Graphical abstract
Keywords: Ivermectin, PLGA nanoparticle, chitosan, RBC-hitchhiking, acute lung injury
Abstract
Recent studies have demonstrated that ivermectin (IVM) exhibits antiviral activity against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative virus of coronavirus disease 2019 (COVID-19). However, the repurposing of IVM for the treatment of COVID-19 has presented challenges primarily due to the low IVM plasma concentration after oral administration, which was well below IC50. Here, a red blood cell (RBC)-hitchhiking strategy was used for the targeted delivery of IVM-loaded nanoparticles (NPs) to the lung. IVM-loaded poly (lactic-co-glycolic acid) (PLGA) NPs (IVM-PNPs) and chitosan-coating IVM-PNPs (IVM-CSPNPs) were prepared and adsorbed onto RBCs. Both RBC-hitchhiked IVM-PNPs and IVM-CSPNPs could significantly enhance IVM delivery to lungs, improve IVM accumulation in lung tissue, inhibit the inflammatory responses and finally significantly alleviate the progression of acute lung injury. Specifically, the redistribution and circulation effects were related to the properties of NPs. RBC-hitchhiked cationic IVM-CSPNPs showed a longer circulation time, slower accumulation and elimination rates, and higher anti-inflammatory activities than RBC-hitchhiked anionic IVM-PNPs. Therefore, RBC-hitchhiking provides an alternative strategy to improve IVM pharmacokinetics and bioavailability for repurposing of IVM to treat COVID-19. Furthermore, according to different redistribution effects of different NPs, RBC-hitchhiked NPs may achieve various accumulation rates and circulation times for different requirements of drug delivery.
1. Introduction
Coronavirus infectious disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), an increasingly prevalent viral contagion at the global scale, causes acute respiratory distress syndrome (ARDS)(Mahmudpour et al., 2020, Mohanty et al., 2021). Numerous clinical trials are being reviewed for the use of different drugs, biologics and vaccines in the treatment of COVID-19 (Mohanty et al., 2021). However, there is currently no dependable treatment or suitable therapeutic for COVID-19 (Kaur et al., 2021).
A recent study found that ivermectin (IVM) inhibits the replication of SARS-CoV-2 in vitro (Caly et al., 2020). IVM is an FDA-approved broad-spectrum antiparasitic agent (González Canga et al., 2008), which also exhibits antiviral activity against various RNA (Zika virus, HIV-1, Dengue virus) as well as DNA viruses (Equine herpesvirus type 1, Pseudorabies virus, Porcine circovirus 2) (Heidary and Gharebaghi, 2020, Li et al., 2021, Surnar et al., 2019). IVM is believed to act through the inhibition of viral proteins, including importin α/β1 heterodimer and integrase protein (Wagstaff et al., 2011, Yang et al., 2020). Furthermore, IVM may have anti-inflammatory properties and could play a supportive adjuvant role to prevent acute lung injury (ALI) in SARS-CoV-2 infection (Formiga et al., 2021, González Canga et al., 2008, Yan et al., 2011, Zhang et al., 2008). However, despite its promising antiviral and preliminary anti-inflammatory activities, the development of IVM formulations presents challenges (Formiga et al., 2021). Numerous IVM clinical trials have been registered and conducted to validate its use in the treatment of COVID-19. The results of some trails have shown a potential decrease in the length of hospital stay and survival benefit (Ahmed et al., 2021, Heidary and Gharebaghi, 2020). In contrast, other trials have shown no significant difference between the IVM group and placebo group (or untreated group) (Krolewiecki et al., 2021, López-Medina et al., 2021). There is evidence that the mean IVM plasma concentration levels correlate with antiviral effect (Krolewiecki et al., 2021). The free plasma concentrations of IVM do not reach the IC50 even for a dose level that is 10x higher than the approved dose due to its high binding rate to plasma proteins (93%) (Jermain et al., 2020; Peña‐Silva et al., 2021; Schmith et al., 2020). Moreover, excessive IVM causes dose-dependent adverse reactions and toxicity in animals, including ataxia, tremor, central nervous system depression, and coma, which usually lead to death (Trailovic et al., 2011, Trailovic and Nedeljkovic, 2011). Considering these challenges, novel delivery strategies are important for the repurposing of IVM for the treatment of COVID-19.
Compared with traditional drug delivery strategies, targeted drug delivery can greatly enhance the efficiency of drug delivery, increase local drug concentration in target organs/tissues, and enhance therapeutic efficacy, while reducing systemic exposure and side-effects of drugs (Croci et al., 2016). Therefore, the delivery of IVM to the lungs may alter drug distribution, increase drug concentration in lungs to overcome the drawbacks of oral administration, and at the same time reduce its side-effects. Nanoparticles (NPs) offer advantages in controlled and sustained release, improved bioavailability and enhanced drug's therapeutic efficacy (Onoue et al., 2014). Moreover, NPs can be easily modified for targeted drug delivery. However, ordinary NPs are rapidly removed from the bloodstream by the mononuclear phagocyte system (MPS) and the targeting capability is limited due to the complex environment in vivo (Liu et al., 2020, Perry et al., 2012, Yang et al., 2021).
Recently, a hybrid delivery strategy, namely red blood cell (RBC)-hitchhiking, has offered an optimal blend of natural and synthetic systems to address these limitations (Anselmo et al., 2013, Villa et al., 2016a, Brenner et al., 2018). NPs adsorb onto the surface of RBCs in a non-covalent manner to form NPs-adsorbed RBCs (RBC-NPs) in vitro. Then, NPs are scraped off the surface of RBCs because they fail to resist the shear stress between RBCs and small capillaries. These desorbed NPs mainly accumulate in the lungs, the first capillary bed where RBC-NPs pass through after intravenous injection (Brenner et al., 2021). RBC-hitchhiking, which combines NPs and RBCs carriers, improves drug solubility and enables parenteral delivery of insoluble drugs. Moreover, the sustained release ability of NPs and the long circulation property of RBCs can greatly prolong the circulation and action time of drugs (Wang et al., 2021, Glassman et al., 2020). Importantly, RBC-hitchhiking endows NPs with lung targeting abilities, changes the drug biodistribution, enhances drug accumulation in the lungs, and increases the drug concentration in regional lung tissue (Anselmo et al., 2013, Zelepukin et al., 2019).
