Abstract
Among thoracic tumors, these include subsets of a relatively newly described and yet to be fully characterized tumor entity: SMARCA4-deficient Undifferentiated Tumor (SMARCA4-dUT). Mutations of SMARCA4 (SWI/SNF-related, matrix-associated, actin-dependent regulator of chromatin, subfamily A, member 4) gene and loss of BRG1 (Brahma-related gene-1) is the underlying molecular hallmark of SMARCA4-dUT. They mostly involved the mediastinum, lung, and/or pleura showing undifferentiated round cell or rhabdoid morphology associated with aggressive clinical behavior. The pathogenesis of these tumors is still not clear. Morphologically, SMARAC4-dUT is differentiated from SMARCA4-dNSCLC by the presence of squamous and solid components in the latter. Immunohistochemically SMARC4-dUT has characteristic loss of SMARCA4 and SMARCA2 and strong expression of SOX2, CD34, and SALL4. Common sites of metastasis include lymph nodes, bones, and adrenal glands but rarely brain metastasis. We present a unique and rare case of a 76-year-old male with a right lung mass with documented pathology of SMARCA4-dUT and was found to have multiple brain metastases.
Keywords: diagnostic testing, hematology oncology, pathology, pulmonary critical care
Introduction
SMARCA4 (SWI/SNF-related, matrix-associated, actin-dependent regulator of chromatin, subfamily A, member 4) gene and loss of BRG1 (Brahma-related gene-1) occurs in approximately 5% of non-small cell lung carcinomas (NSCLCs). 1 In 2015, Le Loarer et al 2 reported a distinct subset of highly aggressive thoracic sarcomas, occurring in mostly young males, with undifferentiated immunophenotype and SMARCA4 loss associated with a very aggressive clinical course and named it as SMARCA4-deficient thoracic sarcoma (SMARCA4-dTS). Lately other than sarcomas, loss of SMARCA4/BRG1 has been associated with another subset of poorly differentiated/undifferentiated carcinomas in adults arising in a wide range of anatomical sites, including the lung3 -6 and are called SMARCA4-dUT. They present in all clinical stages as primary lung parenchymal masses ranging from well-differentiated adenocarcinoma to poorly differentiated malignant tumors. 7 The new 2021 WHO Classification of Tumors of the Lung, Pleura, Thymus, and Heart will use the term “SMARCA4-deficient undifferentiated tumor (SMARCA4-dUT)” for the group of tumors previously described as SMARCA4-dTS, which is considered a separate entity from SMARCA4-deficient NSCLC. 7 Most of the literature documented the metastasis to lymph node (59%-91%), adrenal gland (27%-48%), bone (24%-55%), and lungs (29%) at the time of presentation with no brain metastases.8,9 There was mention of a case in one of the studies done by Sauter et al 10 that presented with seizure and 1.4 cm lung mass and was found to have SMARAC4-dTS with loss of BRG1, BRM but retained INI-1. However, no supportive data were available. We present a unique and rare case of a 76-year-old male with a right lung mass and documented pathology of SMARCA4-dUT with multiple brain metastases evident on MRI (Magnetic Resonance Imaging) brain with contrast study.
