Abstract
Background
Sudden cardiac death (SCD) risk stratification in hypertrophic cardiomyopathy (HCM) currently relies on arrhythmic burden quantification by 24 or 48-hour Holter monitoring. Whether this approach adequately captures arrhythmic burden, compared with longer-term continuous monitoring, is unclear. We sought to assess the long-term incidence of nonsustained ventricular tachycardia (NSVT) in HCM patients at low or moderate SCD risk, using implantable cardiac monitors (ICMs) paired with a novel Bluetooth-enabled 2-way communication platform.
Methods
This prospective, single-arm, observational study enrolled 33 HCM patients. Patients were implanted with an Abbott (Chicago, IL) Confirm Rx ICM and monitored using a protocolized care pathway.
Results
A total of 20 patients (60.6%) had ≥ 1 episode of NSVT recorded on the ICM, the majority of whom had previous Holter monitors that did not identify NSVT (60%, n = 12). A total of 71 episodes of NSVT were detected. Median time to first NSVT detection was 76.5 days (range: 0-553 days). A total of 19 patients underwent primary prevention implantable cardioverter defibrillator implantation during an average follow-up of 544 days (range: 42-925 days). A total of 172,112 automatic transmissions were received, and 65 (0.04%) required clinical follow-up. A total of 325 manual transmissions were received and managed. A total of 14 manual transmissions (4.3%) required follow-up, whereas 311 (95.7%) were managed solely with a text message.
Conclusions
Surveillance and reporting systems utilizing 2-way communication enabled by novel ICMs are feasible and allow remote management of patients with HCM. Prolonged monitoring with ICMs identified more patients with nonsustained arrythmias than did standard Holter monitoring. In many cases, this information impacted both SCD risk stratification and patient management.
Résumé
Contexte
La stratification du risque de mort cardiaque subite (MCS) dans la cardiomyopathie hypertrophique (CMH) dépend actuellement de la quantification de la charge arythmique par une surveillance Holter de 24 ou 48 heures. Il n’est pas clair si cette approche permet d’évaluer adéquatement la charge arythmique, comparativement à une surveillance continue à plus long terme. Nous avons cherché à évaluer la fréquence à long terme de la tachycardie ventriculaire non soutenue (TVNS) chez des patients atteints de CMH à risque faible ou modéré de MCS, au moyen de moniteurs cardiaques implantables (MCI) couplés à une nouvelle plate-forme de communication bidirectionnelle utilisable avec Bluetooth.
Méthodologie
Cette étude par observation prospective comportant un seul groupe a été menée auprès de 33 patients atteints de CMH. Les patients ont reçu un MCI Confirm Rx d’Abbott (Chicago, États-Unis) et ont été surveillés dans le cadre d’un parcours de soins reposant sur un protocole.
Résultats
Au total, 20 patients (60,6 %) ont eu au moins un épisode de TVNS enregistré par le MCI. La majorité de ces patients portaient déjà un moniteur Holter qui n’a pas décelé de TVNS (60 %, n = 12). Au total, 71 épisodes de TVNS ont été détectés. Le temps médian écoulé avant la première détection de TVNS était de 76,5 jours (fourchette : 0-553 jours). Au total, 19 patients se sont fait poser un défibrillateur cardioverteur implantable en prévention primaire pendant un suivi moyen de 544 jours (fourchette : 42-925 jours). En tout, 172 112 transmissions automatiques ont été reçues, et 65 (0,04 %) ont nécessité un suivi clinique. Par ailleurs, 325 transmissions manuelles ont été reçues et traitées. De ce nombre, 14 transmissions (4,3 %) ont nécessité un suivi, tandis que 311 (95,7 %) ont été traitées uniquement au moyen d’un message texte.
Conclusions
Les systèmes de surveillance et de signalement utilisant une communication bidirectionnelle rendue possible grâce aux nouveaux MCI sont réalisables et permettent une prise en charge à distance des patients atteints d’un CMH. La surveillance prolongée par un MCI a permis de déceler plus d’arythmies non soutenues que la surveillance Holter type. Dans de nombreux cas, ces renseignements ont eu un effet positif tant sur la stratification du risque de MCS que sur la prise en charge des patients.
Hypertrophic cardiomyopathy (HCM) is the most common inherited heart muscle disorder and carries a risk of sudden cardiac death (SCD) from ventricular arrhythmias.1 Following a diagnosis of HCM, patients undergo risk stratification for SCD, which informs the need for primary prevention implantable cardioverter defibrillators (ICDs).
Risk stratifying patients for SCD remains a major challenge in the management of HCM. European guidelines use a risk score calculated by 7 risk factors to determine the 5-year risk of SCD.2 Patients are then grouped into 3 risk categories (low, medium, or high) with accompanying primary prevention ICD recommendations (ICD not recommended, can be considered, or recommended, respectively). In contrast, American Heart Association/American College of Cardiology (AHA/ACC) guidelines base decisions for recommendations regarding primary prevention ICDs on the presence of 1 or more major risk factors.3 Changes in AHA/ACC guidelines from 2011 to 2020 include recommendations relating to the emergence of new risk markers, including apical aneurysms, late gadolinium enhancement (LGE) on cardiac magnetic resonance imaging, and decreased left-ventricular (LV) systolic function. The calculation of the impact of nonsustained ventricular tachycardia (NSVT) has also been adjusted to factor in patient age.
NSVT remains an incompletely characterized risk marker, generally defined as ≥ 3 consecutive beats at a rate ≥ 120 beats per minute (bpm) but < 30 seconds in duration.2,3 Traditionally, detection and quantification of NSVT episodes rely on the use of 24- to 48-hour Holter monitoring.4 The challenge with this approach is that NSVT episodes are paroxysmal, infrequent, and may be missed during a single 48-hour period.5 Implantable cardiac monitors (ICMs) have the potential to improve the detection and quantification of NSVT episodes.6 Some ICMs can be paired with patient smart phone–enabled applications and communicate with these devices through Bluetooth technology. Currently, no guidelines indicate how these devices can be used in HCM risk stratification. Furthermore, the amount of data generated by these devices can be overwhelming for clinical teams.7 This pilot study aims to assess the performance of ICMs, characterize the incidence of arrhythmias in HCM patients, and propose a care pathway that includes 2-way smartphone communication between patient and care teams to standardize remote care of patients with HCM.