Through the RBC-hitchhiking strategy, methylprednisolone has been successfully delivered to the lungs in our previous research (Ding et al., 2021). However, the effect of the properties of NPs on the delivery efficiency in vivo is unclear. The lung targeting capability and circulation time of various NPs adsorbed on RBCs may vary greatly. The primary reason for these differences may be related to the binding force between the NPs and RBCs. In this study, various NPs with different materials, sizes and polymer coatings were prepared and the adsorption efficiency of NPs to RBCs was systematically investigated (Fig. 1 ). Furthermore, the in vivo circulation time, lung targeting capabilities, and anti-inflammatory activities of different RBC-hitchhiked NPs were studied.
Fig. 1.
Diagram of the red blood cell (RBC)-hitchhiking strategy. Various (different materials, sizes, and material coatings) ivermectin (IVM)-loaded nanoparticles were prepared and adsorbed onto the RBC surface in a non-covalent manner to form a RBC-nanoparticles complex. The RBC-hitchhiked nanoparticles were targeted for delivery to the lungs to treat lung inflammation. The effects of the different properties of nanoparticles on their adsorption efficiency to RBCs, the circulation times, the lung targeting capabilities, and anti-inflammatory activities were investigated emphatically. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
2. Materials and methods
2.1. Materials and animals
PLGA (LA:GA ratio of 75:25, carboxyl group-terminated [C-75/25]; LA:GA ratio of 75:25, ester group-terminated [E-75/25]; LA:GA ratio of 50:50, carboxyl group-terminated [C-50/50]; LA:GA ratio of 50:50, ester group-terminated [E-50/50]) and NHS-biotin were purchased from Xi'an Ruixi Biological Technology Co., Ltd. (Xi'an, China). IVM was purchased from A'lading Co., Ltd. (Shanghai, China). 1,1′-Dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine iodide (DiI), Cyanine5.5 (Cy5.5) and Cyanine7.5 (Cy7.5) were purchased from Dalian Meilun Biotechnology Co., Ltd. (Dalian, China). Poloxamer188 (MW 8600) was purchased from Harveybio Gene Technology Co., Ltd. (Beijing, China). Lipopolysaccharide (LPS), chitosan (CS, low viscosity) and poly-L-lysine (PLL, 300 KD) were purchased from Sigma-Aldrich (MO, USA). The Na+/K+-ATPase Kit was purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). The interleukin (IL)-6 enzyme linked immunosorbent assay (ELISA) Kit and tumor necrosis factor (TNF)-α ELISA Kit were purchased from Solarbio Biotechnology Co., Ltd. (Beijing, China). The enhanced BCA protein assay kit and Hoechst 33258 were purchased from Beyotime Biotechnology Co., Ltd. (Beijing, China). FITC-streptavidin were purchased from Beijing Bioss Biotechnology Co., Ltd. (Beijing, China). The other excipients were of standard pharmaceutical grade and all chemical reagents used were of analytical or high-performance liquid chromatography (HPLC) grade.
RAW264.7 cells were provided by the IBMS Cell Resource Center (Beijing, China) and cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% inactivated fetal bovine serum (FBS), with 100 U/mL penicillin and 100 μg/mL streptomycin. These cells were maintained in a humidified incubator at 37°C under a 5% CO2 atmosphere.
Male Sprague-Dawley (SD) rats (180 ± 20 g) and male ICR mice (20 ± 2 g) were obtained from Vital River Laboratories (Beijing, China). All animal experiments complied with the code of ethics in research, training and testing of drugs issued by the Animal Care and Use Ethics Committee in Beijing Institute of Pharmacology and Toxicology.
2.2. Preparation and characterization of IVM-NPs
PLGA NPs (PNPs) of different materials and sizes were prepared using the O/W emulsion solvent diffusion method (Li et al., 2019). Briefly, PLGA (30 mg) was dissolved in acetone solution (10, 15, 20 and 30 mg/mL), then IVM (3 mg) or hydrophobic fluorescent probe (Cy5.5 or Cy7.5, 0.4 mg) was added and the mixture was used as the organic phase. When completely dissolved, the organic phase was dropped into 10 mL of 1% (w/v) Poloxamer188 solution under continuous magnetic stirring. Then, the resulting mixture was stirred overnight, centrifuged (18,000 g, 20 min) and washed 3 times with distilled water. IVM-loaded PNPs (IVM-PNPs) of approximately 130 nm, 150 nm, 180 nm, and 200 nm were finally obtained. For the preparation of cationic NPs, CS or PLL was used as the coating material to modify the surface of the PNPs. CS or PLL (1 mg/mL) was added to IVM-PNPs dropwise and CS-coated PNPs (IVM-CSPNPs) or PLL-coated PNPs (IVM-PLLPNPs) were obtained.
After washing, all NPs samples were resuspended in distilled water. The mean sizes and zeta potential were analyzed via dynamic light scattering (DLS, Litesizer 500, Anton Parr, Austria), and the morphology was characterized via transmission electron microscopy (TEM, H-7650, Hitachi, Japan). The kinetics of IVM release from IVM-PNPs and IVM-CSPNPs were studied in a simulated physiological environment (0.01 mM PBS, containing 0.5% sodium dodecyl sulfate). 2 mL of IVM-PNPs or IVM-CSPNPs (containing about 0.5 mg of IVM) was placed in the release medium and shaken at 150 rpm at 37°C. 2 mL of release medium was collected at preset time intervals and replaced with 2 mL of fresh release medium at 37°C. The concentration of IVM in the samples was determine at 245 nm by HPLC ( 1200, Agilent, USA) and the cumulative release rate was calculated. The encapsulation efficiency (EE) and drug loading capacity (DL) were calculated using the following equations:
2.3. Adsorption of NPs to RBCs
Whole blood collected from the retro-orbital sinus was centrifuged (1,000 g, 4°C, 10 min) to separate plasma and RBCs. The packed RBCs were washed extensively with precooling PBS. RBCs at 10% hematocrit were incubated with NPs at 37°C for 30 min, and unattached NPs were removed via centrifugation.
A standard hemagglutination assay was performed using V-shaped 96-well plates to examine the degree of hemagglutination after the adsorption of NPs (Zelepukin et al., 2019). RBCs at 1% hematocrit were mixed with a known quantity of NPs. Then, RBC samples (50 μL) were added to the V-shaped well and incubated at 37°C for 1 h. The aggregated RBCs form a 3D-network that prevents sedimentation to the bottom of V-shape instead of settling into a tight point in the center. Free RBCs without NPs were used as controls.
