Abstract
While One Health initiatives are gaining in popularity, it is unclear if and how they are evaluated when implementation at scale is intended. The main purpose of this scoping review was to describe how One Health initiatives targeting infectious diseases and antimicrobial resistance at a large scale are evaluated. Secondary objectives included identifying the main facilitators and barriers to the implementation and success of these initiatives, and how their impacts were assessed. Twenty-three studies evaluating One Health initiatives were eligible. Most studies included the human (n = 22) and animal (n = 15) sectors; only four included the environment sector. The types of evaluated initiative (non-exclusive) included governance (n = 5), knowledge (n = 6), protection (n = 17), promotion (n = 16), prevention (n = 9), care (n = 8), advocacy (n = 10) and capacity (n = 10). Studies used normative (n = 4) and evaluative (n = 20) approaches to assess the One Health initiatives, the latter including impact (n = 19), implementation (n = 8), and performance (n = 7) analyses. Structural and economic, social, political, communication and coordination-related factors, as well as ontological factors, were identified as both facilitators and barriers for successful One Health initiatives. These results identified a wide range of evaluation methods and indicators used to demonstrate One Health's added values, strengths, and limitations: the inherent complexity of the One Health approach leads to the use of multiple types of evaluation. The strengths and remaining gaps in the evaluation of such initiative highlight the relevance of comprehensive, mixed-method, context-sensitive evaluation frameworks to inform and support the implementation of One Health initiatives by stakeholders in different governance settings.
Keywords: Multisectoral, Participatory, Infectious diseases, Global health, One health, Evaluation
Abbreviations: AMR, antimicrobial resistance; NEOH, Network for Evaluation of One Health; OH, One Health; OHHLEP, One Health High Level Expert Panel; WFPHA, World Federation of Public Health Associations
Graphical abstract
Highlights
-
•
Studies evaluating One Health initiatives were scarce.
-
•
Only One Health initiatives related to infectious diseases were evaluated.
-
•
Evaluations were mainly conducted using quantitative approaches.
-
•
Involvement of the community was identified as a major facilitator.
1. Introduction
The One Health (OH) approach has been the subject of particular attention in recent years, especially since the COVID-19 pandemic emerged. About 62% of human infections are believed to have an animal origin [1,2], and this proportion rises to 75% for emerging infections [3]. Recent emerging infectious diseases underline the need to consider health as the result of complex eco-social determinants and to adopt a more holistic approach to health risk management. According to the One Health High Level Expert Panel (OHHLEP), One Health constitutes “an integrative and systemic approach to health, grounded in the understanding that human health is closely linked to the healthiness of food, animals and the environment, and the healthy balance of their impact on the ecosystems they share, everywhere in the world” [4]. Compared to siloed approaches, OH is reported to increase the efficiency and cost-effectiveness of field interventions, surveillance, and health policies, particularly for zoonoses or antimicrobial resistance (AMR) [[5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15]]. Intersectoral collaborations improve our understanding of the epidemiology of health issues that require an integrated approach [12,16]. Researchers and international organizations are therefore advocating for a broader application of OH in various contexts, and including research, training and education, and, crucially, governance as areas of focus. However, the demonstration of OH benefits has been largely limited to small-scale projects [17], and the success factors for scaling up initiatives need to be assessed, especially since the resources mobilized are significant.
WHO defines health governance as “attempts of governments or other actors to steer communities, countries or groups of countries in the pursuit of health” [18] but convincing governments, and policy- and decision-makers to fund or implement a OH approach in health governance can be challenging [9,11,19,20]. First, OH remains a complex concept, and until very recently, had no consensual definition. This impedes awareness, comprehension, and application of OH [21]. Another obstacle is overcoming siloed thinking and practice in order to build sustainable collaboration across Ministries, disciplines or sectors [19]. Moreover, several authors have underlined the lack of robust evidence to support the added value of the OH approach [5,19]. Evaluating the effectiveness, meaningfulness, feasibility, and implementation of OH initiatives is essential to understand the benefits and weaknesses of these initiatives and to enhance buy-in from decision makers.
Previous literature reviews have focused on the evaluation of specific aspects of OH initiatives, such as their impact or effectiveness [5,22], their level of collaboration or integration [23,24], or their challenges [19]. Most reviews only considered quantitative indicators [5,22], excluding qualitative and mixed-methods evaluations, which are crucial to understand how and why some initiatives are effective or not in specific contexts, and to understand effective governance approaches.
Successful implementation at scale requires both evaluation at scale, and context-sensitive attention to implementation facilitators and barriers [25]. The purpose of this review was therefore to complement previous reviews by describing how larger-scale OH initiatives targeting infectious diseases or AMR were evaluated, and the main facilitators and barriers to their implementation and success.
2. Methods
We conducted a scoping review, following PRISMA scoping review guidelines [26,27], to systematically identify research evidence and gaps [[26], [27], [28]], and to examine the extent, range, and nature of research activity related to our research objectives [29]. The study was registered with the Open Science Framework registry (4NH2A; https://osf.io/4nh2a). OH initiatives are defined here as any empirical intervention, programme, health policy, legislation or governance activity addressing infectious diseases or AMR conducted according to OH principles. Our definition of the OH principles are based on the Berlin Principles and the definition of the OHHLEP [4,30]. Both documents recognize the intrinsic and complex links between human, other animal, plants and environmental health and encourage collaboration across disciplines and sectors. We consider such collaboration in its broader sense since its understanding varies among authors and stakeholders [31,32]. We therefore defined the OH initiatives as any initiatives with evidence of cooperation between at least two of the following fields: human health, domestic animal health, wildlife animal health, and environmental health.
2.1. Search strategy
Articles were identified using medical subject headings, keyword combinations and truncations, and combined using the AND Boolean logic operator: One Health, Governance, and Evaluation (Appendix). The citation searches began on August 7, 2020 and the final citation search was conducted on September 17, 2020. We searched the following databases: PubMed / Medline, Embase, Web of Science, CAB abstract, Global Health, The Lens, Lilacs, ERIC, sociological abstract, PsycINFO, Global Health and Native Health, and CINAHL Database. The citations were imported and treated with Covidence® [33]. Four authors (LD, SM, MS, and JD-R) screened and evaluated the eligibility of the publications. An article was included if two evaluators considered it eligible after full review; any discrepancies were resolved by a third evaluator.
2.2. Eligibility criteria
Studies were eligible if they were peer-reviewed publications evaluating policies, governance, programs, or interventions. Only electronically available full text articles in English, French, Spanish, Italian, Portuguese, Russian, Ukrainian, Dutch, and Arabic, were considered. Databases were searched from their inception until September 2020.
During the first phase, studies were excluded if there was no clear indication that OH principles, as previously defined, had been met, or if the outcome of interest was not related to an infectious disease or AMR. If information in the title and abstract was insufficient to assess the inclusion criteria, the article was retained for full text review. At the full text review stage, only studies conducted at a large-scale, defined as one including a population of at least 10,000 individuals in urban areas or at least 10 villages and 1,000 individuals in rural areas, were included. If the information on scale (size study population or size of intervention target population) was not available, studies were excluded. Studies that did not evaluate the OH initiatives or that sought to evaluate them, but did not use an external comparison group or pre-post or other quasi-experimental design were also excluded.
2.3. Data extraction
Extracted data included reference, context (country/region, years), scale (subnational, national or multi-country), stakeholders involved (human health, animal health, environment, other), type of OH initiatives, issue of interest, type of evaluation (including study designs and methodological approaches, when applicable), species and samples evaluated, evaluated outcome indicators, results of evaluation, barriers and impediments, and promotors and facilitators. Extracted data were entered into Excel (Microsoft Corporation, Redmond, WA, US).
