SUMMARY
Overall survival for patients with squamous cell carcinoma of the head and neck (SCCHN) has not improved appreciably over the past few decades. Novel therapeutic approaches, such as immunotherapy, are under clinical investigation since the standard treatments are toxic and have not successfully controlled this disease with sufficiently high success rates. Cancer immunotherapy describes various techniques to expand and activate the immune system to control tumor growth in vivo, and clinical evaluation has so far demonstrated low toxicity. Immunotherapy appears to have the most applicability in settings of minimal residual disease and to reduce distant metastases after other therapeutic interventions, and its potential clinical value is now receiving intensive evaluation. Emerging forms of SCCHN immunotherapy involve both the use of monoclonal antibodies (mAb) that target growth factor receptors where immune activation appears to contribute to tumor cell lysis, as well as various forms of active vaccination strategies which activate and direct the patient’s cellular immunity against the tumor. This article reviews immunotherapeutic strategies currently in clinical trials or under development for patients with SCCHN.
Keywords: Cancer vaccines, Tumor antigens, Immunotherapy, Dendritic cells, Cytokines
Introduction
The long-term survival for patients with squamous cell carcinoma of the head and neck (SCCHN) is <50%.1 Standard treatments have failed to impact long-term survival in this patient population and are increasingly toxic. Adjuvant therapy has long been considered as a potential treatment modality to eradicate local, regional and metastatic microscopic disease.2,3 Cancer immunotherapy is also being evaluated for adjuvant treatment of SCCHN, involving techniques to utilize the patient’s anti-tumor immune response to recognize and reduce metastasis, recurrence, and incipient second primary tumors.4
Immunotherapeutic approaches usually require identification of tumor antigens (TA) expressed by SCCHN cells. Numerous TA have been identified as potential targets for immunotherapy in SCCHN cells, but very few are tumor specific, and usually there is some level of expression in surrounding tissues. In general, TA are short peptide sequences generated from unique or shared proteins expressed by the cancer cells, recognized by either the humoral or cell mediated components of the immune system.5
Briefly, TA fall into a few broad categories. Some antigens, such as CASP-86, are uniquely expressed by SCC, whereas CEA7 and MAGE8 are associated with different stages or differentiation lineages. A third class of TA are over-expressed in tumor cells, such as p539 or EGFR.10 Immune recognition is possible due to higher levels of wild-type sequence TA peptides expressed by malignant and premalignant cells compared to normal tissues. Still, some antigens are mutated forms of proteins found in normal cells, for example p5311 and CDK4.12 Finally, viral encoded oncoproteins, like the well studied human papillomavirus (HPV)-derived E6 and E713,14 proteins or Epstein barr virus (EBV)-derived antigens15, are unique to SCCHN cells, providing strong rationale for immune targeting through cancer therapy or prevention.
TA should possess certain characteristics such as unique or differential expression on malignant cells. Broadly applicability TA would be expressed at a sufficient level in a majority of patients’ tumors and are important for tumor survival or malignant behavior. This ensures that antigen loss, to avoid immune detection, produces a negative effect on tumor cell growth and survival. Finally, a targeted antigen must generate a potent immunologic response, or a means to augment this response must accompany the immunotherapy. The discovery of the identity, antigenic source, and molecular sequence of TA has led to the design of a number of targeted immunotherapeutics for SCCHN. This article provides a brief review of emerging forms of immunotherapy for SCCHN, with a specific focus on recent advances in anti-tumor vaccines and their effectiveness in oral oncology.
