the age of 65 years. This database contains highly accurate records of all outpatient prescriptions dispensed to patients aged 65 years or older, with an error rate of < 1%.38 Diagnostic and procedural information from all hospitalizations was determined using the Canadian Institute for Health Information Discharge Abstract Database (CIHI-DAD). Diagnostic information from emergency room visits was determined using the National Ambulatory Care Reporting System (NACRS). Information was also obtained from the Ontario Health Insurance Plan (OHIP) database, which contains all claims for inpatient and outpatient physician services. Whenever possible, we defined patient characteristics and outcomes using validated codes. The use of data in this project was authorized under section 45 of Ontario’s Personal Health Information Protection Act, which does not require review by a research ethics board. The reporting of this study follows guidelines for observational studies (see Supplemental Table S2).39
Study design
We compared all DOAC users who received a prescription for a new cardiovascular (CV) drug of interest with all DOAC users who received a prescription for a new active comparator using cohort study designs.40 Individuals were followed until either death, an outcome event, the end of follow-up, DOAC drug switching or discontinuation, or CV medication switching or discontinuation plus 30 days (as treated analysis). The study population included all adults ≥ 66 years of age from June 23, 2009 (first date DOACs were added to the Ontario Drug Formulary) to December 31, 2016, in Ontario, Canada (see Supplemental Fig. S1 for cohort creation). Prescription drug information is available for all adults > 65 years of age in Ontario, and we initiated our cohort at the 66-year age cutoff to allow for a 1-year look-back period for existing medications. We identified an exposed cohort of individuals who received a new prescription for a DOAC (apixaban, dabigatran, rivaroxaban). We then identified a subset of patients who received a new prescription of either amiodarone, diltiazem, or verapamil (exposures of interest), or of metoprolol (active comparator for amiodarone) or amlodipine (active comparator for diltiazem and verapamil; see Supplemental Table S3 for all drug definitions used in this study). Metoprolol is a commonly used cardio-selective beta-blocker used for rate control with atrial fibrillation. Amlodipine, similar to verapamil and diltiazem, is also a calcium channel–blocking agent with weak CYP3A4/P-gp activity. Patients previously on any of the CV medications of interest prior to DOAC use were excluded (new-user design; 1 year look-back).41 Patients on any of the CV medications of interest other than the pair studied (active drug and its comparator) were excluded (120-day look-back). Patients could start a DOAC on the same day as a CV medication of interest. The CV medication dispensing date served as the study index date, and patients with prior use of other potent CYP3A4 or P-gp inhibitors (90-day look-back from index; medications included azole antifungals, tacrolimus, cyclosporine, quinines, and rifampin; see Supplemental Table S4) were excluded.42 Patients were included only once in the study and could not be part of multiple treatment groups if they were started on 2 medications of interest during the study period. Drug discontinuation was defined as no refill within 1.5 times the original prescription duration plus 90 days. Individuals on dialysis or with a kidney transplant were excluded.
Covariates
Potential confounders examined included the following: demographics (age, sex, income, place of residence); index year; comorbid illnesses (history of hemorrhage, hypertension, diabetes, stroke, atrial fibrillation, acute coronary syndrome, heart failure, coronary artery disease, coronary artery bypass grafting, percutaneous coronary intervention, peripheral vascular disease, venous thromboembolism); healthcare utilization (number of hospitalizations and emergency room visits in preceding 5 years); medications (beta-blocker, nonsteroidal anti-inflammatory drugs (NSAIDs); proton pump inhibitors; antiplatelet agents (selective serotonin-reuptake inhibitors, and statins); and DOAC type, dose, and duration of use prior to CV medication.
Outcomes
The study outcome was a hospital admission or emergency room visit with major hemorrhage after dispensing of the CV medication of interest (see Supplemental Table S5 for outcome definitions). The following types of hemorrhage were included in the outcome of major hemorrhage: upper or lower gastrointestinal; intracerebral; subarachnoid; and other nontraumatic intracranial (94% sensitivity; positive predictive value: 87%).43 Hospitalizations with a diagnosis of hemorrhage were identified using the International Classification of Diseases, Tenth Revision, Canada (ICD-10) codes in the CIHI-DAD.
Additional analyses
We conducted a number of further analyses, all of which were planned prior to study initiation. First, we repeated all analyses limited to individuals with available kidney function measures (serum creatinine converted to estimated glomerular filtration rate using the Chronic Kidney Disease Epidemiology Collaboration [CKD-epi] equation), as kidney function influences the risk of hemorrhage and DOAC dosage and use.44 Second, we repeated all analyses excluding individuals with a hospitalization in the 90 days preceding CV medication initiation (to exclude acute illness or cardiac procedures). Third, we repeated our models using a negative outcome (composite of anxiety/depression or fracture). Negative or “dummy” outcomes serve as a method to assess the potential for residual confounding.45 For this, we expect no statistically significant difference between the use of CV medications of interest, and their comparators, in relation to the incidence of anxiety/depression or fractures. Fourth, we examined differences based on DOAC type (dabigatran/apixaban/rivaroxaban) and dosage (“high” defined as full dose; “low” defined as any reduced dose), using interaction terms. Fifth, we repeated all analyses using a liberal definition of hemorrhage that included any bleeding event or receipt of a blood transfusion, with presentation to an emergency room or hospitalization. Sixth, we repeated our models, limiting follow-up to the first 90 days after the initiation of the CV medication of interest to examine if the hemorrhage risk differs in the early drug-use period. This measure specifically focuses on a potential “high-risk” period (shortly after drug initiation). Further, as the cohort is of advanced age and at a significant risk of death, assessing a short follow-up period reduces the effect of informative censoring due to the competing risk of mortality.46
Statistical analysis
For the cohort studies, we used absolute standardized differences to assess baseline characteristics by each CV medication of interest and its comparator(s), for a total of 3 comparison groups. Standardized differences describe differences between group means or proportions relative to the pooled standard deviation and are less sensitive to large sample sizes than traditional hypothesis testing.47 A difference is considered significant if it is 0.10 or greater. We calculated the cumulative incidence of hemorrhage for each individual CV drug–comparator pair. We examined the association of each CV drug vs its comparator(s) and hemorrhage using inverse probability of treatment–weighted (IPTW) Cox proportional hazards models.48 Schoenfeld residuals were examined to test for the proportionality assumption. We estimated the average treatment effect in the IPTW models considering only the first hemorrhage event. For the IPTW, we calculated the weights by including all covariates listed in Table 1, with truncation at the 1st and 99th percentiles. Post-weighting, the comparison groups were assessed for balance using standardized differences. To examine for effect modification by DOAC type (apixaban, dabigatran, or rivaroxaban) and DOAC dose, separate models with interaction terms were examined. We conducted all analyses with Enterprise Guide 7.1 (SAS Institute Inc., Cary, NC). Confidence intervals that did not overlap with 1, and P values ≤ 0.05 were treated as statistically significant.
