Abstract
Background
Physical activity is known to have anti-cancer effects, including immunomodulatory actions. This study investigated the hypothesis that physical activity synergizes with combined lenvatinib plus anti-PD-1 therapy to enhance efficacy in patients with unresectable HCC.
Methods
The physical activity levels of patients with unresectable HCC receiving combined lenvatinib plus anti-PD-1 therapy were recorded by questionnaire. Patients were categorized according to physical activity levels (active vs. sedentary). The primary outcome was overall survival (OS). Secondary outcomes included objective response rate (ORR) and progression-free survival (PFS). A subcutaneous syngeneic HCC model was generated in C57BL/6 mice. Mice were randomized to receive placebo, combined lenvatinib plus anti-PD-1 antibodies or combination therapy plus physical activity. Tumors were measured every 3 days and harvested for immunohistochemistry analysis at 20 mm maximum diameter.
Results
Fifty-nine patients with unresectable HCC were categorized to active (n = 28) or sedentary (n = 31) groups. The active group had higher albumin and des-γ-carboxy prothrombin levels and lower hepatitis B virus load at baseline; other clinical and oncologic characteristics were comparable between the two groups. Patients in the active group had significantly longer OS (HR = 0.220, 95% CI 0.060–0.799) and PFS (HR = 0.158, 95% CI 0.044–0.562) and higher ORR (OR = 4.571, 95% CI 1.482–14.102) than patients in the sedentary group. Regular physical activity was independently associated with OS, PFS and ORR. The mouse model showed that physical activity significantly suppressed tumor growth and prolonged survival of tumor-bearing mice. Furthermore, physical activity inhibited Treg cell infiltration and immune checkpoint expression (including CTLA4, TIGIT and TIM3) induced by long-term combined lenvatinib plus anti-PD-1 therapy, improving efficacy.
Conclusions
Regular physical activity was associated with improved outcomes in unresectable HCC receiving combined lenvatinib plus anti-PD-1 therapy. Physical activity may improve therapeutic efficacy by reprograming the tumor microenvironment from an immunosuppressive to immunostimulatory phenotype.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40164-022-00275-0.
Keyword: Hepatocellular carcinoma, Physical activity, Anti-PD-1, Lenvatinib
Background
Primary liver cancer is the fifth most commonly diagnosed cancer and second leading cause of cancer-related death in China [1]. Hepatocellular carcinoma (HCC) represents 75–85% of primary liver cancer and is a major global health problem [2]. The majority of patients with HCC are diagnosed at an advanced stage, resulting in limited treatment options and poor prognosis [3]. Systemic therapy, including sorafenib, lenvatinib, and immune check point blockade (ICB), should be considered in patients with unresectable HCC. However, none of these treatment options have achieved satisfactory efficacy when used as monotherapy in published trials [4–8]. Recently, a phase 1b trial testing the combination of lenvatinib and pembrolizumab has shown encouraging results, with a response rate of 36% reported [9]. Nevertheless, adaptive resistance is still one of the major hinderances for those patients.
Upregulation of alternative immune checkpoints is considered one mechanism underlying adaptive immune resistance [10]. When resistance to anti-programmed cell death protein 1 (anti-PD-1)/anti-programmed cell death ligand 1 (anti-PD-L1) therapy develops, the proportion of T cells expressing alternative immune checkpoints, including cytotoxic T-lymphocyte-associated protein 4 (CTLA4) and T-cell immunoglobulin mucin-3 (TIM3), increases [11, 12]. Regulatory T (Treg) cells play a pivotal role in both maintaining immune homeostasis and tumor immune escape [13]. Moreover, PD-1 blockade significantly enhances the infiltration of immunosuppressive Treg cells, contributing to adaptive resistance to immunotherapy [14]. It is reported that lenvatinib reduces Treg cell infiltration and activates immune pathways, resulting in reprograming of the tumor microenvironment, which may contribute to improvement of the efficacy of anti-PD-1 therapy [15, 16]. Suppressing the infiltration of Treg cells and alternative immune checkpoints level is therefore an attractive strategy to improve the therapeutic efficacy of combined lenvatinib and anti-PD-1 antibody (Ab) therapy.
Regular physical activity is associated with a lower risk of developing several cancers, including HCC [17, 18]. In addition, physical activity reduces the risk of total deaths and cancer-related deaths in both colon and breast cancer [19–21]. Physical activity may reduce tumor cell proliferation, suppress epithelial-mesenchymal transition (EMT), and promote intra-tumoral perfusion/vascularization [22–24]. Moreover, physical activity elicits anticancer effects by reducing systemic inflammation and countering immunosenescence [25, 26]. We hypothesize that physical activity may synergize with combined lenvatinib plus anti-PD-1 therapy through immunomodulatory effects to enhance the therapeutic efficacy of this treatment regimen.
Methods
Patients
Patients with unresectable HCC treated with combined lenvatinib plus anti-PD-1 antibody at Zhongshan Hospital from June 1, 2018 to September 30, 2020 were included in this retrospective study. Inclusion criteria were as follows: (1) patients met the clinical diagnostic criteria for HCC [27], with or without pathological diagnosis; (2) HCC was unresectable and not suitable for transarterial chemoembolization (TACE); (3) treatment with lenvatinib combined with anti-PD-1 antibody was given as first-line systemic therapy; and, (4) an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1. Patients were excluded if they: (1) had other malignancies; (2) received previous systemic treatment; (3) had no tumor evaluation after initiation of the combined treatment regimen; (4) had incomplete basic information; (5) were unable to specify physical activity details. This study was approved by the Hospital Research Ethics Committee. Informed consent was obtained from all patients.
Grouping and measurement of physical activity
Patients took regular physical activity during combined lenvatinib plus anti-PD-1 treatment were classified into the active group, otherwise they were classified into the sedentary group. Physical activity was measured by questionnaire as previously described [19, 28, 29]. Patients or their immediate family members were questioned by phone survey in December, 2020. They were asked the following questions: (1) Does patient take any leisure time physical activity during pharmacotherapy? (2) What kind of physical activity does patient usually take? (brisk-walk, swimming, ball games, Tai Chi, or other aerobic activity) (3) How many days per week does patient take leisure time activity? (4) What is patient’s total time (minutes) of leisure time physical activity per day? (5) On a scale of 1 to 10, please rate the intensity of patient’s leisure time activity (perceived scale: 1–4 = low intensity, 5–6 = moderate, 7–10 = vigorous). The criteria for regular physical activity were based on the American College of Sports Medicine guidelines [30]. Patients were considered to engage in regular physical activity if they met any of the following criteria: (1) no less than 5 d·wk−1 of moderate aerobic activity for ≥ 30 min·d−1; (2) no less than 3 d·wk−1 of vigorous aerobic activity for ≥ 30 min·d−1; (3) no less than 3–5 d·wk−1 of mixed intensity activity for ≥ 30 min·d−1; or (4) any of the above before or within 1 month after the initiation of combination therapy until 2 months before death or the phone call.
