Abstract
Objectives:
To investigate whether PET–CT or PET–MRI is more appropriate for imaging prostate cancer, in terms of primary tumor detection, local staging and recurrence, as well as lymph nodes and distant metastases.
Methods:
A systematic literature search was conducted on Embase, PubMed/MEDLINE, and the Cochrane Library database. Studies evaluating the diagnostic performance of PET–CT vs PET–MRI in prostate cancer patients were emphasized.
Results:
We reviewed 57 original research articles during the period 2016–2021: 14 articles regarding the radiotracer PSMA; 18 articles regarding the primary tumor detection, local tumor staging, managing local recurrence; 17 articles for managing lymph node metastases; and eight articles for managing bone and other distant metastases. PSMA PET could be complementary to mpMRI for primary prostate cancer localization and is particularly valuable for PI-RADS three lesions. PET–MRI is better than PET–CT in local tumor staging due to its specific benefit in predicting extracapsular extension in MRI-occult prostate cancer patients. PET–MRI is likely superior as compared with PET–CT in detecting local recurrence, and has slightly higher detection rates than PET–CT in lymph node recurrence. PET–CT and PET–MRI seem to have equivalent performance in detecting distant bony or visceral metastases.
Conclusion:
In conclusion, PET–MRI is suitable for local and regional disease, either primary staging or restaging, whereas PET–CT is valuable for managing distant bony or visceral metastasis.
Advances in knowledge:
We reviewed the emerging applications of PET–MRI and PET–CT in clinical aspects. Readers will gain an objective overview on the strength and shortfalls of PET–MRI or PET–CT in the management of prostate cancer.
Introduction
Prostate-specific membrane antigen (PSMA) is a 750 amino acid type II membrane glycoprotein abundantly expressed in prostate cancer cells, either in prostate cancer tissue or at other metastatic sites, with activity depending on tumor dedifferentiation. More than 90% of primary prostate cancer displayed moderate-to-high PSMA expression; 1 therefore, PSMA is considered as a suitable target for prostate cancer positron emission tomography (PET) imaging. 2 Several radiopharmaceuticals, which target PSMA for instance 68Ga-PSMA-11, 3 18F-DCFPyL 4 and 18F-PSMA-1007, 5 have been developed for targeting biological features of prostate cancer that have been widely reviewed. 6 68 Ga-PSMA-11 has been extensively adopted and used globally, that is, data from >15,000 patients have been published till now. 1 The European Association of Nuclear Medicine (EANM) – Society of Nuclear Medicine and Molecular Imaging (SNMMI) guidelines on PSMA PET provide an in-depth information regarding the procedure of 68Ga-PSMA-11 PET. 7 In late 2020, 68Ga-PSMA-11 became the foremost approved PSMA PET imaging agent by the U.S. Food and Drug Administration, and distant suppliers expand the availability of PET radiopharmaceuticals in the early stage of development. However, 68Ga still has some drawbacks including shorter half-life, lower positron yield, and higher positron energy. With the increasing demand for PSMA PET, a trend is emerging toward a shift from 68Ga- to in-house production of 18F-labeled PSMA target compounds in recent years. 8 Herein, we use PSMA to generally describe all the PSMA-based radiotracers in this article. For readers seeking for a comprehensive guidelines for interpretation, we recommend an outstanding review by Hofman et al 9 . Before diving into the scope of prostate cancer, readers might need to consider a number of benign (i.e. hemangioma, Paget disease, and fibrous dysplasia) or malignant (i.e. thyroid cancer, renal cell carcinoma, and hepatocellular carcinoma) lesions also lead to PSMA uptake. 10,11 Characterizing PSMA-avid lesions needs a comprehensive consideration to include clinical context and morphological imaging information as possible. For example, the ureteral peristalsis can be misunderstood as a nodal disease.
Meanwhile, multiparametric MRI (mpMRI)—combining morphological T 1-weighted imaging, T 2-weighted imaging with functional sequences, dynamic contrast-enhanced (DCE) imaging, and diffusion-weighted imaging (DWI)—has become broadly adopted in the past decade for detecting clinically significant cancer and plays a vital role in performing targeted biopsy. 12 Using mpMRI as a triage test before biopsy, MRI-targeted biopsies are better than systematic biopsies in prostate cancer detection, which helps in avoiding overdiagnosis or underdiagnosis. 13 The advancement of hybrid PET with magnetic resonance imaging (PET–MRI) or computed tomography (PET–CT) might potentially transform the approach of imaging prostate cancer. PET–MRI could result in lower exposure (80%) to radiation than PET–CT, but the acquisition time is substantially increased. 14 This longer scanning time is attributed to the additional mpMRI that significantly improves the resolution of the prostate region. The above-mentioned points raise the question of whether PET–CT or PET–MRI is more suitable for imaging prostate cancer.
Evidence acquisition
We searched three different electronic databases: Embase, PubMed/MEDLINE, and the Cochrane Library, using the keywords or search terms “prostate cancer”, “PSMA”, “PET–CT”, “PET/MRI”, and “PET/MR” and filtered it for full-text journal articles in English between January 2016 and May 2021. We selected studies that had defined cohorts with any accepted definition of prostate cancer with baseline or follow-up PET–CT or PET–MRI utilizing PSMA that assessed their performance in diagnosis, staging, and detection of recurrence. Next, we excluded articles that were from case reports and proceedings, articles that were predominantly based on secondary metastasis, articles that did not involve human subjects, articles that used radiotracers other than PSMA, and articles that concentrated on other malignancies and were not focused on prostate cancer.
Primary tumor detection and local staging
PET–CT vs mpMRI
For primary tumor detection, Kalapara et al 15 reported a retrospective study of 205 patients, showing non-significant difference in the localization or detection of primary prostate cancer among PET–CT and mpMRI. In another similar retrospective study of 144 patients published by Donato et al 16 PET–CT detected more secondary cancer foci and smaller lesions than mpMRI, demonstrating a higher sensitivity in lesion detection (90% vs 83%). In a meta-analysis published by Satapathy et al 17 PET–CT had an overall sensitivity (97%) and negative likelihood ratio (0.05) for the early diagnosis of prostate cancer and has potential utility to exclude the clinical suspicion of prostate cancer, thereby avoiding needless biopsies.
PET–MRI vs mpMRI
PET–MRI demonstrates a better primary tumor detection rate as compared with mpMRI. 18 Eiber et al 19 compared the diagnostic performance of PET–MRI for localization of primary prostate cancer against mpMRI and PET alone. From the 53 patients reported, mpMRI, PET, and PET–MRI identified prostate cancer in 66%, 92%, and 98% of the patients, respectively. On a region-based analysis, PET–MRI has higher sensitivity (76%) than mpMRI and PET alone (58 and 64%). In another study, Hicks et al 18 reported that the sensitivity of PET–MRI to detect prostate cancer is better than mpMRI. These results indicate that PSMA PET may be complementary to mpMRI for primary prostate cancer localization. An example of PET–MRI complementary to mpMRI for primary prostate cancer localization is shown in Figure 1. Current paradigm shift in prostate cancer diagnosis heads for improved detection of clinically significant cancer. Recent studies focused on the detection of clinically significant prostate cancer. 16,20,21 PET/ MRI demonstrated a higher specificity (96% vs 71%) while remained the same good sensitivity (89%), as compared with PET–CT in a lesion-based analysis. 20 PET–MRI is valuable for PI-RADS three lesions on mpMRI, leading a correct shift toward higher suspicion of malignancy and enabling correct lesion classification for overall 22 and clinically significant prostate cancers. 20 However, it is still unclear of the additional value of PSMA PET to mpMRI in detecting tumors in mpMRI-difficult area, that is, the transitional zone.
