Abstract
Despite a relatively low incidence of serious side effects, fluoroquinolones and the fluoroquinolone pefloxacin have been reported to occasionally promote tendinopathy that might result in the complication of spontaneous rupture of tendons. In the present study, we investigated in rodents the intrinsic deleterious effect of pefloxacin (400 mg/kg of body weight) on Achilles tendon proteoglycans and collagen. Proteoglycan synthesis was determined by measurement of in vivo and ex vivo radiosulfate incorporation in mice. Collagen oxidative modifications were measured by carbonyl derivative detection by Western blotting. An experimental model of tendinous ischemia (2 h) and reperfusion (3 days) was achieved in rats. Biphasic changes in proteoglycan synthesis were observed after a single administration of pefloxacin, consisting of an early inhibition followed by a repair-like phase. The depletion phase was accompanied by a marked decrease in the endogenous serum sulfate level and a concomitant increase in the level of sulfate excretion in urine. Studies of ex vivo proteoglycan synthesis confirmed the in vivo results that were obtained. The decrease in proteoglycan anabolism seemed to be a direct effect of pefloxacin on tissue metabolism rather than a consequence of the low concentration of sulfate. Pefloxacin treatment for several days induced oxidative damage of type I collagen, with the alterations being identical to those observed in the experimental tendinous ischemia and reperfusion model. Oxidative damage was prevented by coadministration of N-acetylcysteine (150 mg/kg) to the mice. These results provide the first experimental evidence of a pefloxacin-induced oxidative stress in the Achilles tendon that altered proteoglycan anabolism and oxidized collagen.
Fluoroquinolones are widely used in clinical practice because of their excellent antibacterial activity, wide spectrum of activity, and high degree of bioavailability. These antibiotics are generally considered well tolerated, although quinolone-induced chondropathy has been observed in young animals of several species (3, 4, 9, 31).
Since 1992, tendinopathy has been described as another side effect in patients treated with fluoroquinolones, with the tendinopathy sometimes resulting in the rupture of the tendon. The cause of this rare (≤1%) (7) but severe complication remains unexplained (13, 14, 28). Probably both because of the large number of prescriptions for pefloxacin and because of the high level of diffusion of pefloxacin into tissue, pefloxacin has been the subject of several reports on such secondary effects, and the Achilles tendon seems to be especially vulnerable to fluoroquinolone-promoted tendinopathy (14). The low incidence of this tendon-damaging effect suggests that it may result from some intrinsic effects of fluoroquinolones that could be realized as a result of certain factors, such as age, sex (the male-to-female ratio of those affected is 3:1), concomitant corticosteroid therapy, especially in renal graft patients (27), duration of treatment (26), pathological state, and possibly, other unknown aggravating factors.
In order to characterize and amplify the intrinsic harmful effect of pefloxacin on proteoglycans and collagen, which are the main biochemical components of the tendon, several investigations used relatively large doses of pefloxacin administered to rodents. As an oxidative event was observed in cartilage (11, 33), we considered the attractive hypothesis that the pathophysiological effects due to pefloxacin administration result from the same effects on both articular cartilage and tendon. On the one hand, the Achilles tendon and articular cartilage are characterized by a low level of or no blood perfusion, respectively, resulting in a low O2 pressure (18). These conditions render them more susceptible to oxidative stress resulting from an incomplete reduction of oxygen in the mitochondria and the formation of superoxide. In apparent agreement, tendon ruptures occurred at a critical zone which is described as hypovascularized.
In this study, we evaluated the consequences of pefloxacin administration on cellular activity in the Achilles tendon by measuring the anabolism of proteoglycans, which have a fast metabolic turnover rate, after the administration of a single dose of pefloxacin to mice. On the other hand, a possible oxidative stress was assessed by measuring matrix modifications on collagen, which has a low turnover rate and which can retain oxidative alterations for 30 days. Pefloxacin-induced modifications of collagen were compared to those due to ischemia-reperfusion (I-R) of the Achilles tendon in rats. I-R was considered a model of oxidative injury in this tissue, as reactive oxygen species (ROSs) are important mediators of postischemic injury in various tissues (1, 8, 10). Finally, we evaluated the effect of N-acetylcysteine, a known antioxidant, on the modifications to collagen induced by pefloxacin. The results obtained provide new insights into the mechanism of fluoroquinolone-induced tendinopathies.
MATERIALS AND METHODS
Animals.
Four- to 6-week-old male Sprague-Dawley rats (weight, 125 to 150 g) and 3- to 4-week-old male Swiss mice (weight, 15 to 20 g) (Charles River, Saint-Aubin-lès-Elbeuf, France) were housed in solid-bottom plastic cages designed to allow easy access to standard laboratory food and water ad libitum. The animals were kept in a 12-h light and 12-h dark cycle in a temperature-controlled chamber.
