Abstract
Background
COVID-19, a disease caused by infection with the SARS-CoV-2 virus, is asymptomatic or mildly symptomatic in most cases. Some patients, usually burdened with risk factors develop acute respiratory failure and other organ dysfunction. In such cases, the mortality rate is very high despite the use of intensive therapy. Amantadine has complex activity including antiviral, antiinflammatory and dopaminergic effects. This clinical trial will assess the efficacy and safety of amantadine in the prevention of COVID-19 progression toward acute respiratory failure and neurological complications.
Methods and results
The trial will enroll 200 patients who are positive for SARS-CoV-2 infection and have one or more risk factors for worsening the disease. These patients will be included as hospitalized or ambulatory subjects for early treatment of illness. The recruitment will take place in 8 centers covering different regions of Poland. For 14 days they will be given either 200 mg of amantadine a day or placebo. Our hypothesis is a considerable reduction in the number of patients with progression toward respiratory insufficiency or neurological complications thanks to the treatment of amantadine.
Conclusions
Demonstrating the efficacy and safety of amantadine treatment in improving the clinical condition of patients diagnosed with COVID-19 is of great importance in combating the effects of the pandemic. It has potential to influence on the severity and course of neurological complications, which are very common and persist long after the infection as long-COVID syndrome.
Clinical trial registration:www.clinicaltrials.gov identification no. NCT04854759; Eudra CT number: 2021–001144-98 (dated 27 February 2021).
Keywords: COVID-19, Amantadine, Outcome, Neurological complications, Trial, Protocol
1. Introduction
According to the current knowledge a vast majority of people infected with SARS-CoV-2 virus develop disease symptoms, appearing most often after the incubation period of 5 to 7 days. [1,2] Three stages of COVID-19 are described as follows: early infection is characterized by mild to moderate, usually influenza-like symptoms; pulmonary stage is characterized by shortness of breath without or with overt signs of hypoxia and pneumonia-like symptoms detectable radiologically; hyperinflammatory stage is characterized by sepsis of lungs and multi-organ failure [3]. The majority of patients encounter COVID-19 restricted to the early infection phase and do not require hospitalization. Others develop the pulmonary phase but the disease does not progress further. A small, but significant fraction enters the hyperinflammatory stage, with a very high mortality level ranging from 1.5% to 9.8%.
Although the search for efficacious pharmacological treatments related to COVID-19 was very intense, it was focused mainly on the development of preventive vaccines and drugs suited for use in the advanced disease. Much less attention was paid to drugs that could be used as prophylaxis or in the early stage of the disease, in particular to those which are not exclusively antiviral [4].
Only a few clinical studies have been published describing effects on COVID-19 evolution of pharmacological pre-exposure prophylaxis, post-exposure prophylaxis or early treatment. Nonetheless, some promising results have been obtained recently. An example of apparently beneficial pre-exposure prophylaxis is reduced severity of COVID-19 disease and related mortality noted in diabetic and/or overweight patients taking metformin prior to the virus exposure. This effect was attributed to the anti-inflammatory and perhaps also to some antiviral activity of the drug [5, 6]. An example of apparently successful post-exposure prophylaxis and early treatment of COVID-19 is the effect of fluvoxamine, an antidepressant belonging to selective serotonin reuptake inhibitors. There is no data on antiviral activity of fluvoxamine, but in a preclinical experiment it has been demonstrated that the drug is efficacious in preventing lipopolisaccharide-induced sepsis, the effect attributed to targeting the sigma-1 receptor in the endoplasmic reticulum [7]. In a blinded, randomized and placebo-controlled trial and in a subsequent open controlled trial none of the SARS-CoV-2 infected patients taking fluvoxamine for 2 weeks did present COVID-19 symptoms, compared with a significant number of patients not treated with the drug [8,9].
Patients suffering from chronic neurological diseases such as Parkinson's disease (PD) were considered at high risk of succumbing to the severe form of COVID-19 [10]. Therefore, identifying a group of patients with PD (and also with multiple sclerosis) who were infected with SARS-CoV-2 but did not develop clinical manifestations of the infection was surprising. All these patients were treated with amantadine, and it was hypothesized that escaping severe form of COVID-19 could be a consequence of its pharmacological activity targeting both the virus life cycle and the host reaction [11,12].
Amantadine is an old drug with a versatile pharmacodynamic profile [13]. Its antiviral activity has been discovered in 1963, and from 1966 the drug had been widely used for prophylaxis and treatment of influenza A, until the virus become resistant in the first decade of 20th century [14]. Serendipitous observations led to a formal repurposing of amantadine to treat Parkinson's disease and to its off-label use for alleviating fatigue in multiple sclerosis [15,16]. It has also been found that the drug exhibits clinically significant activity in Borna virus-1 infection, attributed both to its antiviral activity and antidepressant effects [17].
