Graphical abstract
Designed by Naomi Whitaker (Emory Rollins School of Public Health).
Keywords: COVID pandemic, COVID-19, Trauma, Trauma surgery, Surgical critical care
Abstract
Background
This study investigated the impact of COVID-19 infection on hospitalized trauma patients.
Methods
A retrospective review of hospitalized trauma patients at a level I trauma center was performed from March–December 2020. Data pertaining to patient demographics, presentation and hospital course was compared between COVID positive and negative trauma patients.
Results
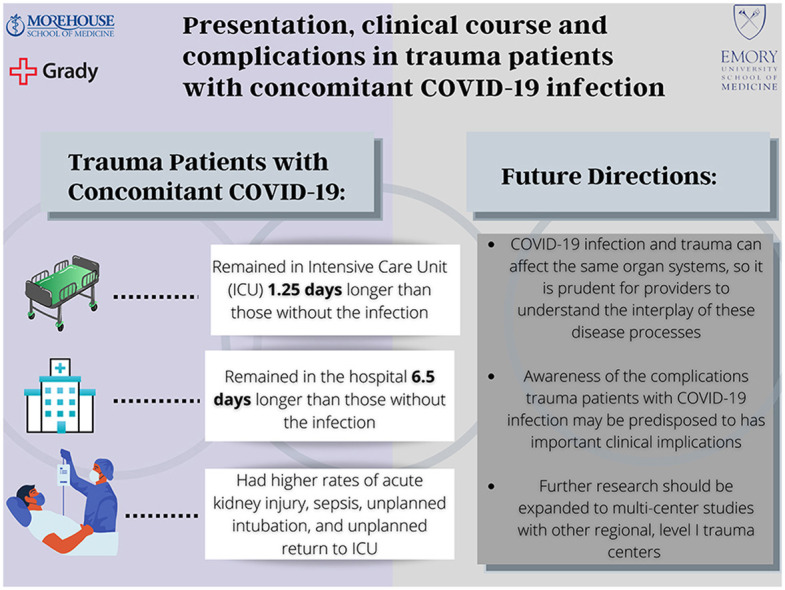
There were 4,912 patients and 179 (3.64%) were COVID-19 positive. Demographics and clinical presentation did not differ significantly between those with and without concomitant COVID-19. However, COVID positive trauma patients had higher rates of acute kidney injury (p = 0.016), sepsis (p = 0.016), unplanned intubation (p = 0.002) and unplanned return to the ICU (p = 0.01). The COVID positive cohort also had longer hospital stays (p < 0.01) with no significant difference in mortality.
Conclusions
In the setting of an ongoing pandemic, awareness of the complications COVID positive trauma patients are predisposed to is important for providers.
1. Introduction
The COVID-19 pandemic has had a significant impact on the field of trauma surgery. On January 20, 2020, the CDC confirmed the first case of COVID-19 infection in the United States.1 In March of 2020, the gravity of the situation became clear, and shelter in place ordinances were enacted, nationally.1 , 2 In metropolitan areas in the United States, it is estimated 2–3% of the population was infected with Sars-Cov-2 virus in the early months of this pandemic.3 These quarantine regulations, in conjunction with a country facing innumerable unknowns and limited resources, led to an immediate decrease in surgical procedures.1 , 2 One study estimated a 48% reduction, nationally, in cases across all surgical specialties in the early phase of the pandemic.2 However, the field of trauma surgery was not amenable to this trend. Given its emergent nature, delaying cases, closing operating rooms and transitioning to telehealth was not a viable nor feasible option. This is supported by the fact that across all surgical specialties, rates of emergent operative cases, including trauma surgery cases, were the least affected in the first year of the pandemic.1 , 2 , 4
While volume remained relatively constant, trauma surgeons did notice a paradigm shift in the patterns of traumatic injury during this period.1 With lock down regulations, there was an initial decrease in overall rates of motor vehicle accidents, pedestrian versus auto accidents and work related injuries.1 However, this was counteracted by a simultaneous, and alarming, rise in the rates of violent, intentional and penetrating injury across the country.1 , 5
A major level I trauma center in Los Angeles, CA had a higher proportion of penetrating trauma in 2020 then the 2 years prior and Philadelphia, PA saw a significant and unprecedented increase in rates of unintentional injury in the weeks following the shelter in place ordinance.1 , 6 Atlanta, GA experienced an 11% increase in the cumulative count of domestic crimes in the 2021 compared to the year prior, with the steepest increase in the spring of 2020.7 On a national scale, police data demonstrated a range of 6–23% increase in gun violence across major US cities in 2021 compared to 2019 and 2018.8
This evidence supports a concerning shift in the pattern of traumatic injury during the early phases of the pandemic. However, there has been little focus in the literature on the impact of COVID-19 infection on these trauma patients once they are hospitalized. It is well established that COVID-19 infection can affect many of the same organ systems commonly prone to complications in hospitalized trauma patients, but the intersection of these two disease processes is not well understood.9
