Abstract
There is increasing interest in the use of multiparametric magnetic resonance imaging (mpMRI) in the prostate cancer pathway. The European Association of Urology (EAU) and the British Association of Urological Surgeons (BAUS) now advise mpMRI prior to biopsy, and the Prostate Imaging Reporting and Data System (PI-RADS) recommendations set out the minimal technical requirements for the acquisition of mpMRI of the prostate.
The widespread and swift adoption of this technique has led to variability in image quality. Suboptimal image acquisition reduces the sensitivity and specificity of mpMRI for the detection and staging of clinically significant prostate cancer.
This critical review outlines the studies aimed at improving prostate MR quality that have been published over the last 5 years. These span from the use of specific MR sequences, magnets and coils to patient preparation. The rates of adherence of prostate mpMRI to technical standards in different cohorts across the world are also discussed.
Finally, we discuss the first standardised scoring system (i.e., Prostate Imaging Quality, PI-QUAL) that has been created to evaluate image quality, although further iterations of this score are expected in the future.
Introduction
Multiparametric magnetic resonance imaging (mpMRI) of the prostate is now an essential component in the diagnostic pathway of prostate cancer. It is also used in active surveillance, treatment planning and in the detection of recurrent local disease. 1
MpMRI of the prostate is a 30 to 45 min scan that includes T2W images (T2-WI) in two/three planes, diffusion-weighted imaging (DWI) and dynamically contrast-enhanced (DCE) sequences. MR spectroscopy is no longer routinely performed according to the latest recommendations. 2
Over the last 10 years, advances in mpMRI have enabled precise detection and characterisation of lesions suspicious for prostate cancer, with level one published evidence for its use in the detection of prostate cancer and targeting of biopsies. 1 More importantly, it has been shown that a negative scan can be used to safely defer biopsy, with a negative predictive value for Gleason Grade Group ≥ 2 and Gleason Grade Group ≥ 3 of 91 and 97%, respectively. 3 However, translating these results from centres with optimised scan quality into clinical practice has been problematic. The widespread use of mpMRI of the prostate has resulted in high variability of scan quality for each sequence, but particularly for the DWI and DCE sequences, and radiologist experience is a key component (i.e., inter reader agreement is poorer for less experienced readers), with minimum requirements recently being proposed in the UK. 4 This is often unrecognised, and the diagnostic accuracy of mpMRI of the prostate is assumed to be that of the published data when in practice this is not the case.
Clinicians who regularly request and perform mpMRI of the prostate are aware that this technique can be extremely useful during daily clinical practice but also recognise the problems from the variability of scan technique between centres. Prostate mpMRI is at critical point in its adoption and MR quality at many sites is disappointingly poor. 2 Clinical decisions will be compromised as prostate MRI quality impacts on patient management, and clinicians will lose confidence in the technique unless this can be improved.
Therefore, a standard for diagnostic scan quality is urgently required.
The first consensus meeting on prostate mpMRI was held in 2011 5 and the panel of prostate cancer experts reached consensus on a number of areas related to the conduct, interpretation and reporting of mpMRI for the detection, localisation and characterisation of prostate cancer.
A year later, the Prostate Imaging Reporting and Data System (PI-RADS) guidelines (v.1), which outlined the minimum technical requirements and standards for prostate mpMRI reporting, were published. 6
These guidelines were subsequently refined in 2015 (v.2) 7 and in 2019 (v.2.1). 8 While maintaining the overall framework described in the previous version, PI-RADS v.2.1 includes revised imaging acquisition parameters and a revised scoring system. As far as technical details are concerned, PI-RADS v.2.1 clarifies that T2-WI should be obtained in the axial plane and in at least one additional orthogonal plane and that a high b-value (≥1,400 s/mm2) DWI should be always acquired. The minimum temporal resolution for DCE acquisitions has been decreased from ≤10 to ≤15 s.
In order to reduce the number of indeterminate lesions, subtle changes to the scoring of the transitional zone (scores 1 to 3) and an update to the scoring of lesions on DWI (scores 2 and 3 for all zones) have been made in PI-RADS v.2.1 and some of the subjective definitions have been clarified (e.g., the term ‘marked’ has been changed to ‘a more pronounced signal change than any other focus in the same zone’).
In the recent years, there has been also interest in the evaluation of inter reader agreement for PI-RADS v.2 and v.2.1 scoring systems with results showing substantial to excellent concordance 9,10 and in the comparison between biparametric MRI (i.e., without DCE acquisitions) and mpMRI, with some evidence 11 suggesting similar sensitivity (94%) and diagnostic accuracy (87%) for both techniques when comparing PI-RADS 1 and 2 lesions vs PI-RADS 3, 4 and 5 lesions.
Table 1 shows the most recent technical requirements for a good quality prostate mpMRI according to the latest version (PI-RADS v.2.1). In addition to this, in 2018 a UK consensus meeting reiterated the importance of high-quality prostate MRI, especially when this technique is used to avoid biopsy. 12
Table 1.
Minimal technical requirements for multiparametric prostate MRI according to the PI-RADS v.2.1 guidelines
|
T
2-weighted imaging (T2-WI) |
Diffusion-weighted imaging (DWI) |
Dynamic contrast-enhanced (DCE) |
|
|---|---|---|---|
| Imaging planes | Same used for DWI and DCE | Same used for T2-WI and DCE | Same used for T2-WI and DWI |
| Slice thickness | 3 mm, no gap | ≤4 mm, no gap | 3 mm, no gap |
| Field of view | 12–20 cm a | 16–22 cm | 12–20 cm a |
| In-plane dimension | ≤0.7 mm (phase) x ≤ 0.4 mm (frequency) | ≤2.5 mm (phase and frequency) | ≤2 mm (phase and frequency) |
| Specific recommendations | |||
| T2-WI acquisition | Axial plane: either straight axial to the patient or in an oblique axial plane matching the long axis of the prostate | - | - |
| At least one additional orthogonal plane (sagittal and/or coronal) | - | - | |
| 3D axial as an adjunct to 2D acquisitions | - | - | |
| Low b-value | - | 0 (preferably 50)–100 sec/mm2 | - |
| Intermediate b-value | - | 800–1000 sec/mm2 | - |
| High b-value | - |
|
- |
| Temporal resolution | - | - | ≤15 s |
| Total observation rate | - | - | >2 min |
| Dose of GBCA | - | - | 0.1 mmol/kg |
| Injection rate | - | - | 2 - 3cc/s |
| Fat suppression/subtraction | - | - | Recommended |
Legend – T2-WI: T 2-weighted imaging; DWI: diffusion-weighted imaging; DCE: dynamic contrast enhanced; GBCA: Gadolinium-based contrast agent
to encompass the entire prostate gland and seminal vesicles
In the last 5 years, several questions have been raised about the best protocol that should be used to obtain prostate mpMRI of adequate diagnostic quality, with good spatial resolution and high signal-to-noise ratio for each sequence. This quest has been complicated by the number of differing machine vendors, the ages of the MR scanners and the differences between acquisition at 1.5 vs 3T.
The aim of our paper is to critically discuss the available literature over the last 5 years in order to i) report those studies which have tried to address the major controversies in prostate MRI quality and ii) provide the readers with an overview of current practice and what is still needed to be done to improve the quality of mpMRI of the prostate.
Methods
Search strategy
We searched MEDLINE/PubMed for manuscripts published up to the 1st of April 2021.
As we know that mpMRI has improved over time and in order to provide updated data from the last 5 years, we excluded all papers published before 2016.
The search terms used were (prostate cancer OR prostate adenocarcinoma) AND (MRI quality OR magnetic resonance imaging quality).
Evidence synthesis
Overall, 890 publications were found, 377 of which published before 2016. Therefore, a total of 513 publications were evaluated and if it was not clear from the abstract whether the paper might contain relevant data, the full paper was assessed. In total, 42 papers were included in the final analysis. (Figure 1)
Figure 1.
Flow diagram showing the outcome of the initial searches resulting in the full studies included in the review.
Results
The quality of mpMRI sequences depends not only on the MR systems and scanning parameters utilised but also on patient-related factors, such as bowel peristalsis, rectal distension, the presence of hip metalwork and post-biopsy haemorrhage.
MR sequences
According to current PI-RADS v.2.1 recommendations 8 , T2-WI should be usually obtained with 2D rapid acquisition with fast-spin-echo or turbo-spin-echo sequences.
For DWI, free-breathing spin echo sequences combined with spectral fat saturation are recommended.
As far as DCE sequences are concerned, the assessment of the enhancement may be improved with fat suppression or subtraction techniques (especially in the presence of blood products that are hyperintense on pre-contrast-enhanced T1-weighted images).
We assessed 21 papers investigating the impact of specific MRI sequences on image quality, and the results are listed in Table 2. 13–33
Table 2.