Antibody therapy
Targeting tumor cells with high-affinity antibodies is successfully utilized in the treatment of SCCHN. mAb with high affinities for TA are relatively feasible to manufacture in large quantities and have been shown to be clinically efficacious16,17 and less toxic in comparison to traditional chemotherapeutic agents.18 These factors have led to the recent use of TA specific mAb immunotherapy targeting the epidermal growth factor receptor (EGFR)10 and vascular endothelial growth factor (VEGF).19
TA specific targeting of EGFR has been accomplished by two different FDA-approved mAb, cetuximab and panitumumab. More than 90% of SCCHN overexpress EGFR, and its importance in cell proliferation and survival, invasion and angiogenesis make it an attractive target for immunotherapy.20,21 Cetuximab, a chimeric mAb targeting EGFR, was approved by the FDA in 2006 for use in combination with radiation therapy for treatment of locally advanced SCCHN. Phase II clinical trials have addressed the efficacy of combination cetuximab and radiation therapy,22 as well as cetuximab and chemotherapy for locally advanced or recurrent SCCHN. In a Phase III trial of combination cetuximab, radiation therapy and cisplatin demonstrated 3 year overall survival, progression free survival and locoregional control rates of 76%, 56% and 71%, respectively.23 Panitumumab has also been used in a Phase I trial with chemoradiation, showing clinical efficacy.24 At the University of Pittsburgh, a Phase II trial of adjuvant panitumumab, cisplatin and radiation (UPCI 06-120) (Ferris, PI) is testing the ability of mAb panitumumab immunotherapy to reduce microscopic disease recurrence in high-risk, resected SCCHN patients.
Antibody mediated TA specific immunotherapy can may function through several mechanisms of action.32,33 First, mAb may inhibit tumor growth by inhibiting signaling pathways involved in proliferation, differentiation and survival.25 Second, mAb may serve as immunostimulants and induce innate (complement-mediated) immunity26 or antibody-dependent cellular cytotoxicity (ADCC),27,28 as well as inducing antigen-specific CTL via cross-priming.29 Third, antibodies may serve as vehicles for the delivery of conjugated chemotherapeutic toxins to the tumor bed.10 Fourth, mAb target SCCHN cell derived TA to DC for enhanced processing and cross-priming of T lymphocytes to broaden the anti-tumor immune response.30,31
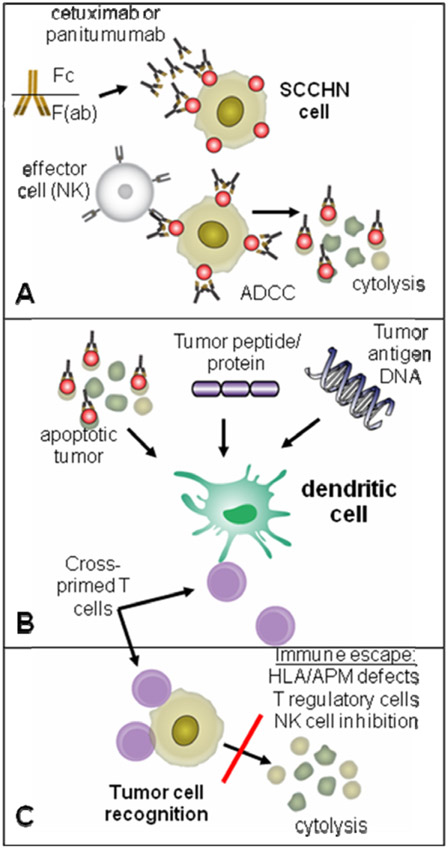
Despite well documented clinical efficacy of EGFR specific mAbs16 their mechanism of action is poorly understood. Clinical response to cetuximab therapy does not correlate with level of expression of EGFR, the targeted TA,25 and likely involves more than simple competitive antagonism of receptor ligands. These mAb and cetuximab, the EGFR specific chimeric IgG1 mAb,27 likely owe their clinical efficacy at least partly to antibody-dependent cell cytotoxicity (ADCC).34,35 Natural killer (NK) cells participate in ADCC through binding of their NK FcγR (see Fig. 1 panel A) and polymorphisms at this FcγR have been implicated in clinical response.34,36 Induction of TA specific T lymphocyte responses may also contribute to clinical responses. A number of investigators have demonstrated the importance of various arms of the immune system in clinical efficacy, and ultimately responses are likely multifactorial.
Figure 1.