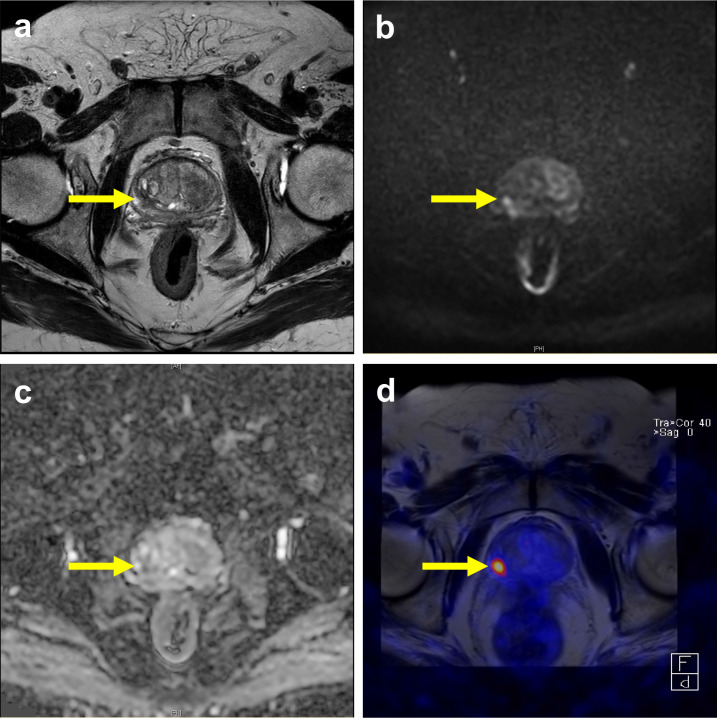
Figure 1.
PSMA PET–MRI complementary to mpMRI for primary prostate cancer localization. A 80-year-old male with slightly increased PSA level 5.66 ng ml−1. (a) A T2 low signal intensity focus was noted at right posterolateral part of right peripheral zone, the greatest dimension was measured as 1.2 cm. (b, c) The lesion showed hyperintense on high b-value DWI and hypointense on ADC, which was considered a PIRADS four lesion. (d) The fused PET/MR image showed high 68Ga-PSMA-11 uptake at same location, and the possibility of extracapuslar extension was excluded. The final biopsy confirmed a Gleason score 4 + 3 prostate cancer lesion.
PET–CT vs PET/MRI
PET/MRI and PET–CT were reported to have a high detection rate for primary prostate cancer (91–98%). 23–25 The sensitivity and specificity of PET–CT and PET/MRI in the detection of primary prostate cancer from articles with pathologic results as reference standard were reported from 67 to 96%, and from 55.5 to 100%, respectively, as summarized in Table 1. 5,16,18,20–22 For primary disease, PSMA PET showed a slightly better detection sensitivity than mpMRI, and the combination may have higher sensitivity than either modality alone. 19
Table 1.
Diagnostic Performance of PSMA PET–CT and PET–MRI in Detection of Primary Prostate Cancer with Pathologic Results as Reference Standard
| Study | Patient | Radiotracer | Data Analysis | Modality | Parameter | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|---|
| Eiber et al 19 e | 53 | 68Ga-PSMA-11 | Region-based | PET/MRI | Score 4–5 as positive a | 76% | 97% |
| Kesch et al 5 e | 10 | 18F-PSMA-1007 | Region-based | PET–CT | Total agreement | 71% | 81% |
| Near-total agreement | 93% | 92% | |||||
| PET/MRI b | Total agreement | 81% | 81% | ||||
| Near-total agreement | 89% | 99% | |||||
| Hicks et al 18 e | 32 | 68Ga-PSMA-11 | Region-based | PET/MRI | Raw stringent approach | 67% | 71% |
| Neighboring approach | 74% | 88% | |||||
| GEE (population-averaged) | 73% | 70% | |||||
| Al-Bayati et al 20 e | 22 | 68Ga-PSMA-11 | Lesion-based | PET/MRI | Score 4–5 as positive a | 88% | 100% |
| Score 3–5 as positive a | 94% | 56% | |||||
| Chen et al 21 e | 54 | 68Ga-PSMA-11 | Lesion-based | PET–CT | 89% c | 71% c | |
| PET–MRI b | 89% c | 96% c | |||||
| Donato et al 16 e | 144 | 68Ga-PSMA-11 | Lesion-based | PET–CT | Equivocal or likely lesions as positive d | 95% c | 93% c |
| Likely lesions as positived | 91%c | 95%c | |||||
| Ferraro et al 25 f | 42 | 68Ga-PSMA-11 | Patient-based | PET–MRI | 96% c | 81% c |
GEE, Generalized estimating equation.
A 5-point Likert-scale
PET–CT in combination with MRI
Diagnostic performance for clinically significant prostate cancer
A 3-point Likert scale
Retrospective study including patients with biopsy-proven prostate cancer
Prospective study including patients with suspected prostate cancer
Besides the detection of primary prostate cancer, the majority of literature discusses how PSMA PET might be a useful tool for local staging of prostate cancer. mpMRI is the imaging modality of choice for pretreatment evaluation of local staging. However, the most updated data showed mpMRI has a sensitivity and specificity of 39 and 56% for detecting extracapsular extension, whereas for seminal vesicle, invasion was 33 and 95%, respectively. 26 Curative surgery is possible when extracapsular extension is absent. Therefore, PET–MRI may improve to define the extracapsular extension or seminal vesicle invasion of prostate cancer, to select the suitable high-risk patients of localized or locally advanced stage for definitive therapy. Based on a prospective study of 80 patients with pretreatment PET–MRI treated with radical prostatectomy published by Grubmüller et al, 25 the accuracy of PET–MRI for T1 stage, T2 stage, T3a stage, and T3b stage were 82.5%, 85%, 79%, and 94%, respectively. In a retrospective study of 40 patients having intermediate- to high-risk prostate cancer who underwent PET–MRI for primary staging followed by radical prostatectomy, Muehlematter et al reported that the diagnostic performance in local staging of PET–MRI and mpMRI was similar, and PET–MRI may improve the sensitivity of MRI to detect extracapsular disease but with reduced specificity. 27 In a similar study published by Brauchli et al, 28 100 patients who underwent radical prostatectomy after mpMRI and PET–CT imaging were reviewed. The results suggest that tumor-capsule interface measured on PET–CT is comparable to mpMRI criteria for predicting the presence of extracapsular extension. PET–MRI may be of specific advantage in predicting extracapsular extension in patients with MRI-occult prostate cancer.
It is important to keep in mind that there are false-positive and false-negative conditions in PSMA PET studies. Despite the term “prostate-specific”, PSMA expression can also be found in other tissues including sympathetic ganglia, inflammation/infection or epithelial cells, and tumor-associated neovascularization, hence causing the false-positivity. 29 Low-to-moderate PSMA expression can also be seen in osteoblastic areas in bony degeneration, osteoarthritis, or insufficiency fractures. 30 On the other hand, a small proportion of prostate cancers display no or minimal uptake at PSMA PET, possibly reflecting low PSMA expression, 9 with an exemplary case shown in Figure 2. In this situation PSMA PET can be false-negative and mpMRI might be helpful for lesion identification.
