Abstract
Mefloquine was found to have bactericidal activity against methicillin- and fluoroquinolone-susceptible and -resistant strains of Staphylococcus aureus and Staphylococcus epidermidis and gentamicin- and vancomycin-resistant strains of Enterococcus faecalis and Enterococcus faecium. The MICs were 16 μg/ml, and the minimal bactericidal concentrations (MBCs) were 16 to 32 μg/ml. These concentrations cannot be achieved in serum. Mefloquine was active at a more achievable concentration against penicillin-susceptible and -resistant Streptococcus pneumoniae, with MICs of 0.2 to 1.5 μg/ml. Mefloquine was not active against gram-negative bacteria and yeasts. In an attempt to find more active derivatives, 400 mefloquine-related compounds were selected from the chemical inventory of The Walter Reed Army Institute of Research. We identified a series of compounds containing a piperidine methanol group attached to pyridine, quinoline, and benzylquinoline ring systems. These had activities similar to that of mefloquine against S. pneumoniae but were far more active against other gram-positive bacteria (MICs for staphylococci, 0.8 to 6.3 μg/ml). They had activities similar to that of amphotericin B against Candida spp. and Cryptococcus neoformans. Combinations of the compounds with gentamicin and vancomycin were additive against staphylococci and pneumococci. The MIC and MBC of gentamicin were decreased by four- to eightfold when this drug was combined with limiting dilutions of the compounds. There was no antagonism with other antimicrobial drugs. The compounds were rapidly bactericidal. They appear to act by disrupting cell membranes. Combinations of the compounds with aminoglycoside antibiotics may have potential for therapeutic use.
The antimicrobial era has reached the point where the emergence of resistant microbes is accelerating while the pace of discovery of new drugs is decelerating (11). Until recently, a new drug or combination arrived just in time to overcome the problem of resistance. Few novel chemical entities have been brought to the market during the past decade. Most new drugs are derivatives of older compounds. Some have increased activity, a broader spectrum of activity, or improved pharmacological properties but can only temporarily overcome the problem of resistance. There is a need for new classes of antimicrobial compounds. This need is particularly critical for infections caused by methicillin- and vancomycin-resistant strains of Staphylococcus aureus, coagulase-negative staphylococci, vancomycin-resistant enterococci, and penicillin-resistant pneumococci.
Quinine and several other antimalarial drugs, including mefloquine, have been reported to exhibit activity against Streptococcus pneumoniae, S. aureus, and Escherichia coli (2, 3, 7, 8). Mefloquine is also active against Mycobacterium avium complex (1). In preliminary experiments, one of the authors (C.M.K.) found that mefloquine exhibited in vitro activity against gram-positive bacteria, including methicillin-resistant staphylococci, pneumococci, and streptococci, but was much less active against gram-negative bacteria. MIC of mefloquine for staphylococci and enterococci was 16 μg/ml, and the minimal bactericidal concentration (MBC) ranged from 16 to 32 μg/ml. These concentrations cannot be achieved in human serum. Mefloquine is highly lipid soluble and has a large volume of distribution (5). In an attempt to find more active derivatives, a collaborative arrangement was developed between The Ohio State University (OSU) and The Walter Reed Army Institute of Research (WRAIR). W.Y.E. selected about 400 mefloquine-related compounds from the WRAIR chemical inventory. Among these were compounds containing a piperidine attached to methanol at its 2 position, which in turn was attached to pyridine, quinoline, or benzylquinoline ring systems. These were found to be far more active than mefloquine against gram-positive bacteria. Some were also active against Candida albicans and Cryptococcus neoformans.
In this report, we describe the antimicrobial spectra of mefloquine and the related compounds, their synergy with gentamicin against staphylococci and pneumococci, and preliminary studies of their mode of action (4a).
MATERIALS AND METHODS
Microorganisms.