Benefits of amantadine in COVID-19 have been suggested in a few case reports and reviews [[18], [19], [20]]. Interaction with SARS-CoV-2 viroprin E channel and with the virus receptor binding domain were proposed as the relevant molecular targets of the drug. [21,22] Of note is also that in the concentration range covered by therapeutic doses amantadine is an effective agonist of sigma-1 receptors, in which it resembles the aforementioned drug fluvoxamine. [13]
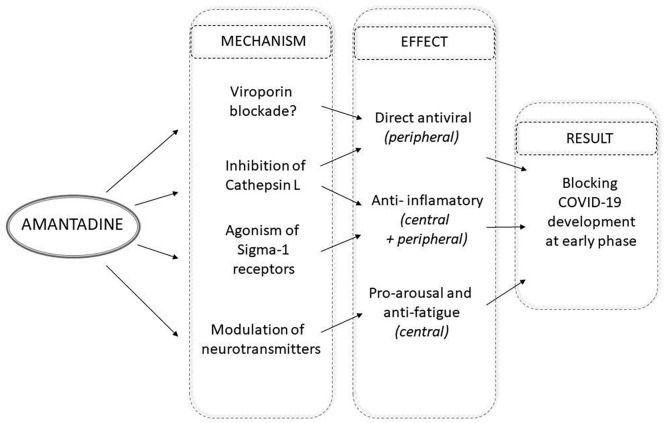
In the context of SARS-CoV-2 and COVID-19 possibly the most important target for amantadine is cathepsin L (CTSL), a lysosomal enzyme implicated in many pathologic conditions including inflammatory status and viral infections, SARS-CoV-2 in particular [23]. In the in vitro screening assay amantadine was found to inhibit expression of cathepsins L and B, which led to the suggestion that the drug may prove useful for prevention of SARS-CoV-2 entry to cells [24]. Very recently it has been found that along with the development of COVID-19 symptoms a rise of CTSL protein level is detected in blood, which is positively correlated with the disease severity. This observation, together with the results of in vitro experiments with a SARS-CoV-2 pseudovirus, led to a hypothesis that enhancement of cellular CTSL expression caused by SARS-CoV-2 infection is a pivotal part of the vicious cycle leading to the clinically severe COVID-19 [25]. Fig. 1 summarizes the aforementioned mechanisms that may participate in putative beneficial effects of amantadine in early phase of COVID-19.
Fig. 1.
Possible mechanisms of amantadine activity in the early phase of COVID-19.
2. Trial design and methods
The study protocol was approved by the referral Ethics Committee (Medical University of Lublin, Lublin, Poland) and the institutional review boards of all participating hospitals. The trial was approved by the Polish Agency of Drugs and Medical Devices (Urząd Rejestracji Produktόw Leczniczych; URPL) as a clinical randomized study with drugs on 30 March 2020.
2.1. Trial objectives
The aim of the project is to determine whether oral intake of amantadine in the early phase of COVID-19 can prevent the development of the disease toward acute respiratory failure and multiple organ failure, reducing the need for oxygen therapy, intubation and respiratory therapy as well as delayed neurological complications.
2.2. Trial design
This is prospective, multicenter, randomized (1:1), double blind, placebo controlled trial (RCT). The trial was designed in accordance with the Declaration of Helsinki, the Convention of the European Council related to human rights and biomedicine, and within the requirements established by Polish legislation in the field of biomedical research, the protection of personal data, and bioethics.
Patients are being randomized and enrolled in 8 centers in Poland, and data are being collected and analyzed in Poland. Study sites are listed in Appendix 1. Study participants will be randomized (1:1) to treatment with amantadine (Arm A) or placebo (Arm B), administered orally at a dose of 100 mg twice daily (morning and noon) for a period of 14 days.
The dose may be modified in the event of poor tolerance (up to 1 × 1 daily 100 mg) or worsening of clinical symptoms (max. 4 × 1 per 100 mg for no longer than 2 days, as a loading dose). The above treatment will be added to the standard care and treatment recommended in the early phase of infection. Open label study is planned for patients participating in the RCT study lasting between Day 15 and Day 210. Treating physician will offer a patient to receive amantadine administered orally at a dose of 100 mg once daily (morning) for a period of additional 14 days based on clinical status and consent of a patient.
The inclusion and exclusion criteria of the study are presented in Table 1 , while study outcomes in Table 2 .
Table 1.
Inclusion and exclusion criteria of the study.
| Inclusion criteria |
|---|
|
1. Men and women aged 18 and over 2. Can give informed consent 3. Confirmed positive result for SARS-CoV-2 within 5 days from the date the result was issued (according to the laboratory report) 4. Patient presently symptomatic with one or more of the following symptoms: fever, cough, myalgia, mild dyspnoea, chest pain, diarrhoea, nausea, vomiting, anosmia, lack of taste, sore throat, nasal congestion 5. At initial screening, the subject will report at least one and no >3 of the following risk factors for clinical worsening: age ≥ 40, obesity, hypertension, diabetes, pulmonary disease (e.g. asthma, chronic obstructive pulmonary disease), immune disorders (e.g. rheumatoid arthritis, lupus) and neurological diseases (e.g. after a distant stroke or trauma to the brain, multiple sclerosis, dementia and other neurodegenerative diseases) 6. Outpatients or hospitalized patients due to meeting the above criteria and requiring observation in a hospital or outpatient. |
| Exclusion criteria |
| 1. Disease severe enough to meet the study's primary endpoint of clinical worsening (e.g. moderate to severe dyspnea with current O2 saturation < 92% with patient exposure to room air, current use of supplemental oxygen to maintain O2 saturation ≥ 92%) 2. WHO score ≥ 4 (requires oxygen therapy during hospitalization) 3. Concomitant diseases which, in the opinion of the attending physician, prevent the patient from participating in the study, such as: decompensated cirrhosis, active ulcer disease, epilepsy and symptomatic convulsions, untreated angle-closure glaucoma determined on the basis of the patient's interview and/or medical documentation. In addition, immunocompromised patients (solid organ transplant, BMT, AIDS, renal failure (patients with renal impairment may develop drug poisoning) or other diseases not mentioned and other diseases treated with biological, immunological and/or steroids in high doses (>20 mg prednisone daily) will not be eligible for the study. 4. Hypersensitivity to any component of the preparation, severe congestive heart failure, cardiomyopathy, myocarditis, II-III degree AV block, bradycardia, clinically significant prolongation of the QT interval, or a family history of congenital long QT syndrome, severe ventricular arrhythmias (including torsade de pointes), concomitant use of drugs that prolong the QT interval, hypokalaemia, hypomagnesaemia 5. Pregnancy, the period of breastfeeding 6. Parallel intake of memantine or other drugs acting on the CNS (neuroleptics, anxiolytics, antiepileptic drugs, antidepressants) 7. Other neurological conditions with agitation or confusion, delirium syndromes or psychoses 8. Receipt of a partial or full vaccination schedule against SARS-CoV-2. |
Abbreviations: OSCI-WHO scale: Ordinal Scale for Clinical Improvement –World Health Organization scale (https://www.who.int/blueprint/priority-diseases/key-action/COVID-19_Treatment_Trial_Design_Master_Protocol_synopsis_Final_18022020.pdf).
