Abstract
Background:
Despite clinical guidelines discouraging the practice, it is well-documented that the concomitant use of benzodiazepines and opioid analgesics occurs regularly. Information on concomitant use of buprenorphine for medication-assisted treatment (MAT) of opioid use disorder (OUD) and benzodiazepines, however, is limited. Thus, we aimed to describe real-world drug dispensing patterns for the concomitant use of buprenorphine products approved for MAT and benzodiazepines.
Methods:
We examined concomitant use of buprenorphine for MAT and benzodiazepines using the 2013 Prescription Behavior Surveillance System data from eight states. For prescription-level analysis, we estimated the proportion of concomitant buprenorphine and benzodiazepine prescriptions and the proportions of concomitant prescriptions prescribed by the same provider (co-prescribing) and dispensed by the same pharmacy (co-dispensing) for each state. For patient-level analysis, we calculated the proportion of patients with ≥ 1 buprenorphine therapy episode overlapping with a benzodiazepine episode, i.e., concomitant users, and the proportion of concomitant users who experienced co-prescribing or co-dispensing.
Results:
In 2013, 1,925,072 prescriptions of buprenorphine products for MAT were dispensed to 190,907 patients in eight states. Approximately 1 in 8 buprenorphine prescriptions was used concomitantly with ≥ 1 benzodiazepine prescription(s). Co-prescribing proportions ranged from 22.2 to 64.6% across states, while codispensing proportions ranged from 54.7 to 91.0%. Approximately 17.7% of patients had > 1 buprenorphine episode overlapping a benzodiazepine episode for ≥ 7 cumulative days’ supply. Among these patients, 33.1–65.2% experienced co-prescribing, and 65.1–93.3% experienced co-dispensing.
Conclusions:
The concomitant use of buprenorphine for MAT and benzodiazepines occurs frequently, with variations by state in co-prescribing and co-dispensing.
Keywords: Benzodiazepines, Buprenorphine, Medication-Assisted treatment, Concomitant use
1. Introduction
Prescription opioid analgesic abuse is associated with significant morbidity and mortality in the United States (Birnbaum et al., 2011). There were over 17,000 fatal overdoses involving a prescription opioid analgesic in the United States in 2015 (Rudd et al., 2016), and nearly two million Americans aged 12 years or older had opioid use disorder (OUD) in 2013 (Hedden, 2015). Prescription opioid analgesic overdoses kill more Americans each year than heroin and cocaine combined (Paulozzi et al., 2014; CDC, 2011).
One of the responses to the opioid overdose crisis has been greater oversight of prescription opioid analgesics through implementation of Prescription Drug Monitoring Programs (PDMPs) in 49 states and the District of Columbia that track prescribing and dispensing data for controlled substances (Brandeis University, 2017; Haffajee et al., 2015). Depending on the state, prescribers and/or dispensers may be legally required to check the PDMP record prior to prescribing a controlled substance to a patient. A recent study showed that state implementation of PDMPs was associated with a reduction in opioid-related overdose deaths compared to states without PDMPs (Patrick et al., 2016).
Many overdoses related to prescription opioid analgesics also involve benzodiazepines (Jones et al., 2012). The frequency of concomitant use of benzodiazepines and opioid analgesics, as well as the consequent increased morbidity and mortality, is well-described in the literature (Gomes et al., 2011; Hwang et al., 2016; Jones and McAninch, 2015; Kim et al., 2016; Larochelle et al., 2015; Peirce et al.,2012. However, the study of concomitant use of buprenorphine for medication-assisted treatment (MAT) of OUD and benzodiazepines is less extensive: many studies that examined opioid-benzodiazepine concomitancy excluded buprenorphine for MAT from the analysis (Jones et al., 2012; Kim et al., 2016; Larochelle et al., 2015). MAT is a mainstay of treatment for OUD and has been shown to be effective in reducing opioid use and opioid craving among affected individuals (Connery, 2015; Fiellin et al., 2006; Fudala et al., 2003; Volkow et al., 2014). Additionally, case reports and epidemiologic studies have shown serious morbidity and mortality can occur when buprenorphine is used with benzodiazepines (Faroqui et al., 1983; Hakkinen et al., 2012; Jones et al., 2012; Lintzeris et al., 2007; Nielsen et al., 2007; Schuman-Olivier et al., 2013). Studies have suggested that the concomitant use of buprenorphine and benzodiazepines has been linked to severe respiratory depression, overdose, unconsciousness, and death (Faroqui et al., 1983; Nielsen et al., 2007).