While we classified the barriers and facilitators through an inductive approach, types of OH initiatives were categorized following the framework of the World Federation of Public Health Associations (WFPHA) [34]. It establishes 8 pillars of public health: governance (e.g., legislation, policy); knowledge (e.g., surveillance, research, dissemination); protection (e.g., control, environmental health, health education); promotion (e.g., health determinants and behaviors); prevention; people-centered care (both were extended to animals); advocacy (e.g., community engagement) and capacity (e.g., workforce development, training). Initiatives could be in more than one category.
For the type of evaluation, we followed the framework proposed by Contandriopoulos et al. [35] This framework considers five components of an initiative: objectives; resources; services, or activities; effects; and a specific context. Evaluation is defined as “making a value judgment about one or more of these components or their interrelationships within the initiative” [35]. We categorized the evaluation types according to the typology defined in this framework, with two main approaches: normative assessment, and evaluative research.
Normative assessment aims to evaluate the degree of conformity of the initiative structures, processes, or results with norms, standards or defined criteria. The standards on which normative evaluations are based are usually derived from evaluative research or experts' opinion [35]. Evaluative research uses more complex methods and approaches and includes six types of analysis: (1) Strategic analysis, which focusses on the relevance of the intervention taking into consideration several elements such the intervention's rationale and the targeted population(s); (2) Intervention analysis, which examines the appropriateness of the relationship between the objectives of the intervention and the means implemented; (3) Productivity analysis looks at how efficiently resources are used to produce services and outputs; (4) Impact analysis is the assessment of effects of the activities conducted within the intervention; (5) Performance analysis aims at comparing the use of resources to the impacts of the intervention (usually conducted via a cost-benefit, cost-effectiveness or cost-utility evaluation); and (6) Implementation analysis, which examines how the degree of implementation as well as the contextual factors influence the effects of an intervention (analogous to process evaluation or implementation research [25]).
3. Results
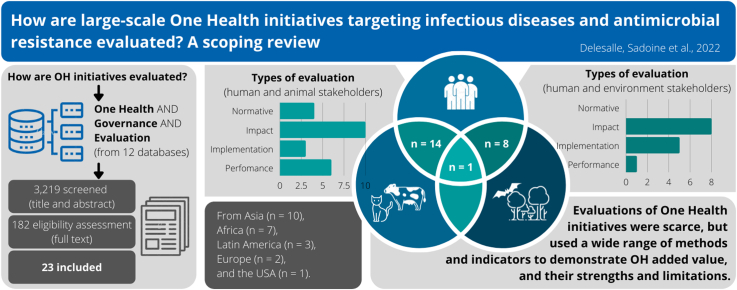
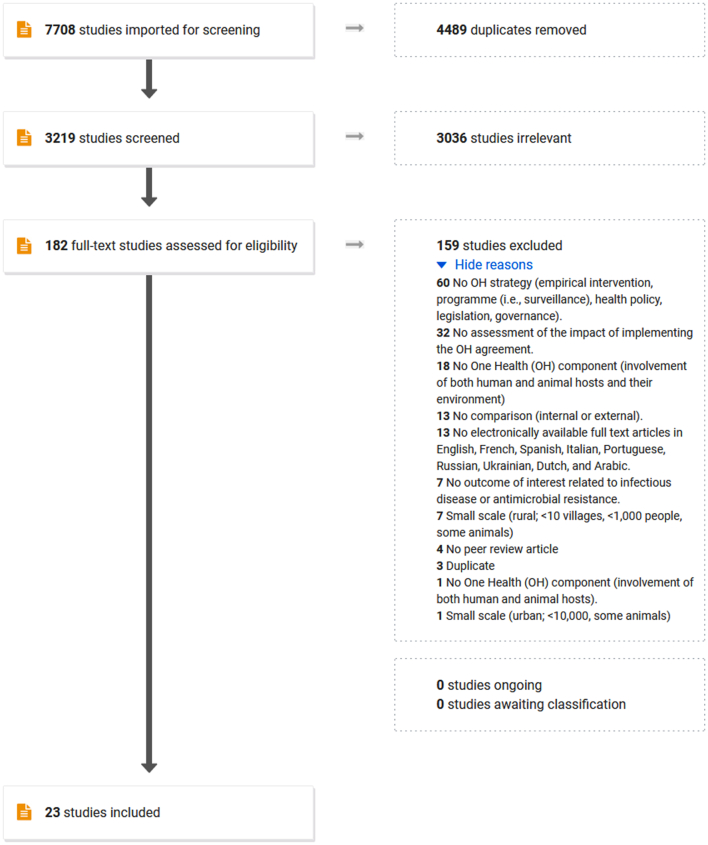
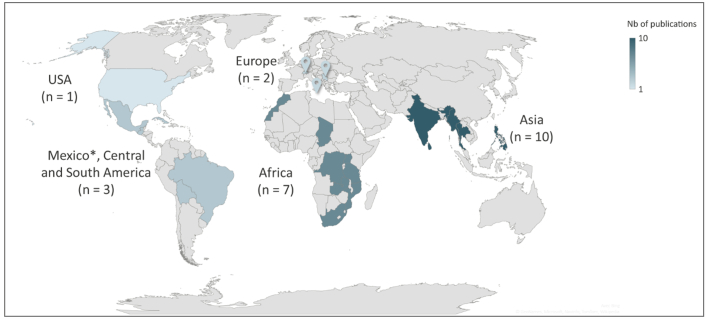
From the database search, 3,219 articles were identified after removing duplicates. Following the review of titles and abstracts, 182 articles were selected for full-text review. We retained 23 articles published between 2011 and 2020 for the scoping review (Fig. 1). Most of the studies selected were carried out in Asian and African countries (Fig. 2), at a subnational scale (Table 1).
Fig. 1.
PRISMA flowchart (obtained from Covidence®) for the scoping review “How are large-scale One Health initiatives targeting infectious diseases and antimicrobial resistance evaluated? A scoping review”.
Fig. 2.
Geographical distribution of the One Health (OH) initiatives of the 23 studies included in a scoping review exploring the evaluation of OH initiatives. Each highlighted country is mentioned in at least one study. As some studies involved multiple countries, the choropleth scale was calculated by continent.
* The study mentioning a OH initiative in Mexico also involved central and south American countries, so Mexico was counted along with this group instead of North America.
Table 1.
Scale and area of implementation of the One Health (OH) initiatives of the 23 studies included in a scoping review exploring the evaluation of OH initiatives.
| n | Reference | ||
|---|---|---|---|
| Scale | |||
| Multi-countries | 1 | [56] | |
| National | 3 | [40,50,51] | |
| Subnational | State | 3 | [36,37,57] |
| Provinces | 3 | [38,48,55] | |
| Districts | 6 | [[45], [46], [47],49,53,54] | |
| Local | Neighbourhood / villages | 7 | [39,[41], [42], [43], [44],52,58] |
| Area | |||
| Urban and/or periurban | 7 | [[41], [42], [43], [44],54,57,58] | |
| Urban and rural | 6 | [36,40,49,51,53,56] | |
| Rural | 8 | [37,39,[45], [46], [47], [48],50,52] | |
| Unknown | 2 | [38,55] | |
3.1. Type of OH initiatives
In most of the studies, multiple initiatives were implemented, each of which could have been classified in multiple categories of the WFPHA framework. Health protection was the most represented category (n = 17), and included vector control and dog population management in 9 studies [17,[36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51]]. Health promotion comprised mainly health education interventions about rabies or vector-borne diseases (n = 16) [17,[36], [37], [38], [39], [40], [41],[43], [44], [45], [46], [47],[49], [50], [51], [52]]. Health advocacy initiatives aimed at community engagement and empowerment about vector control (n = 10) [39,[41], [42], [43], [44], [45], [46],51,53,54]. Prevention, mostly primary but also secondary and tertiary, has been used mostly for rabies through canine vaccination (n = 9) [17,37,38,[47], [48], [49], [50],52,54]. One third of the studies (n = 8) implemented capacity-building initiatives, such as training human or animal health workers, mostly for the management of suspected rabies exposure [38,48,49,51,[53], [54], [55], [56]]. People- or animal-centered healthcare were also provided in eight studies, for example by administrating anti-parasite drugs to human or dogs [17,37,[45], [46], [47],50,52,54]. Six studies meant to improve knowledge, mostly through surveillance and monitoring [[36], [37], [38],40,51,53]. Finally, five studies were focused on governance, such as creating new intersectoral bodies or enforcing new regulations, mainly for bacterial diseases [40,49,[55], [56], [57]].