Summary data for studies investigating the impact of specific MRI sequences on image quality
| Author [ref] | Year | Country | MR system | Endorectal coil | Aim of the study for image quality | MR sequence | Study design | Patients (n) | Scale used to assess prostate MR quality | Inter reader agreement (κ value) |
Key messages on image quality |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Verma et al. 13 | 2016 | USA | 3T | Yes | To compare high b-value acquired DWI with computed DWI obtained using four diffusion models: i) mono-exponential; ii) intravoxel incoherent motion; iii) stretched exponential and iv) diffusional kurtosis. | DWI | Retrospective Three radiologists independently |
94 | Likert (1 to 5) | - | High b-value computed DWI demonstrated higher image quality and lesion conspicuity than acquired DWI except for diffusional kurtosis. |
| Westphalen et al. 14 | 2016 | USA | 3T | Yes | To compare the perceived quality of axial T2-weighted high-resolution 2D and high-resolution 3D fast spin-echo MRI. | T2-WI | Retrospective Six radiologists independently |
85 | Likert (1 to 3) | - | No difference in delineation of the zonal anatomy (p = 0.19), prostatic capsule (p = 0.14) and tumour conspicuity (p = 0.89). No difference when assessing motion artefacts (p = 0.48) and distortion (p = 0.41). 2D images significantly sharper than 3D (p < 0.001) and significantly more likely to exhibit non-motion artefacts (p = 0.002). No difference in confidence in tumour identification. 3D images might be a better option when time saving is crucial. |
| Zhang et al. 15 | 2016 | China | 3T | No | To investigate whether a new readout segmentation of long variable echo-trains (RESOLVE)-based diffusional kurtosis imaging with reduced b-value technique can affect image quality and diagnostic effectiveness of prostate MRI. | DWI | Prospective Two radiologists independently |
120 | - | PI-RADS score: 0.39 to 0.41 Extracapsular extension: 0.86 to 0.89 Seminal vesicle invasion: 0.96 to 1 |
B values significantly influenced image quality, PI-RADS score, and diffusional-kurtosis imaging outputs. |
| Iyama et al. 16 | 2017 | Japan | 3T | No | To compare the quality of fat suppression and image quality between multiecho Dixon technique and spectrally adiabatic inversion recovery (SPAIR). | DCE | Prospective Two radiologists independently |
60 | Likert (1 to 4) | Homogeneity of fat suppression: 0.71 Image noise: 0.77 Image contrast: 0.76 Image sharpness: 0.81 |
Multiecho Dixon technique improved the homogeneity of fat suppression without degrade of image quality. |
| Tamada et al. 17 | 2017 | USA - Japan | 3T | No | To compare image between reduced field-of-view diffusion-weighted imaging (rFOV-DWI) and standard DWI (st-DWI). | DWI | Retrospective Two radiologists independently |
49 | Likert (1 to 5) | Overall image quality: 0.13 Anatomic distortion: 0.07 Visualisation of the prostatic capsule: 0.18 Visualisation of PZ/TZ edge: 0.14 Clarity of the depicted lesion: 0.36 Assigned DWI score: 0.39 Confidence in the assigned DWI score: 0.18 |
Although rFOV-DWI may improve distortion for prostate DWI, only one reader reported significant improvement in image quality using this technique (p < 0.05). |
| Tanaka et al. 18 | 2017 | Japan | 3T | No | To investigate the impact of 3D T2-WI turbo spin-echo imaging (TSE T2-WI) with tissue-specific variable refocusing flip angle (TS-VRFA) on image quality compared to 2D and conventional T2-WI with volume isotropic TSE acquisition (VISTA). |
T2-WI | Retrospective Two radiologists independently |
40 | Likert (1 to 5) | Prostate cancer detection: 0.70 to 0.74 Extraprostatic extension: 0.66 to 0.73 |
TS-VRFA had better image quality than VISTA and equivalent to 2D (p < 0.05). |
| Corcuera-Solano et al. 19 | 2017 | USA | 3T | No | To compare a faster diagonal diffusion-weighted imaging (d-DWI) to conventional three-scan trace DWI (t-DWI) acquisition in terms of image quality, tumour detection/conspicuity and quantitative estimated signal-to-noise ratio (eSNR). | DWI | Retrospective Two radiologists independently |
34 | Likert (1 to 5) | - | eSNR was lower with d-DWI (p < 0.05). d-DWI provided a substantial reduction in acquisition time (30%) with equivalent image quality and tumour detection. |
| Stocker et al. 20 | 2017 | Switzerland | 3T | No | To compare image quality and geometric distortion of four DWI sequences using comparable imaging parameters and similar acquisition times. | DWI | Prospective Two radiologists independently |
10 | Likert (1 to 5) | - | Single-shot spin-echo (Ss-EPI) and prototype single-shot technique applying slice-specific Shimming (iShim-EPI) showed a tendency toward superior image quality and signal-to-noise ratio compared with readout-segmented multishot (rs-EPI) and selective excitation–reduced field of view (sTX-EPI) with no significant differences in geometric distortion. |
| Xi et al. 21 | 2018 | USA | 3T | Yes | To optimise a low-to-high b value DWI ratio approach in terms of visual presentation of prostate cancer and compare it against conventional ADC maps. | DWI | Retrospective Two radiologists independently |
43 | Likert (1 to 5) | Artefacts: 0.39 Cancer conspicuity: 0.72 Image quality: 0.64 |
Optimised DWI ratio images were comparable both quantitatively and qualitatively to ADC maps for the interpretation of DWI data. |
| Ma et al. 22 | 2018 | China | 3T | No | To compare high b value (2,000 s/mm2) reduced field-of-view (rFOV) DWI with a conventional DWI sequence in terms of image quality. | DWI | Retrospective Two radiologists independently |
61 | Likert (1 to 5) | Overall image quality: 0.79 and 0.78 | The rFOV DWI could offer improved image compared to the conventional sequence. |
| Polanec et al. 23 | 2018 | Austria | 3T | No | To determine whether 3D acquisitions provide equivalent image quality compared to 2D acquisitions in T2-WI. | T2-WI | Prospective Two radiologists independently |
150 | Likert (1 to 5) | - | 3D showed equivalent image quality and lesion delineation compared to 2D T2-WI, shortening the MR protocol by 40%. |
| Warndahl et al. 24 | 2018 | USA | 3T | Yes | To compare the image quality and quantitative data provided by a conventional DWI sequence and a limited Field of view Optimised and Constrained Undistorted Single shot (FOCUS) DWI sequence. | DWI | Retrospective Two radiologists independently |
44 | Likert (1 to 5) | - | FOCUS showed significantly better image quality compared to the conventional sequence (p < 0.001). |
| Rosenkrantz et al. 25 | 2018 | USA | 3T | No | To compare standard and reduced field-of-view (rFOV) DWI acquisitions in two patients with hip implants. | DWI | Case reports | 2 | - | - | Improved tumour detection and localisation using the rFOV acquisition scheme improves image quality in patients with hip implants. |
| Czarniecki et al. 26 | 2018 | United Kingdom | 1.5T | No | To compare image quality, artefact, and distortion in standard echo-planar imaging (EPI) with periodically rotated overlapping parallel lines with enhanced reconstruction (PROPELLER) for DWI in patients with previous total hip replacement. | DWI | Retrospective Two radiologists independently |
21 | Likert (1 to 4) and Likert (1 to 5) | T2-WI quality: 0.53 Artefacts on T2-WI: 0.94 Artefacts on EP-DWI: 0.82 Image quality on EP-DWI: 0.73 Image quality for PROPELLER-DWI-FS: 0.75 Artefacts on PROPELLER-DWI-FS: 0.78 Distortion on EP-DWI: 0.20 Distortion on PROPELLER-DWI-FS: 0.3 |
PROPELLER-DWI demonstrated better image quality and decreased both artefact and distortion compared to conventional echo planar sequences in patients with hip metalwork. |
| Meier-Schroers et al. 27 | 2018 | Germany | 3T | No | To evaluate revised PROPELLER (RevPROP) for T2-WI as a substitute for turbo spin echo. |
T2-WI | Prospective Two radiologists in consensus |
50 | Likert (1 to 4) | - | RevPROP showed fewer artefacts and higher image quality (p < 0.001) than turbo spin echo sequences. |
| Jendoubi et al. 28 | 2019 | France | 3T | No | To compare computed high b-value diffusion-weighted images (c-DWI) derived from low b-value DWI images and acquired high b-value DWI (a-DWI), in overall image quality. | DWI | Retrospective Four radiologists independently |
124 | Likert (1 to 5) | - | Computed b 2,000 s/mm2 and b 2,500 s/mm2 images had significant higher overall quality, better background signal suppression, better anatomic clarity and less distortion compared with acquired b 2,000 images (p < 0.001). Greater conspicuity of the index lesions for both computed b 2,000 s/mm2 and computed b 2,500 s/mm2 images (p < 0.001). |
| Kordbacheh et al. 29 | 2019 | USA | 3T | No | To evaluate the impact of complex-averaging on image quality and diagnostic accuracy of acquired and calculated high b-value (aHBV, cHBV) images in DWI. | DWI | Retrospective Two radiologists independently |
84 | Likert (1 to 3) | - | Complex-averaging improved image quality of acquired high b value and calculated high b value images (p < 0.001). The image quality of calculated high b value images was not significantly different than acquired high b value images. Complex-averaging provided better image quality and level of confidence without significant impact on diagnostic accuracy for the detection of significant prostate lesions. |
| Hellms et al. 30 | 2019 | Germany | 3T | No | To evaluate readout-segmented echoplanar (rsEPI) DWI compared to single-shot echoplanar imaging (ssEPI) sequences. | DWI | Prospective Two radiologists independently |
110 | Likert (1 to 5) | - | Anatomic delineation was significantly better and image quality higher with rsEPI than with ssEPI. |
| Klingebiel et al. 31 | 2020 | Germany | 3T | No | To evaluate image quality comparing high resolution readout-segmented (rs) multi shot echo-planar imaging (EPI), parallel transmit (ptx) EPI, and single-shot (ss) EPI with different b-values. | DWI | Retrospective Two radiologists independently |
36 | Likert (1 to 5) | rs-EPI: 0.76 to 0.83 ptx-EPI: 0.83 to 0.92 ss-EPI: 0.86 to 0.94 |
Image quality of rs-EPI is higher compared to ptx-EPI or ss-EPI, at the expense of longer acquisition time. |
| Gassenmaier et al. 32 | 2021 | Germany | 3T | No | To introduce a novel deep learning (DL) T2W TSE imaging (T2DL) and investigate its impact on image quality compared to standard T2W TSE imaging (T2S). | T2-WI | Retrospective Two radiologists independently |
30 | Likert (1 to 4) | Image quality: 0.68 T2 score:
PI-RADS score:
|
Noise levels and overall image quality of T2DL are significantly superior compared to T2S (p < 0.001). |
| Wang et al. 33 | 2021 | USA | 1.5T or 3T | Yes (turned on and turned off) | To evaluate the performance of a deep learning-based reconstruction method (DLR) to T2WI in improving image quality and mitigating artefacts. | T2-WI | Retrospective Three radiologists independently |
31 | Likert (1 to 3) and Likert (1 to 5) | Image quality: 0.58 Artefacts: 0.34 |
The non-endorectal coil (i.e., turned off) DLR was the best series for overall image quality, reduced artefacts and visualisation of anatomical landmarks and tumour. |
The majority of the studies (19/21; 90%) used 3T scanners, with only two (10%) studies using 1.5T systems (one of which using both magnets).