In this study, we used data from the Prescription Behavior Surveillance System (PBSS), a population-based public health surveillance system containing de-identified, longitudinal data from participating state PDMPs, to describe real-world drug dispensing patterns for the concomitant use of buprenorphine products approved for MAT and benzodiazepines.
2. Methods
2.1. Study design and data source
We conducted a cross-sectional study using data from eight states submitting data to PBSS in 2013: California (CA), Ohio (OH), Louisiana (LA), Kentucky (KY), West Virginia (WV), Idaho (ID), Maine (ME), and Delaware (DE) ([dataset]: Kreiner et al., March 14, 2017). These states were selected for this analysis because their PDMPs record prescriptions dispensed to all individuals in the state, including individuals aged 16 years and younger. According to a report by the Centers for Disease Control and Prevention (CDC), five of the eight states included in our analysis were among the top 15 U.S. states with the highest rates of drug overdose deaths in 2013 (WV: 32.2 deaths per 100,000 persons; KY: 23.7 per 100,000; OH: 20.8 per 100,000; DE: 18.7 per 100,000 and LA: 17.8 per 100,000 persons) (Rudd et al., 2016). The other three states had relatively lower rates of drug overdose deaths in 2013 (ID: 13.4 per 100,000; ME: 13.2 per 100,000; and CA: 11.1 per 100,000). At the time of the analysis, the most recent year of complete data for all the analyzed states was 2013. The PBSS is an early warning surveillance and evaluation tool based on de-identified, longitudinal data from participating state PDMPs. It is intended to be used to examine prescribing and dispensing patterns for controlled substances and to identify possible signs of drug misuse and diversion (Morgan et al.,2013). State PDMPs routinely collect information on every prescription for a controlled substance, including those paid for with cash. Participating state PDMPs submit de-identified data quarterly to PBSS, including patient demographics, National Drug Classification (NDC) codes, drug name, fill date, days of supply, pharmacy, and prescriber ID, among other information (Paulozzi et al., 2013). The general characteristics of the PBSS database, methods of data collection, and other data elements are described in more detail in other publications (Finklea et al., 2014; Paulozzi et al., 2013).
We examined the concomitant use of buprenorphine products approved for MAT and benzodiazepines at both prescription- and patient-levels. We used NDC codes to identify prescriptions of all dosage forms of buprenorphine and buprenorphine-naloxone products approved for MAT of OUD (Table S1) as well as benzodiazepine products dispensed in tablets or capsules. We excluded buprenorphine products approved for treatment of pain and benzodiazepines in gel, solution, or suspension forms. The active supply periods of buprenorphine and benzodiazepine prescriptions were defined as fill date plus the days’ supply recorded in the database. Patients were excluded from the analysis if their buprenorphine or benzodiazepine prescriptions had missing, 0, or > 90 days’ supply, as these represent invalid days’ supply.
2.2. Statistical analysis
2.2.1. Prescription-level analysis
We calculated three indicators using different numerators and denominators to assess the concomitant use of buprenorphine for MAT and benzodiazepines: the proportion of overlapping prescriptions (overlapping), the proportion of overlapping prescriptions written by the same provider (co-prescribing), and the proportion of overlapping prescriptions dispensed by the same pharmacy (co-dispensing; Table S2). For a given buprenorphine prescription, if a benzodiazepine days’ supply period overlapped with the days’ supply of a buprenorphine prescription by one day or more, the buprenorphine prescription was counted as a prescription concomitantly used with benzodiazepines (Fig. S1). These indicators were plotted for assessment of state variation.
2.2.2. Patient-level analysis
To ensure the robustness of concomitant use definition, we also conducted patient-level analysis. Using fill dates and days’ supply, we constructed continuous therapy episodes separately for buprenorphine for MAT and benzodiazepines in three steps (Fig. 1):
Fig. 1.
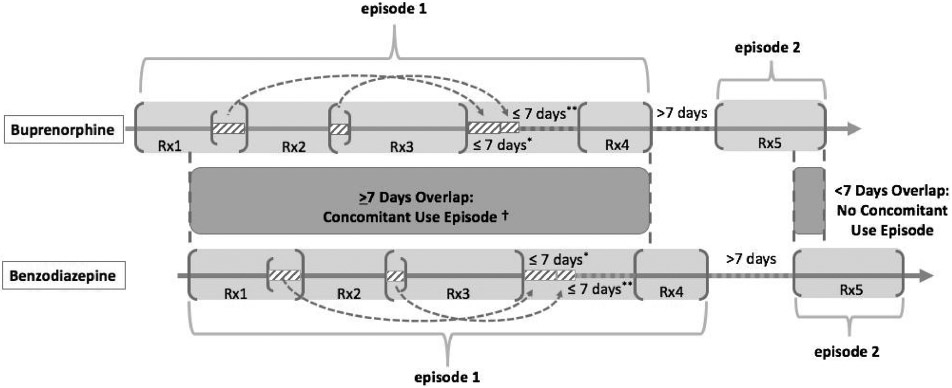
Establishing therapy episodes and concomitant use definition in patient-level analysis.