3.2. Disease of interest
Rabies was the most frequently studied disease (n = 11) [[36], [37], [38],[47], [48], [49],52,54,55,57,58], along with vector-borne diseases (n = 9) such as dengue [[41], [42], [43], [44],53], Chagas disease [39], African trypanosomiasis [50] or opisthorchiasis [45,46]. Only a few initiatives targeted bacterial diseases (brucellosis [40], campylobacteriosis [51], salmonellosis [57] and anthrax [57]) and non-vector borne parasites (soil-transmitted helminths [47], echinococcosis [52]). Five studies looked at more than one infectious disease and none of the included publications presented OH initiatives for AMR.
3.3. Stakeholders and agents involved in the OH initiatives
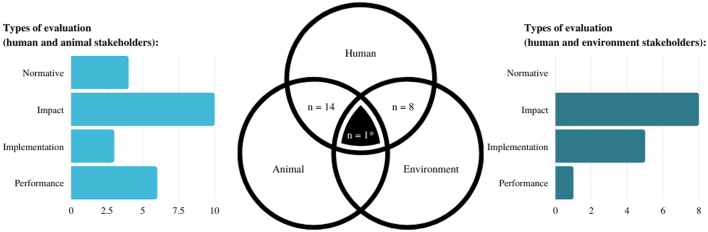
This scoping review classified the agents (those implementing the initiatives) and stakeholders (those targeted by the initiatives) in three sectors based on the principal OH dimensions [59]: human health (including medical and public health), animal health (including domestic animal and animal production), and environment health (including wildlife). As intended by our inclusion criteria, all studies involved at least two of these sectors. Human health agents were involved in all but one of the studied OH initiatives [52]. Animal health agents were the second most represented (n = 15) [[36], [37], [38],40,[47], [48], [49], [50], [51], [52],[54], [55], [56], [57], [58]] and were often involved together with human health agents (n = 13) [[36], [37], [38],40,[47], [48], [49], [50], [51],[54], [55], [56], [57]]. Environment agents were included in only four studies [37,41,42,53]. Five of the initiatives also involved agents from other sectors, including law enforcement or defense (police, army, Interior Ministry, consumer affairs) [37,40,52], education and communication [53] or urban housing and development [37]. When classifying the OH stakeholders the initiatives involved in the studies, all involved the human stakeholders. The studies combined the human and animal stakeholders (n = 14) [[36], [37], [38],40,[47], [48], [49], [50], [51],[54], [55], [56], [57], [58]], the human and environment stakeholders (n = 8) [39,[41], [42], [43], [44], [45], [46],53] and all stakeholders (n = 1) [37]. Just over a third of the included studies mentioned a participatory approach (n = 8) [39,[41], [42], [43], [44],47,53,58], which involved active collaboration with community members, for example through the recruitment, the training of community researchers and experts [60,61]. This contrasted with the top-down approaches that included initiatives that were not developed or delivered in partnership with community members [[36], [37], [38],40,45,46,[48], [49], [50], [51], [52],[54], [55], [56], [57]].
3.4. Type of evaluation and study design
Most studies used more than one type of evaluation, either for different aspects of the study or different measured outcome indicators (Table 2). Normative evaluation was performed in only 4 out of 23 studies, of which two used the Network for Evaluation of One Health (NEOH) framework to assess the “One Health-ness” of integrated programmes [40,56].
Table 2.
Types of evaluation, designs and types of method used to evaluate OH initiatives of the 23 studies included in a scoping review exploring the evaluation of OH initiatives. Categories of evaluation types are drawn from Contandriopoulos et al. [35]. * Registered frameworks.
| Type of evaluation | Design or framework | Methods | n | References |
|---|---|---|---|---|
| Normative evaluations | ||||
| Structure and process appraisal |
|
Mixed | 2 | [40,56] |
|
Qualitative | 1 | [57] | |
| Ethical appraisal |
|
Qualitative | 1 | [58] |
| Evaluative research | ||||
| Impact analysis |
|
Quantitative | 12 | [39,41,43,44,[46], [47], [48], [49],[53], [54], [55],58] |
|
Quantitative | 3 | [38,49,52] | |
|
Quantitative | 3 | [38,48,49] | |
|
Mixed | 1 | [39] | |
|
Mixed | 1 | [36] | |
|
Quantitative | 4 | [37,42,45,50] | |
| Productivity analysis |
|
Quantitative | 1 | [55] |
| Performance analysis |
|
Quantitative | 4 | [43,47,49,52] |
|
Quantitative | 2 | [51,58] | |
|
Quantitative | 2 | [54,58] | |
| Implementation analysis |
|
Mixed | 2 | [43,53] |
|
Mixed | 1 | [39] | |
|
Quantitative | 2 | [38,42] | |
| Mixed | 3 | [44,47,58] | ||
Of the 20 evaluative research studies that used at least one evaluative research method (Table 2), almost all performed at least an impact analysis. Using this type of evaluation, OH initiatives were assessed using randomized trials, comparison groups but no randomization or randomized controlled trials. When randomization was used, randomizing units varied from 8 to 22 clusters (i.e., neighbourhoods or villages). Quantitative pre-post comparative studies were the most common design, whereas only a few studies used time series or longitudinal analyses or a post-test only design. Two studies followed mixed methods protocols: the PRECEDE-PROCEED model designed by Green, Kreuter et al. [62] and the CDC framework for program evaluation [63].
Implementation analyses were conducted in about a third of the included studies. Two of these studies measured the participation of community partners using Rifkin's spidergram, a semi-quantitative framework based on monitoring five factors that influence community participation (needs assessment, leadership, organization, resource mobilization and management) [64].
A third of the studies carried out a performance analysis, with four studies having described the costs of the OH initiative, and three studies performed a cost-benefit or a cost-effectiveness analysis.
The most common type of evaluative research used was impact analysis both in studies involving human and animal sectors, and those involved the human and environment sectors. However, implementation analyses were more often done when the human and environment sectors were involved, and performance analysis was more common when the human and animal sectors were involved (Fig. 3). Additionally, normative assessment was only used when the human and animal sectors were involved.
Fig. 3.
Types of evaluation of One Health (OH) initiatives identified in 23 studies included in a scoping review exploring the evaluation of OH initiatives in a context of intersectoral collaborations (i.e., involving at least two stakeholders among human health, animal health, and environmental health). Studies could use more than one type of evaluation. The types of evaluation were classified as normative assessment, or evaluative research (including impact analysis, implementation analysis, and performance analysis) [35]. The types of evaluation are presented for studies including the human and animal stakeholders, and for studies including the human and environment stakeholders.