Table 2.
Study outcomes
| Primary endpoints (Up to day 15 of follow-up after randomization) |
|---|
| Occurrence of clinical worsening defined as any of the following: a. Moderate or severe dyspnoea b. Drop in O2 saturation (<92% with patient exposure to room air) and/or additional oxygen demand to maintain O2 saturation ≥ 92%) and/or c. Achievement of ≥4 points on the WHO [OSCI-WHO] scale (7-point clinical status assessment scale) Meeting the above criteria qualifies the patient for further treatment in hospital, in accordance with the current recommendations |
| Secondary endpoints (Day 15 or optionally on Day 30 in double blind phase) and 90, 150, 210 (in open label phase) from randomization) |
| a. General Health Assessment (PROMIS Global Health Scale) b. The neurological assessment will include the assessment of neurological functions based on scales for: - fatigue (Fatigue Severity Scale) - depression (Beck Depression Inventory (BDI)), - disorders of smell and taste (Visual Analogue Scale (VAS)), - sleep disorders (The Epworth Sleepiness Scale (ESS)) - quality of life (The Short Form-36 Health Survey Questionnaire (SF-36)) c. Time to clinical deterioration d. Time of Survival |
2.3. Recruitment and patient consent
Information on potential study participants with a positive SARS-CoV-2 test will be disclosed by the medical doctors in the Scientific Board of the study to the study investigators for the purpose of recruitment. Potential study participants will receive an invitation letter, written information on the study and contact information. Information on the study and contact information will also be announced on several digital and non-digital platforms. Non-digital platforms include posters in the health-care sector and digital platforms include hospital internal web pages, healthcare professional information sites.
Informed consent will be obtained from eligible patients or their substitute decision-makers (for patients lacking decision-making capacity) by study physicians. The consent form includes provisions for research data and samples and residual clinical blood samples to be stored for future scientific research on COVID-19.
2.4. Methods of data collection
Data collection will be done by physician or specialist nurse trained in the study protocol. All visits will be performed according study protocol as well as all clinical and laboratory data will be loaded into electronic central CRF system (web based data protection system designed and dedicated for the COV-PREVENT study) by physician or specialist nurse based on patient’ report and clinical and laboratory data.
2.5. Study procedures
In the course of the study the following procedures will be performed as presented in Table 3 .
Table 3.
Study procedures; a) Chest computed tomography (CCT) - changes in the thoracic CT image will be analyzed using the The total severity score (TSS) [Li et al., 2020] or chest X-ray examination (as decided by the attending physician). b) Hematology: hemoglobin, morphology with smear, ESR. c) Biochemistry: C-reactive protein; α-hydroxybutarate dehydrogenase (HBDH); creatinine with GFR (Glomerular Filtration Rate) calculation, pro-calcitonin, LDH, ALT, AST; D-dimers; fibrinogen, ferritin, interleukin-6 d) Only for women of childbearing age e) If not made at diagnosis f) We will conduct a clinical assessment of respiratory fitness every day for 14 days of the study duration with SO2 measurement every 8 h and based on the WHO recommended 7-point scale for clinical improvement (OSCI) (1-line criterion) and general condition assessment questionnaires health (PROMIS Global Health Scale) (2-line criterion). Measurement of SO2 does not apply to patients discharged home. g) ECG during the rest phase and after 1 min of light effort with the assessment of saturation h) ECG should be performed 1 and 3 weeks after the start of therapy (measurement together with QTC determination by Bazett's method). An ECG should also be done before increasing the dose and two weeks after each dose increase. Ultimately, however, it is up to the attending physician to decide. *** Day 30 is a follow-up visit - if all procedures cannot be performed (the patient ends drug / placebo on day 15) or the post-treatment visit in the open part two with a positive SAR-CoV-2 antigen test on day 15 maintenance of symptoms) - treatment could be continued for another 14 days in the open label phase after the decision of the attending physician. This visit does not apply to patients who have completed all Day 15 procedures and have entered the open-label phase. i) In the case of hospitalization of the patient or an outpatient visit, the attending physician will decide to perform the procedure.