2.4. Characterization of RBC-NPs
2.4.1. Morphological characterization
The RBC samples were dehydrated for imaging using a classic fixation technique (Zelepukin et al., 2019). Briefly, RBC samples were fixed in 2.5% glutaraldehyde solution at 4°C overnight and transferred into a gradient ethanol solution for dehydration. Then, the samples were placed on silicon tape and sputter-coated with gold for observation by scanning electron microscopy (SEM, S-4800, Hitachi, Japan). Besides, PNPs or CSPNPs labeled with Cy5.5 were adsorbed to RBCs (RBC-Cy5.5-PNPs or RBC-Cy5.5-CSPNPs, respectively) and then observed via a confocal laser scanning microscopy (CLSM, LSM 880, ZEISS, Germany).
2.4.2. Shear responsiveness
The desorption of IVM-PNPs or IVM-CSPNPs from RBCs performed under shear stress was evaluated using a microfluid-controlled visual rheometer (Fluidicam Rheo, Formulaction, France) (Anselmo et al., 2013). RBC samples were exposed to shear stress (1 Pa or 5 Pa) at 37°C for 15 min, and 10% serum was added to RBC samples to prevent the re-adsorption of NPs. The adsorption efficiency of NPs to RBCs before or after the shear stress challenge was calculated.
2.4.3. In vitro cell uptake
To investigate the anti-phagocytosis ability of RBC-NPs, RAW264.7 cells (105/dish) were seeded into a culture dish for 24 h. After incubation with Cy5.5-PNPs, Cy5.5-CSPNPs, RBC-Cy5.5-PNPs, or RBC-Cy5.5-CSPNPs (10 μM) for 2 h, the cells were washed and fixed with 4% paraformaldehyde solution for 15 min and then incubated with Hoechst 33258 (10 µg/mL) for 10 min to stain the nuclei. The fluorescent images were measured via CLSM.
2.5. Safety evaluation
2.5.1. Osmotic fragility
RBC-IVM-PNPs or RBC-IVM-CSPNPs of 1% hematocrit were resuspended in NaCl concentrations ranging from 0 mM to 154 mM for 30 min at 37°C. RBCs incubated in 154 mM NaCl or distilled water were used as negative (spontaneous lysis) or positive (total lysis) controls of hemolysis, respectively. The RBCs (1,000 g, 5 min) and NPs (18,000 g, 20 min) in the samples were sequentially removed by centrifugation. The hemoglobin in the supernatants was detected immediately with a plate reader by spectrophotometry at a wavelength of 540 nm.
2.5.2. Oxidative fragility
RBC-IVM-PNPs or RBC-IVM-CSPNPs (50 μL) were diluted with PBS (5 mL) containing 3 mM H2O2. Free RBCs subjected to the same condition were used as the negative control of hemolysis, and RBCs incubated in distilled water were used as the positive control (total lysis). All RBC samples were incubated at 37°C for 24 h. Similarly, the RBCs (1,000 g, 5 min) and NPs (18,000 g, 20 min) in the samples were sequentially removed by centrifugation. The hemoglobin in the supernatants was detected immediately at 540 nm with the plate reader.
2.5.3. Na+/K+-ATPase activity assay
The Na+/K+-ATPase activity assay of free RBCs, RBC-IVM-PNPs, and RBC-IVM-CSPNPs was performed according to the protocol of the Na+/K+-ATPase kit (Zhang et al., 2018b). All RBC samples were lysed in 50 × distilled water, and the activity of Na+/K+-ATPase in RBC lysate was measured. Meanwhile, the BCA protein quantification kit was used to measure the protein concentration in equal numbers of RBCs.
2.5.4. Circulation time of RBCs in vivo
To determine the blood clearance of NPs-loaded RBCs, biotin-labeled RBC-IVM-PNPs and RBC-IVM-CSPNPs were prepared, and then injected into the donor mouse. Then serial blood samples were collected from the retro-orbital sinus at certain time intervals and analyzed the survival rate of RBCs by flow cytometry (Mock et al., 2009). Briefly, fresh RBCs were incubated with IVM-PNPs or IVM-CSPNPs as discussed above. Then, the RBC samples were incubated with NHS-biotin (20 μg/mL) at a ratio of 1 to 100 for 1 h. The RBC samples were washed three times with PBS (containing 100 mM glycine) to remove excess NHS-biotin. The biotin-labeled RBC samples were reinfused into the donor mice (n = 3 per group). At 0 h, 1 h, 6 h, 12 h, 24 h, 3 d, 5 d and 7 d after injection, 0.02 mL of blood was collected and washed with PBS. Next, the RBC samples were incubated with FITC-labeled streptavidin at a ratio of 1 to 100 for 1 h. After removal of excess fluorescent reagent, the relative levels of NHS-biotin-streptavidin-FITC binding on RBCs were determined by flow cytometry. The proportion of biotin-labeled RBCs at 0 h after injection was defined as 100% survival.
2.6. In vivo pharmacokinetic (PK) study
Healthy SD rats were randomly divided into five groups, free-IVM, IVM-PNPs, IVM-CSPNPs, RBC-IVM-PNPs, and RBC-IVM-CSPNPs (n = 3). Various IVM-loaded formulations were administered via the caudal vein at 500 µg/kg. Blood samples (0.4 mL) were collected at the scheduled time points from the retro-orbital sinus and centrifuged (3,000 rpm, 10 min) to obtain plasma for analysis.
The IVM concentrations in rat plasma were determined using an Agilent HPLC-electrospray ionization-tandem mass spectrometry (HPLC-MS/MS) system (Pump G1312C, Autosampler 1367E, Degasser G1322A, G6460A triple quad mass spectrometer, Agilent Technologies, USA). The IVM and internal standard (IS, Doramectin) were separated by automated injection of samples (5 μL) into a reversed phase C8 analytical column (Agilent Zorbax SB C8, 2.1 × 100 mm, 3.5 μm, Agilent Technologies, USA) under isocratic conditions, which contained methanol and 10 mM ammonium acetate containing 0.1% (v/v) formic acid (90:10, v/v) at a flow rate of 0.25 mL/min.
The triple-quadrupole mass spectrometer was operated in the positive ionization mode. Detection and quantification were performed using multiple-reaction monitoring (MRM). Tandem mass spectrometry detection was conducted by monitoring the fragmentation of 892.5 → 307.3 (m/z) for IVM and 916.5 → 331.1 (m/z) for IS. The product ions were generated with a collision energy of 20 eV for both IVM and IS. The optimized MS parameters were as follows: fragment voltage, 140 V for IVM and IS; capillary voltage, 4000 kV; gas temperature, 200°C; gas flow, 10 L/min; nebulizer pressure, 30 psi.
