Abstract
Objective
To report a case of pituitary functioning gonadotroph microadenoma accompanied by ovarian hyperstimulation syndrome (OHSS) in a woman of reproductive age.
Design
A case report.
Setting
Tertiary care unit of a university hospital.
Patients
A 42-year-old parous woman with bilateral ovarian enlargement presumed to be secondary to a functioning gonadotroph pituitary microadenoma.
Interventions
Oral contraceptives and endoscopic transsphenoidal surgery for adenoma that initially could not be visualized on a magnetic resonance imaging (MRI) scan.
Main Outcome Measures
Medical and radiographic assessment of endogenously induced OHSS and its resolution after treatment.
Results
The patient was diagnosed with OHSS secondary to elevations in endogenous levels of follicle-stimulating hormone (FSH). The cranial contrast-enhanced MRI scan did not show any apparent tumor in the pituitary gland. She was, therefore, treated with oral contraceptives, which resulted in a modest resolution of ovarian enlargement; however, this treatment became ineffective 3 years later. A small pituitary adenoma (maximum diameter of 8 mm) was suspected on repeated MRI (2 years after the first MRI). Selective adenomectomy was performed, which resulted in normalization of the ovarian size and resumption of regular menstrual cycles.
Conclusions
We report a case of a functional pituitary microadenoma secreting FSH in quantities significant enough to result in OHSS. The excessive FSH production was resistant to medical therapy; however, surgical treatment was ultimately successful.
Key Words: Functioning gonadotroph adenoma, ovarian hyperstimulation syndrome, reproductive-aged woman, transsphenoidal surgery, pituitary microadenoma
Discuss: You can discuss this article with its authors and other readers at https://www.fertstertdialog.com/posts/xfre-d-21-00167
Introduction
Pituitary gonadotroph adenomas (GAs) are usually clinically nonfunctioning adenomas, in which patients present with symptoms due to the mass effect, including visual disturbances, various degrees of hypopituitarism, and headache (1). In contrast, functioning gonadotroph adenomas (FGAs) of the pituitary are rare, accounting for <1% of all pituitary adenomas. The functioning gonadotroph adenomas can secrete luteinizing hormone (LH) (2) and follicle-stimulating hormone (FSH) in biologically active forms. Women of reproductive age with FGAs experience enlarged ovaries, reflecting an excess secretion of FSH (3). Thus, persistent secretion of FSH can lead to ovarian hyperstimulation syndrome (OHSS), consisting of elevated estradiol (E2) levels, enlarged multifollicular ovaries, ascites, and electrolyte imbalance. The first case report on FGA was published in 1974 (4). Since the 1980s, the diagnosis of FGA has mainly relied on pituitary magnetic resonance imaging (MRI), which usually demonstrates large adenomas (macroadenomas) sized >10 mm. To our knowledge, the occurrence of a functioning gonadotroph microadenoma of <10 mm diameter is extremely rare, with only four cases reported in the literature to date (3).
Herein, we report a rare case of an FSH-secreting pituitary microadenoma resulting in ovarian hyperstimulation. The patient gave written informed consent to publish this article.
Case report
A 42-year-old woman was referred to our hospital with transvaginal ultrasonography and abdominal MRI findings that revealed enlargement of the ovaries with a multifollicular appearance, similar to that in patients with OHSS. Her medical and family history was unremarkable. Her age at menarche was 13 years, and her menstrual cycle had been regular since then. She was infertile since marriage at the age of 34 years, and she consulted in a private clinic for her infertility and was diagnosed with unexplained infertility. After unsuccessful treatment by use of intrauterine insemination for >10 cycles, she was treated in the in vitro fertilization and embryo transfer program with ovarian stimulation, and second oocyte retrieval and subsequent embryo transfer resulted in conception. Her mode of parturition was a cesarean section, during which