Schematic representation of ADCC, the effector mAb has a constant fragment [Fc] that interacts with immune effector cells, and a variable fragment [F(ab)] that is antigen (EGFR) specific. During cross presentation, tumor antigens are degraded in the cytoplasm of dendritic cells (DC), and presented to T cells producing a cellular immune response. Panel A: schematic representation of ADCC. The TA specific mAb has a constant fragment [Fc] that interacts with immune effector (NK) cells through a polymorphic FcγR, and a variable fragment [F(ab)] that is TA specific. This binding of mAb coated SCCHN cells to the FcγR-bearing NK cell leads to cytolysis. Panel B: during cross presentation, TA are transferred (taken up) into DC, degraded in the cytoplasm (termed TA processing), and presented to T cells producing a cellular anti-tumor immune response. Panel C: anti-tumor immune activity can be circumvented through multiple immune escape mechanisms, preventing SCCHN cell lysis and tumor outgrowth.
Vaccine strategies for SCCHN
Generation of an anti-tumor immune response involves many elements of the immune system, with T lymphocytes considered critical cellular effectors involved in anti-tumor activity. T cells recognize short peptide fragments (TA of 8–10 amino acids in length), derived from cellular protein antigens. These TA peptides are expressed by tumor cells and are also processed in antigen presenting cells from much larger proteins. These antigen presenting cells, such as dendritic cells (DC), display surface complexes composed of TA peptides bound to human leukocyte antigens (HLA) on the cell surface for recognition by T cells. It is these HLA:peptide complexes and adjacent costimulatory molecules which activate the T cells and ultimately produce anti-tumor activity (see Fig. 1 panel B). A major challenge of T cell based immunotherapies has been to find the both the appropriate TA and optimal method of delivery into professional APC, such as DC, to initiate the most efficient and persistent immune response.
Multiple approaches have been explored to develop clinically efficacious specific immunotherapies, including the use of DNA, bacterial or viral vectors, peptide, whole protein, DC or tumor cell based vaccines. Below we outline current SCCHN vaccine strategies and outcomes undergoing clinical evaluation or in development.
Peptide vaccines
Antigenic peptides can be directly delivered to cancer patients to generate immunologic responses to tumor cells. Precisely designed TA peptides can associate with HLA class I or II molecules on the cell surface of antigen presenting cells (DC) and trigger anti-tumor effector mechanisms by activating helper T cell (Th) or cytotoxic T cells (CTL). Peptide-based vaccines have been shown to be safe and easy to produce on a large scale for clinical grade vaccines. The main obstacle, aside from identifying the appropriate immunogenic epitope on the TA, is the identification of cognate HLA alleles that will bind with the synthetic peptide and generate an immune response. In general high binding to these HLA roughly correlates with in vivo immunogenicity. Disadvantages of peptide vaccines include their relative weak immunogenicity when compared to viral and bacterial vaccine strategies.37 In addition, once a TA peptide is selected, its use is limited to the population of individuals expressing the appropriate restricting HLA molecule, which can present the antigenic peptide to T cells. This HLA restriction associated with peptide-based vaccines is circumvented by the use of larger protein-based or multi-peptide vaccines, where multiple TA peptides are presented in polyvalent fashion.
Currently, a clinical trial at the University of Maryland (NCT00257738) (Strome, PI) is enrolling patients with advanced recurrent SCCHN, using a vaccine containing MAGE-A3 and HPV-16 E7 proteins administered to HLA-A2 positive patients.38 This multi-epitope vaccine contains both CD4 and CD8 epitopes that are cleaved and released by the Golgi apparatus. This strategy utilizes a basic Trojan peptide sequence derived from HIV-I Tat protein to deliver peptide epitopes intracellularly directly into the endoplasmic reticulum, and lead to the generation of CTL and Th responses.39
One of the major benefits of peptide-based vaccines is the ability to monitor specific immunologic response to vaccination; however in most cases the immune response detected has not been correlated with clinical responses. Given the discordance between immunologic and clinical responses, as well as the above mentioned restrictions on HLA haplotype, many researchers are utilizing peptide-pulsed DC, the addition of helper peptides (see below), as well as depletion of regulatory T cell (Treg) subsets to improve the effectiveness of peptide vaccines.