| Screening |
Randomization visit |
Monitoring visits |
Final visit |
|
|---|---|---|---|---|
| Time point | Day −5 to Day 1 | Day 1 (D1) |
Day 2–14 (D2-D14) |
Day 15 (Day 30***) (D15; D30) |
| Procedures | ||||
| Informed consent | x | |||
| Demigraphic and medical history | x | |||
| Inclusion and exclusion criteria assessment | x | x | ||
| Confirmation of positive test for SARS-CoV-2 infection | x | |||
| Physical examination | x | x | xi | x |
| Neurological examination | x | x | x | |
| Vital signs assessment | x | x | xi | x |
| Height | x | |||
| Weight, Body Mass Index (BMI) | x | x | x | |
| Computed tomography/X-ray of chest a | x | x | ||
| Hematologyb | x | x | ||
| Biochmistryc | x | x | ||
| Pregnancy test in blood d | x | |||
| Pregnancy test in urine d | x | x | ||
| SARS-CoV-2 test (PCR or antygen) | xe | x | ||
| SARS-CoV-2 antibodies in serum | x optional | X | ||
| Electrocardiography | x | x | ||
| Vital signs | xg | x | xh | xh |
| Drug compliance | xf | xf | xf | xf |
| PROMIS Global Health Scale | x | x | x*** | |
| WHO scale | x | x | ||
| Fatigue Severity Scale | x | x | x | x |
| Beck Depression Inventory | x | x | ||
| Visual Analogue Scale | x | x | ||
| The Epworth Sleepiness Scale | x | x | ||
| The Short Form-36 Health Survey Questionnaire | x | x | ||
| Adverse effects (AE/ SAE) |
x | x | ||
| Adjunctive treatment review | x | x | x | |
| SARS-CoV-2 test (PCR or antigen) | x | x | x |
2.5.1. Screening and randomization visit for the RCT part of the study
Part of the medical history may be collected remotely by the attending physician for initial assessment. Study-specific screening and randomization procedures may not begin until signed patient consent to participate in the study (PIC) has been obtained and must be completed before the patient is randomized and before the first dose of the drug is administered. During this visit, the patient will be trained on the procedures related to the drug (intake, storage and return schedule). The patient will be hospitalized upon enrollment in the study or kept out-patient based on the assessment of clinical status. Those patients who can be discharged home will be monitored remotely.
2.5.2. Monitoring visits on Days 2–14
Ambulatory or hospitalized patients will be issued with a diary to record temperature, number of breaths / min and heart rate at rest and the PROMIS scale.
During follow-up visits, the main outcome measure will be the PROMIS scale completed by the patient and the assessment of respiratory function (saturation) based on WHO scale [26]. In addition, the attending physician will collect information on concomitant medications and the occurrence of possible adverse events - to be completed in the medical records and the eCRF.
2.5.3. Final visit for the RCT part of the study
The final visit will be performed on Day 15 (D15). On this day, the patient (for patients with a negative SARS-CoV-2 test result) will be issued: a diary to continue records during observation visits and 3 sets of PROMIS and neurological scales. The medication previously delivered to the patient home will also be counted.
Optional visit on Day 30. The Day 30 (D30) visit will take place on two occasions:
(1) persistence of COVID-19 symptoms and a positive virus test on Day 15.
(2) the patient was unable to complete all day 15 procedures (D15) at the end of treatment.
The Day 30 visit includes all Day 15 visit procedures for health assessment at the end of treatment.
2.5.4. Open label part of the study
During Day 15 (or optionally on Day 30) there will be the beginning of the open label part of the study. The treating physician will offer a patient to extend the treatment by 14 days based on clinical status of a patient and his consent to receive amantadine.
Consecutive visits will be performed on Day 90, 150, 210 in order to assess clinical status of the patient based on PROMIS and neurological scales.
2.6. Risk and methods to protect against bias
All patient data will be identified by a unique participant ID number. After obtaining consent from the patient, the investigator will allow the study monitor, independent auditor, or regulatory body personnel to review the portion of the patient's medical records that is directly related to the study. This applies to all relevant documentation of the study, including the patient's medical history to verify his / her eligibility for the study, laboratory test results, information on admission from hospitalization during the patient's participation in the study, as well as the results of post-mortem examinations in the event of the patient's death. During the test (if applicable). All data will be treated confidentially. Clinical trial documentation should be kept at the facility for 25 years. Patient's medical documentation will be managed in accordance with current Polish law. The clinical trial documentation cannot be destroyed without the prior consent of the trial sponsor.
2.7. Randomization and allocation concealment
A secure, web-based randomization system will be used to allocate treatment assignments.
The test is fully blinded, which means that the researcher's team and the patient do not know what preparation has been assigned to them (arm A or arm B). The medication in both arms is identically packed to prevent unblinding. All patients meeting the inclusion criteria and none of the exclusion criteria will be randomized 1: 1 to the study using the central Interactive Web Response System (IWRS). A randomization list will be constructed according to a flowchart inside each site. Before testing is initiated at the facility, each investigator will receive written instructions to explain the IWRS procedure and to perform an emergency unblinding. In the event of a threat to the patient's life or other clinical situation that endangers his safety, the Investigator is obliged to contact the medical examination monitor in order to assess the need for further treatment and possible emergency unblinding. If contact with the medical monitor was not possible, and the unblinding has occurred (procedures in the IWRS system), the Investigator is obliged to report this situation to the Study Sponsor within 24 h. Each patient is identified in the study by an individual number. The number of the patient will be assigned by the IWRS system. The individual number is assigned to the patient during the consent signing process and cannot be used again.