*Overlaps (≤ 7 days) of consecutive prescriptions were added to the end of the last prescription; if the total overlapping days of supply was > 7 days, we added 7 days to the end of the prescription period.
*We allowed a grace period of ≤ 7 days between the active days’ supply periods of two prescription periods while creating therapy episodes.
†Concomitant users were patients with at least one benzodiazepine episode overlapping a buprenorphine episode by ≥ 7 days. If multiple concomitant use episodes occurred for a given patient dispensed buprenorphine, the patient was counted once in the numerator.
Step 1: We created the start date and end date of prescription periods by linking consecutive prescriptions for the same patient with continuous days’ supply.
Step 2: We counted the total overlapping days’ supply in each prescription period. If the total overlapping days’ supply was ≤ 7 days, we added the number of overlapping days to the end of the prescription period. If the total overlapping days of supply was > 7 days, we added 7 days to the end of the prescription period.
Step 3: We created therapy episodes using prescription periods. We permitted a grace period of ≤ 7 days between the active days’ supply of two prescription periods and considered these two prescription periods as parts of the same therapy episode. If the gap between two prescription periods was > 7 days, we considered the two prescription periods as separate therapy episodes.
We defined concomitant users as patients who had at least one benzodiazepine episode that overlapped a buprenorphine episode by ≥ 7 consecutive days (numerator). In each state, we identified patients dispensed at least one buprenorphine product for MAT (denominator). We calculated and plotted the proportion of patients using buprenorphine for MAT and benzodiazepines concomitantly as the first indicator of concomitancy at patient-level.
Among the concomitant users (denominator), we then estimated the proportion of those who received at least one buprenorphine and one benzodiazepine prescription from the same prescriber as well as the proportion of those who received at least one buprenorphine and one benzodiazepine prescription from the same pharmacy. We defined the numerator as the number of concomitant users with at least one buprenorphine prescription with overlapping days’ supply of a benzodiazepine prescribed by the same prescriber (co-prescribing—second indicator of concomitant use at patient level) and the number of concomitant users with at least one buprenorphine prescription with overlapping days’ supply of a benzodiazepine dispensed by the same pharmacy (co-dispensing—third indicator of concomitant use at patient level).
All analyses were conducted using SAS 9.4 software (SAS Institute, Cary, NC); maps showing the results for each of the eight states were created using R software packages. This study was approved by the Prescription Drug Monitoring Program Center of Excellence at Brandeis University.
3. Results
In 2013, 1,925,072 prescriptions of buprenorphine for MAT of opioid use disorder were dispensed to 190,907 patients in eight states; the majority of those patients were aged 18–44 years (Table S3). Compared to patients in other states, patients dispensed buprenorphine in California and Idaho tended to be older, and a greater proportion of patients were aged > 45 years.
In the prescription-level analysis, the days’ supply of approximately one in eight buprenorphine prescriptions dispensed overlapped the days’ supply of at least one benzodiazepine prescription across eight states in 2013. The proportion of overlap was highest in California (20.3%, 95% Confidence Interval [CI]: 20.2–20.4%), which was 1.5 times higher than the lowest proportion in West Virginia ((8.0%, CI: 7.9–8.1%; Fig. S2), interquartile range (IQR) = 10.6–16.6%). Among the overlapping buprenorphine and benzodiazepine prescriptions, the proportion prescribed by the same prescriber ranged from 22.2% (CI: 21.6–22.9%) to 64.6% (CI: 64.1–65.1%), IQR = 30.8–57.3% across the states analyzed (Fig. 2). Likewise, the proportion of overlapping prescriptions dispensed by the same pharmacy ranged from 54.7% (CI: 54.3–55.1%) to 91.0% (CI: 90.7–91.3%), IQR = 61.8–83.7%. Across all states, the proportion of co-dispensing was approximately 1.5–3.5 times greater than the proportion of co-prescribing. We observed higher proportions of all three indicators in California, Louisiana, and Idaho, while Ohio, Kentucky, and West Virginia had relatively lower proportions for all three indicators compared to the proportions of other states. Although Maine had the lowest proportion of co-prescribing (22.2%, CI: 21.6–22.9%), it had a relatively high proportion of co-dispensing (80.8%, CI: 80.2–81.4%) (Fig. 2).