Treatment outcomes
The primary treatment outcome was overall survival (OS). Secondary outcomes included objective response rate (ORR) and progression-free survival (PFS). All outcomes were assessed using magnetic resonance imaging and modified response evaluation criteria in solid tumors (mRECIST) criteria for hepatocellular carcinoma response assessment [31].
Cells and reagents
Hepa1-6 cells, derived from the BW7756 tumor in a C57L mouse, were obtained from the American Type Culture Collection (ATCC) (Manassas, VA, USA). The cells were cultured in high-glucose Dulbecco’s modified eagle medium (DMEM) (BasalMedia Technologies Co., Shanghai, China) supplemented with 10% fetal bovine serum (FBS) (Yeasen Biotechnology Co., Shanghai, China) and 1% penicillin–streptomycin (Yeasen Biotechnology Co., Shanghai, China) at 37 °C under a 5% CO2 atmosphere.
Lenvatinib was obtained from Eisai (Ibaraki, Japan). Anti-mouse PD-1 Ab (anti-PD-1 Ab; clone RMP1- 14) and mouse isotype control IgG (control IgG; clone 2A3) were purchased from Bio X Cell (West Lebanon, NH, USA).
Primary Abs used for immunohistochemical staining included: anti-CD4 Ab (#25229, CST), anti-CD8α Ab (#98941,CST), anti-Forkhead box protein p3 (Foxp3) Ab (ab253297, Abcam), anti-F4/80 Ab (#70076, CST), anti-CTLA4 Ab (A00020, Boster), anti-T cell immunoreceptor with Ig and immunoreceptor tyrosine-based inhibitory motif (ITIM) domains (TIGIT) Ab (A01962, Boster), anti-TIM3 Ab (ab241332, Abcam) and, anti-V domain-containing Ig suppressor of T-cell activation (VISTA) Ab (#54979, CST).
Subcutaneous syngeneic mouse model
A subcutaneous syngeneic HCC model was generated by subcutaneously injecting ~ 3*106 Hep1-6 cells in 100 μl PBS on the backs of 5–6-week-old female C57BL/6 J mice (Charles River Laboratories) (n = 21). Animals were weighed and tumor volume was assessed every three days. Once the maximum diameters reached 10 mm, animals were randomly assigned to receive combination therapy (lenvatinib plus anti-PD-1 Ab; n = 7), combination therapy plus physical activity (n = 7), or placebo (control, drug vehicle plus mouse isotype IgG; n = 7).
Lenvatinib (dissolved in 3 mM HCl, 10 mg/kg) was administered daily by oral gavage [32]. Anti-PD-1 Ab (200 μg/mouse) was intraperitoneally administered every five days [33]. Physical activity was facilitated by placing running wheels in cages; running distance was recorded by electromagnetic sensors in the combination therapy plus physical activity group. Mice were sacrificed and tumors harvested once tumor maximum diameter reached 20 mm. The study was performed in compliance with guidelines for the use of animals established by the institution ethical committee and the “Tumor induction in mice and rats IACUC Guideline” [34].
Immunohistochemical staining and analysis
Immunohistochemical staining was conducted as previously described [35, 36] on tissue microarray. Images were captured using the PANNORAMIC panoramic slice scanner after staining. The Densito quant module in the Quant Center2.1 analysis software was used to quantify the H-Score (H-SCORE = ∑ (PI × I) = (percentage of cells of weak intensity × 1) + (percentage of cells of moderate intensity × 2) + (percentage of cells of strong intensity × 3), where PI represented the percentage of positive signal pixel area; I represented the coloring intensity).
Tumor viability, defined as the proportion of tumors presenting viable cells in a sample (i.e. excluding necrotic regions or granulation tissue), was assessed on hematoxylin and eosin slides. All analyses were performed by an expert pathologist blinded to the treatment arms.
Statistical analysis
All statistical analyses were performed using SPSS (Version 22, Chicago, IL, USA). Continuous variables were compared with the Student’s t-test (equal variances assumed) or Wilcoxon rank sum test (equal variances not assumed). Categorical variables were analyzed by Chi-squared test or Fisher exact test. Kaplan–Meier survival analysis with the log-rank test was used to evaluate the associations between various interventions and survival. Univariate and multivariate logistic regression was used to determine factors affecting ORR. Multivariate Cox regression was used to determine variables associated with survival. For the analysis, a p-value < 0.05 in a two-tailed test was considered statistically significant. Graphs were drawn with Graphpad Prism (Version 8.0.2, San Diego, CA, USA).
Results
Baseline characteristics of patients
Fifty-nine patients were eligible for this study. Twenty-eight patients were classified into the active group and 31 into the sedentary group. Up to June 30, 2021, the median follow-up duration was 13 months (range, 3–28 months). The baseline clinical characteristics are summarized in Table 1. Compared to patients in the sedentary group, patients in the active group had higher albumin and des-γ-carboxy prothrombin (DCP) levels and lower hepatitis B virus (HBV) viral load at baseline (P < 0.05). However, the usage of antiviral drugs showed no significant difference between the two groups (0.796). Other clinical characteristics were comparable between the two groups.
Table 1.