While multiple studies have now been published demonstrating the impact of COVID-19 infection on general surgical patients, much fewer have investigated the outcomes in trauma patients, specifically. To our knowledge, one of the only studies to do so thus far is by Kauman et al., who did find an increased risk of morbidity and mortality in COVID positive trauma patients.10 Therefore, this study sought to further investigate this complex relationship of how concomitant COVID-19 infection impacts the clinical course and outcomes of patients hospitalized after traumatic injury.
2. Methods
A retrospective cohort study was conducted at Grady Memorial Hospital (GMH) in Atlanta, GA. GMH is one of the busiest, urban, academic level I trauma centers in the United States, with over 10,000 trauma activations, annually. The GMH trauma registry includes all hospitalized trauma patients. All patients in the trauma registry from March 1, 2020–December 31, 2020 were included in the study. This time period was selected as it was prior to the widespread availability of the COVID-19 vaccine to the public and therefore avoided potential confounders that may have introduced.
Data on patient demographics, injury patterns, clinical presentation, hospital course and complications were obtained from the trauma registry. Data pertaining to COVID status was also captured in the registry, as GMH instituted a hospital wide policy at the beginning of March for all inpatients to have a COVID-19 test within 24 h of admission. These COVID tests were performed in-house with rapid antigen testing. The COVID positive and COVID negative cohorts were delineated based on positive or negative antigen test. This study did not further differentiate between symptomatic and asymptomatic COVID-19 infections.
The exposure of interest was COVID-19 diagnosis and the primary outcomes included in-hospital mortality, ICU length of stay and overall hospital length of stay. Secondary outcomes included rates of in-hospital complications. We compared these between trauma patients with and without concomitant COVID positive tests using Chi-square (Fisher's exact test) and two-sample t-tests.
Given the relatively small sample size of COVID positive patients in relation to the trauma cohort in its entirety, a multivariate analysis was not feasible. Therefore, propensity score matching was used to investigate COVID positive status as an independent risk factor for the outcomes of interest.
Patients were matched in a 1:20 fashion (1 COVID positive: 20 COVID negative) using calipers (0.2 x standard deviation) = 0.2 x 0.01 = 0.002. The propensity score model was estimated using 8 variables of interest; age, gender, race, frailty, total GCS score, injury type (blunt/penetrating), injury severity score (ISS), shock index, if required MPT, if required emergent OR intervention. This yielded a model with 83 COVID positive patients and 1660 COVID negative patients who were equally matched.
ISS, shock index, requiring MTP and requiring emergent intervention were included as surrogate markers for severity of trauma at the time of admission. Frailty was determined using the mF1-11, which has been validated in the trauma population. It accounts for the presence of the following medical comorbidities: diabetes mellitus (DM), chronic obstructive pulmonary disease (COPD), congestive heart failure (CHF), history of myocardial infarction, history of angina, hypertension (requiring medication), peripheral vascular disease (PVD), history of cerebrovascular accident (CVA) or transient ischemic attack (TIA), history of CVA with neurologic deficits and dementia or altered sensorium. Of note, this data set lacked information on the presence or absence of neurologic deficits after CVA, so the mFI score denominator was 10 instead of the traditional 11. Regardless of this modification, any score ≥0.2 is considered frail.