Methods
Patient enrollment
Thirty-three HCM patients were implanted with Bluetooth-enabled Confirm Rx ICMs (Abbott, Chicago, IL) at the University of British Columbia in Canada. Patients were prospectively offered this option if they were felt to be at moderate risk of SCD by their clinical team. Patients included had low (< 4% risk of SCD in 5 years) or moderate risk (4%-6% risk of SCD in 5 years), according to the European Society of Cardiology (ESC) HCM Risk-SCD calculator, either had a class IIA (ICD reasonable) or IIB (ICD can be considered) recommendation for an ICD, according to AHA/ACC guidelines, or had other risk factors highlighted by the recent AHA guidelines. Patients at high risk who declined an ICD were also enrolled to allow for early notification of high-risk episodes, assess arrhythmia incidence, and provide direct patient feedback for symptomatic episodes. Patients received education related to the indication, use, and planned care pathway from the research team, the Abbott representative, and a customized video tutorial generated by the research team (Video 1
 , view video online). Study protocols were approved by institutional ethics committees (study ID: H17-02707).
, view video online). Study protocols were approved by institutional ethics committees (study ID: H17-02707).
Device-detected automatic transmissions and patient-reported manual transmissions
Automatic transmissions were uploaded directly to Abbott’s patient care network and electrograms (EGMs) were adjudicated by a device-trained nurse (C.M.), with further adjudication conducted by a cardiac electrophysiologist (Z.L.) if necessary. ICMs were programmed to send automatic transmissions following the detection of atrial fibrillation (AF; ≥ 30 seconds of AF, based on the ConfirmRx SharpSense Technology AF Detection Algorithm), ventricular tachyarrhythmias (≥ 140 bpm for ≥ 8 beats), ventricular pauses (> 5 seconds), bradycardia (≤ 40 bpm), or significant ST shifts (> 2 mm).
Patients had the option to use their smartphone app to self-report symptomatic episodes (Supplemental Fig. S1). The device would then upload an EGM corresponding to the time of symptom onset, for adjudication. Manual transmissions were classified as “green,“ “orange,” or “red” alerts, depending on the associated symptom or EGM recordings. Any symptomatic NSVT, ventricular tachycardia, or any self-reported syncopal episodes were indications for a red alert. An NSVT episode, new-onset AF, a pause > 5 seconds, and significant ST changes associated with symptoms were indications for an orange alert. Any other EGM findings were considered a green alert. Regardless of the level of intervention, all patients received a follow-up text message from the care team outlining the expected next steps (Supplemental Fig. S2).
Automatic transmissions were submitted if an episode of AF, tachycardia (either ventricular or supraventricular), bradycardia, or pause was detected by the ICM. These were classified by the research team as green, orange, or red alerts, based on the EGM recordings. The criteria for these classifications were the same as those used for manual transmissions. In contrast to manual transmissions, however, messaging to patients was sent only for automatic transmissions classified as orange or red.
Patients with documented NSVT episodes on extended monitoring had EGMs reviewed with an interdisciplinary team that included 4 or more cardiac electrophysiologists. The EGMs were reviewed in the context of other clinical risk factors, and consensus decisions were made regarding recommendations for ICD intervention. Patients with ICM-detected NSVT episodes were recommended to start beta-blocker treatment if this was not already prescribed.
Statistical analysis
Data for continuous variables are displayed as mean with standard deviation (SD), range, and quartiles, as appropriate. Data for categorical variables are displayed as proportions. Graphs were generated and analyzed using Prism GraphPad 9.0.0 (La Jolla, CA). Unless otherwise stated, statistics for between-group comparisons were calculated using the t test.
Results
Patient demographics
Table 1 lists all patient demographic and baseline risk factors. ICMs were implanted in 33 patients (20 male, 60.6%), with a mean age of 51.8 years upon implantation (range: 23.8-73.6 years). Aggregate data were collected from implantation date until May 1, 2021. Average duration of monitoring was 544 days (range: 42-925 days), or 49 person-years. Most patients (25 of 33, 75.8%) had a discussion with the clinical care team regarding ICD therapy and their assigned risk assessment and status according to current guidelines, upon enrollment in the study. All patients included in the study declined primary prevention ICD therapy and opted for continuous rhythm monitoring.
Table 1.
Clinical characteristics of 33 patients at time of enrollment
| Age, y | 51.8 (SD 12.4) |
| Male | 20 (60.6) |
| Offered ICD prior to study | 25 (75.8) |
| ESC 5-year risk of sudden cardiac death | |
| Low risk (< 4%) | 20 (60.6) |
| Moderate risk (4%–6%) | 9 (27.3) |
| High risk (> 6%) | 4 (12.1) |
| AHA/ACC classification | |
| Class I | 0 (0.0) |
| Class IIA | 18 (54.5) |
| Class IIB | 9 (27.3) |
| Class III | 6 (18.2) |
| Risk factors | |
| Family history of sudden cardiac death | 6 (18.2) |
| Maximum wall thickness, mm | 21.7 (SD 4.9) |
| Unexplained syncope | 8 (24.2) |
| Prior nonsustained ventricular tachycardia | 11 (33.3) |
| Abnormal blood pressure response to exercise | 4 (12.1) |
| Left atrial diameter, mm | 41.6 (SD 7.7) |
| Maximum left ventricular outflow gradient | 30.1 (SD 34.9) |
| Late gadolinium enhancement | 25 (75.8) |
| Left apical aneurysm | 4 (12.1) |
| LVEF | 64% (SD 7.3) |
| Alcohol septal ablation | 1 (3.0) |
| Septal myectomy | 2 (6.1) |
| Genetic variants | |
| Pathogenic | 5 (15.2) |
| Likely pathogenic | 7 (21.2) |
| Variant of unknown significance | 5 (15.2) |
| Medications | |
| Beta-blocker | 17 (51.5) |
| Calcium-channel blocker | 10 (30.3) |
| Disopyramide | 2 (6.1) |
Values are n (%) or mean (SD).
ESC, European Society of Cardiology; AHA/ACC, American Heart Association/American College of Cardiology; ICD, implantable cardioverter defibrillator; LVEF, left ventricular ejection fraction; SD, standard deviation.
