Abstract
Rationale & Objective
Hereditary hypophosphatemic rickets with hypercalciuria (HHRH) is a rare monogenic disorder caused by SLC34A3 pathogenic variants. HHRH is characterized by kidney phosphate wasting, hypophosphatemia, hypercalciuria, an elevated 1,25-dihydroxyvitamin D level, nephrocalcinosis, and urinary stone disease. Previously, we reported a 100% prevalence of kidney cysts in the related CYP24A1 deficiency. Thus, in the current study, we characterized cysts’ presence in HHRH, another monogenic cause of hypercalciuria, nephrocalcinosis, and urinary stone disease.
Study Design
Case series.
Setting & Participants
Medical records from the Mayo Clinic and the Rare Kidney Stone Consortium monogenic stone disease database were queried for patients with a genetically confirmed HHRH diagnosis. The number, sizes, and locations of kidney cysts in each patient were recorded.
Results
Twelve patients with SLC34A3 pathogenic variants were identified (7 monoallelic, 5 biallelic). Of these, 5 (42%) were males, and the median (Q1, Q3) ages were 16 years (13, 35 years) at clinical presentation and 42 years (20, 57 years) at genetic confirmation. Kidney cysts were present in 9 of 12 (75%) patients, and the median (Q1, Q3) age at first cyst detection was 41 years (13, 50 years). The median number of cysts per patient was 2.0 (0.5, 3.5). Fifty percent of adult patients had a cyst number that exceeded the 97.5th percentile of an age- and sex-matched control population. All children had at least 2 or more total cysts. None had a family history of cystic kidney disease.
Limitations
Retrospective study, possible selection bias, single-center experience.
Conclusions
A strong association between HHRH and kidney cysts was observed. Similarities in the biochemical profiles of HHRH and CYP24A1 deficiency suggest elevated active vitamin D and hypercalciuria may be potential cystogenic factors. Further studies are needed to understand how genetic changes in SLC34A3 favor cyst formation.
Index Words: Case series, HHRH, hypercalciuria, hypophosphatemic rickets with hypercalciuria, kidney cyst, nephrocalcinosis, SLC34A3 gene, urinary stone disease
Plain-Language Summary.
Hereditary hypophosphatemic rickets with hypercalciuria (HHRH) is a rare disorder characterized by kidney phosphate wasting, high active vitamin D, hypercalciuria, osteoporosis, rickets, and urinary stone disease. Previously, we reported a high prevalence of kidney cysts associated with CYP24A1 deficiency, a condition with an overlapping phenotypic profile, including elevated active vitamin D, hypercalciuria, and urinary stone disease. By comparing the prevalence, size, and distribution with the transplant donor candidates from a previously published study, we found that the association between HHRH and kidney cysts appears to be higher than expected. These results can help kidney professionals, provide an additional diagnostic clue, enable early recognition of HHRH, and advance the understanding of the full pathophysiology of this rare disease and other diseases with similar biochemical profiles.
Biallelic pathogenic variants in SLC34A3, the gene encoding a sodium-dependent inorganic phosphate transporter expressed in kidney proximal tubular cells, are associated with hereditary hypophosphatemic rickets with hypercalciuria (HHRH).1 This disorder is characterized by kidney phosphate wasting and is characterized by hypophosphatemia, rickets and/or osteopenia, an elevated 1,25-dihydroxyvitamin D [1,25(OH)2D] level, hypercalciuria, nephrocalcinosis, and urinary stone disease.2,3 Most patients are diagnosed with HHRH after presenting with urinary stone disease and/or bone disease (osteopenia and/or rickets, bone pain, and bone fractures). Recent cases of HHRH in patients with confirmed monoallelic pathogenic SLC34A3 changes have been described and often have an attenuated clinical phenotype and present later in life.4 Recently, we reported a high prevalence of kidney cysts in patients with CYP24A1 deficiency, another monogenic disorder characterized by elevated 1,25(OH)2D, hypercalcemia, hypercalciuria, nephrocalcinosis, and urinary stone disease.5 Given the overlap in phenotypes, in the current study we systematically examined the prevalence and characteristics of kidney cysts in a cohort of patients with genetically confirmed HHRH.