Baseline characteristics
| Variables | Sedentary group (n = 31) | Active group (n = 28) |
P value |
|---|---|---|---|
| Age (years), mean ± SD | 55.9 ± 12.4 | 53.5 ± 8.1 | 0.387 |
| Gender | |||
| Male | 26 (83.9%) | 27 (96.4%) | 0.245 |
| Female | 5 (16.1%) | 1 (3.6%) | |
| PS score | |||
| 0 | 12 (38.7%) | 18 (64.3%) | 0.050 |
| 1 | 19 (61.3%) | 10 (35.7%) | |
| Overweight | |||
| No | 30 (96.8%) | 26 (92.9%) | 0.599 |
| Yes | 1 (3.2%) | 2 (7.1%) | |
| Child–Pugh classification | |||
| A | 30 (96.8%) | 27 (96.4%) | 1.000 |
| B | 1 (3.2%) | 1 (3.6%) | |
| BCLC stage | |||
| A | 1 (3.2%) | 3 (10.7%) | 0.520 |
| B | 7 (22.6%) | 6 (21.4%) | |
| C | 23 (74.2%) | 19 (67.9%) | |
| CNLC stage | |||
| I | 1 (3.2%) | 3 (10.7%) | 0.520 |
| II | 7 (22.6%) | 6 (21.4%) | |
| III | 23 (74.2%) | 19 (67.9%) | |
| Tumor size (cm), mean ± SD | 12.3 ± 5.5 | 10.7 ± 5.8 | 0.351 |
| Extra-hepatic metastasis | |||
| No | 26 (83.9%) | 21 (75.0%) | 0.398 |
| Yes | 5 (16.1%) | 7 (25.0%) | |
| Macrovascular invasion | |||
| No | 11 (35.5%) | 14 (50.0%) | 0.260 |
| Yes | 20 (64.5%) | 14 (50.0%) | |
| WBC (× 109/L), mean ± SD | 5.8 ± 2.0 | 5.7 ± 1.9 | 0.828 |
| NLR, mean ± SD | 3.3 ± 1.6 | 2.7 ± 1.2 | 0.124 |
| TB (μmol/L), mean ± SD | 17.5 ± 9.6 | 16.7 ± 7.6 | 0.753 |
| ALB (g/L), mean ± SD | 38.5 ± 5.8 | 41.6 ± 4.0 | 0.020 |
| ALT (U/L), mean ± SD | 42.0 ± 26.3 | 40.8 ± 24.7 | 0.856 |
| AST (U/L), mean ± SD | 73.7 ± 68.4 | 51.6 ± 34.8 | 0.130 |
| GGT (U/L), mean ± SD | 234.9 ± 223.9 | 183.3 ± 211.8 | 0.368 |
| INR, mean ± SD | 1.2 ± 0.1 | 1.1 ± 0.1 | 0.092 |
| AFP (ng/mL) | |||
| < 400 | 13 (41.9%) | 11 (39.3%) | 0.836 |
| ≥ 400 | 18 (58.1%) | 17 (60.7%) | |
| DCP (mAU/mL) | |||
| < 400 | 3 (9.70%) | 10 (35.7%) | 0.036 |
| ≥ 400 | 28 (90.3%) | 18 (64.3%) | |
| HBsAg | |||
| – | 05 (16.1%) | 6 (21.4%) | 0.602 |
| + | 26 (83.9%) | 22 (78.6%) | |
| Antiviral therapies | |||
| Entecavir | 22 (84.6%) | 18 (81.8%) | 0.796 |
| Tenofovir disoproxil fumarate | 4 (15.4%) | 4 (18.2%) | |
| HBV-DNA (IU/mL) | |||
| ≤ 1000 | 13 (41.9%) | 21 (75.0%) | 0.010 |
| > 1000 | 18 (58.1%) | 7 (25.0%) | |
AFP alpha-fetoprotein ,ALB albumin, ALT alanine transaminase, AST aspartate transaminase, BCLC Barcelona Clinic Liver Cancer, CNLC China Liver Cancer, DCP des-γ-carboxy prothrombin, GGT gamma-glutamyl transpeptidase, HBV hepatitis B virus, HbsAg hepatitis B surface antigen, INR international normalized ratio, NLR neutrophil–lymphocyte ratio, PS performance status, TB total bilirubin, WBC white blood count
With respect to the forms of physical activity performed by patients in the active group, brisk walking was the most common one, accounting for 75.0%. In addition to brisk walk, 4 patients chose jogging (14.3%) and 3 patients chose ball games (3.6%), equipment exercising (3.6%) and swimming (3.6%) respectively (Additional file 2: Table S1).
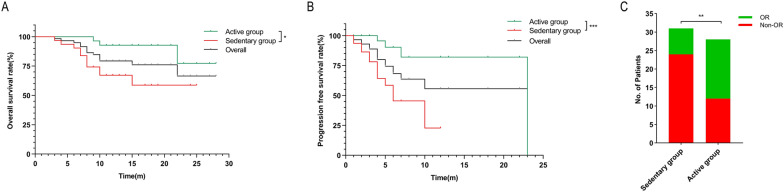
Regular physical activity was associated with improved OS and PFS
Of the 59 patients, 14 died and 17 experienced tumor progression by the last follow-up. The 1- and 2-year OS rates were 92.7% vs. 67.1% and 77.3% vs. 58.7% in the active group and sedentary group, respectively (P < 0.05, Fig. 1A). The 1-year PFS rate was 82.1% in the active group vs. 23.0% in the sedentary group (P < 0.001, Fig. 1B). The median PFS in the active group was 23 months, longer than that in the sedentary group (6 months, P < 0.001). The results of the univariate analysis are presented in Additional file 3: Table S2. Multivariate Cox regression analysis identified that physical activity was independently associated with improved OS (hazard ratio [HR] = 0.203, 95% confidence interval [CI], 0.052–0.794, P = 0.022) and PFS (HR = 0.158, 95% CI 0.044–0.562, P = 0.004) (Table 2).
Fig. 1.
Regular physical activity was associated with improved outcomes in HCC patients receiving combined therapy. In a cohort of patients with unresectable or advanced HCC treated with lenvatinib plus anti-PD-1 antibody combination, regular physical activity was associated with an improved overall survival (A), progression-free survival (B), and high rate of objective response (C). * P < 0.05, ** P < 0.01, *** P < 0.001. HCC hepatocellular carcinoma, OR objective response
Table 2.
Multivariate analysis of the association between baseline factors and treatment outcomes
| Variables | No. of patients | Overall survival | Progression-free survival | Objective response | |||
|---|---|---|---|---|---|---|---|
| Multivariate Cox regression | Multivariate Logistic regression | ||||||
| HR (95% CI) | P value | HR (95% CI) | P value | OR (95% CI) | P value | ||
| Regular physical activity | |||||||
| Yes | 28 | 0.203 | 0.022 | 0.158 | 0.004 | 4.204 | 0.016 |
| No | 31 | (0.052–0.794) | (0.044–0.562) | (1.302–13.569) | |||
| Macrovascular invasion | |||||||
| Yes | 34 | 5.430 | 0.03 | ||||
| No | 25 | (1.183–24.935 | |||||
| INR | |||||||
| > 1.20 | 15 | 0.189 | 0.049 | ||||
| ≤ 1.20 | 44 | (0.036–0.992) | |||||
CI confidence interval, HR hazard ratio, INR international normalized ratio, OR objective response
Regular physical activity was associated with higher ORR
As of June 30, 2021, the ORR in the active group was 57.1% vs. 22.6% in the sedentary group (P < 0.01, Fig. 1C). The results of the univariate analysis are presented in Additional file 3: Table S1. Univariate and multivariate Logistic regression confirmed that regular physical activity and international normalized ratio (INR) were independently associated with OR in patients treated with combination therapy (odds ratio [OR] = 4.204, 95% CI 1.302–13.569, P = 0.016; OR = 0.189, 95% CI 0.036–0.992, P = 0.049, respectively) (Table 2).