Case Presentation
A 76-year-old African American male, chronic heavy active smoker, with a medical history of hypertension, chronic obstructive lung disease, chronic diastolic heart failure presented to the hospital with a complaint of sudden onset right upper extremity weakness. At baseline, he ambulated with the assistance of a cane. His family and surgical history were insignificant. He was admitted for a possible cerebrovascular accident (CVA), and physical examination revealed 3/5 motor weakness in the right upper extremity with the rest of the examination showing no abnormalities. Routine blood work showed white blood cell 5.2 (4.1-10.1 × 1000/µL), hemoglobin 12.9 (12.9-16.7 g/dL), platelet count 241 (153-328 × 1000/uL) blood urea nitrogen 12 (9-20 mg/dL), creatinine 1.5 (0.66-1.25 mg/dL), estimated glomerular filtration rate 60 mL/min, serum protein 7.1 g/dL (6.3-8.2 g/dL). The imaging included MRI brain (Figure 1A, B) and CT (Computer Tomography) scan head that revealed an 8 mm round hyperdense area in the high left frontal white matter with surrounding edema and 2 perhaps 3 directly adjacent peripherally enhancing lesions in the left occipital lobe measuring up to 8 to 10 mm. CT Chest abdomen pelvis without contrast showed 1.8 × 1.7 cm subpleural irregular mass in the anterior right upper lobe with multiple smaller irregular nodules in the distribution of the right middle lobe and enlarged right hilar and mediastinal lymph nodes, with moderate size right pleural effusion (Figure 2). On questioning the patient, he stated that he was aware of this lung mass detected 8 years ago as incidental findings on the CT chest but did not follow up with any physician. Pulmonology evaluated the patient and thoracentesis was done for pleural effusion. Pleural studies showed evidence of mild to moderate predominantly chronic inflammation scattered among histiocytic and mesothelial cells with moderate cellularity noted with no malignant cells. The patient was scheduled for CT-guided biopsy of lung mass, however, left against medical advice. Subsequently, after 1 month he again presented to the hospital with a fall as he had the feeling of his legs giving away. He denied hitting his head or any loss of consciousness. CT head this time showed the same mass in the high left frontal lobe at the vertex and other smaller enhancing masses in the left occipital lobe. MRI brain showed a demonstration of the mass seen on the prior MRI. CT-guided 20G core biopsy of right middle lobe lung nodule was done and sent for pathology. As per the initial preliminary pathology report, the diagnosis of poorly differentiated carcinoma was made but not confirmed whether it was small cell or non-small cell lung cancer given the tumor was necrotic and markers could not be identified. Awaiting the final conclusive pathology report, the decision was taken to treat all the lesions of brain metastasis with IMRT (Intensity-Modulated Radiation Therapy). The final pathology was sent to Sloan Kettering pathology department and finally diagnosed as SMARCA4-deficient undifferentiated tumor of the lung mass (Figure 3). The report revealed a solid and variable cohesive proliferation of malignant epithelioid tumor cells in a background of fibrosis and chronic inflammation (Figure 4A, B). The tumor cells are medium-sized and contain moderate amounts of eosinophilic cytoplasm and have enlarged nuclei with granular chromatin and irregular contours. A subset of tumor cells shows focal rhomboid features with central eosinophilic intracytoplasmic inclusions and eccentrically placed nuclei. Mitotic figures are frequently identified. Immunohistochemical (IHC) stains showed that the tumor cells are positive for CK7 (weak, focal), synaptophysin, but negative for cytokeratin AE1/AE3, TTF-1, p63, CK5/6, and chromogranin. Ki-67 shows a proliferative index of 90% within the tumor cells. IHC stains performed at MSKCC (Memorial Sloan Kettering Cancer Center, New York) shows that the tumor cells are positive for cytokeratin OSCAR, CD34 (focal), SALL4, and SOX2, but negative for claudin-4, INSMI, CD56, NUT, and SOX10. BRGI/SMARCA4 and BRM/SMARCA2 are lost in the tumor cells as shown by the IHC stain in Figure 5A, B, respectively. Given the predominantly undifferentiated morphology and the presence of focal rhabdoid features, consider the possibility of SMARCA4-deficient undifferentiated tumor. Here, we show that the tumor lacks expression for BRGI/SMARCA2, thus supporting the diagnosis of SMARCA4-deficient undifferentiated tumor. In addition, expression of stem cell markers (e.g., CD34, SOX, and SALL4) reduced keratin staining, and lack of claudin-4 further support this consideration. Synaptophysin is another common finding.
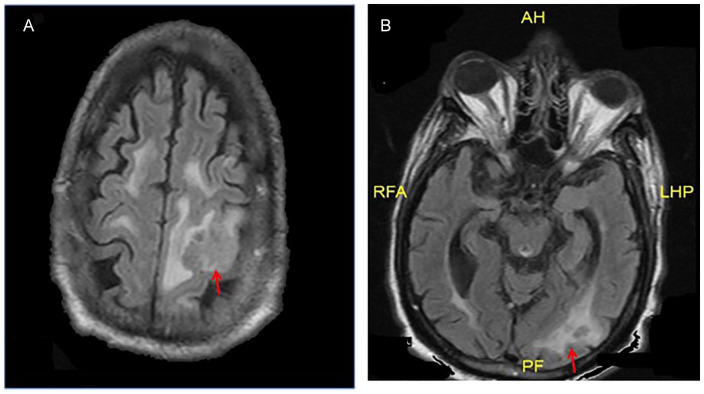
Figure 1.