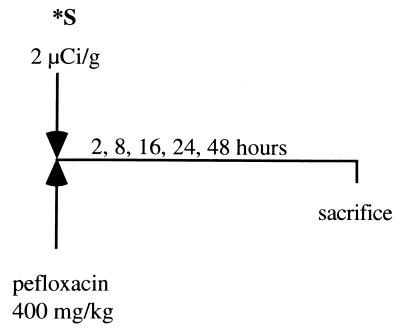
Kinetics of incorporation of 35S in blood and Achilles tendons in vivo in mice: effect of pefloxacin administration. (i) Effects during the first phase (early effects).
Mice received by gavage either a single dose (400 mg/kg of body weight daily; 10 μl/g with saline as the vehicle) of pefloxacin dihydrate mesylate (provided by Bellon Laboratories, Neuilly/Seine, France) or saline solution (as a control) (n = 5 per group) and a simultaneous intraperitoneal injection of Na235SO4 (2 μCi/g body weight). Animals were decapitated 2, 8, 16, 24, or 48 h later (Fig. 1). Blood samples were collected, and the Achilles tendons were dissected out and were placed overnight in 1 ml of cetylpyridinium chloride (Sigma) in phosphate-buffered formalin. They were dissolved overnight in Soluene-350 (Packard, Rungis, France). The amount of [35S]sulfate incorporated into each sample was counted by liquid scintillation spectrometry in 4.5 ml of Hionic Fluor (Packard) as the scintillation fluid. Blood samples (10 μl) were decolored by adding 100 μl of propan-2-ol and 100 μl of H2O2. Results are expressed as the difference between the mean percentage of 35S incorporated into the Achilles tendons from pefloxacin-treated animals and the mean percentage of 35S incorporated into the Achilles tendons from the control animals treated with the vehicle alone.
FIG. 1.
Kinetics of incorporation of 35S in blood and Achilles tendons in vivo in mice: effect of pefloxacin administration during the first phase (early effects of pefloxacin). ∗S, Na235SO4.
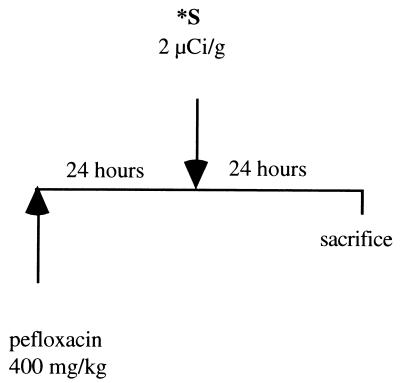
(ii) Effects during the second phase (24 to 48 h).
Weight-matched mice received by gavage a single dose of pefloxacin (400 mg/kg) and 24 h later received an intraperitoneal injection of radioactive sulfate (2 μCi of Na235SO4/g) (n = 5 per group with two experiments). At 48 h after pefloxacin administration, blood was sampled and Achilles tendons were removed as described above (Fig. 2). The 35S contents in the blood of both treated and untreated mice were identical to those in serum derived from the blood. Thus, blood samples were used and were considered equivalent to serum samples for measurement of circulating inorganic radiosulfate contents.
FIG. 2.
Kinetics of incorporation of 35S in blood and Achilles tendon in vivo in mice: effect of pefloxacin administration during the second phase (24 to 48 h). ∗S, Na235SO4.
Measurement ex vivo of proteoglycan synthesis.
An assay for measurement of proteoglycan synthesis was performed as described by Van den Berg et al. (36). Briefly, pefloxacin dihydrate mesylate was administered orally to mice (400 mg/kg daily; 10 μl/g), which were killed by cervical dislocation 24 or 48 h after drug administration. The Achilles tendons were carefully dissected and were incubated as described above by using RPMI culture medium (200 μl/patella or femoral head cap) containing gentamicin (50 μg/ml), l-glutamine (2 mM), and Na235SO4 (10 μCi/ml). After incubation for 2 h at 37°C in a 5% CO2 atmosphere, the tendons were washed with isotonic saline solution and were fixed overnight in 0.5% cetylpyridinium chloride in phosphate-buffered formalin. The samples were then dissolved overnight in Soluene-350.
Experimental model of I-R of Achilles tendons of rats.
In the experiments with the I-R model, the animals were separated into four groups (with five rats per group). Pefloxacin was administered to rats in group 1 (400 mg/kg/day) for 7 days, and I-R was achieved on the 4th day of pefloxacin treatment. Rats in group 2 were submitted to tendon I-R without pefloxacin treatment. Rats in group 3 were given pefloxacin for 7 days and underwent sham surgery. Control rats from group 4 that underwent sham surgery received saline solution. Anesthesia was achieved by intraperitoneal administration of ketamine (Imalgen; 0.1 ml/100 g). Midline incisions of 3 cm were made over the right and the left Achilles tendons, and the tendons were isolated from the surrounding fascia. To promote ischemia, the myotendinous portion and the calcaneal insertion were ligated by a suture technique (with Ethicon sutures). The skin was then sutured. Two hours later, the ligations were removed while the rats were under anesthesia to allow reperfusion and the skin was again sutured.