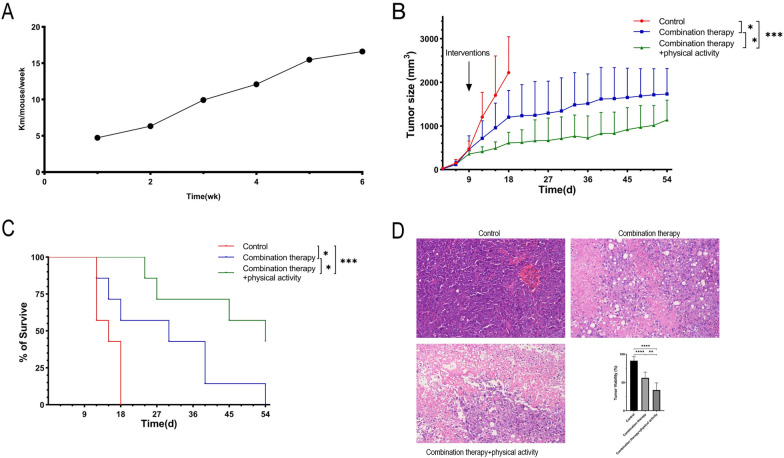
Physical activity improved the effect of combination therapy in syngeneic HCC mice
To elucidate why physical activity improved the outcomes of HCC patients treated with combination therapy, we established a subcutaneous syngeneic HCC mouse model. The average running distance per mouse per week in the combination therapy plus physical activity group is shown in Fig. 2A. Compared to the control group, both combination therapy and combination therapy plus physical activity groups had lower tumor burden (P < 0.05 and P < 0.001, respectively; Fig. 2B). Physical activity synergized with combination therapy to further improve its inhibitory effect on tumor growth (P < 0.05; Fig. 2B).
Fig. 2.
Voluntary running improved the effect of combination therapy in HCC mouse model. A Average running distance per mouse per week in the combination therapy plus physical activity group. B Tumor growth and C survival (time to 20 mm in maximum diameter) of subcutaneously implanted HCC mice. D Tumor viability assessed in Hematoxylin and eosin slides from the subcutaneous HCC mouse model. Representative images captured at 40 ×. * P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. HCC hepatocellular carcinoma
Both combination therapy and combination therapy plus physical activity groups showed prolonged survival (time to 20 mm in maximum diameter) of tumor-bearing mice compared to the control group, and mice in the combination therapy plus physical activity group had the longest lifespan (median survival of 15, 30 and 54 days in control, combination therapy and combination therapy plus physical activity groups, respectively, P < 0.001; Fig. 2C).
Finally, tumor viability was assessed to further understand the synergistic effect of physical activity and combination therapy. The combination therapy plus physical activity group had the lowest tumor viability (P < 0.0001 vs. control group and P < 0.01 vs. combination therapy group), followed by the combination therapy group (P < 0.0001 vs. control group) (Fig. 2D).
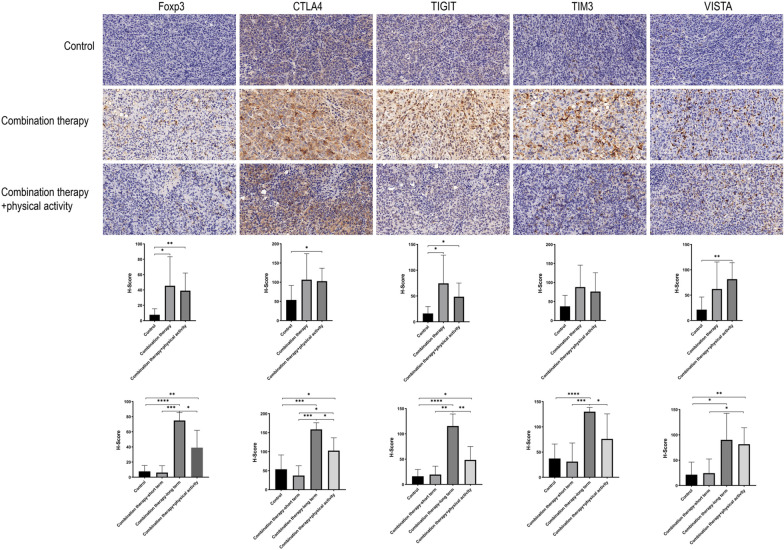
Mechanism of the synergistic effect of physical activity and combination therapy
To elucidate why physical activity improved the efficacy of combination therapy, immunohistochemical staining of major immune cell populations and alternative immune checkpoints was performed. H-scores were generated to quantitatively analyze expression levels among the three groups. Both combination therapy and combination therapy plus physical activity groups showed increased CD4 + T and Treg cell infiltrations and TIGIT expression compared to the control group (Additional file 1: Fig. S1 & Fig. 3). The combination therapy plus physical activity group had elevated immune cell infiltration, including macrophage and CD8 + T cells, and increased CTLA4 and VISTA expression (SAdditional file 1: Fig. S1 & Fig. 3). Compared to the combination therapy group, the combination therapy plus physical activity group tended to exhibit reduced infiltration of Treg cells and expression of several immune checkpoints, including TIGIT and TIM3.
Fig. 3.
Immunohistochemical staining and analysis of subcutaneous tumors. Immunohistochemical staining was conducted on subcutaneous tumor tissue microarray. H-score were calculated to quantify the expression levels of marker proteins. Representative images captured at 40 ×. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Foxp3 forkhead box protein p3, CTLA4 cytotoxic T-lymphocyte-associated protein 4, TIGIT T cell immunoreceptor with Ig and ITIM domains, TIM3 T-cell immunoglobulin mucin-3, VISTA V domain-containing Ig suppressor of T-cell activation
Interestingly, we observed that mice receiving short-term (< 15 days, n = 3) and long-term (≥ 15 days, n = 4) combination therapy showed distinct immunophenotypes. Short-term combination therapy had a similar immunophenotype to the control group, while long-term combination therapy exhibited an immunosuppressive phenotype, with increased infiltration of Treg cells and higher levels of alternative immune checkpoints, including CTLA4, TIGIT, TIM3 and VISTA (Fig. 3). However, the combination therapy plus physical activity group showed reduced infiltration of Treg cells and expression of CTLA4, TIGIT and TIM3 (Fig. 3).