enlarged ovaries were not noted. After completing the lactation period, she remained amenorrheic with lower abdominal pain. Although ovarian swelling was noted, the brain MRI scan obtained 1 year and 6 months after parturition showed no apparent lesion of the pituitary gland. On arrival at our facility, laboratory data showed serum FSH, LH, prolactin (PRL), E2, thyroid-stimulating hormone, and antimüllerian hormone levels of 13.9 mIU/mL, 0.2 mIU/mL, 11 ng/mL, 527.0 pg/mL, 2.36 IU/mL, and 1.28 ng/mL, respectively. Transvaginal ultrasonography revealed a small uterine myoma sized 1 cm and enlarged bilateral ovaries (right: 56.6 × 38.4 mm, left: 35.4 × 24.5 mm, expressed as major axis and orthogonal short diameter), resembling ovaries under controlled ovarian stimulation in a patient undergoing an in vitro fertilization program (Fig. 1A and B). She underwent cranial contrast-enhanced MRI; however, no pituitary lesions, including pituitary tumors, were observed (Fig. 1C and D). Therefore, pituitary surgery was not performed at that time, and a low-dose estrogen-progestin (LEP) containing 20 μg of ethinyl E2 and 3 mg of drospirenone was administered to suppress the secretion of FSH and E2 as done in our previous report (5). The LEP treatment modestly suppressed the secretion of FSH (11.4 mIU/mL). The ovarian volume seemed to be slightly decreased (right: 29.8 × 41.6 mm, left: 20.4 × 24.2 mm); thus, the treatment was continued. However, LEP use was ineffective 3 years after treatment initiation. A repeated detailed pituitary MRI performed 2 years after her first visit led to a suspicion of a small pituitary adenoma with a maximum diameter of <10 mm, located in the center of the pituitary gland (Fig. 1C and D). Moreover, her abdominal distension worsened, and a gonadotropin-releasing hormone (GnRH) antagonist, relugolix (40 mg/d), was administered for 1 month; however, it was ineffective. Subsequently, a gonadotropin-releasing hormone agonist (GnRH-a), nafarelin (400 μg/d), was also attempted for 1 month but was ineffective as well. After the prescription had been abandoned, her abdominal distension worsened; exploratory pituitary surgery was performed, and a microadenoma was found intraoperatively (Fig. 2A). Selective complete tumor resection was performed to resolve OHSS and hypersecretion of FSH and E2 (Fig. 2B). The pathological examination confirmed that the tumor was a pituitary adenoma. Moreover, the immunohistochemical examination showed intense reactivity to FSH in most cells and immunonegativity to LH in some scattered cells (Fig. 2C and D), with immunonegativity to growth hormone, PRL, thyroid-stimulating hormone, and adrenocorticotropic hormone (data not shown). The preoperative laboratory data on the day of the surgery and the postoperative endocrine examination on postoperative day six showed no additional pituitary hormone deficiency; furthermore, there was a decrease in FSH, LH, and E2 levels from 11.1 mIU/mL to 0.8 mIU/mL, 0.4 mIU/mL to <0.3 mIU/mL, and 473 pg/mL to 11.6 pg/mL, respectively. Postoperatively, her menstrual cycle was regular, and the ovarian sizes normalized.
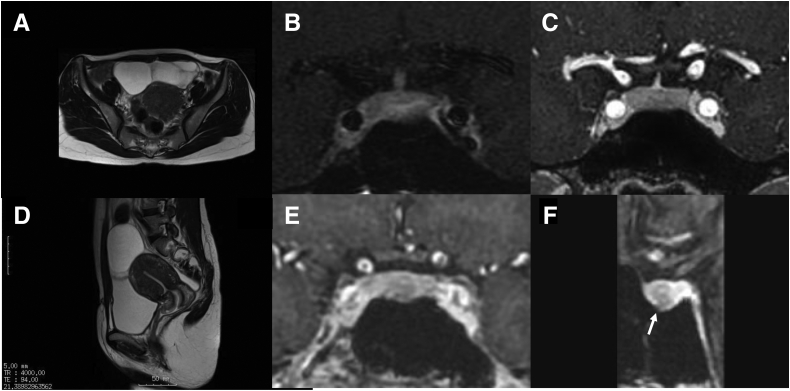
Figure 1.
Abdominal findings on magnetic resonance imaging (MRI) indicate enlarged ovaries, and pituitary MRI scan show scarce but apparent lesions. Abdominal MRI scans obtained before the patient visited our hospital show enlarged and polycystic ovaries of approximately 7 cm in diameter. (A) T2 width, axial view. (B) T2 width, sagittal view. (C) A contrast-enhanced coronal T1-weighted MRI scan obtained 2.5 years before surgery. (D) A contrast-enhanced coronal spoiled gradient recalled acquisition MRI scan obtained 1.25 years before surgery. Coronal (E) and sagittal (F) view images of spoiled gradient echo 3-dimensional T1 sequence obtained 2 months before surgery. No adenoma is detected in the first (C) and second (D) MRI scans; however, the third MRI scan, in which spoiled gradient recalled acquisition was used, shows the presence of a distinct microadenoma located in the middle of the pituitary gland in both coronal and sagittal sections (arrow).