Table 1.
Baseline characteristics comparing initiation of amiodarone/metoprolol, verapamil/amlodipine, and diltiazem/amlodipine among direct oral anticoagulant (DOAC) users
| Characteristic | Amiodarone | Metoprolol | Std Diff | Verapamil | Amlodipine | Std Diff | Diltiazem | Amlodipine | Std Diff |
|---|---|---|---|---|---|---|---|---|---|
| Total N | 4872 | 21,853 | 1284 | 26,043 | 14,638 | 26,176 | |||
| Demographics | |||||||||
| Female | 2319 (47.6) | 11,675 (53.4) | 0.02 | 819 (63.8) | 14,967 (57.5) | 0.03 | 8850 (60.5) | 15,036 (57.4) | 0.00 |
| Age group, y | |||||||||
| 66–75 | 2301 (47.2) | 11,603 (43.4) | 0.01 | 656 (51.1) | 11,774 (45.2) | 0.05 | 6734 (46.0) | 11,866 (45.3) | 0.00 |
| 76–85 | 2015 (41.4) | 11,203 (41.9) | 0.02 | 504 (39.3) | 10,730 (41.2) | 0.01 | 5985 (40.9) | 10,755 (41.1) | 0.00 |
| 86–95 | 536 (11.0) | 3771 (14.1) | 0.00 | 120 (9.3) | 3428 (13.2) | 0.06 | 1844 (12.6) | 3441 (13.1) | 0.00 |
| > 95 | 20 (0.4) | 148 (0.6) | 0.01 | - | 111 (0.4) | 0.00 | 75 (0.5) | 114 (0.4) | 0.00 |
| Income quintiles | |||||||||
| 1 (low) | 873 (17.9) | 4322 (19.8) | 0.00 | 218 (17.0) | 4785 (18.4) | 0.03 | 2780 (19.0) | 4846 (18.5) | 0.00 |
| 2 | 974 (20.0) | 4458 (20.4) | 0.01 | 266 (20.7) | 5455 (20.9) | 0.00 | 3042 (20.8) | 5472 (20.9) | 0.00 |
| 3 | 964 (19.8) | 4429 (20.3) | 0.00 | 251 (19.5) | 5225 (20.1) | 0.01 | 2811 (19.2) | 5236 (20.0) | 0.00 |
| 4 | 993 (20.4) | 4311 (19.7) | 0.00 | 264 (20.6) | 5165 (19.8) | 0.01 | 2885 (19.7) | 5183 (19.8) | 0.00 |
| 5 (high) | 1061 (21.8) | 4278 (19.6) | 0.01 | 279 (21.7) | 5350 (20.5) | 0.00 | 3086 (21.1) | 5377 (20.5) | 0.00 |
| Rural residence | 4872 (100.0) | 21,852 (100.0) | 0.02 | 238 (18.5) | 3457 (12.7) | 0.08 | 2114 (14.4) | 3244 (12.4) | 0.00 |
| Index year | |||||||||
| 2008 | 54 (1.1) | 618 (2.8) | 0.02 | 66 (5.1) | 765 (2.9) | 0.08 | 418 (2.9) | 745 (2.8) | 0.00 |
| 2009 | 505 (10.4) | 5,015 (22.9) | 0.02 | 630 (49.1) | 8192 (31.5) | 0.05 | 4073 (27.8) | 8092 (30.9) | 0.01 |
| 2010 | 139 (2.9) | 856 (3.9) | 0.01 | 58 (4.5) | 1376 (5.3) | 0.12 | 678 (4.6) | 1438 (5.5) | 0.00 |
| 2011 | 230 (4.7) | 1,346 (6.2) | 0.00 | 70 (5.5) | 1741 (6.7) | 0.01 | 880 (6.0) | 1777 (6.8) | 0.01 |
| 2012 | 613 (12.6) | 2,508 (11.5) | 0.01 | 108 (8.4) | 2850 (10.9) | 0.00 | 1648 (11.3) | 2921 (11.2) | 0.00 |
| 2013 | 736 (15.1) | 2,557 (11.7) | 0.01 | 105 (8.2) | 2731 (10.5) | 0.01 | 1612 (11.0) | 2803 (10.7) | 0.00 |
| 2014 | 787 (16.2) | 2,888 (13.2) | 0.00 | 87 (6.8) | 2873 (11.0) | 0.02 | 1702 (11.6) | 2900 (11.1) | 0.00 |
| 2015 | 886 (18.2) | 3,181 (14.6) | 0.00 | 92 (7.2) | 2963 (11.4) | 0.06 | 1853 (12.7) | 2980 (11.4) | 0.01 |
| 2016 | 922 (18.9) | 2,884 (13.2) | 0.02 | 68 (5.3) | 2552 (9.8) | 0.05 | 1774 (12.1) | 2520 (9.6) | 0.01 |
| Comorbid illness | |||||||||
| Major hemorrhage | 67 (1.4) | 307 (1.4) | 0.01 | - | 247 (0.9) | 0.05 | 116 (0.8) | 246 (0.9) | 0.00 |
| Hypertension | 4117 (84.5) | 18,462 (84.5) | 0.04 | 1109 (86.4) | 24,691 (94.8) | 0.15 | 12,695 (86.7) | 24,837 (94.9) | 0.03 |
| Diabetes | 1293 (26.5) | 6483 (29.7) | 0.02 | 307 (23.9) | 8181 (31.4) | 0.06 | 3977 (27.2) | 8303 (31.7) | 0.01 |
| Stroke/TIA | 108 (2.2) | 669 (3.1) | 0.02 | 13 (1.0) | 743 (2.9) | 0.09 | 317 (2.2) | 762 (2.9) | 0.01 |
| Atrial fibrillation/flutter | 2442 (50.1) | 7,697 (35.2) | 0.00 | 102 (7.9) | 2,478 (9.5) | 0.03 | 4864 (33.2) | 2572 (9.8) | 0.04 |