Microorganisms consisted of American Type Culture Collection strains and clinical isolates of methicillin-susceptible and -resistant S. aureus and Staphylococcus epidermidis, Streptococcus pyogenes, gentamicin- and vancomycin-resistant Enterococcus faecalis and Enterococcus faecium, penicillin-susceptible and -resistant strains of S. pneumoniae, E. coli, Enterobacter cloacae, Proteus mirabilis, Klebsiella pneumoniae, Pseudomonas aeruginosa, Haemophilus influenzae, Neisseria gonorrheae, Neisseria meningitidis, C. albicans, Candida spp., and C. neoformans. In addition, 21 clinical isolates of penicillin-susceptible and -resistant strains of S. pneumoniae and 24 clinical isolates of methicillin-susceptible and -resistant strains of S. aureus were provided by Christian Parker of Procter & Gamble Pharmaceuticals, Mason, Ohio.
Chemicals.
Mefloquine (Ro 21-5998-000) was provided by Roche Laboratories, Nutley, N.J. All the antimicrobial drugs were obtained from Sigma Chemical Co., St. Louis, Mo.
Susceptibility tests.
Bacteria were grown overnight at 35°C in Mueller-Hinton broth (MHB) (Difco Laboratories, Detroit, Mich.) and streaked on blood agar plates containing 5% sheep cells. A single colony was isolated and grown in MHB as recommended by the National Committee for Clinical Laboratory Standards (NCCLS) (9) for rapidly growing bacteria. Candida spp. and C. neoformans were incubated overnight at 35°C and assayed according to the NCCLS method (10) in RPMI 1640 medium (YeastOne; Trek Diagnostic Systems, Westlake, Ohio). Neisseriae were tested in Fildes medium (Difco).
The antibiotic susceptibility profile for each bacterial strain was determined with standard microtiter dilution plates obtained from the Clinical Microbiology Laboratory at Ohio State University Hospitals. The panel contained 16 different antimicrobial drugs. Inocula were prepared by suspending 4-h log-phase growth in MHB to a turbidity visually equal to the turbidity of a 0.5 McFarland standard. Inocula were further diluted and added to microdilution trays to achieve a final density of approximately 105 CFU/ml. The trays were incubated for 16 to 20 h at 35°C. The highest dilution at which the wells remained clear was considered MIC.
Screening.
WRAIR shipped the compounds to OSU under code. Some identical compounds were shipped with different code numbers to check reproducibility. The structures were not revealed until screening was completed. The compounds were dissolved in 1 ml of methanol or dimethyl sulfoxide, diluted in distilled water, and used the same day. Antimicrobial activity was determined with two strains of S. aureus. S. aureus ATCC 29213 is highly susceptible to penicillin and other antibacterial drugs. S. aureus T67738 is highly resistant to penicillin, oxacillin, gentamicin, trimethoprim-sulfamethoxazole, and ciprofloxacin but susceptible to imipenem, clindamycin, erythromycin, and vancomycin. The MIC was determined by the twofold dilution microtiter plate method. After 24 h of incubation, 0.01 ml was taken from the last two tubes or wells without visible growth and streaked on Trypticase soy agar plates (Difco). The highest dilution at which 99.9% of the bacterial inoculum was killed was considered the MBC.
Synergy and antagonism.
Serial dilutions of the compounds alone or in combination with tetracycline, trimethoprim, cefazolin, ofloxacin, clarithromycin, rifampin, gentamicin, and vancomycin were prepared in microtiter wells by the checkerboard pattern method. Combinations were considered synergistic if the sum of the fractional MICs was 0.5 or less. They were considered additive if the MIC was half the MIC for both drugs (4). Intermediate results were considered additive.
Effect of compounds on optical density and release of nucleic acids.
S. aureus T67738 was grown for 14 h at 35°C in MHB. The cells were collected by centrifugation and resuspended in 5 ml of a 1:10 dilution of MHB to yield an optical density at 550 nm of 0.400 in a Spectronic 601 spectrophotometer (Milton Roy, Rochester, N.Y.). Compounds at five times the MIC or the same volume of water was added to 2 ml of the suspension. The optical density was determined at intervals of up to 24 h. Samples were removed at 0, 1, and 3 h and centrifuged at 10,000 × g for 5 min. DNA was extracted from the supernatant, applied to an 0.8% agarose gel, and stained with ethidium bromide (12).