Overall, we observed that physical activity inhibited the infiltration of immune-suppressive Treg cells and the adaptive upregulation of alternative immune checkpoints to enhance the therapeutic efficacy of combination therapy.
Discussion
In this study, we found that regular physical activity was associated with improved outcomes in patients with unresectable HCC receiving combined lenvatinib and anti-PD-1 therapy. Compared with the sedentary population, physically active patients had an approximately 80% lower risk of death and progression and four times greater likelihood of reaching objective response criteria. Considering that patients’ basic general status might influence the intensity and frequency of physical activity, we excluded patients with performance score greater than 2. And multivariate analysis shown that the protective effect of regular physical activity was independent from patients’ basic general status. However, considering the small number of patients choosing physical activities other than brisk walk and the short follow-up period, it was difficult to explore the extent to which different physical activities affect survival. Which form of physical activity provides greater benefit to HCC patients required further research.
Previous treatment may affect the efficacy of physical activity and combination therapy. However, in the present study, lenvatinib plus anti-PD-1 was given as first-line systemic treatment, and only one patient in the active group received TACE before combination therapy. Thus, the effect of previous treatment could be excluded.
Ample studies have shown that physically active lifestyles reduce the risk of multiple cancers, including HCC [17, 18, 37]. Following primary treatment, physical activity has been consistently shown to have positive effects on vigor and vitality, cardiorespiratory fitness, quality of life, depression, anxiety, pain and fatigue; physical activity also reduces the overall risk of death and cancer-related death in cancer survivors [19–21, 38]. During primary treatment with lenvatinib, physical activity might enable patients to receive longer-term treatment through improvement of their physical and psychological condition [38]. Moreover, both physical activity and lenvatinib have immunomodulatory effects [15, 16, 25, 26]. Therefore, physical activity might synergize with lenvatinib to enhance the therapeutic efficacy of anti-PD-1 therapy.
To elucidate the reason why physical activity synergizes with combination therapy, we established a subcutaneously implanted HCC mouse model. Since mice are natural runners [39], running wheels were placed in cages of the combination therapy plus physical activity group to facilitate voluntary running. The mouse model showed that physical activity improved the therapeutic efficacy of combination therapy with retarded tumor growth and prolonged survival, consistent with clinical findings in humans.
We found that both combination therapy and combination therapy plus physical activity were associated with an immunosuppressive tumor microenvironment, which contradicts the findings of previous studies [15, 16]. However, the treatment duration in the present study was much longer (median treatment duration of 30 days) than those reported in previous studies [15, 16]. As short-term and long-term treatments showed distinct immunophenotypes, this may account for the contradictory findings of the present study. It is suggested that the immunostimulatory effect of lenvatinib might decrease with prolonged treatment duration.
Our findings suggest that physical activity enhances combined lenvatinib plus anti-PD-1 therapy by counteracting the immunosuppressive tumor microenvironment induced by long-term treatment. Compared to tumors treated with combination therapy, the addition of physical activity inhibited the infiltration of immunosuppressive Treg cells and the expression of several immune checkpoints, including CTLA4, TIGIT and TIM3, reprogramming the tumor immune microenvironment. Notably, the immunomodulatory function of physical activity during long-term treatment postponed tumor progression and prolonged OS. In addition to the modulation of immune microenvironment, physical activity ameliorates immunosenescence through promoting the secretion of several cytokines by skeletal muscle during physical activity, including IL-6, IL-7 and IL-15 [26]. Similarly, a meta-analysis conducted by Micael Deivison de Jesus Alves, et al. found that long-distance running was associated with increased levels of IL-6, IL-1ra, IL-1β, IL-8, IL-10, and TNF-α, and with decreased levels of IL-2, and IFN-γ [40]. In general, physical activity has immune-promoting effect, which may facilitate the anti-tumor immune response.
In addition to immunomodulatory effects, physical activity has been associated with other anti-cancer mechanisms. It was reported that physical activity suppressed tumor growth by promoting p53-driven apoptosis [41]. Our previous study found that moderate swimming inhibited liver cancer progression through suppression of transforming growth factor-beta-induced epithelial-mesenchymal transition [23]. It was also reported that exercise promoted a shift towards a more “normalized” tumor microenvironment by improving intra-tumoral perfusion/vascularization [42, 43]. Multiple mechanisms may therefore account for the anti-cancer effects of physical activity.
These findings add to the evidence supporting physical activity as an important lifestyle intervention in patients with cancer. In recent years, multiple international organizations have published physical activity recommendations for patients living with and beyond cancer, including the American Cancer Society [44], the American College of Sports Medicine [45], Cancer Care Ontario [46], and the Clinical Oncology Society of Australia [47], and Exercise and Sports Science Australia [48]. The specific value of physical activity in promoting an enhanced treatment response in patients receiving combination lenvatinib plus anti-PD-1 therapy should be explored further in this context.
Our study is not without limitations. Firstly, the retrospective design of the study may have introduced bias, such as recall bias and confounding bias. Secondly, the sample size of this retrospective study is small. Thus, a well-designed prospective study with large sample size is required to control bias and quantify the physical activity level. Thirdly, the PD-1 inhibitors used in this study was not unified. All PD-1 inhibitors were off-label therapies for HCC and cannot be reimbursed in China, which made patient’s choice become an important consideration (mostly cost and updated information from clinical trials). Fourthly, the duration of combination treatment might influence the intra-tumor immunophenotype, which was not accounted for at the time of study conception. Further studies are needed to confirm the effect of duration of combination treatment on the tumor microenvironment.
Conclusions
The present study suggests that physical activity improved the therapeutic efficacy of long-term combined lenvatinib plus anti-PD-1 therapy in patients with HCC through reprograming the tumor microenvironment from an immunosuppressive to immunostimulatory phenotype. This study provides evidence for recommending physically active lifestyles to patients with unresectable HCC receiving combined lenvatinib and anti-PD-1 therapy.