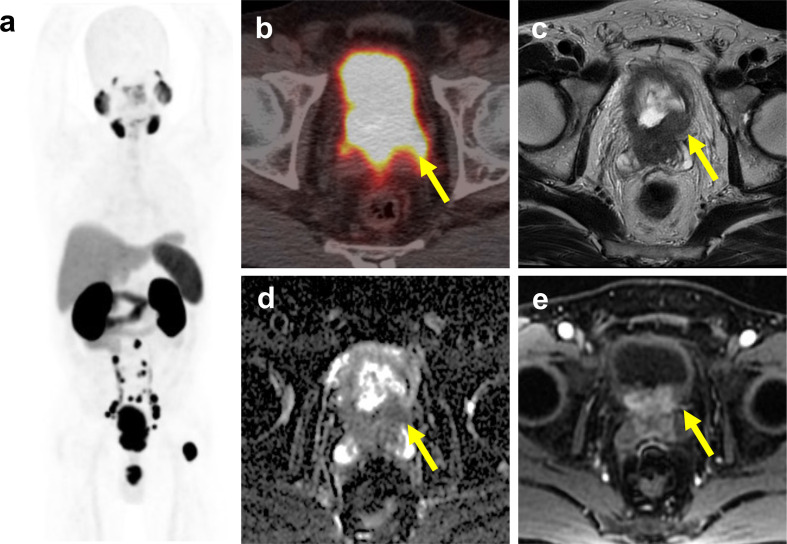
Figure 2.
Low PSMA expression cancer with nodal metastasis depicted by mpMRI. A 73-year-old male with biopsy-proved prostate cancer, the Gleason score was 5 + 4, and the PSA level was 5.73 ng ml−1. (a) The PSMA maximal intensity projection did not reveal any abnormal 68Ga-PSMA-11 uptake. (b) The fused PET–CT image showed low 68Ga-PSMA-11 uptake at right portion of prostate gland and right periprostatic node, labelled by yellow arrows. (c, d, e) The MRI showed a large prostate tumor with right extracapsular extension and a periprostatic lymph node. The lesions showed restricted diffusion on DWI.
Management of local recurrence
Evidence from a prospective multicenter study, 31 with 312 patients underwent PET–CT for restaging due to biochemical relapse with negative/equivocal conventional imaging (i.e., bone scintigraphy, CT and MRI), PET–CT revealed additional lesions in the prostate bed in 32% of patients. Lütje et al 32 compared PET–MRI with PET–CT for detection of recurring prostate cancer. In 25 patients, PET–MRI and PET–CT identified 14 and 9 local resurrecting cases, respectively, showing that PET–MRI was better as compared with PET–CT in the prostate bed recurrences. The major advantage of PET–MRI for detection of tumor recurrence in the prostate bed was the superior soft tissue contrast from MRI-component of PET–MRI. 33 Data from 119 patients with biochemical recurrence after radical prostatectomy underwent both PET–CT and PET–MRI, Freitag et al 34 showed that more cases of local recurrence were diagnosed in MRI-component of PET–MRI as compared to PET-component of PET–CT and PET–MRI (18 vs 9). In addition, detection of local recurrence using the PET-component was considerably influenced by its proximity to the bladder. The above studies suggested that PSMA PET is useful for detection of local recurrence, 31 and PET–MRI is likely superior as compared with PET–CT. 32,34 An example of prostate cancer with bladder involvement better delineated by mpMRI is shown in Figure 3. Since PSMA uptake is strongly influenced by the viable cells percentage, 9,10 and DWI is known to represent cell density, combined PET–MRI might enhance tumor characterization, however not yet been fully evaluated. The 68Ga-PSMA is excreted into urinary tract resulting high radioactivity in the bladder, which might mask lesions close proximity to the bladder and limit the PET detection of local recurrence of prostate cancer. By comparison to 68Ga-PSMA, 18F-PSMA is characterized by hepatobiliary clearance resulting in reducing the bladder radioactivity and has the potential to overcome the limitations. 33
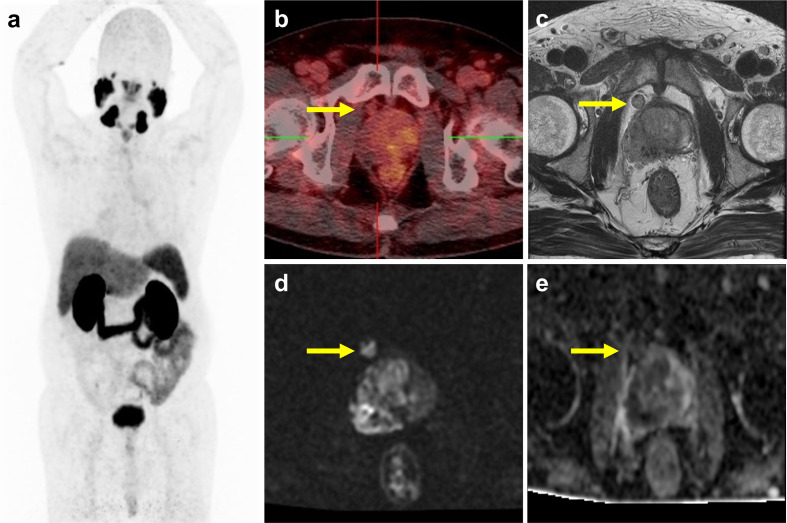
Figure 3.
Prostate cancer with bladder involvement better delineated by mpMRI. A 60-year-old male with prostate cancer initial clinical stage IVB. (a) The maximal intensity projection of PSMA PET showed prostate tumor with pelvic and para-aortic lymph nodes, and left proximal femur bone metastasis. (b) The fused PET–CT image showed high 68Ga-PSMA-11 uptake at prostate gland. However, high radioactivity in the bladder limit the ability of 68Ga-PSMA PET–CT to detect bladder neck invasion by prostate tumor. Prostate cancer with bladder involvement (arrow) and seminal vesicle invasion was clearly delineated on T 2-weighted image (c), ADC map (d), and DCE MRI (e).
Detection of lymph nodes metastases
PET–CT vs mpMRI
A recent meta-analysis compared the diagnostic performance of PET–CT and mpMRI in identifying lymph nodes (LN) metastasis for the intermediate- to high-risk prostate cancer. 35 The per-patient sensitivity and specificity of PET–CT were 65 and 94%, superior to the corresponding values of mpMRI were 41 and 92%, respectively. The area under the receiver-operating characteristic curve for PET–CT and mpMRI were 0.92 and 0.83, respectively. Malaspina et al prospectively compared PET–CT and whole-body MRI in 79 patients having intermediate- or high-risk prostate cancer at primary staging. 36 Pelvic LN metastases were found in 31 (39%) patients. At the patient level, sensitivity/specificity values for PET–CT and whole-body MRI were 87%/98 and 37%/98%, respectively. From the above studies, it can be supported that PET–CT has a significant higher sensitivity than mpMRI in the detection of LN metastasis in the primary staging. We had prospectively enrolled 34 patients with biochemically recurrent prostate cancer followed by robot-assisted radical prostatectomy, in which PET–CT identified more regional LN metastases than the mpMRI (10 vs 8). 37
PET–CT vs conventional CT/MRI
For primary nodal staging before definite therapy, studies have been conducted to assess PSMA studies in detecting regional LN metastasis with histopathological results from pelvic LN dissection as the gold standard. A retrospective study of 130 patients with intermediate- or high-risk prostate cancer revealed PET–CT to outperform CT or MRI in the detection of LN metastasis, with a per-patient sensitivity of 66vs 44% and a specificity of 99vs 85%. 1 Another study of 30 patients having intermediate- or high-risk patients showed PET–CT to have a per-patient sensitivity of 64% and specificity of 95%. 38 The “ProPSMA” study, a prospective randomized study for 302 prostate cancer patients with high-risk features considered for curative therapies from ten centers in Australia revealed PET–CT to provide superior per-patient sensitivity (83% vs 23%) and similar specificity (99% vs 96%) to CT for pelvic LN metastasis. 39
However, the sensitivity of PET–CT for detecting LN metastasis is only moderate, in contrast to the specificity which is higher than 90%. Klingenberg et al. evaluated 691 high-risk patients primary staged by PET–CT. LN metastasis was detected in 31% (217/691). 40 In 117 patients who undertook radical prostatectomy with concomitant pelvic LN dissection, per-patient sensitivity, specificity, positive and negative predictive values, and accuracy for LN metastasis detection on PET–CT were 31, 97, 69, 85, and 83%, respectively. Undetected LN metastases were either micro-metastases located in the LN border or without PSMA expression. Due to the limited resolution of PET, small LN metastases might undergo undetected. 38,41 LN metastases can also be missed due to the lack of PSMA expression in a minority of prostate cancers. 42 It is clear that PET–CT imaging still cannot replace pelvic LN dissection to exclude pelvic LN metastases.
