Abstract
Approximately 3.5 million youth and adolescents in the US play football, a sport with one of the highest rates of concussion. Repeated subconcussive head impact exposure (HIE) may lead to negative neurological sequelae. To understand HIE as an independent predictive variable, quantitative cumulative kinematic metrics have been developed to capture the volume (i.e., number), severity (i.e., magnitude), and frequency (i.e., time-weighting by the interval between head impacts). In this study, time-weighted cumulative HIE metrics were compared with directional changes in diffusion tensor imaging (DTI) metrics. Changes in DTI conducted on a per-season, per-player basis were assessed as a dependent variable. Directional changes were defined separately as increases and decreases in the number of abnormal voxels relative to non-contact sport controls. Biomechanical and imaging data from 117 athletes (average age 11.9 ± 1.0 years) enrolled in this study was analyzed. Cumulative HIE metrics were more strongly correlated with increases in abnormal voxels than decreases in abnormal voxels. Additionally, across DTI sub-measures, increases and decreases in mean diffusivity (MD) had the strongest relationships with HIE metrics (increases in MD: average R2 = 0.1753, average p = 0.0002; decreases in MD: average R2 = 0.0997, average p = 0.0073). This encourages further investigation into the physiological phenomena represented by directional changes.
Keywords: Football, Concussion, Magnetic resonance imaging, Head injury, Youth Athletes, HIT System, Injury Risk
INTRODUCTION
Football is a collision sport known for frequent contact among players. Repeated subconcussive head impact exposure (HIE) from collision sports may lead to negative neurological sequelae.20 Therefore, there is concern for the short- and long-term effects on the brain attributed to accumulation of HIE from football. Youth athletes represent a majority of all football players (numbering approximately 2.8–3.5 million17), yet not much is known about the effects of repeated HIE in the youth population.2 Much of the research related to repeated HIE has been conducted by equipping athletes with sensors that approximate the frequency and magnitude of head impacts they receive. Prior studies have examined the effects of player position, level of play, as well as drills conducted during practice, while others have examined short- and long-term effects of repeated head impacts on clinical outcomes such as neurocognitive testing and neuroimaging.12,14,23,29,34,38,44 The factors that contribute to specific changes in clinical outcome measures are not yet fully understood.
Efforts to understand cumulative HIE arose from neurological changes observed in athletes who citreceived repeated head impacts from contact and collision sports,1,31,37 even after a single season of play.12,13,20,38 Biomechanical metrics quantifying cumulative HIE as the volume and magnitude of impacts sustained over a season were developed.8,45 Recent studies, however, have incorporated the timing of impacts into cumulative HIE metrics.9,32 This technique, known as time-weighting, considers the brain’s capacity to recover from repeated HIE. The efficacy of HIE metrics in evaluating changes in the brain are assessed by investigating their relationship with changes in pre- to post-season clinical outcome measures thought to be correlated with injury. Conventional MRI has been used to identify non-specific abnormalities following sports-related concussions; however, such modalities lack utility in diagnosing or describing certain specific tissue changes.25 Alternatively, studies have employed diffusion tensor imaging (DTI), which offers in vivo measures of molecular water diffusion and can be used to interrogate specific brain microstructural changes associated with mTBI in the brain.13,19 Associations between repeated HIE and DTI-derived changes in the brain have been observed among adolescent football players.12
Total changes in abnormal voxels are often used to evaluate DTI-derived changes in the brain, but few analyses have evaluated the directional changes in DTI measures.7 When compared separately, increases and decreases of DTI metrics have different associations with brain changes. Increased fractional anisotropy (FA) and decreased mean diffusivity (MD) have been associated with axonal swelling reducing space between fibers, cytotoxic edema, and inflammation.40 Meanwhile, decreased FA and increased MD can be indicative of myelin damage, disruption of tissue structure, and axonal damage20,36 Clinical evidence has shown increases in symptoms, impairment measures, and total number of abnormal DTI measures associated with concussion within just a week of the event.16,30 There is also evidence that repeated HIE can lead to long-lasting (several months or years) effects in the brain.1 The mechanisms behind these differences is not fully understood, with one explanation being that they represent acute and chronic phases of injury, separately. Investigating the effects of HIE on directional changes in DTI metrics could further elucidate the effects that subconcussive HIE has on the brain, including the kind of damage that occurs, along with the phase of injury (acute vs. chronic). By comparing time-weighted HIE metrics to directional changes in DTI metrics, the relationship between HIE biomechanics and acute/chronic changes in the brain, as well as other directional changes can be assessed. The objective of this study was to compare cumulative biomechanical HIE metrics that consider volume, severity, and time of impacts, with directional changes in DTI measures.
MATERIALS AND METHODS
Athletes were recruited from three youth football organizations to participate in this study approved by the Wake Forest University Health Sciences Institutional Review Board. The athletes played 11 on 11 full tackle football under the governance of their national organization. Written participant assent and parental consent were acquired for participation in the study. Each enrolled athlete was fitted with a Riddell Speed or Revolution football helmet instrumented with a Head Impact Telemetry (HIT) System MxEncoder, which includes a six-sensor accelerometer array that records and stores head impacts that a participant receives in real-time.4,24 A researcher was present at each game and practice to operate the HIT system, and record video of each event using a time-synchronized camera. Post-season, video was reviewed to validate recorded impacts pre-analysis. This validation involved removal of impacts that did not occur while the players were involved in a practice or game session, including before and after a session, water breaks, and half time. Video of individual impacts exceeding 60 g in magnitude were manually reviewed to confirm whether the event was associated with the player’s involvement in a football-related activity, and remove false events (i.e., dropped helmet). Data for this study was collected across six seasons (2012–2017). Biomechanics data processing methods have been previously described.5,23,47 Participant demographics are provided in Table 1. To obtain control samples, non-contact athletes were recruited from local youth organizations, including baseball, swimming, and tennis. Control participants were screened to ensure they did not have any prior concussions or participation in contact sports. Biomechanical data was only collected from enrolled football players. For all participants, individuals were excluded if they had prior brain surgery or history of traumatic brain injury.