Supplementary Information
Additional file 1: Figure S1. Immunohistochemical staining and analysis of subcutaneous tumors. Immunohistochemical staining was conducted on subcutaneous tumor tissue microarray. H-score were calculated to quantify the expression levels of marker proteins. Representative images captured at 40X. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Additional file 2: Table S1. Distribution of selected physical activities in the active group
Additional file 3: Table S2. Univariate analysis of the association between baseline factors and treatment outcomes. AFP alpha-fetoprotein, ALB albumin, ALT alanine transaminase, AST aspartate transaminase, BCLC Barcelona Clinic Liver Cancer, CI confidence interval, CNLC China Liver Cancer, DCP des-γ-carboxy prothrombin, GGT gamma-glutamyl transpeptidase, HBV hepatitis B virus, HBsAg hepatitis B surface antigen, HR hazard ratio, INR international normalized ratio, NLR neutrophil–lymphocyte ratio, OR odds ratio, PS performance status, TB total bilirubin WBC white blood count.
Acknowledgements
Not applicable.
Abbreviations
- AFP
Alpha-fetoprotein
- ALB
albumin
- ALT
Alanine transaminase
- AST
Aspartate transaminase
- ATCC
American Type Culture Collection
- BCLC
Barcelona Clinic Liver Cancer
- CNLC
China Liver Cancer
- CTLA4
Cytotoxic T-lymphocyte-associated protein 4
- DCP
Des-γ-carboxy prothrombin
- DMEM
Dulbecco’s modified eagle medium
- ECOG
Eastern Cooperative Oncology Group
- FBS
Fetal bovine serum
- Foxp3
Forkhead box protein p3
- GGT
Gamma-glutamyl transpeptidase
- HBV
Hepatitis B virus
- HbsAg
Hepatitis B surface antigen
- HCC
Hepatocellular carcinoma
- IACUC
Institutional Animal Care and Use Committees
- ICB
Immune check point blockade
- INR
International normalized ratio
- ITIM
Immunoreceptor tyrosine-based inhibitory motif
- mRECIST
Modified response evaluation criteria in solid tumors
- NLR
Neutrophil–lymphocyte ratio
- OR
Objective response
- ORR
Objective response rate
- OS
Overall survival
- PD-1
Programmed cell death protein 1
- PDL-1
Programmed cell death ligand 1
- PFS
Progression free survival
- PS
Performance status
- TACE
Transarterial chemoembolization
- TB
Total bilirubin
- TIGIT
T cell immunoreceptor with Ig and ITIM domains
- TIM3
T-cell immunoglobulin mucin-3
- Treg cells
Regulatory T cells
- VISTA
V domain-containing Ig suppressor of T-cell activation
- WBC
White blood count
Author contributions
XFL and XDZ conceived the idea, designed the study, collected the data and wrote the initial draft of the paper. LHF and XLL analyzed the data. BX and KSL conducted the immunohistochemical staining of the tissue microarray and analyzed the results. NX and ML interpreted the results. HCS and ZYT conceived the idea, supervised and supported the study and revise the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the Leading Investigator Program of the Shanghai municipal government (17XD1401100), the National Key Basic Research Program (973 Program; 2015CB554005) from the Ministry of Science and Technology of China, and the National Natural Science Foundation of China (81672326 and 81871928).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Ethical approval was obtained from the Zhongshan Hospital Ethics Committee. Informed consent was obtained from all participants. Animal research was conducted in compliance with guidelines for the use of animals established by the institution ethical committee and the “Tumor induction in mice and rats IACUC Guideline”.
Consent for publication
Not applicable.
Competing interests
HCS has received speaker fees from Hengrui, Bayer, Eisai, and MSD. XDZ has received speaker fees from Eisai and MSD. The other authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xue-Feng Liu and Xiao-Dong Zhu contributed equally to thi work
Contributor Information
Hui-Chuan Sun, Email: sun.huichuan@zs-hospital.sh.cn.
Zhao-You Tang, Email: zytang88@163.com.
References
- 1.Qiu H, Cao S, Xu R. Cancer incidence, mortality, and burden in China: a time-trend analysis and comparison with the United States and United Kingdom based on the global epidemiological data released in 2020. Cancer Commun (Lond) 2021 doi: 10.1002/cac2.12197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–1314. doi: 10.1016/S0140-6736(18)30010-2. [DOI] [PubMed] [Google Scholar]
- 4.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 5.El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling THR, Meyer T, Kang YK, Yeo W, Chopra A, Anderson J, Dela Cruz C, Lang L, Neely J, Tang H, Dastani HB, Melero I. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 7.Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, Breder V, Edeline J, Chao Y, Ogasawara S, Yau T, Garrido M, Chan SL, Knox J, Daniele B, Ebbinghaus SW, Chen E, Siegel AB, Zhu AX, Cheng AL. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020;38(3):193–202. doi: 10.1200/JCO.19.01307. [DOI] [PubMed] [Google Scholar]
- 8.Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, Verslype C, Zagonel V, Fartoux L, Vogel A, Sarker D, Verset G, Chan SL, Knox J, Daniele B, Webber AL, Ebbinghaus SW, Ma J, Siegel AB, Cheng AL, Kudo M. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19(7):940–952. doi: 10.1016/S1470-2045(18)30351-6. [DOI] [PubMed] [Google Scholar]
