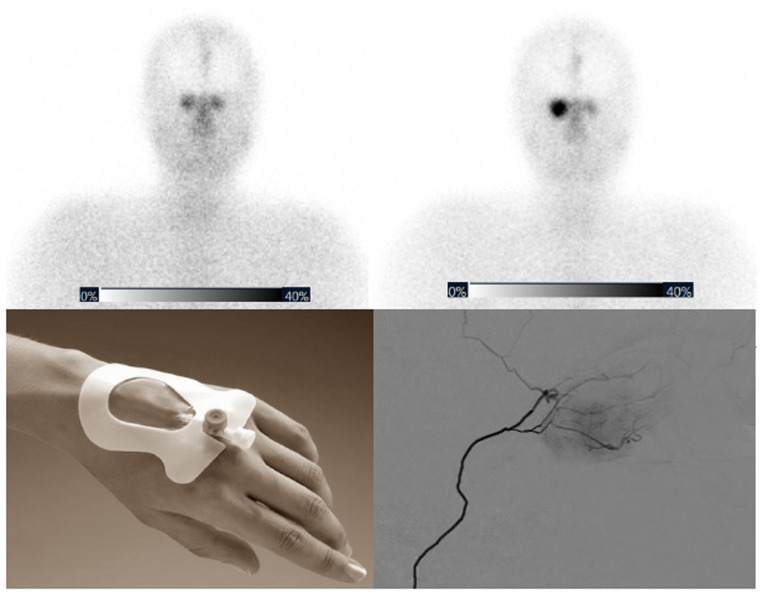
Visual Abstract
Keywords: meningioma, PRRT, intraarterial, somatostatin receptor
Abstract
Intravenous 177Lu-high-affinity (HA)-DOTATATE has shown promising results for the treatment of surgery- and radiotherapy-refractory meningiomas. We aimed to investigate the added value of intraarterial administration. Methods: Patients underwent at least 1 intravenous 177Lu-HA-DOTATATE treatment first and subsequent intraarterial cycles. Inpatient and intrapatient comparison was based on posttreatment 177Lu-HA-DOTATATE imaging 24 h after injection. The technical success rate and adverse events were recorded. Results: Four patients provided informed consent. The technical success rate was 100%, and no angiography-related or unexpected adverse events occurred. Intrapatient comparison showed an increased target lesion accumulation on both planar imaging (mean, +220%) and SPECT/CT (mean, +398%) after intraarterial administration, compared with intravenous administration. No unexpected adverse events occurred during follow-up. Conclusion: Intraarterial peptide receptor radionuclide therapy significantly increases tracer accumulation and is a safe and promising improvement for salvage meningioma patients. Future prospective studies on intraarterial peptide receptor radionuclide therapy are needed to determine the gain in efficacy and survival.
Meningiomas refractory to surgery and external-beam radiation are difficult to treat. The reported outcomes of a wide range of systemic treatments in this end-stage setting are poor, and progression-free survival rates are rather limited (1). Somatostatin receptor is overexpressed in meningiomas and is one of the most specific immunohistochemical markers (2). In recent years, promising results for peptide receptor radionuclide therapy (PRRT) with either 177Lu- or 90Y-labeled compounds in salvage meningioma have been reported, and its potential was recently recognized in the European Association of Neuro-Oncology guidelines on meningioma (3). The number of studies conducted on PRRT in meningioma is limited and far less than for neuroendocrine neoplasms (NENs) (4). Intraarterial administration of 177Lu-HA-DOTATATE may benefit meningioma patients, as it might result in higher tumor uptake, as previously shown in NENs (5,6). This article reports the preliminary results for an intrapatient comparison of intravenous versus intraarterial treatment of salvage meningioma patients.