TABLE 1.
Demographics of football study participants.
| Total n = 117 |
2012 Season (n = 16) |
2013 Season (n = 9) |
2014 Season (n = 12) |
2015 Season (n = 30) |
2016 Season (n = 23) |
2017 Season (n = 27) |
|---|---|---|---|---|---|---|
| Age (years) | 11.4 ± 0.9 | 12.3 ± 1.1 | 12.2 ± 1.0 | 11.4 ± 0.9 | 12.2 ± 1.0 | 11.9 ± 1.1 |
| Height (in.) | n/a | 62.0 ± 3.6 | 61.6 ± 3.1 | 59.2 ± 2.9 | 61.9 ± 3.7 | 61.6 ± 3.9 |
| Weight (lbs) | 104.5 ± 10.2 | 107.6 ± 16.5 | 109.8 ± 8.7 | 101.0 ± 21.8 | 115.3 ± 18.8 | 121.2 ± 33.5 |
Imaging
Pre and postseason brain MRI of football players was acquired on a 3 T Siemens Skyra MRI scanner (mean time between scans was 4.5 ± 0.95 months). Control participants also completed MRI scans at baseline and follow-up 3.98 ± 1.07 months later. Each MRI scan was evaluated by a board certified neuroradiologist for structural abnormalities and motion artifacts. Any scans with clinically relevant abnormalities (e.g., tumors, uncharacteristically high white matter lesions) or motion artifacts were excluded from analysis. MRI acquisition and processing has been previously described and are abbreviated here.13 T1-weighted images as well as diffusion data were acquired. Voxel-wise DTI scalar metrics: FA, MD, linear anisotropy (CL), planar anisotropy (CP), and spherical anisotropy (CS), were computed using DTI-TK. FA is a measure of the uniformity of direction of water molecule flow in white matter, and MD is a measure of the degree of free movement of water molecules.3 These scalar maps were then warped to Montreal Neurologic Imaging space based on the SPM8 normalization from the T1 image. The first DTI protocol parameters included a 10,500 ms/99 ms TR/TE, 90° flip angle, and 2.2 mm × 2.2 mm × 3.0 mm voxel resolution with b-values of 0, 1000, and 2000 s/mm2. The second DTI protocol parameters included a 12,600 ms/100 ms TR/TE, 90° flip angle, a 2.0 mm × 2.0 mm × 2.0 mm voxel resolution with b-values of 0, 1000, and 2000 s/mm2.
MRI scans collected from control participants were used to determine the amount of change from pre- to post-season that was considered abnormal. Each preseason scalar map was subtracted from the post-season map for each player (follow-up scans for control participants), creating a delta map for each metric. The voxel-wise group mean and standard deviation (SD) of the controls’ delta maps were used to calculate voxel-wise z-scores for each football player. A voxel was defined as abnormal if its z-score was at least 2 SD away from the mean of the voxel value in the control sample. These represented abnormally high and low scalar values. A cluster threshold requiring a minimum 1 mL contiguous volume was applied to reduce false positives. The number of abnormal voxels 2 SDs above the mean is defined as the number of increases in abnormal voxels, and the number of abnormal voxels 2 SDs below the mean are defined as the number of decreases in abnormal voxels. Figure 1 shows the process of determining abnormality in voxel maps.
FIGURE 1.

(a–d) Abnormal voxel calculations. (a) For each control subject, baseline images were subtracted from the corresponding follow up images. (b) At each voxel, abnormally high and abnormally low changes were respectively defined as +/− 2 SDs from the control population mean (pink regions under the distribution curve). (c) Postseason minus preseason change in diffusion images were created for each football subject. (d) Diffusion changes for each football subject were compared to that of the control population distribution. Abnormally high or low diffusion changes at each voxel were identified for each football subject. Abnormally high and low voxels for a low, medium, and high HIE subject are displayed in red on the Dartel template image. Note the graded differences in the number of abnormal voxels between subjects.
Biomechanical Metrics
Several biomechanical metrics were investigated as independent variables, including number of impacts, risk-weighted exposure (RWE), and three time-weighted HIE metrics: exponential time-weighted exposure (TWEExp), inverse time-weighted exposure (TWEInv), and time until assessment (TUA) (Table 2). Number of impacts is the total quantity of impacts recorded over a season for an athlete. This metric only considers volume of impacts. RWE combines the magnitude and frequency of impacts by non-linearly weighting each impact by the estimated concussion risk.45 To compute RWE, the risk of concussion for each impact was calculated using the combined probability (CP) risk function and summed to generate RWE for a player’s season.42 The logistic regression equation is provided in Table 2, where β0 is −10.2, β1 is 0.0433, β2 is 0.000873, β3 is −9.2E−7 and ai and αi are the peak resultant linear and rotational acceleration, respectively, for each impact.
TABLE 2.
Biomechanical and cumulative HIE metrics.
| Abbreviation | Description | Equation |
|---|---|---|
| Number of Impacts | Number of impacts | n |
| X | Combined probability injury risk function | |
| RWE | Risk-weighted exposure | |
| TWE Exp | Exponential time-weighted exposure | |
| TWE Inv | Inverse time-weighted exposure | |
| TUA | Time until assessment |
The equation for each time-weighted metric is provided in Table 2 and further described below. In Table 2, ‘XC’ represents the magnitude of the current impact, ‘XP’ represents the magnitude of an impact that occurred before the impact under consideration (i.e., prior to). ‘n’ is the total number of impacts the player experienced, ‘m’ is the number of impacts that occurred in a preceding time window, where the duration of each time window varies per metric, ‘TC’ is the time of the current impact, ‘TP’ is the time of a prior impact. Remaining variables specific to each time-weighted metric are further described below.
