Abstract
Many recent phylogenetic methods have focused on accurately inferring species trees when there is gene tree discordance due to incomplete lineage sorting (ILS). For almost all of these methods, and for phylogenetic methods in general, the data for each locus are assumed to consist of orthologous, single-copy sequences. Loci that are present in more than a single copy in any of the studied genomes are excluded from the data. These steps greatly reduce the number of loci available for analysis. The question we seek to answer in this study is: what happens if one runs such species tree inference methods on data where paralogy is present, in addition to or without ILS being present? Through simulation studies and analyses of two large biological data sets, we show that running such methods on data with paralogs can still provide accurate results. We use multiple different methods, some of which are based directly on the multispecies coalescent model, and some of which have been proven to be statistically consistent under it. We also treat the paralogous loci in multiple ways: from explicitly denoting them as paralogs, to randomly selecting one copy per species. In all cases, the inferred species trees are as accurate as equivalent analyses using single-copy orthologs. Our results have significant implications for the use of ILS-aware phylogenomic analyses, demonstrating that they do not have to be restricted to single-copy loci. This will greatly increase the amount of data that can be used for phylogenetic inference.[Gene duplication and loss; incomplete lineage sorting; multispecies coalescent; orthology; paralogy.]
Species tree inference often requires us to account for the fact that the evolutionary histories of different loci can disagree with each other, as well as with the phylogeny of the species. The reasons for this incongruence include biological causes such as incomplete lineage sorting (ILS) and introgression (broadly interpreted to include all biological processes involving genetic exchange), as well as technical causes such as the misidentification of paralogs as orthologs (“hidden paralogy”; Doolittle and Brown 1994).
The inference of phylogenies can be carried out by concatenating all loci together or by treating each locus separately (reviewed in Bryant and Hahn 2020). While concatenation ignores incongruence, gene tree-based methods allow each locus to take on its own topology. Some gene tree-based methods rely on a model for how these trees evolve within the species phylogeny (in addition to probabilistic models of sequence evolution on the gene trees). The multispecies coalescent (MSC) (Hudson 1983; Takahata 1989; Rannala and Yang 2003; Degnan and Rosenberg 2009) has emerged as the most commonly employed model of such gene genealogies. Indeed, in the last two decades a wide array of methods and computer programs have been developed for species tree inference under the MSC; see Liu et al. (2009), Knowles and Kubatko (2011), Nakhleh (2013), and Liu et al. (2015) for recent reviews and surveys of these methods. Other gene tree-based methods are inspired by the MSC, but do not rely explicitly on this model (e.g., Mirarab et al. 2014). In either case, the goal is for the methods to be robust to incongruence caused by ILS.
Regardless of the method being employed, the inference of species trees usually assumes that the data consist of only orthologous sequences. Indeed, most phylogenetic methods require the identification of orthologs; see Smith and Hahn (2021b) for a review of methods that do not require orthologs. As a result of the common requirement of orthologous loci, before such inference methods are applied to a phylogenomic data set paralogs must be identified and removed from the data. One common approach for removing paralogs is to use graph-based methods to identify homologous gene families, and then to use those gene families present in exactly a single copy in each sampled genome for phylogenetic inference (e.g., Li et al. 2003). Another approach is to use branch-cutting methods to extract orthologs from larger gene families (e.g., Yang and Smith 2014). Neither of these two approaches guarantees that the resulting data set includes only orthologous sequences (Koonin 2005). Furthermore, restricting the data to single-copy genes—which is by far the most common practice in the community—means that much of the data must be excluded from the analysis. In particular, as more species are sampled, the frequency of genes that are present in single-copy across all species will decrease (Emms and Kelly 2018).
Paralogous sequences are often modeled by a process of gene duplication and loss (GDL) (Boussau et al. 2013). This process can also produce incongruence, as every duplication event adds a single branch not found in the species tree (losses cannot generate incongruence). Although the MSC generates a distribution of gene trees due to ILS, it is likely that GDL models induce a distribution that differs from this. An obvious way to handle data sets where ILS and GDL could have simultaneously acted on gene families is to employ models of gene evolution that go beyond the MSC in order to incorporate GDL as well. Indeed, such models are beginning to emerge (Rasmussen and Kellis 2012; Li et al. 2021). However, the more complex the models of gene family evolution, the more computationally prohibitive statistical inference under these models becomes (Du and Nakhleh 2018), rendering their applicability infeasible except for very small data sets in terms of the number of species and gene families.
Given that much progress in terms of accuracy and computational efficiency has been made
on gene tree-based, ILS-aware species tree inference methods, we ask in this paper the
following question: are these inference methods robust to the presence of paralogs in
the data? If they are, then the reach of gene tree-based inference methods is
significantly extended and the exclusion of paralogous loci from phylogenomic data sets
is deemed unnecessary, thus providing more signal for the inference task. To answer this
question, we study the performance of five species tree inference methods, all of which
use gene trees as the input data: The maximum pseudolikelihood method of Yu and Nakhleh (2015) as implemented by the
function  in PhyloNet
(Wen et al. 2018), ASTRAL-III (Zhang et al. 2018), NJ
in PhyloNet
(Wen et al. 2018), ASTRAL-III (Zhang et al. 2018), NJ (Liu and Yu 2011), ASTRAL-Pro (Zhang
et al. 2020), and FastMulRFS (Molloy and
Warnow 2020). The latter two methods were developed with paralogs in mind,
and so should serve as a good baseline for comparison to the MSC-inspired methods. In
particular, ASTRAL-Pro makes use of counts of quartets from speciation, but not
duplication, events. Thus, there is a connection between the ASTRAL-Pro method and
orthology detection.
(Liu and Yu 2011), ASTRAL-Pro (Zhang
et al. 2020), and FastMulRFS (Molloy and
Warnow 2020). The latter two methods were developed with paralogs in mind,
and so should serve as a good baseline for comparison to the MSC-inspired methods. In
particular, ASTRAL-Pro makes use of counts of quartets from speciation, but not
duplication, events. Thus, there is a connection between the ASTRAL-Pro method and
orthology detection.
To test these methods, we use both simulated and real data. We simulate across a wide range of GDL rates and levels of ILS, and use two genome-scale empirical data sets with thousands of loci that contain branches with very different levels of discordance. We also sample the gene family data in multiple ways, in all cases finding that the inferences made by all methods are quite accurate and are mostly identical to the accuracy of the inferences when using only single-copy orthologs. Particularly striking is the finding that these methods infer very accurate species trees when all gene tree incongruence is due to GDL, and ILS is not a factor. We find that gene tree estimation error affects the methods’ performances at a similar, or even higher, level than ILS. We also find that methods designed specifically to take GDL into account, namely ASTRAL-Pro and FastMulRFS, do not generally have higher accuracy than the other methods. Overall, our results support the use of approaches that account for gene tree incongruence, regardless of its causes.
Methods
Species Tree Inference Methods
For species tree inference, we use five different methods. The first three assume that the input data come from single-copy genes:
The maximum pseudolikelihood inference function
 in PhyloNet, which implements the method of Yu and Nakhleh (2015). This method amounts to
running MP-EST (Liu et al. 2010)
when restricted to trees with no reticulations.
in PhyloNet, which implements the method of Yu and Nakhleh (2015). This method amounts to
running MP-EST (Liu et al. 2010)
when restricted to trees with no reticulations.ASTRAL-III (Zhang et al. 2018; Rabiee et al. 2019), Version 5.6.3.
NJ
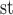 (Liu and Yu 2011).
(Liu and Yu 2011).
While the maximum likelihood method of Yu et al.
(2014) as implemented by the 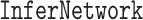 function in PhyloNet (Wen et al. 2018) is
relevant here, it is much more computationally demanding than maximum
pseudolikelihood, so we chose not to run it.
function in PhyloNet (Wen et al. 2018) is
relevant here, it is much more computationally demanding than maximum
pseudolikelihood, so we chose not to run it.
For comparison, we also use two methods that were designed specifically with paralogs in mind:
ASTRAL-Pro (Zhang et al. 2020).
FastMulRFS (Molloy and Warnow 2020).
For the sake of conclusions that we draw from this study, it may be helpful to
highlight the differences between these methods. 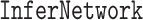 optimizes a pseudolikelihood function that is derived based on the assumptions
of the MSC. This function is very different, for example, from a likelihood
function based on a model of gene duplication and loss (Arvestad et al. 2009). Therefore, its accuracy in inferring
species trees from data with paralogs reflects directly on the performance of
MSC-based methods on such data. None of the other four methods make direct use
of the MSC, though ASTRAL, ASTRAL-Pro, and NJ
optimizes a pseudolikelihood function that is derived based on the assumptions
of the MSC. This function is very different, for example, from a likelihood
function based on a model of gene duplication and loss (Arvestad et al. 2009). Therefore, its accuracy in inferring
species trees from data with paralogs reflects directly on the performance of
MSC-based methods on such data. None of the other four methods make direct use
of the MSC, though ASTRAL, ASTRAL-Pro, and NJ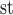 have all been shown to
be statistically consistent under the MSC, at least when both gene lengths and
the number of genes go to infinity. Their accuracy on data with paralogs
therefore reflects the suitability of these methods, rather than the MSC itself,
for analyzing such data. Legried et al.
(2021) proved that ASTRAL-ONE and ASTRAL-multi are statistically
consistent under the GDL model of Arvestad et al.
(2009), whereas Markin and Eulenstein
(2021) and Hill et al. (2020)
proved that ASTRAL-ONE and ASTRAL-multi are statistically consistent under the
unified GDL/ILS model (the DLCoal model) of Rasmussen and Kellis (2012). ASTRAL-Pro is conjectured to be
statistically consistent under the DLCoal model (Zhang et al. 2020). FastMulRFS has been proven to be statistically
consistent under a model of either only duplication or only loss (Molloy and Warnow 2020).
have all been shown to
be statistically consistent under the MSC, at least when both gene lengths and
the number of genes go to infinity. Their accuracy on data with paralogs
therefore reflects the suitability of these methods, rather than the MSC itself,
for analyzing such data. Legried et al.
(2021) proved that ASTRAL-ONE and ASTRAL-multi are statistically
consistent under the GDL model of Arvestad et al.
(2009), whereas Markin and Eulenstein
(2021) and Hill et al. (2020)
proved that ASTRAL-ONE and ASTRAL-multi are statistically consistent under the
unified GDL/ILS model (the DLCoal model) of Rasmussen and Kellis (2012). ASTRAL-Pro is conjectured to be
statistically consistent under the DLCoal model (Zhang et al. 2020). FastMulRFS has been proven to be statistically
consistent under a model of either only duplication or only loss (Molloy and Warnow 2020).