MATERIALS AND METHODS
Patient Population
The institutional medical ethics committee approved this retrospective study, and the requirement to obtain informed consent was waived. All historically referred meningioma patients were included in this retrospective analysis, and only patients treated intraarterially were included. Screening of patients included clinical assessment, laboratory examinations, and 68Ga-DOTATOC PET/CT (Somakit; Advanced Accelerator Applications) to assess the sufficiency of tracer accumulation, in line with current guidelines (7). Treatment with four 7,400-MBq cycles of 177Lu-high-affinity (HA)-DOTATATE (Scintomics) was planned for all patients. At 4–5 wk after each cycle, patients returned to the outpatient clinic for physical examination and laboratory testing. Baseline and follow-up toxicity was recorded according to the Common Terminology Criteria for Adverse Events, version 5 (8).
Procedures
The first treatment cycle was always intravenous, and if logistically possible, subsequent cycles were administered intraarterially. A diagnostic angiogram was performed to identify the tumor-feeding vessel (TFV). If there were multiple TFVs, a single more proximal injection was chosen to cover all TFVs. A single injection position was preferred to avoid microcatheter manipulation and contamination of the angiography suite. In case of multifocal disease, the lesion causing the most complaints was selectively treated (defined as the target lesion). The production and administration methods for 177Lu-HA-DOTATATE have been previously reported (9). The technical success rate and adverse events related to the angiography procedure were registered.
Image Analysis
In line with standard clinical care, posttreatment scintigraphy was performed 24 h after injection. On planar and SPECT/CT imaging, target-to-background ratios were compared between the intravenous and intraarterial cycles. On planar imaging, a background region of interest was drawn in the right hemithorax (diameter 7 cm) for comparison with the lesional region of interest. On SPECT/CT, a background volume of interest was placed in the contralateral neck muscles for comparison with the lesion volume of interest (40% threshold of the maximum pixel value). All regions and volumes of interest were copied to the corresponding images of subsequent treatment cycles. The percentage difference was calculated by dividing the intraarterial target-to-background ratio by the intravenous target-to-background ratio and multiplying by 100. Response was based on target lesions and defined according to Response Assessment in Neuro-Oncology Criteria on gadolinium-enhanced MRI (10) and SUVpeak measurements on 68Ga-DOTATOC PET, both acquired 2 mo after treatment.
RESULTS
Patients
Up to July 2020, 7 patients were referred, of whom 4 patients received intraarterial 177Lu-HA-DOTATATE after initial intravenous 177Lu-HA-DOTATATE. These 4 patients were treated between March 2018 and September 2020 and were included in the current analysis. Baseline and treatment characteristics are shown in Table 1. The first patient was published previously (5). All patients had histopathologic confirmation on the primary resection specimen or an additional biopsy specimen and had progressive disease on MRI before therapy. At baseline, no hematologic or biochemical abnormalities were present, and tumor-related complaints varied (Table 1).
TABLE 1.
Baseline Characteristics
| Characteristic | Data |
|---|---|
| Age (y) | 57 (44–66) |
| Localization* (main complaint) | |
| Temporal (epilepsy) | 2 |
| Falx (nausea) | 1 |
| Optic meningioma (proptosis-induced pain) | 1 |
| Previous treatments | |
| Surgical resection | 4 |
| External-beam radiation therapy | 4 |
| Temozolomide | 1 |
| Unifocal/multifocal | 2/2 |
| Histopathology WHO grade | |
| 1 | 0 |
| 2 | 3 |
| 3 | 1 |
| Median number of treatment cycles | 4 (3–4) |
| Intravenous | 2 (1–2) |
| Intraarterial | 2 (1–3) |
| Median activity per cycle (MBq) | 7,338 (3,668†–7,637) |
| Median interval per cycle (wk) | 6.3 (5.7–10) |
*Largest or most symptomatic lesion only.
†Final cycle of 1 patient was delayed out of precaution because of grade 3 leukopenia, with activity reduction of 50%.
Qualitative data are number; continuous data are median and range.
Angiography Procedures
The technical success rate of intraarterial administration was 100%. A single TFV could be identified in 3 patients and multiple TFVs in 1 patient (proximal injection position, external carotid artery). No administration difficulties and no contamination of the angiography suite occurred.