Only 5/21 (24%) studies used an endorectal coil (one of which comparing the findings with the coil turned on and then off).
Most of the studies (14/21; 67%) were retrospective, followed by 6/21 (29%) prospective studies and by one paper (4%) describing two case reports.
The number of radiologists participating in the studies ranged from two (for 16/21 studies; 76%) to six (for 1/21 study; 5%), the majority of which (19/21; 90%) assessed the images independently.
As far as mpMRI sequences are concerned, 14/21 (67%) studies focussed on DWI, 6/21 (29%) on T2-WI and 1/21 (4%) on DCE sequences.
From the papers included in this section, it is clear that non-patient-related approaches, including post-processing techniques such as reduced field of view sequences, 24 dedicated sequences for the correction of artefacts 26 or read-out segmented echo planar imaging sequences 30 can improve image quality.
A general recommendation is that DWI and DCE acquisitions should always match T2-WI in order to have a synchronous view when assessing all three sequences side by side.
As far as DWI is concerned, diagonal DWI (i.e., a particular acquisition in which all three gradients are turned on simultaneously to maximum strength, enabling a shorter echo time compared to conventional DWI acquisitions where the three gradients are switched on in three mutually perpendicular directions sequentially) has shown a substantial reduction in acquisition time (30%) with equivalent image quality and tumour detection. 19
A matter of particular interest is the role of computed vs acquired high b-value sequences, as mixed results have been published. Verma and colleagues 13 reported that high b-value computed sequences demonstrated higher image quality and lesion conspicuity than acquired DWI. Jendoubi et al 28 reported that computed high b-value (b = 2,000 s/mm2 and b = 2,500 s/mm2) images had significant higher overall quality, better background signal suppression, better anatomic clarity and less distortion compared with acquired (b = 2,000 s/mm2) images (p < 0.001) with a significant impact on lesion conspicuity (p < 0.001).
On the other hand, Kordbacheh and colleagues 29 evaluated the impact of complex-averaging on image quality of acquired and calculated high b-values and found that although complex-averaging improved image quality of both acquired high b-value and calculated high b-value images (p < 0.001), the image quality was not different between the two sequences.
As far as T2-WI is concerned, three studies 14,18,23 reported that 3D images showed similar or higher image quality than 2D acquisitions and that there might be a better option when time saving is crucial.
In addition to this, Meier Schroers and colleagues 27 have shown that revised Periodically Rotated Overlapping ParallEL Lines with Enhanced Reconstruction (PROPELLER) acquisitions (RevPROP) (i.e., a method to reduce motion artefacts) showed fewer artefacts and higher image quality (p < 0.001) than conventional turbo spin echo sequences on T2-WI.
As far as DCE is concerned, a study by Iyama and colleagues 16 has shown that the Multiecho Dixon technique improved the homogeneity of fat suppression without degrading image quality.
Magnets and coils
According to current PI-RADS v.2.1 recommendations, 8 an endorectal coil should be used to improve the signal-to-noise ratio with older 1.5T MR scanners and with larger patients, as the signal-to-noise ratio could be highly reduced hampering the diagnostic quality of the scan.
We assessed nine papers investigating the impact of MR magnets and coils on image quality, and the results are presented in Table 3. 34–42
Table 3.
Summary data for studies investigating the impact of coils and magnets on image quality
| Author [ref] | Year | Country | MR system | Endorectal coil | Aim of the study for image quality | Study design | Patients (n) | Scale used to assess prostate MR quality | Inter-reader agreement (κ value) |
Key messages on image quality |
|---|---|---|---|---|---|---|---|---|---|---|
| Baur et al. 34 | 2016 | Germany | 3T | Yes | To compare image quality and diagnostic performance for the detection of prostate cancer using a pelvic-phased array coil (PAC) and a combined endorectal and pelvic-phased array coil (ERC-PAC). | Prospective Two radiologists independently |
45 | Likert (1 to 5) | - | Overall image quality for T2-WI was significantly improved with an ERC-PAC compared to a PAC (p < 0.05). |
| Barth et al. 35 | 2016 | Switzerland | 3T | Yes | To compare image quality and patient discomfort using a pelvic-phased array (PPA) coil and an endorectal (ER) coil. | Prospective Two radiologists independently |
98 | Likert (1 to 5) | - | Comparable image quality using PPA or ER coil for T2-WI. Better image quality using the ER coil for DWI for one of the two readers. |
| Gawlitza et al. 36 | 2017 | Germany | 3T | Yes | To compare image quality between surface coils only and in combination with an endorectal coil. | Retrospective Two radiologists independently |
41 | Likert (1 to 5) | - | Image quality improved with an endorectal coil. |
| Ullrich et al. 37 | 2017 | Germany | 3T (mpMRI) and 1.5T (bpMRI) | No | To evaluate image quality of 1.5T and 3T MRI without endorectal coil. | Prospective Two radiologists independently |
63 | Likert (1 to 5) | Image quality (3T and 1.5T):
PI-RADS Score (3T and 1.5T):
|
Comparable image quality (i.e., signal to noise ratio and contrast to noise ratio) on T2-WI. Better image quality (i.e., signal to noise ratio and contrast to noise ratio) of DWI at 3T. Subjective image quality of 3T images superior to 1.5T images. |
| Tanaka et al. 38 | 2019 | USA | 1.5T | Yes | To report image quality in patients with cardiac implantable electronic devices (CIEDs). | Retrospective Two radiologists independently |
25 | Likert (1 to 5) | - | Multiparametric 1.5T MRI can be safely performed in selected patients with CIEDs under controlled conditions with acceptable image quality when MR parameters are optimised. |
| O’Donohoe et al. 39 | 2019 | USA | 3T | Yes | To evaluate T2-WI and DWI image quality using a wearable pelvic coil (WPC) compared with an endorectal coil (ERC). | Prospective Two radiologists in consensus |
18 | Likert (1 to 5 and 1 to 4) | - | More artefacts on T2-WI with an ERC than a WPC (p = 0.003). Zonal anatomy distinction superior for ERC compared with WPC (p = 0.018) on DWI. SNR significantly higher for ERC for both T2-WI and DWI. |
| Dhatt et al. 40 | 2020 | Canada | 3T | Yes (with and without) | To compare image quality with and without endorectal coil (ERC) using a combination of T2-WI and DWI. | Prospective Two radiologists independently |
23 | Likert (1 to 5) | - | No significant difference in image quality with and without an ERC. |
| Ullrich et al. 41 | 2020 | Multicentre | 3T | No (Group A) Yes (Group B) |
To compare image quality with a novel flexible surface coil (FSC) and with a conventional endorectal coil (ERC). | Retrospective Six radiologists independently |
150 | Likert (1 to 4) | Moderate for four readers: 0.42, 0.42, 0.48 and 0.53 Substantial for one reader: 0.62 Almost perfect for one reader: 0.90 |
Comparable subjective image quality for prostate MRI with an ERC and the novel FSC. ERC imaging might be valuable for sequences with inherently lower signal-to-noise ratio (e.g., DWI and larger patients). FSC is generally preferred for T2WI, as FSC imaging generates a lower signal-to-noise ratio than with an ERC. |
| Stocker et al. 42 | 2021 | Switzerland | 3T | No | To evaluate the influence of body-phased array receive coil set-ups on signal-to-noise ratio and image quality. | Prospective Two radiologists independently |
10 | Likert (1 to 5) | - | Image quality was similar for different body-phased array receive coil set-ups. Good image quality with 18-channel body-phased array receive coil set-up. Improved signal-to-noise ratio for T2-WI with a 60-channel body-phased array receive coil setup. |
The majority of the studies (7/9; 78%) used 3T scanners, with only two (22%) papers using 1.5T scanners (one of which using both magnets).
Seven studies (78%) used an endorectal coil (two of which comparing the findings with and without).
Six studies out of nine (67%) were retrospective, and the number of radiologists ranged from two (for 8/9 studies; 89%) to six (for 1/9 study; 11%), all but one 39 assessing images independently.
We found mixed results, as a number of studies showed that the use of an endorectal coil improves image quality 34,36 while other studies reported more artefacts on T2-WI but a superior zonal anatomy distinction on DWI with an endorectal coil 39 or no significant differences in image quality with and without an endorectal coil. 40
Interestingly, a study by Barth and colleagues 35 showed comparable image quality using a pelvic-phased array or an endorectal coil for T2-WI, but one of the radiologists of the study reported better image quality using the endorectal coil for DWI.
Another aspect that should be considered is that of patients with cardiac implantable electronic devices. Tanaka and colleagues 38 reported that patients with such devices can be safely studied on 1.5T mpMRI under controlled conditions with acceptable image quality when MR parameters are optimised (while maintaining a specific absorption rate <1.5 W/kg).
It should be pointed out that unlike MR protocols that incorporate endorectal coils, the sole use of body coils can lead to susceptibility artefacts due to the presence of rectal air, and this is particularly evident at high b-values on DWI.