Given a collection of trees corresponding to gene families (one tree per gene family), we generated four types of input to each of the methods:
ONLY: The input consists of trees of only gene families that are present in exactly one copy in each of the species.
ONLY-NoDup: The input consists of trees of ONLY gene families that have no history of gene duplication. These are canonical single-copy orthologs.
ONE: The input consists of trees of all gene families, but where a single copy per species per gene family is selected at random and the remaining copies are removed. If a gene family has no copies at all for some species, then the resulting tree of that gene family also has no copies for that species.
ALL: The input consists of trees of all gene families, but where all copies of a gene in a species are treated as multiple alleles from different individuals within the species. Similar to ONE, if a gene family has no copies at all for some species, then the resulting tree of that gene family also has no copies for that species.
ONLY corresponds to the practice that is followed in many phylogenomic studies,
though it does not necessarily guarantee that the included genes are orthologs.
Instead, “hidden paralogs” (Doolittle and Brown 1994) or “pseudoorthologs” (Koonin 2005) may occur: these are cases in
which complementary losses result in single-copy paralogs present in different
species. ONLY-NoDup corresponds to a scenario where researchers know which genes
have a history of duplication and can exclude them from their analysis. ONE is
likely to have some hidden paralogs in the input, unless GDL does not occur. By
construction, ALL has all orthologs and paralogs as input, but these are
effectively labeled as orthologs with multiple individuals sampled per species,
since 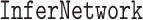 ,
ASTRAL-III, and NJ
,
ASTRAL-III, and NJ were not originally
developed with paralogs in mind.
were not originally
developed with paralogs in mind.
Simulation Setup
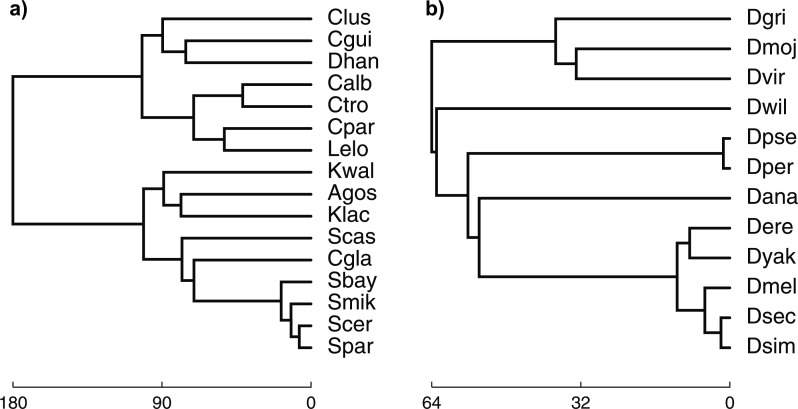
For model species trees, we used the trees of 16 fungal species and 12 fly species reported in Rasmussen and Kellis (2012) and shown in Figure 1. The 16 fungal species are: Candida albicans (Calb), Candida tropicalis (Ctro), Candida parapsilosis (Cpar), Lodderomyces elongisporus (Lelo), Candida guilliermondii (Cgui), Debaryomyces hansenii (Dhan), Candida lusitaniae (Clus), Saccharomyces cerevisiae (Scer), Saccharomyces paradoxus (Spar), Saccharomyces mikatae (Smik), Saccharomyces bayanus (Sbay), Candida glabrata (Cgla), Saccharomyces castellii (Scas), Kluyveromyces lactis (Klac), Ashbya gossypii (Agos), and Kluyveromyces waltii (Kwal). Note that Saccharomyces castellii has since been renamed Naumovozyma castellii (https://www.uniprot.org/taxonomy/27288), Kluyveromyces waltii has since been renamed Lachancea waltii (https://www.uniprot.org/taxonomy/1089441), and Ashbya gossypii has been renamed Eremothecium gossypii (https://www.uniprot.org/taxonomy/33169).
Figure 1.
The species trees reported in Rasmussen and Kellis (2012), which we use as the topologies in the simulations and in the empirical data analysis. a) The species tree of 16 fungal species. b) The species tree of 12 fly species. The species tree topologies and their branch lengths in units of million years are taken from http://compbio.mit.edu/dlcoal/.
The 12 fly species are: Drosophila melanogaster (Dmel), Drosophila simulans (Dsim), Drosophila sechellia (Dsec), Drosophila erecta (Dere), Drosophila yakuba (Dyak), Drosophila ananassae (Dana), Drosophila pseudoobscura (Dpse), Drosophila persimilis (Dper), Drosophila willistoni (Dwil), Drosophila mojavensis (Dmoj), Drosophila virilis (Dvir), and Drosophila grimshawi (Dgri).
To generate gene trees while allowing for ILS and GDL, we used SimPhy (Mallo et al. 2015) with the parameters
specified below (assuming all species are diploid). SimPhy uses the three-tree model
developed in Rasmussen and Kellis (2012) to
simulate data. In this model, a locus tree is simulated within the
branches of the species tree. All incongruence between the locus tree and the
species tree is due to GDL. Then, a gene tree is simulated within
the branches of the locus tree, where all incongruence between the locus tree and
the gene tree is due to ILS. The resulting gene tree differs from the species tree
due to a combination of ILS and GDL. Using the locus trees as input to an inference
method amounts to using data where all incongruence is solely due to GDL (but not
ILS). Setting the rates of GDL to 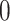 amounts to
generating gene trees where all incongruence is solely due to ILS. Note that SimPhy
makes two further assumptions relevant to the results presented here: first, it
assumes no hemiplasy of new duplication mutations. That is, all new duplicates
immediately fix before they can be lost during a polymorphic phase. Rasmussen and Kellis (2012) found that this
assumption affected 5% of gene families simulated under similar conditions.
Furthermore, hemiplasy results in an excess of apparent gene losses, which should
not affect inferences of species trees. The second assumption is that all gene
families are independent: no events duplicate or delete more than a single gene at a
time. In real data, large-scale events (including whole-genome duplications) can
affect many genes at a time.
amounts to
generating gene trees where all incongruence is solely due to ILS. Note that SimPhy
makes two further assumptions relevant to the results presented here: first, it
assumes no hemiplasy of new duplication mutations. That is, all new duplicates
immediately fix before they can be lost during a polymorphic phase. Rasmussen and Kellis (2012) found that this
assumption affected 5% of gene families simulated under similar conditions.
Furthermore, hemiplasy results in an excess of apparent gene losses, which should
not affect inferences of species trees. The second assumption is that all gene
families are independent: no events duplicate or delete more than a single gene at a
time. In real data, large-scale events (including whole-genome duplications) can
affect many genes at a time.
For the fungal tree simulated data sets, we used five different duplication and loss
rates (assuming duplication and loss rates are equal):  (to investigate the performance
when ILS, but not GDL, acted on the gene families),
(to investigate the performance
when ILS, but not GDL, acted on the gene families), 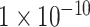 ,
,
 ,
,
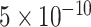 , and
, and
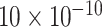 per
generation. We take the case where the rate is
per
generation. We take the case where the rate is  to be similar
similar to the duplication rate of
to be similar
similar to the duplication rate of 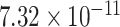 and loss rate of
and loss rate of
 used by
Rasmussen and Kellis (2011), and denote
this rate as “1
used by
Rasmussen and Kellis (2011), and denote
this rate as “1 ”. We used two
effective population sizes:
”. We used two
effective population sizes:  and
and
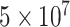 , where the former
was also used by Rasmussen and Kellis (2012)
as the true population size. We assumed 0.9 years per generation as in Rasmussen and Kellis (2012) and used
, where the former
was also used by Rasmussen and Kellis (2012)
as the true population size. We assumed 0.9 years per generation as in Rasmussen and Kellis (2012) and used
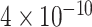 as the
nucleotide mutation rate per site per generation, similar to the rates of
as the
nucleotide mutation rate per site per generation, similar to the rates of
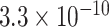 and
and
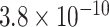 used by Zhang and Wu (2017) and Lang and Murray (2008), respectively.
used by Zhang and Wu (2017) and Lang and Murray (2008), respectively.
For the fly tree simulated data sets, we used five different duplication and loss
rates (assuming duplication and loss rates are equal):  ,
, 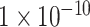 ,
,
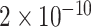 ,
,
 , and
, and
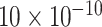 per
generation. A GDL rate of
per
generation. A GDL rate of 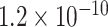 was used in Rasmussen and Kellis (2012); Zhang and Wu (2017) and reported by Hahn et al. (2007); we again denote this rate
as “1
was used in Rasmussen and Kellis (2012); Zhang and Wu (2017) and reported by Hahn et al. (2007); we again denote this rate
as “1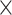 ”. We used two
effective population sizes:
”. We used two
effective population sizes:  and
and
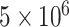 , similar to the
values used in Rasmussen and Kellis (2012)
and the estimated value of
, similar to the
values used in Rasmussen and Kellis (2012)
and the estimated value of 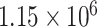 reported in Sawyer and Hartl (1992) and Pollard et al. (2006). We assumed 10
generations per year as in Rasmussen and Kellis
(2012) and Zhang and Wu (2017) and
used
reported in Sawyer and Hartl (1992) and Pollard et al. (2006). We assumed 10
generations per year as in Rasmussen and Kellis
(2012) and Zhang and Wu (2017) and
used 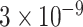 as the mutation
rate per site per generation, similar to the rate of
as the mutation
rate per site per generation, similar to the rate of 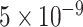 found in Schrider et al. (2013).
found in Schrider et al. (2013).
For each combination of GDL rate and population size, 10,000 gene families (each containing a locus tree and its corresponding gene tree) were simulated in this fashion as one data set. Ten such data sets, each with 10,000 gene families, were generated for each condition. To study the effect of using data sets of varying sizes, for each of the 10 data sets we randomly sampled 10, 50, 100, and 250 gene families from the 10,000 gene families under the ALL, ONE, ONLY, and ONLY-NoDup scenarios. In case the number of available gene families that fits ONLY or ONLY-NoDup is smaller than the desired size, that number of gene families was used (e.g., when only 6 gene family trees are available when data sets of size 10 are desired, the 6 trees are used as input).