Methods
Study Population
This retrospective, observational study was conducted in accordance with recommendations of the Mayo Clinic Institutional Review Board. This work was deemed exempt by the institutional review board, and the need for informed consent was waived due to the use of deidentified information (application 21-005257). Cases of HHRH were identified by searching the Mayo Clinic medical records and the Rare Kidney Stone Consortium for confirmed monoallelic or biallelic pathogenic variants of the SLC34A3 gene with appropriate phenotypes. Demographic, clinical, laboratory, and imaging data were abstracted for the confirmed cases. Kidney function was assessed using the full age spectrum glomerular filtration rate equation because the cohort spans age ranges from childhood to late adulthood.6
Imaging Review
Imaging examinations, including ultrasound (US), contrast-enhanced computed tomography (CCT), and noncontrast computed tomography, were obtained for clinical purposes using standard protocols and were reviewed by a single board-certified radiologist (TAP) with subspecialty training in genitourinary imaging. The presence or absence of cysts and cyst sizes were recorded by kidney for each patient. Preference was given to the last CCT, if available, to assess the number of cysts and cyst sizes, regardless of the timing of the genetic diagnosis. US examinations were also reviewed, and the latest examination was used for cyst number and size if no CCT was available. US examinations routinely included longitudinal cine clips through the kidney, which increase the sensitivity for small cyst detection that may not have been captured or measured on still ultrasound images alone. If a CCT preceded a US (n=1), the subsequent US was also reviewed for concordance in cyst number and sizes. Noncontrast computed tomography was reviewed if it were the only available imaging modality for a given patient. If cysts were detected, earlier examinations were reviewed to determine the earliest date that cysts were present. There were 3, 5, and 4 patients imaged by CCT, US, and noncontrast computed tomography, respectively. To establish an association between HHRH and kidney cysts, we compared the number, sizes, and locations of kidney cysts in our cohort with those in a previously published healthy, age- and sex-controlled population.7 To determine whether patients with HHRH develop liver cysts, we reviewed all imaging studies, which included the liver, with preference again given to CCT, then to US, and then to noncontrast computed tomography.
Statistical Methods
Results were expressed as medians with interquartile ranges of Q1-Q3 (25th-75th percentiles) for continuous variables and as percentages for categorical variables. Comparisons between groups for the continuous variables were performed using the exact Cochran-Mantel-Haenszel test and nonparametric Spearman rank correlation as required, with a P value of <0.05 accepted as significant.
Results
Twelve patients were identified with genetically confirmed pathologic variants in the SLC34A3 gene (Table 1). Monoallelic or biallelic pathogenic variants were detected in 7 of 12 (58%) patients and 5 of 12 (42%) patients of the cohort, respectively. Five of 12 (42%) patients were male, 3 of 12 (25%) patients were children (age <18 years), 11 of 12 (91.7%) patients were diagnosed after presenting with clinical findings, and 1 of 12 (8.3%) patient was diagnosed based on family screening. Additional clinical characteristics are summarized in Table 1. The median age (Q1, Q3) of clinical presentation was 16.5 years (13.8, 35.8 years), and the median age at genetic confirmation was 42.0 years (20.0, 57.3 years). Among the 11 probands that presented with clinical findings, the initial presenting symptom was urinary stone disease in 9 of 11 (82%) patients, bone fracture and urinary stone disease in 1 of 11 (9%) patient, and osteopenia in 1 of 11 (9%) patient. The remaining patient identified by family screening had a medical history of osteopenia and possible stress fractures. Table 2 displays the biochemical characteristics of the cohort. The median estimated glomerular filtration rates (eGFRs; mL/min/1.73 m2) in both children and adults in this cohort were reduced compared to expected average values in the healthy general population.8 The median (Q1, Q3) value was, however, higher in children than in adults, but the difference was not statistically significant (76.0 mL/min/1.73 m2 [72.0, 98.0 mL/min/1.73 m2] vs 64.0 mL/min/1.73 m2 [50.0, 69.0 mL/min/1.73 m2], respectively; P = 0.14). The median 1,25(OH)2D level (Q1, Q3) was higher in children than in adults (160.0 pg/dL [91.0, 178.0 pg/dL] vs 63.0 pg/dL [40.2, 80.0 pg/dL], respectively; P < 0.05), whereas median serum calcium, inorganic phosphorus, intact parathyroid hormone, and 25-hydroxyvitamin D levels were similar between children and adults. The levels of median 24-hour urinary calcium exertion were elevated in children and adults.
Table 1.