| Myocardial infarction | 194 (4.0) | 784 (3.6) | 0.02 | 6 (0.5) | 238 (0.9) | 0.03 | 106 (0.7) | 243 (0.9) | 0.00 |
| Heart failure | 1731 (35.5) | 3961 (18.1) | 0.01 | 61 (4.8) | 2007 (7.7) | 0.05 | 1807 (12.3) | 2041 (7.8) | 0.01 |
| Coronary artery disease | 1616 (33.2) | 5407 (24.7) | 0.00 | 149 (11.6) | 4286 (16.5) | 0.09 | 2293 (15.7) | 4389 (16.8) | 0.00 |
| Coronary artery bypass grafting | 264 (5.4) | 1030 (4.7) | 0.01 | 29 (2.3) | 666 (2.6) | 0.02 | 278 (1.9) | 680 (2.6) | 0.00 |
| Percutaneous cardiac intervention | 422 (8.7) | 1501 (6.9) | 0.00 | 47 (3.7) | 1383 (5.3) | 0.04 | 639 (4.4) | 1403 (5.4) | 0.00 |
| Peripheral vascular disease | 141 (2.9) | 655 (3.0) | 0.01 | 22 (1.7) | 619 (2.4) | 0.02 | 317 (2.2) | 616 (2.4) | 0.00 |
| Venous thromboembolism | 72 (1.5) | 488 (2.2) | 0.01 | 12 (0.9) | 441 (1.7) | 0.05 | 210 (1.4) | 446 (1.7) | 0.01 |
| Healthcare utilization | |||||||||
| Hospitalizations | 1 (1-2) | 1 (1-2) | 0.02 | 0 (0-0) | 0 (0-0) | 0.28 | 0 (0-1) | 0 (0-0) | 0.00 |
| ED visits | 2 (1-3) | 1 (1-2) | 0.02 | 0 (0-0) | 0 (0-1) | 0.22 | 0 (0-1) | 0 (0-1) | 0.01 |
| Medications | |||||||||
| β-blocker | 2939 (60.3) | - | - | 195 (15.2) | 8595 (33.0) | 0.22 | 3577 (24.4) | 8666 (33.1) | 0.00 |
| NSAID | 1649 (7.5) | 262 (5.4) | 0.02 | 116 (9.0) | 2614 (10.0) | 0.01 | 1164 (8.0) | 2649 (10.1) | 0.00 |
| Proton pump inhibitor | 6012 (27.5) | 1556 (31.9) | 0.01 | 283 (22.0) | 6717 (25.8) | 0.04 | 3837 (26.2) | 6849 (26.2) | 0.00 |
| Antiplatelet agent | 1798 (8.2) | 362 (7.4) | 0.01 | 50 (3.9) | 1680 (6.5) | 0.08 | 747 (5.1) | 1709 (6.5) | 0.01 |
| SSRI | 1976 (9.0) | 406 (8.3) | 0.00 | 106 (8.3) | 2273 (8.7) | 0.02 | 1397 (9.5) | 2297 (8.8) | 0.00 |
| Lipid-lowering agent | 8062 (36.9) | 2158 (44.3) | 0.01 | 382 (29.8) | 9582 (36.8) | 0.07 | 4716 (32.2) | 9769 (37.3) | 0.01 |
| DOAC type | |||||||||
| Apixaban | 1816 (37.27) | 7545 (34.53) | 0.00 | 338 (26.32) | 7516 (28.86) | 0.01 | 1816 (37.27) | 7545 (34.53) | 0.09 |
| Dabigatran | 1255 (25.76) | 4976 (22.77) | 0.10 | 284 (22.12) | 4252 (16.33) | 0.13 | 1255 (25.76) | 4976 (22.77) | 0.16 |
| Rivaroxaban | 1801 (36.97) | 9332 (42.7) | 0.09 | 662 (51.56) | 14,275 (54.81) | 0.11 | 1801 (36.97) | 9332 (42.7) | 0.21 |
| Mean daily dose, mean (SD) | |||||||||
| Apixaban | 7.63 (4.24) | 7.51 (2.84) | 0.03 | 7.41 (2.57) | 7.44 (6.36) | 0.01 | 7.62 (3.11) | 7.44 (6.35) | 0.02 |
| Dabigatran | 252.05 (210.2) | 252.11 (152.78) | 0.03 | 238.73 (40.4) | 247.84 (159.61) | 0.07 | 252.37 (183.87) | 247.95 (159.27) | 0.01 |
| Rivaroxaban | 17.64 (3.49) | 16.62 (6.04) | 0.13 | 15.03 (5.79) | 14.53 (5.36) | 0.10 | 16.55 (12.47) | 14.51 (5.36) | 0.12 |
| High daily DOAC dose | 1373 (28.2%) | 5894 (27.0%) | 0.01 | 313 (24.4) | 5834 (22.4%) | 0.03 | 4105 (28.0%) | 5861 (22.4%) | 0.01 |
| DOAC duration prior to CV medications, mean (SD) | 148.4 (292) | 62.3 (204.3) | 0.03 | 43.8 (176.9) | 69 (222.0) | 0.06 | 59.2 (198.9) | 66.6 (217.5) | 0.01 |
| eGFR, ml/min per 1.73 m2, mean (SD) | 64.2 (17.2) | 67.8 (17.1) | 0.21 | 68.9 (16.6) | 68.0 (17.8) | 0.06 | 69.2 (16.6) | 68.0 (17.9) | 0.02 |
Values are n (%) or median (interquartile range), unless otherwise indicated. Post-weighting absolute standardized differences (Std Diff) ≥ 0.1 are statistically significant and are presented in boldface.