(A, B). MRI brain with & without contrast: T1/T2 weighted signal mass identified in the high left frontoparietal lobe, based on the FLAIR (fluid-attenuated inversion recovery) images measured roughly 2.7 × 2.7 cm, previously 2.4 × 1.7 cm with surrounding edema. In addition, 2 perhaps 3 directly adjacent peripherally enhancing lesions in the left occipital lobe which are measuring up to 8 to 10 mm. (AH, RFA, LHP and PF are not related/relevant in the figure)
Figure 2.
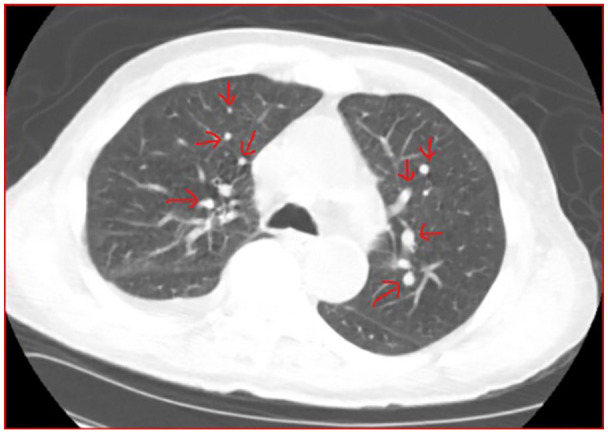
CT scan of the chest/Axial plane soft tissue window shows a 2 cm subpleural irregular mass in the anterior right upper lobe with multiple smaller irregular nodules (shown in red arrows) in the distribution of the right middle lobe and moderate right pleural effusion.
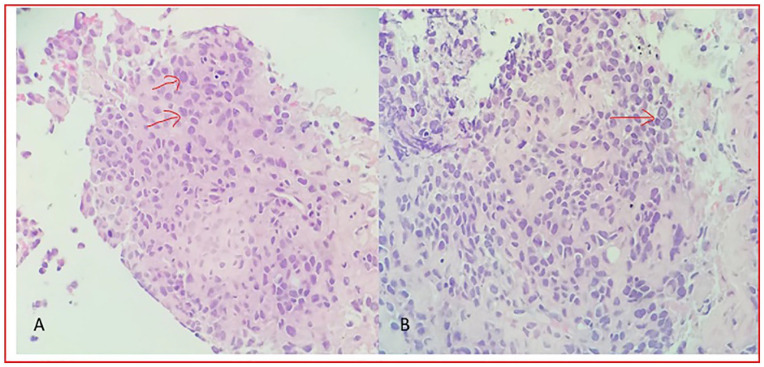
Figure 3.
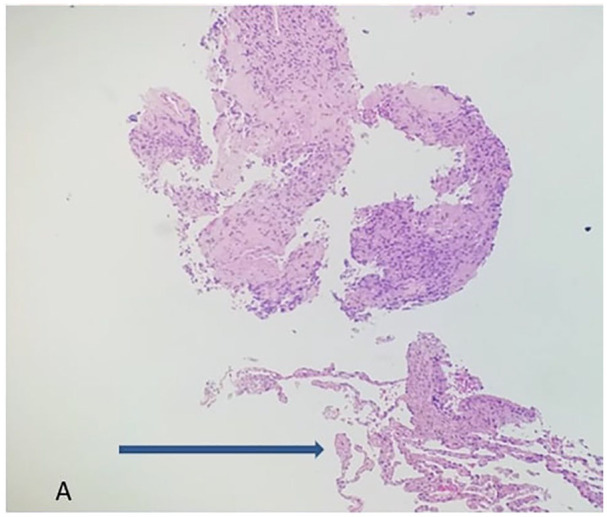
Microscopy of the lung, right middle lobe biopsy revealed scant normal lung shown by the arrow and mostly tumor on the above (hematoxylin-eosin, original magnifications × 10[A].