PET–MRI vs mpMRI
Park et al 38 prospectively enrolled 33 patients with intermediate- or high-risk prostate cancer who were scheduled for radical prostatectomy with pelvic LN dissection, 43 with 12 out of 382 dissected nodes confirmed to harbor metastatic disease in three patients. PSMA PET showed six true-positive and six false-positive LNs while mpMRI showed no true-positive lesion and nine false-positive LNs. It is thus suggested that PET–MRI may better inform the need and extent for pelvic node dissection, yet more evidence is needed.
Management of nodal recurrence
For patients with biochemical failure and planning for salvage lymphadenectomy, Rauscher et al 44 reported data with mixed PET–CT and PET–MRI, detecting LN metastasis in 78% metastatic fields while CT or MRI was positive in only 27%. Specificity of PSMA PET and CT or MRI was 97 and 99%, respectively. The mean size of PET-positive LNs measured by MRI or CT was 8.3 mm (range 4–25 mm). Mandel et al 45 retrospectively analyzed 23 patients with nodal recurrence and found 29/109 (27%) resected fields harbored histologically confirmed LN metastases. The sensitivity and specificity of PSMA PET studies were 76 and 88% in region-based analysis. Linxweiler et al 46 identified 25 patients who underwent through robotic salvage lymphadenectomy for nodal recurrence detected by PET–CT. In total, 43 suspicious spots were detected on PET–CT and 66 histologically positive LNs were removed. In 23 LN-positive patients, PET–CT was correct in 13 (57%) patients, whereas nodal metastatic spread was more extensive in 10 (43%) patients than suggested by imaging. Siriwardana et al 47 retrospectively analyzed 35 patients who underwent robotic salvage lymphadenectomy for nodal recurrence detected by PET–CT. A total of 58 lesions suspicious for LN metastasis were detected and 32 patients (91%) were histopathologically proven. Jilg et al 42 retrospectively analyzed 30 patients with suspicion of exclusively nodal relapse who underwent a template pelvic/retroperitoneal salvage lymphadenectomy after PET–CT. 41 The sensitivity and specificity for main region (pelvic left/right, retroperitoneal) were 93.2 and 100%, and for subregion (common iliac, internal iliac, external iliac, obturator, aortal, vena cava, presacral, and aortic-bifurcation) were 81.2 and 99.5%, respectively. Based on anatomical sub regions containing just one LN metastasis, the essential short diameter of tumor deposits in LN metastasis required to reach a detection rate of 50 and 90% was estimated to be ≥2.3 mm and ≥4.5 mm, respectively.
PET–CT vs PET–MRI
There are few head-to-head comparisons of PET–CT vs PET–MRI in this regard. Domachevsky et al prospectively enrolled 140 successive patients with biopsy-proven prostate cancer to undergo prostate and pelvic PET/MR immediately after 68Ga-PSMA injection followed by whole-body PET–CT at 60 min after injection. 48 PSMA-avid pelvic LNs were noted on PET–CT in 33 patients (24%) and on PET/MR in 32 patients (23%), although no pathological approval was obtained. We have identified four studies with retrievable information for comparing PET–CT and PET–MRI imaging performed on the same day in patients with biochemical failure, with PET–CT acquisition performed around 1 h after PSMA injection and PET–MRI acquisition performed around 3 h after injection. 32,49–51 The patient-based and lesion-based detection rates for LN metastasis are listed in Table 2. An example of PET–MRI demonstrated local, regional, and distant tumor recurrence is shown in Figure 4. Overall PET–MRI seems to have slightly higher detection rates than PET–CT. This may be attributed to the delayed imaging time of PET–MRI and also to the better soft tissue contrast of MRI.
Table 2.
Detection of lymph node metastases in patients by 68Ga-PSMA PET–CT and PET–MRI with biochemically recurrent prostate cancer
| Study | Patient-based Analysis | Lesion-based Analysis | |||||
|---|---|---|---|---|---|---|---|
| Patient number | LN positive patient | PET–CT a | PET–MRI a | LN positive lesion | PET–CT b | PET–MRI b | |
| Afshar-Oromieh et al 49 | 20 | 11 | 100% | 100% | 49 | 100% | 100% |
| Freitag et al 48 | 26 | 20 | 100% | 100% | 64 | 98% | 100% |
| Lütje et al 32 | 25 | 14 | 93% | 100% | 24 | 83% | 96% |
| Guberina et al 50 | 93 | 103 | 95% | 98% | |||
LN, Lymph node.
Patient-based detection rate.
Lesion-based detection rate.
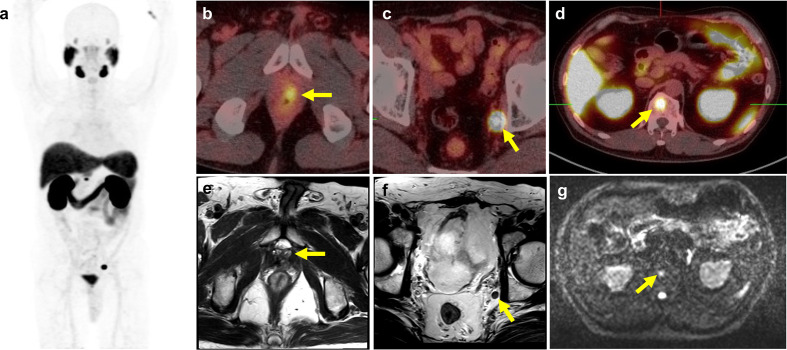
Figure 4.
PSMA PET–MRI demonstrated local, regional and distant tumor recurrence. A 65-year-old male underwent radical prostatectomy 7 years ago, initial staging pT3bN0M0, Gleason score 4 + 3, PSA was slow raising to 4.20 ng ml−1. (a) The maximum intensity projection of PSMA PET shows abnormally-increased 68Ga-PSMA-11 uptake at (b) left prostate fossa, (c) left obturator lymph node, and (d) T12 spine. The yellow arrows on the corresponding multisequence MR imaging demonstrates (e) a focal nodule at left anastomotic site, (f) a left obturator lymph node with short axis diameter measured as 0.6 cm, and (g) a focal hyperintensity on DWI at T12 vertebral body. These lesions were considered as recurrent and metastatic prostate cancer.