- 9.Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, Okusaka T, Kobayashi M, Kumada H, Kaneko S, Pracht M, Mamontov K, Meyer T, Kubota T, Dutcus CE, Saito K, Siegel AB, Dubrovsky L, Mody K, Llovet JM. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol. 2020;38(26):2960–2970. doi: 10.1200/JCO.20.00808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qin S, Xu L, Yi M, Yu S, Wu K, Luo S. Novel immune checkpoint targets: moving beyond PD-1 and CTLA-4. Mol Cancer. 2019;18(1):155. doi: 10.1186/s12943-019-1091-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koyama S, Akbay EA, Li YY, Herter-Sprie GS, Buczkowski KA, Richards WG, Gandhi L, Redig AJ, Rodig SJ, Asahina H, Jones RE, Kulkarni MM, Kuraguchi M, Palakurthi S, Fecci PE, Johnson BE, Janne PA, Engelman JA, Gangadharan SP, Costa DB, Freeman GJ, Bueno R, Hodi FS, Dranoff G, Wong KK, Hammerman PS. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun. 2016;7:10501. doi: 10.1038/ncomms10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kates M, Nirschl TR, Baras AS, Sopko NA, Hahn NM, Su X, Zhang J, Kochel CM, Choi W, McConkey DJ, Drake CG, Bivalacqua TJ. Combined next-generation sequencing and flow cytometry analysis for an anti-PD-L1 partial responder over time: an exploration of mechanisms of PD-L1 activity and resistance in bladder cancer. Eur Urol Oncol. 2021;4(1):117–120. doi: 10.1016/j.euo.2019.01.017. [DOI] [PubMed] [Google Scholar]
- 13.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10(7):490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 14.Kamada T, Togashi Y, Tay C, Ha D, Sasaki A, Nakamura Y, Sato E, Fukuoka S, Tada Y, Tanaka A, Morikawa H, Kawazoe A, Kinoshita T, Shitara K, Sakaguchi S, Nishikawa H. PD-1(+) regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer. Proc Natl Acad Sci USA. 2019;116(20):9999–10008. doi: 10.1073/pnas.1822001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yi C, Chen L, Lin Z, Liu L, Shao W, Zhang R, Lin J, Zhang J, Zhu W, Jia H, Qin L, Lu L, Chen J. Lenvatinib targets FGF receptor 4 to enhance antitumor immune response of anti-programmed cell death-1 in HCC. Hepatology. 2021 doi: 10.1002/hep.32023. [DOI] [PubMed] [Google Scholar]
- 16.Torrens L, Montironi C, Puigveh M, Mesropian A, Leslie J, Haber PK. Immunomodulatory effects of lenvatinib plus anti-PD1 in mice and rationale for patient enrichment in hepatocellular carcinoma. Hepatology. 2021;74:2652. doi: 10.1002/hep.32023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruiz-Casado A, Martín-Ruiz A, Pérez LM, Provencio M, Fiuza-Luces C, Lucia A. Exercise and the hallmarks of cancer. Trends Cancer. 2017;3(6):423–441. doi: 10.1016/j.trecan.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 18.Baumeister SE, Schlesinger S, Aleksandrova K, Jochem C, Jenab M, Gunter MJ, Overvad K, Tjønneland A, Boutron-Ruault MC, Carbonnel F, Fournier A, Kühn T, Kaaks R, Pischon T, Boeing H, Trichopoulou A, Bamia C, La Vecchia C, Masala G, Panico S, Fasanelli F, Tumino R, Grioni S, Bueno de Mesquita B, Vermeulen R, May AM, Borch KB, Oyeyemi SO, Ardanaz E, Rodríguez-Barranco M, Dolores Chirlaque López M, Felez-Nobrega M, Sonestedt E, Ohlsson B, Hemmingsson O, Werner M, Perez-Cornago A, Ferrari P, Stepien M, Freisling H, Tsilidis KK, Ward H, Riboli E, Weiderpass E, Leitzmann MF. Association between physical activity and risk of hepatobiliary cancers: a multinational cohort study. J Hepatol. 2019;70(5):885–892. doi: 10.1016/j.jhep.2018.12.014. [DOI] [PubMed] [Google Scholar]
- 19.Meyerhardt JA, Heseltine D, Niedzwiecki D, Hollis D, Saltz LB, Mayer RJ, Thomas J, Nelson H, Whittom R, Hantel A, Schilsky RL, Fuchs CS. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Clin Oncol. 2006;24(22):3535–3541. doi: 10.1200/JCO.2006.06.0863. [DOI] [PubMed] [Google Scholar]
- 20.Meyerhardt JA, Giovannucci EL, Holmes MD, Chan AT, Chan JA, Colditz GA, Fuchs CS. Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol. 2006;24(22):3527–3534. doi: 10.1200/JCO.2006.06.0855. [DOI] [PubMed] [Google Scholar]
- 21.Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293(20):2479–2486. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- 22.Zhu Z, Jiang W, Zacher JH, Neil ES, McGinley JN, Thompson HJ. Effects of energy restriction and wheel running on mammary carcinogenesis and host systemic factors in a rat model. Cancer Prev Res (Phila) 2012;5(3):414–422. doi: 10.1158/1940-6207.CAPR-11-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang QB, Zhang BH, Zhang KZ, Meng XT, Jia QA, Zhang QB, Bu Y, Zhu XD, Ma DN, Ye BG, Zhang N, Ren ZG, Sun HC, Tang ZY. Moderate swimming suppressed the growth and metastasis of the transplanted liver cancer in mice model: with reference to nervous system. Oncogene. 2016;35(31):4122–4131. doi: 10.1038/onc.2015.484. [DOI] [PubMed] [Google Scholar]
- 24.Betof AS, Lascola CD, Weitzel D, Landon C, Scarbrough PM, Devi GR, Palmer G, Jones LW, Dewhirst MW. Modulation of murine breast tumor vascularity, hypoxia and chemotherapeutic response by exercise. J Natl Cancer Inst. 2015 doi: 10.1093/jnci/djv040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turner JE, Brum PC. Does regular exercise counter T cell immunosenescence reducing the risk of developing cancer and promoting successful treatment of malignancies? Oxid Med Cell Longev. 2017;2017:4234765. doi: 10.1155/2017/4234765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duggal NA, Niemiro G, Harridge SDR, Simpson RJ, Lord JM. Can physical activity ameliorate immunosenescence and thereby reduce age-related multi-morbidity? Nat Rev Immunol. 2019;19(9):563–572. doi: 10.1038/s41577-019-0177-9. [DOI] [PubMed] [Google Scholar]
- 27.Xie DY, Ren ZG, Zhou J, Fan J, Gao Q. 2019 Chinese clinical guidelines for the management of hepatocellular carcinoma: updates and insights. Hepatobiliary Surg Nutr. 2020;9(4):452–463. doi: 10.21037/hbsn-20-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolf AM, Hunter DJ, Colditz GA, Manson JE, Stampfer MJ, Corsano KA, Rosner B, Kriska A, Willett WC. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol. 1994;23(5):991–999. doi: 10.1093/ije/23.5.991. [DOI] [PubMed] [Google Scholar]
- 29.Chasan-Taber S, Rimm EB, Stampfer MJ, Spiegelman D, Colditz GA, Giovannucci E, Ascherio A, Willett WC. Reproducibility and validity of a self-administered physical activity questionnaire for male health professionals. Epidemiology. 1996;7(1):81–86. doi: 10.1097/00001648-199601000-00014. [DOI] [PubMed] [Google Scholar]
- 30.Magal MS. Benefits and risks associated with physical activity. In: Deborah JKE, G Liguori, M Magal, eds. Guidelines for exercise testing and prescription. Philadelphia: Lippincott williams wilkins. 2017. p.1–27.