DNA gyrase inhibition.
DNA gyrase inhibition was determined as described by Inoue et al. (6) with Micrococcus luteus DNA gyrase, topoisomerase I, and the supercoiled plasmid pBR322 (Gibco BRL, Rockville, Md.). Ciprofloxacin served as the positive control.
RESULTS
Antimicrobial activity of mefloquine.
The MIC of mefloquine was 16 μg/ml for all strains of staphylococci, regardless of susceptibility or resistance to penicillin, oxacillin, gentamicin, imipenem, ciprofloxacin, or other antimicrobial drugs within the ranges published by the NCCLS (Table 1). The MBC for all strains of staphylococci was either 16 or 32 μg/ml. At concentrations of 32 μg/ml, mefloquine reduced the count of two strains each of S. aureus and S. epidermidis from 2 × 108 to less than 1 × 101 CFU/ml after 24 h of incubation. Mefloquine was highly active against S. pneumoniae (0.2 to 1.5 μg/ml), was less active against Streptococcus faecalis, and showed poor or no activity against gram-negative bacteria and C. albicans.
TABLE 1.
MICs and MBCs of mefloquine against gram-positive and gram-negative bacteria and C. albicans
| Microorganism | MIC (μg/ml) | MBC (μg/ml) |
|---|---|---|
| Staphylococcus aureus | ||
| ATCC 29213 | 16 | 16 |
| MRSAa (10 clinical isolatesb) | 16 | 16–32 |
| Staphylococcus epidermidis | ||
| ATCC 35547 | 16 | 16 |
| MRSEc (1 penicillin-susceptible clinical isolate) | 16 | 16 |
| Streptococcus pneumoniaed | ||
| ATCC 6301 | 0.8 | 1.5 |
| ATCC 6303 and 49619 | 0.4 | 0.4 |
| Streptococcus faecalis ATCC 29212 | 16 | 64 |
| Escherichia coli ATCC 25922 | 32 | 32 |
| Proteus mirabilis ATCC 7002 | 256 | 256 |
| Pseudomonas aeruginosa ATCC 27853 | 256 | >256 |
| Enterobacter cloacae clinical isolate | 128 | 128 |
| Klebsiella pneumoniae clinical isolate | 64 | 64 |
| Candida albicans ATCC 90028 | >25 |
MRSA, methicillin-resistant S. aureus.
Six of these strains were resistant to ciprofloxacin.
MRSE, methicillin-resistant S. epidermidis.
For 21 penicillin-susceptible and -resistant clinical isolates tested, MICs (micrograms per milliliter) were 0.2 to 1.5 (range), 0.4 (inhibiting 50% of isolates), and 0.8 (inhibiting 90% of isolates).
The effect of various antimicrobial drugs in combination with mefloquine was examined in checkerboard pattern experiments with four strains of S. aureus and three strains of S. epidermidis. There was no antagonism with cefazolin, ofloxacin, trimethoprim, tetracycline, and clarithromycin. An additive effect was noted with vancomycin against most of the strains and with gentamicin and rifampin against some strains.
Screening of mefloquine-related compounds.
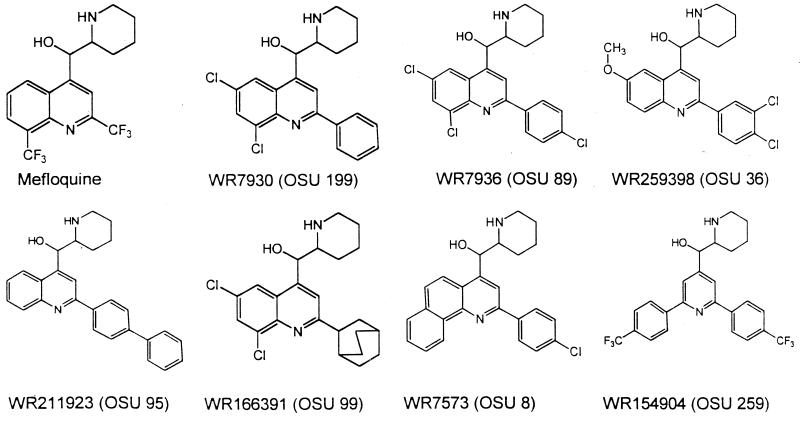
Thirty-six compounds were found in the screening program to be active against both penicillin-susceptible S. aureus ATCC 29213 and methicillin-resistant S. aureus T67738 at concentrations of 0.8 to 1.56 μg/ml and bactericidal at 1.56 to 2.5 μg/ml. These compounds resembled mefloquine. The chemical structures of some of the most active compounds are shown in Fig. 1. They are designated by WRAIR (WR) numbers. The OSU code numbers are used to simplify presentation in the tables.