To study the effect of GDL and ILS on species tree estimates, for each data set of
trees (true gene trees or true locus trees; i.e., trees without estimation error) of
a given size, we fed the data set as input to 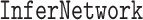 ,
ASTRAL, NJ
,
ASTRAL, NJ , ASTRAL-Pro, and
FastMulRFS and computed the Robinson–Foulds distance (Robinson and Foulds 1981), normalized by the number of internal
branches in the (unrooted) species tree to obtain a value between 0 and 1. This is
the normalized distance between the true and inferred species trees. To study the
further effect of error in the gene tree estimates on species tree estimates, we
simulated the evolution of sequences of length 500 nucleotides on all gene trees
under the HKY model, using Seq-gen (Rambaut and
Grassly 1997). We then inferred gene trees from the simulated sequence
data using IQ-TREE (Nguyen et al. 2014).
Furthermore, to study the effect of error in the locus tree estimates, we treated
the true locus tree as a gene tree and simulated the evolution of sequences of
length 500 nucleotides on all locus trees under the HKY model, again using Seq-gen,
and inferred locus trees from the simulated sequence data using IQ-TREE. It is
important to note that in practice only gene trees, but not locus trees, are
inferrable, as the locus tree is an artifact of the three-tree model and not a
biological entity (Rasmussen and Kellis
2012). However, conducting analysis using inferred locus trees gives a
picture of the performance when all incongruence is due to GDL and gene tree error
only. Finally,
, ASTRAL-Pro, and
FastMulRFS and computed the Robinson–Foulds distance (Robinson and Foulds 1981), normalized by the number of internal
branches in the (unrooted) species tree to obtain a value between 0 and 1. This is
the normalized distance between the true and inferred species trees. To study the
further effect of error in the gene tree estimates on species tree estimates, we
simulated the evolution of sequences of length 500 nucleotides on all gene trees
under the HKY model, using Seq-gen (Rambaut and
Grassly 1997). We then inferred gene trees from the simulated sequence
data using IQ-TREE (Nguyen et al. 2014).
Furthermore, to study the effect of error in the locus tree estimates, we treated
the true locus tree as a gene tree and simulated the evolution of sequences of
length 500 nucleotides on all locus trees under the HKY model, again using Seq-gen,
and inferred locus trees from the simulated sequence data using IQ-TREE. It is
important to note that in practice only gene trees, but not locus trees, are
inferrable, as the locus tree is an artifact of the three-tree model and not a
biological entity (Rasmussen and Kellis
2012). However, conducting analysis using inferred locus trees gives a
picture of the performance when all incongruence is due to GDL and gene tree error
only. Finally,  assumes
that the input gene trees are rooted. In this study, we rooted the gene tree
estimates by minimizing deep coalescences (Maddison
1997; Than and Nakhleh 2009); that
is, we rooted each gene tree in a way that minimizes the number of extra lineages
when reconciled with the true species tree.
assumes
that the input gene trees are rooted. In this study, we rooted the gene tree
estimates by minimizing deep coalescences (Maddison
1997; Than and Nakhleh 2009); that
is, we rooted each gene tree in a way that minimizes the number of extra lineages
when reconciled with the true species tree.
Biological Data
For the fungal data set, we used 2932 gene trees reported in http://compbio.mit.edu/dlcoal/ and estimated with PhyML (Guindon and Gascuel 2003), where 1867 gene
trees fit the ONLY setting. For the fly data set, we used 9233 gene trees from Hahn et al. (2007) reconstructed using the
neighbor-joining algorithm, where 6698 gene trees fit the ONLY setting. For the fly
data set, we removed any gene trees containing polytomies prior to running
NJ . In neither data set
did we attempt to identify single-copy orthologs. We again rooted each gene tree in
the empirical data with respect to the species trees of Figure 1 so as to minimize deep coalescences (Maddison 1997; Than and Nakhleh 2009) using the method of Yu et al. (2011), as implemented by the function
. In neither data set
did we attempt to identify single-copy orthologs. We again rooted each gene tree in
the empirical data with respect to the species trees of Figure 1 so as to minimize deep coalescences (Maddison 1997; Than and Nakhleh 2009) using the method of Yu et al. (2011), as implemented by the function
 in PhyloNet
(Wen et al. 2018). We estimated species
trees using ASTRAL, NJ
in PhyloNet
(Wen et al. 2018). We estimated species
trees using ASTRAL, NJ , maximum
pseudolikelihood, ASTRAL-Pro, and FastMulRFS with these gene trees as input.
, maximum
pseudolikelihood, ASTRAL-Pro, and FastMulRFS with these gene trees as input.
Results
Characteristics of the Simulated Data
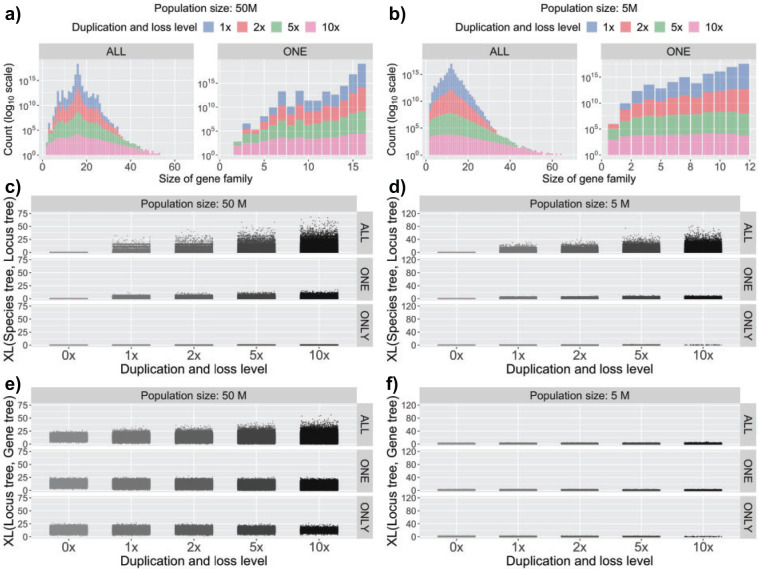
Before we describe the inference results, we discuss the characteristics of the simulated data. First, we investigated the effects of gene duplication and loss on the number of gene copies per species in each gene family. Figure 2a,b and Supplementary Figure S1a,b available on Dryad at https://doi.org/10.5061/dryad.t76hdr81d show data on the sizes (numbers of copies) of gene families in the 16-taxon and 12-taxon data sets, respectively, under the various settings of effective population sizes and duplication and loss rates.
Figure 2.
Characteristics of the simulated data under different settings of the
duplication/loss rates and tree topologies. The duplication/loss rates
are denoted by the rate multiplier (0 ,
1
,
1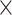 ,
2
,
2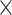 ,
5
,
5 and
10
and
10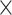 ), where
1
), where
1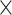 is the rate
found in nature for the clade represented by each species tree topology
(see Methods section). a,b) Distribution of the total number of gene
copies in individual gene families in the 16-taxon and 12-taxon data
sets, respectively. Note that the two tree topologies also have
different simulated effective population sizes in these figures (see
Supplementary
Fig. S1a,b available on Dryad for more conditions). c,d)
Scatter plots of XL (Species tree, Locus tree), the number of extra
lineages when reconciling the true locus trees with the true species
tree, for the 16-taxon and 12-taxon data sets, respectively. These plots
therefore represent the effects of GDL alone. e,f) Scatter plots of XL
(Locus tree, Gene tree), the number of extra lineages when reconciling
the true gene trees with the true locus tree, for the 16-taxon and
12-taxon data sets, respectively. These plots therefore represent the
effects of ILS alone, though note that higher rates of GDL allow there
to be more gene tree branches on which ILS can act.
is the rate
found in nature for the clade represented by each species tree topology
(see Methods section). a,b) Distribution of the total number of gene
copies in individual gene families in the 16-taxon and 12-taxon data
sets, respectively. Note that the two tree topologies also have
different simulated effective population sizes in these figures (see
Supplementary
Fig. S1a,b available on Dryad for more conditions). c,d)
Scatter plots of XL (Species tree, Locus tree), the number of extra
lineages when reconciling the true locus trees with the true species
tree, for the 16-taxon and 12-taxon data sets, respectively. These plots
therefore represent the effects of GDL alone. e,f) Scatter plots of XL
(Locus tree, Gene tree), the number of extra lineages when reconciling
the true gene trees with the true locus tree, for the 16-taxon and
12-taxon data sets, respectively. These plots therefore represent the
effects of ILS alone, though note that higher rates of GDL allow there
to be more gene tree branches on which ILS can act.
Clearly, the higher the GDL rates, the larger the variance in size of gene families. The figure also shows that the average size of a gene family is roughly equal to the number of species, with the largest gene families having 65 copies for the 16-taxon data sets, and 94 copies for the 12-taxon data sets (recall that these trees use different rates of GDL). We then counted the average (over the 10 data sets per setting) number of gene families for each setting that have ONLY one copy per species and the average number of gene families with no history of duplication (i.e., ONLY-NoDup). The results are shown in Table 1. The table shows that as the GDL rates increase, the number of single-copy orthologs decreases. However, as predicted by theory (Smith and Hahn 2021a), there appear to be very few pseudoortholog in the ONLY data set.
Table 1.
The average number of gene families that fit the ONLY/ONLY-NoDup settings out of the 10,000 gene families.
| 16-taxon data | 12-taxon data | |||
|---|---|---|---|---|
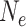
|
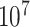
|
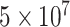
|
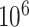
|
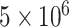
|
| GDL rate | ||||
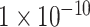
|
7619/7616 | 7585/7583 | 4591/4554 | 4584/4550 |
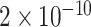
|
5794/5782 | 5787/5775 | 2197/2131 | 2176/2111 |
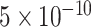
|
2554/2521 | 2538/2508 | 268/226 | 266/222 |

|
689/659 | 688/657 | 12/6 | 13/7 |
We then set out to assess the extent of incongruence in the gene trees due to GDL
and ILS. For every pair of true species tree and true locus tree, we computed
the number of extra lineages (Maddison
1997) using the 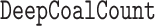 command in PhyloNet (Than and Nakhleh
2009; Wen et al. 2018) as a
proxy for the amount of incongruence in the data. Here, we treated all gene
copies from the same species as different individuals. Zero extra lineages mean
there is no incongruence between the two trees, and the higher the value, the
more incongruence there is. In particular, no incongruence means that all gene
copies from the same species are monophyletic in the locus tree, and when
restricted to a single arbitrary copy per species, the locus tree and species
tree have identical topologies.
command in PhyloNet (Than and Nakhleh
2009; Wen et al. 2018) as a
proxy for the amount of incongruence in the data. Here, we treated all gene
copies from the same species as different individuals. Zero extra lineages mean
there is no incongruence between the two trees, and the higher the value, the
more incongruence there is. In particular, no incongruence means that all gene
copies from the same species are monophyletic in the locus tree, and when
restricted to a single arbitrary copy per species, the locus tree and species
tree have identical topologies.
