Highlights
-
•
A prospective study of neural biomarkers of relapse in remitted depressed patients.
-
•
Assessed neural response to dysphoric mood-induction before and after psychotherapy.
-
•
Relapse over a 2-year follow-up linked to dysphoria-evoked sensory inhibition.
-
•
Relapse risk was lower when dorsolateral prefrontal reactivity decreased over time.
-
•
Depression prophylaxis may involve reducing dysphoria-evoked sensory inhibition.
Keywords: Depression, fMRI, Sensory deactivation, Relapse vulnerability, Mood challenge, Dysphoric reactivity
Abstract
Background
Neural reactivity to dysphoric mood induction indexes the tendency for distress to promote cognitive reactivity and sensory avoidance. Linking these responses to illness prognosis following recovery from Major Depressive Disorder informs our understanding of depression vulnerability and provides engagement targets for prophylactic interventions.
Methods
A prospective fMRI neuroimaging design investigated the relationship between dysphoric reactivity and relapse following prophylactic intervention. Remitted depressed outpatients (N = 85) were randomized to 8 weeks of Cognitive Therapy with a Well-Being focus or Mindfulness Based Cognitive Therapy. Participants were assessed before and after therapy and followed for 2 years to assess relapse status. Neural reactivity common to both assessment points identified static biomarkers of relapse, whereas reactivity change identified dynamic biomarkers.
Results
Dysphoric mood induction evoked prefrontal activation and sensory deactivation. Controlling for past episodes, concurrent symptoms and medication status, somatosensory deactivation was associated with depression recurrence in a static pattern that was unaffected by prophylactic treatment, HR 0.04, 95% CI [0.01, 0.14], p < .001. Treatment-related prophylaxis was linked to reduced activation of the left lateral prefrontal cortex (LPFC), HR 3.73, 95% CI [1.33, 10.46], p = .013. Contralaterally, the right LPFC showed dysphoria-evoked inhibitory connectivity with the right somatosensory biomarker
Conclusions
These findings support a two-factor model of depression relapse vulnerability, in which: enduring patterns of dysphoria-evoked sensory deactivation contribute to episode return, but vulnerability may be mitigated by targeting prefrontal regions responsive to clinical intervention. Emotion regulation during illness remission may be enhanced by reducing prefrontal cognitive processes in favor of sensory representation and integration.
1. Introduction
Relapse and recurrence following guideline indicated pharmacologic (Rush et al., 2006) or psychotherapeutic (Hollon et al., 2005) treatment of Major Depressive Disorder (MDD) remain common and debilitating outcomes (Hardeveld et al., 2009). In contrast to the literature on acute phase treatment, where promising response indicators have been identified (Bartlett et al., 2018, Gadad et al., 2018, Godlewska et al., 2018), efforts to improve clinical prognosis following MDD episode have yielded few indicators of sustained remission (Kennis et al., 2020). Prior episodes and residual depressive symptoms remain the best predictors of future episodes (Eaton et al., 2008, Solomon et al., 2000, Verhoeven et al., 2018), yet these markers do not capture the cognitive and affective dynamics that underlie MDD vulnerability, nor are they sensitive to changes in vulnerability following prophylactic intervention. Identification of dynamic, treatment-responsive indicators of episode recurrence would support greater precision in prophylactic treatment (Kazdin, 2007).
An emerging neural systems model characterizes MDD vulnerability as an overemphasis of dysphoric cognition in response to negative events, to the detriment of sensory integration of novel, depression-incongruent events (Disner et al., 2011, Farb et al., 2015). In response to negative stimuli, acute MDD episodes are often characterized by reduced engagement of cognitive control in brain regions such as the dorsolateral prefrontal cortex (PFC), but elevated engagement of salience detection in medial PFC (Hamilton et al., 2012, Hamilton et al., 2013). The lack of dorsolateral PFC response to negative stimuli may be due to chronic and diffuse hyperactivity observed in resting-state scans of acute MDD patients relative to healthy controls (Hamilton et al., 2013, Sheline et al., 2009). Elevated phasic (stimulus-evoked) dorsomedial PFC activity is also thought to contribute to depressive symptom burden, representing strategic aspects of depressive self-focus that may extend to the lateral PFC (Lemogne et al., 2012). Accordingly, response to depression-specific psychotherapy has been linked to normalization of hyperactivity in the dorsolateral PFC and related regions (Fonseka et al., 2018, Goldapple et al., 2004).
As both dorsolateral and dorsomedial PFC reactivity seems to be provoked by negative stimuli in acute MDD, such reactivity may also indicate increased self-referential processing and rumination in remission, a phenotype repeatedly linked to relapse vulnerability (Kruijt et al., 2013, Segal et al., 2006). Functional connectivity studies corroborate this account, linking MDD to elevated connectivity within prefrontal networks (Wang et al., 2016), and remission to reduced connectivity within these networks (Meyer et al., 2019). Prefrontal hyperactivity may be a lasting consequence of MDD, overwhelming attentional resources for engaging in new learning and behaviour even when symptoms have lessened (Marchetti et al., 2012). Heightened rumination and self-evaluation seem to lead to an expansion of typically dorsomedial PFC activity to include the dorsolateral-PFC, thereby reducing opportunities for adaptive emotion regulation, a foundational premise of the dorsal nexus hypothesis (Sheline et al., 2010). For example, abnormal activity in sensory integration regions such as the anterior insula are commonly observed in MDD, and their normalization may serve as a prognostic marker of treatment response (Dunlop et al., 2015, McGrath et al., 2013). According to the dorsal nexus hypothesis, reducing hyperactive dorsolateral PFC reactivity to negative stimuli in remission may restore emotion regulation capacity.
Despite its promise, a focus on PFC reactivity addresses cognitive features of MDD but speaks less to somatic features. Interoceptive dysfunction, presenting as avoidance of somatic experience, may be a relatively overlooked facet of depression vulnerability (Harshaw, 2015). Dysphoric mood induction has been linked to both activation of the PFC and deactivation of the right middle insula (Farb et al., 2010), a region supporting sensory integration, including awareness of the body’s internal state (Craig, 2002). Insula deactivation rather than PFC activation was associated with concurrent depressive symptoms, affirming the importance of sensory processing in depression. Furthermore, in one of the few prospective neuroimaging studies of MDD recurrence, both PFC activation and sensory deactivation were associated with new MDD episodes over a subsequent 18-month follow-up (Farb et al., 2011). In parallel, emerging connectivity studies of depression suggest that abnormal sensorimotor connectivity may be a powerful but overlooked feature of depressive symptom burden and treatment response (Ray et al., 2021). While small sample sizes limit the generalizability of regional findings, such studies provide initial evidence for characterizing MDD relapse vulnerability as an over-reliance on cognitive elaboration to the detriment of sensory integration.
A well-powered prospective neuroimaging design may better characterize neural biomarkers of cognitive reactivity and sensory deactivation. Biomarkers identified herein could then be evaluated in future studies for their predictive utility. Furthermore, given evidence that prophylactic interventions reduce MDD relapse vulnerability (Guidi and Fava, 2020, Kuyken et al., 2016), biomarkers can also be evaluated for their sensitivity to treatment response. This approach also permits comparing interventions, distinguishing between treatment-specific and trans-therapeutic mechanisms of prophylaxis. The current study therefore aimed to 1) identify static biomarkers of relapse vulnerability over the 24-month clinical follow-up, 2) identify biomarkers sensitive to prophylactic psychological treatment, and 3) explore mechanistic differences between evidence-based prophylactic treatments.
2. Methods
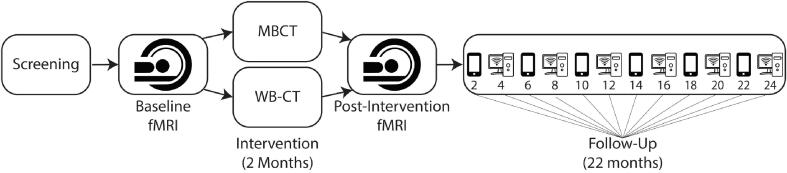
Neuroimaging of a validated dysphoric mood induction task (Farb et al., 2010, Farb et al., 2011) was conducted as part of a broader RCT reporting both clinical and psychometric outcomes (Farb et al., 2018, Segal et al., 2019). In brief, fully remitted participants were randomized to receive either Mindfulness Based Cognitive Therapy (MBCT) (Segal et al., 2012) or Cognitive Behavior Therapy with a Well-Being focus (WB-CT) (Fava, 2016) before entering a 2-year follow-up period; participants performed fMRI and self-report assessment both before and after treatment (Fig. 1). The study protocol was approved by the institutional review board at the Centre for Addiction and Mental Health (CAMH) and registered at clinicaltrials.gov (NCT01178424).
Fig. 1.
Study design summary. Smartphones and computers alternating within the follow-up period indicate bimonthly assessment points, which alternated between phone-based and online eSurvey assessments.
2.1. Participants
Participants were screened for inclusion and exclusion criteria and provided informed consent. Inclusion criteria were: (1) not currently meeting a diagnosis of Major Depressive Disorder (MDD) according to DSM-IV criteria, (2) a score of ≤12 on the Hamilton Depression Rating Scale (HRSD-17), (3) ≥1 previous episode of MDD, (4) between 18 and 65 years of age and (5) English speaking and the ability to provide informed consent. Exclusion criteria were: (1) a current diagnosis of Bipolar Disorder, Substance Abuse Disorder, Schizophrenia or Borderline Personality Disorder, (2) currently receiving psychotherapy or practicing meditation > once per week or yoga > twice per week.