Collagen extraction.
Collagen extraction was performed with rat and mouse Achilles tendons, which were washed for 1 h at 4°C with water and neutral salt solution (0.05 M Tris-HCl, 0.9% NaCl [pH 7.4]) to remove soluble material. The residue was added to a solution of 1 mg of pepsin per ml in 0.5 M acetic acid at a ratio of 1/10 (sample/pepsin), and the mixture was stirred for 2 days at 4°C (24). Undigested solid material was removed by centrifugation at 30,000 × g for 30 min. The operational conditions for pepsin digestion were chosen to be sufficient to extract ≥90% of the collagen, as determined by measuring the hydroxyproline content of the supernatant after pepsin digestion and of the tendons after acid hydrolysis (37). The protein concentrations of the samples were determined by the assay described by Lowry et al. (22) by using bovine serum albumin as the standard.
Protein derivatization with DNPH and by SDS-PAGE.
The fraction extracted from the Achilles tendon (50 μg) was treated with an equal volume of 0.5 mM dinitrophenylhydrazine (DNPH; in 0.1 M sodium phosphate buffer [pH 6.3]; Sigma) for 1 h at room temperature. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) of collagen was performed as described by Laemmli (19) in 1-mm-thick 6% slab gels (16- by 18-cm format). The same amount of collagen (15 or 7 μg) was loaded into all lanes. After SDS-PAGE, the gels were transferred onto Immobilon-P membranes (Sigma) with a Trans-Blot electrophoretic transfer apparatus as described by Towbin et al. (34). Immunochemical detection of protein carbonyls was performed as described elsewhere (16, 29). Briefly, the blots were incubated with 0.1% (wt/vol) Ponceau solution (Sigma) in 5% (vol/vol) acetic acid and were destained in methanol until bands appeared. Then the blots were incubated with bovine serum albumin (3%) for at least 90 min, followed by an incubation at room temperature with rabbit anti-dinitrophenyl antibodies (diluted 1:2,000 in 9 mM Tris-HCl [pH 9.0], 154 mM NaCl, 0.05% [vol/vol] Tween 20 [TBST]; Sigma). The primary antibody was removed, and the blots were washed three times for 10 min each time with TBST. The blots were incubated with alkaline phosphatase-conjugated goat anti-rabbit immunoglobulin G (diluted 1:5,000 in TBST; Sigma) for 90 min at room temperature. After washing the blots with TBST three times for 10 min each time, oxidized proteins were revealed by the addition of 5-bromo-4-chloro-3-indolylphosphate–nitroblue tetrazolium. Optical densities were acquired with an image analysis system (NIH Image 1.54).
Statistical analysis.
Biochemical data are presented as means ± standard errors of the means (SEMs). Groups were compared by a two-way analysis of variance, with a P value of <0.05 taken as the significance level.
RESULTS
In vivo effects of a single dose of pefloxacin on the content of 35S in Achilles tendons of mice. (i) Effects during the first phase (early effects).
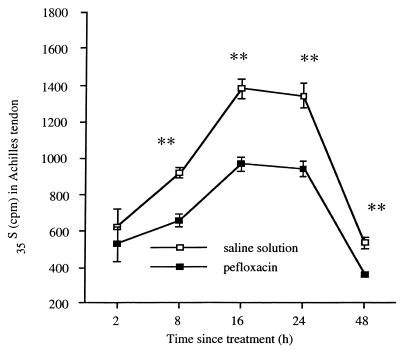
The effect of a single oral dose (400 mg/kg) of pefloxacin on proteoglycan synthesis in mice, as measured by the incorporation in vivo of 35S given intraperitoneally at the same time as pefloxacin, varied with the tissue studied. In both treated and control mice, the amount of radioactivity in blood decreased with time, whereas in the Achilles tendon it increased to a maximum at 16 to 24 h (Fig. 3) and then decreased until 48 h after the injection. The radioactivity in the Achilles tendon was 29% less in treated animals than in control animals 8 h after pefloxacin administration. Similar differences between treated animals and control animals were seen at 16 and 24 h and persisted until 48 h. Throughout these assays, the urinary excretion of 35S was twofold higher in the treated mice than in the control mice (data not shown).
FIG. 3.
Effect in mice of a single oral dose (400 mg/kg) of pefloxacin on the in vivo incorporation of simultaneously administered 35S into the Achilles tendon. Control animals were given saline solution instead of pefloxacin. Values for 35S are means ± SEMs (n = 4 experiments). ∗, P < 0.05, and ∗∗, P < 0.01, versus controls (Student's t test).
(ii) Effects during the second phase (24 to 48 h).