Fig. 2.
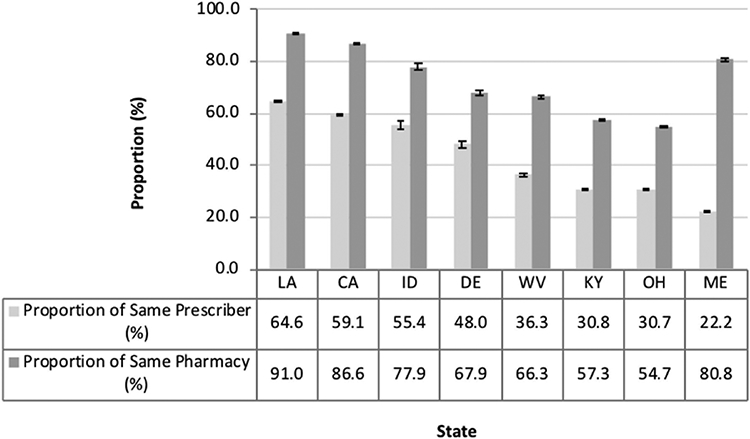
Indicators of co-prescribing and co-dispensing of buprenorphine for medication-assisted treatment of opioid use disorder and benzodiazepines in eight states submitting data to PBSS in 2013.
*Abbreviations: Louisiana (LA), California (CA), Idaho (ID), Delaware (DE), West Virginia (WV), Kentucky (KY), Ohio (OH), and Maine (ME).
In the patient-level analysis, of 190,907 patients with at least one buprenorphine therapy episode across the eight states, 33,840 (17.7%) patients had at least one buprenorphine episode overlapping with a benzodiazepine episode for ≥ 7 days, and an additional 4747 (2.5%) patients had an overlap episode of < 7 days. Across states, the proportion of patients with at least one buprenorphine therapy episode overlapping a benzodiazepine therapy episode for ≥ 7 days (concomitant users) ranged from 13.9% (CI: 13.5–14.2%) in Louisiana to 24.2% (CI: 23.8–24.5%) in California (Fig. 3). The median length of the overlapping period of buprenorphine and benzodiazepine therapy episodes among concomitant users ranged from 29 to 41 days, and the average length of overlap was 41-74 days. The longest average and median overlapping periods were observed in Maine, and the shortest were observed in California (Fig. S3).
Fig. 3.
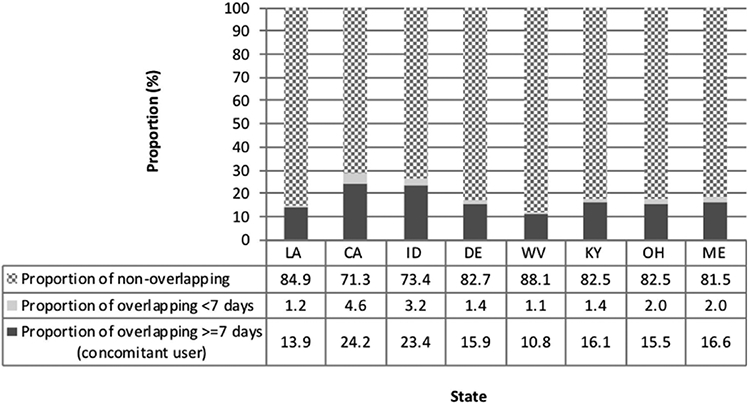
Proportions of patients with at least one buprenorphine therapy episode overlapping a benzodiazepine therapy episode in eight states submitting data to PBSS in 2013.
*Abbreviations: Louisiana (LA), California (CA), Idaho (ID), Delaware (DE), West Virginia (WV), Kentucky (KY), Ohio (OH), and Maine (ME).
In the patient-level analysis, we further examined whether concomitant users’ prescriptions were prescribed by the same prescriber or dispensed by the same pharmacy. The proportion of concomitant users with concomitant benzodiazepine and buprenorphine prescriptions prescribed by the same prescriber varied from 33.1% (CI: 31.9–34.3%) to 65.2% (CI: 63.9–66.5%) (IQR = 34.7–60.3%) across the states analyzed, and the proportion of concomitant users with prescriptions dispensed by the same pharmacy ranged from 65.1% (CI: 63.8–66.3%) to 93.3% (CI: 92.7–94.0%) (IQR = 71.2–89.7%) (Fig. 4). In all states except Maine, states with higher levels of co-prescribing had correspondingly higher levels of co-dispensing. In Maine, we observed a relatively high proportion of concomitant users with prescriptions dispensed by the same pharmacy but a relatively low proportion of prescriptions prescribed by the same prescriber.
