Abstract
Irritable bowel syndrome (IBS) is a chronic functional gastrointestinal disorder characterized by recurring abdominal pain and altered bowel habits without detectable organic causes. This study aims to provide a comprehensive overview of the literature on functional neuroimaging in IBS and to highlight brain alterations similarities with other functional disorders - functional movement disorders in particular. We conducted the bibliographic search via PubMed in August 2020 and included 50 studies following Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines for systematic reviews. Overall, our findings showed an aberrant activation and functional connectivity of the insular, cingulate, sensorimotor and frontal cortices, the amygdala and the hippocampus, suggesting an altered activity of the homeostatic and salience network and of the autonomous nervous system. Moreover, glutamatergic dysfunction in the anterior insula and hypothalamic pituitary axis dysregulation were often reported. These alterations seem to be very similar to those observed in patients with functional movement disorders. Hence, we speculate that different functional disturbances might share a common pathophysiology and we discussed our findings in the light of a Bayesian model framework.
Keywords: Amygdala, Brain, Conversion disorders, Functional neuroimaging, Irritable bowel syndrome
Introduction
Irritable bowel syndrome (IBS) is a chronic functional gastrointestinal disorder (FGID) characterized by recurring abdominal pain associated with altered bowel habits without detectable organic causes.1,2 The definition of IBS is based on the Rome IV criteria: recurrent abdominal pain at least 3 days per month in the last 3 months, associated with a change in stool frequency or form and improvement with defecation.2 IBS is a multi-factorial disorder with a complex bio-psychosocial pathophysiology,3 including altered gastrointestinal motility, visceral hyperalgesia,4 increased intestinal permeability, immune activation,5 altered microbiota,6 and disrupted communication between the gastrointestinal tract and the central nervous system.7 The relevance of bidirectional communication pathways between the brain and the gastrointestinal system, namely the brain-gut-axis, is increasingly acknowledged as underlying pathophysiological mechanism of different somatic and neuropsychiatric disturbances.8 Multiple brain networks (salience, sensorimotor, and executive-control networks) have been reported to mediate the effects of affect, mood, and environmental factors on gut function and pain perception, resulting in visceral hypersensitivity and altered bowel habits9,10 and IBS can be considered an exemplary disorder of brain-gut communication. Therefore, in the recent years, several neuroimaging studies have been conducted to investigate brain alterations associated with IBS. Recent studies implementing quantitative magnetic resonance spectroscopy (qMRS) highlighted an aberrant glutamatergic neurotransmission in limbic structures of IBS patients.11-13 Bednarska et al11 observed that increased concentration of glutamate was also reported in the posterior insula (pINS) of patients with fibromyalgia.14 Moreover, according to a recent case-control study from our group,15 a glutamate increase has been detected in the limbic system of patients suffering from functional movement disorders (FMD, also called conversion disorders) when compared to healthy controls (HC). Based on these observations, herein we conducted a systematic review of the literature focused on functional neuroimaging in the IBS setting. Consequently, we discuss whether these shared brain alterations may represent a common pathophysiology for different functional disturbances.
Methods
We followed Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines for systematic reviews.16 The bibliographic search was conducted on PubMed in August 2020. Moreover, we looked through reference lists of screened articles.
Inclusion criteria were: (1) English-written articles, (2) articles including only human subjects above 18 years old, (3) studies on IBS, and (4) functional neuroimaging studies.
Exclusion criteria were: (1) studies about any other functional somatic disorder, (2) review articles, (3) structural neuroimaging studies, (4) case series (less than 10 patients with IBS), (5) studies without a HC group, and (6) studies including pediatric patients with IBS.
The search was conducted using the following keywords: (irritable bowel syndrome), (spastic colon), (spastic bowel), (mucous colitis), and (nervous colon); and combined on a string with the keywords: (functional neuroimaging) and (functional brain imaging). Supplementary Table shows results for each string on PubMed. We retrieved 911 articles. After removing duplicates, 210 articles remained, which were independently screened by 2 authors (V.N. and R.E.R.) by reading abstracts and full text. Disagreement was resolved by discussion between the 2 independent authors; if no agreement was reached, a third independent party (B.D.) was involved as an arbiter. Fifty studies were selected (Fig. 1). Results are presented divided up by methodology in Table 1 and synthetically described in Table 2.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow diagram illustrating the bibliographic search and the selection process. Adapted from Moher et al.16 IBS, irritable bowel syndrome; HC, healthy control.
Table 1.
Results of the Systematic Review Divided up by Methodology
| First author, year | Participants | Technique | Task | Main results |
|---|---|---|---|---|
| Hong,19 2013 | 60 IBS vs 108 HC | fMRI, ALFF | Resting-state | Female HC and IBS showed hyperactivation in amygdala and hippocampus compared with male HC and IBS. Female IBS showed hyperactivation in INS and hypoactivation in S1 compared to male IBS. |
| Gupta,20 2014 | 58 IBS vs 110 HC | fMRI | Resting-state | History of EAL was associated with altered FC in the salience/executive control network in IBS patients; male IBS patients demonstrated additional EAL-related alterations in the cerebellar network. |
| Hong,26 2014 | 48 IBS vs 48 HC | fMRI | Resting-state | Male IBS showed increased FC than female IBS of the dorsal aINS bilaterally with mPFC and dorsal pINS; (2) female IBS showed greater negative FC than male IBS of L dorsal aINS with L precuneus; clinical characteristics correlated to the FC between INS and dorsal mPFC in male IBS, with the precuneus in female IBS. |
| Ke,30 2015 | 31 IBS vs 32 HC | fMRI, ReHo analysis | Resting-state | IBS patients showed increased FC in S1 and thalamus and decreased FC in the ACC and PFC. |
| Ma,17 2015 | 21 IBS vs 21 HC | fMRI, ALFF | Resting-state | IBS patients showed hypoactivity in the L SFG, R hippocampus, R MFG, bilateral M1, and R superior temporal pole; hyperactivation in the L MCC and L calcarine; increased FC in cingulate and frontal cortex. |
| Qi,18 2016 | 30 IBS vs 31 HC | fMRI, ALFF | Resting-state | IBS showed hypoactivity in DMN regions (mPFC, PCC, bilateral inferior parietal cortices), MFG, R orbital part of the SFG, dorsal ACC, ventral ACC; hyperactivity in bilateral pINS and cuneus; decreased positive FC between mPFC and R orbital SFG, between ventral ACC and PCC; decreased negative FC between mPFC and L pINS; increased negative FC between mPFC and cuneus. |
| Qi,27 2016 | 31 IBS vs 32 HC | fMRI | Resting-state | IBS showed higher positive FC between the amygdala and INS, midbrain, parahippocampal gyrus, S1/M1, and SMA. |
| Qi,21 2016 | 65 IBS vs 67 HC | fMRI, IVMHC | Resting-state | IBS showed higher interhemispheric FC between bilateral thalami, cuneus, PCC, lingual gyri and inferior occipital/cerebellum lobes; lower interhemispheric FC between bilateral ventral ACC and inferior parietal lobules. |
| Qi,24 2016 | 31 IBS vs 32 HC | fMRI | Resting-state | IBS showed decreased DMN inter-regional FC between precuneus and ACC, medial orbital SFG, and MTG, together with decreased DMN global efficiency (E glob). |
| Gupta,23 2017 | 16 IBS vs 16 HC | fMRI | Resting-state | Regions of the salience network, (MCC, MTG, STG) were positively correlated with proinflammatory genes (IL-6 and APOL2) in IBS, but negatively correlated with anti-inflammatory genes (KRT8 and APOA4) in HCs. |
| Icenhour,29 2017 | 41 IBS vs 20 HC | fMRI | Resting-state | H-IBS, compared to N-IBS, showed increased positive FC of pregenual ACC and thalamus and of pINS within the sensorimotor network; N-IBS showed decreased positive FC of amygdala and decreased negative FC in dorsal aINS within the DMN. FC between DMN and sensorimotor network correlated with rectal perception thresholds, while FC in pINS correlated with symptom severity. |
| Longarzo,28 2017 | 19 IBS vs 26 HC | fMRI | Resting-state | In IBS patients, correlation emerged: between hypochondriasis and FC between PCC and L supramarginal gyrus/STG; interoception and FC between L ventral aINS and supramarginal gyrus bilaterally. |
| Weng,22 2017 | 32 IBS vs 32 HC | fMRI | Resting-state | IBS showed decreased long- and short-range FCD in bilateral anterior MCC and inferior parietal lobules; decreased long-range FCD in R aINS; decreased short-range FCD in bilateral PFC, subgenual ACC and caudates; increased long- and short-range FCD in S1/M1; increased long-range FCD in R SMA; increased short-range FCD in occipital lobe. |
| Witt,25 2019 | 32 IBS vs 15 HC | fMRI | Resting-state | IBS patients only showed an increased FC of the R amygdala, correlated with decreased gut permeability. |
| Mertz,31 2000 | 18 IBS vs 16 HC | fMRI | Rectal distention exam | In HC and IBS, rectal stimulation led to a greater activity of ACC, PFC, INS, and thalamus. In IBS pain produced an hyperactivation of the ACC. |
| Yuan,32 2003 | 26 IBS vs 11 HC | fMRI | Rectal distention exam | In IBS and HC, rectal distention stimulation increased the activity of ACC, PFC, INS and thalamus. During painful stimulation, IBS hyperactivated INS, PFC, and thalamus. |
| Wilder-Smith,35 2004 | 10 IBS vs 10 HC | fMRI | Rectal distention exam | IBS-D, during heterotopic stimulation showed significant deactivation in the right aINS; IBS-C showed increased activations of the amygdala and hippocampus; HC only showed deactivation of the PAG. |
| Song,36 2006 | 12 IBS vs 12 HC | fMRI | Rectal distention exam | During rectal stimulation, compared to heterotopic stimulation, HC but not IBS showed greater activation in bilateral aINS, S2 and putamen; IBS showed greater activation during rectal plus heterotopic stimulation, compared to rectal stimulation alone, in bilateral S1, R STG, R inferior lobule and bilateral STG. |
| Berman,33 2008 | 14 IBS vs 12 HC, female only | fMRI | Rectal distention exam | During cued anticipation of distention, HC showed hypoactivity in INS, supragenual ACC, amygdala, and DBS, while IBS patients showed less anticipatory inactivation. Group differences were significant in R pINS and bilateral DBS. During distention, both groups showed activity increases in INS dorsal ACC, and DBS and decreases in the infragenual ACC. The increases were more extensive in patients, in dorsal ACC and DBS. |