Image Analysis and Efficacy
Intrapatient comparison between cycles showed increased accumulation in the target lesion on both planar imaging (mean, +220%) and SPECT/CT (mean, +398%) (Table 2). In patients with multifocal disease, inpatient comparison at a single time point was possible, and a clear distinction could be made between intraarterially and intravenously treated lesions (Fig. 1).
TABLE 2.
Imaging Analysis Results
| Response assessment | Baseline | After therapy | Mean change |
|---|---|---|---|
| Maximum diameter on MRI (mm) | 24 (19–32) | 21 (15–45) | |
| SUVpeak on 68Ga-DOTATOC PET | 6.33 (5.13–8.81) | 7.1 (2.4–8.7)* | |
| Posttreatment imaging assessment (n = 15) | |||
| Planar target-to-background ratio | 1.7 (1.4–2.5) (intravenous) | 3.7 (2.8–5.2) (intraarterial) | +220% |
| SPECT/CT target-to-background ratio | 15.0 (12.7–18.6) (intravenous) | 59.8 (40.5–82.0) (intraarterial) | +398% |
*Not available in the single progressive patient.
Data are median and range.
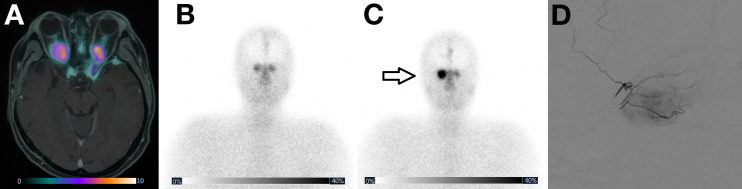
FIGURE 1.
(A) Pretreatment 68Ga-DOTATOC PET fused with MRI showing multiple meningiomas, especially bilateral optic meningiomas, causing blindness and proptosis-induced pain complaints. Posttreatment 177Lu-HA-DOTATATE planar image after intravenous (B) and intraarterial (C) treatment showing clearly increased accumulation in right optical meningioma (arrow) by intraarterial treatment, compared to the contralateral optical meningioma (treated by second pass). (D) Corresponding digital subtraction angiography of intraarterial administration. Patient had stable disease on imaging, and proptosis-induced pain complaints subsided.
One World Health Organization (WHO) grade 3 meningioma patient had progressive disease clinically and on imaging during treatment, and therapy was terminated earlier (after 3 cycles; 2 intravenous and 1 intraarterial). The patient died 6 mo after initiation of 177Lu-HA-DOTATATE therapy. The remaining 3 patients with a WHO grade 2 meningioma completed all 4 treatment cycles: 1 had a partial response and 2 had stable disease according to Response Assessment in Neuro-Oncology Criteria. The baseline clinical complaints of the 3 WHO grade 2 meningioma patients improved after treatment (Fig. 1). Median follow-up was 1.7 y (range, 1–3 y). One of these patients had an additional surgical resection (1 y after treatment), and disease in the others continued to be controlled. No other additional therapies have been initiated.
Toxicity
No significant clinical or biochemical toxicities were reported (Common Terminology Criteria for Adverse Events grade ≥ 3; Table 3). Significant hematologic toxicity was limited to 1 patient, experiencing an isolated grade 3 leukopenia. No unexpected or angiography-related adverse events occurred.
TABLE 3.
Reported Adverse Events During the Whole Treatment, by Common Terminology Criteria for Adverse Events Grade (Version 5)
| Event | Grade 1 | Grade 2 | Grade 3 |
|---|---|---|---|
| Clinical | |||
| Nausea | 1 | ||
| Fatigue | 3 | ||
| Hair loss | 1 | ||
| Hematologic* | |||
| Anemia | 2 | ||
| Leukopenia | 2 | 1 | |
| Biochemical* | |||
| AST | 1 | ||
| ALT | 2 |
*Other laboratory investigations did not show any toxicity during follow-up.
ALT = alanine aminotransferase; AST = aspartate transaminase.