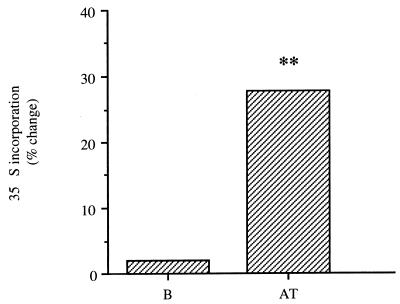
Forty-eight hours after the administration of a single dose of pefloxacin (400 mg/kg) and 24 h after injection of 35S, proteoglycan synthesis was higher in the Achilles tendons of the experimental group than in the Achilles tendons of the control group (27%), as measured by incorporation of 35S (Fig. 4). Identical results (increase by 32%) were obtained with the fibrocartilage of the peripheral part of the patellae (data not shown). The amounts of radioactivity in blood were identical in both experimental mice and control mice.
FIG. 4.
In vivo effects in mice of a single oral dose (400 mg/kg) of pefloxacin on proteoglycan synthesis as indicated by changes in 35S incorporation 48 h after pefloxacin administration and 24 h after intraperitoneal administration of 35S (2 μCi/g). Control animals received saline only. Values are percent differences in 35S incorporation in treated mice and controls (n = 2 experiments each with five mice). B, blood; AT, Achilles tendon. ∗, P < 0.05, and ∗∗, P < 0.01, versus controls (Student's t test).
Ex vivo effects in mice of a single dose of pefloxacin on the content of 35S in Achilles tendon 24 and 48 h after administration of a single oral dose (400 mg/kg).
Pefloxacin decreased by 29% the level of ex vivo incorporation of 35S in the Achilles tendons 24 h after administration (data not shown). In addition, the level of proteoglycan synthesis in the Achilles tendons returned to the control level 48 h after pefloxacin administration.
Influence of pefloxacin treatment (400 mg/kg/day) on carbonyl derivative formation in type I collagen in mice.
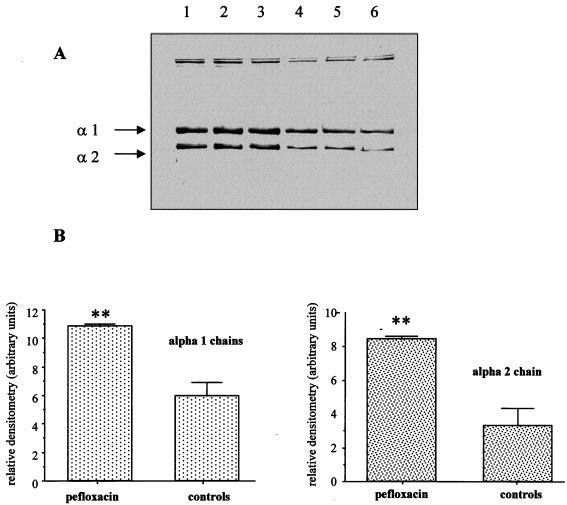
We examined the collagen extracted from the Achilles tendons of both control animals and mice treated with pefloxacin for 7 days. SDS-PAGE and immunoblotting of tendon collagen showed the two characteristic bands from the α1(I) and α2(I) chains of collagen type I. Figures 5A and B show that the collagen extracted from the tendons of mice treated with pefloxacin for 7 days had a higher carbonyl content than the collagen extracted from the tendons of control mice. This was observed for both α1(I) and α2(I) chains.
FIG. 5.
Immunochemical detection of carbonyl groups in Achilles tendon collagen of mice after pefloxacin administration. A total of 10 μg of DNPH-derivatized protein was loaded onto each lane. (A) Lanes 1 to 3, Achilles tendon collagen of mice treated with pefloxacin (400 mg/kg/day) for 7 days; lanes 4 to 6, Achilles tendon collagen of control mice. (B) Densitometric analysis of the immunoblots in panel A. Values are means ± standard deviations. ∗, P < 0.05, and ∗∗, P < 0.01, versus controls (Student's t test).
Influence of pefloxacin treatment (400 mg/kg/day) and of an experimental tendinous I-R on carbonyl derivative formation in type I collagen in rats.
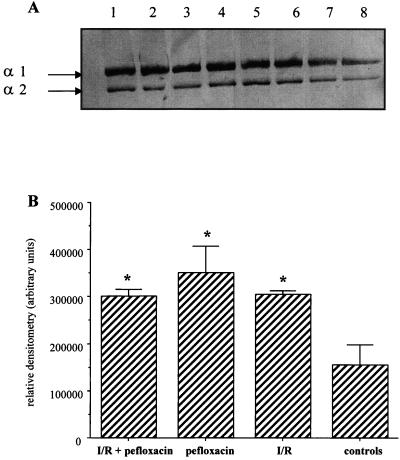
Tendons were submitted to a 2-h ischemia followed by a reperfusion for 3 or 7 days. The tendons were analyzed for their collagen contents under the conditions described above. Before the experimentation with this model, pefloxacin was administered orally to the rats 7 days (twice at 200 mg/kg/day), whereas the experimental I-R was achieved on the 4th day. An increase in the level of carbonyl derivatives from collagen type I was observed in the tendons of rats which received pefloxacin for 7 days, and a similar effect was observed in rat tendons submitted to I-R (Fig. 6A and B). This response was observed for the α1(I) chains only, as there was no significant increase in the carbonyl contents of the α2(I) chains. The same increase was observed when rats were first treated with pefloxacin and then submitted to an ischemia (2 h)-reperfusion (3 days) of the tendon.