CV, cardiovascular; DOAC, direct oral anticoagulant; ED, emergency department; eGFR, estimated glomerular filtration rate; NSAID, nonsteroidal anti-inflammatory drug; SD standard deviation; SSRI, selective serotonin reuptake inhibitor; TIA, transient ischemic attack.
Results
We identified a total of 295,038 DOAC users during the study period, from which 3 study cohorts, one for each study drug of interest and its active comparator, were constructed as follows: (i) 4872 amiodarone users, compared to 21,853 metoprolol users; (ii) 1284 verapamil users, compared to 26,043 amlodipine users; and (iii) 14,638 diltiazem users compared to 26,176 amlodipine users (see Table 1). Roughly 47%, 51%, and 46% of amiodarone, verapamil, and diltiazem users, respectively, were aged 66 to 75 years and were younger relative to those prescribed metoprolol or amlodipine. The most common comorbidities were hypertension (over 80%) and diabetes mellitus (24% to 32%). Comparing amiodarone use to metoprolol use, differences were noted in the following: presence of atrial fibrillation; 86 to 95 years of age; index year of cohort entry; coronary artery disease; emergency room visits; and lipid lowering–agent use (see Supplemental Table S6 for pre- and post-weighting). Verapamil users more commonly were female, younger, and rural residents, with less comorbid illness, and less use of beta-blockers, antplatelets, and lipid-lowering agents, compared with amlodipine users. Diltiazem users were less likely to have a history of hypertension, heart failure, and beta-blocker or lipid lowering–agent prescriptions, with more atrial fibrillation, compared to amlodipine users.
Rivaroxaban was the most commonly used DOAC (37% to 55%), followed by apixaban (26% to 37%) and dabigatran (16% to 26%). Diltiazem users were more commonly on full doses of DOACs, compared with those on amlodipine, whereas no such differences were seen between amiodarone/metoprolol or verapamil/amlodipine pairs. Duration of DOAC use prior to prescription of the CV medication differed between all 3 pairs. Kidney function data were available for over 60% of the cohort, with a mean baseline estimated glomerular filtration rate > 60 ml/min per 1.73 m2 across all groups. Previous warfarin use was higher for amiodarone (32.7%) vs metoprolol (30.0%), verapamil (30.5%) vs amlodipine (22.0%), and diltiazem (30.4%) vs amlodipine (22.2%), relative to the comparator drugs.
A total of 1737 hemorrhagic events occurred that required an emergency room visit or hospitalization (amiodarone, 80 events [1.64%] vs metoprolol, 503 events [2.30%]; verapamil, 32 events [2.49%] vs amlodipine, 406 events [1.56%]; diltiazem, 312 events [2.13%] vs amlodipine, 404 events [1.54%]). Cox proportional hazards models applying IPTW showed no higher risk of hemorrhage with amiodarone (hazard ratio [HR] 0.77, 95% confidence interval [CI] 0.61-0.97), verapamil (HR 1.32, 95% CI 0.88-1.98), or diltiazem (HR 0.99, 95% CI 0.85-1.15; Table 2, Figure 1). Additional analyses are presented in Supplemental Table S7. These findings were consistent in models accounting for kidney function (amiodarone HR 0.85, 95% CI 0.66-1.11; verapamil HR 1.17, 95% CI 0.63-2.21; diltiazem HR 1.04, 95% CI 0.86-1.26) and when we excluded individuals with a hospitalization 90 days prior to cardiac medication initiation (amiodarone HR 0.80, 95% CI 0.59-1.09; verapamil HR 1.45, 95% CI 0.95-2.22; diltiazem HR 0.99, 95% CI 0.83-1.19). No association with hemorrhage was present in a model with additional adjustment for post-weighting differences between verapamil compared to amlodipine (HR 1.34 95% CI 0.89-2.01). No association was identified between a CV medication and a negative outcome (anxiety/depression: amiodarone, 11 events [0.23%] vs metoprolol, 39 events [0.18%]; adjusted HR 0.82, 95% CI 0.37-1.82; fracture: verapamil 57 events [4.44%] vs amlodipine 893 events [3.43%]; adjusted HR 1.09, 95% CI 0.81-1.45; anxiety/depression: diltiazem 28 events [0.19%] vs amlodipine, 32 events [0.12%]; HR 1.36, 95% CI 0.80-2.33]). Fractures were examined for the verapamil/amlodipine pair comparison, as there were no anxiety/depression events in the verapamil group.
Table 2.
The hazard of hemorrhage requiring hospitalization or emergency room visit, comparing initiation of amiodarone vs metoprolol, verapamil vs amlodipine, and diltiazem vs amlodipine, among direct oral anticoagulant users
| Comparison | Number of events | Cumulative incidence (%) | Median follow-up time, d (IQR) | Unweighted HR (95% CI) | Weighted HR∗ (95% CI) |
|---|---|---|---|---|---|
| Amiodarone vs metoprolol | |||||
| Amiodarone | 80 | 1.64 | 193 (398) | 0.80 (0.63–1.01) | 0.77 (0.61–0.97) |
| Metoprolol | 503 | 2.30 | 233 (534) | ||
| Verapamil vs amlodipine | |||||
| Verapamil | 32 | 2.49 | 168 (473) | 1.39 (0.97–1.99) | 1.32 (0.88–1.98) |
| Amlodipine | 406 | 1.56 | 139 (372) | ||
| Diltiazem vs amlodipine | |||||
| Diltiazem | 312 | 2.13 | 257 (641) | 1.04 (0.89–1.20) | 0.99 (0.85–1.15) |
| Amlodipine | 404 | 1.54 | 137 (376) | ||
CI, confidence interval; HR, hazard ratio; IQR, interquartile range.