FIG. 1.
Structures of mefloquine and related compounds (WR, WRAIR catalog number; OSU, OSU code number).
Antibacterial spectrum of mefloquine-related compounds.
The activities of the compounds against gram-positive bacteria are summarized in Table 2. The MICs and MBCs were usually the same. In some instances, particularly with the enterococci, the MBC was one twofold dilution higher. The MIC ranges for penicillin-susceptible and -resistant S. pneumoniae and methicillin-susceptible and -resistant S. aureus were virtually identical and were pooled in Table 2.
TABLE 2.
Activities of mefloquine-related compounds against gram-positive bacteria
| Microorganism and breakdown of MICa | MIC (μg/ml) of OSU compound:
|
||||
|---|---|---|---|---|---|
| 8 | 95 | 99 | 199 | 259 | |
| Staphylococcus aureus | |||||
| ATCC 29213 | 6.3 | 1.5 | 1.5 | 6.3 | 3.2 |
| T67738b | 6.3 | 1.5 | 1.5 | 6.3 | 1.5 |
| Clinical isolates (n = 24)c | 3.2 | 1.5 | 1.5 | 6.3 | 3.2 |
| Range | 0.8–6.3 | 0.8–3.2 | 0.8–3.2 | 3.2–6.3 | 0.8–3.2 |
| 50% | 1.5 | 1.5 | 1.5 | 6.3 | 1.5 |
| 90% | 3.1 | 1.5 | 1.5 | 6.3 | 1.5 |
| Staphylococcus epidermidis ATCC 12228 | 1.5 | 1.5 | 1.5 | 6.3 | 1.5 |
| Streptococcus pneumoniae | |||||
| ATCC 9163 | 0.15 | 0.32 | 0.15 | 1.25 | |
| ATCC 6303 | 0.15 | 0.15 | 0.08 | 0.63 | |
| Clinical isolates (n = 21)d | |||||
| Range | 0.08–0.63 | 0.08–2.5 | 0.08–1.25 | 0.02–5.0 | 0.15–2.5 |
| 50% | 0.15 | 0.15 | 0.32 | 0.15 | 0.63 |
| 90% | 0.32 | 1.25 | 0.63 | 1.25 | 1.25 |
| Streptococcus pyogenes | |||||
| ATCC 12344 | 1.6 | 3.1 | |||
| W61967a | 1.6 | 3.1 | |||
| Enterococcus faecalis | |||||
| ATCC 29212 | 6.3 | 1.5 | 1.5 | 12.5 | 1.5 |
| F21351 (gentamicin resistant)e | 3.1 | 6.3 | |||
| Enterococcus faecium EF-12 (vancomycin resistant)e | 0.8 | 1.5 | 1.5 | 6.3 | 1.5 |
50% and 90%, MICs at which 50 and 90% of isolates were inhibited, respectively.
Multiresistant clinical isolate used in the screening program.
Methicillin-susceptible and -resistant clinical isolates.
Penicillin-susceptible and -resistant clinical isolates.
Clinical isolates.
The compounds were relatively inactive against gram-negative bacteria. MICs (in micrograms per milliliter) of compound 95, which is representative, were as follows: E. coli ATCC 25922, 12.5; P. aeruginosa ATCC, 100; N. meningitidis ATCC 13077, 6.26; and clinical isolates of P. mirabilis, K. pneumoniae, H. influenzae, and N. gonorrheae, 50, 50, 25, and 3.13, respectively.