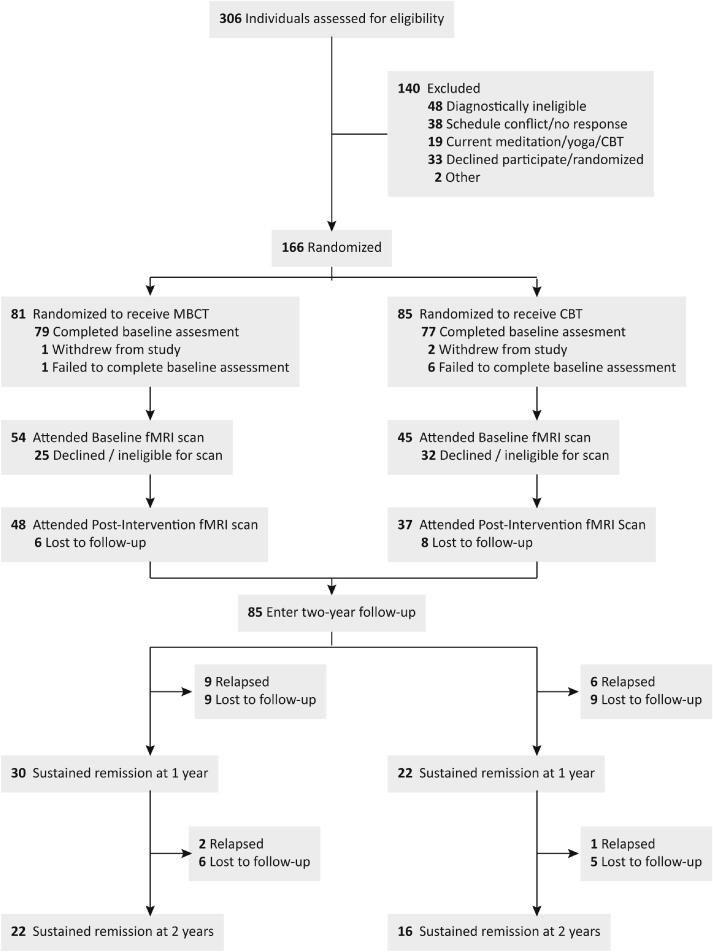
Following enrolment in the RCT (n = 166), 60% of participants (n = 99) opted to attend the pre-intervention neuroimaging scan, of whom 86% (n = 85) returned for the post-intervention scan. Participants who failed to complete assessments were interviewed by the research team to confirm their intention to leave the study. Of the 85 patients who entered the two-year clinical follow up, data on relapse status was available for 81% of the sample at one year and 60% of the sample at the end of two-year follow up. Complete CONSORT information is presented in Fig. 2, and details on the rates of voluntary withdrawal vs. relapse over the study period are available in Table S1. The neuroimaging group did not differ on demographic or clinical history variables compared to the original RCT clinical sample at either pre- or post-intervention scan timepoints (Table 1) (Farb et al., 2018, Segal et al., 2019). Relapse status was not significantly correlated with any of the demographic variables listed in Table 1.
Fig. 2.
Study consort diagram.
Table 1.
Demographic and clinical characteristics by study stage.
| Measure | Clinical Sample n = 166 |
Baseline Sample n = 99 |
Post-Intervention n = 85 |
|---|---|---|---|
| Age, mean (SD) | 40.63 (11.77) | 39.23 (12.00) | 39.08 (12.16) |
| Gender, n (%) Female |
112 (67.5%) |
62 (62.6%) |
58 (68.2%) |
| Ethnicity, n (%) | |||
| Caucasian | 132 (82.0%) | 79 (86.8%) | 73 (86.9%) |
| Afro-Canadian | 8 (5.0%) | 1 (1.1%) | 0 |
| Asian/East-Asian | 12 (7.5%) | 6 (6.6%) | 6 (7.1%) |
| Hispanic | 4 (2.5%) | 1 (1.1%) | 1 (1.2%) |
| Other | 5 (3.1%) | 4 (4.4%) | 4 (4.8%) |
| Education, n (%) | |||
| High school | 27 (16.3%) | 16 (16.2%) | 14 (16.5%) |
| College/University | 110 (66.3%) | 60 (60.6%) | 56 (65.9%) |
| Graduate school | 25 (15.1%) | 16 (16.2%) | 15 (17.6%) |
| Other | 4 (2.4%) | 7 (7.1%) | 0 |
| Employment, n (%) | |||
| Full time job | 91 (59.9%) | 49 (57.0%) | 46 (58.2%) |
| Part time job | 23 (15.1%) | 15 (17.4%) | 15 (19.0%) |
| Unemployed | 29 (19.1%) | 17 (19.8%) | 14 (17.7%) |
| Student/Other | 9 (5.9%) | 5 (5.8%) | 4 (5.1%) |
| Age of onset of first episode of depression, mean (SD) | 22.43 (10.67) | 20.87 (9.45) | 20.46 (9.41) |
| Number of past episodes of depression, mean (SD) | 3.86 (2.34) | 4.17 (2.60) | 4.08 (2.51) |
| Previous hospitalization n (%) | 38 (22.9%) | 27 (27.3%) | 27 (31.8%) |
| Suicide attempts, n (%) | 29 (17.5%) | 19 (19.2%) | 16 (18.8%) |
| Family Hx depression, n (%) | 114 (68.7%) | 67 (67.7%) | 62 (72.9%) |
| Antidepressant at intake, n (%) | 100 (60.2%) | 59 (59.6%) | 55 (64.7%) |
| Previous or current psychotherapy, n (%) | 140 (84.3%) | 77 (77.8%) | 70 (82.4%) |
| Remission achieved via, n (%) | |||
| CBT | 39 (28.7%) | 16 (20.3%) | 16 (21.9%) |
| Psychotherapy | 16 (11.8%) | 10 (12.7%) | 8 (11.0%) |
| Medication | 34 (25.0%) | 23 (29.1%) | 20 (27.4%) |
| Medication & psychotherapy | 26 (19.1%) | 19 (24.1%) | 18 (24.7%) |
| Other | 21 (15.4%) | 11 (13.9%) | 11 (15.1%) |
| Number of treatment sessions attended, mean (SD) | 6.27 (2.00) | 6.21 (2.14) | 6.72 (1.48) |
Chi-square tests (for categorical variables) and t-tests for (continuous variables) across all 3 samples revealed no significant differences. Please note that participants had the option to disclose demographic information at their discretion; as such, the total number of demographic variable responses may be less than the full sample size. Percentages are relative to the total number of respondents for each demographic variable.
2.2. Sample size justification
Required sample size was determined prior to study commencement using the fMRIPower software package (Mumford, 2012), which used data from our prior mood-induction prospective relapse study (Farb et al., 2011) to estimate power to detect relapse from mood induction responses in the prefrontal cortex and sensory cortices. Detection of mood-related activation in the medial (BA32) and lateral (BA 46) prefrontal cortex achieved 80% power with 24 participants, whereas detection of mood-related deactivation in somatosensory, insula, and visual clusters (BA 18) required 35 participants within each group. Assuming up to 30% attrition over the clinical intervention period, it was decided to recruit 100 participants to baseline fMRI assessment.
2.3. Randomization and masking
Eligible patients were randomized in blocks of four, using computer generated quasi-random numbers, to receive eight weekly group sessions of either MBCT or WB-CT. Randomization was performed by the study coordinator via a randomization table; investigators were blind to group allocation during the intervention and follow-up assessment periods.
2.4. Clinical outcomes
The primary outcome measure was time to relapse/recurrence of DSM-IV-TR major depressive episode (the study began prior to DSM-5 publication), using the depression module of the SCID. Symptom assessments were conducted at bimonthly intervals alternating between an eSurvey using the Quick Inventory of Depressive Symptomatology (QIDS-SR) (Rush et al., 2003) and the HAMD-17 during phone interviews (Fig. 1). A QIDS score of ≥12 also triggered a phone assessment with HAMD-17 and SCID. Episode return was defined as a score ≥16 on HAMD and meeting MDD criteria on a subsequent SCID. All interviews were audio-taped and inter-rater agreement was calculated on a subset of HAMD-17 interviews, yielding an intraclass correlation coefficient of 0.94 (n = 18). Similarly, reliability of MDD diagnoses based on the SCID yielded a kappa 0.82 (n = 22). Relapse diagnoses were confirmed by an experienced research psychiatrist.
Survival analysis on the Intention to Treat sample indicated that the 2-year relapse rate was 21% with no differences between the groups (7/37 = 19% in WB-CT and 11/48 = 23% in MBCT), χ2 = 0.2, p = .65. Furthermore, although 64% of participants were on a stable regimen of antidepressant medication over the study period, the rates of antidepressant medication were not significantly different in relapsers (72%) than in non-relapsers (61%), χ2 = 0.35, p = .56. Further details about assessment and follow-up are available in the published trial protocol (Farb et al., 2018).