* One study included the human, animal, and environment stakeholders and was not included in the graphs. The type of evaluation conducted in this study was an impact analysis [37].
3.5. Indicators of success
Several outcome indicators and impacts of the OH initiatives were often measured together. Most of the included studies underlined positive effect of the initiative on knowledge, attitudes, and health behaviors and practices (n = 14/23), for example, improved control of vector reproduction sites (i.e., lids on water container) or an increase in vaccination coverage. Similarly, the implemented OH initiatives were found to have improved human, animal or environmental health indicators (n = 15/23; e.g., decreased dog bites, decrease of mosquito pupae per person index, etc.) [[36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46],50,53,54,58]. Along with direct effect on health, 43% (n = 10/23) of the studies reported the emergence of new intersectoral partnerships, health policies or agendas, or the creation of new integrated governance bodies [36,41,42,45,46,[49], [50], [51],53,56]. Other benefits included the improvement of existing surveillance activities (e.g., increase in case detection, n = 6/23) [38,48,49,53,55,57], or financial savings compared to non-OH initiatives (n = 5/23) [47,49,50,54,58]. In contrast, one descriptive cost analysis showed that the OH approach led to additional costs associated with the increased demand for post-exposure prophylaxis following an effective awareness campaign [55].
As measured in five participatory studies, OH initiatives also had social impacts such as increased community leadership and awareness concerning infectious diseases and enhanced social acceptance of health initiatives. One study reported the creation of training activities or curriculum for students or professional [56], and another assessed the ethical aspects related to dogs and humans to evaluate the utilitarianism of the OH initiative [58].
3.6. Facilitators and barriers
Seven categories of facilitators and barriers for initiatives' implementation and success were identified, of which five were identified as both barriers and facilitators (Table 3). Social factors were the most frequently reported facilitators (n = 8/23). Among them, socio-culturally appropriate OH initiatives, bottom-up approach, strong community support and leadership, were key element to success of initiatives [37,38,43,44,46,47,50,53]. In contrast, a top-down approach and low community participation were mentioned as important barriers in half of these 8 publications [37,43,46,56]. The importance of the communication and coordination among stakeholders has also been underlined [41,47,57], whereas a lack of coordination was cited as a major obstacle [49,56,57]. Factors such as the prior existence of laws, governance bodies, and intersectoral agreements were often mentioned as structural promotors and facilitators, as well as low-cost initiatives [36,38,40,41,50,57]. These facilitators could also be related to the political context as authors mentioned that government perceiving health as a priority ensured a political engagement in their program [36,50] The importance of the communication and coordination among stakeholders has also been underlined [41,47,57], whereas a lack of coordination was cited as a major obstacle [49,56,57]. In four of the included studies, the authors encouraged using innovative methods that are accessible to stakeholders (e.g., the use of mobile phones for timely data collection in disease surveillance) [42,48,49], and recommended to continually review or to revise protocols to adapt the initiative when targeting behavioural changes [46]. Consideration of the traditional practices and valuing local knowledge were key elements mentioned to ensure success and sustainability of behavioural initiatives [49]. Finally, the recognition of OH evidence and “OH-thinking” among the stakeholders were mentioned as facilitators to successfully implement OH initiatives [37,40,56]. The lack of structures, resources, and logistical capacities were the most recurrent barriers to ensure sustainable implementation of OH initiatives. These limitations were related, but not limited to inadequate surveillance systems, staff turnover or shortages, or the little external support and time [36,47,48,56,58]. Gaps in awareness, education, and training among providers and practitioners regarding zoonotic disease threats, reporting requirements and laws, or even sample collection were also obstacles to the implementation of the OH initiatives [37,57].
Table 3.
Barriers and facilitators to One Health (OH) implementation and success in 23 studies included in a scoping review exploring the evaluation of OH initiatives in a context of intersectoral collaborations (i.e., involving at least two sectors among human, animal, environment).
| Category of factors | Facilitators |
Barriers |
||
|---|---|---|---|---|
| Description | References | Description | References | |
| Structural / economic | - Available/existing structures (legislation, chain of supply, easy diagnosis, network) | [36,40,41,57] | - Structures, resources, and logistical limitation (e.g., weak surveillance system, lack of staff, external support, time, long walking distance, change of staff) | [36,43,[47], [48], [49],56,57] |
| - Low cost of intervention / affordable | [38,41,50] | - Border control not sufficient | [40] | |
| - Economic challenges (cost and respect budget allocation) | [49] | |||
| - Decentralization of government services for animal health work | [50] | |||
| Social | - Socially and culturally appropriate | [37,46,50] | - Lack of communication to community | [37] |
| - Strong community support and participation, involvement of specific key actors | [37,38,43,44,46,47,53] | - Low community participation (e.g., affected by historical and current community dynamic or not feeling concerned) | [43,56] | |
| - Strong community organization/leadership | [43] | - Cultural barrier (from community to pursue traditional practices or lack of integration of cultural practices from experts) | [46] | |
| - Engagement with community in a reciprocal learning process | [46] | |||
| Political | - Health perceived as a priority which ensure political engagement / sense of urgency | [36,50] | - Sustainability of activities related to stakeholders (e.g., priorities changes or low risk perception or not enough practice of activities which leads to forgetting procedures) | [37,39,44,48] |
| Communication / coordination | - Collaboration and communication between stakeholders | [41,47,57] | - Lack of coordination or communication among stakeholders | [49,56,57] |
| - Top-down management | [56] | |||
| Methodological | - Continually review and revise protocols | [46] | ||
| - Use of innovative and accessible method | [42,48,49] | |||
| Paradigm / ontology | - Recognition and awareness of OH evidence | [37,40,56] | - No OH thinking | [40,52] |
| Gap of awareness, education / training | - Gap of awareness, education, and training regarding zoonotic disease threat, requirements, and law | [57] | ||
| - Lack of training to appropriately implement activities (e.g., sample collection) | [37] | |||
4. Discussion
This scoping review is the first to describe the methods for evaluating the implementation and impacts of large-scale OH initiatives, while considering governance dimensions. Large-scale OH initiatives for infectious diseases were evaluated using predominantly quantitative approaches with pre-post designs and little attention to the specific context of implementation. While a recent scoping review reported that existing frameworks for evaluating OH initiatives are usually focused on design and implementation [21], we found that many evaluations attempt to assess the impact of OH interventions. Most focused on human and animal health outcomes, with rare consideration of the environmental component. Social factors such as strong stakeholder support and the cultural suitability of OH initiatives were often reported as facilitating elements of a successful implementation of OH initiatives. Conversely, resources (time, structure, economy) were the main reported obstacles to implementation. The large-scale initiatives mainly included field interventions, public awareness or education activities regarding infectious disease, but none focused on AMR, although it has previously been described as a major OH issue [65,66]. Some evidence on the positive impacts of AMR policies has been reported, but limited to human health [22], neglecting the potential impact and interactions with animals and the environment. Quantitative evidence on socio-economic and OH impacts of AMR policies are generally lacking [22]. While the concept of the interconnection between health and environments is not new, the OH approach is innovative in the sense that it seeks increased intersectoral coordination, to break down silos between sectors responsible for the health of humans, animals and the environment. In doing so, it aims to address more effectively the global factors that influence health [67]. Importantly, the environmental component of OH initiatives continue to be neglected, and our findings corroborate this point. Barrett and Bouley [68] have discussed the lack of representation of the environmental component in OH and have suggested different solutions. Human and animal health is impacted both positively and negatively by any environmental changes, which will further intensify with climate change and continued population growth. There is a real and critical need to better integrate environmental components and disciplines into the OH architecture.