For each time-weighted metric, CP is used as the exposure measure to create a metric comparable to RWE. Additionally, for each time-weighted metric, time was measured in units of hours. Each metric is unitless (except number of impacts, which is a count), representing injury risk. Each time-weighted metric is described in detail below.
Exponential Time-Weighted Exposure (TWEExp)
For a given impact (XC), the CP of each prior impact (XP) within a specified time window was weighted based on an exponential decay function ranging from 0 to 1, as a function of time between the given impact and the prior impact. An exponential decay constant (γ = − 0.3) was multiplied with the time interval (i.e., the time between XC and XP) in the exponent (TC − TP). For each head impact, the exponential time-weight was applied to all head impacts occurring less than 72 h prior to the given one; this represents, conservatively, the approximate time for blood biomarker and ion levels associated with traumatic brain injuries to return to normal following concussion.41 This was iteratively computed for every head impact over the season and summed to generate a single value representing the exponential time-weighted cumulative exposure.
Inverse Time-Weighted Exposure (TWEInv)
This method was derived from the work of Merchant-Borna et al., except CP was used as the measure of exposure instead of peak acceleration.32 The time-weighting scale factor employed in this method weights the CP of each impact inversely by time between the given impact (XC) and every previous impact occurring on the same day. The time difference was measured in hours so the smallest scale factor possible is 1/24 (if the two impacts occurred an entire day apart), and the largest scale factor approaches 1/0, if the impacts occur very close together. In this equation, the time difference (TC − TP) is between the current and prior impacts respectively, and ‘b’ represents a constant that has a value of 1 h.
Time Until Assessment (TUA)
This method was also derived from Merchant-Borna et al., but also uses CP as the exposure metric.32 In this approach, impacts are evaluated relative to the post-season assessment. The CP of each impact is multiplied with the inverse of the time difference between the time of post-season assessment (TD) and the time of impact (TC). As a result, impacts closer to the end of the season are weighed more heavily than those that occurred near the beginning. For example, if an impact occurs 3 months (2208 h) before post-season assessment, its CP is multiplied by 1/2208 before being added to the cumulative measure for the season. In this equation, the time difference (TD − TC) is between the time of post season scan and the given impact respectively, and ‘b’ represents a constant that has a value of 1 h.
Statistical Analysis
To evaluate the relationship between cumulative HIE on directional changes in the brain, biomechanical metrics were compared with increases and decreases in abnormal voxels derived from DTI using linear regression analysis. Five diffusion-based metrics were used to represent changes in the brain: FA, MD, CL, CS, and CP. The Fisher–Pearson standardized moment coefficient of skewness was calculated to determine normality of metrics. All DTI metrics, (as well as each time-weighted metric) were logarithmically transformed pre-analysis to improve normality. Number of Impacts and RWE were not log-transformed due to skewness coefficients less than 2.1 (a threshold determined to be indicative of a non-skewed distribution) in addition to visual examination of the distribution. Collinearity of predictor variables was also evaluated. Cook’s distance (4/n, where n is the number of participants) was used to identify and exclude outliers from regressions. Covariate adjustment was conducted to account for differences in age, weight, time between scans, and differences in imaging protocol between seasons. Statistical analysis was completed in SAS v.9.4 (Cary, NC, USA).
RESULTS
95 Athletes were enrolled in this study who played in one or more seasons over the course of six seasons and had complete, quality MRI data, resulting in 117 player-seasons (11.9 ± 1.0 years, 110.6 ± 21.4 lbs). A total of 34,039 verified impacts were recorded, with an average number of impacts per player per season of 290.9 ± 205.8. Summary statistics of biomechanical metrics are provided in Table 3.
TABLE 3.
Summary statistics of biomechanical metrics.
| Metric | Mean (± standard deviation) | Median | 95th Percentile | Skewness coefficient | Coefficient of variation |
|---|---|---|---|---|---|
| Number of impacts | 290.9 ± 205.8 | 222 | 681.8 | 0.999 | 0.707 |
| RWE | 0.44 ± 0.58 | 0.18 | 1.76 | 2.056 | 1.318 |
| TWE Exp | 4.87 ± 9.39 | 1.35 | 22.13 | 3.678 | 1.928 |
| TWE Inv | 1088 ± 1855 | 374 | 5718 | 2.593 | 1.705 |
| TUA | 4.16 × 10−4 ± 8.97 × 10−4 | 1.85 × 10−4 | 1.90 × 10−4 | 6.726 | 2.156 |
Results of linear regressions are provided in Table 4. For regressions with increases and decreases in abnormal voxels, 96 and 64% of comparisons were significant (p < 0.05) respectively. The strongest relationships between biomechanical metrics and abnormal increases were with MD. In these comparisons, the relationship between MD and TWEExp was strongest (R2 = 0.1965), followed by TUA (R2 = 0.1837), number of impacts (R2 = 0.1787), RWE (R2 = 0.1628), and TWEInv (R2 = 0.1550). The comparison between MD and TWEExp was also the strongest relationship observed across all comparisons. To demonstrate the relationships between the various biomechanical metrics and increases and decreases separately, linear regression plots are shown in Figs. 2 and 3.
TABLE 4.