The plasma samples (100 μL) and IS (10 μL, 500 ng/ml) were mixed with ethyl acetate (900 μL) containing 10% (v/v) methyl tert-butyl ether. After vigorous vortex-mixing (3 min) and centrifugation (14,000 rpm, 5 min), the organic layer was transferred and evaporated at 40°C for 30 min. The residue was then reconstituted with the mobile phase (100 μL). After centrifugation (14,000 rpm, 5 min), the supernatants (5 μL) were injected into the HPLC-MS/MS system for analysis.
2.7. In vivo imaging and biodistribution studies
The near-infrared fluorescent probe Cy7.5 labeled PNPs and CSPNPs (Cy7.5-PNPs and Cy7.5-CSPNPs) were applied to evaluate the lung targeting efficiency (Hu et al., 2020). Healthy ICR mice were injected with Cy7.5-PNPs, Cy7.5-CSPNPs, RBC-Cy7.5-PNPs, and RBC-Cy7.5-CSPNPs via the caudal vein (n = 3). After 0.5 h and 2 h, mice were anesthetized with 3% isoflurane and then observed (750/820 nm) via IVIS® Spectrum CT (IVIS® Spectrum, PerkinElmer, USA). After imaging, the mice were sacrificed and their major organs (liver, spleen, kidneys, heart, and lungs) were harvested and imaged.
Additionally, free IVM, IVM-PNPs, IVM-CSPNPs, RBC-IVM-PNPs, or RBC-IVM-CSPNPs were administered into healthy SD rats via the caudal vein (500 μg/kg, n = 3). The rats were anesthetized and sacrificed 0.5 h and 2 h after administration. Then, the major organs were harvested, weighed, and homogenized. IVM concentrations in tissues were detected by HPLC-MS/MS as described above.
2.8. In vivo anti-inflammatory activities
The ALI mouse model was established as reported previously (Fang et al., 2017, Ju et al., 2018). LPS from Escherichia coli (5 mg/kg) or PBS (control) was instilled intratracheally, and 5 h after LPS challenge, mice were injected intravenously with different IVM-loaded formulations (500 μg/kg, n = 5). Mice were sacrificed 24 h after administration. After the right lung was ligated, the left lung bronchoalveolar lavage fluid (BALF) was collected and centrifuged (3,000 rpm, 10 min). Subsequently, the cells in the pellet were counted with a hemocytometer, and the levels of total protein and pro-inflammatory cytokines (TNF-α and IL-6) in the supernatant were determined using an enhanced BCA protein assay and ELISA , respectively (Ju et al., 2018). A part of the right lungs was used for weighing and drying (60°C, 3 d) to calculate the wet-to-dry weight ratio. The remaining right lungs were stained with hematoxylin and eosin (H&E) to assess the degree of lung damage.
2.9. Statistical analysis
The results are expressed as mean ± standard deviation (SD), unless otherwise stated. Statistical analysis of the PK parameters was performed using unpaired two-tailed t-test. One-way analysis of variance (ANOVA) was performed to determine significant differences between different groups. A p-value < 0.05 was considered as statistically significant. p-values < 0.05, < 0.01, and < 0.001 are denoted as *, **, and ***, respectively, and NS: non-significant.
3. Results and discussion
3.1. Characterization of IVM-NPs
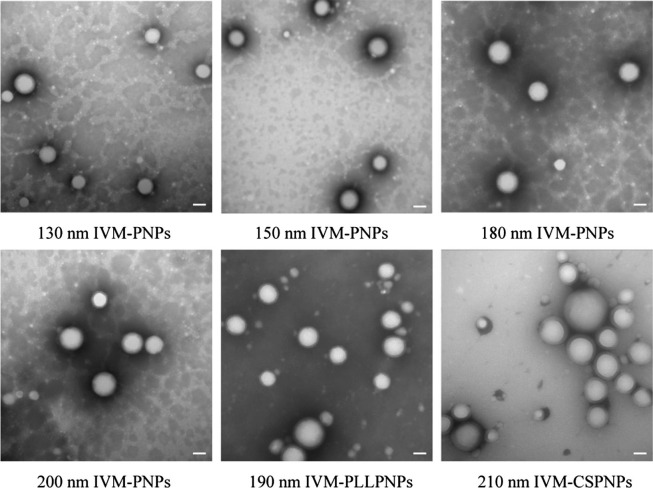
The NPs were successfully prepared and the mean sizes, zeta potential, EE, and DL are shown in Table 1 . The resulting IVM-PNPs exhibited mean particle sizes in the range 132.13 ± 1.08 nm to 201.05 ± 2.58 nm (Fig. S1). Compared to uncoated IVM-PNPs, IVM-CSPNPs and IVM-PLLPNPs exhibited an increase in mean sizes (212.60 ± 2.30 nm and 192.65 ± 2.02 nm vs 180.76 ± 2.64 nm, respectively) and a conversion of zeta potential (+24.10 ± 1.69 mV and +17.93 ± 2.04 mV vs −25.90 ± 0.57 mV, respectively) (Fig. S2). Increased particle sizes and positive potential indicated that IVM-PNPs were successfully coated with CS or PLL. The spherical IVM-PNPs cores and polymer outer rings could be clearly observed via TEM (Fig. 2 ). The kinetics of IVM release was investigated in PBS to mimic in vivo environment. As shown in Fig. S3, both IVM-PNPs and IVM-CSPNPs exhibited sustained release behavior within 48 h. At 24 h, 64% and 59% of IVM were released from IVM-PNPs and IVM-CSPNPs, respectively. Compared to IVM-PNPs, IVM-CSPNPs exhibited a slightly slower rate of IVM release. The sustained release of IVM from formulations blocked the rapid peak of the drug, which might prolong the action time of the drug at the target site, thereby improving the therapeutic effect. Moreover, the EE of all NPs exceeded 90% and the DL was approximately 8%.
Table 1.