| Ringel,48 2008 | 10 IBS vs 10 HC, female, with 5 and 5 respectively having history of abuse | fMRI | Rectal distention exam | In HC and IBS, distention-elicited pain correlated with activation of PCC and MCC, but subjects with IBS and history of sexual abuse showed higher activity in L MCC and PCC, and lower activity in L supragenual ACC. |
| Elsenbruch,34 2010 | 15 IBS vs 12 HC, female only | fMRI | Rectal distention exam | IBS showed hyperactivation in aINS and PFC. Anxiety correlated with pain-induced activation of the anterior MCC and pregenual ACC. Depression correlated with activation of PFC and cerebellar areas. |
| Elsenbruch,41 2010 | 15 IBS vs 12 HC, female only | fMRI | Rectal distention exam | During rectal stimulation, IBS showed more pronounced stress-induced modulation of neural activation in the INS, MCC, VLPFC. During relaxation, IBS patients demonstrated reduced modulation of distension-induced activation in the INS. |
| Hubbard,56 2011 | 14 IBS vs 17 HC, female only | fMRI | Pain expectation + two single oral doses (20 or 200 mg) of the CRF1 antagonist GW876008 vs PLA | During pain expectation, IBS and HC receiving GW876008 showed hypoactivation in the amygdala, hippocampus, INS, ACC, and OFC/mPFC. IBS showed greater BOLD responses in the L locus coeruleus and hypothalamus after PLA compared with HCs, and hypoactivation of the L hypothalamus after drug. |
| Kilpatrick,53 2011 | 26 IBS vs 29 HC, female only | fMRI | Affect-matching paradigm | IBS patients with the C/C genotype had increased amygdala responses to nonemotional stimuli, compared with other subjects with C/C genotype. |
| Labus,1 2011 | 12 HC vs 14 IBS | fMRI | Rectal distention exam | In HC and IBS-C, ATD lead to an increased response to rectal distention of the amygdala and nodes of emotional arousal and homeostatic afferent networks. The effect was greater during high inflation. |
| Aizawa,51 2012 | 30 IBS vs 30 HC | fMRI | Wisconsin Card Sorting Test | IBS showed hypoactivity of the R DLPFC and R hippocampus; hyperactivity of the L pINS at error feedback during set-shifting; less FC from the DLPFC to pre-SMA. |
| Larsson,49 2012 | Total: 44 IBS vs 20 HC; imaging: 18 normosensitive and 15 hypersensitive patients with IBS and 18 controls | fMRI | Rectal distention exam | H-IBS had hyperactivation of INS and hypoactivation in pregenual ACC during noxious rectal distensions, compared to HC and N-IBS. During expectation of rectal distension, N-IBS had showed hyperactivation in R hippocampus than HC. |
| Lee,59 2012 | 17 IBS vs 17 HC | fMRI | Rectal distention exam | IBS and HC showed comparable visceral PLA analgesia, but IBS showed hyperactivity in INS, MCC and VLPFC. VLPFC was also more active during anticipation in IBS. |
| Bouhassira,37 2013 | 10 IBS patients with facilitation and 10 with inhibition of the RIII reflex vs 11 HC, female only | fMRI | Rectal distention exam | Non-painful and painful rectal distension induced similar changes in brain activity in IBS patients with facilitation and inhibition of the RIII reflex. |
| Labus,42 2013 | 47 IBS vs 67 HC | fMRI | Emotion recognition paradigm | Male IBS and HC showed greater overall brain responses to emotional stimuli than females in PFC, INS, and amygdala. |
| Lowén,46 2013 | 44 IBS vs 20 HC, female only | fMRI | Rectal distention exam and hypnotherapy | Hypnotherapy or educational intervention were both effective in reducing pain during rectal distention in both IBS and HC group; after the treatment, the brain response to distension was similar in IBS and in HCs. |
| Rosenberger,38 2013 | 15 IBS vs 15 HC | fMRI | Rectal distention exam | Within IBS, depression scores correlated with non-painful distension-induced activation in the R cerebellum (Crus I, II, and lobule VIIIb) and with painful distension-induced activation in vermal lobule V; anxiety scores correlated with non-painful induced activation in Crus II. |
| Hubbard,52 2015 | 15 IBS vs 14 HC | fMRI | Attention network test | IBS showed shorter reaction times during the alerting and orienting conditions, correlated with hyperactivation of anterior MCC (correlated with duration and severity of GI-symptoms) and INS, and hypoactivation in the R inferior frontal junction and SMA. during the executive control task, IBS showed activation in the dorsal mPFC and deactivation of thalamus. |
| Icenhour,45 2015 | 20 IBS vs 23 HC | fMRI | Rectal distention exam with fear acquisition paradigm | IBS showed: during fear acquisition, hyperactivation of PFC and amygdala; during extinction, hyperactivation of cingulate cortex; during reinstatement, hyperactivation of hippocampus. |
| Lowén,50 2015 | 33 IBS vs 18 HC | fMRI | Rectal distention exam | In the last trials of a series of rectal distension, N-IBS showed decreasing activation in INS, PFC and amygdala, H-IBS showed greater activation in insula, ACC and MCC. |
| Hong,43 2016 | 37 HC vs 37 IBS | fMRI | Pain expectation | Regions within the salience, attention, default mode, and emotional arousal networks were more activated by the cued abdominal threat condition and the uncued condition than in the cued safe condition. During the uncued condition IBS subjects showed hyperactivations in amygdala, aINS, MFG, thalamus and precuneus. |
| Wong,55 2016 | 13 IBS vs 11 HC | fMRI | Rectal distention exam | IBS showed, within the scanner environment, significantly increased visceral, but not somatic, pain perception. |
| Claassen,39 2017 | 17 IBS vs 21 HC | fMRI | Differential fear conditioning paradigm | IBS patients revealed hyperactivity responses to pain-predictive and safety cues in the vermis, intermediate cerebellum (maximum in lobule VIII), and the posterolateral cerebellar hemisphere (maximum in lobule VI). During extinction and reinstatement, no differences emerged between HC and IBS. During visceral pain-related fear conditioning, IBS patients showed hyperactivations in several areas of the medial, intermediate, and lateral cerebellum. |
| Guleria,54 2017 | 20 IBS vs 10 HC | fMRI | Rectal distention exam | IBS patients showed greater cerebral activations in INS, MTG, and cerebellum in the L hemisphere, but lacked of activation in bilateral precuneus/superior parietal lobules. IBS-C activated R MCC, while IBS-D activated L inferior OFC, L calcarine, and bilateral fusiform gyri. |
| Kano,44 2017 | 26 IBS vs 29 HC | fMRI | Rectal distention exam | IBS patients, in uncertain anticipation, showed greater activation of anterior MCC, thalamus, and visual processing areas; the following rectal distention elicited in IBS higher activity in PCC, MCC and the precuneus; lack of rectal distention after the cue of uncertainty lead, in IBS, to a lack in bilateral insula activation. |
| Kano,57 2017 | 28 IBS vs 34 HC | fMRI | Rectal distention exam + intravenous CRF administration (2 μg/kg) | HC, but not IBS, showed a negative correlation between ACTH response to CRF and activity in the pregenual ACC during rectal distention. |
| Wang,40 2017 | 31 IBS vs 20 HC | fMRI | Rectal distention exam | Activation in parietal areas, PFC, cerebellum, ACC, INS and thalamus increased along with increases in rectal balloon dilation, except in women with IBS and patients with disease duration less than 5 years. |
| Kano,47 2019 | 27 IBS vs 33 HC | fMRI | Rectal distention exam | In HC, but not in IBS, a positive correlation between baseline high frequency values of HRV and neural responses to rectal distension was found in the R caudate, bilateral dorsolateral ACC, and pregenual ACC. |
| Kano,58 2020 | 26 IBS vs 35 HC | fMRI | Rectal distention exam | During rectal distention, activity in the R INS was positively associated with alexithymia scores to a greater extent in patients with IBS than in HCs. |
| Nakai,60 2003 | 12 IBS vs 12 HC | PET | Resting-state - 5-HT Synthesis | 5-HT synthesis was greater in female in the R MTG (multimodal sensory association cortex) compared with the female HC. |
| Naliboff,65 2006 | 20 IBS vs 14 HC | PET | Resting-state and rectal distentions exam | IBS, during repeated trials of rectal distention, showed a stable activation of the central pain matrix but a gradually decreasing activation in limbic, paralimbic, and pontine regions; during the anticipation condition, there were significant decreases in amygdala, dorsal ACC, and DBS activation. |
| Berman,63 2012 | 11 IBS vs 11 HC | PET | Auditory oddball vigilance task + double-blind ingestion of the α2AR antagonist YOH, agonist CLO or PLA | IBS showed higher plasma NE levels than HCs before and after ingestion of all drugs. IBS patients appeared downregulated the for functional presynaptic α2AR and showed less YOH-mediated reduction of activity in a central arousal circuit in brainstem and amygdala, which inversely correlated with early life trauma. |
| Niddam,12 2011 | 15 IBS vs 15 HC | qMRS | Resting-state | IBS showed reduced levels of Glx in hippocampus. |
| Bednarska,11 2019 | 30 IBS vs 21 HC, female only | qMRS | Resting-state | IBS showed lower concentrations of Glx in L and R aINS; no group differences for GABA1 concentrations. In IBS, lower R-lateralized Glx concentrations correlated with longer pain duration; lower Glx in L aINS correlated with ess frequent use of adaptive pain-coping. |
| Icenhour,13 2019 | 64 IBS vs 32 HC, female only | qMRS + fMRI | Resting-state | IBS and HC showed similar GABA+ and Glx levels in mPFC. Anxiety was positively associated with mPFC GABA+ concentrations in IBS; Glx was unrelated to psychological or gastrointestinal symptoms. IBS with high anxiety showed increased mPFC GABA+ and lower mPFC FC with ACC. |
IBS, irritable bowel syndrome; HC, healthy control; fMRI, functional magnetic resonance imaging; INS, insula; S1, primary somatosensory cortex; EAL, early adverse life events; aINS, anterior insula; mPFC, medial prefrontal cortex; pINS, posterior insula; FC, functional connectivity; ReHo, regional homogeneity; ACC, anterior cingulate cortex; PFC, prefrontal cortex; ALFF, amplitude of low-frequency fluctuation; L, left; SFG, superior frontal gyrus; R, right; MFG, middle frontal gyrus; M1, primary motor cortex; MCC, mid-cingulate cortex; DMN, default mode network; PCC, posterior cingulate cortex; SMA, supplementary motor area; IVMHC, interhemispheric voxel-mirrored homotopic connectivity; APOL2, apolipoprotein L2 gene; KRT8, keratin 8 gene; APOA4, apolipoprotein A-IV gene; H-IBS, hypersensitive IBS; N-IBS, normosensitive IBS; STG, superior temporal gyrus; FCD, functional connectivity density; IBS-D, diarrhea-predominant IBS; IBS-C, constipation-predominant IBS; PAG, periaqueductal gray; S2, secondary somatosensory cortex; DBS, dorsal brainstem; VLPFC, ventrolateral prefrontal cortex; CRF, corticotropin-releasing factor; OFC, orbito-frontal cortex; BOLD, blood oxygenation level-dependent; ATD, acute tryptophan depletion; DLPFC, dorsolateral prefrontal cortex; PLA, placebo; GI, gastrointestinal; ACTH, adrenocorticotropic hormone; HRV, heart rate variability; PET, positron emission tomopgraphy; 5-HT, serotonin; MTG, middle temporal gyrus; NE, noradrenergic; α2AR, α2-adrenoreceptor; YOH, yohimbine; CLO, clonidine; Glx, glutamate-glutamine; GABA, gamma-aminobutyric acid; qMRS, quantitative magnetic resonance spectroscopy.