DNA vaccines
One of the simplest means of eliciting antigen-specific immune response is through the introduction of naked DNA/RNA. After transfection, nucleic acid vaccines rely on host cellular machinery to generate TA. Nucleic acid vaccines have the advantage of being relatively easy to manufacture in large quantities and have inherent stability in a wide range of conditions over an extended period of time. Furthermore, nucleic acid based vaccines have little inherent risk to the patient and are safe in immunosuppressed individuals. However, nucleic acid based vaccines have their drawbacks. Most notably, DNA vaccines generate relatively weak immune responses when compared to their viral or bacterial vector counterparts.
Several animal models have demonstrated the ability of DNA plasmid-based vaccines to elicit immune responses to the HPV-16 E7 protein.40 Based on these data, an HPV-E7-specific clinical trial has been completed at Johns Hopkins University administering naked DNA encoding HPV-16 E7 linked to M. tuberculosis HSP70 protein to patients with advanced HPV-16 associated SCCHN. Eighteen oropharyngeal SCC patients were vaccinated after chemoradiation and had documented HPV-16+ disease by fluorescence in situ hybridization (FISH). Clinical results are being correlated with induction of E7-specific immunity.
One of the obstacles of DNA vaccine therapy lies in the route of administration of naked nucleic acid vaccines. Most commonly, intramuscular or intratumoral injections are used in the delivery of DNA based vaccines which may not target DC, the optimal cell type for induction of strong anti-tumor immunity. As mentioned, the immune response generated by DNA vaccines may be diminished when compared to viral and bacterial vaccine strategies. However, plasmid DNA has been shown to activate innate immune responses42 through its own cognate immunostimulatory sequences which can stimulate inflammatory signals and cytokines via CpG motifs and toll-like receptors (TLR).41
Dendritic cell based vaccines
Strategies to circumvent drawbacks in peptide and nucleic acid based vaccines have led to autologous DC loaded with tumor peptides, tumor lysates, or tumor DNA for generation of antigen-specific immunity. Given their high level of expression of HLA and costimulatory molecules, these DC become potent stimulators of anti-tumor immune responses. The most commonly used strategy for DC vaccination is the loading of HLA class I and II molecules with peptides from TA.43 However, this strategy has inherent drawbacks including HLA restriction, limited numbers of TA, rapid turnover of exogenous peptide–HLA complexes, and potential for skewing T cells toward tumor-permissive regulatory or terminal differentiation phenotypes. Therefore, loading DC with total antigen preparations, as in apoptotic tumor fed DC has been utilized to circumvent these restrictions, combined with DC cytokine maturation to reduce regulatory T cells (Treg).
Antigen fed dendritic cell vaccines
Two strategies for utilizing polyvalent tumor antigen-loaded DC were investigated at the University of Pittsburgh. The first trial utilized autologous DC incubated for 18 h with surgically resected, irradiated tumor cells. The resulting, cytokine-matured apoptotic tumor fed DC (UPCI 00-046) were delivered intranodally and anti-tumor immune effects measured. As a phase I feasibility vaccine there was little toxicity in the four SCCHN patients treated. Although, immune responses were observed and patients are without evidence of recurrent disease, over 50 patients had to be screened. Frequent bacterial contamination of the DC: tumor cell incubation culture was due to oral flora brought by the tumor cells. Secondly the requirement for sufficient cellular numbers and tumor products limited accrual to the trial. Third after intensive surgery and/or chemoradiation to eradicate their tumor, patients had a psychological resistance to having tumor products delivered back into their body, even after assurance that the tumor cell specimens were lethally irradiated. Lessons learned from this phase I trial led to the design of a currently open trial, utilizing autologous tumor DNA transfected into the patient’s DC. UPCI 04-178 (Johnson, PI) uses lysed resected tumor specimens to obtain tumor DNA, transfected into autologous DC to express a wide variety of public and private TA. However, sufficient numbers of resected tumor cells are still necessary, limiting the applicability for this reason, as well as the logistical issues involved with tumor procurement. In addition the lack of specific identified TA to be monitored for determining vaccine efficacy ultimately limits these approaches.