Figure 2c,d and Supplementary Figure S1c,d available on Dryad show data on the
number of extra lineages in the simulated 16-taxon and 12-taxon data sets,
respectively, under the various settings of effective population sizes and
duplication and loss rates. It is important to note that all incongruence in
this case is exclusively due to GDL (ILS is not a factor in the results in these
two panels). The panels do not have results for the GDL rate of
0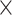 , because in such cases
there is no incongruence at all between the locus tree and the species tree, and
thus there are zero extra lineages. The results show that, unsurprisingly, there
is much more incongruence for the ALL scenario than the ONE scenario. For the
ONLY scenario, there is very little incongruence in either data set. The
incongruence in ONLY would indicate the phenomenon of hidden paralogy:
single-copy genes are paralogs, so that their gene trees do not always agree
with the species tree. Given the small number of hidden paralogs (Table 1),
these results are unsurprising. The ONLY-NoDup data sets are not plotted,
because the number of extra lineages in those locus trees is always zero, as
expected.
, because in such cases
there is no incongruence at all between the locus tree and the species tree, and
thus there are zero extra lineages. The results show that, unsurprisingly, there
is much more incongruence for the ALL scenario than the ONE scenario. For the
ONLY scenario, there is very little incongruence in either data set. The
incongruence in ONLY would indicate the phenomenon of hidden paralogy:
single-copy genes are paralogs, so that their gene trees do not always agree
with the species tree. Given the small number of hidden paralogs (Table 1),
these results are unsurprising. The ONLY-NoDup data sets are not plotted,
because the number of extra lineages in those locus trees is always zero, as
expected.
We also computed the number of extra lineages when reconciling the true gene trees with the true locus trees. Here, incongruence is exclusively due to ILS (GDL is not a factor). Figure 2e,f and Supplementary Figure S1e,f available on Dryad show data on the number of extra lineages in the simulated 16-taxon and 12-taxon data sets, respectively, under the various settings of effective population sizes and duplication and loss rates. When the gene tree topology is identical to the locus tree topology, the number of extra lineages is zero, and the larger the number of extra lineages, the more ILS has an effect on the data. The figure shows that, as expected, the amount of ILS is larger for larger population sizes, and consequently there is much more ILS in the 16-taxon data set than in the 12-taxon data set. One other trend to observe is that, on average, the amount of incongruence due to ILS increases with the increase in the GDL rate. This is a reflection of the fact that for higher GDL rates, the locus trees are larger (more leaves and internal branches), and this naturally results in more branches that can be affected by ILS. Finally, the amount of incongruence due to ILS is generally far lower than the amount due to GDL in the 12-taxon data set, while the levels of incongruence due to GDL and ILS are similar in the 16-taxon data set, especially when the rates of duplication and loss are high.
Results on Simulated Data
We are now in position to describe the inference results. We show figures for the 16-taxon data sets in the main text, while figures for the 12-taxon data sets are all in the Supplementary Figs. S8–S11 available on Dryad. The results for the 12-taxon data sets are consistently better in terms of accuracy, so we chose to focus here on the less-optimal results.
We first ran the inference methods ASTRAL, 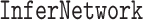 ,
NJ
,
NJ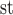 , ASTRAL-Pro, and
FastMulRFS on the true gene trees for all four input scenarios: ALL, ONE, ONLY,
and ONLY-NoDup. In this case, gene tree estimation error is not a cause of gene
tree incongruence. Instead, all incongruence is due to a combination of ILS and
GDL. Results on the full 16-taxon tree are shown in Figure 3 and Supplementary Figure
S4 available on Dryad. Note that, in all cases, using input data
with GDL levels of 0 amounts to inferring a species tree from gene trees whose
incongruence is solely due to ILS.
, ASTRAL-Pro, and
FastMulRFS on the true gene trees for all four input scenarios: ALL, ONE, ONLY,
and ONLY-NoDup. In this case, gene tree estimation error is not a cause of gene
tree incongruence. Instead, all incongruence is due to a combination of ILS and
GDL. Results on the full 16-taxon tree are shown in Figure 3 and Supplementary Figure
S4 available on Dryad. Note that, in all cases, using input data
with GDL levels of 0 amounts to inferring a species tree from gene trees whose
incongruence is solely due to ILS.
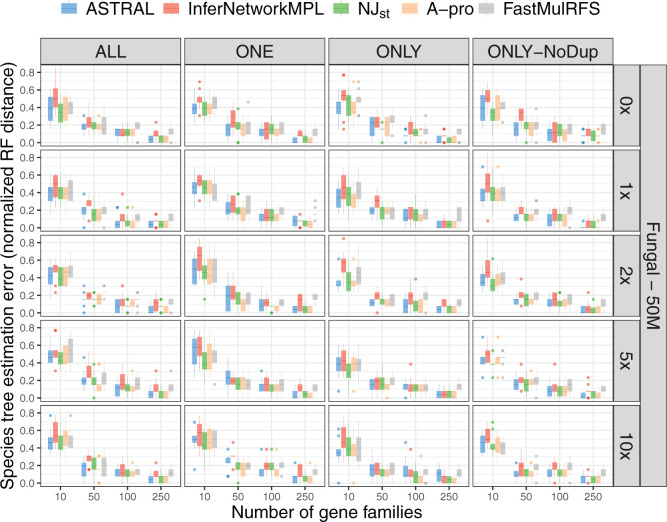
Figure 3.
Species tree estimation error for data simulated from the 16-taxon fungal
tree with a population size of 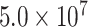 and
varying GDL rates; note that simulations include the effects of both ILS
and GDL (but no gene tree estimation error). Species tree estimation
error was measured as the normalized RF distance between the true
species tree and the ones inferred from true gene trees. The five
inference methods used are ASTRAL,
and
varying GDL rates; note that simulations include the effects of both ILS
and GDL (but no gene tree estimation error). Species tree estimation
error was measured as the normalized RF distance between the true
species tree and the ones inferred from true gene trees. The five
inference methods used are ASTRAL, 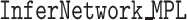 ,
NJ
,
NJ , ASTRAL-Pro
(“A-pro”), and FastMulRFS. The duplication/loss rates are
denoted by the rate multiplier (0
, ASTRAL-Pro
(“A-pro”), and FastMulRFS. The duplication/loss rates are
denoted by the rate multiplier (0 ,
1
,
1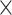 ,
2
,
2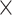 ,
5
,
5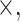 and
10
and
10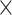 ), where
1
), where
1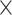 is the rate
estimated in nature for fungi. Each row corresponds to a combination of
population size and GDL rates. The X-axis in each panel represents the
number of gene families used and the Y-axis represents the normalized RF
distance.
is the rate
estimated in nature for fungi. Each row corresponds to a combination of
population size and GDL rates. The X-axis in each panel represents the
number of gene families used and the Y-axis represents the normalized RF
distance.
There are several observations based on these results. First, the accuracy of the inferred 16-taxon trees is much lower in general than that of the inferred 12-taxon trees. In particular, for the 12-taxon data sets, the species trees are perfectly estimated in almost all cases (Supplementary Fig. S3 available on Dryad), whereas the species tree estimation error is high, especially for the larger population sizes, for the 16-taxon data sets. As shown in Figure 2 and Supplementary Figure S1 available on Dryad, both data sets have similar gene family sizes but differ significantly in terms of the amount of ILS in the data, with the 12-taxon data sets having very little ILS. Therefore, the straightforward explanation for the observed differences species tree inference accuracy between the 16- and 12-taxon data sets is the higher level of ILS in the former. Given that the level of incongruence due to GDL is similar between the 16-taxon and 12-taxon data sets (Fig. 2c,d and Supplementary Fig. S1c,d available on Dryad), these results point to the larger role that ILS plays in the methods’ performance than GDL does.
Second, in the case of the 16-taxon data, the performance of all methods improves
as the number of gene families used as input to the method increases. Note also
that the largest data set used here consists of only 250 gene trees, which is
much smaller than the number available in most phylogenomic data sets. While
there is very little difference observed in the performance among the methods on
the 16-taxon data, ASTRAL, ASTRAL-Pro, and NJ are more similar to
each other in terms of performance than either of them is to inference under
maximum pseudolikelihood or FastMulRFS. This makes sense as ASTRAL, ASTRAL-Pro,
and NJ
are more similar to
each other in terms of performance than either of them is to inference under
maximum pseudolikelihood or FastMulRFS. This makes sense as ASTRAL, ASTRAL-Pro,
and NJ are summary methods
that make inference based on statistics derived from the input gene trees,
whereas maximum pseudolikelihood uses calculations based on the multispecies
coalescent directly. The performance of FastMulRFS is similar to that of other
methods, but its error rates remain higher than the other methods when more gene
families are used. Although ASTRAL-Pro and FastMulRFS were developed with gene
duplication and loss in mind, they do not appear to outperform the other summary
methods.
are summary methods
that make inference based on statistics derived from the input gene trees,
whereas maximum pseudolikelihood uses calculations based on the multispecies
coalescent directly. The performance of FastMulRFS is similar to that of other
methods, but its error rates remain higher than the other methods when more gene
families are used. Although ASTRAL-Pro and FastMulRFS were developed with gene
duplication and loss in mind, they do not appear to outperform the other summary
methods.
Third, the level of ILS for a population size of 50M is higher than for a population size of 10M, and this results in lower accuracy of inferred species trees by all methods in the former case (Supplementary Fig. S4 available on Dryad). This behavior is expected for any method, regardless of whether GDL is acting. Notably, FastMulRFS was not developed to deal correctly with ILS and seems to have an inflated error rate with larger population sizes, but not with smaller population sizes (Supplementary Fig. S4 available on Dryad), suggesting that ILS may be the cause of higher error rates in this method.
Lastly, we observe very little difference in the accuracy of inferred species
trees across the four input scenarios: ALL, ONE, ONLY, and ONLY-NoDup. The only
case in which there is a noticeable difference is in the 12-taxon data sets with
the duplication rate 10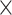 that
found in nature, when only ten genes are used for inference (Supplementary Figs. S8 and S9 available
on Dryad). These results imply that the presence of paralogs in the data, no
matter how they are treated, does not have much of an effect on the performance
of the five methods, unless very few genes are used.
that
found in nature, when only ten genes are used for inference (Supplementary Figs. S8 and S9 available
on Dryad). These results imply that the presence of paralogs in the data, no
matter how they are treated, does not have much of an effect on the performance
of the five methods, unless very few genes are used.