DISCUSSION
Our preliminary results show a clear increase in 177Lu-HA-DOTATATE accumulation after intraarterial administration (+398% on SPECT/CT), compared with intravenous administration in an intrapatient comparison. How these results translate to improved survival or a higher number of objective response rates remains to be seen and should be an aim of future prospective studies on PRRT in meningioma.
A recent metaanalysis by Mirian et al. confirmed durable disease control rates, a limited toxicity profile, and long progression-free survival rates on different kinds of therapeutic somatostatin receptor–targeted radiopharmaceuticals in salvage meningioma patients (4). A pooled analysis of 111 patients showed a 6- and 12-mo progression-free survival of 61% and 53%, respectively, for the entire analyzed group. The poorer outcome of WHO grade 3 meningioma patients was consistent in all publications (6-mo progression-free survival of 94%, 48%, 0% for WHO grade 1, 2, and 3, respectively). Objective response rates, however, are still far from promising, at just 2%. However, as the authors describe, many unknowns exist in treating meningiomas with PRRT. A wide variety of total administered activities was used (1.7–29.8 GBq in 1–6 cycles), and application was either as a monotherapy or concurrent with EBRT. However, compelling evidence of one treatment algorithm over another is lacking. Our used treatment algorithm was based on experience with PRRT in NEN but not proven for meningiomas (11). Unfortunately, as in many other studies, dosimetric analysis with multiple-time-point imaging was unavailable in our cohort. The current European Association of Neuro-Oncology guideline does not give any direction on how to perform PRRT in salvage meningioma and only recommends that it be used for WHO grade 3 tumors, which seems contradictory to the evidence (3,4). As no distinct guideline based on evidence exists, the preferred treatment algorithm, proper patient selection on pretreatment PET/CT, and potential dosimetric thresholds of PRRT in meningiomas remain unknown and an open field of research.
Literature on intraarterial PRRT in neurooncology is sparse. To our knowledge, only 1 recent publication describes the intrapatient comparison of intravenous versus intraarterial 68Ga-DOTATATE in meningioma (n = 4), showing a 2- to 5-fold increase in tumor uptake (12). Outside the scope of meningioma, a study in 2002 already reported results on intraarterial 90Y-lanreotide in 6 astrocytomas and 1 histiocytoma (13). Even though the results of these 2 previous reports and our findings are in line with previous results on intraarterial PRRT in NENs, it is a difficult comparison, as NENs are a completely different entity and the treated organ (brain vs. liver) differs in arterial vascularization. Nonetheless, increased uptake of different radiopharmaceuticals after intraarterial administration seems to be present in NENs (6). However, more recently, Lawhn-Heath et al. published the direct opposite (14). The imaging analysis was based on the surrogate of 68Ga-DOTATOC, comparing intravenous and intraarterial administration, with the intraarterial 68Ga-DOTATOC being simultaneously administered with a single intraarterial cycle of 90Y-DOTATOC. Interestingly, intraarterial administration lead to a lower SUVmax overall—not only in extrahepatic tumor deposition (median ratio, 0.73), for which one might expect this to occur, but also in hepatic tumor deposition (median ratio, 0.90) (14). One possible explanation for their results may be the interference of radiopharmaceuticals during simultaneous infusion, thus making their results difficult to interpret. Currently, 2 other trials are ongoing to define the added benefit of intraarterial over intravenous administration, and with multiple cycles instead of just one ((15) and NCT04544098), hopefully providing definite answers.
The presented data will be used to initiate a new prospective study to determine the clinical benefit of intraarterial PRRT and its effect on objective response and survival in salvage meningioma.
CONCLUSION
Intraarterial PRRT significantly increases tracer accumulation, providing a safe and promising improvement for salvage meningioma patients. Future prospective studies on intraarterial PRRT in meningioma are needed.
DISCLOSURE
Marnix Lam receives research support from AAA/Novartis. No other potential conflict of interest relevant to this article was reported.
KEY POINTS.
QUESTION: Does intraarterial administration increase 177Lu-HA-DOTATATE uptake in meningioma?
PERTINENT FINDINGS: This retrospective, intrapatient comparison shows that intraarterial administration results in a mean 4-fold increase in tumor uptake, without additional side effects.