2.8. Analytic plan
2.8.1. Sample size and power calculations
The primary endpoint of the study is the proportion of patients in each arm who experienced a clinical worsening within 15 days from randomization. The proportion of patients who experienced a clinical worsening within 15 days from randomization will be compared between study arms using Fisher's exact test. It is planned to enroll 200 patients in the study, who will be randomized in the proportion 1:1 to study arms (100 patients in the active arm and 100 patients in placebo arm). Drop-out is assumed to be 10%, thus it is expected to include 180 patients in the statistical analysis. Assuming that the percentage of patients in the placebo group with clinical deterioration within 15 days from randomization will be approximately 20% (based on previous observations in ambulatory patients) [8], if the proportion of patients in the active arm with a clinical worsening in 15 days from randomization is not >5.1%, inclusion of 180 patients in the primary endpoint analysis will provide at least 80% power in detecting the difference in the percentages between the treatment arms as a statistically significant at a significance level set at 0.0492. The minimal detectable difference between study arms of 14.9% was considered a sufficient difference to detect clinically significant differences.
2.8.2. Populations in the statistical analysis
2.8.2.1. Intention to treat population (ITT)
All patients meeting the inclusion criteria, not meeting the exclusion criteria, who were randomized (classification by study arms as randomized will be used in the statistical analysis). The ITT population will be main analysis population in the primary and secondary efficacy endpoints analysis.
2.8.2.2. Per protocol population (PP)
All patients meeting the inclusion criteria, not meeting the exclusion criteria, for who no significant protocol deviation were reported (i.e. protocol deviations that affect a reliable assessment of endpoints). The PP population will be used in the sensitivity analysis of the primary and secondary efficacy endpoints.
2.8.2.3. Safety population (SAS)
All patients enrolled in the study who received at least one dose of study drug or placebo. The SAS population will be analyzed for the safety of treatment according to the treatment used in the individual patient (test drug or placebo).
2.8.3. Demographics and baseline characteristics analysis
Demographics (age, gender) and baseline characteristics (height, weight, BMI, vital signs, symptoms at screening, medical history, results of laboratory and imaging tests performed at screening) will be analyzed using descriptive methods and compared between study arms. The descriptive statistics for categorical variables will include the number and percentage of occurrences. Descriptive statistics for continuous variables will include the mean and standard deviation (SD), the median with the 25th and 75th percentiles (Q1 and Q3), and the minimum and maximum. The distribution of categorical variables will be compared between subgroups using either the Fisher test or the chi-square test, depending on the expected size of the categories. Continuous variables with a normal distribution (the normality of the distribution will be tested with the Shapiro-Wilk test) will be compared between the subgroups using the Student's t-test, otherwise the Mann-Whitney test will be used.
2.8.4. Analysis of primary and secondary outcomes
Statistical analysis plan (SAP) will be prior to the database closure. SAP will cover the description the planned statistical analysis of the data in more details, as well as any modifications to the analysis plan described below. Unless otherwise stated, the tests used will be two-tailed.
One interim analysis is planned to analyze the results for the primary endpoint after collecting the data on primary outcomes for 50% of the planned final number of patients. The following values of statistical significance levels will be used in the analysis of the primary endpoint (O'Brien-Fleming method for group sequential design with one interim analysis, with an overall significance level of 0.05): in the interim analysis 0.0054 and in the final analysis 0.0492. A significance level of 0.05 will be used in the analysis concerning the remaining endpoints of the study. The final primary endpoint analysis will be performed after the primary endpoint data has been collected for all patients included in the study, the remaining endpoint analysis will be performed after the end of the study.
2.8.4.1. Primary endpoint analysis
The primary endpoint of the study is the proportion of patients in each arm of the study who experienced a clinical worsening within 15 days of randomization, defined as dyspnoea and/or a decrease in O2 saturation (<92% with patient exposure to room air) or additional need for oxygen to maintain O2 saturation ≥ 92%) or achieving a OSCI-WHO score ≥ 4 (7-point clinical status scale) [26]. The primary endpoint will be assessed on study Day 15.
The null hypothesis for primary endpoint is that there is no significant difference in the proportion of patients who experienced clinical deterioration within 15 days from randomization between the active and placebo arm of the study. Fisher's exact test will be used to test the null hypothesis. In addition, 95% confidence interval for the percentages will be reported. No data imputation methods will be applied, data will be analyzed as available.
As a part of exploratory analysis, treatment effect on odds for experiencing clinical deterioration within 15 days from randomization adjusted for symptoms severity at baseline, risk factors, age, gender and days since symptoms onset will be assessed using multivariable logistic regression model. To adjust results for the potential mediating effect of hospital care, analysis stratified for hospitalization status at Day 3 will be performed as a part of sensitivity analyses.
2.8.4.2. Secondary efficacy endpoints analysis
Secondary efficacy endpoints of the study are: time to clinical deterioration (assessed up to day 210 of the study), overall survival (assessed up to day 210 of the study), general health assessment on the PROMIS scale, neurological assessment on the fatigue, depression, smell and taste, sleep disturbance, and quality of life (assessed on study days 15 (30), 90, 150 and 210). Data on secondary endpoints will be analyzed using descriptive methods and compared between the study arms (see demographics and baseline characteristics section for description of descriptive methods to be used). Change from baseline for general health assessment on the PROMIS scale, neurological assessment on the fatigue, depression, smell and taste, sleep disturbance, and quality of life in consecutive time points will be reported and compared between study arms. Time to clinical worsening and data on overall survival will be analyzed using survival analysis methods: the Kaplan-Meier survival curve estimator will be used to estimate the median time to event with 95% CI and the probability of event occurrence up to the selected time points with 95% CI; survival curves will be compared between the study arms using the log-rank test; Cox proportional hazards model will be used to estimate hazard ratio for treatment. No data imputation methods will be applied, data will be analyzed as available.