The results thus far raise the important question: does GDL have any effect on
the performance of these five methods? To answer this question, we ran all of
them on the locus trees as input to infer species trees. By the three-tree
model, this amounts to feeding these methods “gene trees” whose
incongruence is solely due to GDL; that is, ILS plays no role in incongruence
here. It is important to point out that locus trees are mathematical constructs
of the three-tree model; in practice, inferring a locus tree is not possible,
unless the data has no ILS at all. We conducted this experiment to study the
performance of methods when GDL, but not ILS, causes all incongruence. Results
on the full 16-taxon data sets are shown in Figure
4 and Supplementary Figure
S5 available on Dryad. As the results show, all methods infer the
species tree perfectly accurately on almost all data sets, regardless of the
parameter settings and the input scenario. In other words, when these
methods—some of which have been developed based on the multispecies
coalescent directly (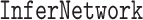 ), some
of which were inspired by the MSC (ASTRAL, ASTRAL-Pro, and
NJ
), some
of which were inspired by the MSC (ASTRAL, ASTRAL-Pro, and
NJ ), and one that does
not deal with ILS at all (FastMulRFS)—are applied to data that have no
ILS but do have paralogs in them, they have almost perfect accuracy in terms of
the species tree topology they infer, under the conditions of our simulations.
Combined with the results summarized in Figure
3 and Supplementary Figure
S4 available on Dryad, these results show, perhaps surprisingly,
that methods developed to handle ILS but not GDL do much better in handling GDL
than they do in handling ILS. Perhaps unsurprisingly, ASTRAL-Pro and FastMulRFS,
methods designed to handle GDL, also perform well on the true locus trees. The
inflated errors seen with FastMulRFS under some settings with gene trees are
absent when true locus trees are used as input, suggesting that, indeed, these
errors were due to ILS. ASTRAL-Pro was designed to deal with both ILS and GDL
and performs well on both true gene trees and true locus trees.
), and one that does
not deal with ILS at all (FastMulRFS)—are applied to data that have no
ILS but do have paralogs in them, they have almost perfect accuracy in terms of
the species tree topology they infer, under the conditions of our simulations.
Combined with the results summarized in Figure
3 and Supplementary Figure
S4 available on Dryad, these results show, perhaps surprisingly,
that methods developed to handle ILS but not GDL do much better in handling GDL
than they do in handling ILS. Perhaps unsurprisingly, ASTRAL-Pro and FastMulRFS,
methods designed to handle GDL, also perform well on the true locus trees. The
inflated errors seen with FastMulRFS under some settings with gene trees are
absent when true locus trees are used as input, suggesting that, indeed, these
errors were due to ILS. ASTRAL-Pro was designed to deal with both ILS and GDL
and performs well on both true gene trees and true locus trees.
Figure 4.
Species tree estimation error for data simulated from the 16-taxon fungal
tree with a population size of 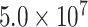 and
varying GDL rates; note that simulations include the effects of GDL only
(no ILS or gene tree estimation error). Species tree estimation error
was measured as the normalized RF distance between the true species tree
and the ones inferred from true locus trees. The five inference methods
used are ASTRAL,
and
varying GDL rates; note that simulations include the effects of GDL only
(no ILS or gene tree estimation error). Species tree estimation error
was measured as the normalized RF distance between the true species tree
and the ones inferred from true locus trees. The five inference methods
used are ASTRAL,  ,
NJ
,
NJ , ASTRAL-Pro
(“A-pro”), and FastMulRFS. The duplication/loss rates are
denoted by the rate multiplier (0
, ASTRAL-Pro
(“A-pro”), and FastMulRFS. The duplication/loss rates are
denoted by the rate multiplier (0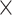 ,
1
,
1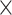 ,
2
,
2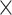 ,
5
,
5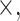 and
10
and
10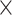 ), where
1
), where
1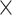 is the rate
estimated in nature for fungi. Each row corresponds to a combination of
population size and GDL rates. The X-axis in each panel represents the
number of gene families used and the Y-axis represents the normalized RF
distance.
is the rate
estimated in nature for fungi. Each row corresponds to a combination of
population size and GDL rates. The X-axis in each panel represents the
number of gene families used and the Y-axis represents the normalized RF
distance.
In practice, gene trees are unknown and are inferred from sequence data. Therefore, to simulate more realistic scenarios, we inferred gene trees and locus trees from simulated sequence data and fed these tree estimates as input to the five methods. In this case, gene tree estimation error is a factor in the observed incongruences. We show the extent of error in the estimated gene and locus trees for the 16-taxon data in Supplementary Figure S2 available on Dryad.
Gene tree estimation error is measured by the normalized RF distance between the true gene tree and the reconstructed gene tree. For the 12-taxon data set, the average gene tree estimation error ranges from 0.456 to 0.648, whereas the average locus tree estimation error is slightly lower, ranging from 0.414 to 0.627 (Supplementary Fig. S3 available on Dryad). For the 16-taxon data set, the average gene tree estimation error ranges between 0.073 to 0.130 while the average locus tree estimation error ranges from 0.065 to 0.099. In other words, there is much less gene tree estimation error in the 16-taxon data sets than in the 12-taxon data sets. Moreover, for the 12-taxon data sets under the ALL and ONLY settings, with increased GDL rate, a decline in error was observed (the average error dropping from 0.614 to 0.477 and 0.615 to 0.489 under ALL and ONE, respectively). Such a pattern, however, was not detected for the 16-taxon data sets.
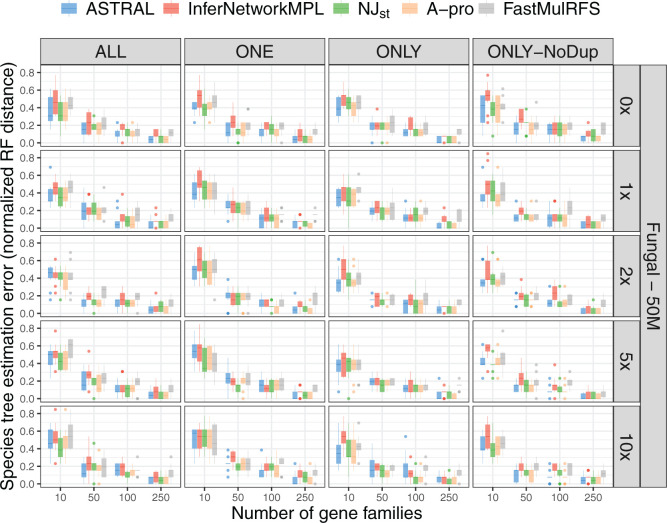
Results of species tree inference using the full 16-taxon data set based on estimated gene trees are shown in Figure 5 and Supplementary Figure S6 available on Dryad; those based on the locus tree estimates are shown in Figure 6 and Supplementary Figure S7 available on Dryad. These results should be contrasted with Figure 3, Supplementary Figure S4 available on Dryad, Figure 4 and Supplementary Figure S5 available on Dryad, respectively, to understand the effect of gene tree estimation error on the accuracy of species tree inference.
Figure 5.
Species tree estimation error for data simulated from the 16-taxon fungal
tree with a population size of 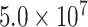 and
varying GDL rates; note that simulations include the effects of ILS, GDL
and gene tree estimation error. Species tree estimation error was
measured as the normalized RF distance between the true species tree and
the ones inferred from estimated gene trees. The five inference methods
used are ASTRAL,
and
varying GDL rates; note that simulations include the effects of ILS, GDL
and gene tree estimation error. Species tree estimation error was
measured as the normalized RF distance between the true species tree and
the ones inferred from estimated gene trees. The five inference methods
used are ASTRAL, 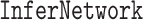 ,
NJ
,
NJ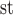 , ASTRAL-Pro
(“A-pro”), and FastMulRFS. The duplication/loss rates are
denoted by the rate multiplier (0
, ASTRAL-Pro
(“A-pro”), and FastMulRFS. The duplication/loss rates are
denoted by the rate multiplier (0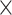 ,
1
,
1 ,
2
,
2 ,
5
,
5 and
10
and
10 ), where
1
), where
1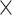 is the rate
estimated in nature for fungi. Each row corresponds to a combination of
population size and GDL rates. The X-axis in each panel represents the
number of gene families used and the Y-axis represents the normalized RF
distance.
is the rate
estimated in nature for fungi. Each row corresponds to a combination of
population size and GDL rates. The X-axis in each panel represents the
number of gene families used and the Y-axis represents the normalized RF
distance.
Figure 6.
Species tree estimation error for data simulated from the 16-taxon fungal
tree with a population size of 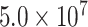 and
varying GDL rates; note that simulations include the effects of GDL and
gene tree estimation error (no ILS). Species tree estimation error was
measured as the normalized RF distance between the true species tree and
the ones inferred from estimated locus trees. The five inference methods
used are ASTRAL,
and
varying GDL rates; note that simulations include the effects of GDL and
gene tree estimation error (no ILS). Species tree estimation error was
measured as the normalized RF distance between the true species tree and
the ones inferred from estimated locus trees. The five inference methods
used are ASTRAL,  ,
NJ
,
NJ , ASTRAL-Pro
(“A-pro”), and FastMulRFS. The duplication/loss rates are
denoted by the rate multiplier (0
, ASTRAL-Pro
(“A-pro”), and FastMulRFS. The duplication/loss rates are
denoted by the rate multiplier (0 ,
1
,
1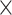 ,
2
,
2 ,
5
,
5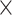 , and
10
, and
10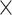 ), where
1
), where
1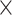 is the rate
estimated in nature for fungi. Each row corresponds to a combination of
population size and GDL rates. The X-axis in each panel represents the
number of gene families used and the Y-axis represents the normalized RF
distance.
is the rate
estimated in nature for fungi. Each row corresponds to a combination of
population size and GDL rates. The X-axis in each panel represents the
number of gene families used and the Y-axis represents the normalized RF
distance.