Variables included in inverse probability of treatment–weighted hazards model are as follows: demographics (age, sex, income, place of residence); index year; comorbid illnesses (history of hemorrhage, hypertension, diabetes, stroke, atrial fibrillation, acute coronary syndrome, heart failure, coronary artery disease, coronary artery bypass grafting, percutaneous coronary intervention, peripheral vascular disease, venous thromboembolism); healthcare utilization (number of hospitalizations and emergency room visits in preceding 5 years); medications (beta-blocker, nonsteroidal anti-inflammatory drug, proton pump inhibitors, antiplatelet agents, selective serotonin reuptake inhibitors, and statins in preceding 1 year); and direct oral anticoagulant type, dose (high/low), and duration.
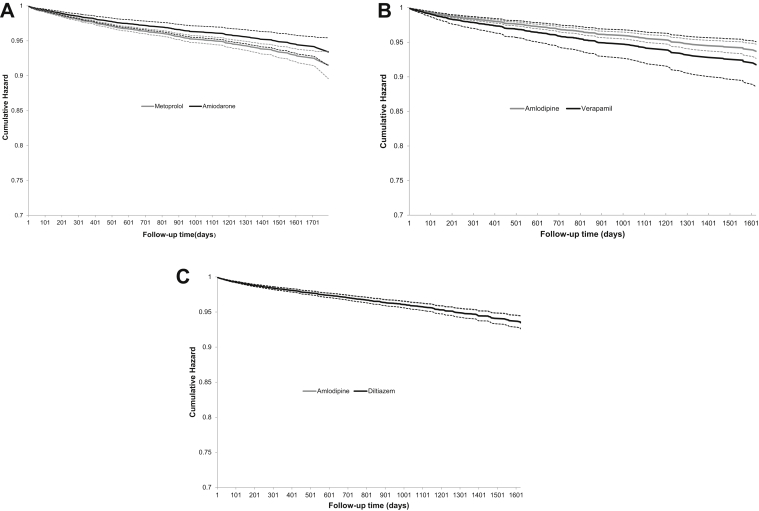
Figure 1.
The cumulative hazard of hemorrhage requiring hospitalization or an emergency room visit in direct oral anticoagulant users prescribed the following: (A) amiodarone vs metoprolol; (B) verapamil vs amlodipine; and (C) diltiazem vs amlodipine. Dashed lines represent 95% confidence intervals. Cumulative hazard was determined using inverse probability treatment–weighted Cox models. Weights were calculated accounting for the following variables: demographics (age, sex, income, place of residence); index year; comorbid illnesses (history of hemorrhage, hypertension, diabetes, stroke, atrial fibrillation, acute coronary syndrome, heart failure, coronary artery disease, coronary artery bypass grafting, percutaneous coronary intervention, peripheral vascular disease, venous thromboembolism); healthcare utilization (number of hospitalizations and emergency room visits in preceding 5 years); medications (beta-blocker, nonsteroidal anti-inflammatory drugs, proton pump inhibitors, antiplatelet agents, selective serotonin reuptake inhibitors, and statins); direct oral anticoagulant type, dose, and duration of use prior to cardiovascular medication.
There was no difference in the hemorrhage risk by DOAC type or dose in any of the 3 comparison groups (interaction P values were nonsignificant for all comparisons).
We further examined a broader definition of hemorrhage, with a total of 7007 hemorrhagic events (amiodarone, 364 events [7.47%] vs metoprolol, 1890 events (8.65%); verapamil, 108 events (8.41%) vs amlodipine, 1680 events (6.45%); diltiazem, 1280 events [8.74%] vs amlodipine, 1685 events [6.44%]). In IPTW models, there was no increase in the hemorrhage risk with amiodarone (HR 0.97, 95% CI 0.87-1.08), verapamil (HR 1.02, 95% CI 0.82-1.27), or diltiazem (HR 0.91, 95% CI 0.85-0.98).
When the follow-up period was limited to 90 days after initiation of a CV medication, a higher risk of hemorrhage was observed with diltiazem, compared with amlodipine (diltiazem 102 events [0.70%] vs amlodipine, 116 events [0.44%] events; HR 1.32, 95% CI 1.01-1.73), whereas no statistical difference for amiodarone or verapamil was detected . Lastly, our results were consistent when our weighted models were additionally adjusted for previous warfarin use.
Discussion
In this retrospective cohort study examining 3 commonly prescribed CV medications that are moderate CYP3A4 and P-gp inhibitors (amiodarone, verapamil, and diltiazem) in DOAC users, the overall rate of major hemorrhage requiring hospitalization, or an emergency room visit, was not statistically higher when compared to that with similar CV medications without CYP3A4 or P-gp activity (metoprolol and amlodipine). These findings were consistent after excluding individuals with a recent hospitalization, when accounting for kidney function, and when using a broader definition of hemorrhage. No association was observed when examining negative controls, indicating that our models accounted for residual confounding. In addition, we did not observe any differences based on the type or dose of DOAC.
Important to note is that in the early follow-up period (90 days), diltiazem, but not amiodarone or verapamil, was associated with a higher rate of hemorrhage, compared to amlodipine. Furthermore amiodarone (compared to metoprolol) may be associated with a lower hemorrhage risk. The results of our study suggest that amiodarone, and verapamil, can be used safely in patients on DOACs, regardless of kidney function or the DOAC selected. However, an elevated hemorrhage risk may be present in the early initiation period with diltiazem that may warrant careful monitoring or consideration of alternative agents.