We know that prostate mpMRI at both 1.5T and 3T can provide adequate and reliable diagnostic examinations when acquisition parameters are optimised.
In addition to this, the increasing shift from 1.5T to 3T MR scanners has resulted in less examinations conducted with endorectal coils (that provide an increase in the signal-to-noise ratio) due to the inherent higher signal-to-noise ratio and superior spatial resolution of 3T scanners using body surface coils.
There are a number of disadvantages to the use of endorectal coils, such as patient discomfort, distortion of the prostate contour, increased acquisition time and cost, near-field flare artefacts in the peripheral zone, and the inducement of rectal wall spasm. 43
Ullrich and colleagues 37 compared image quality of 1.5T and 3T MRI without endorectal coil in 63 consecutive patients. All patients received mpMRI on a 3T (T2-WI, DWI, DCE) and biparametric MRI (T2-WI, DWI) on a 1.5T scanner using body coils. Signal-to-noise ratio and contrast-to-noise ratio of T2-WI were similar at 1.5T and 3T, but significantly lower at 1.5T (p < 0.01). Image quality (using a 5-point Likert scale) was significantly better at 3T for T2-WI and DWI (p < 0.01) but PI-RADS scores were comparable for both field strengths. Inter-reader agreement was excellent for image quality and PI-RADS scoring. The authors concluded that although image quality on 1.5T and 3T MR was different on DWI, the diagnostic performance (i.e., PI-RADS score) was similar with both magnets.
Patient preparation
According to current PI-RADS v.2.1 recommendations, 8 the use of an antispasmodic agent may not be necessary in all patients. The incremental cost and potential for adverse drug reactions should be taken into consideration.
We assessed a total of eight studies focussing on the impact of patient preparation on image quality, and the results are listed in Table 4. 44–51
Table 4.
Summary data for studies investigating the impact of patient preparation on image quality
| Author [ref] | Year | Country | MR system | Endorectal coil | Aim of the study for image quality | Study design | Patients (n) | Scale used to assess prostate MR quality | Inter-reader agreement (κ value) |
Key messages on image quality |
|---|---|---|---|---|---|---|---|---|---|---|
| Caglic et al. 44 | 2017 | UK | 3T | No | To evaluate the effect of rectal distension on image quality. | Retrospective Two radiologists independently |
173 | Likert (1 to 5) | Scoring of rectal loading: 0.82 | Strong correlation between subjective scoring of rectal loading and objectively measured rectal volume (p < 0.001). Significant correlation between increased rectal distension and both reduced DWI quality and increased DWI distortion (p < 0.001). Significant trend for rectal distension to increase artefact at DWI (p = 0.042). Increased rectal distension led to increased motion artefact on T2-WI (p < 0.01). No relationship between rectal distension and DCE image quality (p = 0.693). |
| Slough et al. 45 | 2017 | UK | 3T | No | To evaluate the effect of an i.v. spasmolytic agent on the quality of anatomical and functional imaging. | Retrospective Two radiologists independently |
173 | Likert (1 to 4) and Likert (1 to 5) | T2-WI image quality: 0.67 T2-WI motion artefacts: 0.51 T2-WI blurring: 0.34 DWI image quality: 0.44 DWI distortion 0.74 DWI artefact 0.43 ADC image quality 0.63 |
Administration of an i.v. spasmolytic agent significantly improved the image quality of T2-WI. No significant improvement in DWI or ADC image quality, or DWI degree of distortion or artefact. |
| Ullrich et al. 46 | 2018 | Germany | 3T | No | To evaluate the effect of an i.v. spasmolytic agent on the visualisation of anatomical details and motion-related artefacts. | Prospective Two radiologists independently |
103 | Likert (1 to 5) | Anatomical details: 0.97 (before spasmolytic agent) and 0.96 (after spasmolytic agent) Artefacts: 0.98 (before spasmolytic agent) and 0.95 (after spasmolytic agent) |
Administration of an i.v. spasmolytic agent significantly improved the visualisation of anatomic details and reduced motion-related artefacts. Non-diagnostic MRI studies reduced to <1%. |
| Purysko et al. 47 | 2020 | USA | 3T | No | To evaluate the effect of enema and dietary restrictions on prostate MR image quality. | Retrospective Four radiologists independently |
195 | Likert (1 to 5) | Overall image quality: 0.29 T2-WI artefacts: 0.37 DWI distortion: 0.34 |
Enema and dietary restriction improved the quality of prostate MRI by decreasing rectal distension and distortion of DWI and by increasing reader confidence in image assessment. Inter reader agreement using subjective criteria for analysis of MRI quality was fair. |
| Coskun et al. 48 | 2020 | USA | 3T | No | To investigate the effects of cleansing Fleet’s™ enema (FE) on rectal distention and image quality of DWI. | Retrospective Two radiologists independently |
117 | Likert (1 to 4) and Likert (1 to 5) | Rectal distension: 0.53 (without bowel preparation) and 0.45 (with bowel preparation) DWI distortion: 0.1 (without bowel preparation) and 0.24 (with bowel preparation) Artefacts: 0.08 (without bowel preparation) and 0.1 (with bowel preparation) |
Bowel preparation with enema prior to prostate MRI diminished rectal gas but had modest effects on DWI distortion and overall image quality. |
| Plodeck et al. 49 | 2020 | Germany | 3T | No | To assess whether the application of a preparatory micro-enema reduces gas-induced susceptibility artefacts on DWI. |
Retrospective Two radiologists independently |
114 | Likert (1 to 3) | Artefacts on DWI: 0.8 | The use of a preparatory micro-enema prior to prostate MRI significantly reduced both the incidence and severity of gas-induced artefacts on DWI, improving image quality. |
| Reischauer et al. 50 | 2021 | Switzerland | 3T | No | To compare the impact of enema vs a small catheter on image quality on DWI | Retrospective Two radiologists independently |
200 | Likert (1 to 5) | Artefacts: 0.95 (catheter) and 0.92 (enema) Anatomical structures: 0.95 (catheter) and 0.92 (enema) Overall image quality: 0.94 (catheter) and 0.92 (enema) |
Enema preparation was superior to catheter preparation and yielded substantial improvements in image quality. |
| Schmidt et al. 51 | 2021 | Switzerland | 3T | No | To investigate the value of hyoscine N-butylbromide, microenema and dietary restrictions for artefact reduction and image quality. | Retrospective Two radiologists independently |
180 | Likert (1 to 5) | - | Microenema significantly improved image quality of DWI and the overall impression of image quality (encompassing all aspects of the whole MRI exam, including T2-WI, DWI, ADC and DCE-MRI). Hyoscine N-butylbromide and dietary restrictions did not improve image quality. |
All studies were conducted on 3T scanners without endorectal coils.
Most of the studies (5/6; 83%) were retrospective and the number of radiologists ranged from two (for 5/6 studies; 83%) to four (for 1/6 study; 17%), all assessing images independently.
Three studies 45,46,50 investigated the effect of i.v. administration of spasmolytic agents on image quality. Four studies 44,47–49 investigated the effect of rectal distension, enema and dietary restrictions on image quality. One study 51 compared all of these techniques.
An inverse correlation between image quality and increased rectal distension has been reported by Caglic and colleagues, 44 with a significant increase of artefacts on T2-WI and DWI with increasing rectal distension.
As far as motion-related artefacts due to peristalsis are concerned, a recent study by Schmidt and colleagues 51 compared the use of antispasmodic agents (i.e., hyoscine N-butylbromide) against microenema and dietary restriction. They found that microenema significantly improves both image quality of DWI and the overall impression of image quality (encompassing all aspects of the whole MRI exam, including T2-WI, DWI, and DCE sequences) while hyoscine N-butylbromide and dietary restrictions do not improve image quality.
A significant improvement of image quality using i.v. spasmolytic agents 45,46 has been observed in terms of anatomic details on T2-WI but not for the presence of artefacts on DWI.
Mixed results have been reported as far as the use of preparatory enemas are concerned, with two studies 47,49 showing that this approach improves image quality on DWI by decreasing rectal distension and distortion while another study by Coskun and colleagues 48 showed that the use of a cleansing enema prior to prostate MRI has modest effects on DWI distortion and overall image quality.
It is clear that prostate MRI quality can be significantly degraded by motion-related (due to bowel peristalsis) and susceptibility-related (due to air/tissue interfaces) artefacts, which in turn reduces the ability to rule in and rule out clinically significant disease. 52
In order to overcome this issue, different methods including the administration of antispasmodic agents, rectal enemas or the use of small catheters to remove the air from the rectum have been proposed as potential ways to reduce such artefacts.
Although the current evidence suggests routine use of anti-peristaltic agents prior to prostate mpMRI to optimise image quality, current guidelines are contradictory. 53
Rate of adherence to standard technical requirements
Four papers have evaluated the adherence of prostate mpMRI to minimum technical standards, as shown in Table 5. 54–57
Table 5.