In the case of species tree inferences using data where ILS, GDL, and gene tree estimation error are involved, the error rates of all five species tree inference methods went up, as expected (Fig. 5 and Supplementary Fig. S6 available on Dryad), but only slightly. The accuracy of the species trees improves as the number of gene families increases. As discussed above, the error in gene tree estimates in the 16-taxon data sets is very low. Since gene tree estimation error in the 12-taxon data sets is much higher (because the higher substitution rates result in noisier sequence data), we observe a larger impact of this error on the performance of methods on the 12-taxon data sets (Supplementary Fig. S10 available on Dryad). While the methods had an almost perfect accuracy on true gene trees, species tree estimates now have as high as 50% error when 10 gene family trees are used, and close to 25% error when 250 gene family trees are used (Supplementary Fig. S10 available on Dryad). These results illustrate the large impact gene tree estimation error has on these methods. In the case of the 12-taxon data sets, the impact of gene tree estimation error significantly outweighs that of ILS or GDL.
Figure 6 and Supplementary Figure S7 available on Dryad demonstrate how GDL and gene tree estimation error (but no ILS) impact species tree inference. As with Figure 4 and Supplementary Figure S5 available on Dryad, which show almost perfect performance of species tree inference from true locus trees (i.e., GDL and no ILS), we observe little reduction in performance here due to error in the estimates of gene trees. The results demonstrate that in the absence of ILS, all methods are robust to gene tree estimation error, except when the number of gene families is very small. In the case of the 12-taxon data sets, where locus tree estimation error is much higher, the five species tree inference methods also have comparable, but lower, accuracies (Supplementary Fig. S11 available on Dryad).
All of these results combined point to a very small impact of GDL on the performance of the five studied species tree inference methods and given the simulation parameters used here, regardless of how the paralogs are handled. On the other hand, across all data sets it was evident that gene tree estimation error has a noticeable impact on the methods’ performance, and that ILS often had a substantial impact on accuracy.
Results on Biological Data
We ran all five methods used above on two empirical data sets, each consisting of thousands of gene trees. As the two data sets were the basis for the simulated data presented above, they share many of the same properties as these data.
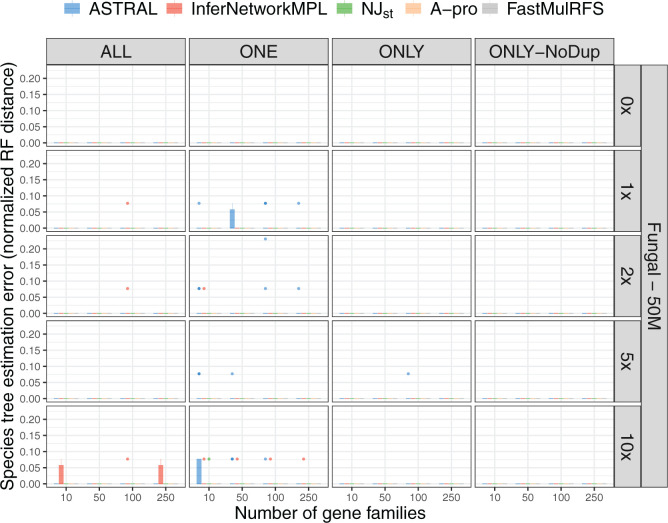
For the 16 fungal genomes, the inferred species trees from all five methods
differ from the tree shown in Figure 1a.
ASTRAL, NJ , ASTRAL-Pro, and
FastMulRFS inferred the same topology depicted in Figure 7c under all three input scenarios (recall that ONLY-NoDup is
not used here, since true orthologs are not known). The same phylogeny is also
inferred by
, ASTRAL-Pro, and
FastMulRFS inferred the same topology depicted in Figure 7c under all three input scenarios (recall that ONLY-NoDup is
not used here, since true orthologs are not known). The same phylogeny is also
inferred by  (ONE).
This inferred tree is topologically different from the tree shown in Figure 1a: in particular, the positions of
Kluyveromyces waltii and Kluyveromyces
lactis have been switched, as have the positions of Candida
glabrata and Saccharomyces castellii (Fig. 7c). The trees inferred by
(ONE).
This inferred tree is topologically different from the tree shown in Figure 1a: in particular, the positions of
Kluyveromyces waltii and Kluyveromyces
lactis have been switched, as have the positions of Candida
glabrata and Saccharomyces castellii (Fig. 7c). The trees inferred by
 (ALL)
and
(ALL)
and  (ONLY)
differ from the reference tree of Figure 1a
in terms of the placement of Candida glabrata and
Saccharomyces castellii, as shown in Figure 7a,b.
(ONLY)
differ from the reference tree of Figure 1a
in terms of the placement of Candida glabrata and
Saccharomyces castellii, as shown in Figure 7a,b.  (ALL)
additionally grouped Saccharomyces cerevisiae and
Saccharomyces mikatae as sisters, and switched the position
of Kluyveromyces waltii and Kluyveromyces
lactis. Interestingly, the position of Candida
glabrata is not a settled issue: Shen et al. (2016) label the relevant branch as
“unresolved” in their analysis of 1233 single-copy orthologs.
Similarly, their results support the same placement of Kluyveromyces
lactis as in Figure 7a,c here.
The placement of these species shown in Figure
1a originally comes from a concatenated analysis of 706 single-copy
genes (Butler et al. 2009).
(ALL)
additionally grouped Saccharomyces cerevisiae and
Saccharomyces mikatae as sisters, and switched the position
of Kluyveromyces waltii and Kluyveromyces
lactis. Interestingly, the position of Candida
glabrata is not a settled issue: Shen et al. (2016) label the relevant branch as
“unresolved” in their analysis of 1233 single-copy orthologs.
Similarly, their results support the same placement of Kluyveromyces
lactis as in Figure 7a,c here.
The placement of these species shown in Figure
1a originally comes from a concatenated analysis of 706 single-copy
genes (Butler et al. 2009).
Figure 7.
Inferred fungal species trees. a) The fungal species tree inferred by
 (ALL).
b) The fungal species tree inferred by
(ALL).
b) The fungal species tree inferred by  (ONLY)
c) The fungal species tree inferred by ASTRAL,
NJ
(ONLY)
c) The fungal species tree inferred by ASTRAL,
NJ ,
ASTRAL-Pro, FastMulRFS, and
,
ASTRAL-Pro, FastMulRFS, and  (ONE).
Differences between the inferred species trees and the tree in Figure 1 are highlighted in red.
(ONE).
Differences between the inferred species trees and the tree in Figure 1 are highlighted in red.
For the 12 fly genomes, all three sampling schemes and all five methods inferred the exact same tree as the species tree shown in Figure 1b.
Discussion
As phylogenomic data sets grow, our ability to use them within the bounds of current analysis paradigms shrinks. One of the main problems is the decreasing number of gene families that are single-copy as the number of sampled species increases (Emms and Kelly 2018). Because most current phylogenetic methods assume that only single-copy orthologs are being used, this restriction means that such methods cannot be used for data sets with even several dozen taxa without severe downsampling or other ad hoc solutions (e.g., Thomas et al. 2020). Here, we set out to ask whether phylogenomic methods intended to deal with incongruence due to ILS can be applied to data containing both orthologs and paralogs, which contain incongruence due to GDL.
On simulated data sets where only ILS acted, and GDL was not a factor, all methods had the expected performance: accurate species tree estimates that improved as the number of gene trees used increases. In the case where the level of ILS was very low (the 12-taxon data), the methods had perfect performance under almost all conditions, regardless of the number of gene trees used. FastMulRFS (Molloy and Warnow 2020) sometimes had high error rates when rates of ILS were high, a result that has been found in previous studies on the accuracy of this method (Zhang et al. 2020). FastMulRFS is also the only method employed here that has not been proven to be statistically consistent under the multispecies coalescent model, in which ILS is the driving forces behind incongruence.
In the cases where both ILS and GDL acted, the performance of the five methods was hardly affected by the type of data set used (ALL, ONE, ONLY, ONLY-NoDup). Within the range of simulation parameters and data sets analyzed here, our results imply that running these methods on data with paralogs will produce species tree topologies at least as accurate as those using single-copy orthologs alone. This is especially important for data sets with a large number of species or high GDL rates.
When the methods were run on the locus tree data, where ILS does not play a role and the data consist of many gene families with multiple copies, the methods produced very accurate species trees. When as few as ten gene trees were used, error rates were elevated in data sets including paralogs (Supplementary Fig. S9 available on Dryad). However, with more than 10 genes, GDL alone did not appear to affect species tree inference under our simulation conditions. This further demonstrates that GDL has very little effect on the performance of these methods.
While at first it may be surprising that these methods performed very well in terms
of accuracy, the majority of signal in any input gene tree reflects species
relationships. Gene duplication—if random across the species
tree—simply adds noise to the data, while at the same time often doubling the
amount of information on the relationships among species carrying an extra gene
copy. Similarly, gene loss does not positively mislead these methods, leading to
accurate reconstructions of the species tree. Nevertheless, upon close inspection,
some of these results are not intuitive, especially for the maximum pseudolikelihood
inference. 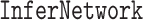 makes
direct use of the MSC, whose assumptions are clearly violated in all data sets
except when the GDL rates are set to 0, whereas all other methods are summary
methods that make no direct use of the MSC. Consequently, one would have expected
that
makes
direct use of the MSC, whose assumptions are clearly violated in all data sets
except when the GDL rates are set to 0, whereas all other methods are summary
methods that make no direct use of the MSC. Consequently, one would have expected
that 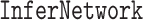 would
be very sensitive to the presence of paralogs in the data, while the others were
less so. However, we largely did not observe this behavior (but see discussion of
the fungal tree below). Using methods designed specifically to deal with duplication
and loss (ASTRAL-Pro and FastMulRFS) also did not lead to lower error rates. In the
case of ASTRAL-Pro, we find performance similar to ASTRAL, as expected given the
statistical consistency of these methods, as discussed above.
would
be very sensitive to the presence of paralogs in the data, while the others were
less so. However, we largely did not observe this behavior (but see discussion of
the fungal tree below). Using methods designed specifically to deal with duplication
and loss (ASTRAL-Pro and FastMulRFS) also did not lead to lower error rates. In the
case of ASTRAL-Pro, we find performance similar to ASTRAL, as expected given the
statistical consistency of these methods, as discussed above.
In practice, gene trees are estimated from sequence data and can be erroneous. Error in the gene tree estimates, rather than ILS, could explain much of the heterogeneity observed in phylogenomic analyses, especially at deeper nodes in a species tree (Scornavacca and Galtier 2017). We showed the gene tree estimation error can indeed impact species tree inference significantly, and that the level of its impact is similar to that of ILS, if not larger. The results from simulations including gene tree error (and from the biological data sets) should be considered the most realistic. However, as more gene trees are used, regardless of levels of ILS or GDL, species tree accuracy increased.