Most patients had low or moderate risk, per HCM-SCD risk scoring (28 of 33, 84.8%; Table 1). A total of 27 patients (81.8%) had either a class IIA or class IIB recommendation for an ICD, according to recent AHA guidelines. Risk factors considered by both ESC and AHA guidelines include family history of SCD (6 patients, 18.2%), unexplained syncope (8 patients, 24.2%), and maximum wall thickness (mean 21.7 mm, SD 4.9), with 2 patients demonstrating maximal wall thicknesses > 30 mm. In addition to these 3 risk factors, 2020 AHA guidelines also consider the presence of apical aneurysm on imaging (3 patients, 9.1%) and ejectiong fraction ≤ 50% (0 patients). The most recent ESC guidelines instead also consider age (mean 51.8 years, SD 12.4), LV outflow gradient (mean 30.1 mm Hg, SD 34.9), left atrial diameter (mean 41.6 mm, SD 7.7), and the presence of NSVT on previous ambulatory Holter monitoring (10 patients, 30.3%).
Cardiac magnetic resonance (CMR) imaging was performed on 29 patients. Magnetic resonance imaging data were available for review by a centralized core imaging facility in 25 patients (P.B., J.L., P.I., E.K.). Quantification of LGE mass as a percentage of the total LV mass was performed using commercially available software and standardized methods (Supplemental Appendix S1). Myocardial fibrosis in the form of LGE was present in 20 of 25 patients (80%) and was quantified as being extensive (> 15% of total LV mass) in 5 of 25 patients (20%; Supplemental Table S1). Genetic testing using an HCM gene panel was done in 24 patients. Variants were identified in 17 of these patients (70.8%), including 7 likely pathogenic and 5 pathogenic variants (Supplemental Table S2). The most common variants found on genetic testing were in MYH7 (8 patients) and MYBPC3 (7 patients), with one patient having both a pathogenic variant in MYBPC3 and a likely pathogenic variant in MYH7.
Automatic and manual transmissions
A total of 325 manual transmissions and 172,112 automatic transmissions were recorded. The average number of manual transmissions received per patient was 9.6 (range: 0-48). A total of 14 of 325 transmissions (4.3%) were considered clinically significant, 7 of 14 (50%) of which corresponded to NSVT episodes from 5 patients. One transmission was associated with a syncopal episode resulting in a red alert. Another significant transmission was an episode of presyncope and dyspnea that corresponded with high-grade atrioventricular block and a 3.4-second pause. Sixteen manual transmissions (4.9%) required adjudication by a cardiac electrophysiologist (Z.L.), after review by a device-trained clinic nurse (Supplemental Fig. S3).
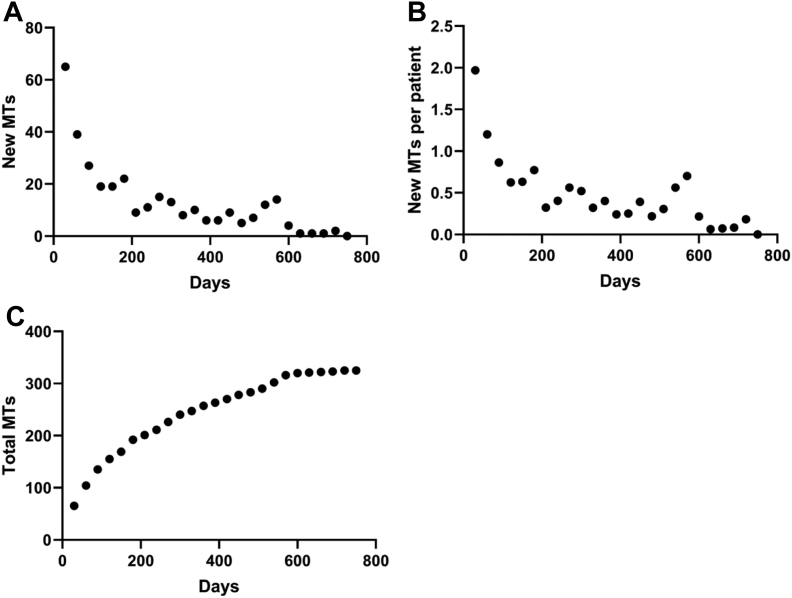
Analysis of manual transmissions per patient over time showed that the number of new manual transmissions decreased with time after device implantation (Fig. 1A). This trend was also observed for the total number of manual transmissions over time (Fig. 1B). Analysis of the rate of manual transmissions per day per patient during quartiles of the total follow-up period showed decreasing rates over time (Table 2). A total of 311 manual transmissions were characterized as green alerts. None of these manual transmissions led to a clinical encounter, and none of the patients contacted the research team, device clinic, or clinical care team for clarification after receiving a green alert.
Figure 1.
(A) New manual transmissions (MTs) for all patients over time. (B) New MTs per patient over time. (C) Total number of MTs over time.
Table 2.
Rate of manual transmissions (MTs) per patient per day across the total study length
| Quartiles of time since device implantation | Rate of MTs per patient per d |
|---|---|
| 1 | 0.033 |
| 2 | 0.015 |
| 3 | 0.016 |
| 4 | 0.007 |
Of 172,112 automatic transmissions, 29,709 (17.3%) of the EGMs were sinus tachycardia (over the detection limit of 8 beats at 140 bpm), and 91,860 (53.4%) were sinus bradycardia (under the detection limit of 40 bpm). Many patients had underlying variations in their baseline electrocardiogram signal, resulting in large numbers of transmissions during sinus rhythm. Review of these transmissions by nurses most often confirmed sinus rhythm (n = 55,150, 32.0%). A total of 64 orange alerts accounted for 0.04% of all automatic transmissions. One automatic transmission was a red alert, and this was for new-onset AF. Of the 65 orange and red transmissions, 57 of 64 (89.1%) were NSVT episodes, 5 of 64 (7.7%) were AF episodes, and 2 of 64 (3.1%) were pauses. All patients were contacted within 1 week for assessment of symptoms during orange alerts, per the predetermined care pathway (Supplemental Fig. S2). A total of 145 automatic transmissions (0.08%) required adjudication by a cardiac electrophysiologist (Z.L.; Supplemental Fig. S3).