FIG. 6.
Immunochemical detection of carbonyl groups in Achilles tendon collagen of rats after pefloxacin administration and ischemia (2 h) and reperfusion (3 days). A total of 15 μg of DNPH-derivatized protein was loaded onto each lane. (A) Lanes 1 and 2, Achilles tendon collagen of rats treated with pefloxacin (400 mg/kg/day) for 7 days; for this group I-R and sham surgery were done on the 4th day of pefloxacin treatment (duplicate loads); lanes 3 and 4, Achilles tendon collagen of rats that underwent sham surgery and that were treated with pefloxacin (400 mg/kg/day) for 7 days (duplicate loads); lanes 5 and 6, Achilles tendon collagen of rats submitted to a tendinous I-R (duplicated loads); lanes 7 and 8, Achilles tendon collagen of rats that underwent sham surgery and that received saline solution for 7 days. (B) Densitometric analysis of the immunoblots in panel A. Values are means ± standard deviations. ∗, P < 0.05, and ∗∗, P < 0.01, versus controls (Student's t test).
Influence of coadministration of pefloxacin (400 mg/kg/day) and N-acetylcysteine (150 mg/kg/day) on carbonyl derivative formation in type I collagen in mice.
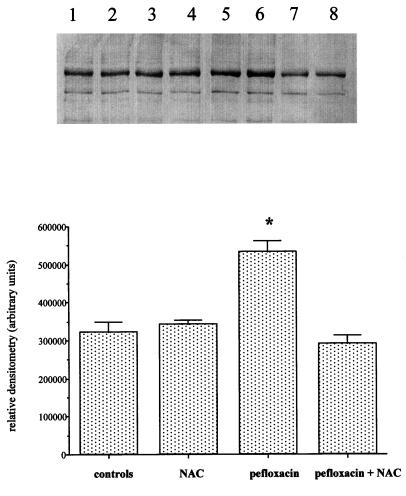
We examined the collagen extracted from the Achilles tendons of both control animals and mice treated with pefloxacin (400 mg/kg/day) and N-acetylcysteine (150 mg/kg/day) for 10 days. The increase in the carbonyl content of collagen observed in mice treated with pefloxacin was prevented by the simultaneous administration of N-acetylcysteine. No changes were observed between control animals and rats which received N-acetylcysteine only (Fig. 7A and B).
FIG. 7.
Immunochemical detection of carbonyl groups in Achilles tendon collagen of mice after coadministration of pefloxacin (400 mg/kg/day) and N-acetylcysteine (NAC; 150 mg/kg/day) for 10 days. A total of 10 μg of DNPH-derivatized protein was loaded onto each lane. (A) Lanes 1 and 2, Achilles tendon collagen of controls receiving saline solution for 10 days (duplicate loads); lanes 3 and 4, Achilles tendon collagen of mice receiving N-acetylcysteine (150 mg/kg/day) for 10 days (duplicate loads); lanes 5 and 6, Achilles tendon collagen of mice receiving pefloxacin (400 mg/kg/day) for 10 days (duplicate loads); lanes 7 and 8, Achilles tendon collagen of mice receiving pefloxacin (400 mg/kg/day) and, simultaneously, N-acetylcysteine (150 mg/kg/day) for 10 days (duplicate loads). (B) Densitometric analysis of the immunoblots in panel A. Values are means ± standard deviations. ∗, P < 0.05 versus controls (Student's t test).
DISCUSSION
Fluoroquinolone derivatives are characterized by good tissue penetration, a broad antibacterial spectrum, and a relatively low incidence of serious side effects. However, quinolones may have adverse effects on the musculoskeletal system, but with a very low incidence (1% or less [32]). Recently, adult or old-age tendinopathy, sometimes resulting in spontaneous rupture, has been considered a side effect of treatment with these drugs. The Achilles tendon seems to be especially affected, but other targets may also be damaged (20). Pefloxacin has been the subject of several studies of such damage, probably both because of the large number of prescriptions for pefloxacin and because of the high level of diffusion of pefloxacin into tissues (5, 15). In this study, we demonstrated for the first time that pefloxacin induces an oxidative stress on proteoglycans and collagen, which are the main constituents of tendon, by using relatively large doses of pefloxacin in order to amplify the drug effects. Measurement of proteoglycan anabolism in mice revealed modifications of the cellular activity after administration of a single dose of pefloxacin, and tissue alterations were confirmed by detection of an increase in the oxidation markers of collagen after several days of treatment of rats.