We found a wide heterogeneity of evaluation methods and indicators, which likely reflects the current lack of consensus on how to evaluate OH initiatives. Only six studies in our review based their assessment on peer-reviewed evaluation tools or frameworks (CDC Framework for Program Evaluation, NEOH framework, PRECEDE-PROCEED Model, Rifkin's spider-gram). The CDC framework is a practical, non-prescriptive tool developed to summarize and organise the elements of public health programme evaluation. The framework guides its users in selecting evaluation strategies that are useful, feasible, ethical, and accurate for their programme. It consists of 6 stages of evaluation, guided by a set of standards approved by the American National Standards Institute and have been endorsed by the American Evaluation Association and 14 other professional organizations [63]. While the CDC framework focuses solely on evaluation, the PROCEDE-PROCEED is a logic model that provides a template for the process of conceiving, planning, implementing, and evaluating a community intervention. PRECEDE is the diagnostic portion of the model while PROCEED is the treatment portion which includes the implementation and evaluation of the educational intervention. It is a community-based and participatory, designed for health programmes but adaptable to other community issues. The model considers the ways in which administrative and policy guidelines can limit or shape an intervention and builds in monitoring of the intervention, allowing for adjustment [69]. Finally, the Rifkin Spider-gram is a tool that focuses on evaluating community participation in primary health care programs. It aims to assess the process of participation rather than its impact. It is based on the evaluation of five factors (needs assessment, leadership, organization, resource mobilization and management) and can be used to compare the same program at different points in time, observations by different evaluators, or perceptions of different participants in the same programmes [64]. Only the NEOH framework aims to address the complex prism of an integrated approach since it specifically targets OH initiatives. Its approach relies on: the description of the initiative and its context, the description of the theory of change that underpins the initiative, including the evaluation of expected and unexpected results, and the evaluation of the process of functioning and supporting infrastructure [70].
Difficulties in performing monitoring and evaluation of OH initiatives have been previously demonstrated [19]. The challenges were related to the lack of guidelines and metrics for OH evaluation. A recent scoping review suggested that OH initiative evaluation should: define the research question explicitly, define the system (inputs, outputs and interactions), and theory of change, and consider appropriate methods that capture the whole system that is impacted by changes through the initiative, and value outcomes accordingly [22]. The need to integrate the stakeholders' objectives into evaluation methods was particularly emphasized, and the authors have proposed cross-sectoral impact evaluations based on multi-level compartmental modelling approach [22]. In addition to this approach, various evaluation frameworks for OH interventions have been proposed [5,71,72]. The existence of diverse frameworks and methods for evaluating OH initiatives is not problematic. On the contrary, it is necessary to have a variety of frameworks and methods adapted to the specific needs targeted by an evaluation. Nevertheless, our study highlights the need to develop better guidance on when and how to use existing evaluation frameworks in the future, and to strengthen training on evaluation approaches for professionals working in the OH field. Using frameworks and methods to evaluate OH implementation, and assess its added value would, in the long run, allow for more robust conclusions about effective implementation strategies, which could then serve as models for decision-makers.
The barriers and facilitators identified in our review are coherent with previous findings [[73], [74], [75], [76]]. Financial, technical, and structural resources are important limiting factors of sustainable initiatives in most low- and middle-income countries [74]. Other studies have shown that these factors not only limit the start-up of projects but also to intervene at each stage of the process (including execution and monitoring and evaluation) [19]. Keys to success require broad political commitment, building the country's workforce capacity through professional training programmes, and developing laboratory capacities both in veterinary and public health [74]. This implies that the conditions at the systemic and local levels should be met to allow the success and sustainability of the initiatives [19]. It is possible that studies about OH initiatives have a publication bias and that initiatives that did not succeed were never shared in the literature. If this is the case, the barriers and facilitators to OH initiatives could be different than the ones identified here. This bias could also change the results in other ways: the type of initiatives that were evaluated and the type of evaluations that were used could also have been different in unsuccessful initiatives.
The results of our review show that studies at the intersection of animal and environmental health are lacking. A possible explanation lies in our selection criterion according to which the interventions had to directly involve at least two of the OH compartments. It is therefore possible that intervention targeting only the environment but with expected results affecting both environmental and animal health were not included in our review. Another reason could be related to our search strategy which incorporated the terms One Health and Ecohealth. The former is a relatively new term (e.g., it was indexed on Medline as MESH in 2018). As a result, studies that did not mention OH but targeted the same components, may have been missed by our research. We therefore suggest that future research incorporate additional terms related to biodiversity, wildlife and ecosystems. Finally, the scope of our review was restricted to infectious diseases and AMR, therefore our results cannot be generalized to other fields to which the OH concept could be applied.
5. Conclusion
The results of this scoping review show the range of evaluation methods and indicators used to evaluate the impacts and added value of OH, as well as their strengths and limitations. Our review highlights what remains to be done in order to gather more robust and context-sensitive evidence regarding OH benefits. Such evidence is needed to inform and support successful implementation at scale.
Funding
This work was supported by the Canadian Institutes of Health Research [NGG-411390 and NGR-167542], the Canada Research Chair in Epidemiology and One Health [CRC 950–231857].
Declarations of Competing Interest
The author JD-R was hired as an independent researcher (consultant) for her contribution to the study and manuscript. She declares no conflict of interest.
Authors' contribution
Léa Delesalle: Formal analysis, Investigation, Data Curation, Writing - original draft, Visualization.
Margaux L. Sadoine: Formal analysis, Investigation, Data Curation, Writing – original draft, Visualization.
Sarah Mediouni: Investigation, Data Curation, Writing – Review & Editing.
José Denis-Robichaud: Conceptualization, Methodology, Data Curation, Writing – Review & Editing, Visualization, Project administration.
Kate Zinszer: Conceptualization, Writing - Review & Editing.
Christina Zarowsky: Conceptualization, Methodology, Writing - review & editing.
Cécile Aenishaenslin: Conceptualization, Methodology, Project administration, Resources, Supervision, Writing – review & editing.
Hélène Carabin: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing – review & editing
Acknowledgements
The authors thank Marie-Claude Poirier for her support with the databases search.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.onehlt.2022.100380.