1824 patients did not meet inclusion criteria for propensity score matching and were therefore excluded from this analysis. 1210 were not tested for COVID, 24 were less than 16 years of age, 84 had no shock index, 36 had no GCS total score and 470 were not candidates for propensity score matching.
Significance for all was set at α = 0.05. Statistical analysis was performed using SAS® version 9.4 (SAS Inc., Cary, NC). Approval for this study was obtained from the Institutional Review Board.
3. Results
From March 2020 through December 2020, there were 4,912 total trauma activations. Of these, 3.64% (n = 179) tested positive for COVID-19 within 24 h of admission [see Table 1 ]. The cohort of COVID positive trauma patients were predominantly male (65.3%) with a median age of 44.08 years [IQR 16–92]. Over two thirds were Black (67.5%) and one quarter were White (25.1%). These baseline demographics did not differ significantly from the cohort of trauma patients without concomitant COVID-19 infection.
Table 1.
Demographics and pre-existing comorbidities of COVID positive versus COVID negative trauma patients.
| COVID + Trauma Patients (n = 179) |
COVID - Trauma Patients (n = 4742) | P-value | |
|---|---|---|---|
| Age (Mean) | 45.95 (16–92) | 44.08 (16–113) | 0.247 |
| % Male | 117 (65.3%) | 3306 (69.7%) | 0.246 |
| Race | 0.535 | ||
| Black or African American | 121 (67.5%) | 3051 (64.3%) | 0.40 |
| White | 45 (25.1%) | 449 (9.4%) | 0.39 |
| Other | 13 (7.26%) | 1242 (26.1%) | 0.81 |
| Pre-existing Comorbidities | |||
| Diabetes | 25 (13.9%) | 460 (9.7%) | 0.079 |
| Hypertension | 58 (32.4%) | 1288 (27.1%) | 0.14 |
| CHF | 10 (5.58%) | 196 (4.13%) | 0.44 |
| Chronic Renal Disease | 2 (1.1%) | 74 (1.56%) | 0.87 |
| COPD | 6 (3.3%) | 197 (4.1%) | 0.735 |
| Current smoker | 56 (31.2%) | 1689 (35.6%) | 0.267 |
| Anticoagulation Use | 13 (7.26%) | 259 (5.4%) | 0.38 |
| BMI | |||
| Lower | 18 (10.1%) | 493 (10.4%) | 0.96 |
| Normal | 67 (37.4%) | 1796 (37.9%) | 0.97 |
| Upper | 94 (52.5%) | 2453 (51.7%) | 0.89 |
The COVID-19 positive cohort had higher rates of pre-existing diabetes (13.9% vs 9.7%), hypertension (32.4% vs 27.1%), chronic heart failure (5.58% vs 4.13%)and anticoagulation use (7.26% vs 5.4%) [see Table 1]. Both cohorts had an approximate 80% to 20% ratio of blunt to penetrating trauma, consistent with the regional average [see Table 2 ]. The two groups also presented with similar vital signs, with no significant differences in terms of mean systolic blood pressure, mean diastolic blood pressure, mean respiratory rate or mean O2 saturation. The COVID-19 positive cohort had a higher mean Injury Severity Score (ISS) at 13.45 compared to 11.9 in the COVID negative group, but this was not statistically significant. Both groups also required similar rates of operative intervention within the first 24 h of admission with a rate of 55.8% in the COVID positive cohort and 51.24% in the COVID negative cohort.
Table 2.
Clinical presentation of COVID positive versus COVID negative trauma patients.