Figure 4.
Microscopy of lung, right middle lobe biopsy: revealed cohesive proliferation of malignant epithelioid tumor cells (shown in red arrows) in a background of fibrosis (A) and chronic inflammation (B) (hematoxylin-eosin, original magnifications × 40 [A] and 40 [B].
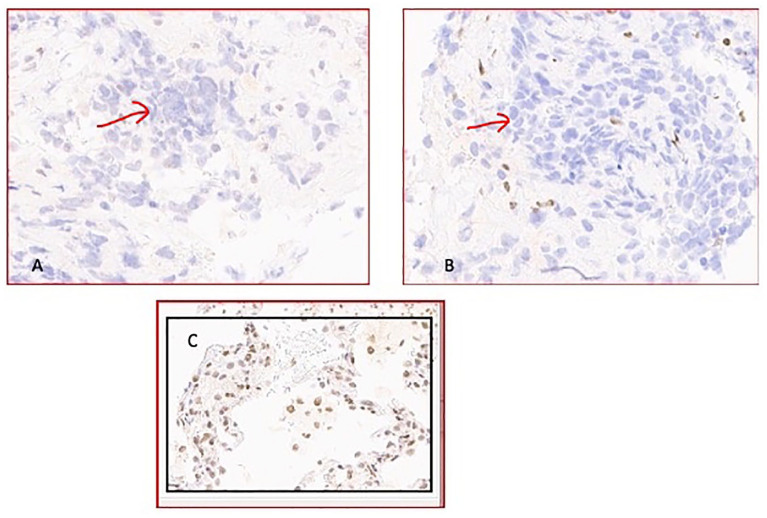
Figure 5.
(A) Loss of SMARCA4/BRG1 demonstrated by IHC stain (tumor cells shown in red arrow), original magnification × 20. (B) Loss of SMARCA2/BRM demonstrated by IHC stain (tumor cells shown in red arrow) original magnification × 20. (C) Positive controls of lung tissue.
Abbreviations: SMARCA4, SWI/SNF-related, matrix-associated, actin-dependent regulator of chromatin, subfamily A, member 4; BRG1, Brahma-related gene-1; IHC, immunohistochemical; BRM, Brahma.
The patient was scheduled for one cycle of systemic chemotherapy with carboplatin, and etoposide however was transferred to another institution for further treatment and lost to follow-up.
Discussion
Thoracic SMARCA4-dUT (formerly called “SMARCA4-deficient thoracic sarcoma/ SMARCA4-dTS”) are aggressive tumors found commonly in the mediastinum of young male smokers.8,11 The hallmark of this newly described and better characterized entity is loss of Brahma (BRM), encoded by SMARCA2 (SWI/SNF-related, matrix-associated, actin-dependent regulator of chromatin, subfamily A, member 2), or Brahma-related gene-1 (BRG1), encoded by SMARCA4 (SWI/SNF-related, matrix-associated, actin-dependent regulator of chromatin, subfamily A, member 4), respectively.3,6 The tumor typically affects younger patients in the age group of 30 to 59 years with a male preponderance of 9:1.11,12 The common site of involvement is the mediastinum followed by the pleura and lung. 7 Morphologically thoracic SMARCA4-dUT appear as diffuse sheets of poorly differentiated cells that are monotonous, ovoid with abundant eosinophilic cytoplasm and prominent nucleoli. 10 Tumor cells grow in a dis-cohesive pattern with interspersed rhabdoid and hepatoid morphology, brisk mitotic activity, and extensive necrosis.13,14
Immunohistochemical diagnosis is made by the loss of SMARAC4 and SMARCA2 with strong expression of SOX2, CD34, and SALL4.2,11,15 The expression of SOX2, CD34, and SALL4 helps to differentiate Thoracic SMARCA4-dUT from SMARCA4-dNSCLC. 6 SMARCA4-dUT are negative for desmin, NUT, S100, WT-1, and p4011,12 also seen in our patient. Inactivating mutations of SMARCA4 were identified in all SMARCA4-dUT with no evidence of any germline mutation. 16 Loss of SMARCA4 leads to overexpression of MYC and undifferentiated gene expression. 17
Metastasis is documented in bones, adrenal glands, and lungs, a pattern similar to NSCLC however unlike NSCLC brain metastases are a rare occurrence.8,10,15 After extensive research through several resources available (PUBMED, COCHRANE, Google scholar, web med), there was mention of one case report of SMARCA4-dUT by Sauter et al 10 that had 1.4 cm lung mass with brain metastasis and seizures; however, no supportive data is available. Our case is unique concerning advanced age presentation, mediastinal lung mass with multiple brain metastasis presenting as upper extremity weakness. Symptoms are based on the extent of disease and site of metastasis as mostly present in advanced stages with common symptoms of chest pain, dyspnea, bone pain, and in rare instances as seizures or extremity weakness.10,11 However, the aggressive phenotype described in the literature may likely be somewhat skewed by observation of the younger male patients, because of our case presented patient diagnosed at an older age and an 8 years history of lung mass with smaller growth but at 8 years later, the diagnosis of multiple brain metastasis.