Antifungal spectrum of mefloquine-related compounds.
The compounds were about as active as amphotericin B against yeasts (Table 3). The MBCs were equal to and in some cases twofold higher than the MICs. In killing curve experiments, the cell counts fell 4 to 5 log10 units within 4 h.
TABLE 3.
Activities of mefloquine-related compounds against yeasts
| Microorganism | MIC (μg/ml) of:
|
|||||
|---|---|---|---|---|---|---|
| OSU compound:
|
Amphotericin B | |||||
| 8 | 95 | 99 | 199 | 259 | ||
| Candida albicans ATCC 90028 | 0.8 | 3.1 | 1.5 | 2.5 | 0.4 | 1.2 |
| Candida glabrata ATCC 90030 | 0.8 | 1.5 | 0.8 | 6.2 | 3.1 | 0.6 |
| Candida kruzei ATCC 6258 | 1.5 | 1.5 | 0.8 | 3.1 | 0.8 | 0.6 |
| Candida parapsilosis ATCC 90018 | 0.4 | 1.5 | 0.8 | 1.5 | 0.4 | 1.2 |
| Cryptococcus neoformans | ||||||
| ATCC 90112 | 0.8 | 1.5 | 0.4 | 1.5 | 0.4 | 0.3 |
| ATCC 90113 | 0.8 | 1.5 | 0.4 | 1.5 | 0.4 | 0.6 |
Interactions of mefloquine-related compounds with gentamicin and vancomycin.
The antimicrobial activities of compounds 95 and 99 in combination with gentamicin and vancomycin were examined by checkerboard pattern experiments with methicillin-susceptible and -resistant strains of S. aureus. The MICs and MBCs of the compounds were decreased fourfold when the compounds were combined with limiting dilutions of gentamicin. The MICs and MBCs of gentamicin were decreased four- to eightfold when gentamicin was combined with limiting dilutions of the compounds. In contrast, there was only a twofold decrease in the MICs and MBCs of the compounds combined with vancomycin.
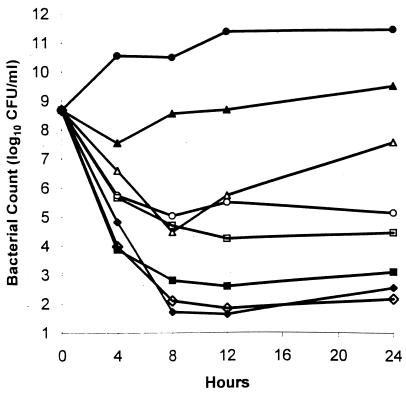
Killing curves were determined with a large inoculum of S. aureus ATCC 29213 (108.6 CFU/ml) (Fig. 2). The MIC of compound 95 was 1 μg/ml. Bactericidal activity was noted at concentrations of 4 and 6 μg/ml. Combinations of 1 μg of compound 95 per ml with 1 μg of gentamicin or vancomycin per ml were more bactericidal than any of the agents alone (Fig. 2). Similar results were obtained with methicillin-resistant S. aureus T67738.
FIG. 2.
Antibacterial activities of compound 95, gentamicin, and vancomycin alone or in combination against penicillin-susceptible S. aureus ATCC 29213. Symbols: ●, control; ▴, vancomycin at 1 μg/ml; ○, gentamicin at 1 μg/ml; ▵, compound 95 at 1 μg/ml; ■, compound 95 at 4 μg/ml; ⧫, compound 95 at 6 μg/ml; □, compound 95 at 1 μg/ml plus vancomycin at 1 μg/ml; and ◊, compound 95 at 1 μg/ml plus gentamicin at 1 μg/ml.
Gentamicin was also noted to augment the activity of the compounds against S. pneumoniae ATCC 6303. Combinations of compounds 95 and 199 with gentamicin and vancomycin were examined by checkerboard pattern experiments. The MICs and MBCs of the compounds fell twofold in combinations with limiting dilutions of gentamicin. The MICs and MBCs of gentamicin fell eightfold (from 10 to 1.25 μg/ml) in combinations with limiting dilutions of either compound. Combinations of the compounds with vancomycin resulted in a twofold decrease in their MICs and MBCs.