A number of studies to date demonstrate an increase in serum concentration levels and/or prolonged clotting times, with the co-prescription of amiodarone, verapamil, or diltiazem with a DOAC.5,7,10,11,16,17,21,22,29,32,34,49 However, few examine clinically relevant hemorrhage events that are reflective of real-world practice. Chang et al., examining a large cohort of DOAC users for drug interactions, reported a higher hemorrhage risk with amiodarone but not with verapamil or diltiazem.6 The study, as opposed to the current work, lacked use of an active comparator drug, thereby increasing the risk of residual confounding and raising concerns about the findings. Pham et al. examined 48,442 DOAC users with normal kidney function for hemorrhage risk with verapamil and diltiazem, compared to amlodipine or metoprolol, and reported a higher hemorrhage risk with dabigatran only.12 The higher risk of hemorrhage was observed with the composite of verapamil/diltiazem and dabigatran on stratified analyses. Our findings further clarify these findings, as they specifically identify diltiazem as possibly being associated with a higher risk, and indicate that the risk is significantly elevated only within the first 90 days of drug initiation. Notably, differences between the current work and previous studies include differences in cohort size (verapamil or diltiazem use was almost 10 times greater, at 15,922 in the current study, relative to use in the Pham et al. study), the inclusion of individuals with reduced kidney function, and examination of temporality of risk (our additional analysis limited to the first 90 days).
Our study findings carry important clinical implications in terms of drug safety, with the potential to alter prescribing practices, regarding not just CV medication selection, but also decisions related to DOAC dose reduction. The early higher hemorrhage risk with diltiazem, if found to be consistent in additional studies, should lead to consideration of alternative CV agents, alternative anticoagulants, more judicious monitoring, and an increased focus on determination of individual bleeding risk. The medications we examined are commonly co-prescribed to patients on DOACs, owing to their use in the treatment of either atrial fibrillation or diseases associated with atrial fibrillation due to shared risk factors. The strengths of our study include the robust sample size in our cohort, the use of active comparators, the new-user design, and the use of IPTW to decrease the risk of bias.40,41,48
The findings of our study should be interpreted with the study limitations kept in mind. First, our cohort included individuals aged 66 years or older, limiting generalizability to younger individuals. Second, although the number of patients included in our cohort was quite large (295,038), the number of absolute hemorrhagic events seen in some categories was small. Possibly, the number of bleeding events was not large enough to allow us to see small differences between our treatment groups. Third, our cohort was not limited to individuals with atrial fibrillation. Fourth, patients may have been exposed to weak or moderate CYP3A4/P-gp inhibitors, as we excluded only strong CYP3A4/P-gp inhibitors. Fifth, although we used an active comparator study design, some differences in treatment indications and therapeutic properties between the CV medication of interest and its comparator may be present. Sixth, although we corrected for all anticipated cofounders, given the observational nature of the study, unknown and unadjusted factors could have introduced confounding bias into our results. As an example, although adjusting for comorbidities is possible, complete removal of the possibility that channeling bias altered prescribing habits in patients subjectively deemed to be “sicker” by prescribers is difficult. Finally, although we can comment on prescription filling, we do not know information regarding patient adherence to treatment. Differences could exist among our groups, in adherence to DOACs, our medications of interest, or both.
Conclusions
In a large retrospective cohort study on adults of advanced age treated with a P-gp and/or CYP3A4 inhibiting medication (verapamil, diltiazem, or amiodarone) while on a DOAC, we observed no difference in the risk of major hemorrhage during the entire follow-up period, compared to use of similar medications. However, diltiazem may be associated with a higher risk of hemorrhage in the first 90 days after initiation, as compared to amlodipine, prompting consideration of more-intensive monitoring with its use, consideration of viable alternatives, and assessment of individual risk vs benefit. The results of our study suggest that patients on DOACs may be treated safely with verapamil or amiodarone, whereas caution may be required with diltiazem initiation. Further confirmatory analyses should be considered to better characterize this possible interaction.
Acknowledgements
Members of the ICES Kidney, Dialysis and Transplantation team conducted the research. Parts of this material are based on data and/or information compiled and provided by the Canadian Institute for Health Information (CIHI) and Service Ontario. However, the analyses, conclusions, opinions and statements expressed in the material are those of the authors and not necessarily those of CIHI. We thank IQVIA Solutions Canada Inc for use of their Drug Information File. The Jindal Research Chair supports M.M.S. for the prevention of kidney disease.
Funding Sources
Funding was received from the Heart and Stroke Foundation, G-20-0029507. ICES supported this study, which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care(MOHLTC), Canada. The ICES Ottawa site completed this study. University of Ottawa, The Ottawa Hospital Research Institute (OHRI) and the Canadian Institutes of Health Research (CIHR) provide core funding for ICES Ottawa. ICES is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC). The analyses, conclusions, opinions, and statements expressed herein are solely those of the authors and do not reflect those of the funding or data sources; no endorsement is intended or should be inferred.
Disclosures
M.M.S. has received speaker fees from AstraZeneca. T-F.W. reported honorary consulting for the advisory board from Pfizer. M.C. reported research funding from BMS, Leo Pharma, and Pfizer, and honoraria from Bayer, Sanofi, Servier, BMS, and Leo Pharma. The other authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: The use of data in this project was authorized under section 45 of Ontario’s Personal Health Information Protection Act, which does not require review by a research ethics board.
See page 322 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2021.11.002.