NSVT
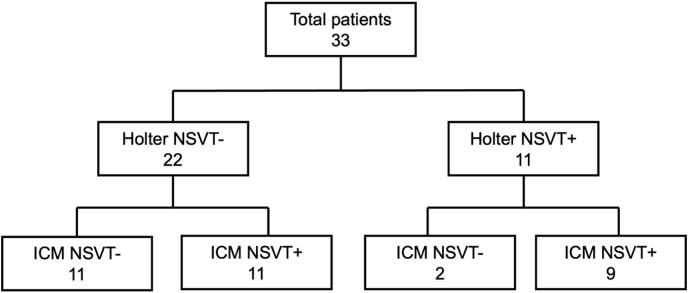
A total of 20 of 33 patients (60.6%) had at least one NSVT episode (≥ 140 bpm for ≥ 8 beats) detected on prolonged monitoring. A total of 71 NSVT runs were detected. All NSVT episodes were monomorphic. Two patients had NSVT previously detected on 24-hour ambulatory monitoring, but no recurrence of NSVT on prolonged ICM monitoring (Fig. 2). Subgroup analysis (Table 3) of patients with vs without NSVT detected on either Holter monitor or ICM revealed that patients with NSVT were older (mean age 54.3 vs 46.8 years, P = 0.13) and were more often male (15 [68.2%] vs 5 [45.5%], P = 0.24), but these findings were not statistically significant. A higher proportion of patients without NSVT had a recent history of syncope (36.4% vs 18.2%, P = 0.25) and a family history of SCD (27.3% vs 13.6%, P = 0.34). These differences were not statistically significant. Gadolinium enhancement in > 15% of myocardial tissue was similar in the 2 patient groups (13.6% vs 18.2%, P = 0.73). All 4 patients with left apical aneurysms on imaging had NSVT detected on ICM.
Figure 2.
Nonsustained ventricular tachycardia (NSVT) detected by implantable cardiac monitor (ICM), separated by prior detection (+ = NSVT detected; – = no NSVT detected) on 24-hour Holter monitoring.
Table 3.
Subgroup analysis comparing patients with [NSVT (+)] vs without [NSVT (-)] nonsustained ventricular tachycardia (NSVT) on implantable cardiac monitoring
| Characteristic | NSVT (+), n = 20 | NSVT (–), n = 11 | P |
|---|---|---|---|
| Age, y | 54.3 (SD 11.8) | 46.8 (SD 13.6) | 0.14 |
| Male | 14 (70) | 5 (45.5) | 0.18 |
| ESC 5-year risk of sudden cardiac death | |||
| Low risk (< 4%) | 12 (60) | 8 (72.7) | 0.75 |
| Moderate risk (4%-6%) | 6 (30) | 2 (18.2) | |
| High risk (> 6%) | 2 (10) | 1 (4.5) | |
| AHA/ACC class | |||
| I | 0 (0) | 0 (0) | 0.57 |
| IIA | 10 (50) | 8 (72.7) | |
| IIB | 6 (30) | 1 (9.1) | |
| III | 4 (20) | 2 (18.2) | |
| Risk factors | |||
| Family history of sudden cardiac death | 3 (15) | 3 (27.3) | 0.41 |
| Maximum wall thickness > 30 mm | 0 (0) | 2 (18.2) | 0.05 |
| Maximum wall thickness, mm | 21.0 (SD 3.7) | 22.5 (SD 6.8) | 0.74 |
| Unexplained syncope | 4 (20) | 4 (36.4) | 0.32 |
| Abnormal blood pressure response to exercise | 2 (10) | 2 (18.2) | 0.52 |
| Left atrial diameter, mm | 41.9 (SD 5.8) | 38.2 (SD 11.0) | 0.51 |
| Maximum left ventricular outflow gradient | 22.8 (SD 31.9) | 21.7 (SD 20.8) | 0.92 |
| Late gadolinium enhancement (> 15%) | 3 (15) | 2 (18.2) | 0.85 |
| Left apical aneurysm | 3 (15) | 0 (0) | 0.11 |
| LVEF, % | 63.6 (SD 8.5) | 65.3 (SD 5.5) | 0.47 |
Values are n (%) or mean (SD), unless otherwise indicated. P values for categorical variables were obtained via χ2 tests, and via t tests for continuous variables.
AHA/ACC, American Heart Association/American College of Cardiology; ESC, European Society of Cardiology; LVEF, left-ventricular ejection fraction; SD, standard deviation.
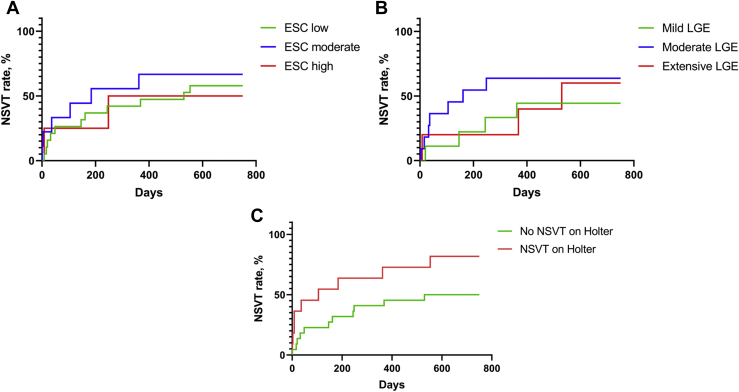
Percentage rates of NSVT episodes were plotted as Kaplan-Meier curves (Fig. 3). Four of the 11 patients (36.4%) with previously detected Holter-detected NSVT had an ICM-detected NSVT episode occurring within the first 10 days of implantation. A total of 7 of 11 patients (63.6%) had more than one NSVT episode on ICM monitoring (mean = 4.3 episodes; range = 2-9). Of 22 patients without previous Holter-detected NSVT, 3 patients had ICM-detected NSVT within the first 30 days of monitoring, and 1 of these episodes was within the first 10 days. No significant difference was found in percentage rates of NSVT episodes between patients grouped by ESC-SCD score, amount of LGE on CMR imaging, or presence of prior Holter-detected NSVT.
Figure 3.