Linear regression analysis of biomechanical metrics and DTI metrics (bolded represents statistically significant relationships p < 0.05).
| Increases | Decreases | |||||
|---|---|---|---|---|---|---|
| Comparisons | Adjusted R2 | Adjusted R2 Rank by DTI Metric | Total Rank | Adjusted R2 | Adjusted R2 Rank by DTI Metric | Total Rank |
| FA vs. # Impacts | 0.1681 | 1 | 4 | 0.1547 | 1 | 3 |
| FA vs. RWE | 0.1489 | 5 | 11 | 0.0492 | 4 | 19 |
| FA vs. TWEExp | 0.1619 | 2 | 6 | 0.1115 | 2 | 7 |
| FA vs. TWEInv | 0.1547 | 4 | 9 | 0.0352 | 5 | 21 |
| FA vs. TUA | 0.1567 | 3 | 7 | 0.0860 | 3 | 14 |
| MD vs. # Impacts | 0.1787 | 3 | 3 | 0.1015 | 3 | 10 |
| MD vs. RWE | 0.1550 | 5 | 8 | 0.1028 | 2 | 9 |
| MD vs. TWEExp | 0.1965 | 1 | 1 | 0.1031 | 1 | 8 |
| MD vs. TWEInv | 0.1628 | 4 | 5 | 0.0920 | 5 | 13 |
| MD vs. TUA | 0.1837 | 2 | 2 | 0.0993 | 4 | 11 |
| CL vs. # Impacts | 0.1536 | 1 | 10 | 0.0824 | 2 | 15 |
| CL vs. RWE | 0.1308 | 5 | 18 | 0.0563 | 4 | 17 |
| CL vs. TWEExp | 0.1403 | 4 | 16 | 0.0925 | 1 | 12 |
| CL vs. TWEInv | 0.1413 | 3 | 14 | 0.0168 | 5 | 23 |
| CL vs. TUA | 0.1417 | 2 | 13 | 0.0694 | 3 | 16 |
| CS vs. # Impacts | 0.1284 | 1 | 21 | 0.1625 | 1 | 1 |
| CS vs. RWE | 0.0630 | 4 | 24 | 0.1491 | 5 | 6 |
| CS vs. TWEExp | 0.1277 | 2 | 22 | 0.1580 | 2 | 2 |
| CS vs. TWEInv | 0.0490 | 5 | 25 | 0.1537 | 3 | 4 |
| CS vs. TUA | 0.0996 | 3 | 23 | 0.1518 | 4 | 5 |
| CP vs. # Impacts | 0.1342 | 3 | 17 | 0.0454 | 2 | 20 |
| CP vs. RWE | 0.1409 | 2 | 15 | 0.0503 | 1 | 18 |
| CP vs. TWEExp | 0.1295 | 4 | 19 | 0.0190 | 3 | 22 |
| CP vs. TWEInv | 0.1478 | 1 | 12 | 0.0031 | 5 | 25 |
| CP vs. TUA | 0.1290 | 5 | 20 | 0.0146 | 4 | 24 |
FIGURE 2.
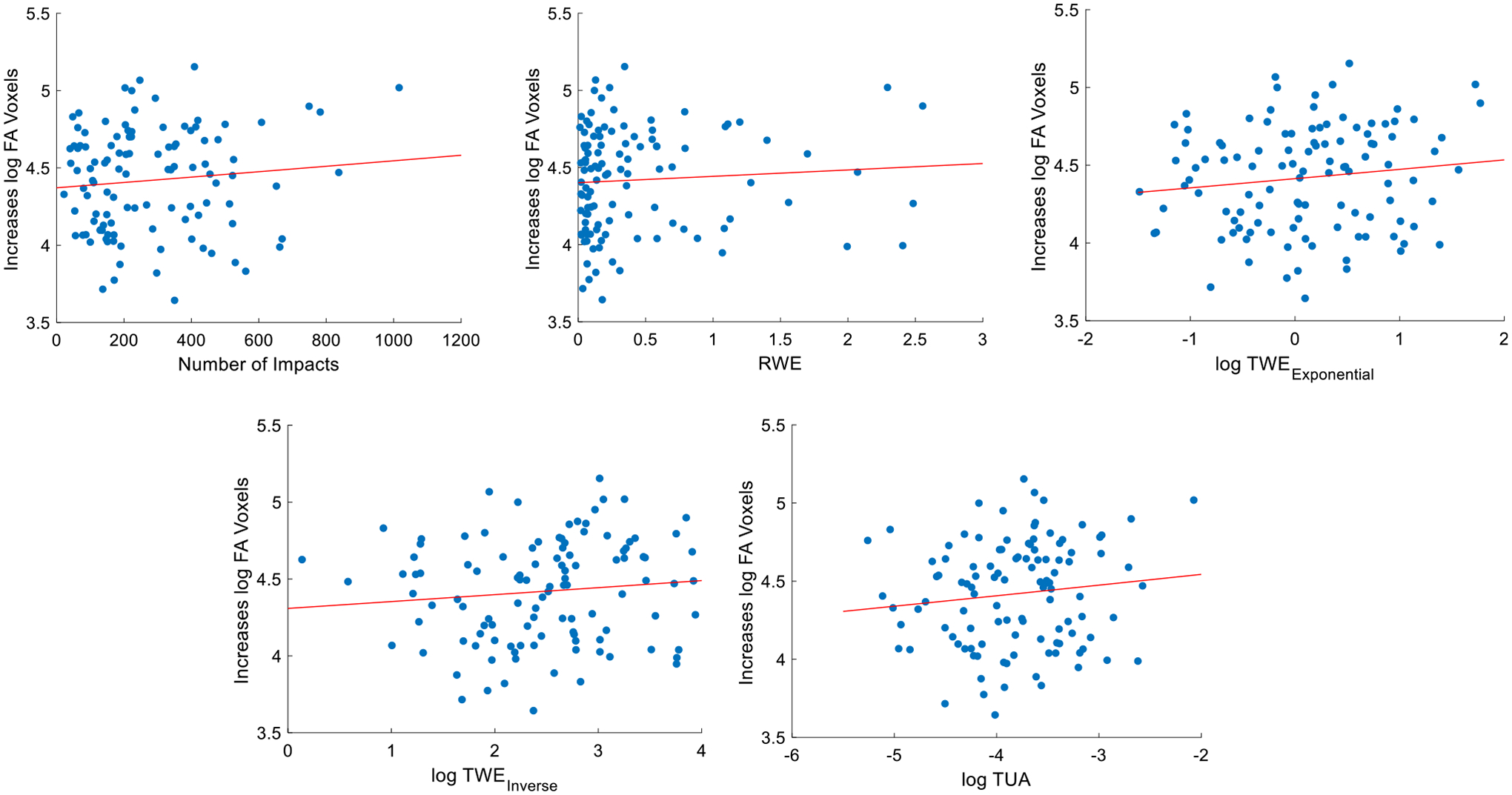
Linear regression between cumulative HIE metrics and increases in abnormal FA voxels.
