Abstract
The enzyme IMP dehydrogenase (IMPDH) catalyzes an essential step in the de novo biosynthesis of guanine nucleotides, namely, the conversion of IMP to XMP. The major event occurring in cells exposed to competitive IMPDH inhibitors such as ribavirin or uncompetitive inhibitors such as mycophenolic acid (MPA) is a depletion of the intracellular GTP and dGTP pools. Ribavirin is approved as an inhaled antiviral agent for treatment of respiratory syncytial virus (RSV) infection and orally, in combination with alpha interferon (IFN-α), for the treatment of chronic hepatitis C virus (HCV) infection. VX-497 is a potent, reversible uncompetitive IMPDH inhibitor which is structurally unrelated to other known IMPDH inhibitors. Studies were performed to compare VX-497 and ribavirin in terms of their cytotoxicities and their efficacies against a variety of viruses. They included DNA viruses (hepatitis B virus [HBV], human cytomegalovirus [HCMV], and herpes simplex virus type 1 [HSV-1]) and RNA viruses (respiratory syncytial virus [RSV], parainfluenza-3 virus, bovine viral diarrhea virus, Venezuelan equine encephalomyelitis virus [VEEV], dengue virus, yellow fever virus, coxsackie B3 virus, encephalomyocarditis virus [EMCV], and influenza A virus). VX-497 was 17- to 186-fold more potent than ribavirin against HBV, HCMV, RSV, HSV-1, parainfluenza-3 virus, EMCV, and VEEV infections in cultured cells. The therapeutic index of VX-497 was significantly better than that of ribavirin for HBV and HCMV (14- and 39-fold, respectively). Finally, the antiviral effect of VX-497 in combination with IFN-α was compared to that of ribavirin with IFN-α in the EMCV replication system. Both VX-497 and ribavirin demonstrated additivity when coapplied with IFN-α, with VX-497 again being the more potent in this combination. These data are supportive of the hypothesis that VX-497, like ribavirin, is a broad-spectrum antiviral agent.
Cells require adequate nucleotide levels which are made available for nucleic acid synthesis via two distinct mechanisms: the salvage pathway and de novo synthesis. Using the salvage pathway, cells recycle nucleosides and nucleobases, whereas with de novo synthesis, the purine or pyrimidine ring systems of the nucleotides are assembled in a stepwise manner (17). Different cell types rely on the two pathways of nucleotide biosynthesis to various degrees. Cells that proliferate relatively rapidly, such as lymphocytes, rely more on the de novo pathway because they require more nucleotides than can be provided by the salvage pathway (1).
IMP dehydrogenase (IMPDH; EC 1.1.1.205) catalyzes the rate-limiting step in the de novo biosynthesis of guanine nucleotides, the NAD+-dependent conversion of IMP to XMP. XMP is aminated in the next biosynthesis step to form GMP. It is crucial for many cellular metabolic and synthetic processes. Two isoforms of human (and mouse) IMPDH (isoforms I and II) have been identified, with each containing 514 amino acids, and they share 84% sequence identity. Forms I and II of human and mouse IMPDHs have 97 and 99% sequence identities, respectively. The native enzyme exists as a homotetramer with a subunit molecular mass of 56 kDa. X-ray crystal structures of mycophenolic acid (MPA) (26), ribavirin monophosphate (27), and an IMP analogue together with an NAD analogue (3) in complex with IMPDH have been determined. Inhibition of IMPDH reduces the level of intracellular guanine nucleotides required for adequate RNA and DNA synthesis. Therefore, IMPDH inhibitors have potential antiproliferative, antiviral, and antiparasitic effects (25, 36).
The pharmacologic effects of IMPDH inhibition have been exploited by a number of marketed products. MPA is a potent, uncompetitive IMPDH inhibitor. Its ester prodrug, mycophenolate mofetil (CellCept), has been approved for use for the prevention of acute rejection in kidney (for a review, see reference 29) and heart (15) transplant recipients when used in combination with steroids and cyclosporine A. Ribavirin (Virazole, Rebetol) is a nucleoside analog which, following intracellular phosphorylation, is a competitive IMPDH inhibitor. Ribavirin is approved as an inhaled antiviral agent for treatment of respiratory syncytial virus (RSV) infection and, orally in combination with alpha interferon (IFN-α), for the treatment of chronic hepatitis C virus (HCV) infection.
Ribavirin is a broad-spectrum antiviral agent with activity against at least 12 DNA-containing viruses and 40 RNA-containing viruses (4, 6, 22, 24). Three different mechanisms for the antiviral activity of ribavirin have been proposed (11). One proposed mechanism is the inhibition of viral RNA transcription and/or elongation. It has been observed that ribavirin triphosphate inhibits vesicular stomatitis virus RNA polymerase (9, 31), La Crosse encephalitis virus polymerase (2, 31), reovirus transcriptase (22), and influenza virus polymerase (8, 33) transcription. Inhibition of the viral RNA polymerase elongation reaction has been proposed for reovirus (22) and influenza virus (33). A second proposed mechanism involves inhibition of the formation of a guanine pyrophosphate “cap” on the 5′ end of viral mRNA by viral mRNA guanylyltransferase. This effect has been observed in vaccinia virus mRNA (12), and the cause of this effect in Sindbis virus mutants resistant to MPA and ribavirin was mapped to a viral gene coding for RNA guanylyltransferase (23). However, the major event occurring in cells exposed to ribavirin and structurally related compounds such as 5-ethynyl-1-β-d-ribofuranosylimidazole-4-carboxamide, tiazofurin, and selenazofurin is a depletion of the intracellular GTP and dGTP pools as a result of the inhibition of IMPDH (for a review, see reference 4). Thus, the antiviral effects of ribavirin and other IMPDH inhibitors can be reversed by the exogenous addition of guanosine but not other nucleosides (6, 34). These results strongly suggest that, analogous to the cytostatic effect of IMPDH inhibitors on rapidly proliferating lymphocyte and tumor cell lines, the de novo rather than the salvage pathway of GTP synthesis may be critical in the supply of precursors for viral RNA and DNA synthesis. Since the antiviral and cytostatic effects of IMPDH inhibitors are established under different conditions (with resting cell monolayers and exponentially growing cells, respectively), they are not mutually exclusive.
IMPDH inhibitors have also been shown to potentiate the effect of purine nucleoside analogs which are inhibitors of human immunodeficiency virus (13) and herpesvirus replication (19). This potentiation is thought to occur via a more efficient phosphorylation of the nucleoside analog that arises from a depletion of dGTP pools by IMPDH inhibition, resulting in an enhancement of antiviral activity (5).