Summary data for studies investigating the rate of adherence to minimum technical requirements
| Author [ref] | Year | Country | MR system | Endorectal coil | Aim of the study for image quality | Guidelines | Study design | Number of scans (n) | Scale used to assess prostate MR quality | Inter reader agreement (κ value) |
Key messages on image quality |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Esses et al. 54 | 2018 | USA | 1.5T and 3T | Yes / No | To assess variability in imaging facilities’ adherence to the minimum technical standards established by PI-RADS v.2 guidelines. | PI-RADS v.2 | Retrospective | 107 | - | - | Lowest adherence on T2-WI for frequency resolution (17%) and phase resolution (49%). Lowest adherence on DWI for field of view (30%). Lowest adherence on DCE imaging for slice thickness (33%) and temporal resolution (31%). Adherence to T2-WI phase resolution and DWI inter slice gap were greater at 3T than at 1.5T (p < 0.05). |
| Burn et al. 55 | 2019 | United Kingdom | 1.5T and 3T | No | To assess image quality and compliance with technical standards between centres in the South West region of the United Kingdom. | PI-RADS v.2 and UK Consensus paper on prostate MRI | Retrospective Two radiologists in consensus |
94 | Likert (1 to 5) and Likert (1 to 4) | - | Patients with diagnostically acceptable scans were 68% for T2-WI, 81% for DWI, and 60% for both T2-WI and DWI. 93% percent of the 45 patients who underwent DCE had diagnostically acceptable studies. By scanner age, the percentage of patients with diagnostically acceptable T2-WI scores was 53% for scanners ≥ 7 years and 80% when < 7 years (p = 0.006). |
| Coskun et al. 56 | 2019 | Turkey | 1.5T and 3T | Yes / No | To determine the compliance with the minimum acceptable technical parameters in tertiary-care centres in Turkey. | PI-RADS v.2 | Retrospective Survey (questionnaire) |
111 tertiary referral centres across Turkey | - | - | Low adherence to voxel dimensions for T2-WI, b values ≥ 1,400 s/mm2 for DWI and temporal resolution for DCE. The adherence to slice thickness, field of view, and in-plane dimension (phase) for T2-WI was higher for 3T. |
| Sackett et al. 57 | 2020 | USA | 1.5T and 3T | NA | To evaluate the adherence to the PI-RADS v.2 minimal technical requirements for T2-WI and DWI. | PI-RADS v.2 | Retrospective Six radiologists independently |
62 | Likert (1 to 5) | Image quality:
|
Lower adherence for DWI. Adherence to PI-RADS v.2 minimum technical standards did not increase the likelihood of having a qualitatively adequate T2-WI or DWI. |
Two studies were conducted in the USA, 54,57 one in the UK 55 and one in Turkey. 56
All studies included both 1.5T and 3T scanners and assessed the image quality and compliance against the technical standards as per PI-RADS v.2 guidelines, 7 although one study 55 did also test image quality making reference to a UK consensus paper on prostate MRI. 12
All studies were retrospective, and one of them (25%) was carried out as a survey using a specific questionnaire that was sent to all radiology departments of tertiary referral hospitals in Turkey. 56
An important aspect of mpMRI of the prostate is the compliance with the minimum technical requirements recommended by national/international guidelines, although we know that this does not necessarily mean that a scan is of adequate diagnostic quality simply adhering to a set of pre-defined technical recommendations.
It is interesting to note that in the study by Esses and colleagues 54 in the USA, the rate of adherence to PI-RADS v.2 guidelines was significantly greater at 3T for some parameters on T2-WI and DWI, and similar findings were observed in the study by Coskun et al for T2-WI in Turkey. 56 In both studies, the adherence to temporal resolution <10 s on DCE was low (31% in 54 and 56% in 56 ). It should be noted that the temporal resolution has been decreased to 15s in PI-RADS v.2.1 recommendations, 8 and this means that the spatial resolution should be optimised and not compromised, especially since curve analysis and quantitative kinetic modelling are no longer recommended for routine clinical use.
Another important aspect that should be taken into account is scanner age. Although this requires more investigation, Burn and colleagues 55 have shown a significant difference in the quality of prostate MRI at a 7-year cut-off for scanner age and this is something that will need to be explicitly addressed in the next iteration of the PI-RADS recommendation.
Discussion
From the evidence gathered in this review, we can reiterate that the quality of mpMRI must be high both at a ‘centre level’ (i.e., good quality images with up-to-date MR scanners and dedicated radiologists/radiographers highly experienced in prostate mpMRI) and at a ‘patient level’ (i.e., patient- related artefacts such as rectal gas or movement should be minimised).
The available data published over the last five years and presented in this review are conflicting and with mixed conclusion in terms of MR sequences, patient preparation, magnet strengths and coils.
The main differences lie in the heterogeneity of the cohorts (e.g., dietary habits of different countries and cultures can influence rectal distension - and therefore image quality) and in the study conduct (e.g., different timing of an enema or different timing and route of antiperistaltic agents administration can affect image quality).
We know from a recent consensus report by the European Society of Urogenital Radiology (ESUR) and the European Association of Urology (EAU) Section of Urologic Imaging (ESUI) that there is still vast inconsistency in the conduct of prostate mpMRI. 58 The panel of experts who outlined these recommendations concluded that quality measures for prostate mpMRI have yet to be clearly defined, and they called for a standardised set of criteria for assessing image quality.
This is corroborated by the fact that each study mentioned in this work included a different subjective scoring system mostly based on a 1–5 Likert scale, although some studies comprised a simplified 1–3 14 or 1–4 45 (for artefacts) scale. This explains why mixed results in the inter-observer agreement have been reported in the studies, with DWI being the diagnostic sequence with the highest variability in terms of artefacts and overall image quality (as reported in Table 2).
What has emerged from this review is the high inconsistency and subjectivity in defining a scan of ‘poor’, ‘acceptable’ or ‘good’ diagnostic quality, and this is indeed due to the lack of standardised criteria for the assessment of image quality.
A first attempt to fill this void has been the recent publication of a dedicated scoring system from the multicentre randomised PRECISION trial, 59 called Prostate Imaging Quality (PI-QUAL). 60 (Table 6)