2.5. Dysphoric mood induction
The dysphoric mood induction task has been previously validated (Farb et al., 2010, Farb et al., 2011), and was programmed for the current study in the Visual Basic programming language (Microsoft Visual Studio 2012; Redmond, WA). During fMRI scanning, participants viewed film clips and rated their sadness between clips. Participants viewed four sets of clips over two fMRI acquisition runs, with each run containing one neutral and one sad set per run. The first run always featured a neutral set followed by a sad set, with a sad set followed by neutral set in the second run. Each set featured an instruction screen (10 sec) prior to viewing (45–50 sec), rating (6 sec), and reflecting upon (50 sec) each of the four film clips in sequence. Participants rated their sadness on a scale of 1 (“Not at All Sad”) to 7 (“Extremely Sad”) using a scanner-compatible button box. A blank screen reflection period followed each rating to allow washout of film viewing effects.
2.6. fMRI data acquisition
Neuroimaging was performed at the Rotman Research Institute using a Siemens Trio 3.0-Tesla scanner, with slew rate of 400 T/m/s and a 12-channel asymmetric gradient head coil. During the pre- and post-treatment scans, 2 runs of 434 functional volumes were collected, for a total of 868 volumes per assessment. Additional details can be found in the supplementary materials.
2.7. Preprocessing
Data preprocessing was performed using fMRIPrep 20.0.6 (Esteban et al., 2019, Esteban et al., 2020), a consensus standard fMRI preprocessing pipeline; complete details are provided in the supplementary materials. In summary, anatomical images from both baseline and post-intervention were segmented and normalized into a standard Montreal Neurological Institute (MNI) space through nonlinear registration. Functional runs were co-registered to the anatomical images, slice-time corrected, and resampled into 2 mm isotropic voxels in MNI space. Preprocessing and analysis following the fMRIPrep pipeline were performed using MATLAB 2018a (MathWorks, Natick, MA, USA) and SPM12 (Wellcome Department of Cognitive Neurology, UK). Functional data were spatially smoothed using an 8 mm Gaussian kernel, as recommended for optimizing group inference (Mikl et al., 2008).
2.8. fMRI analysis
Whole brain, voxelwise analyses were based on participants (N = 85) who were each scanned both pre- and post-intervention. First level analysis featured a block design to model neutral and sad film viewing periods as separate boxcar regressors across two functional runs. The standard six motion parameters (3 translation + 3 rotation) and global CSF signals were included as nuisance regressors, as CSF signals act as a reliable proxy for physiological noise (Birn, 2012, Kong et al., 2012). Participants did not differ in any of the 6 motion parameters as a function of Time or Relapse status (Table S2). Sad and neutral film conditions were contrasted within each participant session.
The second level analysis employed a mixed linear model to analyze the dependent factor of Time (Baseline vs. Post-Intervention), and the independent factors Group (MBCT vs. WB-CT) and Relapse (Relapse vs. Remitted). Given their documented relationship to depression vulnerability, residual symptoms (Paykel, 2008), number of past episodes (Bulloch et al., 2014), and antidepressant medication status (Dobson et al., 2008) were all included as covariates of interest in fMRI factorial models and in all Cox regressions. Residuals symptom scores were obtained concurrently at the time of each fMRI assessment, and calculated from data reduction of previously described psychometric data (Segal et al., 2019).
2.8.1. Statistical thresholds
False positive rates were addressed through statistical thresholding using clusters determined by a voxel height threshold of z > 2.58 (p < .005) and an FWE-corrected cluster threshold of p < .05 (∼750 voxels at 2x2x2 resolution). Despite earlier calls for highly conservative peak threshold of p < .001 (Eklund et al., 2016), more recent research has advocated for an equitable balance between voxel and cluster thresholds, an approach that has proved superior to voxelwise p < .001 alone (Cox, 2019). Furthermore, a focus on cluster thresholding over voxel thresholding appears to lead to superior replicability in larger datasets such as ours (170 scans) (Bossier et al., 2020). While novel adaptive clustering techniques have been introduced to the neuroimaging community during the preparation of this manuscript (Cox, 2019, Smith and Nichols, 2009), we followed an a priori analysis plan to fix voxel threshold at p < .005. Our planned threshold was successfully employed in past studies using this film-based mood induction paradigm (Farb et al., 2010, Farb et al., 2011); control for Type-1 error was still maintained using the cluster correction algorithms built into SPM12.
2.8.2. Relapse biomarker identification
Three a priori analyses supported the study aims:
Static Biomarkers. To identify static biomarkers of relapse vulnerability, the main effect of Relapse (non-relapsers – relapsers) across both assessment time points (Baseline and Post-Intervention) was computed as an unbiased estimator of static relapse vulnerability. To test the stability of the regions identified, post hoc simple effects analyses were conducted for the main effect of Relapse at both baseline and post-intervention, as well as an exploratory conjunction analysis between these separate timepoint whole brain contrasts.
Dynamic Biomarkers. To identify biomarkers of treatment response, all regions demonstrating a main effect of Time [Post-intervention – Baseline] within the non-relapse group were analyzed for their association with future relapse. The analysis was constrained to the non-relapse group to isolate changes that could plausibly be related to treatment-induced prophylaxis again depression, as a more conventional whole-brain Time * Relapse interaction effect might be driven by either baseline differences or changes in the relapse group, neither of which speak to treatment response. Because changes in the non-relapse group could be non-specific effects of time rather than relevant for depression vulnerability, all significant regions of interest were then subjected to post-hoc Time * Relapse interactions to determine their clinical significance. These post-hoc tests were conducted in the R statistical programming environment (R Core Team, 2017), using the ‘lme4′ library for mixed models (Bates et al., 2015, p. 4), using the following equation:
Time * Group interactions within regions identified from the non-relapse group were also explored to identify differential responses to prophylactic intervention associated with future relapse. Again, only the non-relapse group was included in the initial contrasts to provide the most sensitive estimator of treatment response, as the relapse group, by definition, did not experience sufficient change over time to achieve prophylaxis.
Given the typicality of running Relapse × Time interactions in clinical trials rather than focusing on change in the non-relapse group, an exploratory Relapse × Time whole brain analysis was conducted and is described in the supplementary results, but are not reported in the main text as they did not inform treatment-related change.
2.8.3. Survival analysis
To better characterize neural reactivity associated with relapse, survival analysis was performed using Cox proportional hazards models featuring neural reactivity scores were estimated using the ‘survival’ library (Therneau and Grambsch, 2000) in the R statistical programming environment (R Core Team, 2017). Cox models were used to estimate relapse risk at each of the bimonthly time points over the follow-up period, including the activation levels of neural regions-of-interest while controlling for past episodes and concurrent depressive symptoms as covariates. The analysis also modelled censoring of participants due to study withdrawal, so that the most complete dataset available at each time point was used to estimate relapse risk. While neural activity was a continuous variable in the model, median splits were used for generating survival curves using the ‘survminer’ and ‘ggplot2′ libraries (Kassambara et al., 2020, Wickham, 2016, p. 2).
2.8.4. Psychophysiological interaction (PPI) analysis
The neural region-of-interest most significantly associated with relapse (peak MNI location: x = 52, y = −30, z = 64) was used as a seed region in a post-hoc, generalized psychophysiological interaction (gPPI) analysis to measure task-evoked changes in whole-brain functional connectivity with the seed region. Within each participant-session, signal from the seed region was extracted to construct an interaction term with a vector contrasting film task conditions (Sad vs. Neutral). The resulting first-level participant-session maps indicated areas where seed region functional connectivity was significantly altered as a function of film condition. At the group level, relapse status was regressed onto the participant-session maps to identify where mood-related changes in somatosensory connectivity were associated with relapse.
3. Results
3.1. Effects of mood induction
Characterization of dysphoric mood induction effects was conducted prior to analyses supporting the study aims (Table S3). Sadness ratings were greater for sad than neutral clips, β = 1.78, 95% CI [1.57, 2.00], p < .001; relapse status did not interact with evoked sadness ratings, β = −0.11, 95% CI [−0.65, 0.43], p = .69.
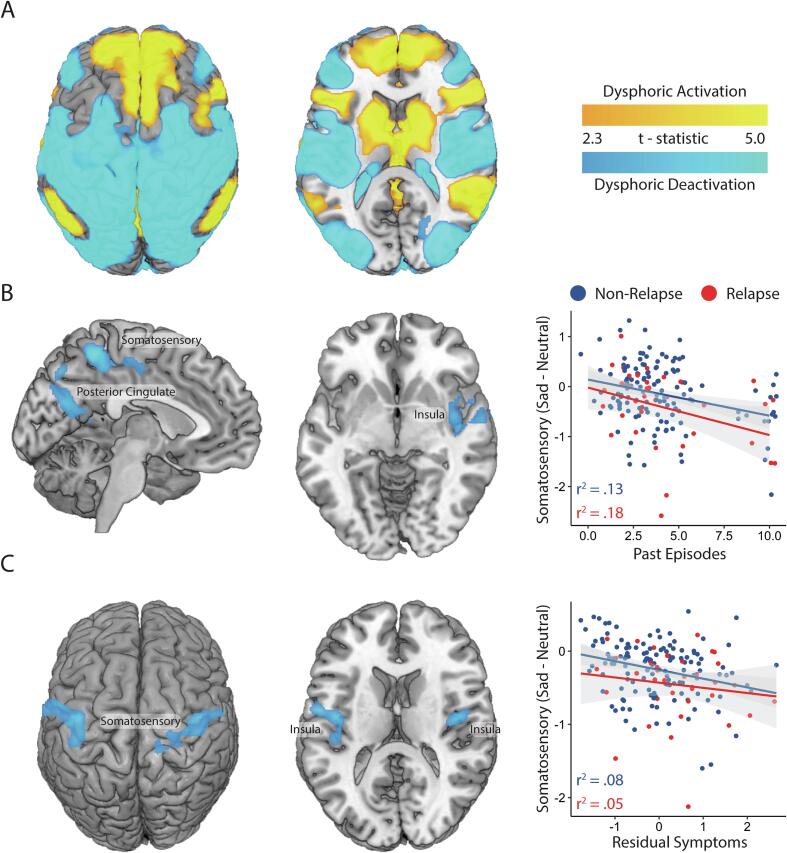
The contrast of [Sad Films – Neutral Films] revealed that dysphoric mood induction was associated with activation along the cortical midline, including the posterior cingulate, striatum, and medial prefrontal cortex, as well as the anterior insula and superior temporal gyrus. Deactivations were apparent across diverse sensory representation regions, including the primary somatosensory cortex, posterior insula, and visual regions such as the fusiform gyrus, as well as aspects of the inferior frontal gyrus (Fig. 3A; Table S4).