The pefloxacin-induced alterations in the Achilles tendon were revealed in mice by measurement of the level of 35S incorporation in vivo, which is a highly sensitive and reproducible assay for proteoglycan synthesis. This approach allows quantification of both the early and the late changes in proteoglycan synthesis induced by pefloxacin by using various time delays between pefloxacin administration and 35S injection. In control mice, very low levels of radioactivity remained in the blood 24 h after 35S injection (30), whereas in the tendon, the radioactivity peaked at between 16 and 24 h. When mice received either pefloxacin or saline solution and a simultaneous intraperitoneal 35S injection, the amount of radioactivity in the tissue studied was significantly lower in pefloxacin-treated mice than in control mice at every time during the first phase (after 8, 16, 24, and 48 h). As demonstrated in a previous work (30), pefloxacin induced a marked fall in the endogenous serum sulfate level. This effect was similar to the effects of salicylate on cartilage, as determined by using different times between drug and radiolabeled sulfate administrations (6). In order to determine whether the decrease in 35S incorporation during the first phase results from a direct effect of pefloxacin or from the lower concentration of sulfate, we evaluated the effects of pefloxacin in ex vivo experiments (data not shown). In these ex vivo studies, by using an identical sulfate concentration for both the controls and the assays, an inhibition of 35S incorporation was observed 24 h after pefloxacin administration (−29%), as was also demonstrated in vivo. Therefore, this depletion phase in proteoglycan synthesis seems to be a direct effect of pefloxacin on tissue metabolism rather than a result of a depletion of the sulfate in serum.
Moreover, 48 h after a single pefloxacin administration (400 mg/kg) and 24 h after 35S injection, pefloxacin induced an increase in the level of 35S in the Achilles tendon (27%) and also in the peripheral fibrocartilage of the patellae (32%; data not shown). Under these conditions, proteoglycan synthesis suggests the onset of a tissue-specific repair process in response to a previous deleterious effect. All these changes suggest a fibroproliferative response, presumably in an attempt to repair the tendon defect resulting from the depletion phase.
The depletion phase in proteoglycan synthesis observed after pefloxacin administration also appeared in articular cartilage. However, 48 h after pefloxacin administration and 24 h after 35S injection, the level of 35S incorporation in cartilage did not differ from that in controls (30). Therefore, we postulate that repair-like responses to the early inhibition of proteoglycan synthesis promoted by pefloxacin are regulated differently in tendon and fibrocartilage than in cartilage. Our results also suggest that a single dose of pefloxacin induced a cellular damage which leads to a biphasic effect on Achilles tendon proteoglycan synthesis: a transitory decrease followed by a repair-like response.
Together, the results of the present study suggest that fluoroquinolone-induced tendinopathies occur by the same pathophysiological pathways as those described for cartilage (12, 24). Previous reports suggested compromised mitochondrial activity, and a precocious stimulation of the oxidative metabolism within immature articular chondrocytes was also described (11, 33), with both of these resulting in the generation of reactive oxygen species. Therefore, we looked for an eventual pefloxacin-induced oxidative stress on the collagen isolated from the Achilles tendon. We observed in mice that after a 7-day pefloxacin treatment, an increase in the level of carbonyl derivatives was observed in type I collagen, indicating oxidative damage. These oxidative changes were observed only after at least 5 days of pefloxacin administration (data not shown). We attach particular importance to the similar effect that appeared during Achilles tendon I-R, which leads to ROS production, which is the main mediator of postischemic injury in various tissues. A synergistic increase in carbonyl formation was not detected after pefloxacin treatment was applied during an I-R, perhaps indicating a saturation of collagen-oxidizable sites. Curiously, pefloxacin induced an oxidative modification of both α1(I) and α2(I) chains in mice, whereas only α1(I) chains were affected in rats. Moreover, this study demonstrated that N-acetylcysteine, a thiol antioxidant, prevented the pefloxacin-induced oxidative modifications of Achilles tendon collagen. We also observed that pefloxacin treatment induced oxidative damage on type II collagen from articular cartilage (30). These results suggest that the hypoxic conditions of the cartilage and tendon could lead fluoroquinolones to particular redox levels, allowing the generation of free radicals which promote cell and tissue damages. In particular, superoxide has been shown to exert direct deleterious effects on collagen fibers and could oxidize susceptible amino acids in collagen and change the protein conformation (24). In the same way, ROSs could be toxic to matrix components or could act as activators of metalloproteinases (2, 21).
In conclusion, the present study provides new insights into fluoroquinolone-induced tendinopathies. A single large dose of pefloxacin promoted a change in proteoglycan synthesis, with a precocious inhibition followed by a repair-like phase. We also reported that pefloxacin induced oxidative damage to collagen that was similar to that resulting from an experimental I-R of the tendon and that was prevented by the simultaneous administration of N-acetylcysteine, therefore suggesting in both cases the involvement of ROSs. Individual factors of susceptibility such as participation in sports, age, or corticosteroid therapy possibly do not allow the tendon to repair adequately, resulting in irreversible matrix alterations that might explain the occurrence of tendinopathies that sometimes result in the rupture of the tendon (35). Accordingly, in vitro experiments with cultured tendon cells have confirmed the clinical observations that the administration of other drugs in parallel with fluoroquinolones can increase the risk of tendon rupture (17). The doses of pefloxacin administered to rodents in this study are much larger than those usually delivered to humans. Nevertheless, the half-lives of this drug range from 1.9 h in mice to 8.6 h in humans (25). Thus, pefloxacin is metabolized more rapidly in mice and this dose may allow a sufficient diffusion into tissue. Therefore, the results presented here should be considered with care in relation to the adverse effects of pefloxacin reported in humans.