As a part of exploratory analysis, treatment effect on time to clinical deterioration and overall survival adjusted for symptoms severity at baseline, risk factors, age, gender, and days since symptoms onset will be assessed using multivariable Cox proportional hazards model. Treatment effect on general health assessment on the PROMIS scale, neurological assessment on the fatigue, depression, smell and taste, sleep disturbance, and quality of life will be additionally assessed using linear mixed effects models for repeated measures, with fixed effects for study arm, time, and study arm and time interaction term, symptoms severity at baseline, risk factors, age, gender, and days since symptoms onset.
2.8.5. Safety analysis
The analysis of the safety profile will be based on the prevalence of AE and SAE and the analysis of safety parameters (vital signs, ECG, laboratory parameters). The analysis will be carried out using descriptive methods, by the measurement time point. The results will be compared between the study arms (see section on demographics and baseline characteristics for description). Additionally, descriptive statistics for the change in the value of selected safety parameters relative to the baseline value will be presented
2.8.6. Interim analysis
One interim analysis for efficacy is planned to evaluate outcomes for the primary endpoint. It will be performed after collecting data on the primary outcomes for 50% of the planned number of patients to be enrolled in the study (i.e. 100 patients). The remaining endpoints will only be assessed in the final statistical analysis. Following values of statistical significance level will be used in the interim and final analysis of the results for the primary endpoint (O'Brien-Fleming method for group sequential design with one interim analysis, with the overall significance level of the study 0.05): in the interim analysis 0.0054 and in the final analysis 0.0492. In the analysis concerning the remaining endpoints of the study, a significance level of 0.05 will be adopted. No stopping criteria for safety reasons are planned, as safety endpoints will not be assessed in the interim analysis
2.9. Risks to the safety of potential participants
Investigators and sponsor are obliged to follow the study protocol, including reporting all adverse events, serious adverse events and suspected unexpected serious adverse reactions to the relevant authorities as outlined by the Polish Health and Medicine Authority and the European Commission
Participants will be thoroughly asked if they have experienced any adverse event during the study period in the online questionnaires. Adverse events will be registered in predefined electronic case report form (eCRFs). All adverse events will be followed until they have abated, or until a stable situation has been reached. Online questionnaires will be evaluated weekly
All adverse events must be evaluated by the principal investigator in order to determine possible causal association with amantadine. At study termination, a final report of registered events in the eCRFs will be included in the Eudra-CT reports
All adverse events and reactions will always be registered in predefined case report forms and thus available to monitoring units. Each adverse event/reaction will be assessed by the investigator, and if indicated further examinations will be initiated and relevant measures taken. However, if these events/reactions are considered mild without need for medical attention or intervention and insignificant to the patient's continuation in the study, the sponsor will not be informed
Serious adverse events must be reported by investigators to the sponsor within 24 h. The investigators are obliged to fill out the relevant reporting file electronically. Serious adverse events should be assessed for possible causality to amantadine, and the sponsor will then assess whether this is expected according to the summary of product characteristics (SPC). If expected, it will be considered a serious adverse reaction. However, if unexpected it will be registered as a Suspected Unexpected Serious Adverse Reaction (SUSAR). In case of death or life-threatening disease, the sponsor will report the SUSAR to the Polish Medicines Agency. Within the following 8 days, a detailed report on the implemented measures in the given case will be made. All other SUSARs will be reported within 15 workdays. All SUSARs will lead to unblinding prior to reporting to the Polish authorities
2.9.1. Study monitoring/committees
The trial steering committee consists of the principal investigators from all centers
Sponsor responsibilities include:
-
-
Monitoring of clinical sites
-
-
Contact to regulatory authorities
-
-
Annual safety reporting to the Scientific Ethical Committee
-
-
Assessment of adverse events and reporting of SUSARS to relevant regulatory entities
-
-
Monitoring and reporting of protocol violations
-
-
Data management
Oversight will be ensured through contracts between sponsor and contributing sites with detailed descriptions of responsibilities, standard operating procedures including description of reporting of adverse events and protocol violations. Moreover, oversight will be ensured though monitoring reports from regular external monitoring visits performed by GCP-units
An independent data safety monitoring board (DMSB) will receive the results of the interim analysis as well as adverse events and serious adverse events reports. Based on previously described trial conclusion parameters the DSMB may recommend stopping the entire trial for superiority, futility or harm. The DSMB is empowered to report independently to health authorities at their discretion
2.10. Estimated duration of the trial
Based on projected enrolment rates we anticipate trial completion within 18 months