Detection of distant metastases
PET–CT vs mpMRI
Although mpMRI may achieve a sensitivity of 95% to detect bone metastasis for the purpose of primary staging, the routine MRI protocol usually limits the scanning field to the pelvifemoral area and lumbar spine and cannot assess the global burden of bone metastasis. 52 For detecting distant metastases at primary staging, the prospective “proPSMA” study revealed PET–CT to provide superior per-patient sensitivity (92% vs 54%) and specificity (99% vs 93%) to conventional imaging consisting of bone scintigraphy and CT. 39 Klingenberg et al 41 evaluated 691 consecutive newly diagnosed high-risk prostate cancer patients with PET–CT. 40 Bone metastases were identified in 17% of males (116/691) and 78% of these patients (90/116) had concurrent LN metastasis. Bone metastases were correlated with increasing PSA levels, clinical stages, and tumor grades, and around 2% of patients (13/691) also presented with visceral metastases. 40 Damjanovic et al retrospectively evaluated PET–CT scans of 739 prostate cancer patients to detect lung metastases and non-solid focal pulmonary opacities. 53 Ninety-one pulmonary metastases, of which 66 (72.5%) were PSMA-positive and 25 (27.5%) were PSMA-negative, and 14 opacities were recognized in 34 patients. Pulmonary opacities revealed a moderate tracer uptake, significantly lower than PSMA-positive lung metastases yet higher than PSMA-negative metastases. The study showed that PET–CT was not able to differentiate between pulmonary opacities and pulmonary metastases. Damjanovic et al also identified 123 liver metastases in 18 patients in these scans, 54 with 8 (78%) metastases being PSMA-positive while 23 (22%) metastases being PSMA-negative. Whole-body PSMA PET can be valuable in the detection of occult distant metastases and help identifying these patients to receive individualized multimodal treatment. Special attention should be taken on interpreting the lesions in the lung and liver.
PET–MRI vs mpMRI
As mpMRI can be simultaneously acquired during a PET–MRI study, PET–MRI is expected to be the one-stop-shop modality for prostate cancer imaging. The combined interpretation of distant lesions with both PSMA PET and mpMRI may decrease the false-positive and false-negative findings. An exploratory cost-effectiveness analysis for prostate cancer patients with biochemical failure suggested the use of PET/MRI to be cost-effective comparative to normal care. 55 Further studies are wanted to determine if PET–MRI can be cost-effective in comparison with PET–CT with mpMRI.
PET–CT vs PET–MRI
In the study conducted by Domachevsky et al of 140 newly diagnosed patients undergoing prostate and pelvic PET/MR followed by whole-body PET–CT, 11 patients (7.8%) were found to have bone metastases. 48 In three patients, bone metastases were detected on PET–CT outside the PET/MR field of view. Two patients had other clinically significant findings on PET–CT (1.4%), including one patient with PSMA-avid cervical and supra clavicular LNs and a second patient with PSMA-avid lung nodules. From the studies for comparing PET–CT and PET–MRI performed on the same day in patients with biochemical failure, the patient-based and lesion-based detection rates for bone metastases are listed in Table 3 injection. 32,49–51 Both modalities seem to have equivalent performance. In the study by Guberina et al 50 , 15 visceral metastases were also detected by both modalities. 51
Table 3.
Detection of bone metastases by 68Ga-PSMA PET–CT and PET–MRI with biochemically recurrent prostate cancer
| Study | Patient-based Analysis | Lesion-based Analysis | |||||
|---|---|---|---|---|---|---|---|
| Patient number | Bone positive patient | PET–CT a | PET/MRI a | Bone positive lesion | PET–CT b | PET/MRI b | |
| Afshar-Oromieh et al 49 | 20 | 6 | 100% | 100% | 23 | 100% | 100% |
| Freitag et al 48 | 26 | 8 | 100% | 100% | 28 | 100% | 100% |
| Lütje et al 32 | 25 | 2 | 100% | 100% | 4 | 100% | 100% |
| Guberina et al 50 | 93 | 10 | 100% | 100% | 23 | 100% | 100% |
LN, Lymph node.
Patient-based detection rate.
Lesion-based detection rate.
Management of distant recurrence
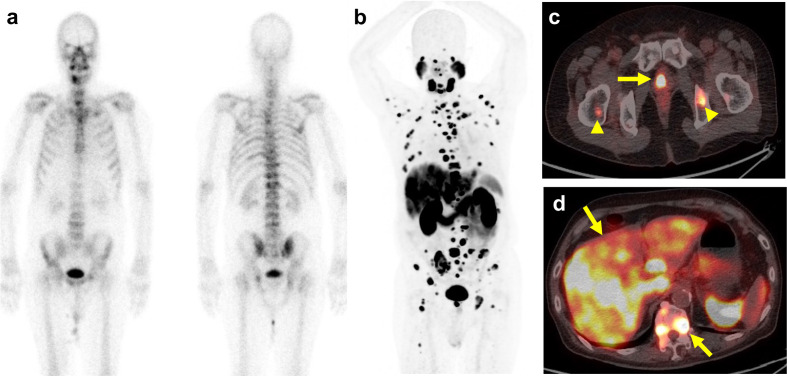
For patients with extensive metastases, PSMA PET may not provide additional impact for initial therapy but may serve as a baseline study to assess the global burden for later utility. In our prospective study of 34 patients with biochemically recurrent prostate cancer, 68Ga-PSMA-11 PET–CT identified non-regional LN metastases and bone metastases in 1 and 5 patients while mpMRI spotted these lesions in 1 and 4 patients, respectively. 37 With recurrent prostate cancer, Verburg et al retrospectively studied 155 patients receiving PET–CT studies. 56 PET–CT was positive in 44%, 79 and 89% patients and detected bone metastasis in 15%, 16 and 39% of patients having PSA levels of ≤1, 1–2 and ≥2 ng ml−1, respectively. Pyka et al retrospectively analyzed 126 patients who underwent either PET–CT or PET–MRI studies and bone scintigraphy with a median interval of 20 days. 57 In the patient-based analysis, with ambiguous scans considered negative, PSMA PET showed a sensitivity and specificity of 100vs57 and 100% vs 96%, respectively, as compared with bone scintigraphy. PSMA PET outperforms bone scintigraphy for the detection of bone metastasis for the primary staging and biochemical recurrence. PSMA PET and bone scintigraphy have equivalent performance even in the subset of metastatic castration-resistant prostate cancer (mCRPC) subgroup, 57 shown as in Figure 5. It can be concluded that additional bone scintigraphy is not needed if PSMA PET has been performed. To date no direct comparison of PET–CT vs PET–MRI has been reported on this aspect.
Figure 5.
PSMA PET–CT for managing castration-resistant prostate cancer (mCRPC). A 73-year-old male with initial staging T3N1M1b with androgen deprivation therapy since 7 years ago, PSA keep progression even after change treatment to new antiandrogen drug, castration-resistant prostate cancer (mCRPC) status was confirmed, and current PSA level was 675 ng ml−1. (a) The bone scintigraphy showed multiple hot spots involving spines, left posterior ribs, and pelvic bones. (b) The PSMA PET showed more extensively bony metastasis, (c) primary prostate cancer lesion labelled by yellow arrow, bony metastasis by arrowheads and (d) liver metastasis.
Conclusions
PSMA PET may be complementary to mpMRI for primary prostate cancer localization and is particularly valuable for PI-RADS three lesions. PET–MRI is superior to PET–CT in local tumor staging, may be of particular benefit in predicting extracapsular extension in patients with MRI-occult prostate cancer. PET–MRI is likely superior as compared with PET–CT in detecting local recurrence, and have slightly higher detection rates than PET–CT in lymph node recurrence. PET–CT and PET–MRI seem to have equivalent performance in detecting distant bony or visceral metastases. PET–MRI is useful for local and regional disease, either primary staging, or restaging. PET–CT is useful for managing distant bony or visceral metastasis. The cost-effective comparison of both modalities is still to be exploited.
Footnotes
Acknowledgements: The authors express thanks to Kuan-Ying Lu for manuscript preparation.
Funding: Grants from Chang Gung Memorial Foundation (CPRPG3G0023 and CLRPG3K0021) and Ministry of Science and Technology, Taiwan (MOST 109-2628-B-182A-007). Chang Gung IRB 104-4855A and 106-6435C.