IMPLICATIONS FOR PATIENT CARE: Efficacy of 177Lu-HA-DOTATATE in meningioma may be improved by intraarterial administration.
REFERENCES
- 1. Kaley T, Barani I, Chamberlain M, et al. Historical benchmarks for medical therapy trials in surgery- and radiation-refractory meningioma: a RANO review. Neuro Oncol. 2014;16:829–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boulagnon-Rombi C, Fleury C, Fichel C, Lefour S, Marchal Bressenot A, Gauchotte G. Immunohistochemical approach to the differential diagnosis of meningiomas and their mimics. J Neuropathol Exp Neurol. 2017;76:289–298. [DOI] [PubMed] [Google Scholar]
- 3. Goldbrunner R, Minniti G, Preusser M, et al. EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol. 2016;17:e383–e391. [DOI] [PubMed] [Google Scholar]
- 4. Mirian C, Duun-Henriksen AK, Maier AD, et al. Somatostatin receptor-targeted radiopeptide therapy in treatment-refractory meningioma: an individual patient data meta-analysis. J Nucl Med. 2021;62:507–513. [DOI] [PubMed] [Google Scholar]
- 5. Braat AJAT, Snijders TJ, Seute T, Vonken EPA. Will 177Lu-DOTATATE treatment become more effective in salvage meningioma patients, when boosting somatostatin receptor saturation? A promising case on intra-arterial administration. Cardiovasc Intervent Radiol. 2019;42:1649–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ebbers SC, Barentsz MW, Braat AJAT, Lam MGEH. Intra-arterial peptide receptor radionuclide therapy for neuroendocrine tumor liver metastases. Dig Dis Interv. 2019;3:81–90. [Google Scholar]
- 7. Bodei L, Mueller-Brand J, Baum RP, et al. The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2013;40:800–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. Cancer Therapy Evaluation Program website. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf. Published November 27, 2017. Accessed October 14, 2021.
- 9. Ebbers SC, Barentsz MW, de Keizer B, Krijger GC, Lam MGEH, Braat AJAT. A rapid and safe infusion protocol for lutetium-177 peptide receptor radionuclide therapy. J Nucl Med. 2021;62:816–822. [DOI] [PubMed] [Google Scholar]
- 10. Huang RY, Bi WL, Weller M, et al. Proposed response assessment and endpoints for meningioma clinical trials: report from the Response Assessment in Neuro-Oncology Working Group. Neuro Oncol. 2019;21:26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Strosberg J, El-Haddad G, Wolin E, et al. Phase 3 trial of 177Lu-DOTATATE for midgut neuroendocrine tumors. N Engl J Med. 2017;376:125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Verburg FA, Wiessmann M, Neuloh G, Mottaghy FM, Brockmann MA. Intraindividual comparison of selective intraarterial versus systemic intravenous 68Ga-DOTATATE PET/CT in patients with inoperable meningioma. Nuklearmedizin. 2019;58:23–27. [DOI] [PubMed] [Google Scholar]
- 13. Buscombe JR, Pigott K. New approaches in targeting intracerebral tumours with 90Y-labelled radiopeptides. Eur J Nucl Med Mol Imaging. 2002;29:1697–1698. [DOI] [PubMed] [Google Scholar]
- 14. Lawhn-Heath C, Fidelman N, Chee B, et al. Intraarterial peptide receptor radionuclide therapy using 90Y-DOTATOC for hepatic metastases of neuroendocrine tumors. J Nucl Med. 2021;62:221–227. [DOI] [PubMed] [Google Scholar]
- 15. Ebbers SC, Braat AJAT, Moelker A, Stokkel MPM, Lam MGEH, Barentsz MW. Intra-arterial versus standard intravenous administration of lutetium-177-DOTA-octreotate in patients with NET liver metastases: study protocol for a multicenter, randomized controlled trial (LUTIA trial). Trials. 2020;21:141. [DOI] [PMC free article] [PubMed] [Google Scholar]