Appendix A. Supplementary data
Supplementary material
References
- 1.Jones K.E., Patel N.G., Levy M.A., Storeygard A., Balk D., Gittleman J.L., Daszak P. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taylor L.H., Latham S.M., Woolhouse M.E. Risk factors for human disease emergence. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2001;356:983–989. doi: 10.1098/rstb.2001.0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.King D.A., Peckham C., Waage J.K., Brownlie J., Woolhouse M.E.J. Epidemiology. Infectious diseases: preparing for the future. Science. 2006;313:1392–1393. doi: 10.1126/science.1129134. [DOI] [PubMed] [Google Scholar]
- 4.One Health High Level Expert Panel . 2021. Tripartite and UNEP support OHHLEP’s definition of “One Health,”.https://www.who.int/news/item/01-12-2021-tripartite-and-unep-support-ohhlep-s-definition-of-one-health (accessed February 8, 2022) [Google Scholar]
- 5.Baum S.E., Machalaba C., Daszak P., Salerno R.H., Karesh W.B. Evaluating one health: are we demonstrating effectiveness? One Health. 2017;3:5–10. doi: 10.1016/j.onehlt.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Bank Group, People, pathogens and our planet: the economics of one health (English) 2022. http://documents.worldbank.org/curated/en/612341468147856529/People-pathogens-and-our-planet-the-economics-of-one-health (accessed July 7, 2020)
- 7.World Health Organization, F. and A.O. of the U. Nations, W.O. for A. Health . World Health Organization; 2012. High-level technical meeting to address health risks at the human-animal ecosystems interfaces: Mexico city, Mexico 15-17 November 2011.https://apps.who.int/iris/handle/10665/78100 (accessed August 12, 2021) [Google Scholar]
- 8.FAO The FAO Action Plan on Antimicrobial Resistance 2016–2020. 2016. http://www.fao.org/3/a-i5996e.pdf
- 9.Gongal G. One Health approach in the South East Asia region: opportunities and challenges. Curr. Top. Microbiol. Immunol. 2013;366:113–122. doi: 10.1007/82_2012_242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lavan R.P., King A.I.M., Sutton D.J., Tunceli K. Rationale and support for a One Health program for canine vaccination as the most cost-effective means of controlling zoonotic rabies in endemic settings. Vaccine. 2017;35:1668–1674. doi: 10.1016/j.vaccine.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 11.Munyua P.M., Njenga M.K., Osoro E.M., Onyango C.O., Bitek A.O., Mwatondo A., Muturi M.K., Musee N., Bigogo G., Otiang E., Ade F., Lowther S.A., Breiman R.F., Neatherlin J., Montgomery J., Widdowson M.-A. Successes and challenges of the One Health approach in Kenya over the last decade. BMC Public Health. 2019;19:465. doi: 10.1186/s12889-019-6772-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.FAO, OIE, WHO . UNICEF; World Bank Group, Contributing to One World, One Health. A Strategic Framework for Reducing Risks of Infectious Diseases at the Animal–Human–Ecosystems Interface: 2008. UN System Influenza Coordination.http://www.fao.org/3/aj137e/aj137e00.htm (accessed September 3, 2021) [Google Scholar]
- 13.Grace D. The business case for One Health. Onderstepoort J. Vet. Res. 2014;81:E1–E6. doi: 10.4102/ojvr.v81i2.725. [DOI] [PubMed] [Google Scholar]
- 14.Zinsstag J., Schelling E., Waltner-Toews D., Whittaker M., Tanner M. CABI; Wallingford: 2015. One Health: The Theory and Practice of Integrated Health Approaches. [DOI] [Google Scholar]
- 15.Zinsstag J., Schelling E., Roth F., Bonfoh B., de Savigny D., Tanner M. Human benefits of animal interventions for zoonosis control. Emerg. Infect. Dis. 2007;13:527–531. doi: 10.3201/eid1304.060381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamara Y., Lamorde M., Mukiibi P. Evaluation of the costs of a comprehensive one health preparedness intervention program in 3 districts in Uganda’s West Nile region. Value Health. 2019;22:S252. doi: 10.1016/j.jval.2019.04.1182. [DOI] [Google Scholar]
- 17.Häsler B., Cornelsen L., Bennani H., Rushton J. A review of the metrics for One Health benefits. Rev. Sci. Tech. Int. Off. Epizoot. 2014;33 doi: 10.20506/rst.33.2.2294. [DOI] [PubMed] [Google Scholar]
- 18.Kickbusch I., Gleicher D. Governance for Health in the 21st Century. 2013. https://www.euro.who.int/en/publications/abstracts/governance-for-health-in-the-21st-century
- 19.Ribeiro C. Dos S., van de Burgwal L.H.M., Regeer B.J. Overcoming challenges for designing and implementing the One Health approach: a systematic review of the literature. One Health Amst. Neth. 2019;7 doi: 10.1016/j.onehlt.2019.100085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dahal R., Upadhyay A., Ewald B. One Health in South Asia and its challenges in implementation from stakeholder perspective. Vet. Rec. 2017;181:626. doi: 10.1136/vr.104189. [DOI] [PubMed] [Google Scholar]
- 21.Lee K., Brumme Z.L. Operationalizing the One Health approach: the global governance challenges. Health Policy Plan. 2013;28:778–785. doi: 10.1093/heapol/czs127. [DOI] [PubMed] [Google Scholar]
- 22.Naylor N.R., Lines J., Waage J., Wieland B., Knight G.M. Quantitatively evaluating the cross-sectoral and One Health impact of interventions: a scoping review and case study of antimicrobial resistance. One Health. 2020;11 doi: 10.1016/j.onehlt.2020.100194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dente M.G., Riccardo F., Nacca G., Ranghiasci A., Escadafal C., Gaayeb L., Jiménez-Clavero M.A., Manuguerra J.-C., Picard M., Fernández-Pinero J., Pérez-Ramírez E., Robert V., Victoir K., Declich S. Strengthening preparedness for arbovirus infections in Mediterranean and Black Sea countries: a conceptual framework to assess integrated surveillance in the context of the one health strategy. Int. J. Environ. Res. Public Health. 2018;15:489. doi: 10.3390/ijerph15030489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Errecaborde K.M., Rist C., Travis D., Ragan V., Potter T., Pekol A., Pelican K., Dutcher T. Evaluating One Health: the role of team science in multisectoral collaboration. Rev. Sci. Tech.-Off. Int. Épizooties. 2019;38:279–289. doi: 10.20506/rst.38.1.2960. [DOI] [PubMed] [Google Scholar]
- 25.Moore G.F., Audrey S., Barker M., Bond L., Bonell C., Hardeman W., Moore L., O’Cathain A., Tinati T., Wight D., Baird J. Process evaluation of complex interventions: Medical Research Council guidance. BMJ. 2015;350 doi: 10.1136/bmj.h1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peters M.D.J., Godfrey C.M., Khalil H., McInerney P., Parker D., Soares C.B. Guidance for conducting systematic scoping reviews. Int. J. Evid. Based Healthc. 2015;13:141–146. doi: 10.1097/XEB.0000000000000050. [DOI] [PubMed] [Google Scholar]