VX-497 (molecular weight, 452.5) is a selective, highly potent, reversible, and uncompetitive inhibitor of the two isoforms of human IMPDH (Kis, 10 and 7 nM for isoforms I and II, respectively), being structurally unrelated to other known IMPDH inhibitors and suitable for oral dosing. Vertex Pharmaceuticals is conducting phase III clinical trials to evaluate VX-497 as a possible treatment for psoriasis or for chronic hepatitis caused by HCV. The aims of the studies described herein were to determine the antiviral effect of VX-497 against a variety of viruses, to compare this activity to that of ribavirin, and to determine the combined antiviral effect of VX-497 with IFN-α. VX-497 had a consistently greater antiviral effect than ribavirin in almost all of the assays performed.
MATERIALS AND METHODS
Compounds.
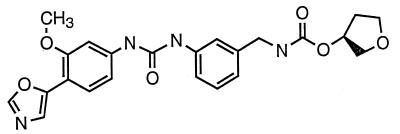
VX-497 (Fig. 1) is a low-molecular-mass (452.5 Da) phenyloxazole derivative (chemical name, (S)-N-3-[3-(3-methoxy-4-oxazol-5-yl-phenyl)-ureido]-benzyl-carbamic acid tetrahydrofuran-3-yl-ester). It was stored frozen in dimethyl sulfoxide as a 50 mM stock. Ribavirin (1-β-d-ribofuranosyl-1H-1,2,4-triazole-3-carboximide; Virazole) was obtained from Sigma (catalog no. R9644) and was stored frozen as a 500 mM stock in dimethyl sulfoxide. IFN-α (mouse, recombinant) was obtained from Calbiochem (catalog no. 407293) as a frozen solution in water. L929 cells (mouse fibroblast CCL-1 cells [American Type Culture Collection]) were maintained in Eagle minimal essential medium containing 10% fetal bovine serum, nonessential amino acids, sodium pyruvate, and l-glutamine. Poly(dI-dC) was obtained from Pharmacia.
FIG. 1.
Chemical structure of VX-497.
Antiviral and cell growth analyses.
The antiviral activities of VX-497 and ribavirin were tested against a variety of DNA viruses (hepatitis B virus [HBV], human cytomegalovirus [HCMV], and herpes simplex virus type 1 [HSV-1]) and a variety of RNA viruses (RSV, parainfluenza-3 virus, bovine viral diarrhea virus [BVDV], Venezuelan equine encephalomyelitis virus [VEEV], dengue virus, yellow fever virus [YFV], coxsackie B3 virus, influenza A virus, and murine encephalomyocarditis virus [EMCV]). These studies either were performed at Vertex Pharmaceuticals or were contracted to ViroMed Laboratories Inc. (Minneapolis, Minn.), Southern Research Institute (Frederick, Md.), or Advanced Biotechnologies Inc. (Columbia, Md.). While the conditions and assays used for each virus differed, the conditions for the ribavirin and VX-497 comparison were identical in each case and were performed in a blinded manner. The appropriate cells were trypsinized, counted, and seeded into 96-well plates. At confluence, serial dilutions of test compounds and test compound combinations were added to the cells, followed by the addition of a predetermined multiplicity of infection for each virus. Appropriate viral, cell, growth medium, and compound cytotoxicity controls were contained within each plate. Each datum point is the average of three determinations. (Standard deviations were, on average, approximately 10% of the mean, with a range typically no more than 20% of the mean.) At an appropriate postinfection time point, an aliquot of the medium from each well was taken (where indicated) and was stored for viral yield determinations or PCR analysis. Each datum point is the average of three determinations. Table 1 shows the results for a single experiment performed by the contracted commercial analysts or in-house, as indicated. Duplications of any work and the mean and standard deviations or range of results are presented in the footnotes to Table 1, where appropriate. The antiviral methodology for each virus including cell type, cytopathic effect (CPE), plaque reduction, or viral yield is indicated in the footnotes to Table 1.
TABLE 1.
Summary of assays with VX-497 and ribavirin for their antiviral activity and cytotoxicitya
| Virus | Ribavirin
|
VX-497
|
||||||
|---|---|---|---|---|---|---|---|---|
| No guanosine
|
With 100 μM guanosine
|
No guanosine
|
With 100 μM guanosine
|
|||||
| IC50 (μM) | CC50 (μM) | IC50 (μM) | CC50 (μM) | IC50 (μM) | CC50 (μM) | IC50 (μM) | CC50 (μM) | |
| HBVb | 43.8 | 96.4 | 32.7 | 128.5 | 0.4 | 5.2 | 1.3 | 8.9 |
| HCMVc | 148.5 | >500 (MTC) | 62.9 | >500 (MTC) | 0.8 | <31 (MTC) | 0.8 | <31 (MTC) |
| RSVd | 20.9 | >500 | 61.3 | >500 | 1.1 | 10.3 | 4.0 | 12.6 |
| HSV-1e | 162 | >500 | >500 | >500 | 6.3 | >31 | >31 | >31 |
| Parainfluenza-3 virusf | 197.9 | >500 | >500 | >500 | 13.8 | >31 | 2.2 | 25.9 |
| BVDVg | 44.6 | >500 | 293.9 | >500 | 12.4 | >31 | >31 | >31 |
| VEEVh | >500 | 446 | >500 | 400 | 19.2 | >31 | >31 | >31 |
| Dengue virusi | 8.3 (Red Inf) | 31 (NTC) | None (Red Inf) | 31 (NTC) | 8 (Red Inf) | 2 (NTC) | None (Red Inf) | 8 (NTC) |
| YFVj | >500 | >500 | >500 | >500 | >31 | 52.9 | >31 | 29.7 |
| Coxsackie B3 virusk | >500 | >500 | >500 | 464 | >31 | >31 | 22.6 | >31 |
| Influenza A virusl | 27.8 | 232 | >500 | 412 | >31 | 4.8 | >31 | >31 |
| EMCVm | 17.0 | >500 | >500 | >500 | 1.0 | 20.0 | 14.0 | >20 |
Antiviral activity is given as IC50, and cytotoxicity is given as CC50. Abbreviations: MTC, minimum toxic concentration; NTC, highest concentration of drug with no cytotoxicity; Red Inf, concentration at which the amount of virus was noticeably reduced without cytotoxicity or with reduced cytotoxicity.
HepG2 2.2.15 cells, PCR assay for antiviral activity (16), XTT assay for cytotoxicity. Performed by Southern Research in a blinded manner. Ribavirin (n = 1) for plus and minus guanosine. VX-497 (n = 2); IC50 = 0.34 ± 0.04 μM (minus guanosine) and 0.8 ± 0.5 μM (plus guanosine).
Towne strain, human foreskin fibroblast cells, plaque assay for antiviral activity, CPE assay for cytotoxicity. Performed by Southern Research in a blinded manner (n = 1).