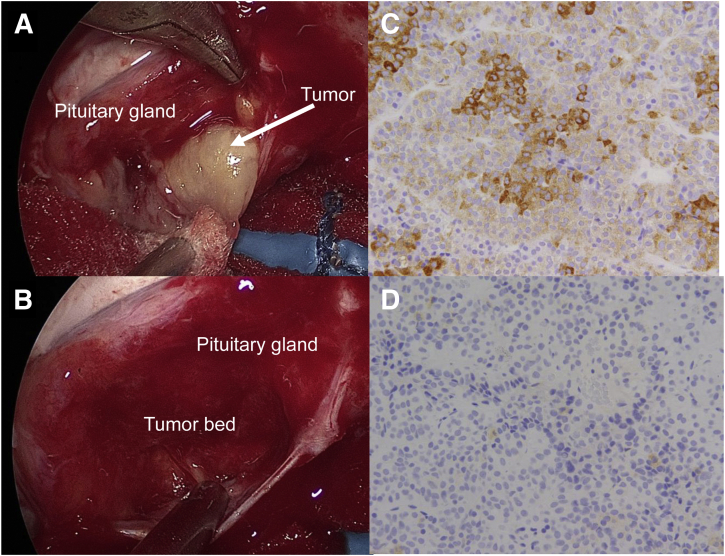
Figure 2.
Operative procedure and histologic findings of the tumor. (A) Exploratory pituitary surgery was performed; a microadenoma (arrow) was found in the center of the pituitary gland. (B) Selective complete tumor resection was performed. On immunohistochemical analysis, most adenoma cells were immunopositive for follicle-stimulating hormone-β, as seen on 3,3′-diaminobenzidine staining (C), although some scattered cells were immunonegative for luteinizing hormone-β (D).
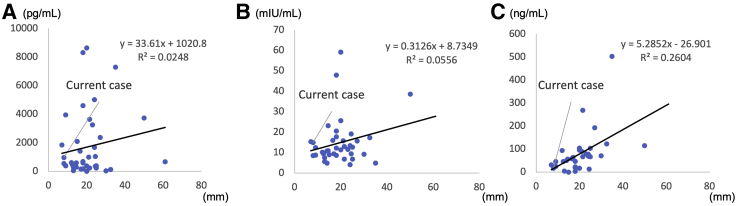
We investigated the correlation between the lesion size and hormonal secretion, and it was suggested that the PRL levels, because of the compression of the pituitary stalk, had been correlated with tumor size (r = 0.4946, P<.01) (Fig. 3).
Figure 3.
Analysis of laboratory data and tumor size from previous cases. (A) Correlation between tumor size and estradiol level. (B) Correlation between tumor size and follicle-stimulating hormone (FSH) level. (C) Correlation between tumor size and prolactin level. The current case is indicated by an arrow, and the values are indicated. Understandably, small lesions can produce excessive amounts of estradiol. The tumor size does not necessarily reflect FSH level, which is reasonable because FSH produced by the tumor may have a biologically different activity. However, the tumor size was well correlated with prolactin levels (r = 0.504, P<.01).
Discussion
Two recent comprehensive reviews (3, 6) presented four cases of OHSS with lesion sizes of <10 mm. However, we present an unusual case in which FSH hypersecretion with consequences was discovered before the development of discernible pituitary enlargement.
Diagnosis of FGAs
Ovarian enlargement is the most significant hallmark symptom of OHSS and occurs after gonadotropin stimulation, known as controlled ovarian stimulation. The controlled ovarian stimulation aims to obtain oocytes for subsequent conception; nevertheless, continuous stimulation via endogenous FSH produces the same clinical feature. Another disease caused by a mutated FSH receptor is the spontaneous OHSS (7), and types I, II, and III OHSS occur due to a mutated FSH receptor, secondary to elevated levels of human chorionic gonadotropin, and after hypothyroidism, respectively. Generally, the FSH levels can be normal or mildly elevated in cases of FGA (8), and the tumor size does not reflect endogenous FSH levels (Fig. 3B).
Cranial MRI is indispensable for the confirmatory diagnosis of OHSS that occurs after FGA. Dynamic contrast-enhanced MRI uses T1-weighted images acquired after intravenous injection of an MRI contrast agent. This can improve the sensitivity of tumor detection but is associated with an increased incidence of false-positive findings. Recently, the spoiled gradient recalled acquisition (SPGR) method has become popular because of its high sensitivity. The section slice is 1–1.2 mm in thickness, and images obtained using the SPGR method are improved due to a superior soft-tissue contrast and resolution. Therefore, we consider the SPGR method superior in detecting tiny microadenomas. The third repeated MRI performed 1.4 years after the first MRI examination could exhibit an adenoma; however, the same SPGR in the second MRI performed 3 months after the first MRI could not demonstrate any distinct adenoma. This may be due to tumor growth during the period before the third MRI was performed.
Treatment of FGAs
Generally, all patients diagnosed with FGA are eligible for transsphenoidal surgery. However, the management of FGA is controversial. Notably, the size of FGA is not a reliable indicator of surgery, as the plot of correlation (Fig. 3A and B) in the current case report clearly shows that small lesions can produce FSH and E2 at levels high enough to cause OHSS. Considering the presence of nonfunctioning pituitary adenomas, immunohistochemical analysis is warranted after tumor resection. Although the role of adjuvant radiotherapy may be considered, there is no conclusive agreement on its use. This is partially attributed to the fact that long-term follow-up data on FGA are not available.