| COVID + Trauma Patients (n = 179) |
COVID - Trauma Patients (n = 4742) | P-value | |
|---|---|---|---|
| Injury | 0.75 | ||
| Blunt | 141 (78.8%) | 3,781 (79.7%) | |
| Penetrating | 38 (21.2%) | 961 (20.3%) | |
| Systolic Blood Pressure (Mean) | 129.2 (0–223) | 129.1 (0–266) | 0.97 |
| Diastolic Blood Pressure (Mean) | 76.2 (0–135) | 76.95 (0–197) | 0.69 |
| Respiratory Rate (Mean) | 17.4 (16–41) | 17.03 (16–58) | 0.43 |
| O2 saturation (Mean) | 92.87 (0–100) | 90.57 (0–100) | 0.14 |
| Injury Severity Score (Mean) | 13.45 (1–54) | 11.9 (0–75) | 0.057 |
| Operative intervention within 24 h of admission | 100 (55.8%) | 2430 (51.24%) | 0.25 |
The cohort of trauma patients with concomitant COVID-19 infection had higher rates of all in-hospital complications studied, with statistically significantly higher rates of acute kidney injury (2.79% vs 0.8%, p = 0.016) and sepsis (2.79% vs 0.8% p = 0.016) [see Table 3 ]. The COVID-19 positive cohort also had higher rates of unplanned intubation (5.02% vs 1.64%, p = 0.002) and unplanned return to the ICU (5.58% vs 2.27%, p = 0.01) (see Table 4, Table 5, Table 6 ).
Table 3.
In-hospital complications in COVID positive versus COVID negative trauma patients.
| COVID + Trauma Patients (n = 179) |
COVID - Trauma Patients (n = 4742) | P-value | |
|---|---|---|---|
| AKI | 5 (2.79%) | 38 (0.8%) | 0.016* |
| Sepsis | 5 (2.79%) | 38 (0.8%) | 0.016* |
| ARDS | 3 (1.67%) | 20 (0.42%) | 0.0632 |
| Pulmonary embolism | 1 (0.55%) | 22 (0.46%) | 1.00 |
| Deep vein thrombosis | 3 (1.67%) | 38 (0.8%) | 0.39 |
| Cardiac Arrest | 6 (3.35%) | 98 (2.07%) | 0.363 |
| Unplanned intubation | 9 (5.02%) | 78 (1.64%) | 0.002* |
| Unplanned return to ICU | 10 (5.58%) | 109 (2.269%) | 0.01* |
| Unplanned return to OR | 1 (0.55%) | 4 (0.08%) | 0.44 |
Table 4.
Clinical course of COVID positive versus COVID negative trauma patients.
| COVID + Trauma Patients (n = 179) |
COVID - Trauma Patients (n = 4742) | P-value | |
|---|---|---|---|
| Total Ventilator Days (Mean) | 1.642 (0–52) | 0.93 (0–127) | 0.12 |
| Total ICU Days (Mean) | 3.4 (0–52) | 2.14 (0–126) | 0.026* |
| Total Hospital Days (Mean) | 13.15 (0–75) | 6.67 (0–133) | <0.001* |
| Overall Mortality | 8 (4.4%) | 321 (6.7%) | 0.2905 |
Table 5.
Propensity score matched COVID positive to COVID negative trauma patients.
| Variable | COVID + Trauma Patients (n = 83) | COVID - Trauma Patients (n = 1660) | P-value |
|---|---|---|---|
| Age, y | 37 (26–56) | 38 (27–56) | 0.9 |
| Race | |||
| Asian/AI/NH/PI/Other | 1 (1.2) | 38 (2.3) | |
| Black | 58 (69.9) | 1084 (65.3) | 0.7 |
| Unknown | 4 (4.8) | 120 (7.2) | |
| White | 20 (24.1) | 418 (25.2) | |
| Gender, male | 53 (63.9) | 1107 (66.7) | 0.6 |
| Frailty | 15 (18.1) | 224 (13.5) | 0.2 |
| Mechanism, blunt | 66 (79.5) | 1267 (76.3) | 0.5 |
| GCS score, total | 15 (14-15 | 15 (15-15 | 0.2 |
| ISS | 10 (5–17) | 10 (5–17) | 0.6 |
| Shock index | 0.7 (0.7–0.9) | 0.7 (0.7–0.9) | 0.8 |
| MTP activated | 1 (1.2) | 16 (1) | 0.8 |
| Emergent operation | 46 (55.4) | 983 (59.2) | 0.5 |
* Patients matched in 1:20 fashion (1 COVID + to 20 COVID -) using calipers (0.2 x standard deviation) = 0.2 x 0.01 = 0.002.