The common over-expression of p53 in many tumor cells44 has led to the development of multi-valent p53 loaded DC vaccines. At the University of Pittsburgh (UPCI 03-156) (Ferris, PI), a total of 17 patients have been treated in the adjuvant setting with a multiepitope, wild-type p53-based vaccine using autologous peptide-loaded DC. To date, it has been used on SCCHN patients of all clinical stages, including early-stage disease, with low toxicity and promising immunologic responses. These p53 peptide-loaded DC are then infused intranodally in SCCHN patients with early or advanced disease at risk for recurrence or second primary tumor. Immunologic responses have been observed in several patients without evidence of residual/recurrent disease in 15/17 vaccinated patients to date.
Viral and bacterial vectors
Delivery of TA has also been achieved using bacterial (Listeriolysin O) or viral vectors.45,46 A recent study demonstrated the feasibility and safety of an autologous tumor cell-New Castle disease virus (ATV-NDV) vaccine for SCCHN.47 In this study, autologous tumor cells from twenty patients with SCCHN were cultured and infected with NDV prior to subcutaneous injection to stimulate cross-reactive TA immunity. Survival of patients with stage III and IV tumors was 61% at five years, and immune monitoring revealed significant anti-tumor delayed type hypersensitivity (DTH) responses. Tumor-reactive T cells were present in peripheral blood up to seven years post-vaccination. However a potential risk of disseminated viral infection exists, though no major side effects were seen in the study. Although bacterial and viral vectors produce robust immunologic responses, the potential presence of neutralizing antibodies and limited ability for repeat treatment, due to potential toxicity, remain their drawbacks. Concern for disseminated infection in immunosuppressed individuals or direct close patient contacts remains a concern.
Hurdles to successful immunotherapy
Tumors are inherently defective targets for immune recognition and their ability to evade recognition by the host immune system, by a variety of mechanisms, is collectively known as tumor immune escape (see Fig. 1 panel C). Alterations in the processing and presentation of endogenous TA represent a major mechanism of tumor immune escape. Downregulation of antigen processing machinery (APM), such as TAP 1/2 and HLA class I antigen leads to ineffective recognition by CTL in SCCHN.48 Clinical efforts to restore APM and HLA class I antigen expression in SCCHN are being studied, in particular, administration of IFN-γ which functions to upregulate APM and HLA molecules and restores the ability of CTL to recognize tumor cells.49 A second tumor escape mechanism with implications in the development of cancer immunotherapies involves Treg cells, which function to downmodulate immune responses. Tumor cells are postulated to recruit Treg and suppress anti-tumor immunity.50 Continuing investigation on the biology of Treg and the potential benefits or toxicities associated with removing regulatory immune elements from the tumor microenvironment will likely contribute to improving cancer immunotherapeutics. As mentioned, the efficacy of antibody therapy relies, at least in part, on ADCC for effective anti-tumor activity. Bevacizumab, a vascular endothelial growth factor (VEGF) specific mAb may enhance dendritic cell (DC) maturation and play a role in tumor escape mechanisms51, supporting an additional immunologic role. Much as cetuximab activity is dependent on the host immune system through the NK expressing FcγR, bevacizumab may increase host anti-tumor activity through the reduction of immunosuppressive effects of VEGF on DC.
Conclusions
Numerous strategies have been and are currently being employed to develop new methods of vaccination as well as augment currently available vaccine delivery systems. The use of immunotherapy has been most commonly in the realm of adjuvant therapy, and may be most promising in early-stage or premalignant SCCHN, given the patient’s lower tumor burden, and decreased the likelihood of tumor-associated immune dysregulation. Preferably the ideal vaccine would be an “off the shelf” product that ultimately reduces recurrence and improves overall survival. Various immunomodulatory strategies are likely necessary to augment current methods of targeting TA in an attempt to optimize the patient’s immune response to tumor vaccines and to determine the most clinically relevant application of immunotherapy in head and neck oncologic care.