Contributor Information
Feng-Yuan Liu, Email: billliufy@gmail.com.
Ting-Wen Sheng, Email: steven.sheng@gmail.com.
Jing-Ren Tseng, Email: b9105019@gmail.com.
Kai-Jie Yu, Email: cgurjay@gmail.com.
Ke-Hong Tsui, Email: tsui2130@gmail.com.
Se-Tong Pang, Email: jacobpang@cgmh.org.tw.
Li-Jen Wang, Email: lijenwang0918@gmail.com.
Gigin Lin, Email: giginlin@cgmh.org.tw, giginlin@gmail.com.
REFERENCES
- 1. Maurer T, Gschwend JE, Rauscher I, Souvatzoglou M, Haller B, Weirich G, et al. Diagnostic efficacy of (68)Gallium-PSMA positron emission tomography compared to conventional imaging for lymph node staging of 130 consecutive patients with intermediate to high risk prostate cancer. J Urol 2016; 195: 1436–43. doi: 10.1016/j.juro.2015.12.025 [DOI] [PubMed] [Google Scholar]
- 2. Afshar-Oromieh A, Babich JW, Kratochwil C, Giesel FL, Eisenhut M, Kopka K, et al. The rise of PSMA ligands for diagnosis and therapy of prostate cancer. J Nucl Med 2016; 57(Suppl 3): 79S–89. doi: 10.2967/jnumed.115.170720 [DOI] [PubMed] [Google Scholar]
- 3. Hoffmann MA, Miederer M, Wieler HJ, Ruf C, Jakobs FM, Schreckenberger M. Diagnostic performance of 68Gallium-PSMA-11 PET–CT to detect significant prostate cancer and comparison with 18FEC PET–CT. Oncotarget 2017; 8: 111073–83. doi: 10.18632/oncotarget.22441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li X, Rowe SP, Leal JP, Gorin MA, Allaf ME, Ross AE, et al. Semiquantitative Parameters in PSMA-Targeted PET Imaging with 18F-DCFPyL: Variability in Normal-Organ Uptake. J Nucl Med 2017; 58: 942–6. doi: 10.2967/jnumed.116.179739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kesch C, Vinsensia M, Radtke JP, Schlemmer HP, Heller M, Ellert E, et al. Intraindividual cComparison of 18F-PSMA-1007 PET–CT, mMultiparametric MRI, and radical prostatectomy specimens in patients with primary prostate cancer: a retrospective, proof-of-concept studyRadical Prostatectomy Specimens in Patients with Primary Prostate Cancer: A Retrospective, Proof-of-Concept Study. J Nucl Med 2017; 58: 1805–10. doi: 10.2967/jnumed.116.189233 [DOI] [PubMed] [Google Scholar]
- 6. Bednarova S, Lindenberg ML, Vinsensia M, Zuiani C, Choyke PL, Turkbey B. Positron emission tomography (PET) in primary prostate cancer staging and risk assessment. Transl Androl Urol 2017; 6: 413–23. doi: 10.21037/tau.2017.03.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fendler WP, Eiber M, Beheshti M, Bomanji J, Ceci F, Cho S, et al. 68Ga-PSMA PET–CT: joint EANM and SNMMI procedure guideline for prostate cancer imaging: version 1.0. Eur J Nucl Med Mol Imaging 2017; 44: 1014–24. doi: 10.1007/s00259-017-3670-z [DOI] [PubMed] [Google Scholar]
- 8. Werner RA, Derlin T, Lapa C, Sheikbahaei S, Higuchi T, Giesel FL, et al. 18F-labeled, PSMA-targeted radiotracers: leveraging the advantages of radiofluorination for prostate cancer molecular imaging. Theranostics 2020; 10: 1–16. doi: 10.7150/thno.37894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hofman MS, Hicks RJ, Maurer T, Eiber M. Prostate-specific membrane antigen PET: clinical utility in prostate cancer, normal patterns, pearls, and pitfalls. Radiographics 2018; 38: 200–17. doi: 10.1148/rg.2018170108 [DOI] [PubMed] [Google Scholar]
- 10. Salas Fragomeni RA, Amir T, Sheikhbahaei S, Harvey SC, Javadi MS, Solnes LB, et al. Imaging of nonprostate cancers using PSMA-targeted radiotracers: rationale, current state of the field, and a call to arms. J Nucl Med 2018; 59: 871–7. doi: 10.2967/jnumed.117.203570 [DOI] [PubMed] [Google Scholar]
- 11. Sheikhbahaei S, Afshar-Oromieh A, Eiber M, Solnes LB, Javadi MS, Ross AE, et al. Pearls and pitfalls in clinical interpretation of prostate-specific membrane antigen (PSMA)-targeted PET imaging. Eur J Nucl Med Mol Imaging 2017; 44: 2117–36. doi: 10.1007/s00259-017-3780-7 [DOI] [PubMed] [Google Scholar]
- 12. Stabile A, Giganti F, Rosenkrantz AB, Taneja SS, Villeirs G, Gill IS, et al. Multiparametric MRI for prostate cancer diagnosis: current status and future directions. Nat Rev Urol 2020; 17: 41–61. doi: 10.1038/s41585-019-0212-4 [DOI] [PubMed] [Google Scholar]
- 13. Kasivisvanathan V, Rannikko AS, Borghi M, Panebianco V, Mynderse LA, Vaarala MH, et al. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med 2018; 378: 1767–77. doi: 10.1056/NEJMoa1801993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Domachevsky L, Bernstine H, Goldberg N, Nidam M, Stern D, Sosna J, et al. Early 68GA-PSMA PET–MRI acquisition: assessment of lesion detectability and PET metrics in patients with prostate cancer undergoing same-day late PET–CT. Clin Radiol 2017; 72: 944–50. doi: 10.1016/j.crad.2017.06.116 [DOI] [PubMed] [Google Scholar]
- 15. Kalapara AA, Nzenza T, Pan HYC, Ballok Z, Ramdave S, O'Sullivan R, et al. Detection and localisation of primary prostate cancer using 68 gallium prostate-specific membrane antigen positron emission tomography/computed tomography compared with multiparametric magnetic resonance imaging and radical prostatectomy specimen pathology. BJU Int 2020; 126: 83–90. doi: 10.1111/bju.14858 [DOI] [PubMed] [Google Scholar]
- 16. Donato P, Morton A, Yaxley J, Ranasinghe S, Teloken PE, Kyle S, et al. 68Ga-PSMA PET–CT better characterises localised prostate cancer after MRI and transperineal prostate biopsy: Is 68Ga-PSMA PET–CT guided biopsy the future? Eur J Nucl Med Mol Imaging 2020; 47: 1843–51. doi: 10.1007/s00259-019-04620-0 [DOI] [PubMed] [Google Scholar]
- 17. Satapathy S, Singh H, Kumar R, Mittal BR. Diagnostic aAccuracy of 68Ga-PSMA PET–CT for initial detection in patients with suspected prostate cancer: a systematic review and meta-analysisInitial Detection in Patients With Suspected Prostate Cancer: A Systematic Review and Meta-Analysis. AJR Am J Roentgenol 2021; 216: 599–607. doi: 10.2214/AJR.20.23912 [DOI] [PubMed] [Google Scholar]
- 18. Hicks RM, Simko JP, Westphalen AC, Nguyen HG, Greene KL, Zhang L, et al. Diagnostic accuracy of 68Ga-PSMA-11 PET–MRI compared with multiparametric MRI in the detection of prostate cancer. Radiology 2018; 289: 730–7. doi: 10.1148/radiol.2018180788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eiber M, Weirich G, Holzapfel K, Souvatzoglou M, Haller B, Rauscher I, et al. Simultaneous 68Ga-PSMA HBED-CC PET–MRI improves the localization of primary prostate cancer. Eur Urol 2016; 70: 829–36. doi: 10.1016/j.eururo.2015.12.053 [DOI] [PubMed] [Google Scholar]