**Continuous variables are presented as medians (IQR).
***Categorical variables are presented as count (percentage).
Table 6.
Complications and clinical course of propensity score matched COVID positive versus COVID negative trauma patients.
| Variable | COVID + Trauma Patients n = 83 | COVID - Trauma Patients n = 1660 | P-value |
|---|---|---|---|
| Any complication | 6 (7.2) | 118 (7.1) | 0.9 |
| Acute Kidney Injury | 1 (1.2) | 13 (0.8) | 0.7 |
| Acute Lung Injury (ARDS) | 1 (1.2) | 8 (0.5) | 0.4 |
| Pneumonia | 0 (0.0) | 23 (1.4) | 0.3 |
| Cardiac Arrest w/CPR | 2 (2.4) | 21 (1.3) | 0.4 |
| Stroke/CVA | 0 (0.0) | 6 (0.4) | 0.6 |
| Myocardial Infarction | 0 (0.0) | 2 (0.1) | 0.8 |
| SSI (deep) | 1 (1.2) | 9 (0.5) | 0.4 |
| SSI (organ) | 1 (1.2) | 7 (0.4) | 0.3 |
| Osteomyelitis | 0 (0.0) | 2 (0.1) | 0.8 |
| Sepsis | 1 (1.2) | 11 (0.7) | 0.6 |
| Urinary Tract Infection | 0 (0.0) | 3 (0.2) | 0.7 |
| Pulmonary Embolism | 0 (0.0) | 11 (0.7) | 0.5 |
| DVT/Thrombophlebitis | 0 (0.0) | 9 (0.5) | 0.5 |
| Unplanned Intubation | 2 (2.4) | 27 (1.6) | 0.6 |
| Unplanned Return to ICU | 2 (2.4) | 52 (3.1) | 0.7 |
| Unplanned Return to OR | 1 (1.2) | 2 (0.1) | 0.02* |
| Mortality | 4 (4.8) | 32 (1.9) | 0.07 |
| Total ICU days | 4.5 (3–11) | 4 (3-8 | 0.5 |
| Total Vent Days | 4.5 (2–7) | 3 (2–8) | 0.7 |
| Hospital LOS | 7 (3–18) | 5 (2–10) | 0.02* |
*Categorical variables are presented as count (percentage).
**Continuous variables are presented as medians (IQR).
When analyzing their overall hospital course, the cohort of trauma patients with concomitant COVID-19 infection spent 3.4 days in the ICU compared to 2.14 days in the COVID-19 negative cohort (p = 0.026). Their overall hospital lengths of stay were 13.15 days compared to 6.67 days in the COVID negative group (p < 0.001). However, there was no statistically significant difference in mortality (4.4% vs 6.7%, p = 0.29).
In comparing the rates of in-hospital complications between these propensity matched two cohorts, a higher proportion of the COVID positive cohort required unplanned return to the OR (1.2% vs 0.1%, p = 0.02). The COVID positive cohort also had significantly longer overall hospital lengths of stay (7 days versus 5 days, p = 0.02).
4. Discussion
Trauma patients represent a complex population, and those with concomitant COVID-19 infection comprise an even more unique subset of patients. Our study demonstrated that while their demographics and clinical presentation are similar to standard trauma patients, their hospital courses and outcomes differ. As COVID-19 infection is still widely prevalent in the general population, awareness of the complications these patients may be predisposed to is important for providers.
In our subset of trauma patients who tested positive for COVID-19 infection, results were obtained within 24 h of admission. Knowledge of the incubation period of the virus leads us to believe they were already infected at the time of their traumatic event. However, we did not see significant differences between the trauma patients with and without COVID-19 infection in terms of patient demographics, injury patterns or clinical presentation. We speculate that concomitant COVID-19 infection did not have a strong impact on pre-hospital trauma factors for multiple reasons. First, it is possible some of these patients were in the early stages of their infection at the time of injury. Second, this cohort was predominately young males with relatively few pre-existing medical comorbidities. Given their baseline health status, it is plausible they would not have developed severe disease from COVID-19 in the absence of trauma.