Kaplan-Meier curves comparing subgroups of patients who had nonsustained ventricular tachycardia (NSVT) episodes as detected by an implantable cardiac monitor. (A) Comparison of European Society of Cardiology (ESC) low (< 4%), moderate (4%-6%), and high (> 6%) risk of 5-year mortality from sudden cardiac death. (Log-rank [Mantel-Cox] test P = 0.73.) (B) Comparison of groups based on amount of late gadolinium enhancement (LGE) on cardiac magnetic resonance imaging (mild, < 5%; moderate, 5%-15%; extensive, > 15%). (Log-rank [Mantel-Cox] test P = 0.56.) (C) Comparison of patients with vs without previously detected NSVT on a 24-hour Holter monitor. (Log-rank [Mantel-Cox] test P = 0.069.).
Holter-detected NSVT episodes were shorter in duration and number of beats, and they were slower in rate compared to ICM-detected episodes (Table 4). Across patients with NSVT identified on both Holter monitor and ICM (n = 9), patients on average had NSVT runs lasting 7 beats longer, and 22 bpm faster recorded on ICM. The longest NSVT episode recorded on ICM was 38 beats; the fastest episode was 213 bpm. A total of 18 patients (90%) had NSVT episodes that were ≥ 170 bpm as detected on ICM. Only 1 patient (5%) had an NSVT episode at > 200 bpm. The mean for heart rates during NSVT detected on ICM was 171 bpm (range: 140-213). The mean for number of beats during NSVT on ICM was 18 beats (range: 8-38). Of 71 NSVT episodes detected on ICM, 11 (15.5%) were symptomatic. The most common symptoms were presyncope/dizziness and palpitations.
Table 4.
Nonsustained ventricular tachycardia (NSVT) episodes recorded by 24-hour ambulatory monitoring (Holter) and implantable cardiac monitor (ICM) monitoring
| Age; sex | AHA/ACC ICD class | ESC risk score, % | Holter- NSVT duration, s | Number of Holter- NSVT beats, beats | Holter- NSVT rate, bpm | Days until first ICM-NSVT | Total number of ICM-NSVT episodes | Total days of ICM monitoring | Duration of ICM-NSVT, s∗ | Beats of ICM-NSVT, beats∗ | HR of ICM-NSVT, bpm∗ |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 53; M | IIB | 8.83 | 2.4 | 5 | 156 | 0 | — | — | — | — | — |
| 56; F | IIB | 4.74 | 3.3 | 5 | 115 | 0 | — | — | — | — | — |
| 46; M | IIB | 5.93 | 2.0 | 5 | 120 | 0 | 9 | 591 | 6.3 (1.2) | 18.2 (5.0) | 169.0 (9.0) |
| 46; F | IIA | 1.02 | — | — | — | 1 | 2 | 417 | 3.5 (0.7) | 12.5 (6.4) | 153 (17.0) |
| 68; F | IIA | 4.40 | 2.4 | 5 | 150 | 3 | 3 | 42 | 7.7 (2.5) | 17.0 (2.6) | 163.3 (14.7) |
| 53; M | IIB | 6.09 | 14.5 | 30 | 140 | 8 | 3 | 222 | 5.7 (0.6) | 14.3 (3.8) | 182.0 (8.5) |
| 63; M | IIA | 3.78 | 2.4 | 5 | 161 | 8 | 3 | 158 | 8.3 (2.1) | 25.0 (6.2) | 172.0 (17.3) |
| 57; M | III | 2.26 | — | — | — | 16 | 3 | 101 | 8.7 (4.2) | 24.7 (11.5) | 179.3 (14.0) |
| 68; F | III | 1.74 | — | — | — | 20 | 9 | 540 | 6.2 (1.7) | 19.4 (4.8) | 172.9 (14.9) |
| 60; M | IIA | 3.00 | — | — | — | 32 | 1 | 72 | 4.0 | 18.0 | 172.0 |
| 42; M | IIB | 5.60 | 2.2 | 4 | 166 | 36 | 3 | 159 | 10.3 (5.0) | 28.7 (11.4) | 184.0 (8.7) |
| 41; M | IIA | 2.60 | — | — | — | 48 | 10 | 265 | 5.4 (1.3) | 15.5 (3.6) | 174.4 (16.1) |
| 50; M | IIB | 5.40 | — | 33 | 121 | 105 | 7 | 385 | 5.7 (2.3) | 18.4 (6.7) | 159.0 (12.2) |
| 46; F | IIA | 3.19 | — | — | — | 146 | 2 | 578 | 5.5 (2.1) | 14.5 (4.9) | 167.0 (25.5) |
| 66; M | III | 2.31 | — | — | — | 161 | 1 | 715 | 12.0 | 25.0 | 162.0 |
| 46; M | IIB | 4.65 | 8 | 16 | 129 | 184 | 1 | 267 | 5.0 | 16.0 | 179.0 |
| 74; F | IIA | 0.92 | — | — | — | 244 | 6 | 844 | 5.8 (1.5) | 14.8 (4.4) | 171.3 (3.6) |
| 43; M | IIA | 8.02 | — | — | — | 248 | 3 | 694 | 4.0 (0.0) | 12.7 (0.6) | 172.0 (0.0) |
| 67; F | IIA | 5.70 | 2.2 | 6 | 225 | 362 | 1 | 837 | 8.0 | 24.0 | 191.0 |
| 37; M | IIB | 2.26 | — | — | — | 368 | 1 | 527 | 11.0 | 29.0 | 189.0 |
| 42; M | III | 2.17 | — | — | — | 530 | 1 | 778 | 4.0 | 11.0 | 176.0 |
| 72; M | IIA | 1.68 | — | 11 | 150 | 553 | 2 | 634 | 7.0 (2.8) | 16.5 (9.2) | 158.0 (38.2) |
Holter indicates detection by a Holter monitor; ICM indicates detection by an implantable cardiac monitor.
AHA/ACC, American Heart Assocation/American College of Cardiology; bpm, beats per minute; ESC, European Society of Cardiology; F, female; HR, heart rate; ICD, implantable cardioverter defibrillator; M, male.
Values are mean (standard deviation).
Atrial fibrillation
A total of 4 of 33 patients (12.1%) had paroxysmal AF documented prior to enrollment in the study. One patient had a transmission of new AF that, given its characteristics, is best identified as an atrial high-rate episode. This episode lasted 49 seconds, with a maximum heart rate of 160 bpm, and was asymptomatic. No action was taken. Two patients with previously documented AF continued to have several paroxysmal AF episodes. Two other patients with previously detected AF had no recorded episodes of AF.