- 27.Tricco A.C., Lillie E., Zarin W., O’Brien K.K., Colquhoun H., Levac D., Moher D., Peters M.D.J., Horsley T., Weeks L., Hempel S., Akl E.A., Chang C., McGowan J., Stewart L., Hartling L., Aldcroft A., Wilson M.G., Garritty C., Lewin S., Godfrey C.M., Macdonald M.T., Langlois E.V., Soares-Weiser K., Moriarty J., Clifford T., Tunçalp Ö., Straus S.E. PRISMA Extension for Scoping Reviews (PRISMA-ScR): checklist and explanation. Ann. Intern. Med. 2018;169:467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 28.Pham M.T., Rajić A., Greig J.D., Sargeant J.M., Papadopoulos A., McEwen S.A. A scoping review of scoping reviews: advancing the approach and enhancing the consistency. Res. Synth. Methods. 2014;5:371–385. doi: 10.1002/jrsm.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levac D., Colquhoun H., O’Brien K.K. Scoping studies: advancing the methodology. Implement. Sci. 2010;5:69. doi: 10.1186/1748-5908-5-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gruetzmacher K., Karesh W.B., Amuasi J.H., Arshad A., Farlow A., Gabrysch S., Jetzkowitz J., Lieberman S., Palmer C., Winkler A.S., Walzer C. The Berlin principles on one health – Bridging global health and conservation. Sci. Total Environ. 2021;764 doi: 10.1016/j.scitotenv.2020.142919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cassidy A. In: Investig. Interdiscip. Collab. Theory Pract. Discip. Frickel S., Albert M., Prainsack B., editors. Rutgers University Press; New Brunswick (NJ): 2016. One medicine? Advocating (inter)disciplinarity at the interfaces of animal health, human health, and the environment.http://www.ncbi.nlm.nih.gov/books/NBK395883/ (accessed February 10, 2022) [PubMed] [Google Scholar]
- 32.St-Cyr Bouchard M., Bouchard C., Oestreicher J.S., Simon A., Saint-Charles J. La pratique de la transdisciplinarité dans les approches écosystémiques de la santé. VertigO - Rev. Électronique En Sci. Environ. 2014 doi: 10.4000/vertigo.14926. [DOI] [Google Scholar]
- 33.Babineau J. Product review: Covidence (systematic review software) J. Can. Health Libr. Assoc. J. Assoc. Bibl. Santé Can. 2014;35:68–71. doi: 10.5596/c14-016. [DOI] [Google Scholar]
- 34.The Commonwealth World Federation of Public Health Associations, A Systems Framework for Health Policy. 2016. https://www.thecommonwealth.io/wp-content/uploads/2020/05/A-Systems-Framework-for-Healthy-Policy.pdf (accessed February 19, 2022)
- 35.Contandriopoulos A.P., Champagne F., Denis J., Mc A. L’évaluation dans le domaine de la santé : concepts et méthodes. Rev. Epidemiol. Sante Publique. 2000;48:517–539. [PubMed] [Google Scholar]
- 36.Abbas S.S., Venkataramanan V., Pathak G., Kakkar M. Roadmap to Combat Zoonoses in India (RCZI) Initiative, Rabies control initiative in Tamil Nadu, India: a test case for the “One Health” approach. Int. Health. 2011;3:231–239. doi: 10.1016/j.inhe.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 37.Byrnes H., Britton A., Bhutia T. Eliminating dog-mediated rabies in Sikkim, India: a 10-year pathway to success for the SARAH program. Front. Vet. Sci. 2017;4:28. doi: 10.3389/fvets.2017.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lapiz S.M.D., Miranda M.E.G., Garcia R.G., Daguro L.I., Paman M.D., Madrinan F.P., Rances P.A., Briggs D.J. Implementation of an intersectoral program to eliminate human and canine rabies: the Bohol Rabies Prevention and Elimination Project. PLoS Negl. Trop. Dis. 2012;6 doi: 10.1371/journal.pntd.0001891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gürtler R.E., Yadon Z.E. Eco-bio-social research on community-based approaches for Chagas disease vector control in Latin America. Trans. R. Soc. Trop. Med. Hyg. 2015;109:91–98. doi: 10.1093/trstmh/tru203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buttigieg S.C., Savic S., Cauchi D., Lautier E., Canali M., Aragrande M. Brucellosis control in Malta and Serbia: a One Health evaluation. Front. Vet. Sci. 2018;5 doi: 10.3389/fvets.2018.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arunachalam N., Tyagi B.K., Samuel M., Krishnamoorthi R., Manavalan R., Tewari S.C., Ashokkumar V., Kroeger A., Sommerfeld J., Petzold M. Community-based control of Aedes aegypti by adoption of eco-health methods in Chennai City, India. Pathog. Glob. Health. 2012;106:488–496. doi: 10.1179/2047773212Y.0000000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sommerfeld J., Kroeger A. Eco-bio-social research on dengue in Asia: a multicountry study on ecosystem and community-based approaches for the control of dengue vectors in urban and peri-urban Asia., Pathog. Glob. Health. 2012;106:428–435. doi: 10.1179/2047773212Y.0000000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caprara A., Lima J.W.D.O., Peixoto A.C.R., Motta C.M.V., Nobre J.M.S., Sommerfeld J., Kroeger A. Entomological impact and social participation in dengue control: a cluster randomized trial in Fortaleza, Brazil. Trans. R. Soc. Trop. Med. Hyg. 2015;109:99–105. doi: 10.1093/trstmh/tru187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kittayapong P., Thongyuan S., Olanratmanee P., Aumchareoun W., Koyadun S., Kittayapong R., Butraporn P. Application of eco-friendly tools and eco-bio-social strategies to control dengue vectors in urban and peri-urban settings in Thailand. Pathog. Glob. Health. 2012;106:446–454. doi: 10.1179/2047773212Y.0000000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sripa B., Tangkawattana S., Sangnikul T. The Lawa model: a sustainable, integrated opisthorchiasis control program using the EcoHealth approach in the Lawa Lake region of Thailand. Parasitol. Int. 2017;66:346–354. doi: 10.1016/j.parint.2016.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sripa B., Tangkawattana S., Laha T., Kaewkes S., Mallory F.F., Smith J.F., Wilcox B.A. Toward integrated opisthorchiasis control in Northeast Thailand: the Lawa project. Acta Trop. 2015;141:361–367. doi: 10.1016/j.actatropica.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lankester F., Davis A., Kinung’hi S., Yoder J., Bunga C., Alkara S., Mzimbiri I., Cleaveland S., Palmer G.H. An integrated health delivery platform, targeting soil-transmitted helminths (STH) and canine mediated human rabies, results in cost savings and increased breadth of treatment for STH in remote communities in Tanzania. BMC Public Health. 2019;19:1398. doi: 10.1186/s12889-019-7737-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lushasi K., Steenson R., Bernard J., Changalucha J.J., Govella N.J., Haydon D.T., Hoffu H., Lankester F., Magoti F., Mpolya E.A., Mtema Z., Nonga H., Hampson K. One health in practice: using integrated bite case management to increase detection of rabid animals in Tanzania. Front. Public Health. 2020;8:13. doi: 10.3389/fpubh.2020.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mpolya E.A., Lembo T., Lushasi K., Mancy R., Mbunda E.M., Makungu S., Maziku M., Sikana L., Jaswant G., Townsend S., Meslin F.-X., Abela-Ridder B., Ngeleja C., Changalucha J., Mtema Z., Sambo M., Mchau G., Rysava K., Nanai A., Kazwala R., Cleaveland S., Hampson K. Toward elimination of dog-mediated human rabies: experiences from implementing a large-scale demonstration project in southern Tanzania. Front. Vet. Sci. 2017;4:21. doi: 10.3389/fvets.2017.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Welburn S.C., Coleman P. In: One Health Theory Pract. Integr. Health Approaches. Zinsstag J., Schelling E., Waltner-Toews D., Whittaker M., Tanner M., editors. CABI; Wallingford: 2015. Human and animal African trypanosomiasis; pp. 201–221. [DOI] [Google Scholar]
- 51.Martins S.B., Rushton J., Stärk K.D.C. Economics of zoonoses surveillance in a “One Health” context: an assessment of Campylobacter surveillance in Switzerland. Epidemiol. Infect. 2017;145:1148–1158. doi: 10.1017/S0950268816003320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.El Berbri I., Mahir W., Shaw A., Ducrotoy M.J., Lhor Y., Dehhaoui M., Petavy A.F., Dakkak A., Bouslikhane M., Boué F., O. Fassi Fihri, Evaluation of integrated control of three dog transmitted zoonoses: rabies, visceral leishmaniasis and cystic echinococcosis, in Morocco. Acta Trop. 2020;212 doi: 10.1016/j.actatropica.2020.105689. [DOI] [PubMed] [Google Scholar]