For patients with an ambiguous diagnosis of FGA, as in the current case report, medical management with oral contraceptives can be considered the first-line therapy. We previously reported a case in which a patient with FGA was treated with oral contraceptives for 1 year (5), and LEP can be a good choice of treatment because the incidence of side effects is insignificant and modest hypothalamic–pituitary–gonadal axis suppression is expected. The use of dopamine agonists can reduce FSH levels; however, the role of tumor shrinkage is generally not expected (3). The GnRH analogs may also be prescribed for patients with GAs. However, GnRH-a exhibits a flare-up function soon after administration. This is a completely undesirable effect that may stimulate tumor growth and E2 secretion. The recent development of oral GnRH antagonists could be another choice of treatment because of its adherence. After the intake of oral GnRH antagonists, the secretion level of E2 decreases within 4 hours, which makes this class of drugs superior to the currently available GnRH-a.
Surgical treatment should be considered for patients with FGA in the following two scenarios: complications after aberrant secretion of biologically active hormones and mass effect, including visual field disturbances and hypopituitarism. Our study patient showed relatively elevated FSH and E2 levels, reflecting the pathophysiology of OHSS. Our patient demonstrated OHSS but was initially treated medically because a tumor was undetectable. Altogether, our findings indicate that the ovarian appearance is reliable to predict FSH hypersecretion, the PRL level can be reliable to confirm the presence of pituitary lesions, and small pituitary lesions should not be neglected. A repetitive investigation of the pituitary gland and cranial MRI should be performed in patients with difficult-to-identify FGA lesions.
Acknowledgments
The authors thank Naoko Inoshita, MD, PhD, for providing patient pathological data. The authors also thank Editage for language editing.
Footnotes
O.W.-H. has nothing to disclose. S.Y. has nothing to disclose. Y.O. has nothing to disclose.
Supported by the Ministry of Health, Labour and Welfare (19FB1001, 20FB1001, and large-scale demonstration project on prevention and health promotion).
References
- 1.Ntali G., Capatina C., Grossman A., Karavitaki N. Clinical review: functioning gonadotroph adenomas. J Clin Endocrinol Metab. 2014;99:4423–4433. doi: 10.1210/jc.2014-2362. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y., Chen C., Lin M., Deng K., Zhu H., Ma W., et al. Successful pregnancy after operation in an infertile woman caused by luteinizing hormone-secreting pituitary adenoma: case report and literature review. BMC Endocr Disord. 2021;21:15. doi: 10.1186/s12902-020-00677-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hasegawa H., Nesvick C.L., Erickson D., Cohen S.C., Yolcu Y.U., Khan Z., et al. Gonadotroph pituitary adenoma causing treatable infertility and ovarian hyperstimulation syndrome in female patients: neurosurgical, endocrinologic, gynecologic, and reproductive outcomes. World Neurosurg. 2021;150:e162–e175. doi: 10.1016/j.wneu.2021.02.115. [DOI] [PubMed] [Google Scholar]
- 4.Woolf P.D., Schenk E.A. An FSH-producing pituitary tumor in a patient with hypogonadism. J Clin Endocrinol Metab. 1974;38:561–568. doi: 10.1210/jcem-38-4-561. [DOI] [PubMed] [Google Scholar]
- 5.Hirano M., Wada-Hiraike O., Miyamamoto Y., Yamada S., Fujii T., Osuga Y. A case of functioning gonadotroph adenoma in a reproductive aged woman. Endocr J. 2019;66:653–656. doi: 10.1507/endocrj.EJ19-0066. [DOI] [PubMed] [Google Scholar]
- 6.Halupczok J., Kluba-Szyszka A., Bidzinska-Speichert B., Knychalski B. Ovarian hyperstimulation caused by gonadotroph pituitary adenoma-review. Adv Clin Exp Med. 2015;24:695–703. doi: 10.17219/acem/25212. [DOI] [PubMed] [Google Scholar]
- 7.De Leener A., Montanelli L., Van Durme J., Chae H., Smits G., Vassart G., et al. Presence and absence of follicle-stimulating hormone receptor mutations provide some insights into spontaneous ovarian hyperstimulation syndrome physiopathology. J Clin Endocrinol Metab. 2006;91:555–562. doi: 10.1210/jc.2005-1580. [DOI] [PubMed] [Google Scholar]
- 8.Broughton C., Mears J., Williams A., Lonnen K. A clinically functioning gonadotroph adenoma presenting with abdominal pain, ovarian hyperstimulation and fibromatosis. Endocrinol Diabetes Metab Case Rep. 2018;2018 doi: 10.1530/EDM-18-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]