Fig. 4.
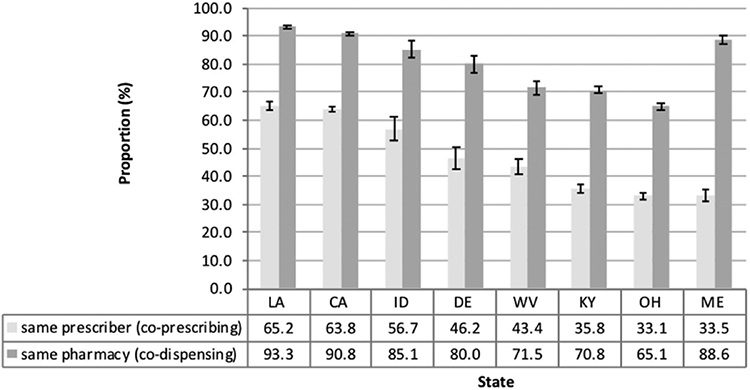
Proportions of concomitant users (therapy episodes overlapping ≥ 7 days) with both products prescribed by the same prescriber or dispensed by the same pharmacy in eight states submitting data to PBSS in 2013.
*Abbreviations: Louisiana (LA), California (CA), Idaho (ID), Delaware (DE), West Virginia (WV), Kentucky (KY), Ohio (OH), and Maine (ME).
4. Discussion
To our knowledge, this is the first large PDMP-based study that has examined the concomitant use of buprenorphine for MAT and benzodiazepines. Our analyses show that despite clinical guidelines cautioning against the prescribing of benzodiazepines in patients also using buprenorphine for MAT (Kampman and Jarvis, 2015), these drug classes are commonly prescribed and dispensed to the same patient. We observed that one-fifth to two-thirds of these concomitant drugs were prescribed by the same prescriber, more than half of these drugs were dispensed from the same pharmacy, and there were variations by state in both prescription- and patient-level analyses.
Several guidelines on prescribing opioids recognize the concomitant use of opioid products and benzodiazepines as a high-risk combination that can result in adverse events including respiratory depression and death (Chou et al., 2009; Dowell et al., 2016; Olsen et al., 2013). However, concomitant use still occurs frequently. In the patient-level analysis, we found that more than one in six patients dispensed buprenorphine for MAT used benzodiazepines concomitantly. Our findings are similar to the findings in a previous study based on Veterans Health Administration records (Park et al., 2014) which observed that 20.2% (median) of veteran patients dispensed buprenorphine also received benzodiazepines concomitantly. The authors also identified considerable variation (IQR = 15.3–24.3%) based on the sample from 21 Veterans Integrated Service Networks, which is consistent with the state variation we observed (IQR = 14.7–20.0%).
We observed variation in the proportions of co-prescribing and co-dispensing of benzodiazepine and buprenorphine products for MAT among the eight states. The proportion of co-prescribing of buprenorphine for MAT and benzodiazepines in Louisiana (highest) was more than twice that of Maine and Ohio (lowest). Further, observed trends of co-prescribing were generally higher (33.1–65.2%) in states with higher proportions of co-dispensing (65.1–93.3%). Substantial variations in co-prescribing of benzodiazepine and buprenorphine across states may indicate differential accessibility to health services including OUD treatment providers. Because buprenorphine for OUD can only be prescribed by providers who have obtained a waiver under provisions of the Drug Addiction Treatment Act (DATA 2000) to provide office-based treatment, the availability of buprenorphine prescribers in the community setting varies by state (Knudsen, 2015; Stein et al., 2015). Maine had the highest number of DATA-waived physicians per 100,000 residents in 2013 (Knudsen, 2015) and the lowest proportion of co-prescribing of buprenorphine for MAT and benzodiazepines (Fig. 2). In contrast, among analyzed states, those with lowest number of DATA-waived physicians per 100,000 residents – California, Louisiana, and Idaho – had the highest proportions of co-prescribing. Additional reasons for regional variation could include the implementation of community public health and education programs, state and municipal law initiatives aimed at curbing opioid abuse, state funding priorities for treatment and prevention of prescription drug misuse, and localized efforts encouraging providers to obtain a DATA 2000 Waiver (Gugelmann and Perrone, 2011; Reifler et al., 2012). In addition to cases of co-prescribing by a single prescriber, patients may also have buprenorphine for MAT concomitantly prescribed with benzodiazepines by different prescribers and dispensed by different pharmacies. Some of these cases may be indicative of drug-seeking behaviors such as “doctor shopping” or may be the result of lack of coordination of care among prescribers.