HEP-2 cells, plaque assay for antiviral activity, MTA assay for cytotoxicity. Performed independently by Southern Research, Viromed, and Advanced Biotechnologies in a blinded manner (n = 3). Ribavirin IC50 = 26.0 ± 9.6 μM (minus guanosine) and 39.1 ± 20.8 μM (plus guanosine), with CC50s ranging from 125 to >500 μM (minus guanosine) to >500 μM (plus guanosine). VX-497 IC50 = 1.3 ± 0.25 μM (minus guanosine) and 2.8 ± 1.1 μM (plus guanosine), with CC50s ranging from <8 to 10.3 μM (minus guanosine) to 12.6 to >31 μM (plus guanosine).
Strain E377, Vero cells, 6-day plaque assay for antiviral activity, MTT assay for cytotoxicity. Performed by Southern Research in a blinded manner (n = 1). In-house estimates are as follows: for ribavirin (n = 1) IC50 = 90 μM and CC50 = >500 μM, for VX-497 IC50 = 4.5 ± 1.7 μM and CC50 = >31 μM (minus guanosine) (n = 4) and IC50 = 12 μM and CC50 = >31 μM (plus guanosine) (n = 1).
Vero cells, plaque assay for antiviral activity, MTA assay for cytotoxicity. Performed independently by Southern Research and Advanced Biotechnologies in a blinded manner (n = 2). Ribavirin, IC50 = 129.6 ± 96.7 μM and CC50 = >500 μM (minus guanosine), VX-497 IC50 = 22.4 ± 12.2 μM and CC50 = >31 μM (minus guanosine) and IC50 = >31 μM and CC50 = >31 μM (plus guanosine).
BT cells, plaque assay for antiviral activity, MTT assay for cytotoxicity. Performed by Viromed in a blinded manner (n = 1).
Vero cells, MTT assay for antiviral activity and cytotoxicity. Performed by Southern research in a blinded manner (n = 1).
Hawaii strain, LLC-MK2 cells, 10-day CPE assay for antiviral activity and cytotoxicity; performed by Advanced Biotechnologies in a blinded manner (n = 1).
Vero cells, 2% IFCS (irradiated fetal calf serum) MTT assay for antiviral activity and cytotoxicity. Performed by Southern Research in a blinded manner (n = 1).
Vero cells, MTT assay for antiviral activity and cytotoxicity. Performed by Southern Research in a blinded manner (n = 1).
MDCK cells, MTT assay for antiviral activity and cytotoxicity. Performed by Southern Research in a blinded manner (n = 1).
L929 cells, MTS assay for cytotoxicity, viral yield assay for antiviral activity. Performed in-house. Ribavirin IC50 = 64 ± 42.6 μM and CC50 = >500 μM (minus guanosine) (n = 3) and IC50 = >500 μM and CC50 = >500 μM (plus guanosine) (n = 1). VX-497 IC50 = 0.85 ± 0.14 μM and CC50 = 21.7 ± 2.9 μM (minus guanosine) (n = 3) and IC50 = 14 μM and CC50 = >20 μM (plus guanosine) (n = 1).
CPE assay.
3-(4,5-Dimethylthiazol-2-yl)-5-(3-carboxymethylphenyl)-2-(4-sulfophenyl)-2H-tetrazolium salt and phenazine ethosulfate (MTS) (or MTT, MTA, or XTT) reagent was added to each well, and the plate was incubated at 37°C before reading of the formazan levels in a microplate reader (490 nm). The data were analyzed to generate cell viability profiles and, where indicated, CPE reduction profiles for each compound and combination. Cell viability was measured as MTS, MTT, MTA, or XTT conversion relative to that for the cell control. Antiviral activity was measured as MTS, MTT, MTA, or XTT conversion relative to the differential between those for cell and viral controls (CPE reduction).
Virus yield and plaque reduction assays.
The virus yield assay was performed with stored aliquots of media from the VX-497, ribavirin, or combination antiviral studies. The aliquots were serially diluted in medium prior to addition to the wells containing confluent cells (in duplicate or triplicate). The virus were allowed to adsorb for 30 min, followed by washing with phosphate-buffered saline and replacement with serum-containing medium and incubation overnight. After an appropriate period of time for plaque formation, stain was added and the plaques were counted. For the plaque reduction assay, a known amount (in PFU) of virus was allowed to adsorb to the appropriate cell line, followed by washing with phosphate-buffered saline and addition of growth medium with and without a test compound(s). Following an appropriate period of time, the medium was removed, the cells were stained, and plaque size and number were recorded.
PCR analysis.
Virion-associated HBV DNA present in the tissue culture supernatant was amplified by PCR with primers derived from HBV strain ayw (16). PCR-amplified DNA was detected in real time by monitoring increases in fluorescence signals that result from exonucleolytic degradation of a quenched fluorescent probe molecule following hybridization of the probe to the amplified HBV DNA. The TaqMan probe molecule, designed with the aid of Primer Express (PE-Applied Biosystems) software, is complementary to the DNA sequences present in the HBV DNA region. A total of 3 μl of clarified supernatant was analyzed directly (without DNA extraction) in a 50-μl PCR mixture. Reagents and conditions used in the quantitative PCR were performed as described by the manufacturer (PE-Applied Biosystems). The standard curve (for a 1.2-kbp HBV ayw subgenomic fragment) ranged from 106 to 101 nominal copy equivalents per PCR mixture.
Reversibility with guanosine.
Studies to compare the antiviral and cytotoxic activities of VX-497 and ribavirin were performed in the presence and absence of 100 μM guanosine to assess whether the antiviral effect was reversible and, hence, could be attributed to inhibition of IMPDH. The effect of the added guanosine on GTP and dGTP pools in the presence of guanosine and VX-497 was not measured in these experiments, and the level of guanosine required for reversal of IMPDH inhibition is cell line dependent. Thus, any decrease in antiviral efficacy of VX-497 in the presence of 100 μM guanosine indicates that IMPDH inhibition is an important component of the antiviral mechanism of action; however, the absence of an effect does not exclude involvement of IMPDH inhibition.
Gel shift assay. (i) Cell culture.
The murine fibroblast L929 cell line was cultured in Eagle minimal essential medium supplemented with 10% fetal bovine serum, nonessential amino acids, 50 U of penicillin per ml, 50 μg of streptomycin per ml, and 2 mM l-glutamine. EMCV was infected at 500 PFU/107 L929 cells. Cells were left untreated or were treated with different concentrations of murine IFN-α alone, VX-497 alone, or combinations thereof.
(ii) EMSAs.