Table 6.
Assessment of the diagnostic quality of multiparametric MRI scans using the PI-QUAL score.
| PI-QUAL score | Criteria | Clinical Implications |
|---|---|---|
| 1 | All mpMRI sequences are below the minimum standard for diagnostic quality | It is NOT possible to rule in all significant lesions
a
It is NOT possible to rule out all significant lesions a |
| 2 | Only one mpMRI sequence is of acceptable diagnostic quality | |
| 3 | At least two mpMRI sequences taken together are of diagnostic quality | It is possible to rule in all significant lesions It is NOT possible to rule out all significant lesions |
| 4 | Two or more mpMRI sequences are independently of diagnostic quality | It is possible to rule in all significant lesions It is possible to rule out all significant lesions |
| 5 | All mpMRI sequences are of optimal diagnostic quality |
PI-QUAL, Prostate Imaging QUALity; PI-RADS, Prostate Imaging Reporting and Data System; mpMRI, multiparametric magnetic resonance imaging.
Reprinted from Giganti F, Allen C, Emberton M, MooreCM, Kasivisvanathan V, for the PRECISION study group (2020) Prostate ImagingQuality (PI-QUAL): A New Quality Control S2588-9311(20)30085-7 (in press) doi:10.1016/j.euo.2020.06.007. Copyright (2020), with permission from Elsevier (https://euoncology.europeanurology.com).
Therefore reports should not include PI-RADS or Likertscores
The PI-QUAL score is built on a 1–5 Likert scale derived by evaluating each mpMRI sequence against a defined set of objective quality criteria in line with the PI-RADS guidelines and also using a subjective assessment of the image.
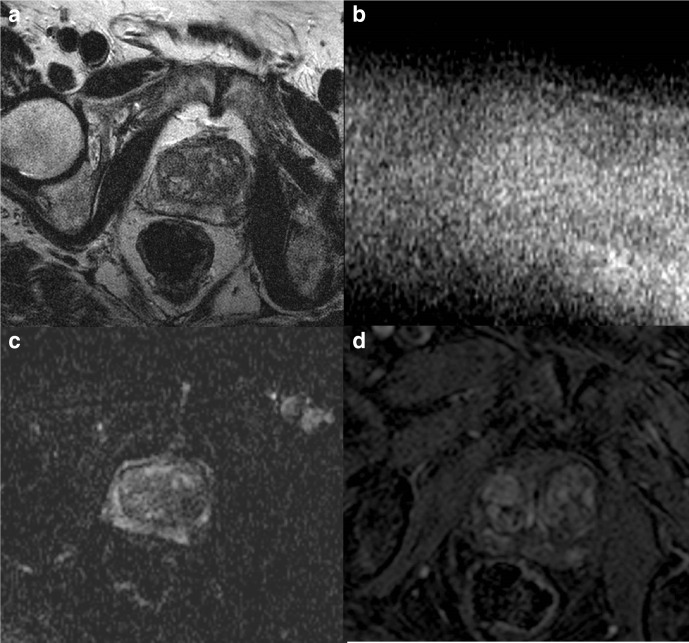
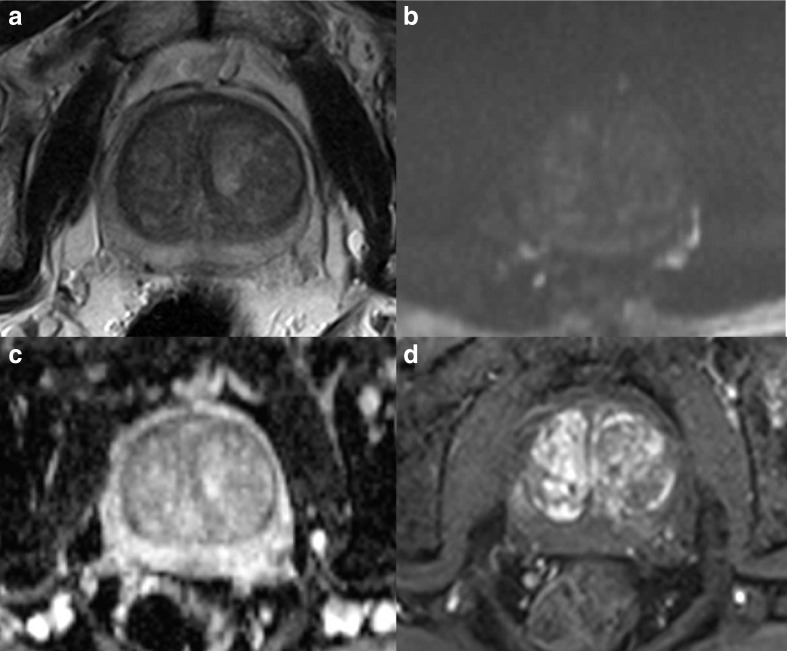
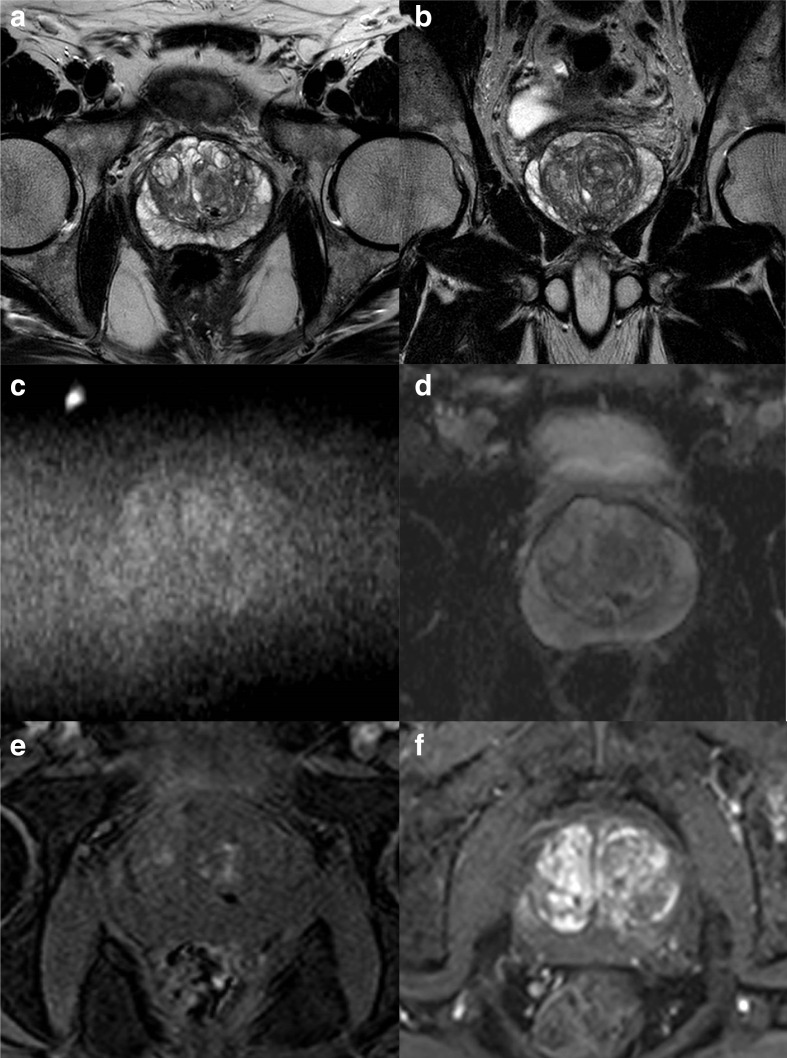
A PI-QUAL score of 1 or 2 (Figure 2) means that it is not possible to rule in and rule out clinically significant prostate cancer, a PI-QUAL score of 3 (Figure 3) entails that it is possible to rule in but not to rule out clinically significant disease, while a PI-QUAL score of 4 or 5 (Figures 4 and 5) corresponds to high-quality mpMRI of the prostate and therefore it is possible both to rule in and to rule out clinically significant prostate cancer. 60
Figure 2.
A 3T multiparametric MRI study of the prostate showing low spatial resolution on axial T2-WI (A), high b-value (b = 2,000 s/mm2) (B) and ADC map (C), and on DCE sequences (D). The overall image quality is suboptimal and only T2-WI is of acceptable diagnostic quality. The scan is scored PI-QUAL 2.
Figure 3.
A 1.5T multiparametric MRI study of the prostate showing artefacts from rectal distension on axial T2-WI (A), high b-value (b = 1,400 s/mm2) (B) and ADC map (C), and on DCE sequences (D). There is a lesion in the left peripheral zone at 4 o’clock but only T2-WI and DCE sequences taken together are of sufficient diagnostic quality. The scan is scored PI-QUAL 3.
Figure 4.
A 1.5T multiparametric MRI study of the prostate showing minor artefacts from bowel peristalsis on axial T2-WI (A) but the image quality is adequate on high b-value (b = 1,400 s/mm2) (B) and ADC map (C), and on DCE sequences (D). The scan is scored PI-QUAL 4.
Figure 5.
A 3T multiparametric MRI study of the prostate of optimal diagnostic quality on axial (A) and coronal (B) T2-WI, on high b-value (b = 2,000 s/mm2) (C) and ADC map (D; note the clear differentiation between the transitional and peripheral zone), and on pre- (E) and post- (F) contrast DCE sequences. The scan is scored PI-QUAL 5.
It should be pointed out that the PI-QUAL score, in the same way as the PI-RADS score, assesses the images but it does not take into account clinical details such as Prostate Specific Antigen (PSA) or PSA density. Also, at present, the PI-QUAL score does not make any clear recommendation whether a scan should be repeated (e.g., if there is a clear PI-RADS 5 lesion that can still be seen with a poor-quality scan, or a PI-QUAL 3 scan in which it is not possible to rule out clinically significant prostate cancer, but the patient is of low clinical risk) or not.
It is important to stress that scans from non-specialist centres usually show suboptimal imaging quality due to a lack of awareness of the quality achieved in high-volume, academic centres and PI-QUAL represents the first effort to inform the radiological community of the importance of prostate MR quality. Therefore, the widespread adoption of this scoring system and its future iterations could help to drive improvements when the imaging quality is inadequate.
It is essential that we reduce the variability in the conduct and quality of mpMRI of the prostate so that clinicians can be confident to use it in the prostate diagnosis and treatment pathways.
Further research on which criteria should be incorporated in the technical guidelines and the creation of a shared sequence bank for widespread improvement of mpMRI quality (including the use of automated methods) is strongly advocated. 61,62
Conclusions
Increasing evidence on the importance of prostate MR quality has been accumulated since the publication of PI-RADS v.2 in 2015.
There is compelling evidence to support the standardisation of prostate MRI quality using objective and pre-defined criteria and the PI-QUAL score is the first attempt to bridge this gap. The application of the PI-QUAL score (and its future iterations) in a ‘real-world’ scenario and the assessment of its effect on patient-level outcomes with prospective trials are planned.
Footnotes
Disclosures: Francesco Giganti is a recipient of the 2020 Young Investigator Award funded by the Prostate Cancer Foundation / CRIS Cancer Foundation.
Veeru Kasivisvanathan is funded by the UK National Institute for Health Research (NIHR).
Alex Kirkham is supported by the UCLH/UCL Biomedical Research Centre.
Shonit Punwani receives research support from the United Kingdom’s
National Institute of Health Research (NIHR) UCLH/UCL Biomedical Research Centre.
Mark Emberton is a United Kingdom National Institute of Health Research (NIHR) Senior Investigator and receives research support from the UCLH/UCL NIHR Biomedical Research Centre. He was conferred NIHR Senior Investigator Status in 2013.
Caroline M Moore is supported by the UKNIHR, Movember, PCUK and the EAU Research Foundation.
Clare Allen: none
Contributor Information
Francesco Giganti, Email: f.giganti@ucl.ac.uk.
Veeru Kasivisvanathan, Email: veeru.kasi@ucl.ac.uk.
Alex Kirkham, Email: alexkirkham@yahoo.com.
Shonit Punwani, Email: shonit.punwani@gmail.com.
Mark Emberton, Email: m.emberton@ucl.ac.uk.
Caroline M Moore, Email: caroline.moore@ucl.ac.uk.
Clare Allen, Email: allenclar@googlemail.com.