ACKNOWLEDGMENT
This study was supported by a grant from Bellon, a division of Rhône-Poulenc Rorer.
REFERENCES
- 1.Birch H L, Rutter G A, Goodship A E. Oxidative energy metabolism in equine tendon cells. Res Vet Sci. 1997;62:93–97. doi: 10.1016/s0034-5288(97)90127-2. [DOI] [PubMed] [Google Scholar]
- 2.Brenneisen P, Briviba K, Wlaschek M, Wenk J, Scharffetter-Kochanek K. Hydrogen peroxide (H2O2) increases the steady-state mRNA levels of collagenase/MMP-1 in human dermal fibroblasts. Free Radical Biol Med. 1997;22:515–524. doi: 10.1016/s0891-5849(96)00404-2. [DOI] [PubMed] [Google Scholar]
- 3.Burkhardt J E, Hill M A, Carlton W W, Kesterson J W. Histologic and histochemical changes in articular cartilages of immature beagle dogs dosed with difloxacin, a fluoroquinolone. Vet Pathol. 1990;27:162–170. doi: 10.1177/030098589002700303. [DOI] [PubMed] [Google Scholar]
- 4.Christ W, Lehnert T, Ullbrich B. Specific toxicologic aspects of the quinolones. Rev Infect Dis. 1988;10(Suppl.):S144–S146. doi: 10.1093/clinids/10.supplement_1.s141. [DOI] [PubMed] [Google Scholar]
- 5.Desbordes J M, Bouquet S, Breux J, Chapelle G, Bataille B, Becq Giraudon B, Fusciardi J. Comparative penetration of 3 fluoroquinolones into human cranial bone tissue after administration of a single intravenous dose. Drugs. 1993;45(Suppl. 3):448–449. [Google Scholar]
- 6.De Vries B J, van den Berg W B, van de Putte L B A. Salicylate-induced depletion of endogenous inorganic sulfate. Arthritis Rheum. 1985;28:922–929. doi: 10.1002/art.1780280812. [DOI] [PubMed] [Google Scholar]
- 7.Donck J B, Segaert M F, Vanrenterghem Y F. Fluoroquinolones and Achilles tendinopathy in renal transplant recipients. Transplantation. 1994;58:736–737. [PubMed] [Google Scholar]
- 8.Farrell A J, Williams R B, Stevens C R, Lawrie A S, Cox N L, Blake D R. Exercise induced release of von Willebrand factor: evidence for hypoxic reperfusion microvascular injury in rheumatoid arthritis. Ann Rheum Dis. 1992;51:1117–1122. doi: 10.1136/ard.51.10.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gough A W, Kasali O B, Sigler R E, Baragi V. Quinolone arthropathy-acute toxicity to immature articular cartilage. Toxicol Pathol. 1992;20:436–450. doi: 10.1177/019262339202000313. [DOI] [PubMed] [Google Scholar]
- 10.Halliwell B. Oxygen radicals and metal ions: potential antioxidant intervention strategies. Ann Intern Med. 1987;107:526–530. doi: 10.7326/0003-4819-107-4-526. [DOI] [PubMed] [Google Scholar]
- 11.Hayem G, Petit P X, Levacher M, Gaudin C, Kahn M F, Pocidalo J J. Cytofluorometric analysis of chondrotoxicity of fluoroquinolone antimicrobial agents. Antimicrob Agents Chemother. 1994;38:243–247. doi: 10.1128/aac.38.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hildebrand H, Kempka G, Schlüten G, Schmidt M. Chondrotoxicity of quinolones in vivo and in vitro. Arch Toxicol. 1993;67:411–415. doi: 10.1007/BF01977402. [DOI] [PubMed] [Google Scholar]
- 13.Jorgensen C, Anaya J M, Didry C, Canovas F, Serre I, Baldet P, Ribard P, Kahn M F, Sany J. Arthropathies et tendinopathie Achilléenne induites par la péfloxacine. Rev Rhum. 1991;58:623–625. [PubMed] [Google Scholar]
- 14.Kahn M F, Hayem G. Tendons et fluoroquinolones: des problèmes non résolus. Rev Rheum. 1997;64:511–513. [Google Scholar]
- 15.Kashida Y, Kato M. Characterization of fluoroquinolone-induced Achilles tendon toxicity in rats: comparison of toxicities of 10 fluoroquinolones and effects of anti-inflammatory compounds. Antimicrob Agents Chemother. 1997;41:2389–2393. doi: 10.1128/aac.41.11.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keller R J, Halmes N C, Hinson J A, Pumford N R. Immunochemical detection of oxidized proteins. Chem Res Toxicol. 1993;6:430–433. doi: 10.1021/tx00034a007. [DOI] [PubMed] [Google Scholar]
- 17.Kempka G, Ahr H J, Rüther W, Schlüter G. Effects of fluoroquinolones and glucocorticoids on cultivated tendon cells in vitro. Toxicol In Vitro. 1996;10:743–754. doi: 10.1016/s0887-2333(96)00050-1. [DOI] [PubMed] [Google Scholar]