To date, less than 100 cases of SMARCA4-UT have been reported. 1 The most extensively studied project on SMARCA4-deficient tumor is small cell carcinoma of the ovary-hypercalcemic type (SCCOTH) that has overlapping morphologic, immunophenotypic, and molecular features with thoracic SMARCA4-DUT. 18 Patients with SMARCA4-dUT have an overall median survival of 4 to 7 months (range 1-13 months), 8 with 2 years overall survival being 12.5%. 10 Rapid disease progression and relapse occur in essentially all patients with most of them succumbing to death due to local complications.2,8 Limited treatment modalities are available with inadequate response to chemotherapy and surgery; however, few case reports showed that SMARCA4-dNSCLC may benefit from treatment with immune checkpoint inhibitors 19 or platinum-based chemotherapy. 20 Immunotherapy especially Pembrolizumab may also benefit SMARCA4-dUT with PD-L1 expression, especially after failed chemotherapy.9,21 Promising clinical trials with inhibitors of Enhancer of Zeste Homolog 2 histone methyltransferase (EZH2 inhibitor) are underway for tumors related to the SWI/SNF complex (NCT03213665; NCT02875548; NCT02601950) emphasizing the importance of an accurate diagnosis of this tumor.2,22 EZH2 Inhibitors have shown activity linked to SMARCB1 or dual SMARCA4/A2 inactivation that has gained attention in recent times as potential therapeutic agents for SMARCA4-dUT.23,24 BETi (bromodomain and extra-terminal motif protein inhibitors) have been used as a phase 1 trial in the treatment of multiple myeloma, acute leukemia, and NUT midline carcinoma and time would tell of their therapeutic benefits in SMARCA4-dUT.25,26
Conclusion
SMARCA4-dUT is a distinctive clinicopathological entity with undifferentiated rhabdoid morphology, loss of BRG1/BRM, and aggressive behavior. It is still unclear whether they represent undifferentiated NSCLC or BRG1/BRM deficient rhabdoid tumors. Brain metastasis is very rarely seen with SMARCA4-dUT unlike NSCLC and affects the overall prognosis. The accurate diagnosis needs morphological, immunohistochemical, and if feasible molecular testing of these uncommon tumors, to differentiate between similar presenting entities namely SMARCA4-dUT, SMARCA4-dNSCLC, and NUT carcinomas. Our case report depicts the expanding spectrum of this recently defined clinical entity. Despite the lack of effective treatment modalities, definitive diagnosis of these lethal tumors is necessary to have the availability of better therapeutic options and improve the overall prognosis by early detection.
Acknowledgments
Immunohistochemical (IHC) stains and a final review of pathology slides were performed at MSKCC (Memorial Sloan Kettering Cancer Center, New York).
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval: Ethics approval to report this case was obtained from Brookdale Hospital Institutional Review Board.
Informed Consent: Informed consent for patient information to be published in this article was obtained.