Once admitted, however, our subset of COVID-19 positive patients had higher rates of all in-hospital complications studied, with statistical significance in their rates of AKI, sepsis, unplanned intubation and unplanned return to the ICU. According to Kakodkar et al., Sars-CoV-2 virus can have respiratory, cardiovascular, hematologic, renal, gastrointestinal and immunologic involvement.9 It is well established in the literature that traumatic injury can also affect these organ systems.11 It is therefore not surprising that our subset of COVID-19 positive trauma patients demonstrated higher rates of not only respiratory complications, but also renal, immune and cardiac.
Interestingly, while COVID-19 causes a hypercoagulable state and traumatic injury predisposes to prothrombotic complications, we did not see higher rates of deep vein thrombosis of pulmonary embolism in this cohort. We propose that given the severity of illness in this patient population and their limited ability to travel out of the ICU for imaging studies, it is possible these complications were underdiagnosed. Furthermore, our hospital protocols during the time of the study required tiered levels of DVT prophylaxis and anti-coagulation for COVID positive patients based on severity of illness. For more severe infections, this protocolized dose was higher than what is used for standard prophylaxis in trauma patients. It is therefore possible this contributed to the lower rates of prothrombotic complications in this cohort.
The cohort of trauma patients with concomitant COVID-19 infections remained in the ICU 1.25 days longer and in the hospital 6.5 days longer than those without COVID infection. This prolonged length of stay was likely due to the complexity of these patients’ clinical course and their higher proportion of in-hospital complications. Furthermore, when controlling for confounding variables through the propensity score model, this difference in overall hospital length of stay remained significant, indicating COVID positive status alone may be an independent predictor for this outcome.Our propensity score model also demonstrated a higher rate of unplanned return to the operating room in the COVID positive cohort. The etiology of this is likely multifactorial and may be associated with their longer and more complex hospital stays.
Despite this, the cohort of COVID-positive trauma patients did not have a higher overall rate of mortality. We speculate this interesting finding may have been due to a number of factors. First, this may be due, in part, to the baseline health status of this population. It is well established in the literature that young, healthy trauma patients have better outcomes, even in the setting of prolonged hospital courses.11 It is plausible to consider the same may hold true for trauma patients with concomitant COVID-19 infection. Additionally, early in the pandemic, our institution began following regimented protocols for COVID-19 patients which included therapies such as tiered levels of anti-coagulation prophylaxis, aggressive adherence to lung protective ventilator settings, strict compliance with contact precautions and no visitors. While not directly controlled for in our study, it is reasonable to consider that these protocols, additional therapies and staff hypervigilance to the care of COVID positive patients may have impacted mortality rates.
To our knowledge, this is one of the first studies to investigate the impact of COVID-19 infection on hospitalized trauma patients. Our results were similar to the Kaufman et al. study in Pennsylvania, which also found significant increases in complication rates among hospital COVID positive trauma patients as well as longer lengths of stay.10 With COVID-19 infection still widespread in the general population, these findings have practice changing implications. The results of this study support that COVID-19 infection, in the setting of traumatic injury can lead to higher rates of in-hospital complications prolonged clinical courses.
There are several limitations to this study the authors would like to acknowledge. First, it was retrospective in nature, leading to certain confounding variables we did not control for such as pre-hospital interventions, standardization of COVID-19 PCR diagnostic testing protocol and consistencies in practice among the various trauma and critical care providers involved. Additionally, we did not obtain data pertaining to the COVID-19 symptoms experienced by patients with a positive test. Therefore, these results could not be stratified further by severity of COVID-19 infection, and it is plausible to consider that a portion of patients were positive tests had an entirely asymptomatic infection that had no impact on their hospital course.
Furthermore, our study did not distinguish between variants of the Sars-COV-2 virus, therefore the virulence and effect of specific variants on clinical outcomes in trauma patients remains poorly understood. Further, this study was performed at a single institution. This may lead to internal biases in our practices and may limit the extent to which these results are externally valid. Lastly, we recognize the small sample size of the COVID-19 positive patients in relation to the large number of total trauma patients, limiting the number of statistical analysis that could be derived.