Table 2.
Main Findings of the Systematic Review Divided up by Brain Areas
| Network | Main findings |
|---|---|
| Sensorimotor network | Resting state: S1 hypoactivation, M1 hyperactivation, increased FC between S1 and thalamus, and aberrant FC between the sensorimotor network and affective-interoceptive areas (amygdala and insula in particular). H-IBS showed increased FC between sensorimotor network and pINS, compared to N-IBS. |
| Affective and interoceptive areas | Resting state: aberrant FC between the cingulate and the frontal cortices, and between amygdala, insula, sensorimotor network, and hippocampal/para-hippocampal gyri. Amygdala: hyperactivity at resting state; aberrant activation during fear acquisition and during the formation of abdominal pain-related memories. Higher FC of R amygdala positively correlated with pain intensity and decreased gut permeability; FC between L amygdala, bilateral insula, and midbrain positively correlated with symptom severity. Bilateral INS: aberrant activation and FC with limbic and cortical areas at resting-state and during painful and nonpainful rectal distention. R INS: aberrant activation correlated with pain intensity. L INS: abnormal activation correlated with symptom severity and interoceptive awareness; aINS hyperactive during pain anticipation. |
| Attentional areas | Resting state: decreased activity of the DMN. Rectal stimulation: ACC and PFC hyperactivity. Pain anticipation: mPFC and MFG aberrant activation. Painful distension: greater stress-induced activation in insula and VLPFC, and hypoactivation of DLPFC and subgenual ACC. History of adverse life event was associated with altered FC in the salience network and in the executive control network. Regions of the salience network (MCC, MTG, and STG) were positively correlated with proinflammatory genes (IL-6 and APOL2). |
S1, primary somatosensory cortex; M1, primary motor cortex; FC, functional connectivity; H-IBS, hypersensitive IBS; pINS, posterior insula; N-IBS, normosensitive IBS; INS, insula; R, right; L, left; aINS, anterior insula; DMN, default mode network; ACC, anterior cingulate corte; PFC, prefrontal cortex; mPFC, medial prefrontal cortex; MFG, middle frontal gyrus; VLPFC, ventrolateral prefrontal cortex; DLPFC, dorsolateral prefrontal cortex; MCC, mid-cingulate cortex; MTG, middle temporal gyrus; STG, superior temporal gyrus; APOL2, apolipoprotein L2 gene.
Results
Functional Magnetic Resonance Imaging at Resting-state
Compared to HC, patients with IBS showed hypoactivity at resting-state in: (1) the left superior frontal gyrus (SFG), the right hippocampus, the bilateral post-central gyrus (primary somatosensory cortex [S1]), and the right superior temporal pole17; (2) the right middle frontal gyrus (MFG)17,18 and the right orbital part of the SFG18; (3) several default mode network (DMN) regions: the medial prefrontal cortex (mPFC), the posterior cingulate cortex (PCC), and the bilateral inferior parietal cortices.18 Hyperactivity was found in the left median cingulate cortex (MCC),17 the calcarine,17 the bilateral pINS,18 and cuneus.18 Duration of the IBS disease positively correlated with the right MFG activity and negatively correlated with the left MCC activity17; dorsal and ventral anterior cingulate cortex (ACC) hypoactivity disappeared after controlling for anxiety and depression, leading the authors to hypothesize that high anxious-depressive symptomatology may explain the intrinsic decreased brain activity in regions involved in affective processing (the ACC).18 Focusing on gender- and disease-related differences, Hong and colleagues19 found that: (1) female IBS patients, compared to HC, showed hyperactivity in the amygdala and hippocampus, and hypoactivity in sensorimotor regions; (2) both female HC and female IBS patients showed higher activity in the amygdala and hippocampus than male HC and male IBS patients, respectively; female IBS patients, compared with males, showed insular hyperactivity; and (3) in female patients, altered activity correlated with abdominal discomfort ratings.19
In summary, these studies show a global frontal, sensorimotor and DMN hypoactivity, and a cingulate, insular and amygdala hyperactivity in patients with IBS at resting state, compared to HC.
Resting-state functional connectivity
In IBS patients, increased connectivity was found between: (1) the left MCC and left SFG;17 (2) the right MFG, left SFG, and left PCC17; and (3) the mPFC and cuneus (negative FC18). Decreased connectivity was found between: (1) the right superior parietal gyrus and left rectus17, (2) mPFC and the right orbital part of the SFG (positive connectivity18), (3) ventral ACC and PCC (positive connectivity18), and (4) mPFC and left pINS (negative connectivity18). Qi and colleagues18 found that FC was not influenced by anxiety and depression, although other studies did not confirm this finding.20,21 Moreover, Qi and colleagues21 showed that IBS patients, compared to HC, had higher interhemispheric FC between thalamus (bilateral), PCC, cuneus, lingual gyri, and cerebellar lobes, together with lower interhemispheric FC between bilateral ventral ACC and inferior parietal lobules. Controlling for anxiety and depression, connectivity differences in the ventral ACC were abolished. Weng and colleagues22 calculated the long-range and short-range FC density (LR- and SR-FCD: the amount of distant and local functional connections of cortical areas). Compared to HC, IBS patients showed several FCD alteration in areas deputed to sensorimotor, homeostatic, emotional and cognitive regulation: (1) decreased LR- and SR-FCD in bilateral anterior MCC and inferior parietal lobules; (2) decreased LR-FCD in the right anterior insula (aINS), positively correlated with severity of IBS symptoms; (3) decreased SR-FCD in bilateral PFC, subgenual ACC and caudate, positively correlated with disease duration; (4) increased LR- and SR-FCD in S1; (5) increased LR-FCD in right supplementary motor area (SMA); and (6) increased SR-FCD in occipital lobe. Moreover, they found altered FC in IBS patients’ pain matrix, in particular: increased FC between the anterior MCC, right aINS, and superior prefrontal cortices; decreased FC between anterior MCC, right aINS, and PCC/precuneus. Postcentral cortices with stronger FCD showed: (1) increased FC with bilateral pre-central cortices, PCC/precuneus and mPFC; and (2) decreased FC with anterior MCC, aINS, bilateral dorsolateral prefrontal cortex (DLPFC), and inferior parietal lobule. Pre-central cortices with stronger FCD showed increased FC with bilateral PFC.
Gupta and colleagues20 found that IBS patients presented a greater within-network connectivity than HC in: (1) the salience network (putamen, insula, ACC, MCC, and supramarginal gyrus); (2) the left frontal parietal network (putamen, ACC, thalamus, and frontal cortex); (3) the DMN (precuneus, inferior parietal gyrus and angular gyrus). Within IBS patients only, early adverse life events positively correlated with (1) FC within the salience network, and (2) FC within the cerebellar network for male subjects only. The same group found that regions of the salience network, including MCC, and mid and superior temporal gyrus (STG) positively correlated with pro-inflammatory genes (IL-6 and APOL2) in patients with IBS, but negatively correlated with anti-inflammatory genes (KRT8 and APOA4) in HC, suggesting that, in IBS, a peripheral pro-inflammatory state is maintained by chronically activated stress signalling pathways.23
Several studies further examined the DMN.24,25 IBS patients presented decreased DMN inter-regional FC between the precuneus and (1) the ACC, (2) the middle temporal gyrus, and (3) the medial orbital SFG. Moreover, the average DMN FC negatively correlated with IBS symptom severity.24 The DMN showed altered FC with sensorimotor regions, the right amygdala, the left orbital frontal cortex, the bilateral middle, and inferior frontal gyri, and regions deputed to pain processing (periaqueductal grey and rostral ventral medulla). Interestingly, patients with IBS only showed increased FC in the right amygdala, correlated with decreased gut permeability.25
Two studies focused on FC in 2 main regions of interoception and emotion regulation, the insula26 and the amygdala.27 Hong et al26 found that male patients, compared to females, presented increased positive connectivity of the bilateral dorsal aINS with the mPFC, which positively correlated with scores of visceral sensitivity; conversely, female patients had greater negative connectivity of the left dorsal aINS with the left precuneus than males, which positively correlated with symptom intensity. Moreover, disease-related differences in the FC between the bilateral dorsal aINS and the dorsal mPFC were observed in female subjects. As regards the amygdala, Qi and colleagues27 found that IBS patients, compared to controls, had: (1) higher left amygdala positive FC with right insula, midbrain, right precentral gyrus (primary motor cortex [M1]), left S1/M1, left parahippocampal gyrus, and bilateral SMA; and (2) higher right amygdala positive FC with the right insula, midbrain, left parahippocampal gyrus, bilateral M1, and right SMA. These results hold when controlling for anxiety and depression. Pain intensity positively correlated with the FC between the right amygdala, the right M1 and right insula; symptom severity positively correlated with FC between the left amygdala, bilateral insula and the midbrain, and with FC between the right amygdala and right insula. Significant clinical correlations emerged also in the study of Longarzo and colleagues,28 which showed that the FC between PCC and left supramarginal gyrus/STG negatively correlated with attitude, fear and beliefs associated with hypochondriac behavior, and that the FC between left ventral aINS and bilateral supramarginal gyrus positively correlated with interoceptive awareness, the ability to perceive signals from within the body.
Icenhour and colleagues29 made a step further by evaluating FC within the salience, sensorimotor and DMN in a sample of selected IBS patients with and without visceral hypersensitivity (a distinction presumably subtended by differences in the processing of visceral afferent signals), compared to HC. Hypersensitive IBS (H-IBS) patients showed increased positive FC of pregenual ACC and thalamus in the salience network and of pINS in the sensorimotor network than normosensitive IBS (N-IBS). N-IBS patients (compared to H-IBS and HC) showed less positive amygdala FC and less negative dorsal aINS FC in the DMN. DMN and sensorimotor network FC were associated with rectal perception thresholds, and FC in pINS correlated with IBS symptom severity.