The nucleotide sequences of the oligonucleotides used as probes in the electrophoretic mobility shift assays (EMSAs) were 5′-CATGCCTCGGGAAAGGGAAACCGAAACTGAAGCC-3′ for probe WT ISRE (interferon-sensitive response element), 5′-CATGCCTCGGGACAGGGACACCGACACTGAAGCC-3′ for probe MUT ISRE (nucleotides that differ from those in the wild-type sequence are underlined), and 5′-CATGTTATGCATATTCCTGTAAGTG-3′ for probe WT GAS (gamma interferon-activated sequence). The oligonucleotides were annealed and end labeled with [γ-32P]ATP and polynucleotide kinase and were purified on 12% acrylamide gels. Small-scale nuclear extracts were made from 107 L929 cells by a modification of the method of Dignam et al. (7). The total protein in nuclear extracts was estimated with the bicinchoninic acid protein assay kit (Pierce). For each EMSA binding reaction, 10,000 cpm (∼0.2 to 0.5 ng) of end-labeled probe was incubated with 6 to 12 μg of nuclear extract in the presence of 2.5 of μg sheared poly(dI-dC). The binding reaction mixtures were incubated at room temperature for 20 min and were then electrophoresed on 4% nondenaturing acrylamide gels at room temperature. The gels were exposed to a Fuji phosphorimager for quantification of the radioactivity in EMSA bands.
Synergy analysis.
Analysis of the antiviral activity of IFN-α with ribavirin or IFN-α with VX-497 with the EMCV-L929 system was undertaken by using the independent effects model (Macsynergy II, version 1) (21). In this model values of synergy or antagonism are presented in synergy volume units, typically as square micromolar percent, for the two compounds and the activity is measured. In this case the synergy volume unit is micromolar unit percent (micromolar for ribavirin or VX-497, unit for IFN-α, and percent for antiviral activity). Values under 25 μM unit %, at 95% confidence, should be regarded as insignificant. Values between 25 and 50 μM unit % should be considered minor but significant, while values between 50 and 100 μM unit % indicate moderate synergy, with the possibility of importance in vivo. Values over 100 μM unit % indicate strong synergy with a probability of importance in vivo.
RESULTS
VX-497 antiviral efficacy.
The effect of VX-497 on virus replication in the assays described above (Table 1) fell into three groups. VX-497 is most potent against the first group of viruses, which includes HBV, HCMV, EMCV, and RSV, with 50% inhibitory concentrations (IC50s) of 0.38, 0.80, 1.0, and 1.14 μM, respectively. VX-497 has intermediate antiviral activity against a second group of viruses, which includes HSV-1, parainfluenza-3 virus, BVDV, VEEV, and dengue virus, with IC50s ranging from 6 to 19 μM. VX-497 did not demonstrate antiviral efficacy against the third group of viruses, as measured by its inability to achieve an IC50 measurement for YFV, coxsackie B3 virus, or influenza A virus at 31 μM, the highest concentration tested.
Antiviral efficacy of ribavirin.
The effect of ribavirin on virus replication in the assays described above could also be divided into three groups. Ribavirin is most potent against a first group of viruses, which includes dengue virus and EMCV, with IC50s of 8 and 17 μM, respectively. Ribavirin has intermediate antiviral activity against a second group of viruses, which includes HBV, HCMV, RSV, HSV-1, parainfluenza-3 virus, and influenza A virus, with IC50s ranging from 20 to 198 μM. Ribavirin did not demonstrate antiviral efficacy, failing to achieve an IC50 at the highest concentration tested (500 μM), for the third group of viruses, namely, coxsackie B3 virus, YFV, and VEEV.
Comparison of VX-497 and ribavirin antiviral potencies.
As summarized in Table 2, VX-497 was 10- to 100-fold more potent than ribavirin against HBV, HCMV, RSV, HSV-1, EMCV, parainfluenza-3 virus, and VEEV. The only virus against which VX-497 was less potent than ribavirin was influenza A virus, against which VX-497 had no activity at the highest concentration (31 μM) tested. Table 2 also demonstrates that the therapeutic index of VX-497 was better than that of ribavirin for HBV (13.7 versus 2.2) and HCMV (38.8 versus >3.4). The therapeutic index of VX-497 was similar to that of ribavirin for HSV-1, EMCV, and parainfluenza-3 virus but was three- to fourfold lower for BVDV and RSV.
TABLE 2.
Comparison of antiviral potencies of VX-497 and ribavirin
| Virusa | Ribavirin
|
VX-497
|
Fold increased potency of VX-497b | ||
|---|---|---|---|---|---|
| IC50 (μM) | Therapeutic indexc | IC50 (μM) | Therapeutic index | ||
| HBV | 43.8 | 2.2 | 0.4 | 13.7 | 110 |
| HCMV | 148.5 | >3.4 | 0.8 | 38.8 | 186 |
| RSV | 20.9 | 24.0 | 1.1 | 9.0 | 19 |
| HSV-1 | 162 | >3.1 | 6.3 | >4.9 | 25.7 |
| Parainfluenza-3 virus | 197.9 | 2.5 | 13.8 | >2.2 | 14.4 |
| BVDV | 44.6 | 11.2 | 12.4 | 2.5 | 3.6 |
| VEEV | >500 | <1 | 19.2 | 1.6 | >26 |
| EMCV | 17.0 | >29.4 | 1.0 | 20.0 | 17 |
The assays with the viruses are described in the footnotes to Table 1.
Fold increase in potency of VX-497 = IC50 of ribavirin divided by the IC50 of VX-497.
Therapeutic index = cytotoxicity measurement (CC50, highest concentration of drug with no cytotoxicity, or minimum toxic concentration) divided by the IC50.
Effects of VX-497 and ribavirin on HBV replication.
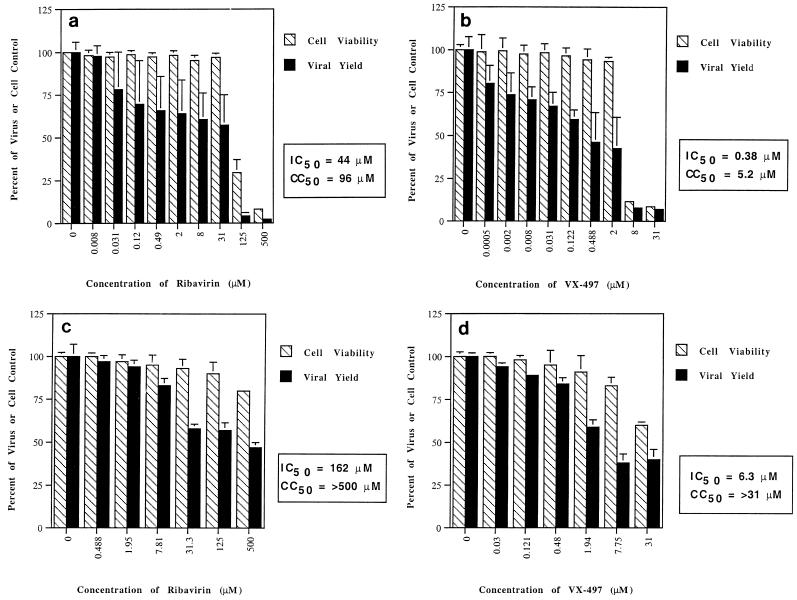
More detailed graphs of the results of testing of ribavirin and VX-497 against HBV are presented in Fig. 2a and b, respectively. Against HBV infection in vitro, ribavirin had an IC50 of 44 μM with a corresponding 50% cytotoxic concentration (CC50) of 96 μM, for a therapeutic or selectivity index of approximately 2. In contrast, VX-497 was 100-fold more potent, with an IC50 of 380 nM and a corresponding CC50 of 5.2 μM, for a therapeutic index of 14. The antiviral activity of VX-497 in HepG2.2.2.15 cells was reversed threefold by the addition of guanosine (Table 1), suggesting that IMPDH inhibition plays a role in the antiviral effect of VX-497 on an HBV-infected liver cell line in vitro.