Funding source:
R01 DE19727
Footnotes
Conflict of Interest Statement
Robert Ferris is a consultant for Merck and has received research funding from Amgen.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin 2007;57(1):43–66. [DOI] [PubMed] [Google Scholar]
- 2.Bernier J, Cooper JS, Pajak TF, et al. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (#9501). Head Neck 2005;27(10):843–50. [DOI] [PubMed] [Google Scholar]
- 3.Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med 2004;350(19):1937–44. [DOI] [PubMed] [Google Scholar]
- 4.Vikram B, Strong EW, Shah JP, Spiro R. Second malignant neoplasms in patients successfully treated with multimodality treatment for advanced head and neck cancer. Head Neck Surg 1984;6(3):734–7. [DOI] [PubMed] [Google Scholar]
- 5.Ferris RL, Whiteside TL, Ferrone S. Immune escape associated with functional defects in antigen-processing machinery in head and neck cancer. Clin Cancer Res 2006;12(13):3890–5. [DOI] [PubMed] [Google Scholar]
- 6.Mandruzzato S, Brasseur F, Andry G, Boon T, van der Bruggen P. A CASP-8 mutation recognized by cytolytic T lymphocytes on a human head and neck carcinoma. J Exp Med 1997;186(5):785–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kass ES, Greiner JW, Kantor JA, et al. Carcinoembryonic antigen as a target for specific antitumor immunotherapy of head and neck cancer. Cancer Res 2002;62(17):5049–57. [PubMed] [Google Scholar]
- 8.Kienstra MA, Neel HB, Strome SE, Roche P. Identification of NY-ESO-1, MAGE-1, and MAGE-3 in head and neck squamous cell carcinoma. Head Neck 2003;25(6):457–63. [DOI] [PubMed] [Google Scholar]
- 9.Ferris RL. Progress in head and neck cancer immunotherapy: can tolerance and immune suppression be reversed? ORL J Otorhinolaryngol Relat Spec 2004;66(6):332–40. [DOI] [PubMed] [Google Scholar]
- 10.Modjtahedi H, Moscatello DK, Box G, et al. Targeting of cells expressing wild-type EGFR and type-III mutant EGFR (EGFRvIII) by anti-EGFR MAb ICR62: a two-pronged attack for tumour therapy. Int J Cancer 2003;105(2):273–80. [DOI] [PubMed] [Google Scholar]
- 11.Balz V, Scheckenbach K, Gotte K, Bockmuhl U, Petersen I, Bier H. Is the p53 inactivation frequency in squamous cell carcinomas of the head and neck underestimated? Analysis of p53 exons 2–11 and human papillomavirus 16/18 E6 transcripts in 123 unselected tumor specimens. Cancer Res 2003;63(6):1188–91. [PubMed] [Google Scholar]
- 12.Patel S, Wang FH, Whiteside TL, Kasid U. Identification of seven differentially displayed transcripts in human primary and matched metastatic head and neck squamous cell carcinoma cell lines: implications in metastasis and/or radiation response. Oral Oncol 1997;33(3):197–203. [DOI] [PubMed] [Google Scholar]
- 13.Gillison ML, Koch WM, Capone RB, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst 2000;92(9):709–20. [DOI] [PubMed] [Google Scholar]
- 14.Albers A, Abe K, Hunt J, et al. Antitumor activity of human papillomavirus type 16 E7-specific T cells against virally infected squamous cell carcinoma of the head and neck. Cancer Res 2005;65(23):11146–55. [DOI] [PubMed] [Google Scholar]
- 15.Chen G, Shankar P, Lange C, et al. CD8 T cells specific for human immunodeficiency virus, Epstein-Barr virus, and cytomegalovirus lack molecules for homing to lymphoid sites of infection. Blood 2001;98(1):156–64. [DOI] [PubMed] [Google Scholar]
- 16.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 2006;354(6):567–78. [DOI] [PubMed] [Google Scholar]
- 17.Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med 2008;359(11):1116–27. [DOI] [PubMed] [Google Scholar]
- 18.Monji M, Senju S, Nakatsura T, et al. Head and neck cancer antigens recognized by the humoral immune system. Biochem Biophys Res Commun 2002;294(3):734–41. [DOI] [PubMed] [Google Scholar]