The properties of nanoparticles.
| PLGA materials | Coating | Particle size nm |
PDI % |
Potential mV |
EE % |
DL % |
|---|---|---|---|---|---|---|
| C-75/25 | Uncoated | 180.76 ± 2.64 | 12.10 ± 3.53 | −25.90 ± 0.57 | 92.43 ± 0.36 | 8.40 ± 0.03 |
| C-50/50 | Uncoated | 186.07 ± 1.57 | 10.27 ± 1.28 | −18.63 ± 0.78 | 93.22 ± 0.71 | 8.26 ± 0.02 |
| E-75/25 | Uncoated | 184.13 ± 1.29 | 9.77 ± 1.13 | −24.47 ± 0.95 | 92.42 ± 0.21 | 8.40 ± 0.02 |
| E-50/50 | Uncoated | 189.91 ± 2.09 | 9.70 ± 2.19 | −16.50 ± 1.61 | 92.86 ± 0.54 | 8.35 ± 0.10 |
| C-75/25 | Uncoated | 132.13 ± 1.08 | 10.30 ± 1.43 | −25.53 ± 0.82 | 90.65 ± 0.46 | 8.24 ± 0.04 |
| C-75/25 | Uncoated | 149.37 ± 1.34 | 13.43 ± 3.71 | −25.97 ± 1.54 | 92.52 ± 0.89 | 8.41 ± 0.08 |
| C-75/25 | Uncoated | 201.05 ± 2.58 | 15.67 ± 1.36 | −25.03 ± 1.31 | 93.19 ± 1.09 | 8.47 ± 0.10 |
| C-75/25 | Chitosan | 212.60 ± 2.30 | 15.90 ± 2.30 | +24.10 ± 1.69 | 92.22 ± 0.07 | 8.27 ± 0.01 |
| C-75/25 | Poly-L-lysine | 192.65 ± 2.02 | 14.27 ± 1.76 | +17.93 ± 2.04 | 92.43 ± 0.36 | 8.40 ± 0.03 |
Fig. 2.
Morphological appearance of IVM-PNPs, IVM-CSPNPs, and IVM-PLLPNPs. Scale bars: 100 nm.
3.2. Adsorption of NPs to RBCs
The interaction forces between NPs and RBCs are mainly non-covalent interactions, such as electrostatic and hydrophobic interactions (Anselmo et al., 2013). The properties of NPs had an important effect on the adsorption efficiency of NPs to RBCs. NPs with different materials, sizes and polymer coatings were prepared for investigation of the adsorption efficiency of NPs to RBCs. First, four PNPs with different composition (75:25 or 50:50) and surface chemistry (carboxyl group-terminated or ester group-terminated) were used to evaluate the influence of NPs materials on the adsorption efficiency (Fig. 3 A). Among the four groups, IVM-PNPs with 75:25 carboxyl group-terminated exhibited the highest adsorption efficiency (44.24% ± 2.26%). Notably, compared to ester group-terminated IVM-PNPs, carboxyl group-terminated IVM-PNPs exhibited higher adsorption efficiency because the carboxyl end could form more hydrogen bonds with the RBC membrane than the ester end (Öcal et al., 2014). Moreover, 75:25 IVM-PNPs, which had higher hydrophobicity (Ibrahim et al., 2013), exhibited higher adsorption efficiency compared to 50:50 IVM-PNPs.
Fig. 3.
Investigation of NPs adsorption to RBCs. The influence of PLGA materials (A), sizes (B), and polymer coatings (C) on the adsorption efficiency of NPs to RBCs. C-75/25: PLGA with an LA:GA ratio of 75:25, carboxyl group-terminated; E-75/25: PLGA with an LA:GA ratio of 75:25, ester group-terminated; C-50/50: PLGA with an LA:GA ratio of 50:50, carboxyl group-terminated; E-50/50: PLGA with an LA:GA ratio of 50:50, ester group-terminated. The results are expressed as mean ± SD. p-values < 0.05, < 0.01, and < 0.001 are denoted as *, **, and ***, respectively, and NS: non-significant. (D) RBC morphology following incubation with IVM-PNPs, IVM-CSPNPs, and IVM-PLLPNPs. Scale bars: 35 μm. (E) Detection of hemagglutination caused by NPs adsorption via V-shaped 96-well plates, in which non-aggregated RBCs precipitated into a tight point, while aggregated RBCs formed a 3D-network that prevents sedimentation to the bottom of V-shape (n = 3). (i): RBC-IVM-PNPs; (ii): RBC-IVM-CSPNPs; (iii): RBC-IVM-PLLPNPs.
Then, IVM-PNPs of different sizes (130 nm, 150 nm, 180 nm, and 200 nm) prepared with 75:25 carboxyl-terminated PLGA were used to characterize the effect of NPs size on the adsorption efficiency (Fig. 3B). The adsorption efficiency increased with the IVM-PNPs sizes. Generally, small NPs had reduced surface contact with RBCs and were easy to desorb from the RBC surface during the washing process (Zelepukin et al., 2019). However, once the size of NPs exceeded 450 nm, the number of NPs adsorbed on RBCs decreased rapidly as the size of the NPs increased (Chambers and Mitragotri, 2004). Therefore, IVM-PNPs with a particle size of 200 nm were selected for further research.
Next, the effect of polymer coatings on the adsorption efficiency was investigated using cationic IVM-CSPNPs or IVM-PLLPNPs. Interestingly, the adsorption efficiency of IVM-CSPNPs and IVM-PLLPNPs increased by 117.12% and 100.42%, respectively (Fig. 3C). However, hemagglutination was observed under some experimental conditions (Fig. 3D). IVM-PNPs caused hemagglutination at a ratio of 8 mg NPs per 1 mL RBCs (8 mg/mL RBCs), showing the lowest agglutination effect (Fig. 3E). The RBCs agglutinated when the ratio of IVM-CSPNPs to RBCs exceeded 4 mg/mL RBCs. However, IVM-PLLPNPs caused hemagglutination at all experimental ratios, even at an excessively low concentration of 0.4 mg/mL RBCs. Generally, cationic NPs led to more severe hemagglutination than anionic NPs (Yu et al., 2011). Therefore, IVM-PLLPNPs and IVM-CSPNPs showed stronger hemagglutination effect than IVM-PNPs. Compared to PLL, CS had higher reliability due to its good biocompatibility and low toxicity (Ahmed and Aljaeid, 2016, Hoemann et al., 2022). Based on the above results, IVM-PNPs and IVM-CSPNPs were selected for further research and the ratio was selected as 4 mg/mL RBCs.
3.3. Characterization of RBC-IVM-PNPs and RBC-IVM-CSPNPs
The morphology of RBC-PNPs and RBC-CSPNPs was characterized using SEM and CLSM. Compared to IVM-PNPs, more IVM-CSPNPs were adsorbed to the surface of RBCs (Fig. 4 A&C), which agreed with the results of the adsorption experiment.