- 20. Al-Bayati M, Grueneisen J, Lütje S, Sawicki LM, Suntharalingam S, Tschirdewahn S, et al. Integrated 68Gallium labelled prostate-specific membrane antigen-11 positron emission tomography/magnetic resonance imaging enhances discriminatory power of multi-parametric prostate magnetic resonance imaging. Urol Int 2018; 100: 164–71. doi: 10.1159/000484695 [DOI] [PubMed] [Google Scholar]
- 21. Chen M, Zhang Q, Zhang C, Zhao X, Marra G, Gao J, et al. Combination of 68Ga-PSMA PET–CT and multiparametric MRI improves the detection of clinically significant prostate cancer: a lesion-by-lesion analysis. J Nucl Med 2019; 60: 944–9. doi: 10.2967/jnumed.118.221010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Uprimny C, Kroiss AS, Decristoforo C, Fritz J, von Guggenberg E, Kendler D, et al. 68Ga-PSMA-11 PET–CT in primary staging of prostate cancer: PSA and Gleason score predict the intensity of tracer accumulation in the primary tumour. Eur J Nucl Med Mol Imaging 2017; 44: 941–9. doi: 10.1007/s00259-017-3631-6 [DOI] [PubMed] [Google Scholar]
- 23. Sachpekidis C, Kopka K, Eder M, Hadaschik BA, Freitag MT, Pan L, et al. 68Ga-PSMA-11 dynamic PET–CT imaging in primary prostate cancer. Clin Nucl Med 2016; 41: e473–9. doi: 10.1097/RLU.0000000000001349 [DOI] [PubMed] [Google Scholar]
- 24. Grubmüller B, Baltzer P, Hartenbach S, D'Andrea D, Helbich TH, Haug AR, et al. Psma ligand PET–MRI for primary prostate cancer: staging performance and clinical impact. Clin Cancer Res 2018; 24: 6300–7. doi: 10.1158/1078-0432.CCR-18-0768 [DOI] [PubMed] [Google Scholar]
- 25. Ferraro DA, Becker AS, Kranzbühler B, Mebert I, Baltensperger A, Zeimpekis KG, et al. Diagnostic performance of 68Ga-PSMA-11 PET–MRI-guided biopsy in patients with suspected prostate cancer: a prospective single-center study. Eur J Nucl Med Mol Imaging 2021; 48: 3315-3324. doi: 10.1007/s00259-021-05261-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee T, Hoogenes J, Wright I, Matsumoto ED, Shayegan B. Utility of preoperative 3 tesla pelvic phased-array multiparametric magnetic resonance imaging in prediction of extracapsular extension and seminal vesicle invasion of prostate cancer and its impact on surgical margin status: experience at a Canadian academic tertiary care centre. Can Urol Assoc J 2017; 11: E174–8. doi: 10.5489/cuaj.4211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Muehlematter UJ, Burger IA, Becker AS, Schawkat K, Hötker AM, Reiner CS, et al. Diagnostic accuracy of multiparametric MRI versus 68Ga-PSMA-11 PET–MRI for extracapsular extension and seminal vesicle invasion in patients with prostate cancer. Radiology 2019; 293: 350–8. doi: 10.1148/radiol.2019190687 [DOI] [PubMed] [Google Scholar]
- 28. Brauchli D, Singh D, Chabert C, Somasundaram A, Collie L. Tumour-capsule interface measured on 18F-DCFPyL PSMA positron emission tomography/CT imaging comparable to multi-parametric MRI in predicting extra-prostatic extension of prostate cancer at initial staging. J Med Imaging Radiat Oncol 2020; 64: 829–38. doi: 10.1111/1754-9485.13084 [DOI] [PubMed] [Google Scholar]
- 29. de Galiza Barbosa F, Queiroz MA, Nunes RF, Costa LB, Zaniboni EC, Marin JFG, et al. Nonprostatic diseases on PSMA PET imaging: a spectrum of benign and malignant findings. Cancer Imaging 2020; 20: 23. doi: 10.1186/s40644-020-00300-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shetty D, Patel D, Le K, Bui C, Mansberg R. Pitfalls in Gallium-68 PSMA PET–CT Interpretation-A pictorial review. Tomography 2018; 4: 182–93. doi: 10.18383/j.tom.2018.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Roach PJ, Francis R, Emmett L, Hsiao E, Kneebone A, Hruby G, et al. The impact of 68Ga-PSMA PET–CT on management intent in prostate cancer: results of an Australian prospective multicenter study. J Nucl Med 2018; 59: 82–8. doi: 10.2967/jnumed.117.197160 [DOI] [PubMed] [Google Scholar]
- 32. Lütje S, Cohnen J, Gomez B, Grüneisen J, Sawicki L, Rübben H, et al. Integrated 68Ga-HBED-CC-PSMA-PET–MRI in patients with suspected recurrent prostate cancer. Nuklearmedizin 2017; 56: 73–81. doi: 10.3413/Nukmed-0850-16-09 [DOI] [PubMed] [Google Scholar]
- 33. Freitag MT, Kesch C, Cardinale J, Flechsig P, Floca R, Eiber M, et al. Simultaneous whole-body 18F-PSMA-1007-PET–MRI with integrated high-resolution multiparametric imaging of the prostatic fossa for comprehensive oncological staging of patients with prostate cancer: a pilot study. Eur J Nucl Med Mol Imaging 2018; 45: 340–7. doi: 10.1007/s00259-017-3854-6 [DOI] [PubMed] [Google Scholar]
- 34. Freitag MT, Radtke JP, Afshar-Oromieh A, Roethke MC, Hadaschik BA, Gleave M, et al. Local recurrence of prostate cancer after radical prostatectomy is at risk to be missed in 68Ga-PSMA-11-PET of PET–CT and PET–MRI: comparison with mpMRI integrated in simultaneous PET–MRI. Eur J Nucl Med Mol Imaging 2017; 44: 776–87. doi: 10.1007/s00259-016-3594-z [DOI] [PubMed] [Google Scholar]
- 35. Wu H, Xu T, Wang X, Yu YB, Fan ZY, Li DX, et al. Diagnostic performance of 68Gallium labelled prostate-specific membrane antigen positron emission tomography/computed tomography and magnetic resonance imaging for staging the prostate cancer with intermediate or high risk prior to radical prostatectomy: a systematic review and meta-analysis. World J Mens Health 2020; 38: 208–19. doi: 10.5534/wjmh.180124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Malaspina S, Anttinen M, Taimen P, Jambor I, Sandell M, Rinta-Kiikka I. Prospective comparison of 18F-PSMA-1007 PET–CT, whole-body MRI and CT in primary nodal staging of unfavourable intermediate- and high-risk prostate cancer. Eur J Nucl Med Mol Imaging 2021; 13: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tseng J-R, Yu K-J, Liu F-Y, Yang L-Y, Hong J-H, Yen T-C, et al. Comparison between 68Ga-PSMA-11 PET–CT and multiparametric magnetic resonance imaging in patients with biochemically recurrent prostate cancer following robot-assisted radical prostatectomy. J Formos Med Assoc 2021; 120(1 Pt 3): 688–96. doi: 10.1016/j.jfma.2020.07.029 [DOI] [PubMed] [Google Scholar]