Fig. 3.
Neural reactivity to dysphoric mood induction. A) Main Effect of Task (Sad – Neutral); B) past episodes covariate of neural reactivity, with a scatterplot of the relationship between past episodes and the peak covariate region located in the medial somatosensory cortex; C) residual symptom covariate of neural reactivity, with a scatterplot of the relationship between residual symptoms and the peak covariate region in the right somatosensory cortex and posterior insula. Scatterplots use data from both timepoints (baseline and post-intervention) and show linear fit within both the non-relapser and relapser sub-groups to illustrate the consistency of the relationship. Gray shaded areas around the fit lines are 95% confidence intervals.
3.2. Covariates of interest
Three common indicators of depression relapse vulnerability, past episodes, residual symptoms and antidepressant status were entered as covariates to the dysphoric-reactivity analysis. Antidepressant medication status was not associated with neural reactivity to dysphoric mood provocation.
However, a greater number of past episodes was associated with greater sadness-evoked deactivation in the medial somatosensory cortex, right anterior insula, and precuneus (Table S5), accounting for 14.4% of the variance in mean signal from these regions. Post-hoc analysis confirmed that this relationship was significant both within non-relapsers, R2 = 0.130, p < .001, and for relapsers, R2 = 0.182, p = .027 (Fig. 3B).
Furthermore, greater levels of residual symptoms were associated with greater sadness-evoked deactivation bilaterally within the somatosensory cortex and posterior insula (Fig. 3C; Table S4), accounting for 7.6% of variance in these regions. Post-hoc analysis confirmed this relationship was significant for non-relapsers, R2 = 0.075, p = .002, but failed to reach significance for relapsers, R2 = 0.046, p = .198.
3.3. Static (Treatment-Invariant) relapse biomarkers
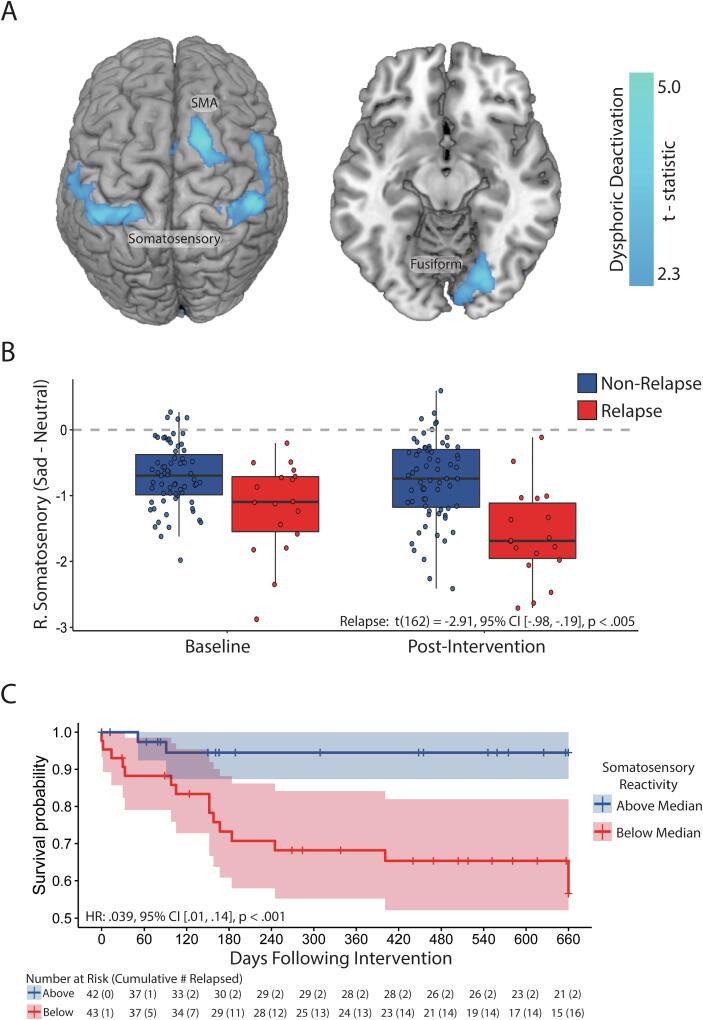
To identify treatment-invariant (static) neural biomarkers of relapse (Aim 1), dysphoric neural reactivity across both timepoints (baseline and post-intervention) was regressed onto relapse status over the 24-month follow-up period. The contrast of [Non-Relapsers – Relapsers] revealed that relapse was not associated with greater activation in any region, but were associated with greater deactivation in bilateral somatosensory cortex, supplementary motor area (SMA), and fusiform gyrus (Fig. 4A, Table S6). Post-hoc analysis confirmed that all four regions were significantly associated with relapse at both time points (Table S7), and an exploratory conjunction analysis that examined the overlap of relapse associations estimated separately at each time point replicated these findings (Fig. S1).
Fig. 4.
Main effects of future relapse status on neural reactivity to dysphoric mood induction. A) Regions sensitive to future relapse status; B) Boxplot of right somatosensory reactivity at both timepoints with 95% confidence intervals; C) survival plot for participants over the follow-up period as a function of average right somatosensory reactivity (sad – neutral film clip viewing) across both time-points. Cross-hatches indicate participants censored due to relapse or being lost to follow-up. Please note that due to the context of sadness-evoked deactivation, ‘Above Median’ scores indicate less deactivation, whereas ‘Below Median’ scores indicate greater deactivation.
To evaluate the potential independence of the four static relapse biomarkers, stepwise Cox regressions were performed, beginning with a baseline model containing number of past episodes and residual symptoms. The right somatosensory cortex region was the most significant correlate of relapse, demonstrating significantly greater deactivation in relapsers compared to non-relapsers, β = −0.65 [−0.87, −0.44], p < .001 (Fig. 4B). Including average right somatosensory reactivity in the Cox model significantly improved model fit, from R2 = 0.201 to R2 = 0.837, χ2(2) = 28.5, p < .001. Survival probability at the end of the follow-up period was much higher for participants above the median level of somatosensory deactivation, p = .945; 95% CI [0.874; 1.00], than for those below the median, p = .566; 95% CI [0.419; 0.765] (Fig. 4C). Including additional regions did not improve model fit (Table S8), so only the right somatosensory region was retained as a static vulnerability marker.
3.4. Dynamic (Treatment-Varying) relapse biomarkers
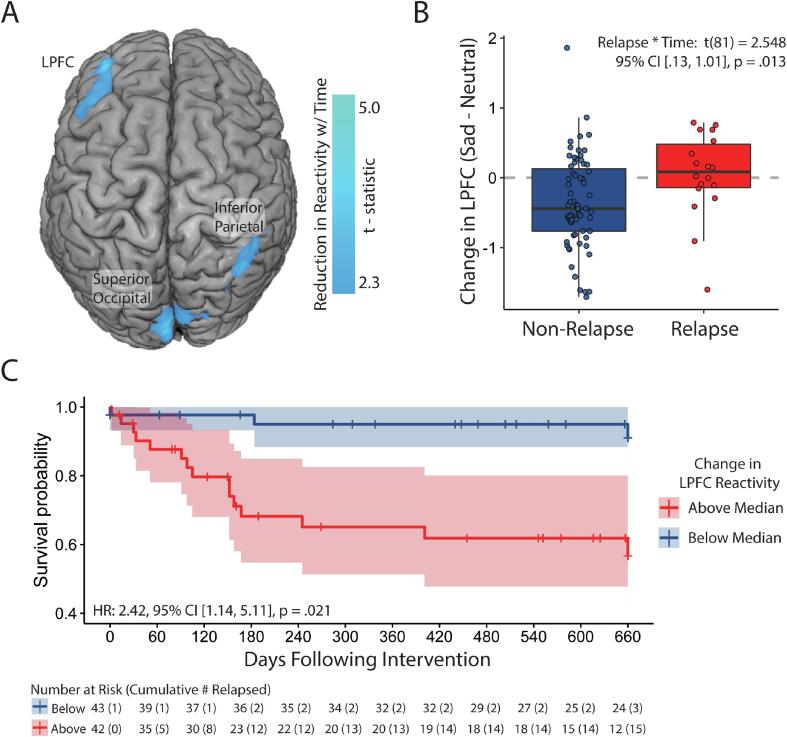
To identify treatment-varying (dynamic) neural biomarkers of relapse (Aim 2), the effect of Time within non-relapsers was explored. Time-related reductions were observed in the right inferior parietal lobe, left DLPFC and left superior occipital regions (Fig. 5A, Table S9). Change scores from all three regions were entered into separate Cox regressions to model relapse risk, controlling for past episodes. Concurrent depressive symptoms and antidepressant status. Of the three regions, only change in the left DLPFC was associated with future relapse status (Table S10).