- 19.Caponigro F, Formato R, Caraglia M, Normanno N, Iaffaioli RV. Monoclonal antibodies targeting epidermal growth factor receptor and vascular endothelial growth factor with a focus on head and neck tumors. Curr Opin Oncol 2005;17(3):212–7. [DOI] [PubMed] [Google Scholar]
- 20.Mendelsohn J Antibody-mediated EGF receptor blockade as an anticancer therapy: from the laboratory to the clinic. Cancer Immunol Immunother 2003;52(5):342–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Modjtahedi H, Eccles S, Sandle J, Box G, Titley J, Dean C. Differentiation or immune destruction: two pathways for therapy of squamous cell carcinomas with antibodies to the epidermal growth factor receptor. Cancer Res 1994;54(7):1695–701. [PubMed] [Google Scholar]
- 22.Shin DM, Donato NJ, Perez-Soler R, et al. Epidermal growth factor receptor-targeted therapy with C225 and cisplatin in patients with head and neck cancer. Clin Cancer Res 2001;7(5):1204–13. [PubMed] [Google Scholar]
- 23.Pfister DG, Laurie SA, Weinstein GS, et al. American society of clinical oncology clinical practice guideline for the use of larynx-preservation strategies in the treatment of laryngeal cancer. J Clin Oncol 2006;24(22):3693–704. [DOI] [PubMed] [Google Scholar]
- 24.Wirth LJ, Posner MR, Tishler RB, Haddad RI, Clark JR, Goguen L, et al. Phase I study of panitumumab + chemoradiotherapy (CRT) for head and neck cancer (HNC). J Clin Oncol 2008;26(May 20 Suppl):abstr 6007. [Google Scholar]
- 25.Kim S, Grandis JR, Rinaldo A, Takes RP, Ferlito A. Emerging perspectives in epidermal growth factor receptor targeting in head and neck cancer. Head Neck 2008;30(5):667–74. [DOI] [PubMed] [Google Scholar]
- 26.Di Gaetano N, Cittera E, Nota R, et al. Complement activation determines the therapeutic activity of rituximab in vivo. J Immunol 2003;171(3):1581–7. [DOI] [PubMed] [Google Scholar]
- 27.Lopez-Albaitero A, Ferris RL. Immune activation by epidermal growth factor receptor specific monoclonal antibody therapy for head and neck cancer. Arch Otolaryngol Head Neck Surg 2007;133(12):1277–81. [DOI] [PubMed] [Google Scholar]
- 28.Musolino A, Naldi N, Bortesi B, et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J Clin Oncol 2008;26(11):1789–96. [DOI] [PubMed] [Google Scholar]
- 29.Dhodapkar KM, Krasovsky J, Williamson B, Dhodapkar MV. Antitumor monoclonal antibodies enhance cross-presentation of Cellular antigens and the generation of myeloma-specific killer T cells by dendritic cells. J Exp Med 2002;195(1):125–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Banerjee D, Matthews P, Matayeva E, Kaufman JL, Steinman RM, Dhodapkar KM. Enhanced T-cell responses to glioma cells coated with the anti-EGF receptor antibody and targeted to activating FcγRs on human dendritic cells. J Immunother 2008;31(2):113–20. [DOI] [PubMed] [Google Scholar]
- 31.Ferris R, Whiteside TL, Ferrone S. Clinical significance of downregulated antigen processing machinery in head and neck cancer. Clin Cancer Res 2006;12(13):3890–5. [DOI] [PubMed] [Google Scholar]
- 32.Weng WK, Levy R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J Clin Oncol 2003;21(21):3940–7. [DOI] [PubMed] [Google Scholar]
- 33.Kushner BH, Kramer K, Cheung NK. Phase II trial of the anti-G(D2) monoclonal antibody 3F8 and granulocyte-macrophage colony-stimulating factor for neuroblastoma. J Clin Oncol 2001;19(22):4189–94. [DOI] [PubMed] [Google Scholar]
- 34.Zhang W, Gordon M, Schultheis AM, et al. FCGR2A and FCGR3A polymorphisms associated with clinical outcome of epidermal growth factor receptor expressing metastatic colorectal cancer patients treated with single-agent cetuximab. J Clin Oncol 2007;25(24):3712–8. [DOI] [PubMed] [Google Scholar]