ORCID iD: Jen C. Wang  https://orcid.org/0000-0002-9623-6645
https://orcid.org/0000-0002-9623-6645
References
- 1. Nambirajan A, Jain D. Recent updates in thoracic SMARCA4-deficient undifferentiated tumor. Semin Diagn Pathol. 2021;38(5):83-89. doi: 10.1053/j.semdp.2021.06.001. [DOI] [PubMed] [Google Scholar]
- 2. Le Loarer F, Watson S, Pierron G, et al. SMARCA4 inactivation defines a group of undifferentiated thoracic malignancies transcriptionally related to BAF-deficient sarcomas. Nat Genet. 2015;47(10):1200-1205. doi: 10.1038/ng.3399. [DOI] [PubMed] [Google Scholar]
- 3. Herpel E, Rieker RJ, Dienemann H, et al. SMARCA4 and SMARCA2 deficiency in non-small cell lung cancer: immunohistochemical survey of 316 consecutive specimens. Ann Diagn Pathol. 2017;26:47-51. doi: 10.1016/j.anndiagpath.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 4. Agaimy A, Fuchs F, Moskalev EA, Sirbu H, Hartmann A, Haller F. SMARCA4-deficient pulmonary adenocarcinoma: clinicopathological, immunohistochemical, and molecular characteristics of a novel aggressive neoplasm with a consistent TTF1neg/CK7pos/HepPar-1pos immunophenotype. Virchows Arch Int J Pathol. 2017;471(5):599-609. doi: 10.1007/s00428-017-2148-5. [DOI] [PubMed] [Google Scholar]
- 5. Matsubara D, Kishaba Y, Ishikawa S, et al. Lung cancer with loss of BRG1/BRM, shows epithelial mesenchymal transition phenotype and distinct histologic and genetic features. Cancer Sci. 2013;104(2):266-273. doi: 10.1111/cas.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Naito T, Umemura S, Nakamura H, et al. Successful treatment with nivolumab for SMARCA4-deficient non-small cell lung carcinoma with a high tumor mutation burden: a case report. Thorac Cancer. 2019;10(5):1285-1288. doi: 10.1111/1759-7714.13070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chatzopoulos K, Boland JM. Update on genetically defined lung neoplasms: NUT carcinoma and thoracic SMARCA4-deficient undifferentiated tumors. Virchows Arch. 2021;478(1):21-30. doi: 10.1007/s00428-020-03011-3. [DOI] [PubMed] [Google Scholar]
- 8. Crombé A, Alberti N, Villard N, et al. Imaging features of SMARCA4-deficient thoracic sarcomas: a multi-centric study of 21 patients. Eur Radiol. 2019;29(9):4730-4741. doi: 10.1007/s00330-019-06017-x. [DOI] [PubMed] [Google Scholar]
- 9. Takada K, Sugita S, Murase K, et al. Exceptionally rapid response to pembrolizumab in a SMARCA4-deficient thoracic sarcoma overexpressing PD-L1: a case report. Thorac Cancer. 2019;10(12):2312-2315. doi: 10.1111/1759-7714.13215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sauter JL, Graham RP, Larsen BT, Jenkins SM, Roden AC, Boland JM. SMARCA4-deficient thoracic sarcoma: a distinctive clinicopathological entity with undifferentiated rhabdoid morphology and aggressive behavior. Mod Pathol. 2017;30(10):1422-1432. doi: 10.1038/modpathol.2017.61. [DOI] [PubMed] [Google Scholar]
- 11. Rekhtman N, Montecalvo J, Chang JC, et al. SMARCA4-deficient thoracic sarcomatoid tumors represent primarily smoking-related undifferentiated carcinomas rather than primary thoracic sarcomas. J Thorac Oncol. 2020;15(2):231-247. doi: 10.1016/j.jtho.2019.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Perret R, Chalabreysse L, Watson S, et al. SMARCA4-deficient thoracic sarcomas: clinicopathologic study of 30 cases with an emphasis on their nosology and differential diagnoses. Am J Surg Pathol. 2019;43(4):455-465. doi: 10.1097/PAS.0000000000001188. [DOI] [PubMed] [Google Scholar]