FIGURE 3.

Linear regression between cumulative HIE metrics and decreases in abnormal FA voxels.
The strongest relationships between decreases in abnormal voxels and biomechanical metrics were with CS and number of impacts, followed by CS and TWEExp, then FA and number of impacts. The strongest relationship overall was between CS and number of impacts (R2 = 0.1625). Five of the top six ranking relationships involving decreases in abnormal voxels included CS. In this set of biomechanical metrics (all significant relationships), time-weighted metrics all outrank RWE, but number of impacts ranks highest. For comparisons between decreases in FA and biomechanical metrics, only three relationships are significant: number of impacts (R2 = 0.1547), TWEExp (R2 value = 0.1115), and TUA (R2 = 0.0860). For comparisons between decreases in MD and biomechanical metrics, all relationships were significant: TWEExp (R2 = 0.1031), RWE (R2 = 0.1028), number of impacts (R2 = 0.1015), TUA (R2 = 0.0993), and TWEInv (R2 = 0.0920). Overall, comparisons between cumulative biomechanical metrics and increases in abnormal voxels (average R2 = 0.1410 ± 0.0328) feature stronger and more significant relationships than comparisons between cumulative biomechanical metrics and decreases in abnormal voxels (average R2 = 0.0864 ± 0.0499).
DISCUSSION
The objective of this study was to evaluate the relationship between cumulative HIE metrics and increases and decreases in abnormal voxels from DTI metrics. The focus of this study was in youth athletes for whom effects of cumulative HIE is not well understood and potential long-term implications of exposure are concerning.2,23,38,43 The results support a relationship between cumulative HIE and changes in the brain associated with directional changes in DTI metrics. Overall, the strength of the associations was weak (highest R2 of 0.2), comparisons involving abnormal increases tended to be stronger and more significant than comparisons involving abnormal decreases. Additionally, of all DTI measures, the number of abnormal MD voxels tended to have the strongest relationships with time-weighted metrics. The results of this study motivate further investigation into the properties of HIE (including volume, magnitude, and timing) that affect directional changes in the brain attributed to repeated impacts.
DTI differs from standard structural MRI (i.e., T1-weighted imaging) as it measures random translational motion of water at a microscopic level.19 This allows DTI metrics to be more sensitive to changes in white matter integrity associated with head impact induced changes in the brain.15 The two primary DTI metrics used were FA and MD. FA represents a scalar quantity measuring the fraction of the diffusion tensor that can be ascribed to anisotropic diffusion, while MD represents a scalar quantity that measures the average amount of diffusion in a voxel. CL, CP, and CS each represent sub-measures of FA. Abnormal decreases in FA may reflect chronic phases of injury19 while abnormal increases in FA may be associated with acute phases of injury.28 One explanation for this phenomenon is that during the acute phase of injury, axonal swelling occurs, causing movement of water into axons and limiting direction of flow. In this study, we found the strongest relationship among correlations with MD. This may be because unlike FA, which is uniformly low in gray matter and suitable for interrogating changes in white matter fibers, MD is appropriate for describing diffusion in both white and gray matter.6 Our analysis used all voxels in the brain, including gray and white matter. Accordingly, changes in MD may have been a better measure of global brain changes related to HIE than other anisotropy measures. The stronger relationships with MD, a metric known to be more sensitive in gray matter than other DTI metrics, suggest that gray matter may be the more susceptible tissue type. Of the anisotropic measures, increases in FA, CL, and CP, and decreases in CS demonstrated the strongest significant relationships with HIE measures. These directional trends may represent unknown histological changes in gray matter, as well as axonal swelling or cytotoxic edema in white matter.26 In addition to tissue-specific alterations, some diffusion changes may reflect the time course of microstructural change post-trauma. Some abnormal DTI-increases correlate with acute changes post-trauma (increases in FA and CL), while others correlate with chronic changes (increases in MD and CP).18 The differences in what structural elements these metrics represent (gray/white matter, axons, myelin sheath, etc.) in the brain could explain how time-weighted metrics can be used to explain certain types of changes in the brain (including those associated with acute and chronic phases of injury). Future investigations should consider region of interest (ROI)-based DTI metrics, including comparisons of changes observed in gray and white matter separately.
One important finding of the present study is, when isolating directional changes, cumulative HIE was more strongly correlated with increases than decreases. Participants performed MRI scans at the beginning and end of each season. However, the neural effects of subconcussive hits at the beginning of a season may be lost by a post-season scan as the brain recovers.27 The accumulation of impacts may have long-lasting effects on the brain which become evident in the distant future.39 Past work has demonstrated that indicators of injury degrade over time (concussion scores, blood biomarkers, etc.) which support the idea of increasing biomechanical data collection frequency.41 There may be a benefit to performing more frequent in-season imaging of participants to understand the effects of HIE on acute changes in the brain. This may also inform recovery time-windows following subconcussive impacts which are important in the development of robust time-weighted cumulative HIE metrics. Additionally, further work could enhance biomechanical metrics by considering the biomechanics of the head impact in the prescribed decay (e.g., varying which impacts decay and at what rate) and combining when impacts occur relative to one another (TWEExp) with when the impacts occur relative to the post-season assessment (TUA).