The frequent concomitant use of buprenorphine and benzodiazepines observed in our study is concerning. However, there are several plausible clinical explanations for our findings. One study noted that patients dispensed MAT for OUD may request benzodiazepines to mitigate unpleasant opioid withdrawal symptoms (Lintzeris and Nielsen, 2010), although this is not a recommended clinical practice. It is also possible that patients dispensed benzodiazepines and buprenorphine may be receiving treatment for concurrent anxiety or insomnia disorders and OUD or for simultaneous dependence on both benzodiazepines and opioids. In September 2017, the U.S. Food and Drug Administration urged caution about withholding opioid addiction medications, including buprenorphine and methadone, from patients taking benzodiazepines or other central nervous system depressants through a Drug Safety Communication (Food and Drug Administration, 2016). Healthcare providers were advised to treat patients through careful medication management to reduce the risks associated with concomitant use.
4.1. Limitations
This study has several limitations. Due to the nature of the secondary analysis of existing data, estimates of concomitant use are subject to the concomitancy definitions used in our analyses. To address concerns about measurement bias, we used both prescription- and patient-level analyses to assess the concomitant use of benzodiazepines and buprenorphine products. The two analyses produced similar results. Additionally, the state-by-state variations in concomitant use we observed may have resulted from variation in data capture and data quality for each state. We think this is unlikely, however, because PBSS monitors the capture and quality of data submitted by each state quarterly with corrections (Paulozzi et al., 2013). Finally, because the PBSS database is a de-identified dataset that does not link to medical diagnosis or health outcomes, we were unable to determine the indications for the use of benzodiazepines and buprenorphine products or examine potential adverse consequences associated with concomitant use of benzodiazepines and buprenorphine in patients prescribed both products. However, this study demonstrates the value of the PBSS, a data source that may facilitate the analysis of controlled substance prescribing patterns beyond state lines regardless of payment type. Future studies can build upon the treatment patterns we observed to further examine the outcomes related to concomitant use, perhaps with linkages to medical records or death registries.
5. Conclusion
In conclusion, based on both prescription- and patient-level analyses, the concomitant use of buprenorphine for MAT and benzodiazepines occurs regularly and with state-by-state variation. In both analyses, approximately one-fifth to two-thirds of the prescriptions of overlapping benzodiazepine and buprenorphine were prescribed by the same physician, and more than half of the overlapping prescriptions were dispensed by the same pharmacy.
Supplementary Material
Acknowledgements
We would like to acknowledge the support from Institute for Behavioral Health at Brandeis University, specifically the Prescription Behavior Surveillance System (PBSS) team, Dr. Peter Kreiner, Dr. Gail Strickler, Ms. Erin Doyle and Mr. Lee Panas, as well as the state Prescription Drug Monitoring Programs in California, Ohio, Louisiana, Kentucky, West Virginia, Idaho, Maine, and Delaware.
Role of funding source
This project was supported by the United States Food and Drug Administration internally and the Bureau of Justice Assistance [grant number 2011-D6-BX-K052, 2011]. The views presented in this paper are that of the authors and not necessarily those of the United States Food and Drug Administration. The Bureau of Justice Assistance is a component of the U.S. Department of Justice’s Office of Justice Programs, which also includes the Bureau of Justice Statistics, the National Institute of Justice, the Office of Juvenile Justice and Delinquency Prevention, the Office for Victims of Crime, and the SMART Office. Points of view or opinions in this document are those of the authors and do not necessarily represent the official position or policies of the U.S. Department of Justice.
Footnotes
Conflict of interest
The authors have no financial conflicts to report.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.drugalcdep.2018.02.019.