ICD implantation
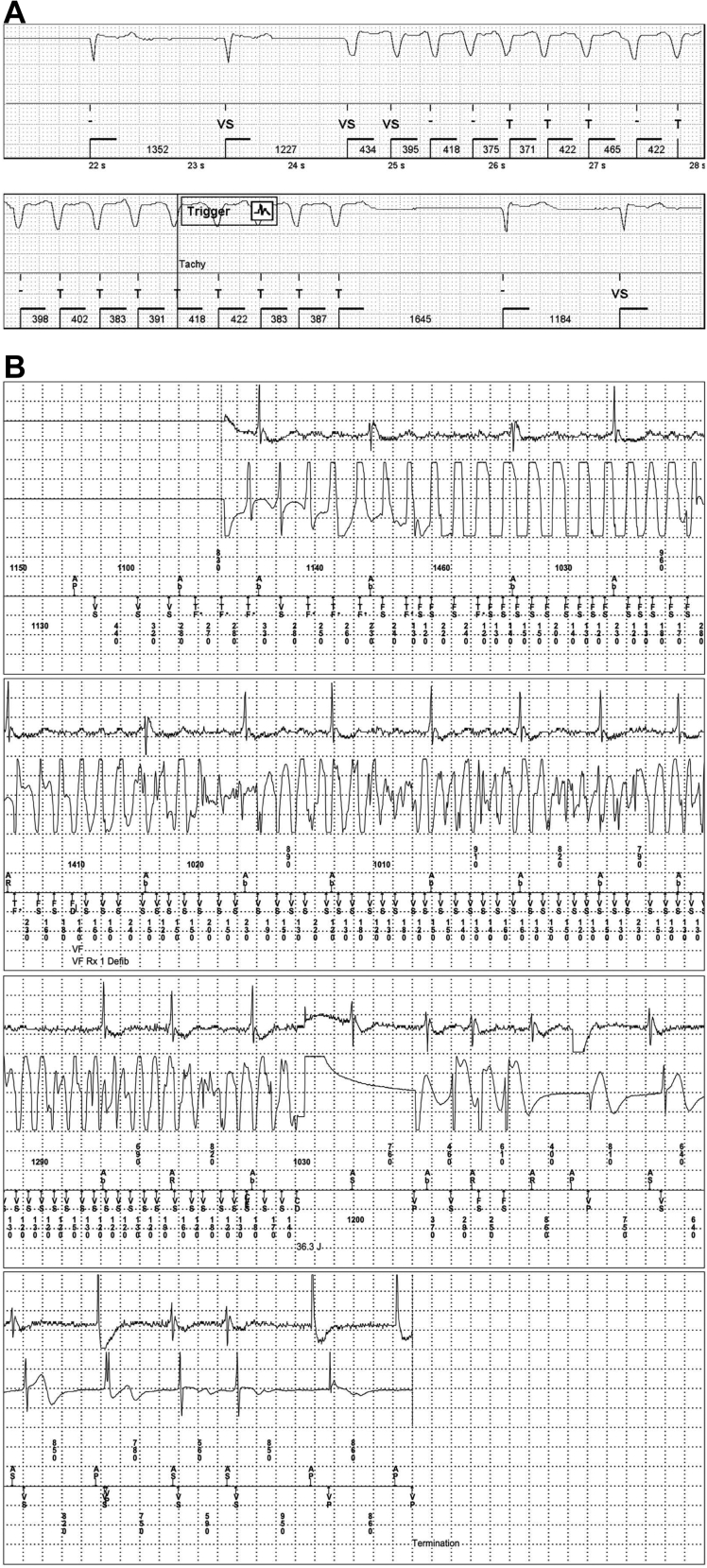
A total of 19 of 33 patients (57.6%) went on to receive a primary prevention ICD. One female patient (age 68 years) had an HCM–SCD risk of 4.4% upon enrollment. Risk markers prior to enrollment included Holter-detected NSVT (5 beats, 150 bpm), LV apical aneurysm, and 5% LGE. She had 3 episodes of NSVT during the first month of prolonged monitoring. One was a run of NSVT (maximum: 152 bpm; 18 beats; 10 seconds) identified 21 days after ICM implantation (Fig. 4A). One week later, her fastest ICM-detected NSVT episode was recorded, at 180 bpm, 19 beats, lasting 8 seconds. Given the recurrent nature of her NSVT episodes, she decided to undergo ICD implantation 1 month later. This patient went on to have appropriate ICD therapy, which successfully terminated ventricular fibrillation recorded at 250 bpm in her sleep, without complication (Fig. 4B). No other patients implanted with an ICD received appropriate therapy during the available follow-up.
Figure 4.
(A) Electrogram from implantable cardiac monitor –detected nonsustained ventricular tachycardia. This female patient (age 67 years) had a hypertrophic cardiomyopathy–sudden cardiac death risk of 4.4%. This run of nonsustained ventricular tachycardia (maximum 152 bpm, 18 beats, 10 seconds) was identified 21 days after implantation of an implantable cardiac monitor. (B) Electrogram traces from an implantable cardioverter defibrillator implant in the same patient, showing ventricular fibrillation onset and termination due to implantable cardioverter defibrillator therapy.
Discussion
This study demonstrates the potential utility of ICM technology and smartphone-enabled 2-way communication in routine HCM management. NSVT was identified in 20 patients (60.6%) in our study, the majority of whom (11) had no previous episodes documented on Holter monitoring. A total of 16 patients (80%) underwent ICD implantation because of arrhythmias detected on ICM, of whom 14 (87.5%) were AHA class IIA or IIB. From the 25 patients who declined ICDs prior to enrollment, patients with an ICM-detected NSVT were more likely to opt for an ICD post-enrollment than were those without an ICM-detected NSVT (87.5% vs 22.2%, P = 0.002). These findings suggest that data generated from ICMs influence patient and provider decision-making. A care pathway utilizing 2-way smartphone communication between patients and care teams enables remote care of patients with HCM. Our current data suggest that this approach would be most clinically indicated for enhanced risk stratification of AHA class IIA or IIB patients.