Ke et al30 showed that IBS patients presented higher FC in the bilateral S1, calcarine, vermis, right thalamus, left superior parietal lobule, and less FC in the bilateral ACC and MCC, PFC, right caudate, and angular gyrus (not influenced by anxiety and depression). Correlations emerged between (1) disease duration and FC in the right S1 (positive correlation) and FC in the right anterior MCC/SMA and bilateral insula (negative correlation), (2) IBS symptoms severity and FC in the left thalamus (positive correlation) and FC in the right ventral mPFC and MFG (negative correlation), and (3) pain intensity and FC in the left S1 (positive correlation) and with FC in right dorsal mPFC and left MFG (negative correlation). The authors concluded that the abnormal synchronization in spontaneous brain activity may reflect symptoms manifestation.
In summary, overall, aberrant FC in IBS patients was found in the salience, sensorimotor and DMN; limbic regions (amygdala and cingulate cortex in particular) showed abnormal FC with sensorimotor, insular and frontal cortical regions. These findings were associated with severity of IBS symptoms, gut permeability, and inflammatory genes, and with the presence of adverse life events. Contradictive results emerged about the influence of anxiety and depression.
Functional Magnetic Resonance Imaging With Tasks
To assess abnormalities in visceral pain processing in IBS, several studies performed functional magnetic resonance imaging (fMRI) during non-painful and painful rectal distension. Overall, patients with IBS rate rectal distension as more painful than HC31-34 and, when combined with painful heterotopic stimulation (eg, a foot in ice-cold water), rectal pain is significantly decreased in HC but not in patients with IBS.35,36 Rectal stimulation generally elicits in both IBS and HC the activation of ACC, PFC, insula, and thalamus,31,32,36,37 together with the cerebellum.36-39 Brain activation of these areas apparently increase along with rectal balloon dilation, but not in women with IBS and in patients whose symptoms lasted more than 5 years.40 More specifically, in IBS subjects, Mertz and colleagues31 found that painful stimulation led to a greater activation in the ACC, while Yuan et al32 found it in the insula, PFC, and thalamus, suggesting a heightened pain sensitivity of the brain-gut axis in IBS, with a normal pattern of activation. Anxiety and depression appeared associated with subjective stimulus ratings and with cerebral activations34,37: anxiety correlated with pain-induced activation of the right anterior MCC and pregenual ACC, and depression with pain-induced activation of left PFC and cerebellar areas. To further understand the role of emotional modulation of neural responses to visceral stimuli, the same group combined rectal stimulation with a psychological distraction (either stress or relaxation).41 During painful distension, patients with IBS showed a greater stress-induced increase in the insula and the ventrolateral prefrontal cortex (VLPFC), and a lower activation of the DLPFC and the subgenual ACC compared to HC, suggesting that HC presented greater up-regulation during stress in these areas. Relaxation brought to a reduced modulation of the activation in the right insula, the cerebellum, the putamen, and temporal regions during non-painful distensions, and in the VLPFC, precuneus and thalamus during painful distensions. However, when controlling for levels of anxiety, only activations in the cerebellum and precuneus survived the correction.41 Similarly, Rosenberger et al38 found that in IBS patients, cerebellar activations induced by non-painful distension (right cerebellum) and by painful distension (intermediate cerebellum) correlated with depression, while non-painful induced cerebellar activations correlated with anxiety. Labus and colleagues42 aimed to evaluate sex- and disease-related differences in brain activation of IBS patients during an emotion recognition paradigm (fear and anger): male subjects (IBS and HC), showed greater activation than females in PFC, insula, and amygdala, as well as stronger connectivity between ACC, amygdala, and insula; female subjects showed stronger connectivity to and from the PFC.
Combining rectal and heterotopic stimulation (to elicit a simultaneous activation of endogenous descending pathways of nociceptive inhibition), Song et al36 found that (1) during rectal stimulation alone, HC showed greater activation bilaterally in the aINS, in the secondary somatosensory cortex (S2), and putamen than patients with IBS; and (2) during rectal plus heterotopic stimulation, HC showed greater activation in bilateral S1 and right STG, while IBS patients in bilateral STF and right inferior lobules.
Berman and colleagues33 added to this task the evaluation of brain changes during rectal distension anticipation, to assess anomalies in preparatory brain activity before painful pelvic visceral distension. In the anticipation phase, HC showed reduced activity in the insula, supragenual ACC, amygdala, and dorsal brainstem; IBS patients showed less anticipatory inactivation, particularly in the right pINS and dorsal brainstem; moreover, the anticipatory decrease in the pons (dorsal brainstem) was associated with higher stimulation-induced activation in right orbitofrontal areas and bilateral supragenual ACC, suggesting that deficits in preparatory inhibition of dorsal brainstem may interfere with descending corticolimbic inhibition. Expectation of pain was investigated also by Hong and colleagues,43 who performed fMRI during cued and uncued pain expectation. In absence of a cue about the occurrence of abdominal pain stimulus (ie, in front of an uncertain painful prospect), IBS subjects (especially females, compared to HC) showed greater brain activations in the affective (amygdala), interoceptive (aINS), and attentional (MFG) regions, together with the thalamus and precuneus. The authors interpreted this hyperactivation as a possible evidence that IBS subjects tend to overestimate the likelihood of painful somatic occurrence and engage brain networks involved in affective and sensory processing. Similarly, Kano and colleagues44 found that IBS patients, compared to HC, in a condition of uncertain anticipation of painful stimulation showed greater activation of anterior MCC, thalamus, and visual processing areas, and a higher activity in PCC/MCC and precuneus induced by the following rectal distension; if, after the uncertain cue, rectal distention was not performed, IBS patients, contrary to HC, lacked to show bilateral insula activation (but the authors note how this effect might have been due to the elevated bilateral insular responses during non-distention after the safe cue, the comparison condition). The authors conclude that cue-dependent alterations in brain responses may underlie hypervigilance to visceral sensations in IBS patients. To investigate the learning processes shaping the expectation of pain in IBS, Icenhour et al45 performed fMRI during the formation, extinction, and reactivation of abdominal pain-related memories in IBS patients and HC, through a fear conditioning paradigm. IBS patients showed: (1) during the fear acquisition phase, differential activation of PFC, amygdala, and cerebellum34; (2) during extinction, enhanced differential cingulate activation; and (3) during reinstatement, greater differential hippocampal activation.45 These results shed another light on the factors that could play a role in the pathophysiology of chronic abdominal pain and suggest that extinction-based interventions might have therapeutic effects on IBS. As regards therapeutic interventions, in fact, Lowen and colleagues46 showed, in a longitudinal fMRI study, that 16 sessions of hypnotherapy or educational intervention were both effective in reducing pain during rectal distention in both IBS and HC groups; after the treatment, brain response to distension was similar between IBS and HC, possibly indicating that this treatment may have normalized the anomalies in the central processing of visceral signals in IBS.
Kano et al47 made a step further by examining, during colorectal distention, brain activation via fMRI and heart rate variability. Patients with IBS, although all constipation-predominant (IBS-C), displayed blunted sympathovagal balance in response to colorectal distention compared to HC; additionally, in the HC but not in the IBS group, brain activation in the right caudate, bilateral dorsolateral ACC and pregenual ACC in response to rectal distention positively correlated with baseline high frequency values.
Several studies performed the same task by dividing IBS subjects according to specific criteria. Patients with diarrhea-predominant IBS, during heterotopic stimulation, showed deactivation in the right aINS; IBS-C showed hyperactivation of amygdala and hippocampus; HC showed deactivation of the periaqueductal grey, suggesting a different abnormal endogenous pain inhibitory mechanism.35 IBS patients with a history of sexual abuse showed hyperactivation in the left MCC and PCC, hypoactivation in the left supragenual ACC, and greater pain, than subjects who did not suffer abuse48; the authors hypothesized that adverse life events may enhance the activation of dorsal cingulate regions implicated in homeostatic afferent processing. IBS patients presenting inhibition or facilitation of the RIII nociceptive spinal reflex induced by rectal distension did not present differences in brain activity associated with non-painful distensions, while differences associated with painful distensions (in frontal areas, the MCC and aINS) were influenced by levels of anxiety, depression, and tendency of catastrophizing.37 Larsson and colleagues49 showed that H-IBS patients, during painful rectal distensions, had greater activation of insula and reduced deactivation in pregenual ACC than HC and patients with N-IBS (which did not differ to each other); during expectation of rectal distension, N-IBS presented more activation in right hippocampus than controls. Similarly, Lowen and colleagues50 compared H-IBS and N-IBS in their ability to engage endogenous pain modulation mechanism during habituation to repeated visceral painful stimuli. In the last trials of a series of rectal distension, N-IBS showed hypoactivation in insula and amygdala, and H-IBS showed greater activation than N-IBS and HC in insula, ACC, and MCC; this suggests a lack of habituation to repeated delivery of rectal stimuli, in terms of both sensitization of sensory pathways and habituation of emotional arousal, consistent with IBS well-known gut-related hypervigilance.
Given the reported aberrant activation of the insula and of the DLPFC, Aizawa et al51 assessed IBS patients’ cognitive flexibility through the Wisconsin Card Sorting Test; they presented more perseverative errors than HC, together with: (1) reduced activity of right DLPFC and right hippocampus, (2) increased activity of left pINS, (3) diminished FC from DLPFC to pre-SMA. Similarly, Hubbard and colleagues52 tested attentional abilities in IBS, by employing a form of flanker task during fMRI. Patients with IBS, with respect to HC, had shorter reaction times during the alerting and orienting conditions, associated with: (1) greater MCC and insula activation, and (2) less activity in the right inferior frontal junction and SMA; also, they showed activation in the dorsal mPFC together with hypoactivation of the thalamus. This aberrant activity correlated with gastrointestinal-specific anxiety, pain catastrophizing, and symptom severity.
Only 1 study performed genetic analysis53: via fMRI, they compared brain response during an affect-matching paradigm in IBS patients and HC, further dividing participants according to their genotype (the C/C genotype of the c.-42C>T polymorphism in serotonin (5-HT) receptor 3, associated with aberrant amygdalar activity while processing emotional faces, compared to T-carrier). The C/C genotype, in both IBS and HC, appeared to correlate with increased anxiety and amygdala responsiveness during emotional and non-emotional tasks; furthermore, the polymorphism was associated with severity of IBS symptoms, and only a few IBS subjects with the C/C genotype presented significantly increased amygdala responses to nonemotional stimuli.