References
- Birnbaum HG, White AG, Schiller M, Waldman T, Cleveland JM, Roland CL, 2011. Societal costs of prescription opioid abuse, dependence, and misuse in the United States. Pain Med. 12, 657–667. [DOI] [PubMed] [Google Scholar]
- Brandeis University, 2017. The Heller School for Social Policy and Management. Prescription Drug Monitoring Program Center of Excellence Frequently Asked Questions, http://www.pdmpassist.org/content/prescription-drug-monitoring-frequently-asked-questions-faq. [Google Scholar]
- enter for Disease Control (CDC), 2011. Vital Signs: Prescription Painkiller Overdoses in the US. Center for Disease Control, Atlanta, GA. https://www.cdc.gov/vitalsigns/painkilleroverdoses/index.html. [Google Scholar]
- Chou R, Fanciullo GJ, Fine PG, Adler JA, Ballantyne JC, Davies P, Donovan MI, Fishbain DA, Foley KM, Fudin J, 2009. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J. Pain 10, 113–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connery HS, 2015. Medication-assisted treatment of opioid use disorder: review of the evidence and future directions. Harvard Rev. Psychiatry 23, 63–75. [DOI] [PubMed] [Google Scholar]
- Dowell D, Haegerich TM, Chou R, 2016. CDC guideline for prescribing opioids for chronic pain – United States, 2016. MMWR Morb. Mortal. Wkly. Rep 65, 1–49. [DOI] [PubMed] [Google Scholar]
- Faroqui MH, Cole M, Curran J, 1983. Buprenorphine, benzodiazepines and respiratory depression. Anaesthesia 38, 1002–1003. [DOI] [PubMed] [Google Scholar]
- Fiellin DA, Pantalon MV, Chawarski MC, Moore BA, Sullivan LE, O'Connor PG, Schottenfeld RS, 2006. Counseling plus buprenorphine-naloxone maintenance therapy for opioid dependence. N. Engl. J. Med 355, 365–374. [DOI] [PubMed] [Google Scholar]
- Finklea K, Sacco LN, Bagalman E, 2014. Prescription drug monitoring programs. J. Drug Addict. Educ. Erad 10, 481. [Google Scholar]
- Food and Drug Administration (FDA), 2016. Drug Safety Communication: FDA Urges Caution About Withholding Opioid Addiction Medications from Patients Taking Benzodiazepines or CNS Depressants: Careful Medication Management can Reduce Risks. Silver Spring, MD. [Google Scholar]
- Fudala PJ, Bridge TP, Herbert S, Williford WO, Chiang CN, Jones K, Collins J, Raisch D, Casadonte P, Goldsmith RJ, Ling W, Malkerneker U, McNicholas L, Renner J, Stine S, Tusel D, 2003. Buprenorphine/Naloxone Collaborative Study, G.,. Office-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxone. N. Engl. J. Med 349, 949–958. [DOI] [PubMed] [Google Scholar]
- Gomes T, Mamdani MM, Dhalla IA, Paterson JM, Juurlink DN, 2011. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch. Intern. Med 171, 686–691. [DOI] [PubMed] [Google Scholar]
- Gugelmann HM, Perrone J, 2011. Can prescription drug monitoring programs help limit opioid abuse? JAMA 306, 2258–2259. [DOI] [PubMed] [Google Scholar]
- Haffajee RL, Jena AB, Weiner SG, 2015. Mandatory use of prescription drug monitoring programs. JAMA 313, 891–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakkinen M, Launiainen T, Vuori E, Ojanpera I, 2012. Benzodiazepines and alcohol are associated with cases of fatal buprenorphine poisoning. Eur. J. Clin. Pharmacol 68, 301–309. [DOI] [PubMed] [Google Scholar]
- Hedden SL, 2015. Behavioral Health Trends in the United States: Results from the 2014 National Survey on Drug Use and Health. Substance Use and Mental Health Services Administration, Rockville, MD. [Google Scholar]
- Hwang CS, Kang EM, Kornegay CJ, Staffa JA, Jones CM, McAninch JK, 2016. Trends in the concomitant prescribing of opioids and benzodiazepines, 2002–2014. Am. J. Prev. Med 51, 151–160. [DOI] [PubMed] [Google Scholar]
- Jones CM, McAninch JK, 2015. Emergency department visits and overdose deaths from combined use of opioids and benzodiazepines. Am. J. Prev. Med 49, 493–501. [DOI] [PubMed] [Google Scholar]
- Jones JD, Mogali S, Comer SD, 2012. Polydrug abuse: a review of opioid and benzodiazepine combination use. Drug Alcohol Depend. 125, 8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampman K, Jarvis M, 2015. American Society of Addiction Medicine (ASAM) national practice guideline for the use of medications in the treatment of addiction involving opioid use. J. Addict. Med 9, 358–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HS, McCarthy DM, Mark Courtney D, Lank PM, Lambert BL, 2016. Benzodiazepine-opioid co-prescribing in a national probability sample of emergency department encounters. Am. J. Emerg. Med 35, 458–464. [DOI] [PubMed] [Google Scholar]