Supplementary Data
References
- 1.Macle L., Cairns J., Leblanc K., et al. 2016 focused update of the Canadian Cardiovascular Society Guidelines for the Management of Atrial Fibrillation. Can J Cardiol. 2016;32:1170–1185. doi: 10.1016/j.cjca.2016.07.591. [DOI] [PubMed] [Google Scholar]
- 2.Witt D.M., Nieuwlaat R., Clark N.P., et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: optimal management of anticoagulation therapy. Blood Adv. 2018;2:3257–3291. doi: 10.1182/bloodadvances.2018024893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sardar P., Chatterjee S., Chaudhari S., Lip G.Y.H. New oral anticoagulants in elderly adults: evidence from a meta-analysis of randomized trials. J Am Geriatr Soc. 2014;62:857–864. doi: 10.1111/jgs.12799. [DOI] [PubMed] [Google Scholar]
- 4.Ntaios G., Papavasileiou V., Makaritsis K., et al. Real-world setting comparison of nonvitamin-K antagonist oral anticoagulants versus vitamin-K antagonists for stroke prevention in atrial fibrillation. Stroke. 2017;48:2494–2503. doi: 10.1161/STROKEAHA.117.017549. [DOI] [PubMed] [Google Scholar]
- 5.Flaker G., Lopes R.D., Hylek E., et al. Amiodarone, anticoagulation, and clinical events in patients with atrial fibrillation: insights from the ARISTOTLE trial. J Am Coll Cardiol. 2014;64:1541–1550. doi: 10.1016/j.jacc.2014.07.967. [DOI] [PubMed] [Google Scholar]
- 6.Chang S., Chou I., Yeh Y., et al. Association between use of non–vitamin K oral anticoagulants with and without concurrent medications and risk of major bleeding in nonvalvular atrial fibrillation. JAMA. 2017;318:1250–1259. doi: 10.1001/jama.2017.13883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Avendano R., Romero J., Lupercio F., et al. Clinical outcomes in patients with atrial fibrillation receiving amiodarone on NOACs vs. warfarin. J Interven Card Electrophysiol. 2019;54:73–80. doi: 10.1007/s10840-018-0427-y. [DOI] [PubMed] [Google Scholar]
- 8.Walenga J.M., Adiguzel C. Drug and dietary interactions of the new and emerging oral anticoagulants. Int J Clin Pract. 2010;64:956–967. doi: 10.1111/j.1742-1241.2009.02286.x. [DOI] [PubMed] [Google Scholar]
- 9.Gulilat M., Keller D., Linton B., et al. Drug interactions and pharmacogenetic factors contribute to variation in apixaban concentration in atrial fibrillation patients in routine care. J Thromb Thrombolysis. 2020;49:294–303. doi: 10.1007/s11239-019-01962-2. [DOI] [PubMed] [Google Scholar]
- 10.Conen D. Edoxaban and amiodarone: interactions on multiple levels. Eur Heart J. 2015;36:2210–2211. doi: 10.1093/eurheartj/ehv245. [DOI] [PubMed] [Google Scholar]
- 11.Steffel J., Giugliano R.P., Braunwald E., et al. Edoxaban vs. warfarin in patients with atrial fibrillation on amiodarone: a subgroup analysis of the ENGAGE AF-TIMI 48 trial. Eur Heart J. 2015;36:2239–2245. doi: 10.1093/eurheartj/ehv201. [DOI] [PubMed] [Google Scholar]
- 12.Pham P., Schmidt S., Lesko L., Lip G.Y.H., Brown J.D. Association of oral anticoagulants and verapamil or diltiazem with adverse bleeding events in patients with nonvalvular atrial fibrillation and normal kidney function. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forbes H.L., Polasek T.M. Potential drug-drug interactions with direct oral anticoagulants in elderly hospitalized patients. Ther Adv Drug Saf. 2017;8:319–328. doi: 10.1177/2042098617719815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vranckx P., Valgimigli M., Heidbuchel H. The significance of drug-drug and drug-food interactions of oral anticoagulation. Arrhythm Electrophysiol Rev. 2018;7:55–61. doi: 10.15420/aer.2017.50.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hill K., Sucha E., Rhodes E., et al. Risk of hospitalization with hemorrhage among older adults taking clarithromycin vs azithromycin and direct oral anticoagulants. JAMA Intern Med. 2020;180:1052–1060. doi: 10.1001/jamainternmed.2020.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheong E.J.Y., Goh J.J.N., Hong Y., Kojodjojo P., Chan E.C.Y. Rivaroxaban with and without amiodarone in renal impairment. J Am Coll Cardiol. 2018;71:1395–1397. doi: 10.1016/j.jacc.2018.01.044. [DOI] [PubMed] [Google Scholar]
- 17.Frost C.E., Byon W., Song Y., et al. Effect of ketoconazole and diltiazem on the pharmacokinetics of apixaban, an oral direct factor Xa inhibitor. B J Clin Pharmacol. 2015;79:838–846. doi: 10.1111/bcp.12541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steinberg B.A., Hellkamp A.S., Lokhnygina Y., et al. Use and outcomes of antiarrhythmic therapy in patients with atrial fibrillation receiving oral anticoagulation: results from the ROCKET AF trial. Heart Rhythm. 2014;11:925–932. doi: 10.1016/j.hrthm.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frost C., Shenker A., Gandhi M.D., et al. Evaluation of the effect of naproxen on the pharmacokinetics and pharmacodynamics of apixaban. Br J Clin Pharmacol. 2014;78:877–885. doi: 10.1111/bcp.12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brüggemann R.J.M., Alffenaar J.-W.C., Blijlevens N.M.A., et al. Clinical relevance of the pharmacokinetic interactions of azole antifungal drugs with other coadministered agents. Clin Infect Dis. 2009;48:1441–1458. doi: 10.1086/598327. [DOI] [PubMed] [Google Scholar]
- 21.Ismail M., Lee V.H., Chow C.R., Rubino C.M. Minimal physiologically based pharmacokinetic and drug-drug-disease interaction model of rivaroxaban and verapamil in healthy and renally impaired subjects. J Clin Pharmacol. 2018;58:541–548. doi: 10.1002/jcph.1044. [DOI] [PubMed] [Google Scholar]