REFERENCES
- 1. Stabile A, Giganti F, Rosenkrantz AB, Taneja SS, Villeirs G, Gill IS, et al. Multiparametric MRI for prostate cancer diagnosis: current status and future directions. Nat Rev Urol 2020; 17: 41–61. doi: 10.1038/s41585-019-0212-4 [DOI] [PubMed] [Google Scholar]
- 2. Giganti F, Allen C. Imaging quality and prostate Mr: it is time to improve. Br J Radiol 2021; 94: 20200934. doi: 10.1259/bjr.20200934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sathianathen NJ, Omer A, Harriss E, Davies L, Kasivisvanathan V, Punwani S, et al. Negative predictive value of multiparametric magnetic resonance imaging in the detection of clinically significant prostate cancer in the prostate imaging reporting and data system era: a systematic review and meta-analysis. Eur Urol 2020; 78: 402–14. doi: 10.1016/j.eururo.2020.03.048 [DOI] [PubMed] [Google Scholar]
- 4. Barrett T, Padhani AR, Patel A, Ahmed HU, Allen C, Bardgett H, et al. Certification in reporting multiparametric magnetic resonance imaging of the prostate: recommendations of a UK consensus meeting. BJU Int 2021; 127: 304–6. doi: 10.1111/bju.15285 [DOI] [PubMed] [Google Scholar]
- 5. Dickinson L, Ahmed HU, Allen C, Barentsz JO, Carey B, Futterer JJ, et al. Magnetic resonance imaging for the detection, localisation, and characterisation of prostate cancer: recommendations from a European consensus meeting. Eur Urol 2011; 59: 477–94. doi: 10.1016/j.eururo.2010.12.009 [DOI] [PubMed] [Google Scholar]
- 6. Barentsz JO, Richenberg J, Clements R, Choyke P, Verma S, Villeirs G, et al. ESUR prostate Mr guidelines 2012. Eur Radiol 2012; 22: 746–57. doi: 10.1007/s00330-011-2377-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weinreb JC, Barentsz JO, Choyke PL, Cornud F, Haider MA, Macura KJ, et al. PI-RADS Prostate Imaging - Reporting and Data System: 2015, Version 2. Eur Urol 2016; 69: 16–40. doi: 10.1016/j.eururo.2015.08.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Turkbey B, Rosenkrantz AB, Haider MA, Padhani AR, Villeirs G, Macura KJ, et al. Prostate imaging reporting and data system version 2.1: 2019 update of prostate imaging reporting and data system version 2. Eur Urol 2019; 76: 340–51. doi: 10.1016/j.eururo.2019.02.033 [DOI] [PubMed] [Google Scholar]
- 9. Brembilla G, Dell'Oglio P, Stabile A, Damascelli A, Brunetti L, Ravelli S, et al. Interreader variability in prostate MRI reporting using prostate imaging reporting and data system version 2.1. Eur Radiol 2020; 30: 3383–92. doi: 10.1007/s00330-019-06654-2 [DOI] [PubMed] [Google Scholar]
- 10. Ahmed HM, Ebeed AE, Hamdy A, et al. Interobserver agreement of prostate Imaging–Reporting and data system (PI-RADS-v2. Egypt J Radiol Nucl Med 2021;;:: 5 52. [Google Scholar]
- 11. EL-Adalany MA, EL-Razek AAEL-khalekA, EL-Diasty T, EL-Hendy A, EL-Metwally D, et al. Comparison between biparametric and multiparametric MR imaging of prostate imaging reporting and data system version 2.1 in detection of prostate cancer. Egypt J Radiol Nucl Med 2021; 52: 68. doi: 10.1186/s43055-021-00443-y [DOI] [Google Scholar]
- 12. Brizmohun Appayya M, Adshead J, Ahmed HU, Allen C, Bainbridge A, Barrett T, et al. National implementation of multi-parametric magnetic resonance imaging for prostate cancer detection - recommendations from a UK consensus meeting. BJU Int 2018; 122: 13–25. doi: 10.1111/bju.14361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Verma S, Sarkar S, Young J, Venkataraman R, Yang X, Bhavsar A, et al. Evaluation of the impact of computed high b-value diffusion-weighted imaging on prostate cancer detection. Abdom Radiol 2016; 41: 934–45. doi: 10.1007/s00261-015-0619-1 [DOI] [PubMed] [Google Scholar]
- 14. Westphalen AC, Noworolski SM, Harisinghani M, Jhaveri KS, Raman SS, Rosenkrantz AB, et al. High-Resolution 3-T endorectal prostate MRI: a Multireader study of radiologist preference and perceived interpretive quality of 2D and 3D T2-weighted fast spin-echo Mr images. AJR Am J Roentgenol 2016; 206: 86–91. doi: 10.2214/AJR.14.14065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang Y-D, Wu C-J, Bao M-L, Li H, Yan X, Liu X-S, et al. New RESOLVE-Based diffusional Kurtosis imaging in MRI-Visible prostate cancer: effect of reduced B value on image quality and diagnostic effectiveness. AJR Am J Roentgenol 2016; 207: 330–8. doi: 10.2214/AJR.15.15990 [DOI] [PubMed] [Google Scholar]
- 16. Iyama Y, Nakaura T, Kidoh M, Katahira K, Namimoto T, Morishita S, et al. Fat suppressed contrast-enhanced T1-weighted dynamic magnetic resonance imaging at 3T: comparison of image quality between spectrally adiabatic Iversion recovery and the Multiecho Dixon technique in imaging of the prostate. J Comput Assist Tomogr 2017; 41: 382–7. doi: 10.1097/RCT.0000000000000540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tamada T, Ream JM, Doshi AM, Taneja SS, Rosenkrantz AB. Reduced Field-of-View diffusion-weighted magnetic resonance imaging of the prostate at 3 tesla: comparison with standard Echo-Planar imaging technique for image quality and tumor assessment. J Comput Assist Tomogr 2017; 41: 949–56. doi: 10.1097/RCT.0000000000000634 [DOI] [PubMed] [Google Scholar]
- 18. Tanaka U, Ueno Y, Morinaga Y, Miyake H, Kyotani K, Ueda Y, et al. Value of three-dimensional T2-weighted turbo spin-echo imaging with tissue-specific variable refocusing FLIP angle for 3-T magnetic resonance imaging of prostate cancer: comparison with conventional two- and three-dimensional T2-weighted turbo spin-echo imaging. Jpn J Radiol 2017; 35: 707–17. doi: 10.1007/s11604-017-0684-1 [DOI] [PubMed] [Google Scholar]
- 19. Corcuera-Solano I, Wagner M, Hectors S, Lewis S, Titelbaum N, Stemmer A, et al. Dwi of the prostate: comparison of a faster diagonal acquisition to standard three-scan trace acquisition. J Magn Reson Imaging 2017; 46: 1767–75. doi: 10.1002/jmri.25705 [DOI] [PubMed] [Google Scholar]
- 20. Stocker D, Manoliu A, Becker AS, Barth BK, Nanz D, Klarhöfer M, et al. Image quality and geometric distortion of modern diffusion-weighted imaging sequences in magnetic resonance imaging of the prostate. Invest Radiol 2018; 53: 200–6. doi: 10.1097/RLI.0000000000000429 [DOI] [PubMed] [Google Scholar]
- 21. Xi Y, Liu A, Olumba F, Lawson P, Costa DN, Yuan Q, et al. Low-to-high B value DWI ratio approaches in multiparametric MRI of the prostate: feasibility, optimal combination of B values, and comparison with ADC maps for the visual presentation of prostate cancer. Quant Imaging Med Surg 2018; 8: 557–67. doi: 10.21037/qims.2018.06.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ma S, Xu K, Xie H, Wang H, Wang R, Zhang X, et al. Diagnostic efficacy of b value (2000 s/mm2) diffusion-weighted imaging for prostate cancer: Comparison of a reduced field of view sequence and a conventional technique. Eur J Radiol 2018; 107: 125–33. doi: 10.1016/j.ejrad.2018.08.028 [DOI] [PubMed] [Google Scholar]
- 23. Polanec SH, Lazar M, Wengert GJ, Bickel H, Spick C, Susani M, et al. 3D T2-weighted imaging to shorten multiparametric prostate MRI protocols. Eur Radiol 2018; 28: 1634–41. doi: 10.1007/s00330-017-5120-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Warndahl BA, Borisch EA, Kawashima A, Riederer SJ, Froemming AT. Conventional vs. reduced field of view diffusion weighted imaging of the prostate: comparison of image quality, correlation with histology, and inter-reader agreement. Magn Reson Imaging 2018; 47: 67–76. doi: 10.1016/j.mri.2017.10.011 [DOI] [PubMed] [Google Scholar]
- 25. Rosenkrantz AB, Taneja SS. Use of reduced field-of-view acquisition to improve prostate cancer visualization on diffusion-weighted magnetic resonance imaging in the presence of hip implants: report of 2 cases. Curr Probl Diagn Radiol 2018; 47: 125–7. doi: 10.1067/j.cpradiol.2017.03.004 [DOI] [PubMed] [Google Scholar]
- 26. Czarniecki M, Caglic I, Grist JT, Gill AB, Lorenc K, Slough RA, et al. Role of PROPELLER-DWI of the prostate in reducing distortion and artefact from total hip replacement metalwork. Eur J Radiol 2018; 102: 213–9. doi: 10.1016/j.ejrad.2018.03.021 [DOI] [PubMed] [Google Scholar]
- 27. Meier-Schroers M, Marx C, Schmeel FC, Wolter K, Gieseke J, Block W, et al. Revised propeller for T2-weighted imaging of the prostate at 3 tesla: impact on lesion detection and PI-RADS classification. Eur Radiol 2018; 28: 24–30. doi: 10.1007/s00330-017-4949-y [DOI] [PubMed] [Google Scholar]
- 28. Jendoubi S, Wagner M, Montagne S, Ezziane M, Mespoulet J, Comperat E, et al. Mri for prostate cancer: can computed high b-value DWI replace native acquisitions? Eur Radiol 2019; 29: 5197–204. doi: 10.1007/s00330-019-06085-z [DOI] [PubMed] [Google Scholar]
- 29. Kordbacheh H, Seethamraju RT, Weiland E, Kiefer B, Nickel MD, Chulroek T, et al. Image quality and diagnostic accuracy of complex-averaged high B value images in diffusion-weighted MRI of prostate cancer. Abdom Radiol 2019; 44: 2244–53. doi: 10.1007/s00261-019-01961-0 [DOI] [PubMed] [Google Scholar]