- 31.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60. doi: 10.1055/s-0030-1247132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kimura T, Kato Y, Ozawa Y, Kodama K, Ito J, Ichikawa K, Yamada K, Hori Y, Tabata K, Takase K, Matsui J, Funahashi Y, Nomoto K. Immunomodulatory activity of lenvatinib contributes to antitumor activity in the Hepa1-6 hepatocellular carcinoma model. Cancer Sci. 2018;109(12):3993–4002. doi: 10.1111/cas.13806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang Y, Wang C, Sun H, Jiang Z, Zhang Y, Pan Z. Apatinib prevents natural killer cell dysfunction to enhance the efficacy of anti-PD-1 immunotherapy in hepatocellular carcinoma. Cancer Gene Ther. 2020 doi: 10.1038/s41417-020-0186-7. [DOI] [PubMed] [Google Scholar]
- 34.IACUC. Tumor Induction in mice and rats IACUC Guideline. 2021; https://iacuc.ucsf.edu/guidelines. Accessed 20 October 2021.
- 35.Zhang W, Zhu XD, Sun HC, Xiong YQ, Zhuang PY, Xu HX, Kong LQ, Wang L, Wu WZ, Tang ZY. Depletion of tumor-associated macrophages enhances the effect of sorafenib in metastatic liver cancer models by antimetastatic and antiangiogenic effects. Clin Cancer Res. 2010;16(13):3420–3430. doi: 10.1158/1078-0432.CCR-09-2904. [DOI] [PubMed] [Google Scholar]
- 36.Gao Q, Qiu SJ, Fan J, Zhou J, Wang XY, Xiao YS, Xu Y, Li YW, Tang ZY. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol. 2007;25(18):2586–2593. doi: 10.1200/JCO.2006.09.4565. [DOI] [PubMed] [Google Scholar]
- 37.Leitzmann M, Powers H, Anderson AS, Scoccianti C, Berrino F, Boutron-Ruault MC, Cecchini M, Espina C, Key TJ, Norat T, Wiseman M, Romieu I. European code against cancer 4th edition: physical activity and cancer. Cancer Epidemiol. 2015;39(Suppl 1):S46–55. doi: 10.1016/j.canep.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 38.Jones LW, Demark-Wahnefried W. Diet, exercise, and complementary therapies after primary treatment for cancer. Lancet Oncol. 2006;7(12):1017–1026. doi: 10.1016/S1470-2045(06)70976-7. [DOI] [PubMed] [Google Scholar]
- 39.Melo L, Hagar A. How to train a mouse-methodological issues in pre-clinical exercise oncology. Am J Cancer Res. 2019;9(6):1246–1253. [PMC free article] [PubMed] [Google Scholar]
- 40.Alves MDJ, Silva DDS, Pereira EVM, Pereira DD, de Sousa Fernandes MS, Santos DFC, Oliveira DPM, Vieira-Souza LM, Aidar FJ, de Souza RF. Changes in cytokines concentration following long-distance running: a systematic review and meta-analysis. Front Physiol. 2022;13:838069. doi: 10.3389/fphys.2022.838069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Higgins KA, Park D, Lee GY, Curran WJ, Deng X. Exercise-induced lung cancer regression: mechanistic findings from a mouse model. Cancer. 2014;120(21):3302–3310. doi: 10.1002/cncr.28878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jones LW, Antonelli J, Masko EM, Broadwater G, Lascola CD, Fels D, Dewhirst MW, Dyck JR, Nagendran J, Flores CT, Betof AS, Nelson ER, Pollak M, Dash RC, Young ME, Freedland SJ. Exercise modulation of the host-tumor interaction in an orthotopic model of murine prostate cancer. J Appl Physiol (Bethesda, Md: 1985) 2012;113(2):263–272. doi: 10.1152/japplphysiol.01575.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCullough DJ, Stabley JN, Siemann DW, Behnke BJ. Modulation of blood flow, hypoxia, and vascular function in orthotopic prostate tumors during exercise. J Natl Cancer Inst. 2014;106(4):dju036. doi: 10.1093/jnci/dju036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rock CL, Doyle C, Demark-Wahnefried W, Meyerhardt J, Courneya KS, Schwartz AL, Bandera EV, Hamilton KK, Grant B, McCullough M, Byers T, Gansler T. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62(4):243–274. doi: 10.3322/caac.21142. [DOI] [PubMed] [Google Scholar]
- 45.Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvão DA, Pinto BM, Irwin ML, Wolin KY, Segal RJ, Lucia A, Schneider CM, von Gruenigen VE, Schwartz AL. American college of sports medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42(7):1409–1426. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- 46.Segal R, Zwaal C, Green E, Tomasone JR, Loblaw A, Petrella T. Exercise for people with cancer: a clinical practice guideline. Curr Oncol. 2017;24(1):40–46. doi: 10.3747/co.24.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cormie P, Atkinson M, Bucci L, Cust A, Eakin E, Hayes S, McCarthy S, Murnane A, Patchell S, Adams D. Clinical oncology society of Australia position statement on exercise in cancer care. Med J Aust. 2018;209(4):184–187. doi: 10.5694/mja18.00199. [DOI] [PubMed] [Google Scholar]
- 48.Hayes SC, Newton RU, Spence RR, Galvão DA. The exercise and sports science Australia position statement: exercise medicine in cancer management. J Sci Med Sport. 2019;22(11):1175–1199. doi: 10.1016/j.jsams.2019.05.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Immunohistochemical staining and analysis of subcutaneous tumors. Immunohistochemical staining was conducted on subcutaneous tumor tissue microarray. H-score were calculated to quantify the expression levels of marker proteins. Representative images captured at 40X. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Additional file 2: Table S1. Distribution of selected physical activities in the active group
Additional file 3: Table S2. Univariate analysis of the association between baseline factors and treatment outcomes. AFP alpha-fetoprotein, ALB albumin, ALT alanine transaminase, AST aspartate transaminase, BCLC Barcelona Clinic Liver Cancer, CI confidence interval, CNLC China Liver Cancer, DCP des-γ-carboxy prothrombin, GGT gamma-glutamyl transpeptidase, HBV hepatitis B virus, HBsAg hepatitis B surface antigen, HR hazard ratio, INR international normalized ratio, NLR neutrophil–lymphocyte ratio, OR odds ratio, PS performance status, TB total bilirubin WBC white blood count.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.