One of the main limits of the aforementioned studies is the high prevalence of female subjects. Guleria et al54 performed the same task in a group of male IBS patients and, consistently with the other studies, found that they hyperactivated the insula, MTG, and cerebellum in the left hemisphere compared to HC, but did not activate the bilateral precuneus/superior parietal lobules. Moreover, it emerged that patients with IBS-C activated the right MCC while patients with IBS-D activated the left inferior orbito-frontal cortex, left calcarine, and bilateral fusiform gyri. A second limit of the majority of the fMRI studies with tasks is pointed out by Wong et al,55 as they showed that performing rectal stimulation within the scanner environment significantly increases visceral, but not somatic, pain perception in both IBS patients and HC (compared to the same exam performed outside the MRI scanner).
Taken together, these results point in the direction of a higher activation of sensitive, interoceptive and frontal areas in IBS patients following painful rectal stimulation; its anticipation is linked to an aberrant activation of attentional (mPFC and MFG) and affective-interoceptive (amygdala, aINS, and ACC) regions. Patients with IBS also resulted in more alertness and presented a reduced attentional shift, when compared to HC, which was associated with an abnormal frontal activation.51,52
Pharmacological functional magnetic resonance imaging studies
To test the hypothesis that alterations in 5-HT signaling within the brain-gut axis may be implicated in IBS pathophysiology, Labus and colleagues1 examined brain responses via fMRI at the rectal distension task after performing acute tryptophan depletion (ATD) in a group of IBS patients (all female, IBS-C) and HC. In both groups, ATD resulted in increased response of an extensive brain network to balloon distension, including the amygdala and nodes of emotional arousal and homeostatic afferent networks. These findings were consistent with an ATD-mediated disinhibition of an emotional arousal network during aversive stimulation, and increased connectivity within the same network, suggesting that 5-HT dysregulation may play a role in central pain amplification and, ultimately, in IBS pathophysiology. Also alterations in corticotropin-releasing factor (CRF) signaling pathways have been implicated in IBS pathophysiology: the CRF receptor is present in many sites of the central nervous system, and is able to influence the activity of many areas, regardless of its effect on the hypothalamus-pituitary-adrenal (HPA) axis. Hubbard and colleagues56 administered the selective CRF receptor 1 antagonist GW876008 or a placebo to a group of HC and a group of IBS patients, while performing fMRI during expectation of abdominal pain. During pain expectation, both IBS patients and HC who received the CRF receptor 1 antagonist showed hypoactivation in amygdala, hippocampus, insula, ACC, and orbito-medial PFC, compared to participants who received placebo. Under placebo, patients showed higher activity in the hypothalamus and locus coeruleus (noradrenergic pathway involved in arousal responses, alarm, and visceral body preparation reactions) than HC, suggesting a greater baseline reactivity in patients; activity at the hypothalamic level decreases after administration of the drug only in patients having average and high levels of state anxiety. More recently, Kano and colleagues57 found in HC, but not in patients with IBS, a negative correlation between adrenocorticotropic hormone response to administered CRF and the activity in the pregenual ACC during rectal distention; authors, therefore, hypothesized that impaired top-down inhibitory input from the pregenual ACC to the HPA-axis may lead to altered neuroendocrine and gastrointestinal responses to CRF in patients with IBS. In a subsequent study,58 they also found that individuals (IBS and HC) with higher levels of alexithymia showed stronger adrenocorticotropic hormone responses to CRF administration, and that brain responses to rectal distention in the right insula, putamen, right pallidum, thalamus, orbital part of inferior frontal gyrus, and left inferior temporal gyrus positively associated with alexithymia scores to a greater extent in patients with IBS than in HC.
When interpreting the results of pharmacological studies, it should be considered that Lee and colleagues59 found that the placebo condition evoked more activity in affective and cognitive regions (insula, MCC, and VLPFC) in IBS patients than in HC (although their visceral placebo analgesia was comparable).
In summary, these data show, from a biochemical perspective, an important role of 5-HT in central modulation (exerted by the amygdala on the emotional arousal circuits), higher basal activity of the noradrenergic centers, and a dysregulation of the corticotropic axis in patients with IBS.
Quantitative Magnetic Resonance Spectroscopy
Bednarska and colleagues11 found that concentrations of glutamate + glutamine (Glx) were lower in bilateral aINS of women with IBS, compared with HC; furthermore, a lateralization pattern emerged, with the lower Glx concentrations of the right aINS being predicted by longer pain duration, and those of the left aINS being predicted by less use of adaptive pain-coping strategies. At the same time, GABA concentrations where comparable between the 2 groups, showing overall a reduced excitatory but unaltered inhibitory neurotransmitter (NT) levels in aINS in IBS patients. Niddam and colleagues12 found a reduction in Glx in IBS patients’ hippocampus, with a concentration inversely related to emotional stress indicators. Furthermore, a functional lateralization was observed as glutamate concentrations in the left, but not the right hippocampus of IBS patients negatively correlated with anxiety, pain catastrophizing, and pain duration. Icenhour and colleagues13 found that IBS patients overall did not differ from HC with respect to mPFC GABA+ or Glx levels; only IBS patients with higher anxiety levels showed increased GABA+ in mPFC, together with lower FC between mPFC and ACC.
In summary, these studies, although sharing the same limitations of the fMRI studies (a female-predominant sample of IBS patients), showed a reduced excitatory NT level in aINS and hippocampus, an unvaried level of inhibitory NT in aINS, and an influence of anxiety on the levels of inhibitory NT in mPFC.
Positron Emission Tomography
Nakai and colleagues60 sought for disease-related and gender-related effect in HT synthesis, and found that female patients with IBS, compared to HC, presented a greater 5-HT synthesis in the right medial temporal gyrus, a multimodal sensory association cortex, widely connected to the PFC and to the limbic system through the cingulate gyrus, parahippocampus and insula.61-62 To investigate the noradrenergic activity in a sample of IBS patients, compared to HC, Berman et al63 performed double-blind study where participants had to perform an auditory vigilance task after intake of the α2-adrenoreceptor (α2AR) antagonist yohimbine (YOH), the α2AR agonist clonidine, or placebo. The α2AR is a presynaptic receptor that controls the release of catecholamine from the synaptic terminal: agonists like clonidine inhibit the release of norepinephrine, while antagonists like YOH facilitates the release of catecholamine from the synapse, which ultimately leads to a reduction in cortical metabolism.64 First, they showed that IBS patients had higher plasma noradrenergic levels than HC before and after ingestion of all drugs. Second, IBS patients showed less YOH-mediated reduction of activity in the central arousal circuit, consistent with a downregulation (ie, fewer functional presynaptic α2AR). YOH-mediated reduction of activity in the brainstem and amygdala negatively associated with adverse early life event. Third, in HC only, the activation of anterior MCC negatively correlated with the activation of the amygdala and the subgenual ACC. One longitudinal positron emission tomography study which evaluated brain responses to rectal distention showed a stable activation in the central pain matrix but a decreased activity in limbic, paralimbic, and pontine regions, suggesting a habituation of visceral perception and central arousal in patients with IBS linked to repeated exposure to experimental aversive visceral stimuli.65
Overall, these data underline the role of specific NTs in central modulation in patients with IBS: 5-HT, which is shown to be increased in brain areas closely interconnected with the limbic circuit especially in women; norepinephrine, which seems to subtend a greater basal activity in the arousal circuits in IBS patients, especially in those with a history of early emotional trauma.
Discussion
This study aims at reviewing the literature on functional neuroimaging in IBS patients. Most of the studies included suggested an involvement of specific areas of the brain (amygdala, insula, prefrontal, and cingulate cortex) in the pathophysiology of IBS, although many studies shared the limitations of female predominance and the presence of confounding factors (such as psychiatric comorbidities).
A first group of studies analysed brain activation and FC at resting-state via fMRI. They highlighted an aberrant activation and FC of the insular, cingulate and frontal cortices, the amygdala, and the hippocampus, suggesting an altered activity of the homeostatic and salience network and of the autonomous nervous system (possibly accounting, respectively, for IBS patients’ hypervigilance and HPA-axis dysregulation). In parallel, hypoactivity in the DMN emerged at the resting state: since the DMN is the brain network presenting the highest activity in the idling brain, its hypoactivity in IBS patients was interpreted as a further hint of their continuous hypervigilance and spontaneous perception of pain.18
Most studies implemented fMRI while the experimental groups were performing different tasks, with a particular focus on painful and non-painful rectal distention. Rectal stimulation seems to elicit in both IBS patients and HC the activation of ACC, PFC, insula, thalamus, and cerebellum31,32,36-39; this activation seems higher in IBS patients,31,32 as confirmed by a recent meta-analysis.66 Aberrant activation of attentional (mPFC and MFG) and affective-interoceptive (amygdala, aINS, and ACC) regions, as well as of the dorsal brainstem, are reported during anticipation of pain33,43,44,65 and during the learning process shaping the expectation of pain,39,45 accounting, again, for an alteration in hypervigilance and in descending corticolimbic inhibition of pain––further underlined by patients’ enhanced activity of noradrenergic centres.56,64 Several studies showed similarities between dysfunctional cerebral activation of IBS patients and patients with other functional disorders, such as functional dyspepsia,67 fibromyalgia,14,68 and other conditions characterized by chronic pain, such as endometriosis.69 Here, we suggest that similarities with the neural activity of patients with functional neurological disorders, FMD in particular, should be also highlighted. In a recent study,70 our group reviewed the literature on functional neuroimaging in patients with FMD: we found a decreased activation in the primary motor cortex contralateral to the symptoms and in the parietal lobe, an aberrant activation of the amygdala, and an increased temporo-parietal junction activity. Most importantly, functional connectivity findings highlighted aberrant connections between the amygdala and motor areas (including the SMA, involved in motor programming), the temporo-parietal junction and the insula. We propose a model where amygdala functional alterations have a crucial role in producing and maintaining FMD, and the aberrant FC between amygdala and the aforementioned brain regions may explain specific secondary co-causative etiopathogenetic FMD mechanisms, respectively: (1) the impaired motor conceptualization, motor preparation and the inhibition of motor execution; (2) the altered sense of agency; and (3) the deficit in processing of the affected body parts at a cognitive level. Here, as well, a predominant role of the amygdala in maintaining IBS symptoms may be highlighted, given its aberrant activation in anticipation33,43,44,65 and regulation11,56,60 of pain. In IBS, a major role of the insula (recognized as site of self-awareness, interoception, self-regulation of emotions and of integration between emotion and motor control), must be acknowledged: bilateral insula showed aberrant activation and FC with limbic and cortical areas at resting-state26,30,45 and during painful and nonpainful rectal distention exam (see above). Moreover, a lateralization pattern emerged, with right insular aberrant activation often correlating with pain intensity,27 and left insular abnormal activation correlating with symptom severity26,27 and levels of interoceptive awareness.28 The activity of insula in FMD was less studied, although first evidence suggests that the insula is aberrantly activated at resting-state71 and when the patient imagines or tries to move the paralyzed leg,72 and hypoactivated during emotional tasks.73 Overall, these findings suggest that the aberrant FC between amygdala and the insula may play a role in the production of IBS symptoms, while the aberrant FC between amygdala and the motor cortices may play a role in the production of functional motor symptoms. Moreover, the insula seems to have a role in Psychogenic Non-Epileptic Seizure (PNES – another widespread subtype of functional neurological disorder): in fact, in patients with PNES, the insula was found to be hyperactivated and its FC with motor areas to be greatly aberrant, suggesting that an aberrant insular-motor interaction may account for some psychological features underlying PNES such as dissociation.74
At the NT level, qMRS studies highlighted a dysregulation in the glutamatergic system of patients with functional disorders. Reduced Glx levels in aINS and hippocampus, unvaried levels of GABA+ in aINS, and an influence of anxiety in GABA+ levels in mPFC were found in IBS patients.11-13 Conversely, an increase of Glx in the limbic system has been found in patients with FMD,15 in the somatosensory cortex of patients with functional dyspepsia,67 and in the pINS of patients with fibromyalgia.14 FMD patients also showed lower cerebrospinal fluid levels of glutamate than HC, suggesting that a glutamatergic dysfunction plays a role in FMD pathophysiology.75
HPA axis dysregulation, widely documented in IBS patients, has been reported in FMD patients as well,76 although future studies should further investigate this area.