Clinical Characteristics of Genetically Confirmed HHRH Cohort
| Clinical Features | |
|---|---|
| N (male/female) | 12 (5/7) |
| Children, n (%) | 3 (25) |
| Indication for genetic testing, n (%) | |
| Family screening | 1 (8.3) |
| Clinical suspicion | 11 (91.7) |
| Age at first clinical presentation, y | |
| Median (Q1, Q3) | 16.5 (13.8, 35.8) |
| Clinical symptoms at presentation, n (%) | |
| Urinary stone disease | 9 (82) |
| Urinary stone disease and bone fractures | 1 (9) |
| Osteopenia | 1 (9) |
| Imaging study findings at presentation | |
| Urinary stone disease, n (%) | 5 (41.6) |
| Nephrocalcinosis, n (%) | 1 (8.3) |
| Urinary stone disease and nephrocalcinosis, n (%) | 6 (50) |
| Age at confirmed pathogenic variants, y | |
| Median (Q1, Q3) | 42.0 (20.0, 57.3) |
| Variants type, n (%) | |
| Monoallelic | 7 (58.3) |
| Biallelic | 5 (41.7) |
| Presence of kidney cysts, n (%) | |
| Yes | 9 (75) |
| No | 3 (25) |
| Age at first kidney cyst(s) detection, y | |
| Median (Q1, Q3) | 41 (13.0, 50.5) |
| No. of total kidney cysts per patienta | |
| Median (Q1, Q3) | 2.0 (0.5, 3.5) |
| Largest cyst size, mm | |
| Median (Q1, Q3) | 20.0 (9.0, 30.0) |
| No. of cysts ≥ 5 mm | |
| Median (Q1, Q3) | 2.0 (0.5, 3.5) |
Abbreviation: HHRH, hypophosphatemic rickets and hypercalciuria.
Preference was given to the last available imaging study.
Table 2.
Biochemical Characteristics of Genetically Confirmed HHRH Cohort
| Biochemical Features | P Value | |
|---|---|---|
| eGFR,a mL/min/1.73 m2, median (Q1, Q3) | 68.5 (55.0, 81.5) | 0.14 |
| eGFR children, median (Q1, Q3) | 76 (72.0, 98.0) | |
| eGFR adult, median (Q1, Q3) | 64.0 (50.0, 69.0) | |
| Serum Ca, mg/dL, median (Q1, Q3) | 9.9 (9.7, 10.1) | 0.59 |
| Children, median (Q1, Q3) | 9.8 (9.7, 9.9) | |
| Adult, median (Q1, Q3) | 9.9 (9.7, 10.2) | |
| Serum Pi, mg/dL, median (Q1, Q3) | 3.1 (2.9, 3.7) | 0.84 |
| Children, median (Q1, Q3) | 3.1 (2.9, 4.3) | |
| Adult, median (Q1, Q3) | 33.0 (2.9, 3.4) | |
| iPTH, pg/dL, median (Q1, Q3) | 13.5 (9.1, 25.5) | 0.35 |
| Children, median (Q1, Q3) | 13.0 (8.1, 15.0) | |
| Adult, median (Q1, Q3) | 14.0 (10.0, 26.0) | |
| 1,25(OH)2D, pg/dL, median (Q1, Q3) | 75.0 (41.6, 99.0) | 0.009 |
| Children, median (Q1, Q3) | 160.0 (491.9, 178.0) | |
| Adult, median (Q1, Q3) | 63.0 (40.2, 80.0) | |
| 25(OH)D, ng/dL, median (Q1, Q3) | 29.0 (21.8, 45.5) | 0.76 |
| Children, median (Q1, Q3) | 22.0 (20.0, 52.0) | |
| Adult, median (Q1, Q3) | 30.0 (25.0, 39.0) | |
| Pi, 24U, mg/d, adult | ||
| Median (Q1, Q3) | 780.0 (700.0, 901.0) | |
| Ca, 24U, mg/d, adult | ||
| Median (Q1, Q3) | 315.0 (273.0, 331.0) | |
| Ca, 24U, mg/k/d, children | ||
| Median (Q1, Q3) | 5.9 (4.5, 7.2) | |
| Pi, 24U, mg/k/d, children | ||
| Median (Q1, Q3) | 14.0 (14.0, 14.1) | |
Note: A normal eGFR value is ≥90; a normal Pi value is 3.5-5.4 mg/dL in children and 2.5-4.5 mg/dL in adults; a normal iPTH value is 15-65 pg/dL; a normal 1,25(OH)2D value is 16-65 pg/dL; and a normal 25(OH)D value is 20-50 ng/dL. Conversion factors for units: SCa in mg/dL to mmol/L, ×0.2495; SPi in mg/dL to mmol/L, ×0.3229; iPTH in pg/mL to pmol/L, ×0.106; 1,25(OH)2D in pg/dL to pmol/L, ×2.6; and 25(OH)D in ng/dL to nmol/L, ×2.496.