In analyses of two biological data sets where a species tree has been inferred using
hundreds or thousands of loci, we found high accuracy of the methods using paralogs.
All methods accurately inferred the published fly species tree. For the fungal
species tree, no methods inferred the species tree we initially assumed to be true,
which is originally based on a concatenated analysis of 706 single-copy genes (Butler et al. 2009). All methods, applied to all
data sets, disagreed with this published tree with respect to the relative positions
of C. glabrata and S. castellii (Fig. 7). Interestingly, the position of
S. castellii in Butler et al.
(2009) was constrained prior to tree search based on several rare genomic
changes; an unconstrained search produced a topology consistent with the one found
here. Shen et al. (2016), using a data set of
1233 single-copy orthologs, could not confidently determine the relationships among
these species. Here, by more than doubling the number of gene trees, we find a
species tree with a local posterior probability of 1.0 for the topology shown in
Figure 7. Furthermore, the results of Shen et al. (2016) support the placement of
K. lactis found here. The only sets of relationships that
appears to differ with up-to-date fungal phylogenies are the ones inferred by
 (ALL)
and
(ALL)
and  (ONLY).
This may be because
(ONLY).
This may be because  explicitly models data according to the MSC.
explicitly models data according to the MSC.
As we highlighted above, we used SimPhy to generate synthetic data, and this tool
makes simplifying assumptions including no hemiplasy of new duplicates and that all
gene families are independent. Under the conditions of our simulations and on the
two biological data sets used here, our results point to a clear message: running
species tree inference methods intended to deal with ILS on gene trees with paralogs
yields highly accurate results. This conclusion is powerful for at least two
reasons. First, it implies that orthology assignment and paralogy removal are not
necessary for running gene tree-based species tree inference; simply treating all
copies as different individuals or randomly selecting a single copy would yield very
accurate species tree topologies. Nevertheless, accurate orthology inference prior
to species tree inference could be helpful under evolutionary scenarios not captured
by our simulations. Second, in many practical cases, too few single-copy genes are
available to ensure good performance of species tree inference from those data
alone. In these cases, our results suggest a ready source of more phylogenetic
signal. Summary methods that do not explicitly use the MSC model (i.e., ASTRAL,
ASTRAL-Pro, FastMulRFS, and NJ ) are
expected to be more robust in the presence of GDL than methods that explicitly use
the model—some of these methods have even been found to be statistically
consistent under a model of GDL and ILS, as discussed above.
) are
expected to be more robust in the presence of GDL than methods that explicitly use
the model—some of these methods have even been found to be statistically
consistent under a model of GDL and ILS, as discussed above.
While our study focused on the accuracy of the inferred species tree topology, using paralogs for inference would clearly have an impact on the estimated branch lengths of the species tree for methods designed with orthologs in mind. In particular, under the ALL setting, there could be much more incongruence due to the large number of lineages, and, consequently, methods that use incongruence (and assume all incongruence is due to ILS) to estimate branch lengths would give values that are shorter than they truly are. For this reason, branch lengths inferred by such methods should not be used. Branch lengths estimated in ASTRAL-Pro should be accurate assuming that the rooting-and-tagging algorithm used is accurate, but, to our knowledge, the accuracy of branch length estimates using this approach has not been evaluated. When users wish to estimate branch lengths using a method designed for use with paralogs, an alternative approach is needed. The results of our analyses point to the following potential approach for inferring accurate species trees (topologies and branch lengths) by utilizing as much of the phylogenomic data as possible:
Use all available gene trees as input, whether or not they are single-copy in all species.
Feed all gene trees to a gene tree-based method to obtain a species tree topology.
Using a smaller subset of truly single-copy genes, and fixing the species tree topology obtained from Step (2), optimize the branch lengths of the species tree.
For Steps (1) and (2), one option is to repeat the random sampling of single copies from each species used to generate multiple “ONE” data sets. Then, these inferred species trees could be scored under some criterion that combines the MSC with a model of gene duplication/loss. This would overcome the issue of fixing a single species tree as input to Step (3), and avoids searching species tree space while evaluating a likelihood function that is very complex and computationally very demanding to compute. As an alternative to using only single-copy orthologs in Step (3), one could also use a statistical model that combines the MSC and GDL models (e.g., Rasmussen and Kellis 2012). Such methods allow for paralogy detection and orthology assignment, conditional on the fixed species tree (or species trees), by using a more detailed evolutionary model and the full signal in the sequence data. For example, the orthology assignment could be “integrated out” or sampled, depending on the desired outcomes of the analysis. Unfortunately, while full Bayesian methods exist that model GDL alone (Boussau et al. 2013) or that model ILS alone (Ogilvie et al. 2017), none that we know of can model both.
Conclusions
In this article, we set out to study how gene tree-based species tree inference would perform on data with paralogs. The motivation for exploring this question was two-fold. First, as methods for dealing with incongruence due to ILS have become commonplace, and as practitioners are almost never certain that their data contain no paralogs, it is important to understand the effect of hidden paralogy on the quality of the inference. Second, as larger phylogenomic data sets become available, insistence on single-copy genes would mean throwing away most of the data and potentially keeping a number of loci that may be inadequate for suitably complex species tree inference methods to perform well. We investigated this question through a combination of simulations and biological data analyses. Our results show that gene tree-based inference is robust to the presence of paralogs in the data, at least under the simulation conditions and on the empirical data sets we investigated.
Our results highlight the issue that gene tree-based inference could result in very accurate species trees even when ILS is not a factor or not the only factor. This finding implies that orthology detection and restricting data to single-copy genes as a requirement for employing gene tree-based inference can be mostly eliminated, thus making use of as much of the data as possible (cf. Smith and Hahn 2021b). In particular, for very large data sets (in terms of the number of species), eliminating all but single-copy genes might leave too few loci for the species tree to be inferred accurately. Our findings show that this data exclusion could be an unnecessary practice. It is important to note however, that our results do not apply to concatenated analyses, and in such cases, the presence of paralogs may indeed have a large, negative effect (Brown and Thomson 2016). Species tree inference from a concatenation of the sequences with gene families is challenging in the presence of paralogs for at least two reasons. First, when gene families have different numbers of copies across species, the concatenated alignment will have very large gaps. Second, correct orthology detection is still required, so that orthologous gene copies are placed in correct correspondence across the multiple genomes in the concatenated alignment. This issue is very important to examine so as to avoid aligning non-orthologous sequences in the concatenated data set.
In our simulations, we generated gene families under a neutral model and with GDL rates that were the same across all families. It is well known that the functional implications of gene duplication and the ways in which they are fixed and maintained in the genome result in much more complex scenarios than those captured in our simulations (Hahn 2009; Innan and Kondrashov 2010). However, analyses of the two biological data sets yield results with very similar trends to those observed in our simulations.
Finally, while we did not discuss or incorporate gene flow in our study, it is possible that all three processes—ILS, GDL, and gene flow—are simultaneously involved in the evolution of some clades. Studies of the robustness of gene tree-based species tree inference under some models of gene flow exist (Roch and Snir 2012; Steel et al. 2013; Davidson et al. 2015; Solís-Lemus et al. 2016; Zhu et al. 2016; Long and Kubatko 2018), but, to the best of our knowledge, such studies under scenarios that incorporate all the aforementioned processes do not exist yet. It is important to highlight, as well, that great strides have been made in developing methods for phylogenetic network inference in the presence of ILS (Elworth et al. 2019), but no probabilistic methods currently incorporate gene duplication and loss (see Li et al. (2021) for a very interesting alternative approach). We believe methods along the lines described in the previous section could be promising for accurate and scalable phylogenomic inferences without sacrificing much of the data.
Contributor Information
Zhi Yan, Department of Computer Science, Rice University, 6100 Main Street, Houston, TX 77005, USA.
Megan L Smith, Department of Biology and Department of Computer Science, Indiana University, 1001 East Third Street, Bloomington, IN 47405, USA.
Peng Du, Department of Computer Science, Rice University, 6100 Main Street, Houston, TX 77005, USA.
Matthew W Hahn, Department of Biology and Department of Computer Science, Indiana University, 1001 East Third Street, Bloomington, IN 47405, USA.
Luay Nakhleh, Department of Computer Science, Rice University, 6100 Main Street, Houston, TX 77005, USA; Department of BioSciences, Rice University, 6100 Main Street, Houston, TX 77005, USA.
Supplementary Material
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.t76hdr81d.
Funding
The National Science Foundation [DBI-1355998, CCF-1514177, CCF-1800723, and DMS-1547433 to L.N.] and [DBI-1564611 and DEB-1936187 to M.W.H.].