Relationships between cumulative HIE and directional changes in DTI metrics were observed in this study; however, the strength of the relationships observe were relatively weak and explain a small amount of variance in the data. Several factors including diet, stress, genetics, disease, etc. could conceivably contribute to measurable changes in the brain. Additionally, the complex biomechanical response of the brain may not be fully captured with the metrics calculated in this study. Future studies should consider strain-based metrics derived from finite element modeling that represent the dynamic response of the brain to each impact.33 Furthermore, the HIT system was used in this study. Video review was used to verify times when athletes were helmeted and remove false events, but impacts below 60 g were not individually verified. The HIT system has some error relating to impact detection and acceleration measurements. Nevertheless, previous studies have used the HIT system and verified its usefulness for HIE measurement.4 Finally, the risk function used in this study was originally created for use with adult populations. There may be benefit in repeating this analysis in the future using newer risk functions such as those created for youth populations as well as sport-specific risk functions,10 and exploring the effects of different helmet types.11
The objective of this study was to ascertain the relationship between time-weighted cumulative HIE metrics and directional changes in DTI measures. The results indicate that time-weighted cumulative HIE metrics better relate to abnormal increases, and they tended to have stronger relationships with directional DTI metrics than risk-based metrics that do not consider time; however, number of impacts ranked the highest correlate in a majority of cases. For abnormal increases in DTI metrics, time-weighted HIE metrics rank higher than RWE for FA, MD, and CL. For abnormal decreases, time-weighted HIE metrics rank higher than RWE for CS. For both increases and decreases, time-weighted HIE metrics never rank higher than number of impacts for FA and CS. The findings of this study motivate further evaluation of time-weighted cumulative HIE metrics to understand the effects of repeated subconcussive head impacts. The clinical impact of this research lies in mitigating incidence of brain injury by identifying the underlying factors behind it. The biomechanical metrics use different methods of weighting the contribution of impacts. By computing and comparing their values with each other and with imaging metrics, it may be possible to determine which strategy best describes the relationship between repeated HIE and changes in clinical outcome measures. Additionally, by cross-referencing these results with literature informing determinants of HIE, new policies or procedures can be implemented to mitigate injury in the future.21,46 If the specific features of HIE that contribute to injury could be identified and understood, modifications could be made to how football is played or the protective equipment that is worn in order to reduce the risk of injury. Future studies should consider investigating in-season assessments to better understand the effects of time-weighted HIE on changes in the brain associated with directional DTI metrics.
ACKNOWLEDGMENTS
Funding was provided by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health via Grants R01NS094410 and R01NS082453. The authors would also like to thank Brian Tomblin as well as the youth football organizations, the Childress Institute for Pediatric Trauma, and the Wake Forest School of Medicine Dean’s Award for supporting this study.
REFERENCES
- 1.Alosco ML, Kasimis AB, Stamm JM, Chua AS, Baugh CM, Daneshvar DH, Robbins CA, Mariani M, Hayden J, Conneely S, Au R, Torres A, McClean MD, McKee AC, Cantu RC, Mez J, Nowinski CJ, Martin BM, Chaisson CE, Tripodis Y, and Stern RA. Age of first exposure to American football and long-term neuropsychiatric and cognitive outcomes. Transl. Psychiatry 7:e1236, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barber Foss KD, Yuan W, Diekfuss JA, Leach J, Meehan W, DiCesare CA, Solomon G, Schneider DK, MacDonald J, Dudley J, Cortes N, Galloway R, Halstead M, Walker G, and Myer GD. Relative head impact exposure and brain white matter alterations after a single season of competitive football: a pilot comparison of youth versus high school football. Clin. J. Sports Med 29:442–450, 2019. [DOI] [PubMed] [Google Scholar]
- 3.Bazarian JJ, Zhu T, Zhong J, Janigro D, Rozen E, Roberts A, Javien H, Merchant-Borna K, Abar B, and Blackman EG. Persistent, long-term cerebral white matter changes after sports-related repetitive head impacts. PLoS ONE 9:e94734, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beckwith JG, Greenwald RM, and Chu JJ. Measuring head kinematics in football: correlation between the head impact telemetry system and hybrid III headform. Ann. Biomed. Eng 40:237–248, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellamkonda S, Woodward SJ, Campolettano E, Gellner R, Kelley ME, Jones DA, Genemaras A, Beckwith JG, Greenwald RM, Maerlender AC, Rowson S, Duma SM, Urban JE, Stitzel JD, and Crisco JJ. Head impact exposure in practices correlates with exposure in games for youth football players. J Appl Biomech 34(5):354–360, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benedetti B, Charil A, Rovaris M, Judica E, Valsasina P, Sormani MP, and Filippi M. Influence of aging on brain gray and white matter changes assessed by conventional, MT, and DT MRI. Neurology 66:535–539, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Brett BL, Wu Y, Mustafi SM, Saykin AJ, Koch KM, Nencka AS, Giza CC, Goldman J, Guskiewicz KM, Mihalik JP, Duma SM, Broglio SP, Mcallister TW, Mccrea MA, and Meier TB. The association between persistent white-matter abnormalities and repeat injury after