Abbreviations: Ca, calcium; eGFR, estimated glomerular filtration rate; HHRH, hypophosphatemic rickets and hypercalciuria; iPTH, intact parathyroid hormone; Pi, inorganic phosphorus; 25(OH)D, 25-hydroxyvitamin D; 24U, 24-hour urine output.
Biochemical data were obtained at the time of HHRH evaluation.
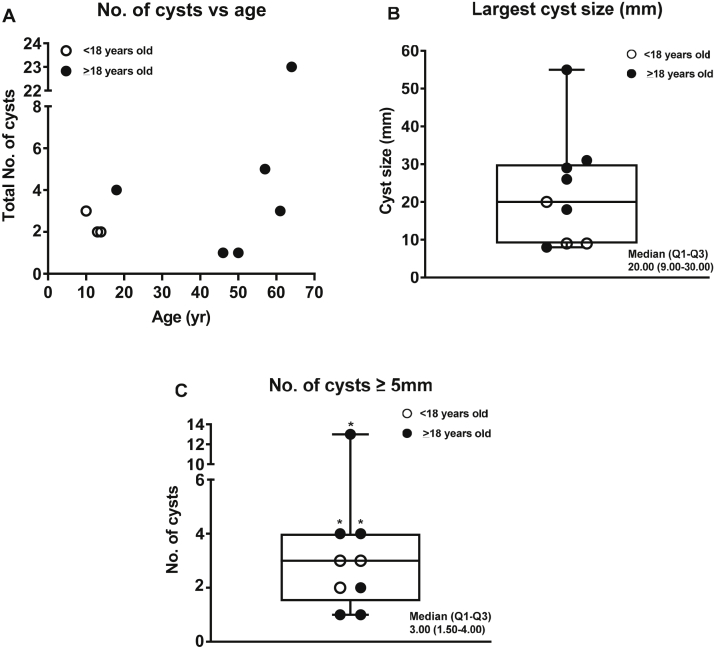
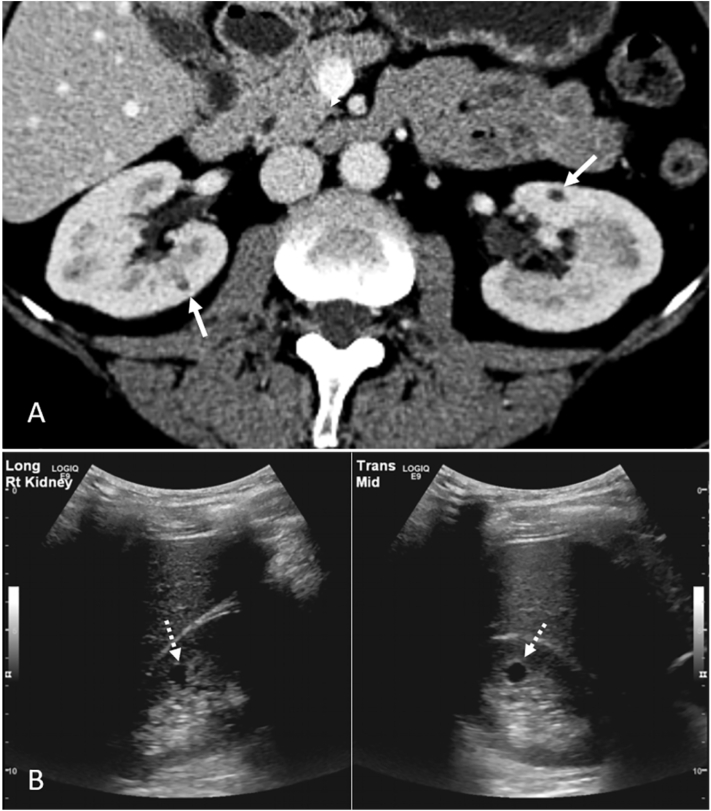
Kidney cysts were present in 9 of 12 (75%) patients of the HHRH cohort. Among the 9 patients with kidney cysts (3 children, 6 adults), the median (Q1, Q3) age at the first available kidney imaging was 41.0 years (13.0, 49.0 years) and the median age at first kidney cyst detection was 41.0 years (13.0, 50.5 years). Four adult patients without cysts on initial imaging developed kidney cysts by a subsequent follow-up. The median (Q1, Q3) number of cysts per patient was 2 (0.5, 3.5). The median (Q1, Q3) number of cysts of ≥5 mm in size per patient was 2 (0.5, 3.5; Table 1). All patients had no known family history of cystic kidney disease. Detailed kidney cyst characteristics based on various imaging modalities in the genetically confirmed patients are described in Table 3. The number of kidney cysts in relation to age and box plots of key cyst variables (median; Q1, Q3; and range), including largest cyst size and number of cysts of ≥5 mm in size, are shown in Fig 1. The number of cysts of ≥5 mm in diameter was above the 97.5th percentile of an age- and sex-matched control population in 3 of 6 50% adult patients with HHRH (Fig 1C).7 At least 2 cysts of ≥5 mm in size were found in all 3 children with HHRH. Cysts were in the kidney cortex, medulla, or corticomedullary junction, with representative imaging by CCT and US displayed in Fig 2. No hepatic cysts were observed in 6 of 7 patients (age range, 18-64 years) with available liver imaging. The remaining patient, 57 years of age at the time of imaging, had an 8-mm simple liver cyst via CCT.
Table 3.
Specific Cyst Characteristics in the HHRH Cohort