Fig. 5.
Effects of time (baseline vs. post-intervention) on neural reactivity to dysphoric mood induction within the non-relapse group. A) Regions demonstrating reduced reactivity over time; B) Boxplot of left lateral prefrontal cortex (LPFC) change scores over time with 95% confidence intervals; C) survival plot for participants over the follow-up period as a function of change in left LPFC change scores. Cross-hatches indicate participants censored due to relapse or being lost to follow-up. Please note that due to the context of reduced reactivity over time, ‘Above Median’ scores indicate a failure to reduce reactivity, whereas ‘Below Median’ scores indicate reduced reactivity.
Follow-up multilevel modelling of Time × Relapse effects suggested that reductions in left DLPFC reactivity were less pronounced for relapsers than non-relapsers, Relapse × Time β = 0.40 [0.06, 0.73], p = .022 (Fig. 5B). The interaction was driven by a significant reduction for non-relapsers, β = −0.36 [−0.51, −0.20], p < .001, but no evidence of change for relapsers, β = 0.04 [−0.24, 0.32], p = .773 (Fig. S2).
Compared to the baseline Cox model of past episodes, residual symptoms and antidepressant status, including change in left DLPFC reactivity significantly improved model fit, from R2 = 0.201 to R2 = 0.410, χ2(2) = 5.44, p = .020. Including additional regions did not improve model fit (Table S10), so only the left DLPRC region was retained as a dynamic vulnerability marker. Survival probability at the end of the follow-up period was much higher for participants below the median level of DLPFC reactivity change, p =.910; 95% CI [0.815; 1.00], than for those above the median, p =.567; 95% CI [0.416; 0.773] (Fig. 5C).
An exploratory, whole-brain Relapse × Time interaction analysis suggested an additional dynamic region in the right cerebellum (Fig. S3A), but follow-up analysis revealed that this interaction was driven only by change in the relapse group (Fig. S3B, Table S11), and therefore not a good candidate biomarker of treatment-related change.
3.5. Interactions with treatment group
To identify distinctive neural mechanisms between the MBCT and WB-CT groups (Aim 3), the overall Group × Time interaction, Group × Time interaction within non-relapsers, and Group × Time × Relapse interactions were explored. However, no group effects were observed.
3.6. Combined relapse model
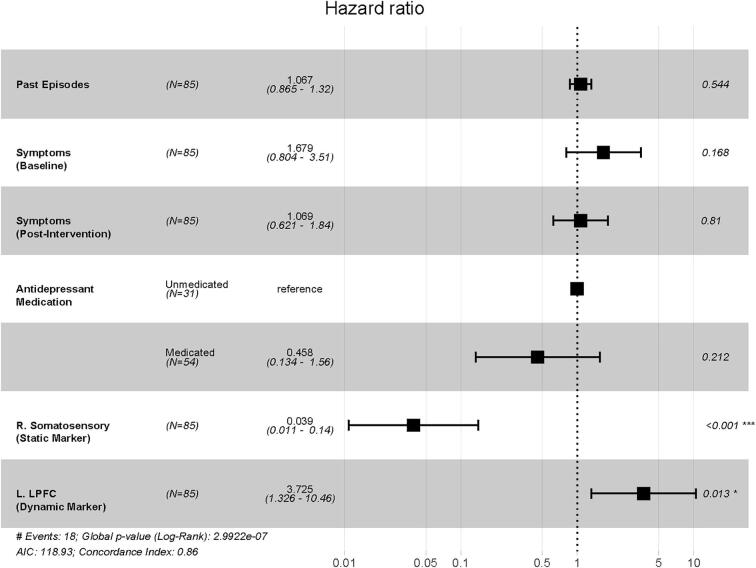
To explore biomarker independence, the candidate relapse biomarkers were combined into a single Cox regression model, controlling for past episodes, depressive symptoms, and antidepressant medication status. The static somatosensory and dynamic DLPFC biomarkers both independently contributed to model fit (Fig. 6), with the combined model accounting for 89.7% of the variance in relapse status, with excellent concordance (C = 0.86).
Fig. 6.
Summary model of relapse risk. The Hazard Ratios for the combined Cox regression model for Relapse that includes neural biomarker activity from both the static marker of relapse (right somatosensory cortex) and the dynamic marker, wherein activity changed over the intervention period (left lateral prefrontal cortex), controlling for past episodes, concurrent depressive symptoms, and antidepressant medication status.
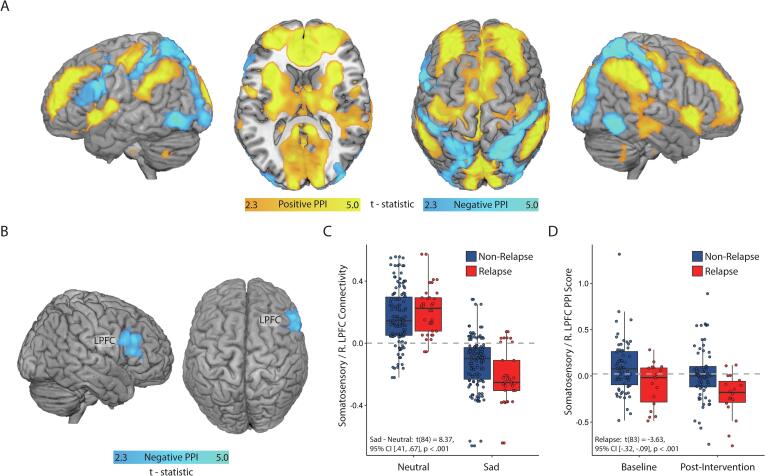
3.7. Psychophysiological interaction (PPI) analysis
Finally, a psychophysiological interaction analysis (PPI) was conducted to explore the whole-brain network arising around sadness-evoked somatosensory deactivation. The right somatosensory region identified as a static biomarker of relapse was employed as a seed region. The analysis indicated that dysphoric mood induction introduced an inhibitory relationship between the prefrontal cortex/cortical midline and posterior sensory regions including the somatosensory, auditory, and visual cortices (Fig. 7A; Table S12). In response to mood induction, relapse was associated with a shift from positive to negative connectivity between the somatosensory seed and a right lateral PFC region (Fig. 7B, Table S12). Examination of the raw (non-PPI) functional connectivity scores between the right somatosensory seed region and the right LPFC indicated positive functional connectivity during neutral mood conditions, but negative (inhibitory) connectivity under sad mood (Fig. 7C). Participants for whom sadness evoked a greater shift from positive to negative connectivity were more likely to relapse (Fig. 7D).
Fig. 7.
Effects of PPI analysis. A) Regions with altered connectivity to the right somatosensory cortex as a function of mood context (sad vs. neutral). Orange areas are FWE-corrected positive PPI score areas, whereas blue areas are negative scores. B) Sadness-evoked connectivity change with the right lateral prefrontal cortex (LPFC) is significantly related to future relapse status. C) Connectivity between the somatosensory cortex and right LPFC is responsive to dysphoric mood induction. D) The magnitude of sadness-evoked deactivation between right LPFC and somatosensory cortex distinguishes relapsers from non-relapsers. Interpretation of how this connectivity relates to the right somatosensory seed region may be challenging given that main effect within the somatosensory region was a deactivation; by this logic, orange areas were more negatively associated with the somatosensory cortex during sad-mood induction, whereas blue areas were more positively associated with the somatosensory cortex, sharing in the deactivation pattern. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
Prior research has focused on elevated prefrontal activity in acute phase depression (Lemogne et al., 2012), which has been interpreted as a loss of cognitive control over emotion (Sheline et al., 2009), potentially due to the recruitment of cognitive resources in depressive rumination (Segal et al., 2006). Functional connectivity studies support this view, as cognitive control networks including the LPFC are observed less frequently in patients remitted from MDD (Figueroa et al., 2019), whereas non-relapsers tend to show increased connectivity of the LPFC with executive control regions following antidepressant discontinuation (Berwian et al., 2020). While less often the focus of neuroimaging research, emerging mechanistic accounts of depression have also implicated sensorimotor dysfunction a reliable correlate of symptom burden (Ray et al., 2021), which is consistent with established findings linking experiential avoidance to depression vulnerability (Barnhofer et al., 2014, Panayiotou et al., 2015). The present findings affirm the role of sensory deactivation in characterizing depression relapse vulnerability, broadening accounts of depression vulnerability to also sensorimotor deactivation as a contributing vulnerability biomarker.
Here, neural responses to dysphoric mood induction in remitted depressed patients implicated sensory deactivation in past, present, and future depression. Greater numbers of past episodes and concurrent residual symptoms were each linked with greater deactivation of the somatosensory cortex and insula, replicating prior findings (Farb et al., 2010). After controlling for these effects, sensory deactivation further accounted for episode return over a 24-month follow-up, characterized by the deactivation of somatosensory, motor, and visual cortices, replicating our earlier exploratory work (Farb et al., 2011). Importantly, the use of two scanning sessions before and after prophylactic treatment allowed for novel distinctions between static (time-invariant) and treatment-responsive (time-varying) vulnerability biomarkers. The pattern of sadness-evoked sensory deactivation in somatomotor and visual cortices was invariant with respect to treatment in its characterization of depressive relapse, with right somatosensory deactivation providing the strongest measure of association.