- 30. Hellms S, Gutberlet M, Peperhove MJ, Pertschy S, Henkenberens C, Peters I, et al. Applicability of readout-segmented echoplanar diffusion weighted imaging for prostate MRI. Medicine 2019; 98: e16447. doi: 10.1097/MD.0000000000016447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Klingebiel M, Ullrich T, Quentin M, Bonekamp D, Aissa J, Mally D, et al. Advanced diffusion weighted imaging of the prostate: comparison of readout-segmented multi-shot, parallel-transmit and single-shot echo-planar imaging. Eur J Radiol 2020; 130: 109161. doi: 10.1016/j.ejrad.2020.109161 [DOI] [PubMed] [Google Scholar]
- 32. Gassenmaier S, Afat S, Nickel D, Mostapha M, Herrmann J, Othman AE. Deep learning–accelerated T2-weighted imaging of the prostate: reduction of acquisition time and improvement of image quality. Eur J Radiol 2021; 137: 109600. doi: 10.1016/j.ejrad.2021.109600 [DOI] [PubMed] [Google Scholar]
- 33. Wang X, Ma J, Bhosale P, Ibarra Rovira JJ, Qayyum A, Sun J, et al.;in press Novel deep learning-based noise reduction technique for prostate magnetic resonance imaging. Abdom Radiol 2021; 201. doi: 10.1007/s00261-021-02964-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Baur ADJ, Daqqaq T, Wagner M, Maxeiner A, Huppertz A, Renz D, et al. T2- and diffusion-weighted magnetic resonance imaging at 3T for the detection of prostate cancer with and without endorectal coil: an Intraindividual comparison of image quality and diagnostic performance. Eur J Radiol 2016; 85: 1075–84. doi: 10.1016/j.ejrad.2016.03.014 [DOI] [PubMed] [Google Scholar]
- 35. Barth BK, Cornelius A, Nanz D, Eberli D, Donati OF. Comparison of image quality and patient discomfort in prostate MRI: pelvic phased array coil vs. endorectal coil. Abdom Radiol 2016; 41: 2218–26. doi: 10.1007/s00261-016-0819-3 [DOI] [PubMed] [Google Scholar]
- 36. Gawlitza J, Reiss-Zimmermann M, Thörmer G, Schaudinn A, Linder N, Garnov N, et al. Impact of the use of an endorectal coil for 3 T prostate MRI on image quality and cancer detection rate. Sci Rep 2017; 7: 40640. doi: 10.1038/srep40640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ullrich T, Quentin M, Oelers C, Dietzel F, Sawicki LM, Arsov C, et al. Magnetic resonance imaging of the prostate at 1.5 versus 3.0T: a prospective comparison study of image quality. Eur J Radiol 2017; 90: 192–7. doi: 10.1016/j.ejrad.2017.02.044 [DOI] [PubMed] [Google Scholar]
- 38. Tanaka T, Froemming AT, Panda A, Edmonson HA, Pooley RA, Carter RE, et al. Safety and image quality of 1.5-T endorectal coil multiparametric MRI of the prostate or prostatectomy fossa for patients with pacemaker or implantable cardioverter-defibrillator. AJR Am J Roentgenol 2019; 212: 815–22. doi: 10.2214/AJR.18.20266 [DOI] [PubMed] [Google Scholar]
- 39. O'Donohoe RL, Dunne RM, Kimbrell V, Tempany CM. Prostate MRI using an external phased array wearable pelvic coil at 3T: comparison with an endorectal coil. Abdom Radiol 2019; 44: 1062–9. doi: 10.1007/s00261-018-1804-9 [DOI] [PubMed] [Google Scholar]
- 40. Dhatt R, Choy S, Co SJ, Ischia J, Kozlowski P, Harris AC, , et al. Mri of the prostate with and without endorectal coil at 3 T: correlation with Whole-Mount histopathologic Gleason score. AJR Am J Roentgenol 2020; 215: 133–41. doi: 10.2214/AJR.19.22094 [DOI] [PubMed] [Google Scholar]
- 41. Ullrich T, Kohli MD, Ohliger MA, Magudia K, Arora SS, Barrett T, et al. Quality comparison of 3 tesla multiparametric MRI of the prostate using a flexible surface receiver coil versus conventional surface coil plus endorectal coil setup. Abdom Radiol 2020; 45: 4260–70. doi: 10.1007/s00261-020-02641-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stocker D, Manoliu A, Becker AS, Barth BK, Nanz D, Klarhöfer M, et al. Impact of different phased-array coils on the quality of prostate magnetic resonance images. Eur J Radiol Open 2021; 8: 100327. doi: 10.1016/j.ejro.2021.100327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Heijmink SWTPJ, Fütterer JJ, Hambrock T, Takahashi S, Scheenen TWJ, Huisman HJ, et al. Prostate cancer: body-array versus endorectal coil MR imaging at 3 T--comparison of image quality, localization, and staging performance. Radiology 2007; 244: 184–95. doi: 10.1148/radiol.2441060425 [DOI] [PubMed] [Google Scholar]
- 44. Caglic I, Hansen NL, Slough RA, Patterson AJ, Barrett T. Evaluating the effect of rectal distension on prostate multiparametric MRI image quality. Eur J Radiol 2017; 90: 174–80. doi: 10.1016/j.ejrad.2017.02.029 [DOI] [PubMed] [Google Scholar]
- 45. Slough RA, Caglic I, Hansen NL, Patterson AJ, Barrett T. Effect of hyoscine butylbromide on prostate multiparametric MRI anatomical and functional image quality. Clin Radiol 2018; 73: 216.e9–216.e14. doi: 10.1016/j.crad.2017.07.013 [DOI] [PubMed] [Google Scholar]
- 46. Ullrich T, Quentin M, Schmaltz AK, Arsov C, Rubbert C, Blondin D, et al. Hyoscine butylbromide significantly decreases motion artefacts and allows better delineation of anatomic structures in mp-MRI of the prostate. Eur Radiol 2018; 28: 17–23. doi: 10.1007/s00330-017-4940-7 [DOI] [PubMed] [Google Scholar]
- 47. Purysko AS, Mielke N, Bullen J, Nachand D, Rizk A, Stevens E, et al.;in press Influence of enema and dietary restrictions on prostate Mr image quality: a Multireader study. Acad Radiol 2020;05 Nov 2020. doi: 10.1016/j.acra.2020.10.019 [DOI] [PubMed] [Google Scholar]
- 48. Coskun M, Mehralivand S, Shih JH, Merino MJ, Wood BJ, Pinto PA, et al. Impact of bowel preparation with Fleet’s™ enema on prostate MRI quality. Abdom Radiol 2020; 45: 4252–9. doi: 10.1007/s00261-020-02487-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Plodeck V, Radosa CG, Hübner H-M, Baldus C, Borkowetz A, Thomas C, et al. Rectal gas-induced susceptibility artefacts on prostate diffusion-weighted MRI with epi read-out at 3.0 T: does a preparatory micro-enema improve image quality? Abdom Radiol 2020; 45: 4244–51. doi: 10.1007/s00261-020-02600-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Reischauer C, Cancelli T, Malekzadeh S, Froehlich JM, Thoeny HC.;in press How to improve image quality of DWI of the prostate-enema or catheter preparation? Eur Radiol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schmidt C, Hötker A, Muehlematter UJ, Burger IA, Donati OF, Barth BK.;in press Value of bowel preparation techniques for prostate MRI: a preliminary study. Abdom Radiol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Caglic I, Barrett T. Optimising prostate mpMRI: prepare for success. Clin Radiol 2019; 74: 831–40. doi: 10.1016/j.crad.2018.12.003 [DOI] [PubMed] [Google Scholar]
- 53. Brennan DL, Lazarakis S, Lee A, Tan TH, Chin KY, Oon SF.;in press Do antispasmodics or rectal enemas improve image quality on multiparametric prostate MRI? An 'Evidence-Based Practice' review of the literature. Abdom Radiol 2021;19 Jan 2021. doi: 10.1007/s00261-020-02916-6 [DOI] [PubMed] [Google Scholar]
- 54. Esses SJ, Taneja SS, Rosenkrantz AB. Imaging facilities' adherence to PI-RADS V2 minimum technical standards for the performance of prostate MRI. Acad Radiol 2018; 25: 188–95. doi: 10.1016/j.acra.2017.08.013 [DOI] [PubMed] [Google Scholar]
- 55. Burn PR, Freeman SJ, Andreou A, Burns-Cox N, Persad R, Barrett T. A multicentre assessment of prostate MRI quality and compliance with UK and international standards. Clin Radiol 2019; 74: 894.e19–894.e25. doi: 10.1016/j.crad.2019.03.026 [DOI] [PubMed] [Google Scholar]
- 56. Coşkun M, Sarp AF, Karasu Şebnem, Gelal MF, Türkbey B. Assessment of the compliance with minimum acceptable technical parameters proposed by PI-RADS V2 guidelines in multiparametric prostate MRI acquisition in tertiary referral hospitals in the Republic of turkey. Diagn Interv Radiol 2019; 25: 421–7. doi: 10.5152/dir.2019.18537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sackett J, Shih JH, Reese SE, Brender JR, Harmon SA, Barrett T, et al. Quality of prostate MRI: is the PI-RADS standard sufficient? Acad Radiol 2021; 28: 199–207. doi: 10.1016/j.acra.2020.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. de Rooij M, Israël B, Tummers M, Ahmed HU, Barrett T, Giganti F, et al. ESUR/ESUI consensus statements on multi-parametric MRI for the detection of clinically significant prostate cancer: quality requirements for image acquisition, interpretation and radiologists' training. Eur Radiol 2020; 30: 5404–16. doi: 10.1007/s00330-020-06929-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kasivisvanathan V, Rannikko AS, Borghi M, Panebianco V, Mynderse LA, Vaarala MH, et al. MRI-Targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med 2018; 378: 1767–77. doi: 10.1056/NEJMoa1801993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Giganti F, Allen C, Emberton M, Moore CM, Kasivisvanathan V, Quality PI, .PRECISION study group . Prostate imaging quality (PI-QUAL): a new quality control scoring system for multiparametric magnetic resonance imaging of the prostate from the precision trial. Eur Urol Oncol 2020; 3: 615–9. doi: 10.1016/j.euo.2020.06.007 [DOI] [PubMed] [Google Scholar]
- 61. Giannarini G, Valotto C, Girometti R, Dal Moro F, Briganti A, Padhani AR. Measuring the quality of diagnostic prostate magnetic resonance imaging: a urologist's perspective. Eur Urol 2021; 79: 440–1. doi: 10.1016/j.eururo.2020.09.015 [DOI] [PubMed] [Google Scholar]
- 62. Turkbey B, Choyke PL. PI-QUAL, a new system for evaluating prostate magnetic resonance imaging quality: is beauty in the eye of the beholder? Eur Urol Oncol 2020; 3: 620–1. doi: 10.1016/j.euo.2020.07.003 [DOI] [PubMed] [Google Scholar]