FIG. 2.
(a) Effect of ribavirin on viability of HepG2 2.2.15 cells and HBV replication. (b) Effect of VX-497 on viability of HepG2 2.2.15 cells and HBV replication. (c) Effect of ribavirin on viability of Vero cells and HSV-1 replication. (d) Effect of VX-497 on viability of Vero cells and HSV-1 replication. Cell viability (striped bars) is measured (in percent) relative to cell growth in the absence of drug. Antiviral activity (solid bars) is measured (in percent) relative to viral replication in the absence of drug. Results are the means of triplicate determinations, with standard deviations shown.
Effects of VX-497 and ribavirin on HSV-1 replication.
In the absence of guanosine, VX-497 was ∼26-fold more potent than ribavirin against HSV-1, with an IC50 of 6.3 μM compared to a ribavirin IC50 of 162 μM (Fig. 2c and d). In the presence of guanosine, VX-497 had a greater than sixfold reduced antiviral activity, suggesting a role for IMPDH inhibition in the antiviral effect on HSV-1 infection in vitro. The effect of guanosine on the reversal of the antiviral activities of ribavirin and VX-497 was also observed in the HSV-1 in vitro infection model (Table 1).
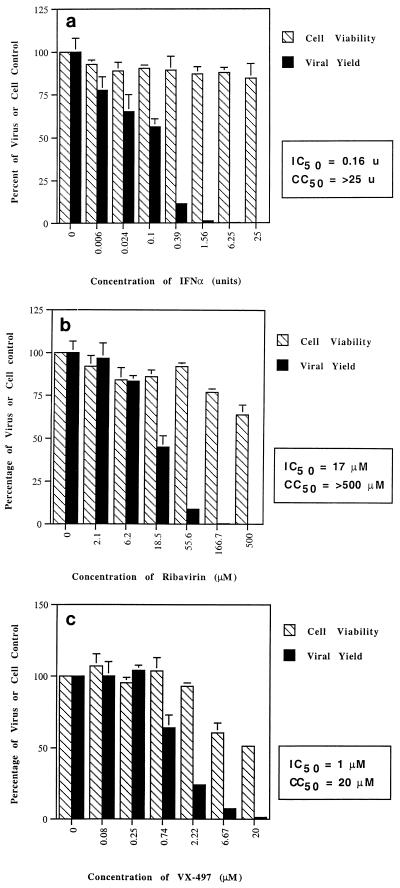
Effects of IFN-α, VX-497, and ribavirin on EMCV replication.
EMCV, while generally regarded as a mouse virus, has a wide host range, including humans. Infection of the mouse cell line L929 with EMCV has routinely been used to evaluate and standardize batches of IFN-α and was used in this work as a means of comparing the antiviral activities of VX-497 and ribavirin when combined with IFN-α. IFN-α demonstrated expected levels of activity against EMCV, with an IC50 of 0.16 units when measured as a viral yield reduction (Fig. 3a) or 1.5 units when measured as a reduction in CPE (data not shown). Little if any cytotoxicity was observed for IFN-α in this system. In the absence of guanosine, VX-497 was 17-fold more potent than ribavirin against EMCV, with IC50s of 1 and 17 μM, respectively (Fig. 3b and c). In the presence of 100 μM guanosine, VX-497 had a 14-fold reduction in antiviral activity and ribavirin had a greater than 29-fold reduction in antiviral activity (Table 1), again suggesting a role for IMPDH inhibition in the antiviral effect.
FIG. 3.
Effects of IFN-α (units per 100-μl well) (a), ribavirin (b), and VX-497 (c) on the viability of L929 cells and EMCV replication. Cell viability (striped bars) is measured (in percent) relative to cell growth in the absence of drug. Viral yield (solid bars) is measured (in percent) relative to viral replication in the absence of drug. Results are the means of triplicate determinations, with standard deviations shown.
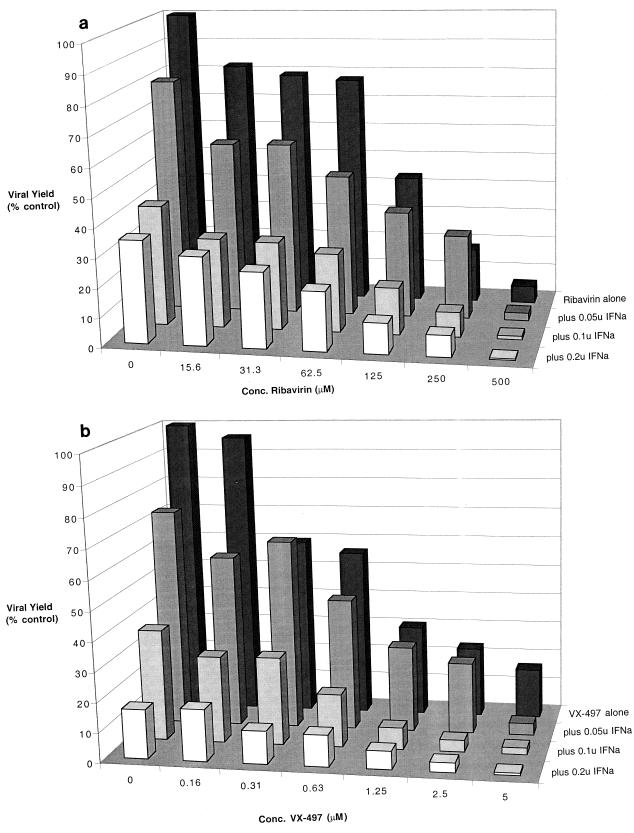
Combinations of VX-497 and IFN-α and of ribavirin and IFN-α were tested in the EMCV-L929 system to determine if the antiviral activities obtained with the single agents could be combined and whether the resulting activity of the combined pair would be additive or synergistic. To this end, a series of experiments was performed in which the level of IFN-α was fixed (0.2, 0.1, 0.05, and 0 units) and the level of VX-497 (or ribavirin) was titrated into the system. Amounts of IFN-α were chosen to be suboptimal (0.2 unit and below) to allow the detection of the antiviral activity of the added agent. The data are presented as a three-dimensional graph showing changes in viral yield for each of the combinations of ribavirin with IFN-α (Fig. 4a) and VX-497 with IFN-α (Fig. 4b). The cell viability profiles for each compound were not noticeably altered by the presence of any of the doses of IFN-α (data not shown). The reduction in viral yield is correlated with the combination of ribavirin with IFN-α or of VX-497 with IFN-α in an additive fashion. Evaluation of the data by using an independent effects model (Macsynergy II, version 1) (21) generated synergy volumes of 33 and 27 μM unit % for the combinations of IFN-α with VX-497 and of IFN-α with ribavirin, respectively. Values in this range (see Materials and Methods), near the threshold of 25 μM unit %, are at the very lower limits of significant synergy. While there may be pockets of marginal synergy or marginal antagonism within the combination profiles for both pairs of reagents, a reasonable interpretation of the data indicates a general trend toward additivity. While this additivity is not perfectly quantitative, particularly at saturating levels of either pair, the trend toward additivity is both apparent and similar for both pairs of reagents. Deviations of individual datum points from the expected linearity for each dimension within the three-dimensional graph reflect the experimental errors inherent in such a multistep quantitative assay for viruses. The principle difference between ribavirin and VX-497 in this assay in which they are used in combination with IFN-α is their relative potencies, as indicated by the numerical range of effective drug concentration for each; VX-497 was again the more potent of the two compounds.