3. Current Status
The trial commenced recruitment on 3 Apr 2021 according to protocol version V1.0 (1 Mar 2021) and all study centres are active
4. Discussion
Whereas effective vaccination against SARS-CoV-2 is of utmost importance for containment of the COVID-19 pandemic, a substantial fraction of the general population will not benefit vaccines. The reasons range from reluctance to vaccinate to inadequate response to a vaccine, development of mutated virus resistance to available vaccines, etc. Drugs effective in preventing unfavourable outcome of the disease are undoubtedly needed
It is frequently believed that a drug to be effective in a viral disease should directly target virus life cycle. However, an alternative approach termed the host-directed therapy (HDT) could also be effective. In HDT drugs modify disease progression through targeting host factors, for example by interfering with mechanisms required for productive replication of a pathogen, inhibiting pathways perturbed by a pathogen that lead to hyperinflammation, and other mechanisms [27]. Interestingly, in COVID-19 amantadine may act both as a sensu stricte antiviral drug and a HDT [12]
Progression of COVID-19 toward severe and potentially fatal stage of the disease starts in vulnerable patients shortly after the occurrence of infection. [28,29] Amantadine is potentially well suited for early, prehospital treatment of COVID-19 because it is well established drug which may be applied orally, and which side effects are well known and mild in the majority of patients. If benefit (in terms of preventing disease progression) of early amantadine treatment of patients infected with SARS-CoV-2 is shown, a positive risk-benefit ratio of such treatment will certainly ensue. In addition, the novelty of the trial is to examine and follow neurological signs and symptoms in the course of COVID-19 and to demonstrate whether centrally acting CNS drug might prevent them in extended follow-up period [30]
The study has some limitations which should be considered. The rapidly evolving nature of the pandemic, with new treatments, vaccinations, and virus variants will likely result in a heterogenous population enrolled in the trial. The sensitivity analyses in the various subgroups detailed above will be used to identify if any of these factors significantly affected the results. Currently, increasing number subject among population are after vaccination but small proportion of subjects get infected. Our study will not include such patients
In conclusion, demonstrating the efficacy and safety of amantadine treatment in improving the clinical condition of patients diagnosed with COVID-19 is of great importance in combating the effects of the pandemic. It has potential to influence on the severity and course of neurological complications, which are very common and persist long after the infection as long-COVID syndrome
Sources of funding
The study was funded by a grant no 2020/ABM/COVID19/SPSK4 from the Medical Research Agency in Poland.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
Acknowledgements
The authors thank Dr. Paweł Pinkosz for help with site selection and contracting and administrative support. CRO CCTS Sp. z o.o. team was instrumental with all administrative and registration process for the study
Appendix 1
Study sites:
Samodzielny Publiczny Szpital Kliniczny Nr 4 w Lublinie; Uniwersytet Medyczny w Lublinie;
Prof. dr hab. n. med. Konrad Rejdak - Principal Investigator
Uniwersyteckie Centrum Kliniczne; Warszawskiego Uniwersytetu Medycznego w Warszawie
Prof. dr hab. Piotr Fiedor - Principal Investigator
Regionalny Szpital Specjalistyczny im. dr. Władysława Biegańskiego; Samodzielny Publiczny Zakład Opieki Zdrowotnej w Grudziądzu
Dr. n. med. Robert Bonek - Principal Investigator
Samodzielny Publiczny Zespół Zakładów Opieki Zdrowotnej w Wyszkowie
Lek. med. Waldemar Chełstowski - Principal Investigator
Kliniczny Szpital Wojewódzki Nr 2 im. Św. Jadwigi Królowej w Rzeszowie
Dr. n. med. Agnieszka Gala-Błądzińska- Principal Investigator
Samodzielny Publiczny Szpital Wojewódzki im. Jana Bożego w Lublinie
Dr. n. med. Sławomir Kiciak- Principal Investigator
SPZOZ Kalwaria Zebrzydowska, Kalwaria Zebrzydowska, Poland
Lek. med. Mateusz Dec – Principal Investigator
Centralny Szpital Kliniczny Ministerstwa Spraw Wewnętrznych i Administracji
Dr. n. med. Zbigniew J. Król - Principal Investig
References
- 1.Buitrago-Garcia D., Egli-Gany D., Counotte M.J., et al. Occurrence and transmission potential of asymptomatic and presymptomatic SARS-CoV-2 infections: A living systematic review and meta-analysis. PLoS Med. 2020;17:e1003346. doi: 10.1371/journal.pmed.1003346. Sep 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rai B., Shukla A., Dwivedi L.K. Incubation period for COVID-19: a systematic review and meta-analysis. Z Gesundh Wiss. 2021:1–8. doi: 10.1007/s10389-021-01478-1. Feb 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siddiqi H.K., Mehra M.R. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J. Heart Lung Transplant. 2020;39:405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim P.S., Read S.W., Fauci A.S. Therapy for early COVID-19: a critical need. JAMA. 2020;324:2149–2150. doi: 10.1001/jama.2020.22813. [DOI] [PubMed] [Google Scholar]