Finally, most of the studies included in this review corrected their results for the levels of anxious-depressive symptomatology,13,17,18,21 usually assessed through self-report questionnaires. At the resting state, several studies reported that anxiety and depression influenced the activity and FC of the ACC13,18,21,34,35 involved in affective processing,18 but not amygdala activity and FC.27 During painful rectal distension, the differences between IBS and HC in frontal areas, the MCC and aINS were found to be influenced by levels of anxiety, depression, and tendency of catastrophizing.37 Moreover, anxiety correlated with pain-induced activation of the right anterior MCC and pregenual ACC, and depression with pain-induced activation of left PFC and cerebellar areas.34,37 When combining rectal stimulation and a psychological distraction (a task implemented to understand the role of emotional modulation of neural responses to visceral stimuli), Elsenbruch and colleagues41 found that during painful distension, IBS patients showed a greater stress-induced increase in the insula and the VLPFC, and a lower activation of the DLPFC and the subgenual ACC compared to HC, suggesting that HC presented greater up-regulation during stress in these areas.41
In the attempt to find a unified theoretical approach for functional disorders, recent advances in cognitive neuroscience and neurobiology in a hierarchical Bayesian framework may help. According to this approach, perception arises from the comparison of a knowledge-driven probabilistic inference on the external causes of sensory signals (top-down generative models) and the correspondent bottom-up signals which convey eventual error to the aforementioned predictions (prediction errors).77 Through prediction errors, probabilistic predictive models are re-computed and refined, in consonance with Bayesian principles; otherwise, through active inference, agents suppress prediction errors by performing actions to modify the environment and make it fit with its predictions. In fact, according to the “Free Energy Principle”, each organism tries to minimize the differences between their predicted models of the environment (priors) and the sensations conveyed by their sense organs.78 This comparison results in a posterior distribution corresponding to the percept (posterior belief). This framework was applied also to psychosomatic medicine. Edwards and colleagues79 recently proposed that functional motor and sensory symptoms arise because of the presence of an abnormal prior belief at an intermediate level in the cortical hierarchy. This belief is granted too much precision through an abnormal allocation of attention, and inevitably bias the outcome of the entire process towards itself, regardless the bottom-up input. In functional sensory symptoms, an abnormal prior in sensory cortices would predict, for example, anaesthesia or pain; in functional motor symptoms, an aberrant prior in SMA would imply a wrong expectation about the proprioceptive consequence of a movement which, in a higher level of the hierarchy (eg, pre-SMA), would be explained as a failure to realize that the movement was intended (active inference). Similarly, it has been proposed80 that an abnormal prior in the control regions of the intestines, highly precise, may produce a perception of intestine pain and discomfort, although intestines sensory signals are normal. Alterations of motility of the intestine, resulting in alterations in defecation, may be a strategy to minimize prediction error.
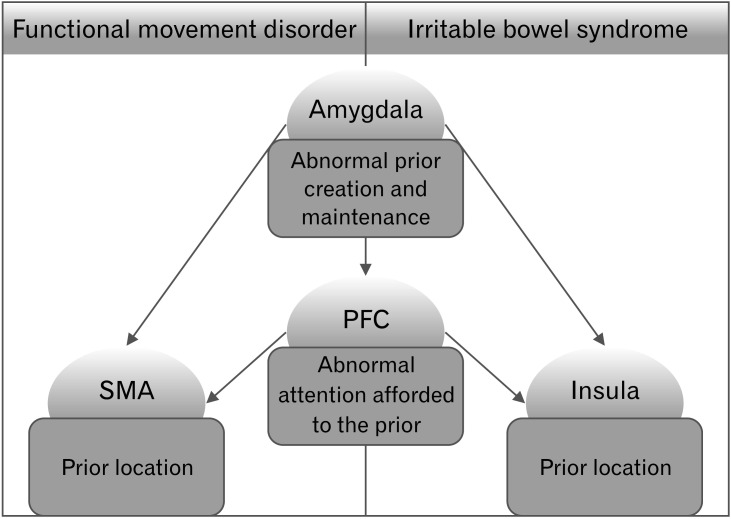
The results of our systematic review fit perfectly within this framework (Fig. 2). The aberrant frontal activation shown in patients with FMD and with IBS may account for the abnormal attention allocated to the aberrant priors. These priors may be located in SMA in FMD, and in the insula and S2 in IBS.79,81 Anterior INS has been hypothesized by several authors as a possible neural substrate for the comparator system between predictions and errors in the interoceptive domain.82,83 Finally, the amygdala may have a predominant role in the creation and maintenance of these priors. Edwards et al79 proposed that pathological expectations, which lead to the construction of the abnormal prior when afforded too much attention and precision, may originate through a multitude of factors, from a traumatic salient event to personal and cultural beliefs, negative effects and cognitive biases. The role of the amygdala in modulating perception and attention, in the elaboration of a perceptual stimuli with a high emotional valence84 and in its retention in memory,85 is well-known. In a predictive coding framework, it has been proposed that the amygdala, with the ACC, may work as a comparator of predictions and predictions errors, ultimately leading to dysfunctional behaviours.80 Therefore, the aberrant activation and FC of the amygdala in functional syndromes may explain, to a certain extent, the maintenance of abnormal priors in specific brain area, producing a continuous discrepancy in the comparison between top-down and bottom-up signals, ultimately leading to each patient specific symptomatology (either motor or sensitive-visceral). This may be supported by the findings of Icenhour and colleagues45 who showed that during the formation of abdominal pain-related memories, IBS patients presented a different activity in the amygdala, compared to HC.
Figure 2.
A common pathophysiology for irritable bowel syndrome (IBS) and functional movement disorders (FMD) in a Bayesian perspective. Recent hypotheses in psychosomatic medicine suggest that functional symptoms might arise when excessive attention is granted to abnormal prior beliefs, located at an intermediate level in the cortical hierarchy. In line with this framework, here we propose that the aberrant frontal activation shown by both patients with FMD and with IBS may account for the abnormal attention allocated to the aberrant priors, which may be located in supplementary motor area (SMA) in FMD, and in the insula and secondary somatosensory cortex (S2) in IBS.79,81 Anterior insula has been hypothesized by several authors as a possible neural substrate for the comparator system between predictions and errors in the interoceptive domain.82,83 Finally, the amygdala may have a predominant role in the creation and maintenance of these priors.79 PFC, prefrontal cortex.
Limitations and Future Perspectives
Our study has the following limitations. First, we discussed functional disorders only: it is possible that the brain alterations that we highlighted here are shared by several other organic diseases, representing either an epiphenomenon of the disorders or a contributing factor to their pathophysiology, as reported for pelvic pain associated with endometriosis.69 Second, although we mentioned other functional disorders such as functional dyspepsia, fibromyalgia and PNES, we mostly focused on FMD; next steps in the research agenda would be (1) to revise the literature about neuroimaging in other functional disorders, and (2) to conduct experimental neuroimaging studies, comparing patients with different functional symptoms, in order to investigate both their common core and their specificity. Third, we included mostly cross-sectional studies: hence, we cannot exclude the hypothesis that these common brain alterations are only common epiphenomena of the functional disorders, and we cannot confirm our hypothesis that they may account for IBS (and functional disorders) pathophysiology. Fourth, we did not conduct a meta-analysis or quantitative analysis, which would have reinforced our claim; future meta-analytic studies should assess whether this similarity is confirmed when focusing on the sub-regions of the aforementioned common areas.
Conclusions
Functional neuroimaging helps detecting, in patients with IBS, several brain alterations which appear very similar to the ones observed in patients with other functional disorders, such as FMD. Overall, our findings lead us to speculate, under a theoretical and neurobiological perspective, that different functional disturbances may share a common pathophysiology.
Supplementary Material
Note: To access the supplementary table mentioned in this article, visit the online version of Journal of Neurogastroenterology and Motility at http://www.jnmjournal.org/, and at https://doi.org/10.5056/jnm21079.
Acknowledgements
Aldo Ravelli Research Center partially supported the study.
Footnotes
Financial support: None.
Conflicts of interest: None.
Author contributions: Conception and design of the study: Veronica Nisticò, Roberta E Rossi, Andrea M D’Arrigo, and Benedetta Demartini; acquisition and analysis of data: Veronica Nisticò, Roberta E Rossi, Andrea M D’Arrigo, and Benedetta Demartini; drafting the manuscript or figures: Veronica Nisticò, Roberta E Rossi, and Andrea M D’Arrigo; and revising the manuscript for intellectual content: Alberto Priori, Orsola Gambini, and Benedetta Demartini.