FIG. 4.
Effects of the combination of ribavirin and IFN-α (a) or of VX-497 and IFN-α on the yield of EMCV in L929 cells. Viral yield is measured (in percent) relative to viral replication in the absence of either drug. Results are the means of triplicate determinations. (Standard deviations were on average 10% of the mean, with a range no more than 20% of the mean.) IFNa, IFN-α.
Does VX-497 have an effect on the IFN-α signaling pathway?
We wished to determine whether VX-497 has any direct or indirect effect on the IFN-α signaling pathway. To this end L929 cells and EMCV-infected L929 cells were treated with IFN-α alone and with IFN-α supplemented with VX-497. Nuclear extracts were prepared, normalized for protein concentration, and tested for binding to an interferon-sensitive response element (ISRE) oligonucleotide or, as a control, a gamma interferon-activated sequence (GAS) oligonucleotide. Two radiolabeled ISRE gel-retarded bands (data not shown) were detected in nuclear extracts of L929 cells (EMCV-infected or uninfected cells) treated with IFN-α (range, 0.01 to 10 units per 100-μl well) which were competed by an excess of unlabeled oligonucleotide. No noticeable difference in pattern or intensity of gel-retarded bands (data not shown) was observed when the experiments were repeated with the addition of VX-497 (range, 100 to 500 nM). This suggests that VX-497 does not alter, positively or negatively, the IFN-α signaling pathway in L929 cells and that the antiviral effect of VX-497 in the EMCV replication system is indeed independent. This is consistent with the simple additivity seen with the IFN-α–VX-497 combination described herein.
DISCUSSION
The aim of this study was to evaluate the antiviral activity of VX-497 against a variety of viruses in vitro and to compare its potency to that of ribavirin. Ribavirin is an established broad-spectrum antiviral agent which has recently been approved for use, in combination with IFN-α, for the treatment of chronic hepatitis resulting from HCV infection. As a result of this, we also wished to investigate the potential of combining VX-497 with IFN-α. Since there is no adequate in vitro assay for HCV replication, we decided to use EMCV replication in L929 cells (a mouse fibroblast cell line) as a robust and rapid viral assay system in which to perform this comparison. Since this system is routinely used to evaluate and standardize IFN-α, it was considered a valid and meaningful assay for the combination studies.
Ribavirin is a competitive inhibitor of IMPDH as ribavirin-5′-monophosphate (Ki = 250 nM). It has demonstrated in vitro activity against a broad spectrum of DNA and RNA viruses and has shown clinical efficacy against influenza A and B viruses, RSV, parainfluenza virus, and Lassa fever virus. It has also been demonstrated to have antiproliferative activity as well as an ability to inhibit proinflammatory mediators induced by viral infection (20, 30). A side effect of ribavirin is anemia, which results from the accumulation of the triphosphate form of the drug in erythrocytes. Ribavirin has recently been approved for use in combination with IFN-α for the treatment of HCV-induced hepatitis. Therapeutically, it appears to act synergistically with IFN-α (for a review, see references 18 and 32). IFN-α has been approved for use in the treatment of a variety of human malignancies and viral diseases and as an immunomodulator (10, 14). It is currently approved for use in the treatment of chronic viral hepatitis (caused by HBV and HCV) and has been approved for use in combination with ribavirin.
In these studies, the antiviral potency of ribavirin was confirmed and the potency of VX-497 was established against a number of viruses as individual agents. The additive antiviral effect of each agent with IFN-α was demonstrated in the L929-EMCV system. For the viruses tested in this study, the activity of VX-497 was consistently more potent than that of ribavirin against all viruses tested except influenza A virus. The reasons for the improved antiviral potency of VX-497 can be considered a function of the relative abilities of either compound to inhibit IMPDH. It should be noted that of the variously phosphorylated intracellular forms of ribavirin, only the monophosphate form significantly inhibits IMPDH. The amount of the monophosphate form as a percentage of all phosphorylated forms of ribavirin has been measured to be in the range of 5 to 12% in 3T3 cells, 4 to 9% in Vero cells, and 4 to 9% in MA-104 cells (28). This makes the IMPDH-inhibitory form of ribavirin a minor intracellular component. It is also interesting that the relative affinities of VX-497 and ribavirin for IMPDH are about 35-fold (Kis = 7 and 250 nM, respectively), with VX-497 again being the more potent agent. The greater potency of VX-497 relative to that of ribavirin in the viral assays demonstrates a similar ratio, having a range of 10- to 100-fold increased potency depending on the particular viral replication system tested. The role of IMPDH in the antiviral activities of both compounds is strengthened by the reversibility of the antiviral effects achieved with guanosine. While the extent of this reversibility is variable and is probably cell line dependent, it does indicate that GTP pool depletion is an important component of the viral reduction mechanism and is consistent with a mechanism of action involving the inhibition of IMPDH. A recent in vitro study (17a) determined that HCV and classical swine fever virus NS5B (an RNA template-dependent RNA polymerase and a key component in the viral replicase complex) is selectively and significantly stimulated by high (admittedly, unphysiologically high) levels of GTP. This stimulation is not seen with ATP, CTP, UTP, GDP, or GMP. Perhaps a reduction in physiologically relevant GTP levels that arises, for instance, from IMPDH inhibition could account for an antiviral effect in vivo.