- 35.Lopez-Albaitero A, Lee S, Morgan S, et al. Role of polymorphic Fc gamma receptor (FcγR)IIIa and EGFR expression level in cetuximab mediated, NK cell dependent in vitro cytotoxicity of head and neck squamous cell carcinoma cells. Cancer Immunol Immunother 2009, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taylor RJ, Chan SL, Wood A, et al. FcγRIIIa polymorphisms and cetuximab induced cytotoxicity in squamous cell carcinoma of the head and neck. Cancer Immunol Immunother 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tomson TT, Roden RB, Wu TC. Human papillomavirus vaccines for the prevention and treatment of cervical cancer. Curr Opin Investig Drugs 2004;5(12):1247–61. [PubMed] [Google Scholar]
- 38.Lu J, Wettstein PJ, Higashimoto Y, Appella E, Celis E. TAP-independent presentation of CTL epitopes by Trojan antigens. J Immunol 2001;166(12):7063–71. [DOI] [PubMed] [Google Scholar]
- 39.Becker-Hapak M, McAllister SS, Dowdy SF. TAT-mediated protein transduction into mammalian cells. Methods 2001;24(3):247–56. [DOI] [PubMed] [Google Scholar]
- 40.Devaraj K, Gillison ML, Wu TC. Development of HPV vaccines for HPV-associated head and neck squamous cell carcinoma. Crit Rev Oral Biol Med 2003;14(5):345–62. [DOI] [PubMed] [Google Scholar]
- 41.Hemmi H, Takeuchi O, Kawai T, et al. A Toll-like receptor recognizes bacterial DNA. Nature 2000;408(6813):740–5. [DOI] [PubMed] [Google Scholar]
- 42.O’Malley BW Jr, Li D, McQuone SJ, Ralston R. Combination nonviral interleukin-2 gene immunotherapy for head and neck cancer: from bench top to bedside. Laryngoscope 2005;115(3):391–404. [DOI] [PubMed] [Google Scholar]
- 43.Gilboa E, Nair SK, Lyerly HK. Immunotherapy of cancer with dendritic-cell-based vaccines. Cancer Immunol Immunother 1998;46(2):82–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sidransky D, Von Eschenbach A, Tsai YC, et al. Identification of p53 gene mutations in bladder cancers and urine samples. Science 1991;252(5006):706–9. [DOI] [PubMed] [Google Scholar]
- 45.Souders NC, Sewell DA, Pan ZK, et al. Listeria-based vaccines can overcome tolerance by expanding low avidity CD8+ T cells capable of eradicating a solid tumor in a transgenic mouse model of cancer. Cancer Immunol 2007;7:2. [PMC free article] [PubMed] [Google Scholar]
- 46.Borysiewicz LK, Fiander A, Nimako M, et al. A recombinant vaccinia virus encoding human papillomavirus types 16 and 18, E6 and E7 proteins as immunotherapy for cervical cancer. Lancet 1996;347(9014):1523–7. [DOI] [PubMed] [Google Scholar]
- 47.Karcher J, Dyckhoff G, Beckhove P, et al. Antitumor vaccination in patients with head and neck squamous cell carcinomas with autologous virus-modified tumor cells. Cancer Res 2004;64(21):8057–61. [DOI] [PubMed] [Google Scholar]
- 48.Ferris R, Hunt J, Ferrone S. Human leukocyte antigen (HLA) class I defects in head and neck cancer: molecular mechanisms and clinical significance. Immunol Res 2005;33(2):113–33. [DOI] [PubMed] [Google Scholar]
- 49.Lopez-Albaitero A, Nayak JV, Ogino T, et al. Role of antigen-processing machinery in the in vitro resistance of squamous cell carcinoma of the head and neck cells to recognition by CTL. J Immunol 2006;176(6):3402–9. [DOI] [PubMed] [Google Scholar]
- 50.Strauss L, Bergmann C, Gooding W, Johnson JT, Whiteside TL. The frequency and suppressor function of CD4+CD25highFoxp3+ T cells in the circulation of patients with squamous cell carcinoma of the head and neck. Clin Cancer Res 2007;13(21):6301–11. [DOI] [PubMed] [Google Scholar]
- 51.Gabrilovich DI, Chen HL, Girgis KR, et al. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med 1996;2(10):1096–103. [DOI] [PubMed] [Google Scholar]