| Allelism | Case, Pedigree | Agea at First Cyst Detection | Age at Cyst Data Collection, Imaging Modalityb | No. of Cysts, Location | Largest Cyst, mm | No. of Cysts ≥5 mm |
|---|---|---|---|---|---|---|
| Biallelic | 1, 1 | 9 | 10, US | 3, M | 9 | 3 |
| 2, 2 | 13 | 13, US | 2, CMJ | 9 | 2 | |
| 3, 3 | 13 | 13, US | 2, U | 20 | 2 | |
| 4, 4 | N/A | 18, NCCT | 0, N/A | N/A | N/A | |
| 5, 5 | 47 | 64, CCT | 23, C/CMJ | 55 | 13 | |
| Monoallelic | 6, 6 | N/A | 23, NCCT | 0, N/A | N/A | N/A |
| 7, 7 | 18 | 18, CCT | 4, CMJ | 31 | 4 | |
| 8, 2 | 50 | 50, US | 1, C | 26 | 1 | |
| 9, 8 | N/A | 55, NCCT | 0, N/A | N/A | N/A | |
| 10, 9 | 41 | 46, US | 1, M | 8 | 1 | |
| 11, 10 | 52 | 57, CCT | 5, C/CMJ | 18 | 4 | |
| 12, 11 | 61 | 61, NCCT | 3, C | 29 | 3 |
Abbreviations: C, cortex; CCT, contrast computed tomography; CMJ, corticomedullary junction; HHRH, hypophosphatemic rickets and hypercalciuria; M, medulla; N/A, not applicable;NCCT, noncontrast computed tomography; U, unknown; US, ultrasound.
All ages are reported in years.
Preference was given to the last CCT or US available and then to NCCT if it was the only available imaging modality.
Figure 1.
Kidney cyst characteristics in 9 of 12 patients from the HHRH cohort. (A) Number of kidney cysts versus age in the genetically confirmed HHRH cohort. (B) Box plot describing the largest cyst size in mm for each case. (C) Box plot describing the number of cysts of ≥5 mm in size in each case. The number of cysts of ≥5 mm in size in 50% (3 of 6) of adult patients (asterisk) with a confirmed deficiency was above the 97.5th percentile of an age- and sex-matched control population. Abbreviation: HHRH, hypophosphatemic rickets with hypercalciuria.
Figure 2.
Kidney cysts in 2 representative patients with hypophosphatemic rickets with hypercalciuria using imaging modality. (A) Contrast-enhanced computed tomography: bilateral cysts at the corticomedullary junction (white arrows). (B) Ultrasound: medullary cyst (white dashed arrows) seen as an anechoic thin-walled structure in longitudinal and transverse views of the right kidney.