- 18.Kirkendall D T, Garrett W E. Function and biomechanics of tendons. Scand J Med Sci Sports. 1997;7:62–66. doi: 10.1111/j.1600-0838.1997.tb00120.x. [DOI] [PubMed] [Google Scholar]
- 19.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 20.Le Huec J C, Schaeverbeke T, Chauveaux D, Moinard M, Rivel J, Le Rebeller A. Epicondylites induites par les fluoroquinolones chez le sportif. J Chir (Paris) 1994;131:408–412. [PubMed] [Google Scholar]
- 21.Lo Y Y, Conquer J A, Grinstein S, Cruz T F. Interleukin-1 beta induction of C-fos and collagenase expression in articular chondrocytes: involvement of reactive oxygen species. J Cell Biochem. 1998;69:19–29. doi: 10.1002/(sici)1097-4644(19980401)69:1<19::aid-jcb3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 22.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 23.Miller E J. Structural studies on cartilage collagen employing limited cleavage and solubilization with pepsin. Biochemistry. 1972;11:4903–4909. doi: 10.1021/bi00776a005. [DOI] [PubMed] [Google Scholar]
- 24.Monboisse J C, Poulin G, Braquet P, Randoux A, Ferradini C, Borel J P. Effect of oxygen radicals on several types of collagen. Int J Tissue React. 1984;6:385–390. [PubMed] [Google Scholar]
- 25.Montay G, Goueffon Y, Roquet F. Absorption, distribution, metabolic fate, and elimination of pefloxacin mesylate in mice, rats, dogs, monkeys, and humans. Antimicrob Agents Chemother. 1984;25:463–472. doi: 10.1128/aac.25.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pierfitte C, Gillet P, Royer R J. More on fluoroquinolone antibiotics and tendon rupture. N Engl J Med. 1995;332:193. doi: 10.1056/NEJM199501193320319. [DOI] [PubMed] [Google Scholar]
- 27.Rolland E, Saillant G. Tendinopathies achilléennes et fluoroquinolones. J Traumatol Sport. 1993;10:175–178. [Google Scholar]
- 28.Royer R J, Pierfitte C, Netter P. Features of tendon disorders with fluoroquinolones. Therapie. 1994;49:75–76. [PubMed] [Google Scholar]
- 29.Shacter E, Williams J A, Lim M, Levine R. Differential susceptibility of plasma proteins to oxidative modification: examination by Western blot immunoassay. Free Radical Biol Med. 1994;17:429–437. doi: 10.1016/0891-5849(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 30.Simonin M A, Pottie P, Minn A, Gillet P, Netter P, Terlain B. Proteoglycan and collagen biochemical variations during fluoroquinolone-induced chondrotoxicity in mice. Antimicrob Agents Chemother. 1999;43:2915–2921. doi: 10.1128/aac.43.12.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stahlmann R, Förster C, Shakibaei M, Vormann J, Günther T, Merker H J. Magnesium deficiency induces joint cartilage lesions in juvenile rats which are identical to quinolone-induced arthropathy. Antimicrob Agents Chemother. 1995;39:2013–2018. doi: 10.1128/aac.39.9.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stahlmann R, Lode H. Safety overview: toxicity, adverse effects and drug interaction. In: Andriole V, editor. The quinolones. London, United Kingdom: Academic Press; 1988. pp. 201–235. [Google Scholar]
- 33.Thuong-Guyot M, Domarle O, Pocidalo J J, Hayem G. Effects of fluoroquinolones on cultured articular chondrocytes. Flow cytometric analysis of free radical production. J Pharmacol Exp Ther. 1994;271:1544–1549. [PubMed] [Google Scholar]
- 34.Towbin H, Stahelin T, Gordon J. Electrophoretic transfer of proteins from acrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1985;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tuite D J, Renström P A F H, O'Brien M. The aging tendon. Scand J Med Sci Sports. 1997;7:72–77. doi: 10.1111/j.1600-0838.1997.tb00122.x. [DOI] [PubMed] [Google Scholar]
- 36.Van den Berg W B, Kruijsen M W M, van de Putte L B A. The mouse patella assay: an easy method of quantitating articular cartilage chondrocyte function in vivo and in vitro. Rheumatol Int. 1982;1:165–169. [Google Scholar]
- 37.Woessner J F. The determination of hydroxyproline in tissue and protein samples containing small proportions of the amino acid. Biochem Biophys Acta. 1961;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]