While mortality rates were not higher for the afflicted patient population, awareness of the complications they may be predisposed to is important for providers. Potential risk stratification could lead to areas for earlier intervention and prevention and has implications for improved patient outcomes as well as decreased ICU and overall hospital lengths of stay. In the setting of an ongoing pandemic, which has highlighted the importance of appropriate resource allocation in medicine, this is a relevant area for further investigation.
5. Conclusions and future directions
Overall, this study demonstrates that trauma patients with concomitant COVID-19 infection are at higher risk for a multitude of complications as well as longer ICU and hospital courses. This should be further investigated in multi-center study with other regional, urban, level I trauma centers. Such data could be used to generate risk stratification or complication predictive models, allowing for improved management of this unique patient population. Additionally, future research should be directed towards understanding the complex physiologic interplay of COVID-19 infection in the setting of traumatic injury to better understand the etiology of these specific complications and target areas for intervention. The results of such research have practice changing implications, from both a patient outcome and financial perspective.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors of this manuscript have no conflicts of interest to declare. All co-authors have seen and agree with the contents of the manuscript and there is no financial interest to report. We certify that the submission is original work and is not under review for other publication.
Acknowledgements
The authors would like to acknowledge the Trauma Registry team at Grady Memorial Hospital for their timely assistance with data extraction and management.
References
- 1.Ghafil C., Matsushima K., Ding L., Henry R., Inaba K. Trends in trauma admissions during the COVID-19 pandemic in Los Angeles county, California. JAMA Netw Open. 2021;4(2) doi: 10.1001/jamanetworkopen.2021.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mattingly A.S., Rose L., Eddington H.S., et al. Trends in US surgical procedures and health care system response to policies curtailing elective surgical operations during the COVID-19 pandemic. JAMA Netw Open. 2021;4(12) doi: 10.1001/jamanetworkopen.2021.38038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pei S., Yamana T.K., Kandula S., Galanti M., Shaman J. Burden and characteristics of covid-19 in the United States during 2020. Nature. 2021;598:338–341. doi: 10.1101/2021.02.15.21251777. [DOI] [PubMed] [Google Scholar]
- 4.Chao G.F., Li K.Y., Zhu Z., et al. Use of telehealth by surgical specialties during the COVID-19 pandemic. JAMA Surgery. 2021;156(7):620. doi: 10.1001/jamasurg.2021.0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sutherland M., McKenney M., Elkbuli A. Gun violence during COVID-19 pandemic: paradoxical trends in New York city, Chicago, Los Angeles and Baltimore. Am J Emerg Med. 2021;39:225–226. doi: 10.1016/j.ajem.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abdallah H.O., Zhao C., Kaufman E., et al. Increased firearm injury during the COVID-19 pandemic: a hidden urban burden. J Am Coll Surg. 2021;232(2):159–168. doi: 10.1016/j.jamcollsurg.2020.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans D.P., Hawk S.R., Ripkey C.E. Domestic violence in Atlanta, Georgia before and during COVID-19. Violence Gend. 2021;8(3):140–147. doi: 10.1089/vio.2020.0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ssentongo P., Fronterre C., Ssentongo A.E., et al. Gun violence incidence during the COVID-19 pandemic is higher than before the pandemic in the United States. Sci Rep. 2021;11(1) doi: 10.1038/s41598-021-98813-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kakodkar P., Kaka N., Baig M.N. A comprehensive literature review on the clinical presentation, and management of the Pandemic Coronavirus Disease 2019 (covid-19) Cureus. 2020 doi: 10.7759/cureus.7560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaufman E.J., Ong A.W., Cipolle M.D., et al. The impact of COVID-19 infection on outcomes after injury in a state trauma system. Journal of Trauma and Acute Care Surgery. 2021;91(3):559–565. doi: 10.1097/ta.0000000000003310. [DOI] [PubMed] [Google Scholar]
- 11.Ong A.W., Omert L.A., Vido D., et al. Characteristics and outcomes of trauma patients with ICU lengths of stay 30 Days and greater: a Seven-year retrospective study. Crit Care. 2009;13(5) doi: 10.1186/cc8054. [DOI] [PMC free article] [PubMed] [Google Scholar]