References
- 1.Labus JS, Mayer EA, Jarcho J, et al. Acute tryptophan depletion alters the effective connectivity of emotional arousal circuitry during visceral stimuli in healthy women. Gut. 2011;60:1196–203. doi: 10.1136/gut.2010.213447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mearin F, Lacy BE, Chang L, et al. Bowel disorders. Gastroenterology. 2016;150:1393–1407. e5. doi: 10.1053/j.gastro.2016.02.031. [DOI] [PubMed] [Google Scholar]
- 3.Tanaka Y, Kanazawa M, Fukudo S, Drossman DA. Biopsychosocial model of irritable bowel syndrome. J Neurogastroenterol Motil. 2011;17:131–139. doi: 10.5056/jnm.2011.17.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boeckxstaens G, Camilleri M, Sifrim D, et al. Fundamentals of neurogastroenterology: physiology/motility-sensation. Gastroenterology. 2016;150:1292–1304. e2. doi: 10.1053/j.gastro.2016.02.030. [DOI] [PubMed] [Google Scholar]
- 5.Vanner S, Greenwood-Van Meerveld B, Mawe G, et al. Fundamentals of neurogastroenterology: basic science. Gastroenterology. 2016;150:1280–1291. doi: 10.1053/j.gastro.2016.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mayer EA, Tillisch K, Gupta A. Gut/brain axis and the microbiota. J Clin Invest. 2015;125:926–938. doi: 10.1172/JCI76304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Oudenhove L, Levy RL, Crowell MD, et al. Biopsychosocial aspects of functional gastrointestinal disorders: how central and environmental processes contribute to the development and expression of functional gastrointestinal disorders. Gastroenterology. 2016;150:1355–1367. doi: 10.1053/j.gastro.2016.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al Omran Y, Aziz Q. The brain-gut axis in health and disease. Adv Exp Med Biol. 2014;817:135–153. doi: 10.1007/978-1-4939-0897-4_6. [DOI] [PubMed] [Google Scholar]
- 9.Elsenbruch S. Abdominal pain in irritable bowel syndrome: a review of putative psychological, neural and neuro-immune mechanisms. Brain Behav Immun. 2011;25:386–394. doi: 10.1016/j.bbi.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 10.Mayer EA, Tillisch K. The brain-gut axis in abdominal pain syndromes. Annu Rev Med. 2011;62:381396. doi: 10.1146/annurev-med-012309-103958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bednarska O, Icenhour A, Tapper S, et al. Reduced excitatory neurotransmitter levels in anterior insulae are associated with abdominal pain in irritable bowel syndrome. Pain. 2019;160:2004–2012. doi: 10.1097/j.pain.0000000000001589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niddam DM, Tsai SY, Lu CL, Ko CW, Hsieh JC. Reduced hippocampal glutamate-glutamine levels in irritable bowel syndrome: preliminary findings using magnetic resonance spectroscopy. Am J Gastroenterol. 2011;106:1503–1511. doi: 10.1038/ajg.2011.120. [DOI] [PubMed] [Google Scholar]
- 13.Icenhour A, Tapper S, Bednarska O, et al. Elucidating the putative link between prefrontal neurotransmission, functional connectivity, and affective symptoms in irritable bowel syndrome. Sci Rep. 2019;9:13590. doi: 10.1038/s41598-019-50024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris RE, Sundgren PC, Craig AD, et al. Elevated insular glutamate in fibromyalgia is associated with experimental pain. Arthritis Rheum. 2009;60:3146–3152. doi: 10.1002/art.24849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Demartini B, Gambini O, Uggetti C, et al. Limbic neurochemical changes in patients with functional motor symptoms. Neurology. 2019;93:e52–e58. doi: 10.1212/WNL.0000000000007717. [DOI] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group, author. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PloS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097.335182623f2e4facbc3552f35ab7aec7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma X, Li S, Tian J, et al. Altered brain spontaneous activity and connectivity network in irritable bowel syndrome patients: a resting-state fMRI study. Clin Neurophysiol. 2015;126:1190–1197. doi: 10.1016/j.clinph.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Qi R, Liu C, Ke J, et al. Intrinsic brain abnormalities in irritable bowel syndrome and effect of anxiety and depression. Brain Imaging Behav. 2016;10:1127–1134. doi: 10.1007/s11682-015-9478-1. [DOI] [PubMed] [Google Scholar]
- 19.Hong JY, Kilpatrick LA, Labus J, et al. Patients with chronic visceral pain show sex-related alterations in intrinsic oscillations of the resting brain. J Neurosci. 2013;33:11994–12002. doi: 10.1523/JNEUROSCI.5733-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta A, Kilpatrick L, Labus J, et al. Early adverse life events and resting-state neural networks in patients with chronic abdominal pain: evidence for sex differences. Psychosom Med. 2014;76:404–412. doi: 10.1097/PSY.0000000000000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qi R, Liu C, Weng Y, et al. Disturbed interhemispheric functional connectivity rather than structural connectivity in irritable bowel syndrome. Front Mol Neurosci. 2016;9:141. doi: 10.3389/fnmol.2016.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weng Y, Qi R, Liu C, et al. Disrupted functional connectivity density in irritable bowel syndrome patients. Brain Imaging Behav. 2017;11:1812–1822. doi: 10.1007/s11682-016-9653-z. [DOI] [PubMed] [Google Scholar]
- 23.Gupta A, Cole S, Labus JS, et al. Gene expression profiles in peripheral blood mononuclear cells correlate with salience network activity in chronic visceral pain: a pilot study. Neurogastroenterol Motil. 2017;29:e13027. doi: 10.1111/nmo.13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qi R, Ke J, Schoepf UJ, et al. Topological reorganization of the default mode network in irritable bowel syndrome. Mol Neurobiol. 2016;53:6585–6593. doi: 10.1007/s12035-015-9558-7. [DOI] [PubMed] [Google Scholar]
- 25.Witt ST, Bednarska O, Keita ÅV, et al. Interactions between gut permeability and brain structure and function in health and irritable bowel syndrome. Neuroimage Clin. 2019;21:101602. doi: 10.1016/j.nicl.2018.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hong JY, Kilpatrick LA, Labus JS, et al. Sex and disease-related alterations of anterior insula functional connectivity in chronic abdominal pain. J Neurosci. 2014;34:14252–14259. doi: 10.1523/JNEUROSCI.1683-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qi R, Liu C, Ke J, et al. Abnormal amygdala resting-state functional connectivity in irritable bowel syndrome. AJNR Am J Neuroradiol. 2016;37:1139–1145. doi: 10.3174/ajnr.A4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Longarzo M, Quarantelli M, Aiello M, et al. The influence of interoceptive awareness on functional connectivity in patients with irritable bowel syndrome. Brain Imaging Behav. 2017;11:1117–1128. doi: 10.1007/s11682-016-9595-5. [DOI] [PubMed] [Google Scholar]
- 29.Icenhour A, Witt ST, Elsenbruch S, et al. Brain functional connectivity is associated with visceral sensitivity in women with irritable bowel syndrome. Neuroimage Clin. 2017;15:449–457. doi: 10.1016/j.nicl.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ke J, Qi R, Liu C, et al. Abnormal regional homogeneity in patients with irritable bowel syndrome: a resting-state functional MRI study. Neurogastroenterol Motil. 2015;27:1796–1803. doi: 10.1111/nmo.12692. [DOI] [PubMed] [Google Scholar]
- 31.Mertz H, Morgan V, Tanner G, et al. Regional cerebral activation in irritable bowel syndrome and control subjects with painful and nonpainful rectal distention. Gastroenterology. 2000;118:842–848. doi: 10.1016/S0016-5085(00)70170-3. [DOI] [PubMed] [Google Scholar]
- 32.Yuan YZ, Tao RJ, Xu B, et al. Functional brain imaging in irritable bowel syndrome with rectal balloon-distention by using fMRI. World J Gastroenterol. 2003;9:1356–1360. doi: 10.3748/wjg.v9.i6.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berman SM, Naliboff BD, Suyenobu B, et al. Reduced brainstem inhibition during anticipated pelvic visceral pain correlates with enhanced brain response to the visceral stimulus in women with irritable bowel syndrome. J Neurosci. 2008;28:349–359. doi: 10.1523/JNEUROSCI.2500-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elsenbruch S, Rosenberger C, Enck P, Forsting M, Schedlowski M, Gizewski ER. Affective disturbances modulate the neural processing of visceral pain stimuli in irritable bowel syndrome: an fMRI study. Gut. 2010;59:489–495. doi: 10.1136/gut.2008.175000. [DOI] [PubMed] [Google Scholar]
- 35.Wilder-Smith CH, Schindler D, Lovblad K, Redmond SM, Nirkko A. Brain functional magnetic resonance imaging of rectal pain and activation of endogenous inhibitory mechanisms in irritable bowel syndrome patient subgroups and healthy controls. Gut. 2004;53:1595–1601. doi: 10.1136/gut.2003.028514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song GH, Venkatraman V, Ho KY, Chee MW, Yeoh KG, Wilder-Smith CH. Cortical effects of anticipation and endogenous modulation of visceral pain assessed by functional brain MRI in irritable bowel syndrome patients and healthy controls. Pain. 2006;126:79–90. doi: 10.1016/j.pain.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 37.Bouhassira D, Moisset X, Jouet P, Duboc H, Coffin B, Sabate JM. Changes in the modulation of spinal pain processing are related to severity in irritable bowel syndrome. Neurogastroenterol Motil. 2013;25:623–e468. doi: 10.1111/nmo.12123. [DOI] [PubMed] [Google Scholar]
- 38.Rosenberger C, Thürling M, Forsting M, Elsenbruch S, Timmann D, Gizewski ER. Contributions of the cerebellum to disturbed central processing of visceral stimuli in irritable bowel syndrome. Cerebellum. 2013;12:194–198. doi: 10.1007/s12311-012-0413-3. [DOI] [PubMed] [Google Scholar]
- 39.Claassen J, Labrenz F, Ernst TM, et al. Altered cerebellar activity in visceral pain-related fear conditioning in irritable bowel syndrome. Cerebellum. 2017;16:508–517. doi: 10.1007/s12311-016-0832-7. [DOI] [PubMed] [Google Scholar]
- 40.Wang D, Zhang X, Zhang X, Huang Z, Song Y. Magnetic resonance imaging analysis of brain function in patients with irritable bowel syndrome. BMC Gastroenterol. 2017;17:148. doi: 10.1186/s12876-017-0673-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elsenbruch S, Rosenberger C, Bingel U, Forsting M, Schedlowski M, Gizewski ER. Patients with irritable bowel syndrome have altered emotional modulation of neural responses to visceral stimuli. Gastroenterology. 2010;139:1310–1319. doi: 10.1053/j.gastro.2010.06.054. [DOI] [PubMed] [Google Scholar]