Workflow efficiency and data integration are shown to be barriers to digital health adoption.8 In this pilot study, all 172,437 transmissions were reviewed by an experienced device nurse, and if necessary, further adjudicated by 1 cardiac electrophysiologist. Of the 172,112 automatic transmissions that were received, 65 (0.04%) required clinical follow-up. A total of 325 manual transmissions were received, of which 14 (4.3%) required follow-up. The other 311 (95.7%) were managed solely with a text message. These findings suggest that this care pathway can be employed with minimal resources. Moreover, newer signal-detection algorithms will improve the signal-to-noise ratio, and possibly the rhythm classification of automatic transmissions, in future ICM studies, which may incorporate machine learning and/or artificial intelligence.9 Our current results also show a trend toward decreasing rates of manual transmissions over time (Fig. 1; Table 2). Data collected by our team on patient experiences with ICMs suggest a correlation of ICM use with reduced patient anxiety over time, allaying concerns about data management and volume.10
The utility of NSVT as a risk marker in HCM is controversial, as some studies have found no association between NSVT on 24- to 48-hour ambulatory monitoring and SCD.11 Wang et al. recently identified that NSVT runs that are faster than 200 bpm, longer than 7 beats, and repetitive (> 1 run over ≥ 6 months) are predictive for ICD-treated ventricular arrhythmias.12 Given our NSVT cutoffs, all patients with NSVT had episodes longer than 7 beats. However, only 1 patient (5%) had an NSVT run faster than 200 bpm, and only 13 patients (65%) had repetitive (> 1 run over ≥ 6 months) NSVT episodes. Thus, all of our patients met 1 of the criteria described by Wang et al., 13 patients (65%) met 2 criteria, and 1 (5%) patient met all 3 criteria.12 Findings from other authors also have supported the notion that longer and faster NSVT runs are predictive of appropriate ICD therapy.13 Specifically, a study involving 60 patients with prophylactic ICDs found that rapid NSVT (≥ 150-200 bpm for 4-16 beats) detected by the ICD was associated with a hazard ratio of 6.2 (P = 0.01) for appropriate ICD therapy (antitachycardia pacing and shocks) for sustained ventricular arrhythmias.14 Of all patients who went on to have appropriate ICD therapy for sustained ventricular arrhythmias, 77.8% (7 of 9) also had ICD-detected rapid NSVT.
Previous studies published using ICMs in HCM patients predominantly have involved either a low- or moderate-risk population,15,16 or patients who are post–alcohol septal ablation.17 Magnusson and Mörner15 studied a low-risk HCM population (mean HCM-SCD risk score of 2.3%) and found the rates of AF and NSVT to be 30.0% and 23.3%, respectively. Sakhi et al.16 designed a prospective observational study in low- and moderate-risk HCM patients (mean HCM-SCD risk score < 6%). The rate of NSVT in the ICM group was 23%, vs 47% in the Holter monitor group, with similar characteristics of NSVT runs between groups. Most of the ICM-detected NSVT runs in this study came from manually reported symptomatic transmissions (4 of 5). Importantly, the tachycardia rate cutoff in both studies is higher than the cutoff set in our study (rate ≥ 160 bpm, 8 beats and ≥ 176 bpm, 16 beats). Most patients included in our study (28 of 33, 84.8%) were low or moderate risk, per HCM-SCD risk scoring. The tachycardia rate cutoff in our study was ≥ 140 bpm for ≥ 8 beats. This cutoff is the most sensitive available for device programming based on manufacturer settings and likely contributed to an abundance of sinus tachycardia reports on automatic transmissions. Using our lower rate cutoff, 8 patients (40%) had NSVT episodes at heart rates between 140 and 160 bpm, but all (8 of 8) had at least 1 episode at a rate faster than 160 bpm. In total, 14 of 72 of NSVT episodes (19.4%) were at rates in the range of 140-160 bpm. Although we targeted sensitivity over specificity in this pilot study, our data suggest that tachycardia cutoff rates of > 160 bpm may be used.
Apical aneurysms and LGE are new risk markers for SCD in HCM.3 All 4 patients with apical aneurysms in our cohort had documented NSVT (1 on prior Holter monitor, 3 on ICM monitoring). LV apical aneurysms were present in 3 of the oldest patients in our cohort—68, 72, and 72 years of age. A recent analysis of the 1700 patient Tufts Hypertrophic Cardiomyopathy Institute cohort, including 118 patients with LV apical aneurysms, found that rates of NSVT on ambulatory monitoring were higher in HCM patients with apical aneurysms than in those without (66% vs 20%, P < 0.001).18 The authors of this study also note that in patients aged ≥ 60 years with apical aneurysms, the risk of SCD is 8-fold higher than that in patients without apical aneurysms.
LGE is a marker for myocardial fibrosis. Studies suggest that the degree of LGE assessed on CMR imaging plays an important role in the pathogenesis of ventricular arrhythmias in HCM.19 A recent study using 14-day Holter monitoring found that LGE extent was associated with both increased frequency and length of NSVT runs.19 Other authors report that attending to the amount of LGE improves the discriminative power of using both AHA/ACC and ESC criteria.20 LGE, measured as percent of total LV myocardial mass, is presented in Supplemental Table S1. Interesting to note is that neither of 2 patients with ≥ 20% LGE had any detected NSVT. Both were monitored for over 1 year. Fourteen patients (70%) with any amount of LGE detected on CMR (total n = 20) had NSVT runs identified, either on Holter or ICM monitoring.
Study limitations
The association between arrhythmias detected on continuous monitoring and SCD risk is still unclear. Findings from this study require replication in larger cohorts linked to long-term and clinically relevant outcomes. Specifically, follow-up trials including appropriate ICD therapy as an endpoint are crucial in approximating the prognostic value of NSVT detected on ICM monitoring. An additional study limitation is the inclusion of study patients from a broad referral base and different referring centres that did not prospectively determine the number of HCM patients referred for ICD; thus, the total number of participants assessed for inclusion and exclusion parameters cannot be reported.
Conclusions
In this pilot study, we demonstrate the potential utility of ICM monitoring in patients with HCM. We find that prolonged and continuous cardiac monitoring identifies more patients with NSVT than does current guideline-recommended Holter monitoring. Detection of these arrhythmias had a significant impact on SCD management and ICD uptake.
Acknowledgments
Funding Sources
Funding was provided via an unrestricted research grant from Abbott via funds paid to and administered by the University of British Columbia.
Disclosures
M.W.D. reports receiving honoraria from Abbott. The other authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: Study protocols were approved by institutional ethics committees (study ID: H17-02707).
See page 314 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2021.10.010.