- 38. Park SY, Zacharias C, Harrison C, Fan RE, Kunder C, Hatami N, et al. Gallium 68 PSMA-11 PET/MR imaging in patients with intermediate- or high-risk prostate cancer. Radiology 2018; 288: 495–505. doi: 10.1148/radiol.2018172232 [DOI] [PubMed] [Google Scholar]
- 39. van Leeuwen PJ, Emmett L, Ho B, Delprado W, Ting F, Nguyen Q, et al. Prospective evaluation of 68Gallium-prostate-specific membrane antigen positron emission tomography/computed tomography for preoperative lymph node staging in prostate cancer. BJU Int 2017; 119: 209–15. doi: 10.1111/bju.13540 [DOI] [PubMed] [Google Scholar]
- 40. Hofman MS, Lawrentschuk N, Francis RJ, Tang C, Vela I, Thomas P, et al. Prostate-Specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet 2020; 395: 1208–16. doi: 10.1016/S0140-6736(20)30314-7 [DOI] [PubMed] [Google Scholar]
- 41. Klingenberg S, Jochumsen MR, Ulhøi BP, Fredsøe J, Sørensen KD, Borre M, et al. 68Ga-PSMA PET–CT for Primary Lymph Node and Distant Metastasis NM Staging of High-Risk Prostate Cancer. J Nucl Med 2021; 62: 214–20. doi: 10.2967/jnumed.120.245605 [DOI] [PubMed] [Google Scholar]
- 42. Jilg CA, Drendel V, Rischke HC, Beck T, Vach W, Schaal K, et al. Diagnostic accuracy of Ga-68-HBED-CC-PSMA-ligand-PET–CT before salvage lymph node dissection for recurrent prostate cancer. Theranostics 2017; 7: 1770–80. doi: 10.7150/thno.18421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Keidar Z, Gill R, Goshen E, Israel O, Davidson T, Morgulis M, et al. 68Ga-PSMA PET–CT in prostate cancer patients - patterns of disease, benign findings and pitfalls. Cancer Imaging 2018; 18: 39. doi: 10.1186/s40644-018-0175-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rauscher I, Maurer T, Beer AJ, Graner F-P, Haller B, Weirich G, et al. Value of 68Ga-PSMA HBED-CC PET for the assessment of lymph node metastases in prostate cancer patients with biochemical recurrence: comparison with histopathology after salvage lymphadenectomy. J Nucl Med 2016; 57: 1713–9. doi: 10.2967/jnumed.116.173492 [DOI] [PubMed] [Google Scholar]
- 45. Mandel P, Tilki D, Chun FK, Pristupa E, Graefen M, Klutmann S, et al. Accuracy of 68Ga-prostate-specific membrane antigen positron emission tomography for the detection of lymph node metastases before salvage lymphadenectomy. Eur Urol Focus 2020; 6: 71–3. doi: 10.1016/j.euf.2018.07.025 [DOI] [PubMed] [Google Scholar]
- 46. Linxweiler J, Saar M, Al-Kailani Z, Janssen M, Ezziddin S, Stöckle M, et al. Robotic salvage lymph node dissection for nodal-only recurrences after radical prostatectomy: perioperative and early oncological outcomes. Surg Oncol 2018; 27: 138–45. doi: 10.1016/j.suronc.2018.02.010 [DOI] [PubMed] [Google Scholar]
- 47. Siriwardana A, Thompson J, van Leeuwen PJ, Doig S, Kalsbeek A, Emmett L, et al. Initial multicentre experience of 68 gallium-PSMA PET–CT guided robot-assisted salvage lymphadenectomy: acceptable safety profile but oncological benefit appears limited. BJU Int 2017; 120: 673–81. doi: 10.1111/bju.13919 [DOI] [PubMed] [Google Scholar]
- 48. Freitag MT, Radtke JP, Hadaschik BA, Kopp-Schneider A, Eder M, Kopka K, et al. Comparison of hybrid (68)Ga-PSMA PET–MRI and (68)Ga-PSMA PET–CT in the evaluation of lymph node and bone metastases of prostate cancer. Eur J Nucl Med Mol Imaging 2016; 43: 70–83. doi: 10.1007/s00259-015-3206-3 [DOI] [PubMed] [Google Scholar]
- 49. Afshar-Oromieh A, Haberkorn U, Schlemmer HP, Fenchel M, Eder M, Eisenhut M, et al. Comparison of PET–CT and PET–MRI hybrid systems using a 68Ga-labelled PSMA ligand for the diagnosis of recurrent prostate cancer: initial experience. Eur J Nucl Med Mol Imaging 2014; 41: 887–97. doi: 10.1007/s00259-013-2660-z [DOI] [PubMed] [Google Scholar]
- 50. Guberina N, Hetkamp P, Ruebben H, Fendler W, Grueneisen J, Suntharalingam S, et al. Whole-body integrated [68Ga]PSMA-11-PET–MR imaging in patients with recurrent prostate cancer: comparison with whole-body PET–CT as the standard of reference. Mol Imaging Biol 2020; 22: 788–96. doi: 10.1007/s11307-019-01424-4 [DOI] [PubMed] [Google Scholar]
- 51. Woo S, Kim SY, Kim SH, Cho JY. Journal Club: identification of bone metastasis with routine prostate MRI: a study of patients with newly diagnosed prostate cancer. AJR Am J Roentgenol 2016; 206: 1156–63. doi: 10.2214/AJR.15.15761 [DOI] [PubMed] [Google Scholar]
- 52. Gordon LG, Elliott TM, Joshi A, Williams ED, Vela I. Exploratory cost-effectiveness analysis of 68Gallium-PSMA PET–MRI-based imaging in patients with biochemical recurrence of prostate cancer. Clin Exp Metastasis 2020; 37: 305–12. doi: 10.1007/s10585-020-10027-1 [DOI] [PubMed] [Google Scholar]
- 53. Verburg FA, Pfister D, Heidenreich A, Vogg A, Drude NI, Voo S, et al. Extent of disease in recurrent prostate cancer determined by [(68)Ga]PSMA-HBED-CC PET–CT in relation to PSA levels, PSA doubling time and Gleason score. Eur J Nucl Med Mol Imaging 2016; 43: 397–403. doi: 10.1007/s00259-015-3240-1 [DOI] [PubMed] [Google Scholar]
- 54. Pyka T, Okamoto S, Dahlbender M, Tauber R, Retz M, Heck M, et al. Comparison of bone scintigraphy and 68Ga-PSMA PET for skeletal staging in prostate cancer. Eur J Nucl Med Mol Imaging 2016; 43: 2114–21. doi: 10.1007/s00259-016-3435-0 [DOI] [PubMed] [Google Scholar]
- 55. Damjanovic J, Janssen J-C, Furth C, Diederichs G, Walter T, Amthauer H, et al. 68 Ga-PSMA-PET–CT for the evaluation of pulmonary metastases and opacities in patients with prostate cancer. Cancer Imaging 2018; 18: 20. doi: 10.1186/s40644-018-0154-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Damjanovic J, Janssen J-C, Prasad V, Diederichs G, Walter T, Brenner W, et al. 68Ga-PSMA-PET–CT for the evaluation of liver metastases in patients with prostate cancer. Cancer Imaging 2019; 19: 37. doi: 10.1186/s40644-019-0220-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Domachevsky L, Bernstine H, Goldberg N, Nidam M, Catalano OA, Groshar D. Comparison between pelvic PSMA-PET–MR and whole-body PSMA-PET–CT for the initial evaluation of prostate cancer: a proof of concept study. Eur Radiol 2020; 30: 328–36. doi: 10.1007/s00330-019-06353-y [DOI] [PubMed] [Google Scholar]