- 5.Bramante C.T., Buse J., Tamaritz L., et al. Outpatient metformin use is associated with reduced severity of COVID-19 disease in adults with overweight or obesity. J. Med. Virol. 2021;93:4273–4279. doi: 10.1002/jmv.26873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lally M.A., Tsoukas P., Halladay C.W., et al. Metformin is associated with decreased 30-day mortality among nursing home residents infected with SARS-CoV2. J. Am. Med. Dir. Assoc. 2021;22:193–198. doi: 10.1016/j.jamda.2020.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hashimoto K. Repurposing of CNS drugs to treat COVID-19 infection: targeting the sigma-1 receptor. Eur. Arch. Psychiatry Clin. Neurosci. 2021;271:249–258. doi: 10.1007/s00406-020-01231-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lenze E.J., Mattar C., Zorumski C.F., et al. Fluvoxamine vs placebo and clinical deterioration in outpatients with symptomatic COVID-19: a randomized clinical trial. JAMA. 2020;324:2292–2300. doi: 10.1001/jama.2020.22760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seftel D., Boulware D.R. Prospective cohort of fluvoxamine for early treatment of Coronavirus Disease 19. Open Forum Infect Dis. 2021;8:ofab050. doi: 10.1093/ofid/ofab050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhidayasiri R., Virameteekul S., Kim J.M., et al. COVID-19: an early review of its global impact and considerations for Parkinson’s disease patient care. J Mov Disord. 2020;13:105–114. doi: 10.14802/jmd.20042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rejdak K., Grieb P. Adamantanes might be protective from COVID-19 in patients with neurological diseases: multiple sclerosis, parkinsonism and cognitive impairment. Mult Scler Relat Disord. 2020;42:102163. doi: 10.1016/j.msard.2020.102163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grieb P., Rejdak K. Are central nervous system drugs displaying anti-inflammatory activity suitable for early treatment of COVID-19? Folia Neuropathol. 2021;59(2):113–120. doi: 10.5114/fn.2021.107572. [DOI] [PubMed] [Google Scholar]
- 13.Danysz W., Dekundy A., Scheschonka A., et al. Amantadine: reappraisal of the timeless diamond-target updates and novel therapeutic potentials. J. Neural Transm. (Vienna) 2021;128:127–169. doi: 10.1007/s00702-021-02306-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hussain M., Galvin H.D., Haw T.Y., et al. Drug resistance in influenza a virus: the epidemiology and management. Infect Drug Resist. 2017;10:121–134. doi: 10.2147/IDR.S105473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paik J., Keam S.J. Amantadine extended-release (GOCOVRITM): a review in levodopa-induced dyskinesia in Parkinson’s disease. CNS Drugs. 2018;32:797–806. doi: 10.1007/s40263-018-0552-2. [DOI] [PubMed] [Google Scholar]
- 16.Perez D.Q., Espiritu A.I., Jamora R.D.G. Efficacy and safety of amantadine for the treatment of fatigue in multiple sclerosis: a systematic review and meta-analysis. Neurodegener Dis Manag. 2020;10:383–395. doi: 10.2217/nmt-2020-0030. [DOI] [PubMed] [Google Scholar]
- 17.Dietrich D.E., Bode L., Spannhuth C.W., et al. Antiviral treatment perspective against Borna disease virus 1 infection in major depression: a double-blind placebo-controlled randomized clinical trial. BMC Pharmacol. Toxicol. 2020;21:12. doi: 10.1186/s40360-020-0391-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiménez-Jiménez F.J., Alonso-Navarro H., García-Martín E., et al. Anti-inflammatory effects of amantadine and Memantine: possible therapeutics for the treatment of Covid-19? J Pers Med. 2020;10:217. doi: 10.3390/jpm10040217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cortés-Borra A., Aranda-Abreu G.E. Amantadine in the prevention of clinical symptoms caused by SARS-CoV-2. Pharmacol. Rep. 2021:1–4. doi: 10.1007/s43440-021-00231-5. Feb 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aranda-Abreu G.E., Aranda-Martínez J.D., Araújo R. Use of amantadine in a patient with SARS-CoV-2. J. Med. Virol. 2021;93:110–111. doi: 10.1002/jmv.26179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mandala V.S., McKay M.J., Shcherbakov A.A., et al. Structure and drug binding of the SARS-CoV-2 envelope protein transmembrane domain in lipid bilayers. Nat. Struct. Mol. Biol. 2020;27:1202–1208. doi: 10.1038/s41594-020-00536-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baig A.M., Khaleeq A., Syeda H. Docking prediction of amantadine in the receptor binding domain of spike protein of SARS-CoV-2. ACS Pharmacol Transl Sci. 2020;3:1430–1433. doi: 10.1021/acsptsci.0c00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gomes C.P., Fernandes D.E., Casimiro F., et al. Cathepsin L in COVID-19: from pharmacological evidences to genetics. Front. Cell. Infect. Microbiol. 2020;10:589505. doi: 10.3389/fcimb.2020.589505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smieszek S.P., Przychodzen B.P., Polymeropoulos M.H. Amantadine disrupts lysosomal gene expression: a hypothesis for COVID19 treatment. Int. J. Antimicrob. Agents. 2020;55:106004. doi: 10.1016/j.ijantimicag.2020.106004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao M.M., Yang W.L., Yang F.Y., et al. Cathepsin L plays a key role in SARS-CoV-2 infection in humans and humanized mice and is a promising target for new drug development. Signal Transduct Target Ther. 2021;6:134. doi: 10.1038/s41392-021-00558-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.WHO COVID-19 Clinical management: living guidance. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected (accessed 21 August 2021)
- 27.Kaufmann S.H.E., Dorhoi A., Hotchkiss R.S. Host-directed therapies for bacterial and viral infections. Nat. Rev. Drug Discov. 2018;17:35–56. doi: 10.1038/nrd.2017.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grieb P., Świątkiewicz M., Prus K., et al. Amantadine for COVID-19. J. Clin. Pharmacol. 2021;61:412–413. doi: 10.1002/jcph.1802. [DOI] [PubMed] [Google Scholar]
- 29.Ayres J.S. A metabolic handbook for the COVID-19 pandemic. Nat Metab. 2020;2:572–585. doi: 10.1038/s42255-020-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rejdak K., Grieb P. Fluvoxamine and amantadine: central nervous system acting drugs repositioned for COVID-19 as early intervention. Curr. Neuropharmacol. 2022;20:777–778. doi: 10.2174/1570159X19666210729123734. 29 Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]