Fig. 4.
Characterization of RBC-NPs in vitro. The morphology of RBC-PNPs and RBC-CSPNPs under CLSM (A) and SEM (C). (i): Free RBCs; (ii): RBC-IVM-PNPs; (iii): RBC-IVM-CSPNPs (green: PNPs or CSPNPs; scale bars: 2 μm.) (B) NPs desorption from the surface of RBCs under different shear stress (1 Pa, 5 Pa). The results are expressed as mean ± SD. p-values < 0.05, < 0.01, and < 0.001 are denoted as *, **, and ***, respectively, and NS: non-significant. Representative fluorescence images of RAW264.7 cells following incubation with Cy5.5-PNPs and RBC-Cy5.5-PNPs (D), or Cy5.5-CSPNPs and RBC-Cy5.5-CSPNPs (E) for 2 h. (green: PNPs or CSPNPs, blue: nuclei; scale bars: 10 μm.). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
RBC-hitchhiked NPs desorbed from RBCs when exposed to the physiological shear stress. In capillaries, such as pulmonary capillaries, RBCs were subjected to high shear force (∼5 Pa) because of vascular stenosis, while in normal blood vessels, RBCs were subjected to low shear force (∼1 Pa) (Malek et al., 1999). To simulate different stress environments in vivo, RBCs were subjected to low shear (∼1 Pa) and high shear (∼5 Pa). The percentage of NPs desorbed from RBCs at 5 Pa was higher than that of NPs desorbed from RBCs at 1 Pa for RBC-IVM-PNPs and RBC-IVM-CSPNPs (Fig. 4B). Additionally, compared to IVM-CSPNPs, IVM-PNPs were easier to desorb from the RBC surface under high shear challenge.
NPs adsorbed to RBCs could avoid MPS uptake, which prolonged their circulation time. A macrophage uptake experiment was used to evaluate the anti-phagocytosis abilities of RBC-PNPs and RBC-CSPNPs (Yao et al., 2021). The fluorescence intensity of the Cy5.5-PNPs group and Cy5.5-CSPNPs group were significantly higher than those of the RBC-Cy5.5-PNPs group and RBC-Cy5.5-CSPNPs group, implying rapid clearance of PNPs and CSPNPs in vivo (Fig. 4E&F). RBC-Cy5.5-PNPs and RBC-Cy5.5-CSPNPs significantly reduced the phagocytosis of RAW264.7 cells (Fig. 4E&F). Overall, RBC-hitchhiking inhibited the phagocytosis of RAW264.7 cells regardless of the surface charge of NPs. RBCs were negatively charged due to the sialic acid groups on the surface of the RBC membrane (Weiss and Zeigel, 1971), which made it possible to form a strong electrostatic interaction with cationic CSPNPs (Anselmo and Mitragotri, 2014). Therefore, compared to PNPs, CSPNPs exhibited a stronger interaction with RBCs, higher RBCs adsorption efficiency in vitro. Additionally, cationic NPs were easier to escape from lysosome after internalization, whereas negatively and neutrally charged NPs tended to accumulate in lysosomes (Xia et al., 2008).
3.4. Safety evaluation
The adsorption of NPs to RBCs might cause damage to RBCs, and the clearance of damaged RBCs by the spleen will shorten the circulation time and affect drug delivery (Lee et al., 2011, Villa et al., 2016b, Villa et al., 2015). Therefore, the damage of RBCs caused by NPs adsorption was evaluated in terms of osmotic fragility, oxidative fragility, Na+/K+-ATPase activity and survival time in vivo.
The osmotic fragility test was performed to evaluate the sensitivity of RBC-IVM-PNPs and RBC-IVM-CSPNPs to the hypotonic solutions (Foroozesh et al., 2011; Pan et al., 2016). Free RBCs, RBC-IVM-PNPs, and RBC-IVM-CSPNPs exhibited similar levels of hemolysis (Fig. 5 A). Regarding oxidative fragility, H2O2 challenge induced 23.21% ± 1.77%, 23.28% ± 0.68%, and 25.95% ± 2.39% hemolysis in the free RBCs group, RBC-IVM-PNPs group, and RBC-IVM-CSPNPs group, respectively. Na+/K+-ATPase is an important RBC membrane enzyme, which is mainly involved in the active transport of Na+ and K+, and is closely related to the deformability of RBCs (Zhang et al., 2018b). The adsorption of NPs on RBCs may lead to a decrease in Na+/K+-ATPase activity, thereby reducing the function and biological activity of RBC carriers. The Na+/K+-ATPase activity of RBC-IVM-PNPs and RBC-IVM-CSPNPs (31.40 ± 2.34 μmolpi·gHb-1·h−1 and 31. 78 ± 2.24 μmolpi·gHb-1·h−1, respectively) was not significantly (p > 0.05) reduced compared to that of free RBCs (32.70 ± 0.61 μmolpi·gHb-1·h−1).
Fig. 5.
Safety evaluation of RBC-IVM-PNPs and RBC-IVM-CSPNPs. (A) Osmotic fragility curves of free RBCs, RBC-IVM-PNPs, and RBC-IVM-CSPNPs. (B) Circulation time of biotin-labeled free RBCs, RBC-IVM-PNPs, and RBC-IVM-CSPNPs in vivo (n = 3). The results are expressed as mean ± SD.
These results indicated that the adsorption of IVM-CSPNPs exhibited certain side effects on RBCs under extreme conditions in vitro. To confirm the influences of NPs adsorption on RBCs, the circulation time of RBC carriers in vivo was studied. Compared to free RBCs, there were no significant differences in RBC-IVM-PNPs or RBC-IVM-CSPNPs (Fig. 5B). Therefore, NPs adsorption may not significantly affect the circulation time of RBCs in vivo. The decrease in biotin-RBCs in the bloodstream was most likely due to multiple sampling and fluorescence attenuation (Chambers and Mitragotri, 2004).