To identify relapse-relevant effects of prophylactic treatment, we identified changes in reactivity over the intervention period in patients who sustained remission across the 24-month follow-up (non-relapsers). Time-related changes in the remission group likely also include non-specific effects of time such as practice effects, so regions identified as changing over time were then screened for their association with future relapse status. Decreased reactivity was observed in the left DLPFC, occipital cortex and temporal/parietal junction, but only change in the left DLPFC indicated future relapse. Sustained remission was linked to a decrease in DLPFC reactivity over the treatment period that was not apparent in patients who relapsed. DLPFC reductions were most often observed in patients with elevated baseline DLPFC reactivity (Fig. S2), consistent with findings of both greater baseline PFC activity indicating acute phase treatment response (Godlewska et al., 2018, Goldapple et al., 2004, Lemogne et al., 2012), and reductions in cortical midline activity following prophylactic intervention (Williams et al., 2020). Together, these findings support a model of prefrontal hyperactivity as a dynamic, treatment-modifiable marker of maladaptive cognitive reactivity.
The association of relapse with exaggerated DLPFC activation and somatosensory deactivation also supports a model of depression vulnerability based on an over-reliance on prefrontal processes such as cognitive elaboration to the detriment of sensory integration. Accordingly, exploratory PPI analyses using the right somatosensory biomarker as a seed region illustrated that dysphoric mood induction introduced an inhibitory relationship with the right DLPFC, contralateral to the left LPFC dynamic biomarker. This inhibition is understandable given that strategies such as distraction or reappraisal feature prominently in treating acute phase depression (Cuijpers et al., 2013, Markowitz, 2008). However, continued attempts to avoid negative affect or down-regulate negative events may leave patients at insensitive to periods of relative symptom quiescence, preventing identification of symptom improvement (Farb et al., 2015, Mellick et al., 2019). A parallel can be found in the use of somatically-informed approaches to support patients in recovery from addiction or in the long term management of chronic pain (Garland, 2016) and therapies delivered during depression remission, such as MBCT and WB-CT, encourage the regulation of negative affect via exposure to its somatic and cognitive features. It is possible that relapsers were less flexible than non-relapsers in adapting their regulatory strategies; this view is supported here by reductions in DLPFC reactivity being limited to the non-relapse group, and in the literature by findings of increased perseveration in remitted depressed patients (Stange et al., 2020).
The final study aim sought to distinguish MBCT and WB-CT mechanisms. However, consistent with psychometric analysis of the larger clinical trial (Farb et al., 2018, Segal et al., 2019), no neural evidence of process dissociation was observed. Although at a procedural level, MBCT and WB-CT emphasize divergent therapeutic strategies, reduction of DLPFC-related cognitive reactivity represents a common prophylactic marker. The attenuation of sensory deactivation would also clearly be a favorable outcome for prophylaxis, but these biomarkers were not impacted by prophylactic treatment. It remains possible that sensory deactivation might be addressed as treatment-acquired skills are consolidated over time (Segal et al., 2019), a potential longitudinal consequence of reduced prefrontal reactivity.
4.1. Limitations and constraints on generalizability
There are several limitations to consider in this work. First, while the study implicates novel biomarkers of depression vulnerability, it is limited by the lack of an independent sample by which to test these biomarkers’ predictive utility (Poldrack et al., 2017). Second, we utilized a dual-criterion threshold for determining clinical relapse (SCID and HRSD); while commonly used (Klein et al., 2004, Reynolds et al., 2006), this approach prioritizes confidence in relapse events at the expense of excluding partial relapse phenomena. Third, this study characterizes MDD vulnerability only in the context of dysphoric reactivity and treatment-response to MBCT and WB-CT. A more comprehensive account would require integrating data from pharmacotherapy and neurostimulation treatments, along with a passive control for the time elapsed between our baseline and post-intervention scans. Fourth, while dysphoric-mood induction proved to be a fruitful approach, probing other aspects of MDD such as anhedonia or self-blame may also expand the neural profiles of relapse and remission (Lythe et al., 2015). Finally, future research should explore ecologically valid indicators of cognitive reactivity or sensory deactivation to characterize dynamic regulatory responses to momentary negative affect.
5. Conclusions
Neural responses to dysphoric mood-induction in remitted depressed patients support a 2-factor model of MDD relapse vulnerability, in which i) enduring patterns of dyphoria-evoked sensory deactivation contribute to MDD vulnerability, but ii) vulnerability may be mitigated by targeting prefrontal regions where elevated DLPFC reactivity seems responsive to clinical intervention. These findings have the potential to inform evaluation of prophylactic treatment response and to spur the development of interventions designed to consolidate clinical remission.
CRediT authorship contribution statement
Norman A.S. Farb: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. Philip Desormeau: Conceptualization, Data curation, Formal analysis, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. Adam K. Anderson: Conceptualization, Funding acquisition, Investigation, Methodology, Resources, Software, Supervision, Writing – original draft, Writing – review & editing. Zindel V. Segal: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Acknowledgments
Acknowledgments
Arun Ravindran, MD facilitated clinical access to study patients and Robert Levitan, MD rated the SCID relapse interviews. The study was funded by the Canadian Institute of Health Research (Grant# 243812).
Disclosures
NF, AA, and PD reported no biomedical financial interests or potential conflicts of interest. ZS disclosed book royalties from Guilford Press, workshop fees from the Centre for Mindfulness studies, and revenue from online sales at Mindful Noggin Inc., which are all related to his work as a co-founder of Mindfulness-Based Cognitive Therapy (MBCT).
Data Sharing Statement
De-identified psychometric and group level neuroimaging data, including data dictionaries, are available upon request. The study protocol is available through https://clinicaltrials.gov, #NCT01178424. Institutional collaboration for publication requires a signed data-access agreement with the University of Toronto.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2022.102969.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Barnhofer T., Brennan K., Crane C., Duggan D., Williams J.M.G. A comparison of vulnerability factors in patients with persistent and remitting lifetime symptom course of depression. J. Affect. Disord. 2014;152–154:155–161. doi: 10.1016/j.jad.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett E.A., DeLorenzo C., Sharma P., Yang J., Zhang M., Petkova E., Weissman M., McGrath P.J., Fava M., Ogden R.T., Kurian B.T., Malchow A., Cooper C.M., Trombello J.M., McInnis M., Adams P., Oquendo M.A., Pizzagalli D.A., Trivedi M., Parsey R.V. Pretreatment and early-treatment cortical thickness is associated with SSRI treatment response in major depressive disorder. Neuropsychopharmacology. 2018;43(11):2221–2230. doi: 10.1038/s41386-018-0122-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D., Mächler M., Bolker B., Walker S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015;67(1):1–48. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- Berwian I.M., Wenzel J.G., Kuehn L., Schnuerer I., Kasper L., Veer I.M., Seifritz E., Stephan K.E., Walter H., Huys Q.J.M. The relationship between resting-state functional connectivity, antidepressant discontinuation and depression relapse. Sci. Rep. 2020;10(1):22346. doi: 10.1038/s41598-020-79170-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn R.M. The role of physiological noise in resting-state functional connectivity. NeuroImage. 2012;62(2):864–870. doi: 10.1016/j.neuroimage.2012.01.016. [DOI] [PubMed] [Google Scholar]