The additivity of the antiviral effects of VX-497 and IFN-α was demonstrated in these studies as a reduction in EMCV replication and was compared to that of ribavirin and IFN-α. Again, VX-497 was the more potent of the two agents over the same range of IFN-α concentrations tested. The simple additivity seen indicates that the antiviral mechanisms elicited by VX-497 and IFN-α are independent of each other in this system and likely involve depletion of GTP pools for VX-497 and one of the mechanisms indicated above for IFN-α. This viewpoint is consistent with the observation made in the studies presented here that VX-497 had no effect on the IFN-α signaling pathway, as determined by gel shift assays with ISRE oligonucleotides.
In summary, VX-497 exhibits 10- to 100-fold more potency than ribavirin against HBV, HCMV, RSV, HSV-1, parainfluenza 3 virus, EMCV, and VEEV infections in cell culture. These data are supportive of the hypothesis that VX-497, like ribavirin, is a broad-spectrum antiviral agent. In vitro testing for activity against HCV is problematic, since a robust in vitro system does not exist for HCV replication. The potential for the use of VX-497 in the treatment of hepatitis C is premised on the demonstrated clinical activity of ribavirin in the treatment of chronic hepatitis C, particularly in combination with IFN-α, and the demonstrated broad-spectrum antiviral activity of VX-497 shown herein. Since ribavirin's activity against HCV may be significantly mediated by IMPDH inhibition, VX-497 may also have activity in the treatment of hepatitis C. We have demonstrated that VX-497 has in vitro antiviral activity that is comparable or superior to that of ribavirin against a number of viruses, and we have demonstrated that the additive antiviral effect of VX-497 and IFN-α is similar to but more potent than that of ribavirin and IFN-α. Given the synergistic activity of ribavirin and IFN-α in the treatment of HCV infection seen in the clinic, the additive effect of ribavirin or VX-497 with IFN-α demonstrated in these studies, and the greater overall potency of VX-497 relative to that of ribavirin, it is possible that a VX-497–IFN-α combination will prove to be a potential alternative therapy. To this end, phase II clinical trials of VX-497 for the treatment of HCV infection have recently concluded (the trials were conducted by Vertex Pharmaceuticals Inc.) (35).
ACKNOWLEDGMENTS
We thank R. A. Byrn for synergy analysis and E. Nimmesgern for input and discussions during the course of this work; the IMPDH Chemistry Team (D. M. Armistead, M. C. Badia, R. S. Bethiel, C. A. Frank, P. M. Novak, S. M. Ronkin, and J. O. Saunders) for generating and providing VX-497; and V. Sato and M. Su for critical reading of the manuscript.
REFERENCES
- 1.Allison A C, Hovi T, Watts R W E, Webster A D B. The role of de novo purine synthesis in lymphocyte transformation. Ciba Found Symp. 1977;48:207–224. doi: 10.1002/9780470720301.ch13. [DOI] [PubMed] [Google Scholar]
- 2.Cassidy L F, Patterson J L. Mechanism of La Crosse virus inhibition by ribavirin. Antimicrob Agents Chemother. 1989;33:2009–2011. doi: 10.1128/aac.33.11.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colby T D, Vanderveen K, Strickler M D, Markham G D, Goldstein B M. Crystal structure of human type II inosine monophosphate dehydrogenase: implications for ligand binding and drug design. Proc Natl Acad Sci USA. 1999;96:3531–3536. doi: 10.1073/pnas.96.7.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Clercq E. Antiviral agents: characteristic activity spectrum depending on the molecular target with which they interact. New York, N.Y: Academic Press, Inc.; 1993. pp. 1–55. [DOI] [PubMed] [Google Scholar]
- 5.De Clercq E. Trends in the development of new antiviral agents for the chemotherapy of infections caused by herpes viruses and retroviruses. Rev Med Virol. 1995;5:149–164. [Google Scholar]
- 6.De Clercq E, Cools M, Balzarini J, Snoeck R, Andrei G, Hosoya M, Shigeta S, Ueda T, Minakawa N, Matsuda A. Antiviral activities of 5-ethynl-1-β-ribofuranosylimidazole-4-carboxamide and related compounds. Antimicrob Agents Chemother. 1991;35:679–684. doi: 10.1128/aac.35.4.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dignam J D, Lebovitz R M, Roeder R G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eriksson B, Helgstrand B, Johansson N G, Larsson A, Misiorny A, Noren J O, Phillipson L, Stenberg K, Stening G, Stridh S, Oberg B. Inhibition of influenza virus ribonucleic acid polymerase by ribavirin triphosphate. Antimicrob Agents Chemother. 1977;11:946–951. doi: 10.1128/aac.11.6.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandez-Larsson R, O'Connell K, Koumans E, Patterson J L. Molecular analysis of the inhibitory effect of phosphorylated ribavirin on the vesicular stomatitis virus in vitro polymerase reaction. Antimicrob Agents Chemother. 1989;33:1668–1673. doi: 10.1128/aac.33.10.1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gale M, Jr, Katze M G. Molecular mechanisms of interferon resistance mediated by viral-directed inhibition of PKR, the interferon-induced protein kinase. Pharmacol Ther. 1998;78:29–46. doi: 10.1016/s0163-7258(97)00165-4. [DOI] [PubMed] [Google Scholar]
- 11.Gilbert B E, Knight V. Biochemistry and clinical applications of ribavirin. Antimicrob Agents and Chemother. 1986;30:201–205. doi: 10.1128/aac.30.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goswami B B, Borek E, Sharma O K. The broad spectrum antiviral agent ribavirin inhibits capping of mRNA. Biochem Biophys Res Commun. 1979;89:830–836. doi: 10.1016/0006-291x(79)91853-9. [DOI] [PubMed] [Google Scholar]