Data were analyzed regarding cyst-related variables with allelism. Using the exact Cochran-Mantel-Haenzel test, there was no evidence found between monoallelic versus biallelic HHRH disease and the total number of cysts, age at imaging for study data collection, the number of cysts of ≥5 mm in size, or eGFR (Table 4). The Spearman rank correlation test was used to study the relationship between cyst-related variables and eGFR. A significant positive correlation was found between eGFR and the total number of cysts (correlation coefficient, 0.74; P < 0.05), in addition to eGFR and the number of cysts of ≥5mm in size (correlation coefficient, 0.75; P < 0.05; Table 5).
Table 4.
Medians and Quartiles of Cyst-related Variables by Allelism
| Biallelic (n=5) | Monoallelic (n=7) | P Valuea | |
|---|---|---|---|
| Total number of cysts | 0.36 | ||
| Median (Q1, Q3) | 2.0 (2.0, 3.0) | 1.0 (0.0, 4.0) | |
| Age at imaging for study data collection | 0.12 | ||
| Median (Q1, Q3) | 13.0 (13.0, 18.0) | 50.0 (23.0, 57.0) | |
| No. of cysts ≥ 5 mm | 0.42 | ||
| Median (Q1, Q3) | 2.0 (2.0, 3.0) | 1.0 (0.0, 4.0) | |
| GFR | 0.80 | ||
| Median (Q1, Q3) | 72.0 (68.0, 76.0) | 64.0 (50.0, 87.0) |
Abbreviation: GFR, glomerular filtration rate.
P values are from the exact Cochran-Mantel-Haenzel test.
Table 5.
Spearman Rank Correlation Between Cyst-related Variables and GFR
| GFR | P Value | |
|---|---|---|
| Total number of cysts | 0.006 | |
| Correlation coefficient | 0.74 | |
| Age at imaging for study data collection | 0.36 | |
| Correlation coefficient | −0.29 | |
| No. of cysts ≥ 5 mm | 0.006 | |
| Correlation coefficient | 0.75 |
Abbreviation: GFR, glomerular filtration rate.
Discussion
In this study, we found a strong association between HHRH and cortical, medullary, or corticomedullary junction simple kidney cysts, with an overall prevalence of 75% in a cohort of genetically confirmed cases. Hepatic cysts were detected in only 1 patient among 7 patients for whom liver imaging was available. Thus, the cysts associated with HHRH appear to be primarily limited to the kidney.
The significance of simple kidney cysts varies depending on the person’s age and total number of cysts.9 Rule et al7 described the expected number of cysts of ≥5 mm in size in a cohort of 1,948 potential adult kidney donors that had available CCT data and lacked a family history of cystic kidney disease and calculated the upper 97.5th percentile of expected cysts by age and sex. Using these data as an age- and sex-matched control population, we found that 50% of the adults who developed kidney cysts in our HHRH cohort exceeded this upper 97.5th percentile, suggesting these are not coincidentally discovered, simple kidney cysts. All 3 children with confirmed HHRH had at least 2 cysts of ≥5 mm in size, which is also significant compared with the control adult population (age, 18-29 years).7 The average eGFR in children and adults was also slightly reduced in this cohort, although there are no previously published data regarding the long-term implications of HHRH for kidney function. Thus, our data suggest that there may be an increased risk of chronic kidney disease.