- 13. Chetty R, Serra S. SMARCA family of genes. J Clin Pathol. 2020;73(5):257-260. doi: 10.1136/jclinpath-2020-206451. [DOI] [PubMed] [Google Scholar]
- 14. Matsushita M, Kuwamoto S. Cytologic features of SMARCA4-deficient thoracic sarcoma: a case report and comparison with other SWI/SNF complex-deficient tumors. Acta Cytol. 2018;62(5-6):456-462. doi: 10.1159/000493335. [DOI] [PubMed] [Google Scholar]
- 15. Yoshida A, Kobayashi E, Kubo T, et al. Clinicopathological and molecular characterization of SMARCA4-deficient thoracic sarcomas with comparison to potentially related entities. Mod Pathol. 2017;30(6):797-809. doi: 10.1038/modpathol.2017.11. [DOI] [PubMed] [Google Scholar]
- 16. Schaefer I-M, Cote GM, Hornick JL. Contemporary sarcoma diagnosis, genetics, and genomics. J Clin Oncol. 2018;36(2):101-110. doi: 10.1200/JCO.2017.74.9374. [DOI] [PubMed] [Google Scholar]
- 17. Romero OA, Setien F, John S, et al. The tumour suppressor and chromatin-remodelling factor BRG1 antagonizes Myc activity and promotes cell differentiation in human cancer. EMBO Mol Med. 2012;4(7):603-616. doi: 10.1002/emmm.201200236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Karnezis AN, Wang Y, Ramos P, et al. Dual loss of the SWI/SNF complex ATPases SMARCA4/BRG1 and SMARCA2/BRM is highly sensitive and specific for small cell carcinoma of the ovary, hypercalcaemic type. J Pathol. 2016;238(3):389-400. doi: 10.1002/path.4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schoenfeld AJ, Bandlamudi C, Lavery JA, et al. The genomic landscape of SMARCA4 alterations and associations with outcomes in patients with lung cancer. Clin Cancer Res. 2020;26(21):5701-5708. doi: 10.1158/1078-0432.CCR-20-1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bell EH, Chakraborty AR, Mo X, et al. SMARCA4/BRG1 is a novel prognostic biomarker predictive of cisplatin-based chemotherapy outcomes in resected non-small cell lung cancer. Clin Cancer Res. 2016;22(10):2396-2404. doi: 10.1158/1078-0432.CCR-15-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Henon C, Blay J-Y, Massard C, et al. Long lasting major response to pembrolizumab in a thoracic malignant rhabdoid-like SMARCA4-deficient tumor. Ann Oncol. 2019;30(8):1401-1403. doi: 10.1093/annonc/mdz160. [DOI] [PubMed] [Google Scholar]
- 22. Chan-Penebre E, Armstrong K, Drew A, et al. Selective killing of SMARCA2- and SMARCA4-deficient small cell carcinoma of the ovary, hypercalcemic type cells by inhibition of EZH2: in vitro and in vivo preclinical models. Mol Cancer Ther. 2017;16(5):850-860. doi: 10.1158/1535-7163.MCT-16-0678. [DOI] [PubMed] [Google Scholar]
- 23. Orlando KA, Nguyen V, Raab JR, Walhart T, Weissman BE. Remodeling the cancer epigenome: mutations in the SWI/SNF complex offer new therapeutic opportunities. Expert Rev Anticancer Ther. 2019;19(5):375-391. doi: 10.1080/14737140.2019.1605905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Januario T, Ye X, Bainer R, et al. PRC2-mediated repression of SMARCA2 predicts EZH2 inhibitor activity in SWI/SNF mutant tumors. Proc Natl Acad Sci U S A. 2017;114(46):12249-12254. doi: 10.1073/pnas.1703966114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Armon S, Hofman P, Ilié M. Perspectives and issues in the assessment of SMARCA4 deficiency in the management of lung cancer patients. Cells. 2021;10(8):1920. doi: 10.3390/cells10081920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shorstova T, Marques M, Su J, et al. SWI/SNF-compromised cancers are susceptible to bromodomain inhibitors. Cancer Res. 2019;79(10):2761-2774. doi: 10.1158/0008-5472.CAN-18-1545. [DOI] [PubMed] [Google Scholar]