- Knudsen HK, 2015. The supply of physicians waivered to prescribe buprenorphine for opioid use disorders in the United States: a state-level analysis. J. Stud. Alcohol Drugs 76, 644–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreiner P, Strickler G, Panas L, Doyle E, 2017. Prescription Behavior Surveillance System Data. Brandeis University PDMP Center of Excellence, Waltham, MA: (dataset). [Google Scholar]
- Larochelle MR, Zhang F, Ross-Degnan D, Wharam JF, 2015. Trends in opioid prescribing and co-prescribing of sedative hypnotics for acute and chronic musculoskeletal pain: 2001–2010. Pharmacoepidemiol. Drug Saf 24, 885–892. [DOI] [PubMed] [Google Scholar]
- Lintzeris N, Nielsen S, 2010. Benzodiazepines, methadone and buprenorphine: interactions and clinical management. Am. J. Addict 19, 59–72. [DOI] [PubMed] [Google Scholar]
- Lintzeris N, Mitchell TB, Bond AJ, Nestor L, Strang J, 2007. Pharmacodynamics of diazepam co-administered with methadone or buprenorphine under high dose conditions in opioid dependent patients. Drug Alcohol Depend. 91, 187–194. [DOI] [PubMed] [Google Scholar]
- Morgan L, Weaver M, Sayeed Z, Orr R, 2013. The use of prescription monitoring programs to reduce opioid diversion and improve patient safety. J. Pain Palliat. Care Pharmacother 27, 4–9. [DOI] [PubMed] [Google Scholar]
- Nielsen S, Dietze P, Lee N, Dunlop A, Taylor D, 2007. Concurrent buprenorphine and benzodiazepines use and self-reported opioid toxicity in opioid substitution treatment. Addiction 102, 616–622. [DOI] [PubMed] [Google Scholar]
- Olsen Y, Adams J, Alvanzo A, 2013. Clinical Guidelines for the Use of Benzodiazepines Among Patients Receiving Medication Assisted Treatment for Opioid Dependence. Baltimore Sustance Abuse Systems, Baltimore, MD. [Google Scholar]
- Park TW, Bohnert AS, Austin KL, Saitz R, Pizer SD, 2014. Datapoints: regional variation in benzodiazepine prescribing for patients on opioid agonist therapy. Psychiatr. Serv 65 (4-4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick SW, Fry CE, Jones TF, Buntin MB, 2016. Implementation of prescription drug monitoring programs associated with reductions in opioid-related death rates. Health Aff. 35, 1324–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulozzi LJ, Strickler GK, Kreiner PW, Koris CM, 2013. Centers for Disease Control and Prevention, 2015. Controlled substance prescribing patterns—Prescription behavior surveillance system, eight states. MMWR Surveill. Summ 64, 1–14. [DOI] [PubMed] [Google Scholar]
- Paulozzi LJ, Mack KA, Hockenberry JM, 2014. Vital signs: variation among states in prescribing of opioid pain relievers and benzodiazepines— United States, 2012. MMWR Morb. Mortal. Wkly. Rep 63, 563–568. [PMC free article] [PubMed] [Google Scholar]
- Peirce GL, Smith MJ, Abate MA, Halverson J, 2012. Doctor and pharmacy shopping for controlled substances. Med. Care 50, 494–500. [DOI] [PubMed] [Google Scholar]
- Reifler LM, Droz D, Bailey JE, Schnoll SH, Fant R, Dart RC, Bartelson BB, 2012. Do prescription monitoring programs impact state trends in opioid abuse/misuse? Pain Med. 13, 434–442. [DOI] [PubMed] [Google Scholar]
- Rudd RA, Seth P, David F, Scholl L, 2016. Increases in drug and opioid-involved overdose deaths – United States: 2010–2015. MMWR Morb. Mortal. Wkly. Rep 65, 1445–1452. [DOI] [PubMed] [Google Scholar]
- Schuman-Olivier Z, Hoeppner BB, Weiss RD, Borodovsky J, Shaffer HJ, Albanese MJ, 2013. Benzodiazepine use during buprenorphine treatment for opioid dependence: clinical and safety outcomes. Drug Alcohol Depend. 132, 580–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein BD, Gordon AJ, Dick AW, Burns RM, Pacula RL, Farmer CM, Leslie DL, Sorbero M, 2015. Supply of buprenorphine waivered physicians: the influence of state policies. J. Subst. Abuse Treat 48, 104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Frieden TR, Hyde PS, Cha SS, 2014. Medication-assisted therapies—tackling the opioid-overdose epidemic. N. Engl. J. Med 370, 2063–2066. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