- 13.Hartman N R, Gurpreet S A, Cooney D A, Mitsuya H, Kageyama S, Fridland A, Broder S, Johns D G. Inhibitors of IMP dehydrogenase stimulate the phosphorylation of the anti-human immunodeficiency virus nucleosides 2′,3′-dideoxyadenosine and 2′,3′-dideoxyinosine. Mol Pharmacol. 1991;40:118–124. [PubMed] [Google Scholar]
- 14.Jaramillo M L, Abraham N, Bell J C. The interferon system: a review with emphasis on the role of PKR in growth control. Cancer Invest. 1995;13:327–338. doi: 10.3109/07357909509094468. [DOI] [PubMed] [Google Scholar]
- 15.Kobashigawa J, Miller L, Renlund D, Mentzer R, Alderman E, Bourge R, Costanzo M, Eisen H, Dureau G, Ratkovec R, Hummel M, Ipe D, Johnson J, Keogh A, Mamelok R, Mancini D, Smart F, Valantine H. A randomized active-controlled trial of mycophenolate mofetil in heart transplants. Transplantation. 1998;66:507–515. doi: 10.1097/00007890-199808270-00016. [DOI] [PubMed] [Google Scholar]
- 16.Korba B F, Gerin J L. Use of a standardized cell culture assay to assess activities of nucleoside analogs against hepatitis B virus replication. Antivir Res. 1992;19:55–70. doi: 10.1016/0166-3542(92)90056-b. [DOI] [PubMed] [Google Scholar]
- 17.Kornberg A, Baker T A. DNA replication. New York, N.Y: W. H. Freeman & Co.; 1992. pp. 53–100. [Google Scholar]
- 17a.Lohmann, Overton V H, Bartenschlager R. Selective stimulation of hepatitis C virus and pestivirus NS5B RNA polymerase activity by GTP. J Biol Chem. 1999;274:10807–10815. doi: 10.1074/jbc.274.16.10807. [DOI] [PubMed] [Google Scholar]
- 18.Main J, McCarron B, Thomas H C. Treatment of chronic viral hepatitis. Antivir Chem Chemother. 1998;9:449–460. doi: 10.1177/095632029800900601. [DOI] [PubMed] [Google Scholar]
- 19.Neyts J, Andrei G, De Clercq E. The novel immunosuppressive agent mycophenolate mofetil markedly potentiates the antiherpes activities of acyclovir, gancyclovir, and penciclovir in vitro and in vivo. Antimicrob Agents Chemother. 1998;42:216–222. doi: 10.1128/aac.42.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ning Q, Brown D, Parodo J, Cattral M, Gorczynski R, Cole E, Fung L, Ding J W, Liu M F, Rotstein O, Phillips M J, Levy G. Ribavirin inhibits viral-induced macrophage production of TNF, IL-1, the procoagulant fgl2 prothrombinase and preserves Th1 cytokine production but inhibits Th2 cytokine response. J Immunol. 1998;22:3487–3493. [PubMed] [Google Scholar]
- 21.Prichard M N, Shipman C. A three-dimensional model to analyze drug-drug interactions. Antivir Res. 1992;14:181–206. doi: 10.1016/0166-3542(90)90001-n. [DOI] [PubMed] [Google Scholar]
- 22.Rankin J T, Eppes S B, Antczak J B, Joklik W K. Studies on the mechanism of the antiviral activity of ribavirin against reovirus. Virology. 1989;168:147–158. doi: 10.1016/0042-6822(89)90413-3. [DOI] [PubMed] [Google Scholar]
- 23.Scheidel L M, Stollar V. Mutations that confer resistance to mycophenolic acid and ribavirin on sindbis virus map to the nonstructural protein nsP1. Virology. 1991;181:490–499. doi: 10.1016/0042-6822(91)90881-b. [DOI] [PubMed] [Google Scholar]
- 24.Sidwell R W, Huffman J H, Khare G P, Allen L B, Witkowski J T, Robins R K. Broad-spectrum antiviral activity of virazole: 1-β-d-ribofranosyl-1,2,4-triazole-3-carboxamide. Science. 1972;177:705–706. doi: 10.1126/science.177.4050.705. [DOI] [PubMed] [Google Scholar]
- 25.Silverman Kitchin J E, Keltz Pomeranz M, Pak G, Washenik K, Shupack J L. Rediscovering mycophenolic acid: a review of its mechanism, side effects, and potential uses. J Am Acad Dermatol. 1997;37:445–449. doi: 10.1016/s0190-9622(97)70147-6. [DOI] [PubMed] [Google Scholar]
- 26.Sintchak M D, Fleminh M A, Futer O, Raybuck S A, Chambers S P, Caron P R, Murcko M A, Wilson K P. Structure and mechanism of inosine monophosphate dehydrogenase in complex with the immunosuppressant mycophenolic acid. Cell. 1996;85:921–930. doi: 10.1016/s0092-8674(00)81275-1. [DOI] [PubMed] [Google Scholar]
- 27.Sintchak M D, Badia M C, Futer O, Nimmesgern E. X-ray crystal structure of the antiviral drug ribavirin monophosphate bound to IMP dehydrogenase. Antivir Res. 1999;41:A56. [Google Scholar]
- 28.Smee D F, Huggins J W. Mode of action of ribavirin against cowpox and monkeypox viruses. Antivir Res. 1999;41:A52. [Google Scholar]
- 29.Sollinger H W. Update on preclinical and clinical experience with mycophenolate mofetil. Transplant Proc. 1995;28:24–29. [PubMed] [Google Scholar]
- 30.Tam R C, Pai B, Bard J, Lim C, Averett D R, Phan U T, Milovanovic T. Ribavirin polarizes human T cell responses towards a type 1 cytokine profile. J Hepatol. 1999;30:376–382. doi: 10.1016/s0168-8278(99)80093-2. [DOI] [PubMed] [Google Scholar]
- 31.Toltzis P, O'Connell K, Patterson J L. Effect of phosphorylated ribavirin on vesicular stomatitis virus transcription. Antimicrob Agents Chemother. 1988;32:492–497. doi: 10.1128/aac.32.4.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wedemeyer H, Caselmann W H, Manns M P. Combination therapy of chronic hepatitis C: an important step but not the final goal! J Hepatol. 1998;29:1010–1014. doi: 10.1016/s0168-8278(98)80133-5. [DOI] [PubMed] [Google Scholar]
- 33.Wray S K, Gilbert B E, Knight V. Effect of ribavirin triphosphate on primer generation and elongation during influenza virus transcription in vitro. Antivir Res. 1985;5:39–48. doi: 10.1016/0166-3542(85)90013-0. [DOI] [PubMed] [Google Scholar]
- 34.Wray S K, Gilbert B E, Noall M W, Knight V. Mode of action of ribavirin: effect of nucleotide pool alterations on influenza virus ribonucleoprotein synthesis. Antivir Res. 1985;5:29–37. doi: 10.1016/0166-3542(85)90012-9. [DOI] [PubMed] [Google Scholar]
- 35.Wright T, Shiffman M L, Knox S, Ette E, Kauffman R S, Alam J. Dose-ranging study of VX-497, a novel, oral IMPDH inhibitor, in patients with hepatitis C. Hepatology. 1999;30(Suppl.):122A. [Google Scholar]
- 36.Wu J C. Mycophenolate mofetil: molecular mechanisms of action. Perspect Drug Disc Design. 1994;2:185–204. [Google Scholar]