3.5. In vivo PK study
The average plasma concentrations of IVM versus time following a single dose of intravenous administration of free IVM, IVM-PNPs, IVM-CSPNPs, RBC-IVM-PNPs, and RBC-IVM-CSPNPs are exhibited in Fig. 6 . The PK parameters results (Table 2 ) showed that the area under curve (AUC), mean residence time (MRT) and t1/2 of the IVM-PNPs group and IVM-CSPNPs group were significantly higher than those of the IVM injection group. However, there was no significant difference in these parameters between IVM-PNPs and IVM-CSPNPs. Moreover, the MRT and t1/2 of the RBC-IVM-PNPs group and RBC-IVM-CSPNPs group were remarkably higher than those of the homologous NPs groups. Importantly, compared to RBC-IVM-PNPs, the AUC, MRT and t1/2 of RBC-IVM-CSPNPs were significantly increased.
Fig. 6.
Mean plasma IVM concentration–time profiles following a single dose intravenous administration of free IVM, IVM-PNPs, IVM-CSPNPs, RBC-IVM-PNPs, and RBC-IVM-CSPNPs in rats (n = 3). The results are expressed as mean ± SD.
Table 2.
Summary of PK parameters for IVM-loaded formulations.
| Parameters | T 1/2z (h) |
MRT0-t (h) |
MRT0-∞ (h) |
AUC0-t (mg/L·h) |
AUC0-∞ (mg/L·h) |
Cmax (mg/L) |
|---|---|---|---|---|---|---|
| Free IVM | 8.35 ± 0.88 | 9.08 ± 1.24 | 9.80 ± 1.26 | 3403.88 ± 357.95 | 3453.8 ± 359.21 | 1099.95 ± 88.57 |
| IVM-PNPs | 11.36 ± 1.26* | 12.89 ± 1.56* | 13.98 ± 1.61* | 4401.72 ± 496.25 | 4444.59 ± 496.78 | 930.79 ± 99.14 |
| RBC- IVM-PNPs |
15.39 ± 1.63**# | 16.21 ± 1.84**# | 16.46 ± 1.87**# | 4881.19 ± 504.73* | 5023.6 ± 507.61* | 806.52 ± 63.73* |
| IVM-CSPNPs | 12.09 ± 1.06* | 12.4 ± 0.98* | 13.64 ± 1.07* | 5077.56 ± 423.52* | 5136.55 ± 424.26* | 1633.25 ± 179.06* |
| RBC- IVM-CSPNPs |
20.42 ± 1.89**†† | 21.33 ± 1.92**†† | 22.24 ± 2.01**†† | 6419.37 ± 578.26**† | 6830.98 ± 584.35**† | 1435.10 ± 130.15* |
*p < 0.05 and **p < 0.01 vs free IVM; #p < 0.05 vs IVM-PNPs; †p < 0.05 and ††p < 0.01 vs IVM-CSPNPs.
NPs can increase the circulation time due to their unique physicochemical and biological properties (Bertrand et al., 2017). However, when the NPs entered the body, a protein crown was quickly formed around the NPs (<0.5 min) (Tenzer et al., 2013, Zhang et al., 2018a), resulting in rapid clearance by MPS in the bloodstream (Casals et al., 2010). For IVM-CSPNPs, the hydrophilic surfaces may prevent the NPs from serum absorption and undergo relatively less opsonization and clearance by MPS (Acharya and Sahoo, 2011). Therefore, compared to IVM-PNPs, IVM-CSPNPs could extend the circulation time in vivo. RBC-hitchhiking could effectively reduce NPs uptake by MPS (Fig. 4D&E) and consequently prolong the circulation time (Chambers and Mitragotri, 2004, Zelepukin et al., 2019). As expected, the PK profiles of RBC-IVM-PNPs and RBC-IVM-CSPNPs were significant different. The higher affinity with RBCs and lower desorption rate of IVM-CSPNPs could contribute to a longer circulation time and higher bioavailability.
3.6. In vivo imaging and biodistribution
Improving the distribution of IVM in the lungs has always been a challenge. RBC-hitchhiking provides a new strategy for lung-targeting drug delivery. To evaluate the biodistribution of RBC-hitchhiked NPs, Cy7.5-labeled RBC-PNPs and RBC-CSPNPs were prepared for imaging (Fig. 7 A&B). The individual Cy7.5-PNPs and Cy7.5-CSPNPs were mainly concentrated in the liver, which was mainly attributed to the phagocytosis by the MPS (Gustafson et al., 2015). RBC-Cy7.5-PNPs and RBC-Cy7.5-CSPNPs showed obvious lung-targeting effects in a short period of 0.5 h. Interestingly, the fluorescence intensity in the lung of the RBC-Cy7.5-PNPs group was higher than that of the RBC-Cy7.5-CSPNPs group at 0.5 h. However, the fluorescence accumulation in the lung of the RBC-Cy7.5-CSPNPs group was higher than that of the RBC-PNPs group at 2 h.
Fig. 7.
In vivo imaging and biodistribution studies. Biodistribution of Cy7.5-labeled formulations detected by in vivo fluorescent imaging (A) and ex vivo fluorescent imaging of organs (B) after 0.5 h (up) and 2 h (down) administration. The scale bars for in vivo fluorescent imaging and ex vivo fluorescent imaging range from 3 × 107 to 3 × 108 and from 1 × 108 to 1 × 109, respectively. The unit for scale bars is [p s−1 cm−2 sr−1] [ μW cm−2] −1. The biodistribution of IVM-loaded formulations after 0.5 h (C) and 2 h (D) administration in rats, n = 3. The results are expressed as mean ± SD. p-values < 0.05, < 0.01, and < 0.001 are denoted as *, **, and ***, respectively, and NS: non-significant.
Similar results were obtained in the biodistribution of RBC-IVM-PNPs and RBC- IVM-CSPNPs (Fig. 7C&D). Additionally, 0.5 h after administration, the IVM concentrations in lungs of the RBC-IVM-PNPs group and RBC-IVM-CSPNPs group were remarkably higher than those of the IVM-PNPs group and IVM-CSPNPs group. Moreover, compared to RBC-IVM-CSPNPs, a significant increase in lung accumulation was observed for RBC-IVM-PNPs. However, the IVM concentrations in the lungs of the RBC-IVM-PNPs group significantly decreased after 2 h, which was lower than that of the RBC-IVM-CSPNPs group. Furthermore, RBC-hitchhiked IVM-CSPNPs in the liver decreased by 35% compared to IVM-CSPNPs at 0.5 h after administration. However, there was no significant difference in liver accumulation between RBC-IVM-PNPs and IVM-PNPs.