Supplementary Material
Patient education video detailing the use and planned care pathway of the ICM (implantable cardiac monitor) system.
References
- 1.Semsarian C., Ingles J., Maron M.S., Maron B.J. New perspectives on the prevalence of hypertrophic cardiomyopathy. J Am Coll Cardiol. 2015;65:1249–1254. doi: 10.1016/j.jacc.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 2.Elliott P.M., Anastasakis A., Borger M.A., et al. 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy. Eur Heart J. 2014;35:2733–2779. doi: 10.1093/eurheartj/ehu284. [DOI] [PubMed] [Google Scholar]
- 3.Ommen S.R., Mital S., Burke M.A., et al. 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2020;142:e558–631. doi: 10.1161/CIR.0000000000000937. [DOI] [PubMed] [Google Scholar]
- 4.Monserrat L., Elliott P.M., Gimeno J.R., et al. Non-sustained ventricular tachycardia in hypertrophic cardiomyopathy: an independent marker of sudden death risk in young patients. J Am Coll Cardiol. 2003;42:873–879. doi: 10.1016/s0735-1097(03)00827-1. [DOI] [PubMed] [Google Scholar]
- 5.Solomon M.D., Yang J., Sung S.H., et al. Incidence and timing of potentially high-risk arrhythmias detected through long term continuous ambulatory electrocardiographic monitoring. BMC Cardiovasc Disord. 2016;16:35. doi: 10.1186/s12872-016-0210-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weissler-Snir A., Adler A., Williams L., Gruner C., Rakowski H. Prevention of sudden death in hypertrophic cardiomyopathy: bridging the gaps in knowledge. Eur Heart J. 2017;38:1728–1737. doi: 10.1093/eurheartj/ehw268. [DOI] [PubMed] [Google Scholar]
- 7.Vogtmann T., Stiller S., Marek A., et al. Workload and usefulness of daily, centralized home monitoring for patients treated with CIEDs: results of the MoniC (Model Project Monitor Centre) prospective multicentre study. Europace. 2013;15:219–226. doi: 10.1093/europace/eus252. [DOI] [PubMed] [Google Scholar]
- 8.Whitelaw S., Pellegrini D.M., Mamas M.A., Cowie M., Van Spall H.G.C. Barriers and facilitators of the uptake of digital health technology in cardiovascular care: a systematic scoping review. Eur Heart J Digit Health. 2021;2:62–74. doi: 10.1093/ehjdh/ztab005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krittanawong C., Rogers A.J., Johnson K.W., et al. Integration of novel monitoring devices with machine learning technology for scalable cardiovascular management. Nat Rev Cardiol. 2021;18:75–91. doi: 10.1038/s41569-020-00445-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies B., Forman J., Joe H., et al. Patient experience of an implanted cardiac monitor for hypertrophic cardiomyopathy. Heart Rhythm. 2021;18:S191. [Google Scholar]
- 11.Elliott P.M., Poloniecki J., Dickie S., et al. Sudden death in hypertrophic cardiomyopathy: identification of high risk patients. J Am Coll Cardiol. 2000;36:2212–2218. doi: 10.1016/s0735-1097(00)01003-2. [DOI] [PubMed] [Google Scholar]
- 12.Wang N., Xie A., Tjahjono R., et al. Implantable cardioverter defibrillator therapy in hypertrophic cardiomyopathy: an updated systematic review and meta-analysis of outcomes and complications. Ann Cardiothorac Surg. 2017;6:298–306. doi: 10.21037/acs.2017.07.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Francia P., Santini D., Musumeci B., et al. Clinical impact of nonsustained ventricular tachycardia recorded by the implantable cardioverter-defibrillator in patients with hypertrophic cardiomyopathy. J Cardiovasc Electrophysiol. 2014;25:1180–1187. doi: 10.1111/jce.12492. [DOI] [PubMed] [Google Scholar]
- 14.Viswanathan K., Suszko A.M., Das M., et al. Rapid device-detected nonsustained ventricular tachycardia in the risk stratification of hypertrophic cardiomyopathy. Pacing Clin Electrophysiol. 2016;39:642–651. doi: 10.1111/pace.12861. [DOI] [PubMed] [Google Scholar]
- 15.Magnusson P., Mörner S. EvaLuation Using Cardiac Insertable Devices And TelephonE in Hypertrophic CardioMyopathy (ELUCIDATE HCM): a prospective observational study on incidence of arrhythmias. J Cardiovasc Electrophysiol. 2021;32:129–135. doi: 10.1111/jce.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakhi R., Huurman R., Theuns D.A.M.J., et al. Incremental value of an insertable cardiac monitor in patients with hypertrophic cardiomyopathy with low or intermediate risk for sudden cardiac death. Cardiology. 2021;146:1–6. doi: 10.1159/000512656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bleszynski P.A., Goldenberg I., Fernandez G., et al. Risk of arrhythmic events after alcohol septal ablation for hypertrophic cardiomyopathy using continuous implantable cardiac monitoring. Heart Rhythm. 2021;18:50–56. doi: 10.1016/j.hrthm.2020.08.013. [DOI] [PubMed] [Google Scholar]
- 18.Rowin E.J., Maron B.J., Chokshi A., Maron M.S. Left ventricular apical aneurysm in hypertrophic cardiomyopathy as a risk factor for sudden death at any age. Pacing Clin Electrophysiol. 2018;41:1031–1033. doi: 10.1111/pace.13413. [DOI] [PubMed] [Google Scholar]
- 19.Weissler-Snir A., Hindieh W., Spears D.A., et al. The relationship between the quantitative extent of late gadolinium enhancement and burden of nonsustained ventricular tachycardia in hypertrophic cardiomyopathy: a delayed contrast-enhanced magnetic resonance study. J Cardiovasc Electrophysiol. 2019;30:651–657. doi: 10.1111/jce.13855. [DOI] [PubMed] [Google Scholar]
- 20.Freitas P., Ferreira A.M., Arteaga-Fernández E., et al. The amount of late gadolinium enhancement outperforms current guideline-recommended criteria in the identification of patients with hypertrophic cardiomyopathy at risk of sudden cardiac death. J Cardiovasc Magn Reson. 2019;21:50. doi: 10.1186/s12968-019-0561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Patient education video detailing the use and planned care pathway of the ICM (implantable cardiac monitor) system.