References
- Arvestad L., Lagergren J., Sennblad B.. 2009. The gene evolution model and computing its associated probabilities. J. ACM 56:7. [Google Scholar]
- Boussau B., Szöllősi G.J., Duret L., Gouy M., Tannier E., Daubin V.. 2013. Genome-scale coestimation of species and gene trees. Genome Res. 23:323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J.M., Thomson R.C.. 2016. Bayes factors unmask highly variable information content, bias, and extreme influence in phylogenomic analyses. Syst. Biol. 66:517–530. [DOI] [PubMed] [Google Scholar]
- Bryant D., Hahn M.W.. 2020. The concatenation question. In: Scornavacca C., Delsuc F., Galtier N, editors. Phylogenetics in the genomic era. No commercial publisher | Authors open access book. p. 3.4:1–3.4:23. [Google Scholar]
- Butler G., Rasmussen M.D., Lin M.F., Santos M.A., Sakthikumar S., Munro C.A., Rheinbay E., Grabherr M., Forche A., Reedy J.L.. et al. 2009. Evolution of pathogenicity and sexual reproduction in eight candida genomes. Nature 459:657–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson R., Vachaspati P., Mirarab S., Warnow T.. 2015. Phylogenomic species tree estimation in the presence of incomplete lineage sorting and horizontal gene transfer. BMC Genomics 16:S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degnan J.H., Rosenberg N.A.. 2009. Gene tree discordance, phylogenetic inference and the multispecies coalescent. Trends Ecol. & Evol. 24:332–340. [DOI] [PubMed] [Google Scholar]
- Doolittle W.F., Brown J.R.. 1994. Tempo, mode, the progenote, and the universal root. Proc. Natl. Acad. Sci. USA 91:6721–6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du P., Nakhleh L.. 2018. Species tree and reconciliation estimation under a duplication-loss-coalescence model. In: Boucher C., Li J., Liu H., Pop M., editors. Proceedings of the 9th ACM Conference on Bioinformatics, Computational Biology, and Health Informatics. ACM Digital Library. p. 376–385. [Google Scholar]
- Elworth R.L., Ogilvie H.A., Zhu J., Nakhleh L.. 2019. Advances in computational methods for phylogenetic networks in the presence of hybridization. In: Warnow T., editor. Bioinformatics and phylogenetics. Springer. p. 317–360. [Google Scholar]
- Emms D., Kelly S.. 2018. STAG: species tree inference from all genes. bioRxiv p. 267914. Available from: https://www.biorxiv.org/content/10.1101/267914v1. [Google Scholar]
- Guindon S., Gascuel O.. 2003. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 52:696–704. [DOI] [PubMed] [Google Scholar]
- Hahn M.W. 2009. Distinguishing among evolutionary models for the maintenance of gene duplicates. J. Heredity 100:605–617. [DOI] [PubMed] [Google Scholar]
- Hahn M.W., Han M.V., Han S.-G.. 2007. Gene family evolution across 12 drosophila genomes. PLoS Genetics 3:e197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M., Legried B., Roch S.. 2020. Species tree estimation under joint modeling of coalescence and duplication: sample complexity of quartet methods. arXiv preprint arXiv:2007.06697. Available from: https://arxiv.org/abs/2007.06697. [Google Scholar]
- Hudson R.R. 1983. Testing the constant-rate neutral allele model with protein sequence data. Evolution 37:203–217. [DOI] [PubMed] [Google Scholar]
- Innan H., Kondrashov F.. 2010. The evolution of gene duplications: classifying and distinguishing between models. Nat. Rev. Genet. 11:97–108. [DOI] [PubMed] [Google Scholar]
- Knowles L.L., Kubatko L.S.. 2011. Estimating species trees: practical and theoretical aspects. Wiley. [Google Scholar]
- Koonin E.V. 2005. Orthologs, paralogs, and evolutionary genomics. Annu. Rev. Genet. 39:309–338. [DOI] [PubMed] [Google Scholar]
- Lang G.I., Murray A.W.. 2008. Estimating the per-base-pair mutation rate in the yeast Saccharomyces cerevisiae. Genetics 178:67–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legried B., Molloy E.K., Warnow T., Roch S.. 2021. Polynomial-time statistical estimation of species trees under gene duplication and loss. J. Comput. Biol. 28(5):452–468. [DOI] [PubMed] [Google Scholar]
- Li L., Stoeckert C.J. Jr., Roos D.S.. 2003. OrthoMCL: identification of ortholog groups for eukaryotic genomes. Genome Res. 13:2178–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Scornavacca C., Galtier N., Chan Y.-B.. 2021. The multilocus multispecies coalescent: a flexible new model of gene family evolution. Syst. Biol. 70(4):822–837. [DOI] [PubMed] [Google Scholar]
- Liu L., Xi Z., Wu S., Davis C.C., Edwards S.V.. 2015. Estimating phylogenetic trees from genome-scale data. Ann. N. Y. Acad. Sci. 1360:36–53. [DOI] [PubMed] [Google Scholar]
- Liu L., Yu L.. 2011. Estimating species trees from unrooted gene trees. Syst. Biol. 60:661–667. [DOI] [PubMed] [Google Scholar]
- Liu L., Yu L., Edwards S.V.. 2010. A maximum pseudo-likelihood approach for estimating species trees under the coalescent model. BMC Evol. Biol. 10:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Yu L.L., Kubatko L., Pearl D.K., Edwards S.V.. 2009. Coalescent methods for estimating phylogenetic trees. Mol. Phylogenet. Evol. 53:320–328. [DOI] [PubMed] [Google Scholar]
- Long C., and Kubatko L.. 2018. The effect of gene flow on coalescent-based species-tree inference. Syst. Biol. 67:770–785. [DOI] [PubMed] [Google Scholar]
- Maddison W.P. 1997. Gene trees in species trees. Syst. Biol. 46:523–536. [Google Scholar]
- Mallo D., de Oliveira Martins L., Posada D.. 2015. SimPhy: phylogenomic simulation of gene, locus, and species trees. Syst. Biol. 65:334–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markin A., Eulenstein O.. 2021. Quartet-based inference methods are statistically consistent under the unified duplication-loss-coalescence model. Bioinformatics. doi: 10.1093/bioinformatics/btab414/6287614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirarab S., Reaz R., Bayzid M.S., Zimmermann T., Swenson M.S., Warnow T.. 2014. ASTRAL: genome-scale coalescent-based species tree estimation. Bioinformatics 30:i541–i548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy E.K., Warnow T.. 2020. FastMulRFS: fast and accurate species tree estimation under generic gene duplication and loss models. Bioinformatics 36:i57–i65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhleh L. 2013. Computational approaches to species phylogeny inference and gene tree reconciliation. Trends Ecol. Evol. 28:719–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen L.-T., Schmidt H.A., von Haeseler A., Minh B.Q.. 2014. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 32:268–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogilvie H.A., Bouckaert R.R., Drummond A.J.. 2017. StarBEAST2 brings faster species tree inference and accurate estimates of substitution rates. Mol. Biol. Evol. 34:2101–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard D.A., Iyer V.N., Moses A.M., Eisen M.B.. 2006. Widespread discordance of gene trees with species tree in Drosophila: evidence for incomplete lineage sorting. PLoS Genetics 2:e173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabiee M., Sayyari E., Mirarab S.. 2019. Multi-allele species reconstruction using astral. Mol. Phylogenet. Evol. 130:286–296. [DOI] [PubMed] [Google Scholar]
- Rambaut A., Grassly N.C.. 1997. Seq-Gen: an application for the Monte Carlo simulation of DNA sequence evolution along phylogenetic trees. Bioinformatics 13:235–238. [DOI] [PubMed] [Google Scholar]
- Rannala B., Yang Z.. 2003. Bayes estimation of species divergence times and ancestral population sizes using DNA sequences from multiple loci. Genetics 164:1645–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen M.D., Kellis M.. 2011. A Bayesian approach for fast and accurate gene tree reconstruction. Mol. Biol. Evol. 28:273–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen M.D., Kellis M.. 2012. Unified modeling of gene duplication, loss, and coalescence using a locus tree. Genome Res. 22:755–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D.F., Foulds L.R.. 1981. Comparison of phylogenetic trees. Math. Biosci. 53:131–147. [Google Scholar]
- Roch S., Snir S.. 2012. Recovering the tree-like trend of evolution despite extensive lateral genetic transfer: a probabilistic analysis. J. Comput. Biol. 20:93–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer S.A., Hartl D.L.. 1992. Population genetics of polymorphism and divergence. Genetics 132:1161–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrider D.R., Houle D., Lynch M., Hahn M.W.. 2013. Rates and genomic consequences of spontaneous mutational events in Drosophila melanogaster. Genetics 194:937–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scornavacca C., Galtier N.. 2017. Incomplete lineage sorting in mammalian phylogenomics. Syst. Biol. 66:112–120. [DOI] [PubMed] [Google Scholar]
- Shen X.-X., Zhou X., Kominek J., Kurtzman C.P., Hittinger C.T., Rokas A.. 2016. Reconstructing the backbone of the Saccharomycotina yeast phylogeny using genome-scale data. G3: Genes, Genomes, Genetics 6:3927–3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M.L., Hahn M.W.. 2021a. The frequency and topology of pseudoorthologs. bioRxiv p. 10.1101/2021.02.17.431499. Available from: https://www.biorxiv.org/content/10.1101/2021.02.17.431499v1. [Google Scholar]
- Smith M.L., Hahn M.W.. 2021b. New approaches for inferring phylogenies in the presence of paralogs. Trends Genet. 37:174–187. [DOI] [PubMed] [Google Scholar]
- Solís-Lemus C., Yang M., Ané C.. 2016. Inconsistency of species tree methods under gene flow. Syst. Biol. 65:843–851. [DOI] [PubMed] [Google Scholar]
- Steel M., Linz S., Huson D., Sanderson M.. 2013. Identifying a species tree subject to random lateral gene transfer. J. Theor. Biol. 322:81–93. [DOI] [PubMed] [Google Scholar]
- Takahata N. 1989. Gene genealogy in three related populations: consistency probability between gene and population trees. Genetics 122:957–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Than C., Nakhleh L.. 2009. Species tree inference by minimizing deep coalescences. PLoS Comput. Biol. 5:e1000501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G.W., Dohmen E., Hughes D.S., Murali S.C., Poelchau M., Glastad K., Anstead C.A., Ayoub N.A., Batterham P., Bellair M.. et al. 2020. Gene content evolution in the arthropods. Genome Biol. 21:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen D., Yun Y., Zhu J., Nakhleh L.. 2018. Inferring phylogenetic networks using PhyloNet. Syst. Biol. 67:735–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Smith S.A.. 2014. Orthology inference in nonmodel organisms using transcriptomes and low-coverage genomes: improving accuracy and matrix occupancy for phylogenomics. Mol. Biol. Evol. 31:3081–3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y., Dong J., Liu K.J., Nakhleh L.. 2014. Maximum likelihood inference of reticulate evolutionary histories. Proc. Natl. Acad. Sci. USA 111:16448–16453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y., Nakhleh L.. 2015. A maximum pseudo-likelihood approach for phylogenetic networks. BMC Genomics 16:S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y., Warnow T., Nakhleh L.. 2011. Algorithms for MDC-based multi-locus phylogeny inference: beyond rooted binary gene trees on single alleles. J. Comput. Biol. 18:1543–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Wu Y.-C.. 2017. Coestimation of gene trees and reconciliations under a duplication-loss-coalescence model. In: Cai Z., Daescu O., Li M., editors. International Symposium on Bioinformatics Research and Applications. Cham: Springer. p. 196–210. [Google Scholar]
- Zhang C., Rabiee M., Sayyari E., Mirarab S.. 2018. ASTRAL-III: polynomial time species tree reconstruction from partially resolved gene trees. BMC Bioinformatics 19:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Scornavacca C., Molloy E.K., Mirarab S.. 2020. ASTRAL-Pro: quartet-based species-tree inference despite paralogy. Mol. Biol. Evol. 37:3292–3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Yu Y., Nakhleh L.. 2016. In the light of deep coalescence: revisiting trees within networks. BMC Bioinformatics 17:415. [DOI] [PMC free article] [PubMed] [Google Scholar]