Kidney cysts have been previously reported in 2 patients with HHRH. The first report, from 2014, described a 6-year-old boy with a homozygous variant in the SLC34A3 gene who presented with kidney stones and nephrocalcinosis and at the age of 13 was found to have an incidental 15-mm left kidney cyst on a kidney US.1 The other report described a female with a homozygous variant in the gene, history of genu varum at the age of 10, urinary stone disease at the age of 19, and incidental bilateral kidney cysts noted on a computed tomography scan at the age of 22.4
A high prevalence (100%) of kidney cysts has been previously described in our recently published study of 16 patients (adult and children) with CYP24A1 deficiency, with the number of cysts of ≥5 mm in size above the 97.5th percentile of an age- and sex-matched control population in 55% of patients.5 The presence of kidney cysts in CYP24A1 deficiency was first reported from our center in 2012, when Tebben et al10 described a 44-year-old man (included in our CYP24A1 cohort) with small bilateral kidney cysts by abdominal computed tomography. There are few published data regarding the long-term implications of CYP24A1 deficiency for kidney function, although our data suggest that chronic kidney disease may be a late outcome.5
A study from 2009 reported a high prevalence of simple kidney cysts in patients with primary hyperparathyroidism compared with healthy controls (34.9% vs 16.2%, respectively; P < 0.01).11 The study concluded that cystogenesis might be related to the action of elevated circulating and filtered parathyroid hormones on tubular epithelial cells. Our HHRH and CYP24A1 deficiency cohorts’ circulating parathyroid hormone levels were low to low normal, as expected; thus, parathyroid hormone does not appear to be a likely cause of cystogenesis in these cohorts. The common biochemical findings shared among HHRH and CYP24A1 deficiency cohorts and patients with primary hyperparathyroidism include hypercalciuria and elevated circulating 1,25 (OH)2D levels. Thus, it is possible that sustained hypercalciuria or exposure to increased 1,25(OH)2D levels could be factors in kidney cyst development. To date, we are not aware of studies examining the frequency of cysts in other diseases associated with genetic or acquired hypercalciuria.
Our observations suggest that the underlying mechanism of cystogenesis in HHRH and CYP24A1 deficiency is likely kidney specific. Recent research in animal models suggests that calcium oxalate crystal deposition in kidney tubules can trigger rapid tubular dilatation, activate polycystic kidney disease–associated signaling pathways, and thus accelerate cystogenesis in polycystic kidney disease and disease progression.12 The authors concluded that the presence of hypercalciuria and crystal deposition could contribute to the development of kidney cysts. Additional studies will be needed to establish the relevance of this hypothesis to patients with HHRH and/or CYP24A1 deficiency.
Our study has several strengths and limitations. To our knowledge, this is the first case series of affected patients with HHRH with genetically confirmed diagnoses that systematically examined the prevalence and characteristics of kidney cysts. However, this retrospective analysis of Rare Kidney Stone Consortium clinical registry data relies on a voluntary data set that is inherently incomplete. Screening was not performed in all family members, and it is possible that there are cases with HHRH who, indeed, manifest a milder phenotype. Some of the cases were monoallelic, and we did not have complete information to verify that they were disease-causing in these instances. Additionally, the imaging availability and modality were heterogeneous, especially with regard to the liver. Although large and numerous kidney cysts are typically detectable on noncontrast computed tomography, it has reduced sensitivity for small cysts.
Kidney cysts have not been widely recognized as a clinical manifestation associated with HHRH. Thus, in the current study, we characterized the cyst presence in HHRH, another monogenic cause of hypercalciuria, nephrocalcinosis, and urinary stone disease. The presence of kidney cysts may provide an additional diagnostic clue, thus facilitating early recognition of HHRH and the early initiation of treatment, as well as advancing the understanding of the full pathophysiology of this rare disease and other diseases with similar biochemical profiles.
In conclusion, this study suggests a high prevalence of kidney cysts in patients with HHRH. Similarities in the biochemical profiles of HHRH and CYP24A1 deficiency suggest elevated active vitamin D and/or hypercalciuria may be potential factors in cyst formation. Further studies are needed to evaluate the role of SLC34A3 pathogenic variants in kidney cyst formation and determine whether cysts enhance the chronic kidney disease risk in patients or modify nephrocalcinosis and urinary stone disease risks in HHRH.
Article Information
Authors’ Full Names and Academic Degrees
Christian Hanna, MD, MS, Theodora A. Potretzke, MD, Maroun Chedid, MD, Laureano J. Rangel, MS, Jennifer Arroyo, PhD, Dalia Zubidat, MD, Peter J. Tebben, MD, Andrea G. Cogal, BS, Vicente E. Torres, MD, PhD, Peter C. Harris, PhD, David J. Sas, DO, John C. Lieske, MD, Dawn S. Milliner, MD, and Fouad T. Chebib, MD
Authors’ Contributions
Research idea and study design: CH, TAP, FTC, DSM; data acquisition: CH, TAP, FTC, JA, AGC; genetic analysis: AGC, JA, JCL, PCH; data analysis and interpretation: CH, LJR, TAP, MC, DZ, FTC; statistical analysis: CH, LJR; supervision and mentorship: DSM, PCH, JCL, VET, DJS, PJT. CH and TAP contributed equally to this work. DSM and FTC contributed equally to this work. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
This work was funded by the Rare Kidney Stone Consortium (U54DK83908), which is part of Rare Diseases Clinical Research Network, an initiative of the Office of Rare Diseases Research, National Center for Advancing Translational Sciences and R21TR003174. The Rare Kidney Stone Consortium was funded through collaboration between National Center for Advancing Translational Sciences and the National Institute of Diabetes and Digestive and Kidney Diseases.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Acknowledgements
We thank the staff of the Mayo Clinic and the Rare Kidney Stone Consortium (RKSC), referring physician Dr. David S. Goldfarb, and the many physicians and patients who have contributed to the study.