Preliminary studies of mode of action.
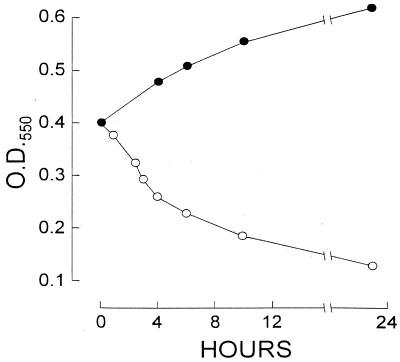
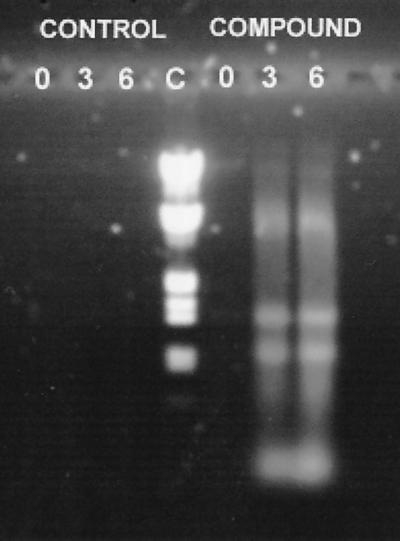
The compounds appeared to lyse staphylococci, as evidenced by a rapid fall in optical spectroscopy and release of DNA into the medium (Fig. 3 and 4). None of six compounds tested inhibited DNA gyrase activity.
FIG. 3.
Change in the optical density at 550 nm (OD550) of methicillin-resistant S. aureus T67738 incubated at 37°C in MHB. Symbols: ●, control; ○, compound 199 at 15 μg/ml (five times the MIC).
FIG. 4.
Agarose gel stained with ethidium bromide to visualize nucleic acids that leaked from methicillin-resistant S. aureus T67738 incubated at 37°C in MHB (control) and with 15 μg of compound 199 per ml (three times the MIC). The numbers refer to hours of incubation. C, nucleic acid standards.
DISCUSSION
We found that mefloquine was bactericidal against gram-positive bacteria, including staphylococci, pneumococci, and enterococci. It had poor activity against gram-negative enteric bacteria and yeasts. There was no cross-resistance or antagonism with beta-lactam antibiotics, quinolones, and other antimicrobial drugs. No intrinsically resistant strains were isolated from large inocula exposed to the drug. Mefloquine is highly lipid soluble. It has a large volume of distribution and a long serum half-life, is bound to serum proteins, and accumulates intracellularly (1, 5). Peak levels in blood following standard therapeutic and prophylactic doses weekly are in the range of 979 to 1,500 ng/ml (13, 14). These values are far lower than the MIC of 16 μg/ml for staphylococci but are close to the MIC for S. pneumoniae.
The WRAIR screening program yielded a series of mefloquine-related compounds that possess a piperidine attached to methanol at its 2 position, which in turn was attached to a variety of ring structures. Compounds without the piperidine methanol group were inactive. Their antimicrobial spectrum resembled that of mefloquine. Their activity against S. pneumoniae was similar to that of mefloquine, but they were more active against staphylococci, enterococci, and yeasts.
The compounds appear to act by disrupting the microbial cell membrane. Increased membrane permeability may account for their ability to augment the activity of gentamicin. The precise mechanism of action is unclear, but quinine has been shown to specifically inhibit the membrane-associated F0F1 H+-ATPase of S. pneumoniae (8). The compounds did not inhibit DNA gyrase activity by the method used in this study and were active against quinolone-resistant staphylococci.
High lipid solubility and serum protein binding may limit the potential therapeutic efficacy of mefloquine and its related compounds when used alone against extracellular infections. Subinhibitory concentrations of the mefloquine-related compounds in combination with gentamicin might be effective against pneumococcal, staphylococcal, and enterococcal infections.