- Bossier H., Roels S.P., Seurinck R., Banaschewski T., Barker G.J., Bokde A.L.W., Quinlan E.B., Desrivières S., Flor H., Grigis A., Garavan H., Gowland P., Heinz A., Ittermann B., Martinot J.-L., Artiges E., Nees F., Orfanos D.P., Poustka L., Fröhner Dipl-Psych J.H., Smolka M.N., Walter H., Whelan R., Schumann G., Moerkerke B. The empirical replicability of task-based fMRI as a function of sample size. NeuroImage. 2020;212:116601. doi: 10.1016/j.neuroimage.2020.116601. [DOI] [PubMed] [Google Scholar]
- Bulloch A., Williams J., Lavorato D., Patten S. Recurrence of major depressive episodes and previous episodes. Depress. Anxiety. 2014;31(1):72–76. doi: 10.1002/da.22173. [DOI] [PubMed] [Google Scholar]
- Cox R.W. Equitable thresholding and clustering: A novel method for functional magnetic resonance imaging clustering in AFNI. Brain Connect. 2019;9(7):529–538. doi: 10.1089/brain.2019.0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig A.D. How do you feel? Interoception: The sense of the physiological condition of the body. Nat. Rev. Neurosci. 2002;3(8):655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Cuijpers P., Berking M., Andersson G., Quigley L., Kleiboer A., Dobson K.S. A meta-analysis of cognitive-behavioural therapy for adult depression, alone and in comparison with other treatments. Can. J. Psychiatry Revue Canadienne De Psychiatrie. 2013;58(7):376–385. doi: 10.1177/070674371305800702. [DOI] [PubMed] [Google Scholar]
- Disner S.G., Beevers C.G., Haigh E.A.P., Beck A.T. Neural mechanisms of the cognitive model of depression. Nat. Rev. Neurosci. 2011;12(8):467–477. doi: 10.1038/nrn3027. [DOI] [PubMed] [Google Scholar]
- Dobson K.S., Hollon S.D., Dimidjian S., Schmaling K.B., Kohlenberg R.J., Gallop R.J., Rizvi S.L., Gollan J.K., Dunner D.L., Jacobson N.S. Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the prevention of relapse and recurrence in major depression. J. Consult. Clin. Psychol. 2008;76(3):468–477. doi: 10.1037/0022-006X.76.3.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop B.W., Kelley M.E., McGrath C.L., Craighead W.E., Mayberg H.S. Preliminary findings supporting insula metabolic activity as a predictor of outcome to psychotherapy and medication treatments for depression. J. Neuropsychiatry Clin. Neurosci. 2015;27(3):237–239. doi: 10.1176/appi.neuropsych.14030048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton W.W., Shao H., Nestadt G., Lee B.H., Bienvenu O.J., Zandi P. Population-based study of first onset and chronicity in major depressive disorder. Arch. Gen. Psychiatry. 2008;65(5):513. doi: 10.1001/archpsyc.65.5.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund A., Nichols T.E., Knutsson H. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. PNAS. 2016;113(28):7900–7905. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban O., Ciric R., Finc K., Blair R.W., Markiewicz C.J., Moodie C.A., Kent J.D., Goncalves M., DuPre E., Gomez D.E.P., Ye Z., Salo T., Valabregue R., Amlien I.K., Liem F., Jacoby N., Stojić H., Cieslak M., Urchs S., Halchenko Y.O., Ghosh S.S., De La Vega A., Yarkoni T., Wright J., Thompson W.H., Poldrack R.A., Gorgolewski K.J. Analysis of task-based functional MRI data preprocessed with fMRIPrep. Nat. Protoc. 2020;15(7):2186–2202. doi: 10.1038/s41596-020-0327-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban O., Markiewicz C.J., Blair R.W., Moodie C.A., Isik A.I., Erramuzpe A., Kent J.D., Goncalves M., DuPre E., Snyder M., Oya H., Ghosh S.S., Wright J., Durnez J., Poldrack R.A., Gorgolewski K.J. fMRIPrep: A robust preprocessing pipeline for functional MRI. Nat. Methods. 2019;16(1):111–116. doi: 10.1038/s41592-018-0235-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farb N.A., Anderson A.K., Bloch R.T., Segal Z.V. Mood-linked responses in medial prefrontal cortex predict relapse in patients with recurrent unipolar depression. Biol. Psychiatry. 2011;70(4):366–372. doi: 10.1016/j.biopsych.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farb N.A., Anderson A.K., Mayberg H., Bean J., McKeon D., Segal Z.V. Minding one’s emotions: Mindfulness training alters the neural expression of sadness. Emotion. 2010;10(1):25–33. doi: 10.1037/a0017151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farb, N. A., Irving, J. A., Anderson, A. K., Segal, Z. V. 2015. A two-factor model of relapse/recurrence vulnerability in unipolar depression. J. Abnorm. Psychol., 1–17. [DOI] [PMC free article] [PubMed]
- Farb N., Anderson A., Ravindran A., Hawley L., Irving J., Mancuso E., Gulamani T., Williams G., Ferguson A., Segal Z.V. Prevention of relapse/recurrence in major depressive disorder with either mindfulness-based cognitive therapy or cognitive therapy. J. Consult. Clin. Psychol. 2018;86(2):200–204. doi: 10.1037/ccp0000266. [DOI] [PubMed] [Google Scholar]
- Fava G.A. Well-being therapy: current indications and emerging perspectives. Psychother. Psychosom. 2016;85(3):136–145. doi: 10.1159/000444114. [DOI] [PubMed] [Google Scholar]
- Figueroa C.A., Cabral J., Mocking R.J.T., Rapuano K.M., van Hartevelt T.J., Deco G., Expert P., Schene A.H., Kringelbach M.L., Ruhé H.G. Altered ability to access a clinically relevant control network in patients remitted from major depressive disorder. Hum. Brain Mapp. 2019;40(9):2771–2786. doi: 10.1002/hbm.24559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseka T.M., MacQueen G.M., Kennedy S.H. Neuroimaging biomarkers as predictors of treatment outcome in Major Depressive Disorder. J. Affect. Disord. 2018;233:21–35. doi: 10.1016/j.jad.2017.10.049. [DOI] [PubMed] [Google Scholar]
- Gadad B.S., Jha M.K., Czysz A., Furman J.L., Mayes T.L., Emslie M.P., Trivedi M.H. Peripheral biomarkers of major depression and antidepressant treatment response: Current knowledge and future outlooks. J. Affect. Disord. 2018;233:3–14. doi: 10.1016/j.jad.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland E.L. Restructuring reward processing with Mindfulness-Oriented Recovery Enhancement: Novel therapeutic mechanisms to remediate hedonic dysregulation in addiction, stress, and pain: Mindfulness and hedonic regulation. Ann. N. Y. Acad. Sci. 2016;1373(1):25–37. doi: 10.1111/nyas.13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godlewska B.R., Browning M., Norbury R., Igoumenou A., Cowen P.J., Harmer C.J. Predicting treatment response in depression: the role of anterior cingulate cortex. Int. J. Neuropsychopharmacol. 2018;21(11):988–996. doi: 10.1093/ijnp/pyy069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldapple K., Segal Z., Garson C., Lau M., Bieling P., Kennedy S., Mayberg H. Modulation of cortical-limbic pathways in major depression: treatment-specific effects of cognitive behavior therapy. Arch. Gen. Psychiatry. 2004;61(1):34. doi: 10.1001/archpsyc.61.1.34. [DOI] [PubMed] [Google Scholar]
- Guidi J., Fava G.A. Sequential combination of pharmacotherapy and psychotherapy in major depressive disorder: A systematic review and meta-analysis. JAMA Psychiatry. 2020;78(3):261. doi: 10.1001/jamapsychiatry.2020.3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J.P., Chen M.C., Gotlib I.H. Neural systems approaches to understanding major depressive disorder: An intrinsic functional organization perspective. Neurobiol. Dis. 2013;52:4–11. doi: 10.1016/j.nbd.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J.P., Etkin A., Furman D.J., Lemus M.G., Johnson R.F., Gotlib I.H. Functional neuroimaging of major depressive disorder: A meta-analysis and new integration of base line activation and neural response data. Am. J. Psychiatry. 2012;169(7):693–703. doi: 10.1176/appi.ajp.2012.11071105. [DOI] [PubMed] [Google Scholar]
- Hardeveld F., Spijker J., De Graaf R., Nolen W.A., Beekman A.T.F. Prevalence and predictors of recurrence of major depressive disorder in the adult population: Recurrence of major depressive disorder. Acta Psychiatr. Scand. 2009;122(3):184–191. doi: 10.1111/j.1600-0447.2009.01519.x. [DOI] [PubMed] [Google Scholar]
- Harshaw C. Interoceptive dysfunction: Toward an integrated framework for understanding somatic and affective disturbance in depression. Psychol. Bull. 2015;141(2):311–363. doi: 10.1037/a0038101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollon S.D., DeRubeis R.J., Shelton R.C., Amsterdam J.D., Salomon R.M., O’Reardon J.P., Lovett M.L., Young P.R., Haman K.L., Freeman B.B., Gallop R. Prevention of relapse following cognitive therapy vs medications in moderate to severe depression. Arch. Gen. Psychiatry. 2005;62(4):417. doi: 10.1001/archpsyc.62.4.417. [DOI] [PubMed] [Google Scholar]
- Kassambara, A., Kosinski, M., & Biecek, P. (2020). survminer: Drawing Survival Curves using “ggplot2”.https://CRAN.R-project.org/package=survminer.