Peer Review
Received August 12, 2021. Evaluated by 2 external peer reviewers, with direct editorial input by an Associate Editor and the Editor-in-Chief. Accepted in revised form December 2, 2021.
Footnotes
Complete author and article information provided before references.
References
- 1.Dasgupta D., Wee M.J., Reyes M., et al. Mutations in SLC34A3/NPT2c are associated with kidney stones and nephrocalcinosis. J Am Soc Nephrol. 2014;25(10):2366–2375. doi: 10.1681/ASN.2013101085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tieder M., Modai D., Samuel R., et al. Hereditary hypophosphatemic rickets with hypercalciuria. N Engl J Med. 1985;312(10):611–617. doi: 10.1056/NEJM198503073121003. [DOI] [PubMed] [Google Scholar]
- 3.Tieder M., Modai D., Shaked U., et al. ‘Idiopathic’ hypercalciuria and hereditary hypophosphatemic rickets. Two phenotypical expressions of a common genetic defect. N Engl J Med. 1987;316(3):125–129. doi: 10.1056/NEJM198701153160302. [DOI] [PubMed] [Google Scholar]
- 4.Chen A., Ro H., Mundra V.R.R., et al. Description of 5 novel SLC34A3/NPT2c mutations causing hereditary hypophosphatemic rickets with hypercalciuria. Kidney Int Rep. 2019;4(8):1179–1186. doi: 10.1016/j.ekir.2019.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanna C., Potretzke T.A., Cogal A.G., et al. High prevalence of kidney cysts in patients with CYP24A1 deficiency. Kidney Int Rep. 2021;6(7):1895–1903. doi: 10.1016/j.ekir.2021.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pottel H., Hoste L., Dubourg L., et al. An estimated glomerular filtration rate equation for the full age spectrum. Nephrol Dial Transplant. 2016;31(5):798–806. doi: 10.1093/ndt/gfv454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rule A.D., Sasiwimonphan K., Lieske J.C., Keddis M.T., Torres V.E., Vrtiska T.J. Characteristics of renal cystic and solid lesions based on contrast-enhanced computed tomography of potential kidney donors. Am J Kidney Dis. 2012;59(5):611–618. doi: 10.1053/j.ajkd.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inker L.A., Astor B.C., Fox C.H., et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis. 2014;63(5):713–735. doi: 10.1053/j.ajkd.2014.01.416. [DOI] [PubMed] [Google Scholar]
- 9.Lippert M.C. In: Adult and Pediatric Urology. 4th ed. Gillenwater J.Y., Grayhack J.T., Howards S.S., Michell M.E., editors. Lippincott, Williams & Wilkins; 2002. Renal cystic disease; p. 8589. [Google Scholar]
- 10.Tebben P.J., Milliner D.S., Horst R.L., et al. Hypercalcemia, hypercalciuria, and elevated calcitriol concentrations with autosomal dominant transmission due to CYP24A1 mutations: effects of ketoconazole therapy. J Clin Endocrinol Metab. 2012;97(3):E423–E427. doi: 10.1210/jc.2011-1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corbetta S., Eller-Vainicher C., Vicentini L., et al. High prevalence of simple kidney cysts in patients with primary hyperparathyroidism. J Endocrinol Invest. 2009;32(8):690–694. doi: 10.1007/BF03345742. [DOI] [PubMed] [Google Scholar]
- 12.Torres J.A., Rezaei M., Broderick C., et al. Crystal deposition triggers tubule dilation that accelerates cystogenesis in polycystic kidney disease. J Clin Invest. 2019;129(10):4506–4522. doi: 10.1172/JCI128503. [DOI] [PMC free article] [PubMed] [Google Scholar]