sport-related concussion. Front. Neurol 10:1–7, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Broglio SP, Eckner JT, Martini D, Sosnoff JJ, Kutcher JS, and Randolph C. Cumulative head impact burden in high school football. J Neurotrauma 28(10):2069–2078, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broglio SP, Lapointe A, O’Connor KL, and McCrea M. Head impact density: a model to explain the elusive concussion threshold. J. Neurotrauma 34:2675–2683, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campolettano ET, Gellner RA, Smith EP, Bellamkonda S, Tierney CT, Crisco JJ, Jones DA, Kelley ME, Urban JE, Stitzel JD, Genemaras A, Beckwith JG, Greenwald RM, Maerlender AC, Brolinson PG, Duma SM, and Rowson S. Development of a concussion risk function for a youth population using head linear and rotational acceleration. Ann. Biomed. Eng 48:92–103, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campolettano ET, Gellner RA, Sproule DW, Begonia MT, and Rowson S. Quantifying youth football helmet performance: assessing linear and rotational head acceleration. Ann. Biomed. Eng 48:1640–1650, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davenport EM, Apkarian K, Whitlow CT, Urban JE, Jensen JH, Szuch E, Espeland MA, Jung Y, Rosenbaum DA, Gioia GA, Powers AK, Stitzel JD, and Maldjian JA. Abnormalities in diffusional kurtosis metrics related to head impact exposure in a season of high school varsity football. J. Neurotrauma 33:2133–2146, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davenport EM, Whitlow CT, Urban JE, Espeland MA, Jung Y, Rosenbaum DA, Gioia GA, Powers AK, Stitzel JD, and Maldjian JA. Abnormal white matter integrity related to head impact exposure in a season of high school varsity football. J. Neurotrauma 31:1617–1624, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DiGuglielmo DM, Kelley ME, Espeland MA, Gregory ZA, Payne TD, Jones DA, Filben TM, Powers AK, Stitzel JD, and Urban JE. The Effect of Player Contact Characteristics on Head Impact Exposure in Youth Football Games. J. Appl. Biomech 37:145–155, 2021. [DOI] [PubMed] [Google Scholar]
- 15.Farrell JAD, Landman BA, Jones CK, Smith SA, Prince JL, Van Zijl PCM, and Mori S. Effects of signal-to-noise ratio on the accuracy and reproducibility of diffusion tensor imaging-derived fractional anisotropy, mean diffusivity, and principal eigenvector measurements at 1.5 T. J. Magn. Reson. Imaging 26:756–767, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia G-GP, Broglio SP, Lavieri MS, Mcallister T, and CARE Consortium Investigators. Quantifying the value of multidimensional assessment models for acute concussion: an analysis of data from the NCAA-DoD CARE Consortium. Sport Med 48:1739–1749, 2018. [DOI] [PubMed] [Google Scholar]
- 17.Gellner RA, Campolettano ET, Smith EP, and Rowson S. Are specific players more likely to be involved in high-magnitude head impacts in youth football? J. Neurosurg. Pediatr 24:47–53, 2019. [DOI] [PubMed] [Google Scholar]
- 18.Herrera JJ, Bockhorst K, Kondraganti S, Stertz L, Quevedo J, and Narayana PA. Acute white matter tract damage after frontal mild traumatic brain injury. J. Neurotrauma 34:291–299, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inglese M, Makani S, Johnson G, Cohen BA, Silver JA, Gonen O, and Grossman RI. Diffuse axonal injury in mild traumatic brain injury: a diffusion tensor imaging study. J. Neurosurg 103:298–303, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Jang I, Chun IY, Brosch JR, Bari S, Zou Y, Cummiskey BR, Lee TA, Lycke RJ, Poole VN, Shenk TE, Svaldi DO, Tamer GG, Dydak U, Leverenz LJ, Nauman EA, and Talavage TM. Every hit matters: white matter diffusivity changes in high school football athletes are correlated with repetitive head acceleration event exposure. NeuroImage Clin 24:101930, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelley ME, Espeland MA, Flood WC, Powers AK, Whitlow CT, Maldjian JA, Stitzel JD, and Urban JE. Comparison of head impact exposure in practice drills among multiple youth football teams. J. Neurosurg. Pediatr 23:381–389, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelley ME, Kane JM, Espeland MA, Miller LE, Powers AK, Stitzel JD, and Urban JE. Head impact exposure measured in a single youth football team during practice drills. J. Neurosurg. Pediatr 20:489–497, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelley ME, Urban JE, Jones DA, Davenport EM, Miller LE, Snively BM, Powers AK, Whitlow CT, Maldjian JA, and Stitzel JD. Analysis of longitudinal head impact exposure and white matter integrity in returning youth football players. J. Neurosurg. Pediatr 2021. 10.3171/2021.1.PEDS20586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelley ME, Urban JE, Miller LE, Jones DA, Espeland MA, Davenport EM, Whitlow CT, Maldjian JA, and Stitzel JD. Head impact exposure in youth football: comparing age- and weight-based levels of play. J. Neurotrauma 34:1939–1947, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klein AP, Tetzlaff JE, Bonis JM, Nelson LD, Mayer AR, Huber DL, Harezlak J, Mathews VP, Ulmer JL, Sinson GP, Nencka AS, Koch KM, Wu YC, Saykin AJ, DiFiori JP, Giza CC, Goldman J, Guskiewicz KM, Mihalik JP, Duma SM, Rowson S, Brooks A, Broglio SP, McAllister T, McCrea MA, and Meier TB. Prevalence of potentially clinically significant magnetic resonance imaging findings in athletes with and without sport-related concussion. J. Neurotrauma 36:1776–1785, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ling JM, Klimaj S, Toulouse T, and Mayer AR. A prospective study of gray matter abnormalities in mild traumatic brain injury. Neurology 81:2121–2127, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MacDonald CL, Dikranian K, Bayly P, Holtzman D, and Brody D. Diffusion tensor imaging reliably detects experimental traumatic axonal injury and indicates approximate time of injury. Neurobiol. Dis 27:11869–11876, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mayer AR, Ling J, Mannell MV, Gasparovic C, Phillips JP, Doezema D, Reichard R, and Yeo RA. A prospective diffusion tensor imaging study in mild traumatic brain injury. Neurology 74:643–650, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McAllister TW, Ford JC, Flashman LA, Maerlender A, Greenwald RM, Beckwith JG, Bolander RP, Tosteson TD, Turco JH, Raman R, and Jain S. Effect of head impacts on diffusivity measures in a cohort of collegiate contact sport athletes. Neurology. American Academy of Neurology 82(1):63–69, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mccrea M, Guskiewicz KM, Marshall SW, Barr W, Randolph C, Cantu RC, Onate JA, and Kelly JP. Acute effects and recovery time following concussion in collegiate football players. JAMA 290:2556–2563, 2003. [DOI] [PubMed] [Google Scholar]