- 53.Díaz C. In: Ecohealth Res. Charron D.F., editor. Pract. Innov. Appl. Ecosyst. Approach Health; Springer, New York, NY: 2012. Preventing dengue at the local level in Havana City; pp. 163–171. [DOI] [Google Scholar]
- 54.Mindekem R., Lechenne M.S., Naissengar K.S., Oussiguéré A., Kebkiba B., Moto D.D., Alfaroukh I.O., Ouedraogo L.T., Salifou S., Zinsstag J. Cost description and comparative cost efficiency of post-exposure prophylaxis and canine mass vaccination against rabies in N’Djamena, Chad. Front. Vet. Sci. 2017;4:38. doi: 10.3389/fvets.2017.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barroga T.R., Gordoncillo M.J., Lagayan M.G., Bernales R., Caniban M., Lopez E., Abila R. Practical inter-sectoral linking: Tool to rabies One Health coordination to the grass-roots level, ZOONOSES PUBLIC. Health. 2018;65:805–814. doi: 10.1111/zph.12502. [DOI] [PubMed] [Google Scholar]
- 56.Hanin M.C.E., Queenan K., Savic S., Karimuribo E., Rüegg S.R., Häsler B. A one health evaluation of the southern African Centre for infectious disease surveillance. Front. Vet. Sci. 2018;5:33. doi: 10.3389/fvets.2018.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Allen H.A. Governance and one health: exploring the impact of federalism and bureaucracy on zoonotic disease detection and reporting. Vet. Sci. 2015;2:69–83. doi: 10.3390/vetsci2020069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Häsler B., Hiby E., Gilbert W., Obeyesekere N., Bennani H., Rushton J. A one health framework for the evaluation of rabies control programmes: a case study from Colombo City, Sri Lanka. PLoS Negl. Trop. Dis. 2014;8 doi: 10.1371/journal.pntd.0003270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.World Health Organization . World Health Organization; 2019. Food and Agriculture Organization of the United Nations, World Organisation for Animal Health, Taking a Multisectoral, One Health Approach: A Tripartite Guide to Addressing Zoonotic Diseases in Countries. https://apps.who.int/iris/handle/10665/325620 (accessed August 14, 2021) [Google Scholar]
- 60.Israel B.A., Schulz A.J., Parker E.A., Becker A.B. Review of community-based research: assessing partnership approaches to improve public health. Annu. Rev. Public Health. 1998;19:173–202. doi: 10.1146/annurev.publhealth.19.1.173. [DOI] [PubMed] [Google Scholar]
- 61.Minkler M. Using participatory action research to build healthy communities, public health rep. Wash. DC 1974. 2000;115:191–197. doi: 10.1093/phr/115.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Green L., Kreuter M. McGraw-Hill Education; 2005. Health Program Planning: An Educational and Ecological Approach. [Google Scholar]
- 63.CDC Framework for Program Evaluation. 2021. https://www.cdc.gov/eval/framework/index.htm (accessed August 14, 2021)
- 64.Rifkin S.B., Muller F., Bichmann W. Primary health care: on measuring participation. Soc. Sci. Med.1982. 1988;26:931–940. doi: 10.1016/0277-9536(88)90413-3. [DOI] [PubMed] [Google Scholar]
- 65.Anderson M., Clift C., Schulze K., Sagan A., Nahrgang S., Ait Ouakrim D., Mossialos E. European Observatory on Health Systems and Policies, Copenhagen (Denmark) 2019. Averting the AMR crisis: What are the avenues for policy action for countries in Europe?http://www.ncbi.nlm.nih.gov/books/NBK543406/ (accessed August 14, 2021) [PubMed] [Google Scholar]
- 66.Robinson T.P., Bu D.P., Carrique-Mas J., Fèvre E.M., Gilbert M., Grace D., Hay S.I., Jiwakanon J., Kakkar M., Kariuki S., Laxminarayan R., Lubroth J., Magnusson U., Thi Ngoc P., Van Boeckel T.P., Woolhouse M.E.J. Antibiotic resistance is the quintessential One Health issue. Trans. R. Soc. Trop. Med. Hyg. 2016;110:377–380. doi: 10.1093/trstmh/trw048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kelly T.R., Machalaba C., Karesh W.B., Crook P.Z., Gilardi K., Nziza J., Uhart M.M., Robles E.A., Saylors K., Joly D.O., Monagin C., Mangombo P.M., Kingebeni P.M., Kazwala R., Wolking D., Smith W., Mazet J.A.K., PREDICT Consortium Implementing One Health approaches to confront emerging and re-emerging zoonotic disease threats: lessons from PREDICT, One Health. Outlook. 2020;2:1. doi: 10.1186/s42522-019-0007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barrett M.A., Bouley T.A. Need for enhanced environmental representation in the implementation of one health. EcoHealth. 2015;12:212–219. doi: 10.1007/s10393-014-0964-5. [DOI] [PubMed] [Google Scholar]
- 69.The Community Tool Box . 2022. Chapter 2. Other Models for Promoting Community Health and Development | Section 2. PRECEDE/PROCEED.https://ctb.ku.edu/en/table-contents/overview/other-models-promoting-community-health-and-development/preceder-proceder/main (accessed February 8, 2022) [Google Scholar]
- 70.Rüegg S.R., McMahon B.J., Häsler B., Esposito R., Nielsen L.R., Speranza C.I., Ehlinger T., Peyre M., Aragrande M., Zinsstag J., Davies P., Mihalca A.D., Buttigieg S.C., Rushton J., Carmo L.P., Meneghi D.D., Canali M., Filippitzi M.E., Goutard F.L., Ilieski V., Milićević D., O’Shea H., Radeski M., Kock R., Staines A., Lindberg A. A blueprint to evaluate one health. Front. Public Health. 2017;5 doi: 10.3389/fpubh.2017.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rüegg S.R., Häsler B., Zinsstag J. Wageningen Academic Publishers; Wageningen: 2018. Integrated Approaches to Health: A Handbook for the Evaluation of One Health. [DOI] [Google Scholar]
- 72.Aenishaenslin C., Häsler B., Ravel A., Parmley E.J., Mediouni S., Bennani H., Stärk K.D.C., Buckeridge D.L. Evaluating the integration of one health in surveillance Systems for Antimicrobial use and Resistance: a conceptual framework. Front. Vet. Sci. 2021;8:169. doi: 10.3389/fvets.2021.611931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Purwo Suseno P., Rysava K., Brum E., De Balogh K., Ketut Diarmita I., Fakhri Husein W., McGrane J., Rasa F. Sumping Tjatur, Schoonman L., Crafter S., Sumantra I. Putu, Hampson K. Lessons for rabies control and elimination programmes: a decade of One Health experience from Bali, Indonesia. Rev. Sci. Tech. Int. Off. Epizoot. 2019;38:213–224. doi: 10.20506/rst.38.1.2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Murphy S.C., Negron M.E., Pieracci E.G., Deressa A., Bekele W., Regassa F., Wassie B.A., Afera B., Hajito K.W., Walelign E., Abebe G., Newman S., Rwego I.B., Mutonga D., Gulima D., Kebede N., Smith W.A., Kramer L.M., Kibria A., Bonnenfant Y.T., Mortenson J.A., Vieira A.R., Kadzik M., Sugerman D., Amare B., Kanter T., Walke H., Belay E., Gallagher K. One Health collaborations for zoonotic disease control in Ethiopia. Rev. Sci. Tech. Int. Off. Epizoot. 2019;38:51–60. doi: 10.20506/rst.38.1.2940. [DOI] [PubMed] [Google Scholar]
- 75.Okello A.L., Bardosh K., Smith J., Welburn S.C. One Health: past successes and future challenges in three African contexts. PLoS Negl. Trop. Dis. 2014;8 doi: 10.1371/journal.pntd.0002884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nguyen H.T.T., Afriyie D.O., Tran C.H., Dang A.D., Tran D.N., Dang T.Q., Otsu S., Urabe M.I., Pham T.N., Nguyen H.T., Nguyen T.T.T., Nguyen T.N., Padungtod P., Nguyen H.T., Nguyen T.T.T., Nguyen H.V., Le H.T., Nguyen H.T. Progress towards rabies control and elimination in Vietnam. Rev. Sci. Tech. Int. Off. Epizoot. 2019;38:199–212. doi: 10.20506/rst.38.1.2953. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material