- Kazdin A.E. Mediators and mechanisms of change in psychotherapy research. Annu. Rev. Clin. Psychol. 2007;3(1):1–27. doi: 10.1146/annurev.clinpsy.3.022806.091432. [DOI] [PubMed] [Google Scholar]
- Kennis M., Gerritsen L., van Dalen M., Williams A., Cuijpers P., Bockting C. Prospective biomarkers of major depressive disorder: A systematic review and meta-analysis. Mol. Psychiatry. 2020;25(2):321–338. doi: 10.1038/s41380-019-0585-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein D.N., Santiago N.J., Vivian D., Blalock J.A., Kocsis J.H., Markowitz J.C., McCullough J.P., Rush A.J., Trivedi M.H., Arnow B.A., Dunner D.L., Manber R., Rothbaum B., Thase M.E., Keitner G.I., Miller I.W., Keller M.B. Cognitive-behavioral analysis system of psychotherapy as a maintenance treatment for chronic depression. J. Consult. Clin. Psychol. 2004;72(4):681–688. doi: 10.1037/0022-006X.72.4.681. [DOI] [PubMed] [Google Scholar]
- Kong Y., Jenkinson M., Andersson J., Tracey I., Brooks J.C.W. Assessment of physiological noise modelling methods for functional imaging of the spinal cord. NeuroImage. 2012;60(2):1538–1549. doi: 10.1016/j.neuroimage.2011.11.077. [DOI] [PubMed] [Google Scholar]
- Kruijt A.-W., Antypa N., Booij L., de Jong P.J., Glashouwer K., Penninx B.W.J.H., Van der Does W., Gray M. Cognitive reactivity, implicit associations, and the incidence of depression: a two-year prospective study. PLoS ONE. 2013;8(7):e70245. doi: 10.1371/journal.pone.0070245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuyken W., Warren F.C., Taylor R.S., Whalley B., Crane C., Bondolfi G., Hayes R., Huijbers M., Ma H., Schweizer S., Segal Z., Speckens A., Teasdale J.D., Van Heeringen K., Williams M., Byford S., Byng R., Dalgleish T. Efficacy of mindfulness-based cognitive therapy in prevention of depressive relapse: an individual patient data meta-analysis from randomized trials. JAMA Psychiatry. 2016;73(6):565–574. doi: 10.1001/jamapsychiatry.2016.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemogne C., Delaveau P., Freton M., Guionnet S., Fossati P. Medial prefrontal cortex and the self in major depression. J. Affect. Disord. 2012;136(1–2):e1–e11. doi: 10.1016/j.jad.2010.11.034. [DOI] [PubMed] [Google Scholar]
- Lythe K.E., Moll J., Gethin J.A., Workman C.I., Green S., Lambon Ralph M.A., Deakin J.F.W., Zahn R. Self-blame-selective hyperconnectivity between anterior temporal and subgenual cortices and prediction of recurrent depressive episodes. JAMA Psychiatry. 2015;72(11):1119. doi: 10.1001/jamapsychiatry.2015.1813. [DOI] [PubMed] [Google Scholar]
- Marchetti I., Koster E.H., Sonuga-Barke E.J., De Raedt R. The default mode network and recurrent depression: A neurobiological model of cognitive risk factors. Neuropsychol. Rev. 2012;22(3):229–251. doi: 10.1007/s11065-012-9199-9. [DOI] [PubMed] [Google Scholar]
- Markowitz J.C. Evidence-based psychotherapies for depression. J. Occup. Environ. Med. 2008;50(4):437–440. doi: 10.1097/JOM.0b013e318168f76e. [DOI] [PubMed] [Google Scholar]
- McGrath C.L., Kelley M.E., Holtzheimer P.E., Dunlop B.W., Craighead W.E., Franco A.R., Craddock R.C., Mayberg H.S. Toward a neuroimaging treatment selection biomarker for major depressive disorder treatment-specific biomarker for major depression treatment-specific biomarker for major depression. JAMA Psychiatry. 2013;70(8):821–829. doi: 10.1001/jamapsychiatry.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellick W.H., Mills J.A., Kroska E.B., Calarge C.A., Sharp C., Dindo L.N. Experiential avoidance predicts persistence of major depressive disorder and generalized anxiety disorder in late adolescence. J. Clin. Psychiatry. 2019;80(6) doi: 10.4088/JCP.18m12265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer B.M., Rabl U., Huemer J., Bartova L., Kalcher K., Provenzano J., Brandner C., Sezen P., Kasper S., Schatzberg A.F., Moser E., Chen G., Pezawas L. Prefrontal networks dynamically related to recovery from major depressive disorder: A longitudinal pharmacological fMRI study. Transl. Psychiatry. 2019;9(1):64. doi: 10.1038/s41398-019-0395-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikl M., Mareček R., Hluštík P., Pavlicová M., Drastich A., Chlebus P., Brázdil M., Krupa P. Effects of spatial smoothing on fMRI group inferences. Magn. Reson. Imaging. 2008;26(4):490–503. doi: 10.1016/j.mri.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Mumford J.A. A power calculation guide for fMRI studies. Soc. Cogn. Affect. Neurosci. 2012;7(6):738–742. doi: 10.1093/scan/nss059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panayiotou G., Leonidou C., Constantinou E., Hart J., Rinehart K.L., Sy J.T., Björgvinsson T. Do alexithymic individuals avoid their feelings? Experiential avoidance mediates the association between alexithymia, psychosomatic, and depressive symptoms in a community and a clinical sample. Compr. Psychiatry. 2015;56:206–216. doi: 10.1016/j.comppsych.2014.09.006. [DOI] [PubMed] [Google Scholar]
- Paykel E.S. Partial remission, residual symptoms, and relapse in depression. Dial. Clin. Neurosci. 2008;10(4):431–437. doi: 10.31887/DCNS.2008.10.4/espaykel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack R.A., Baker C.I., Durnez J., Gorgolewski K.J., Matthews P.M., Munafò M.R., Nichols T.E., Poline J.-B., Vul E., Yarkoni T. Scanning the horizon: Towards transparent and reproducible neuroimaging research. Nat. Rev. Neurosci. 2017;18(2):115–126. doi: 10.1038/nrn.2016.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. (2017). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. https://www.R-project.org/.
- Ray D., Bezmaternykh D., Mel’nikov M., Friston K.J., Das M. Altered effective connectivity in sensorimotor cortices is a signature of severity and clinical course in depression. Proc. Natl. Acad. Sci. U.S.A. 2021;118(40) doi: 10.1073/pnas.2105730118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds C.F., Dew M.A., Pollock B.G., Mulsant B.H., Frank E., Miller M.D., Houck P.R., Mazumdar S., Butters M.A., Stack J.A., Schlernitzauer M.A., Whyte E.M., Gildengers A., Karp J., Lenze E., Szanto K., Bensasi S., Kupfer D.J. Maintenance treatment of major depression in old age. N. Engl. J. Med. 2006;354(11):1130–1138. doi: 10.1056/NEJMoa052619. [DOI] [PubMed] [Google Scholar]
- Rush A.J., Trivedi M.H., Ibrahim H.M., Carmody T.J., Arnow B., Klein D.N., Markowitz J.C., Ninan P.T., Kornstein S., Manber R., Thase M.E., Kocsis J.H., Keller M.B. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): A psychometric evaluation in patients with chronic major depression. Biol. Psychiatry. 2003;54(5):573–583. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- Rush A.J., Trivedi M.H., Wisniewski S.R., Nierenberg A.A., Stewart J.W., Warden D., Niederehe G., Thase M.E., Lavori P.W., Lebowitz B.D., McGrath P.J., Rosenbaum J.F., Sackeim H.A., Kupfer D.J., Luther J., Fava M. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: A STAR* D report. Am. J. Psychiatry. 2006;163(11):1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- Segal Z.V., Anderson A.K., Gulamani T., Dinh Williams L.-A., Desormeau P., Ferguson A., Walsh K., Farb N.A.S. Practice of therapy acquired regulatory skills and depressive relapse/recurrence prophylaxis following cognitive therapy or mindfulness based cognitive therapy. J. Consult. Clin. Psychol. 2019;87(2):161–170. doi: 10.1037/ccp0000351. [DOI] [PubMed] [Google Scholar]
- Segal Z.V., Kennedy S., Gemar M., Hood K., Pedersen R., Buis T. Cognitive reactivity to sad mood provocation and the prediction of depressive relapse. Arch. Gen. Psychiatry. 2006;63(7):749–755. doi: 10.1001/archpsyc.63.7.749. [DOI] [PubMed] [Google Scholar]
- Segal Z.V., Williams J.M.G., Teasdale J.D. Guilford Press; 2012. Mindfulness-based Cognitive Therapy for Depression. [Google Scholar]
- Sheline Y.I., Barch D.M., Price J.L., Rundle M.M., Vaishnavi S.N., Snyder A.Z., Mintun M.A., Wang S., Coalson R.S., Raichle M.E. The default mode network and self-referential processes in depression. Proc. Natl. Acad. Sci. U. S. A. 2009;106(6):1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline Y.I., Price J.L., Yan Z., Mintun M.A. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc. Natl. Acad. Sci. U. S. A. 2010;107(24):11020–11025. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S., Nichols T. Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage. 2009;44(1):83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Solomon D.A., Keller M.B., Leon A.C., Mueller T.I., Lavori P.W., Shea M.T., Coryell W., Warshaw M., Turvey C., Maser J.D., Endicott J. Multiple recurrences of major depressive disorder. Am. J. Psychiatry. 2000;157(2):229–233. doi: 10.1176/appi.ajp.157.2.229. [DOI] [PubMed] [Google Scholar]
- Stange J.P., Hamilton J.L., Shepard R., Wu J., Fresco D.M., Alloy L.B. Inflexible autonomic responses to sadness predict habitual and real-world rumination: A multi-level, multi-wave study. Biol. Psychol. 2020;153 doi: 10.1016/j.biopsycho.2020.107886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therneau T.M., Grambsch P.M. Springer; 2000. Modeling survival data: Extending the Cox model. [Google Scholar]
- Verhoeven F.E.A., Wardenaar K.J., Ruhé H.G.E., Conradi H.J., de Jonge P. Seeing the signs: Using the course of residual depressive symptomatology to predict patterns of relapse and recurrence of major depressive disorder. Depress. Anxiety. 2018;35(2):148–159. doi: 10.1002/da.22695. [DOI] [PubMed] [Google Scholar]
- Wang X., Öngür D., Auerbach R.P., Yao S. Cognitive vulnerability to major depression: view from the intrinsic network and cross-network interactions. Harvard Rev. Psychiatry. 2016;24(3):188–201. doi: 10.1097/HRP.0000000000000081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham, H. (2016). ggplot2: Elegant Graphics for Data Analysis (2nd ed. 2016). Springer International Publishing: Imprint: Springer. https://doi.org/10.1007/978-3-319-24277-4.
- Williams K., Elliott R., McKie S., Zahn R., Barnhofer T., Anderson I.M. Changes in the neural correlates of self-blame following mindfulness-based cognitive therapy in remitted depressed participants. Psychiatry Res.: Neuroimag. 2020;304 doi: 10.1016/j.pscychresns.2020.111152. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.