- 31.McKee AC, Cantu RC, Nowinski CJ, Hedley-Whyte ET, Gavett BE, Budson AE, Santini VE, Lee H-S, Kubilus CA, and Stern RA. Chronic Traumatic Encephalopathy in Athletes: Progressive Tauopathy after repetitive head injury. J Neuropathol Exp Neurol 68(7):709–735, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Merchant-Borna K, Asselin P, Narayan D, Abar B, Jones CMC, and Bazarian JJ. Novel method of weighting cumulative helmet impacts improves correlation with brain white matter changes after one football season of subconcussive head blows. Ann. Biomed. Eng 44:3679–3692, 2016. [DOI] [PubMed] [Google Scholar]
- 33.Miller LE, Urban JE, Davenport EM, Powers AK, Whitlow CT, Maldjian JA, and Stitzel JD. Brain strain: computational model-based metrics for head impact exposure and injury correlation. Ann. Biomed. Eng 49:1083–1096, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller LE, Urban JE, Kelley ME, Powers AK, Whitlow CT, Maldjian JA, Rowson S, and Stitzel JD. Evaluation of brain response during head impact in youth athletes using an anatomically accurate finite element model. J. Neurotrauma 36:1561–1570, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller LE, Urban JE, and Stitzel JD. Estimation of 6 degrees-of-freedom accelerations from head impact telemetry system outputs for computational modeling. 2019, pp. 121–130. 10.1007/978-3-030-23073-9_8. [DOI]
- 36.Molina ISM, Salo RA, Abdollahzadeh A, Tohka J, Gröhn O, and Sierra A. In vivo diffusion tensor imaging in acute and subacute phases of mild traumatic brain injury in rats. eNeuro 7:1–18, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Montenigro PH, Alosco ML, Martin BM, Daneshvar DH, Mez J, Chaisson CE, Nowinski CJ, Au R, McKee AC, Cantu RC, McClean MD, Stern RA, and Tripodis Y. Cumulative head impact exposure predicts later-life depression, Apathy, Executive Dysfunction, and Cognitive impairment in former high school and college football players. J Neurotrauma. Mary Ann Liebert Inc 34(2):328–340, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murugesan G, Saghafi B, Davenport EM, Wagner B, Urban J, Kelley M, Jones D, Powers A, Whitlow C, Stitzel J, Maldjian J, and Montillo A. Single season changes in resting state network power and the connectivity between regions: distinguish head impact exposure level in high school and youth football players. Proc. SPIE Int. Soc. Opt. Eng 2018. 10.1117/12.2293199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Omalu BI, Hamilton RL, Kamboh MI, Dekosky ST, and Bailes J. Chronic traumatic encephalopathy (CTE) in a National Football League Player: Case report and emerging medicolegal practice questions Case report and emerging medicolegal practice questions. J. Forensic Nurs 6:40–46, 2010. [DOI] [PubMed] [Google Scholar]
- 40.Pan J, Connolly ID, Dangelmajer S, Kintzing J, Ho AL, and Grant G. Sports-related brain injuries: connecting pathology to diagnosis. Neurosurg. Focus 40:1–16, 2016. [DOI] [PubMed] [Google Scholar]
- 41.Papa L, Brophy GM, Welch RD, Lewis LM, Braga CF, Tan CN, Ameli NJ, Lopez MA, Haeussler CA, Mendez Giordano DI, Silvestri S, Giordano P, Weber KD, Hill-Pryor C, and Hack DC. Time course and diagnostic accuracy of glial and neuronal blood biomarkers GFAP and UCH-L1 in a large cohort of trauma patients with and without mild traumatic brain injury. JAMA Neurol 73:551–560, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rowson S, and Duma SM. Brain injury prediction: assessing the combined probability of concussion using linear and rotational head acceleration. Ann. Biomed. Eng 41:873–882, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stamm JM, Bourlas AP, Baugh CM, Fritts NG, Daneshvar DH, Martin BM, McClean MD, Tripodis Y, and Stern RA. Age of first exposure to football and later-life cognitive impairment in former NFL players. Neurology 84(11):1114–1120, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Talavage TM, Nauman EA, Breedlove EL, Yoruk U, Dye AE, Morigaki KE, Feuer H, and Leverenz LJ. Functionally-detected cognitive impairment in high school football players without clinically-diagnosed concussion. J Neurotrauma 31(4):327–338, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Urban JE, Davenport EM, Golman AJ, Maldjian JA, Whitlow CT, Powers AK, and Stitzel JD. Head impact exposure in youth football: high school ages 14 to 18 years and cumulative impact analysis. Ann. Biomed. Eng 41:2474–2487, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Urban JE, Flood WC, Zimmerman BJ, Kelley ME, Espeland MA, McNamara L, Davenport EM, Powers AK, Whitlow CT, Maldjian JA, and Stitzel JD. Evaluation of head impact exposure measured from youth football game plays. J. Neurosurg. Pediatr 2019. 10.3171/2019.2.PEDS18558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Urban JE, Kelley ME, Espeland MA, Davenport EM, Whitlow CT, Powers AK, Maldjian JA, and Stitzel JD. In-season variations in head impact exposure among youth football players. J. Neurotrauma 36:275–281, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]