The mefloquine-related compounds were highly active against C. neoformans at MICs similar to those of amphotericin B. Their lipid solubility favors penetration into the central nervous system and cells. Mefloquine has already been shown to be active against intracellular infections caused by M. avium (1). The activities of the mefloquine-related compounds against Histoplasma capsulatum and other intracellular microorganisms warrant further study.
The pharmacology, toxicology, and potential efficacy of the mefloquine-related compounds need to be determined. Water-soluble derivatives, metabolites, or prodrugs might be advantageous. Combinations of the compounds with aminoglycoside antibiotics offer the greatest potential for therapeutic use. The WRAIR inventory should be further explored for interesting antibacterial and antifungal compounds.
ACKNOWLEDGMENTS
This study was supported by grants from the Department of Internal Medicine and The Ohio State University College of Medicine and Public Health.
We thank Hua Hua Tong and Da Neng Li for excellent technical assistance.
REFERENCES
- 1.Bermudez L E, Kolonoski P, Wu M, Aralar P A, Inderlied C B, Young L S. Mefloquine is active in vitro and in vivo against Mycobacterium avium complex. Antimicrob Agents Chemother. 1999;43:1870–1875. doi: 10.1128/aac.43.8.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bromke B J, McGinn M. In vitro susceptibility of selected non-protozoa to mefloquine. Acta Microbiol Hung. 1993;40:387–389. [PubMed] [Google Scholar]
- 3.Brown R E, Stancato F A, Wolfe A D. The effects of mefloquine on Escherichia coli. Life Sci. 1979;25:1857–1864. doi: 10.1016/0024-3205(79)90434-x. [DOI] [PubMed] [Google Scholar]
- 4.Cleeland R, Grunberg E. Laboratory evaluation of new antibiotics in vitro and in experimental animal infections. In: Lorian V, editor. Antibiotics in laboratory medicine. 2nd ed. Baltimore, Md: The Williams & Wilkins Co.; 1986. pp. 833–834. [Google Scholar]
- 4a.Ellis, W. Y., and C. M. Kunin. October 1999. U.S. patent 5,965,572.
- 5.Gilman A G, et al., editors. Goodman and Gilman's the pharmacologic basis of therapeutics. 8th ed. New York, N.Y: McGraw-Hill Book Co.; 1993. [Google Scholar]
- 6.Inoue Y, Kenichi S, Fujii T, Hirai K, Inoue M, Iyobe S, Mitsuhashi S. Some properties of subunits of DNA gyrase from Pseudomonas aeruginosa PAO1 and its nalidixic acid-resistant mutant. J Bacteriol. 1987;169:2322–2325. doi: 10.1128/jb.169.5.2322-2325.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehaffy P C, Barrett M S, Putnam S D, Jones R N. Antigonococcal activity of 11 drugs used for therapy or prophylaxis of malaria. Diagn Microbiol Infect Dis. 1995;23:11–13. doi: 10.1016/0732-8893(95)00148-4. [DOI] [PubMed] [Google Scholar]
- 8.Muñoz R, Garcia E, De La Campa A G. Quinine specifically inhibits the proteolipid subunit of the F0F1 H+-ATPase of Streptococcus pneumoniae. J Bacteriol. 1996;178:2455–2458. doi: 10.1128/jb.178.8.2455-2458.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A2. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1990. [Google Scholar]
- 10.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard M27-A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 11.Neu H C. The crisis in antibiotic resistance. Science. 1992;157:1064–1073. doi: 10.1126/science.257.5073.1064. [DOI] [PubMed] [Google Scholar]
- 12.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 13.Todd G D, Hopperus Buma A P, Green M D, Jaspers C A, Lobel H O. Comparison of whole blood and serum levels of mefloquine and its carboxylic acid derivative. Am J Trop Med Hyg. 1997;57:399–402. doi: 10.4269/ajtmh.1997.57.399. [DOI] [PubMed] [Google Scholar]
- 14.Weidekamm E, Rusing G, Caplain H, Sorgel F, Crevoisier C. Lack of bioequivalence of a generic mefloquine tablet with the standard product. Eur J Clin Pharmacol. 1998;54:615–619. doi: 10.1007/s002280050523. [DOI] [PubMed] [Google Scholar]