- 42.Labus JS, Gupta A, Coveleskie K, et al. Sex differences in emotion-related cognitive processes in irritable bowel syndrome and healthy control subjects. Pain. 2013;154:2088–2099. doi: 10.1016/j.pain.2013.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hong JY, Naliboff B, Labus JS, et al. Altered brain responses in subjects with irritable bowel syndrome during cued and uncued pain expectation. Neurogastroenterol Motil. 2016;28:127–138. doi: 10.1111/nmo.12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kano M, Muratsubaki T, Morishita J, et al. Influence of uncertain anticipation on brain responses to aversive rectal distension in patients with irritable bowel syndrome. Psychosom Med. 2017;79:988–999. doi: 10.1097/PSY.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 45.Icenhour A, Langhorst J, Benson S, et al. Neural circuitry of abdominal pain-related fear learning and reinstatement in irritable bowel syndrome. Neurogastroenterol Motil. 2015;27:114–127. doi: 10.1111/nmo.12489. [DOI] [PubMed] [Google Scholar]
- 46.Lowén MB, Mayer EA, Sjöberg M, et al. Effect of hypnotherapy and educational intervention on brain response to visceral stimulus in the irritable bowel syndrome. Aliment Pharmacol Ther. 2013;37:1184–1197. doi: 10.1111/apt.12319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kano M, Yoshizawa M, Kono K, et al. Parasympathetic activity correlates with subjective and brain responses to rectal distension in healthy subjects but not in non-constipated patients with irritable bowel syndrome. Sci Rep. 2019;9:7358. doi: 10.1038/s41598-019-43455-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ringel Y, Drossman DA, Leserman JL, et al. Effect of abuse history on pain reports and brain responses to aversive visceral stimulation: an FMRI study. Gastroenterology. 2008;134:396–404. doi: 10.1053/j.gastro.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 49.Larsson MB, Tillisch K, Craig AD, et al. Brain responses to visceral stimuli reflect visceral sensitivity thresholds in patients with irritable bowel syndrome. Gastroenterology. 2012;142:463–472. e3. doi: 10.1053/j.gastro.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lowén MB, Mayer E, Tillisch K, et al. Deficient habituation to repeated rectal distensions in irritable bowel syndrome patients with visceral hypersensitivity. Neurogastroenterol Motil. 2015;27:646–655. doi: 10.1111/nmo.12537. [DOI] [PubMed] [Google Scholar]
- 51.Aizawa E, Sato Y, Kochiyama T, et al. Altered cognitive function of prefrontal cortex during error feedback in patients with irritable bowel syndrome, based on FMRI and dynamic causal modeling. Gastroenterology. 2012;143:1188–1198. doi: 10.1053/j.gastro.2012.07.104. [DOI] [PubMed] [Google Scholar]
- 52.Hubbard CS, Hong J, Jiang Z, et al. Increased attentional network functioning related to symptom severity measures in females with irritable bowel syndrome. Neurogastroenterol Motil. 2015;27:1282–1294. doi: 10.1111/nmo.12622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kilpatrick LA, Labus JS, Coveleskie K, et al. The HTR3A polymorphism c. -42C>T is associated with amygdala responsiveness in patients with irritable bowel syndrome. Gastroenterology. 2011;140:1943–1951. doi: 10.1053/j.gastro.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guleria A, Karyampudi A, Singh R, et al. Mapping of brain activations to rectal balloon distension stimuli in male patients with irritable bowel syndrome using functional magnetic resonance imaging. J Neurogastroenterol Motil. 2017;23:415–427. doi: 10.5056/jnm16148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wong RK, Van Oudenhove L, Li X, Cao Y, Ho KY, Wilder-Smith CH. Visceral pain perception in patients with irritable bowel syndrome and healthy volunteers is affected by the MRI scanner environment. United European Gastroenterol J. 2016;4:132–141. doi: 10.1177/2050640615580888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hubbard CS, Labus JS, Bueller J, et al. Corticotropin-releasing factor receptor 1 antagonist alters regional activation and effective connectivity in an emotional-arousal circuit during expectation of abdominal pain. J Neurosci. 2011;31:12491–12500. doi: 10.1523/JNEUROSCI.1860-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kano M, Muratsubaki T, Van Oudenhove L, et al. Altered brain and gut responses to corticotropin-releasing hormone (CRH) in patients with irritable bowel syndrome. Sci Rep. 2017;7:12425. doi: 10.1038/s41598-017-09635-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kano M, Muratsubaki T, Yagihashi M, et al. Insula activity to visceral stimulation and endocrine stress responses as associated with alexithymia in patients with irritable bowel syndrome. Psychosom Med. 2020;82:29–38. doi: 10.1097/PSY.0000000000000729. [DOI] [PubMed] [Google Scholar]
- 59.Lee HF, Hsieh JC, Lu CL, et al. Enhanced affect/cognition-related brain responses during visceral placebo analgesia in irritable bowel syndrome patients. Pain. 2012;153:1301–1310. doi: 10.1016/j.pain.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 60.Nakai A, Kumakura Y, Boivin M, et al. Sex differences of brain serotonin synthesis in patients with irritable bowel syndrome using alpha-[11C]methyl-L-tryptophan, positron emission tomography and statistical parametric mapping. Can J Gastroenterol. 2003;17:191–196. doi: 10.1155/2003/572127. [DOI] [PubMed] [Google Scholar]
- 61.Nieuwenhuys R, Voogd J, van Huijzen C. The human central nervous system. 3rd ed. Springer-Verlag Inc; New York: 1988. [DOI] [Google Scholar]
- 62.Mesulam MM. From sensation to cognition. Brain. 1998;121(pt 6):1013–1052. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- 63.Berman S, Suyenobu B, Naliboff BD, et al. Evidence for alterations in central noradrenergic signaling in irritable bowel syndrome. Neuroimage. 2012;63:1854–1863. doi: 10.1016/j.neuroimage.2012.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bremner JD, Innis RB, Ng CK, et al. Positron emission tomography measurement of cerebral metabolic correlates of yohimbine administration in combat-related posttraumatic stress disorder. Arch Gen Psychiatry. 1997;54:246–254. doi: 10.1001/archpsyc.1997.01830150070011. [DOI] [PubMed] [Google Scholar]
- 65.Naliboff BD, Berman S, Suyenobu B, et al. Longitudinal change in perceptual and brain activation response to visceral stimuli in irritable bowel syndrome patients. Gastroenterology. 2006;131:352–365. doi: 10.1053/j.gastro.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 66.Tillisch K, Mayer EA, Labus JS. Quantitative meta-analysis identifies brain regions activated during rectal distension in irritable bowel syndrome. Gastroenterology. 2011;140:91–100. doi: 10.1053/j.gastro.2010.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mak AD, Northoff G, Yeung DK, et al. Increased glutamate in somatosensory cortex in functional dyspepsia. Sci Rep. 2017;7:3926. doi: 10.1038/s41598-017-04405-1.58c92f9e91cf44a0a84411757a79e763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.De Paepe B, Smet J, Baeken C, Van Oosterwijck J, Meeus M. A capital role for the brain's insula in the diverse fibromyalgia-associated symptoms. Med Hypotheses. 2020;143:110077. doi: 10.1016/j.mehy.2020.110077. [DOI] [PubMed] [Google Scholar]
- 69.As-Sanie S, Kim J, Schmidt-Wilcke T, et al. Functional connectivity is associated with altered brain chemistry in women with endometriosis-associated chronic pelvic pain. J Pain. 2016;17:1–13. doi: 10.1016/j.jpain.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Demartini B, Nisticò V, Edwards MJ, Gambini O, Priori A. The pathophysiology of functional movement disorders. Neurosci Biobehav Rev. 2021;120:387–4500. doi: 10.1016/j.neubiorev.2020.10.019. [DOI] [PubMed] [Google Scholar]
- 71.Stone J, Zeman A, Simonotto E, et al. FMRI in patients with motor conversion symptoms and controls with simulated weakness. Psychosom Med. 2007;69:961–969. doi: 10.1097/PSY.0b013e31815b6c14. [DOI] [PubMed] [Google Scholar]
- 72.Saj A, Raz N, Levin N, Ben-Hur T, Arzy S. Disturbed mental imagery of affected body-parts in patients with hysterical conversion paraplegia correlates with pathological limbic activity. Brain Sci. 2014;4:396–404. doi: 10.3390/brainsci4020396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Espay AJ, Maloney T, Vannest J, et al. Dysfunction in emotion processing underlies functional (psychogenic) dystonia. Mov Disord. 2018;33:136–145. doi: 10.1002/mds.27217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mcsweeney M, Reuber M, Levita L. Neuroimaging studies in patients with psychogenic non-epileptic seizures: a systematic meta-review. Neuroimage Clin. 2017;16:210–221. doi: 10.1016/j.nicl.2017.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Demartini B, Invernizzi RW, Campiglio L, et al. Cerebrospinal fluid glutamate changes in functional movement disorders. NPJ Parkinsons Dis. 2020;6:37. doi: 10.1038/s41531-020-00140-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Apazoglou K, Mazzola V, Wegrzyk J, Frasca Polara G, Aybek S. Biological and perceived stress in motor functional neurological disorders. Psychoneuroendocrinology. 2017;85:142–150. doi: 10.1016/j.psyneuen.2017.08.023. [DOI] [PubMed] [Google Scholar]
- 77.Clark A. Whatever next? Predictive brains, situated agents, and the future of cognitive science. Behav Brain Sci. 2013;36:181–204. doi: 10.1017/S0140525X12000477. [DOI] [PubMed] [Google Scholar]
- 78.Friston K. The free-energy principle: a rough guide to the brain? Trends Cogn Sci. 2009;13:293–301. doi: 10.1016/j.tics.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 79.Edwards MJ, Adams RA, Brown H, Pareés I, Friston KJ. A Bayesian account of 'hysteria'. Brain. 2012;135(pt 11):3495–3512. doi: 10.1093/brain/aws129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ohira H. Regulation of functions of the brain and body by the principle of predictive coding: implications for Impairments of the brain-gut axis. Psychological Topics. 2018;27:1–15. doi: 10.31820/pt.27.1.1. [DOI] [Google Scholar]
- 81.Wiech K, Ploner M, Tracey I. Neurocognitive aspects of pain perception. Trends Cogn Sci. 2008;12:306–313. doi: 10.1016/j.tics.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 82.Seth AK. Interoceptive inference, emotion, and the embodied self. Trends Cogn Sci. 2013;17:565–573. doi: 10.1016/j.tics.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 83.Seth AK, Critchley HD. Extending predictive processing to the body: emotion as interoceptive inference. Behav Brain Sci. 2013;36:227–228. doi: 10.1017/S0140525X12002270. [DOI] [PubMed] [Google Scholar]
- 84.Vuilleumier P. The role of the human amygdala in perception and attention. The Guilford Press; 2009. [DOI] [Google Scholar]
- 85.McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.