- 22.Kim M., Son H., Noh K., et al. Effects of verapamil and diltiazem on the pharmacokinetics and pharmacodynamics of rivaroxaban. Pharmaceutics. 2019;11:133. doi: 10.3390/pharmaceutics11030133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mendell J., Zahir H., Matsushima N., et al. Drug-drug interaction studies of cardiovascular drugs involving P-glycoprotein, an efflux transporter, on the pharmacokinetics of edoxaban, an oral factor Xa inhibitor. Am J Cardiovasc Drugs. 2013;13:331–342. doi: 10.1007/s40256-013-0029-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao Y., Hu Z.Y. Physiologically based pharmacokinetic modelling and in vivo [I]/K(i) accurately predict P-glycoprotein-mediated drug-drug interactions with dabigatran etexilate. Br J Pharmacol. 2014;171:1043–1053. doi: 10.1111/bph.12533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stöllberger C., Finsterer J. Relevance of P-glycoprotein in stroke prevention with dabigatran, rivaroxaban, and apixaban. Herz. 2015;40(suppl 2):140–145. doi: 10.1007/s00059-014-4188-9. [DOI] [PubMed] [Google Scholar]
- 26.Australian PI—ELIQUIS® (apixaban) http://secure.healthlinks.net.au/content/bms/pi.cfm?product=bqpeliqu11112 Available at:=
- 27.Summary of product characteristics for pradaxa. http://www.ema.europa.eu/docs/en_GB/document_library/ EPAR_-_Product_Information/human/000829/WC500041059.pdf Available at:
- 28.XARELTO (rivaroxaban)–official patient site. EMA product report. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR__Product_Information/human/000944/WC500057108.pdf Available at:
- 29.Lupercio F., Romero J., Peltzer B., et al. Efficacy and safety outcomes of direct oral anticoagulants and amiodarone in patients with atrial fibrillation. Am J Med. 2018;131:573. doi: 10.1016/j.amjmed.2017.11.047. e571-e578. [DOI] [PubMed] [Google Scholar]
- 30.Oladiran O., Segal J., Nwosu I., Nazir S. A rare case of spontaneous cardiac tamponade induced by concomitant use of rivaroxaban and amiodarone. Case Rep Cardiol. 2018;2018:1650716. doi: 10.1155/2018/1650716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Härtter S., Sennewald R., Nehmiz G., Reilly P. Oral bioavailability of dabigatran etexilate (Pradaxa(®) ) after co-medication with verapamil in healthy subjects. Br J Clin Pharmacol. 2013;75:1053–1062. doi: 10.1111/j.1365-2125.2012.04453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fountzilas C., George J., Levine R. Dabigatran overdose secondary to acute kidney injury and amiodarone use. N Zea Med J. 2013;126:110–112. [PubMed] [Google Scholar]
- 33.Sennesael A.-L., Larock A.-S., Douxfils J., et al. Rivaroxaban plasma levels in patients admitted for bleeding events: insights from a prospective study. Thromb J. 2018;16:28. doi: 10.1186/s12959-018-0183-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Washam J.B., Hellkamp A.S., Lokhnygina Y., et al. Efficacy and safety of rivaroxaban versus warfarin in patients taking nondihydropyridine calcium channel blockers for atrial fibrillation (from the ROCKET AF trial) Am J Cardiol. 2017;120:588–594. doi: 10.1016/j.amjcard.2017.05.026. [DOI] [PubMed] [Google Scholar]
- 35.Thrombosis Canada Clinical guides. DOACs∗: Comparisons And Frequently Asked Questions. https://thrombosiscanada.ca/clinicalguides/# Available at:
- 36.Steffel J., Verhamme P., Potpara T.S., et al. The 2018 European Heart Rhythm Association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J. 2018;39:1330–1393. doi: 10.1093/eurheartj/ehy136. [DOI] [PubMed] [Google Scholar]
- 37.Statistics Canada Population by year, by province and territoruy (number) 2012 https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1710000901 Available at: Accessed July, 2020. [Google Scholar]
- 38.Levy A.B., O’Brien B.J., Sellors C., Grootendorst P., Willison D. Coding accuracy of administrative drug claims in the Ontario Drug Benefit Database. Can J Clin Pharmacol. 2003;10:67–71. [PubMed] [Google Scholar]
- 39.Benchimol E.I., Smeeth L., Guttmann A., et al. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) Statement. PLoS Med. 2015;12 doi: 10.1371/journal.pmed.1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoshida K., Solomon D.H., Kim S.C. Active-comparator design and new-user design in observational studies. Nat Rev Rheumatol. 2015;11:437–441. doi: 10.1038/nrrheum.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson E.S., Bartman B.A., Briesacher B.A., et al. The incident user design in comparative effectiveness research. Pharmacoepidemiol Drug Saf. 2013;22:1–6. doi: 10.1002/pds.3334. [DOI] [PubMed] [Google Scholar]
- 42.Lin J.H., Lu A.Y.H. Inhibition and Induction of cytochrome P450 and the clinical implications. Clin Pharmacokin. 1998;35:361–390. doi: 10.2165/00003088-199835050-00003. [DOI] [PubMed] [Google Scholar]
- 43.Arnason T., Wells P.S., van Walraven C., Forster A.J. Accuracy of coding for possible warfarin complications in hospital discharge abstracts. Thromb Res. 2006;118:253–262. doi: 10.1016/j.thromres.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 44.Molnar A.O., Bota S.E., Garg A.X., et al. The risk of major hemorrhage with CKD. J Am Soc Nephrol. 2016;27:2825–2832. doi: 10.1681/ASN.2015050535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lousdal M.L., Lash T.L., Flanders W.D., et al. Negative controls to detect uncontrolled confounding in observational studies of mammographic screening comparing participants and non-participants. Int J Epidemiol. 2020;49:1032–1042. doi: 10.1093/ije/dyaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hsu J.Y., Roy J.A., Xie D., et al. Statistical methods for cohort studies of CKD: survival analysis in the setting of competing risks. Clin J Am Soc Nephrol. 2017;12:1181–1189. doi: 10.2215/CJN.10301016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Austin P.C. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun StatSimul Comp. 2009;38:1228–1234. [Google Scholar]
- 48.Austin P.C. The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med. 2014;33:1242–1258. doi: 10.1002/sim.5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bartlett J.W., Renner E., Mouland E., et al. Clinical safety outcomes in patients with nonvalvular atrial fibrillation on rivaroxaban and diltiazem. Ann Pharmacother. 2019;53:21–27. doi: 10.1177/1060028018795140. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.