Therefore, both RBC-IVM-PNPs and RBC-IVM-CSPNPs could significantly enhance IVM delivery to lungs and increase the concentration of IVM in the lungs. However, due to differences in the properties of NPs (NPs materials, surface charge), the redistribution effects of RBC-IVM-PNPs and RBC-IVM-CSPNPs in the lungs were different (Zelepukin et al., 2019). The NPs adsorbed on the RBCs were squeezed and sheared when passing through the pulmonary capillaries, before desorption from RBCs. Cationic IVM-CSPNPs possessed a stronger bonding force and adsorption efficiency with RBCs compared to anionic IVM-PNPs. Therefore, the desorption rate of IVM-CSPNPs might be slower than that of IVM-PNPs in vivo. Moreover, the adhesiveness of IVM-CSPNPs could promote retention of IVM-CSPNPs in the lungs and enhance internalization. Consequently, the accumulation and elimination rates of RBC-hitchhiked IVM-CSPNPs in the lungs were slower than that of RBC-hitchhiked IVM-PNPs. In contrast, anionic IVM-PNPs would easily desorb from RBCs and exhibit rapid accumulation and elimination in the lungs.
3.7. In vivo anti-inflammatory activities
In addition to antiviral activities, IVM also had anti-inflammatory activities, which may also be necessary to prevent ALI in SARS-CoV-2 infection. The anti-inflammatory activities of various formulations were investigated in an ALI mouse model induced by LPS. ALI was mainly characterized by pro-inflammatory cytokine production, inflammatory cell infiltration, alveolar barrier disruption, and severe pathological damage, such as edema, hemorrhage, and thickened alveolar walls (Matthay et al., 2012, Mokra and Kosutova, 2015). LPS challenge significantly increased the lung wet-to-dry ratio (an indicator of pulmonary edema, Fig. 8 A), cell counts (Fig. 8B), protein concentration (Fig. 8C), and levels of pro-inflammatory factors (TNF-α [Fig. 8D] and IL-6 [Fig. 8E]) in BALF compared to those of the control group. Administration of IVM-loaded formulations attenuated the severity of these indicators to varying degrees. Especially, compared to individual IVM-PNPs and IVM-CSPNPs, RBC-hitchhiked IVM-PNPs and IVM-CSPNPs significantly suppressed these indicators induced by LPS. However, IVM-free RBC-NPs (RBC-PNPs and RBC-CSPNPs) or free IVM mixed RBC-NPs did not exhibit significant therapeutic effects compared to control or free IVM, respectively (Fig. S4). Notably, RBC-IVM-CSPNPs exhibited a stronger inhibitory effect on these indicators than RBC-IVM-PNPs.
Fig. 8.
Anti-inflammatory activities of IVM-loaded formulations in lipopolysaccharide (LPS)-induced ALI model mice. Five hours after LPS challenge, mice were injected intravenously with PBS, free IVM, IVM-PNPs, IVM-CSPNPs, RBC-IVM-PNPs, and RBC-IVM-CSPNPs, respectively (500 μg/kg, n = 5). The mice were sacrificed 24 h after administration. The lung wet-to-dry weight ratio (A), cell counts (B), protein (C), TNF-α(D), and IL-6 in BALF (E) were measured. The results are expressed as mean ± SD. p-values < 0.05, < 0.01, and < 0.001 are denoted as *, **, and ***, respectively, and NS: non-significant. (G) Lung tissue from each experimental group was processed for histological evaluation. The black arrows indicate inflammatory cell infiltration. Scale bar: 50 μm.
Furthermore, the histopathological changes were investigated to determine the therapeutic efficacy of various IVM-loaded formulations at the tissue level (Fig. 8F). LPS challenge induced severe inflammatory cell infiltration (black arrows) and interstitial edema (dark purple) in ALI mice. The degree of improvement of the alveolar structure in each administration group was consistent with the inhibitory trend of the inflammatory response. Compared to control or free IVM, IVM-free RBC-NPs or free IVM mixed RBC-NPs did not attenuate inflammatory damage, respectively (Fig. S5). Moreover, RBC-hitchhiked IVM-PNPs and IVM-CSPNPs significantly reduced infiltrating inflammatory cells and relieved the severity of lung lesions compared to homologous IVM-PNPs and IVM-CSPNPs. For the RBC-IVM-CSPNPs groups, the alveoli were almost intact (light purple) and the inflammatory cells disappeared, exhibiting a significant therapeutic effect on ALI.
LPS triggers the innate immune response and activates the redox-sensitive transcription factor nuclear factor kappa-B (NF-κB), which in turn induces the expression of various pro-inflammatory mediators, including TNF-α and IL-6 (Cohen, 2002). IVM could inhibit the LPS-induced production of pro-inflammatory cytokines by suppressing the NF-κB pathway (Zhang et al., 2008). Therefore, the administration of IVM-loaded formulations could inhibit the inflammatory response and ultimately lead to the alleviation of ALI. Notably, RBC-hitchhiked IVM-PNPs and IVM-CSPNPs showed a significant therapeutic effect on ALI, mainly due to their extended circulation times (Fig. 6) and increased IVM concentration in the lungs (Fig. 7). Furthermore, compared to RBC-IVM-PNPs, slower accumulation and elimination rates of RBC-hitchhiked IVM-CSNPs in the lungs could improve the treatment effects.
4. Conclusions
This study investigated the potential of an RBC-hitchhiking strategy to deliver IVM to lungs. After modifying PNPs with chitosan, the optimized CSPNPs could significantly improve the adsorption efficiency of NPs to RBCs. RBC-hitchhiking enhanced lung delivery, increased IVM accumulation in the lungs and prolonged the circulation time of NPs in vivo. Importantly, the redistribution and circulation effects were related to the properties of NPs. The accumulation and elimination rates of RBC-hitchhiked IVM-CSPNPs in the lungs were remarkably slower than those of RBC-hitchhiked IVM-PNPs.
Therefore, RBC-hitchhiking provides an alternative strategy to improve IVM pharmacokinetics and bioavailability for repurposing of IVM for the treatment of COVID-19. Furthermore, according to different redistribution effects of different NPs adsorbed on RBCs, RBC-hitchhiked NPs may achieve various accumulation rates and circulation times for different requirements of drug delivery.
Ethics approval and consent to participate.
All procedures involving the care and handling of animals carried out with the approval of the Animal Care and Use Ethics Committee of the Beijing Institute of Pharmacology and Toxicology (Beijing, China). This committee also approved all animal-related experiments in the current study (No. IACUC-DWZX-2020–646).
Consent for publication.
All authors agreed to submit this manuscript.
Availability of data and materials.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
CRediT authorship contribution statement
Jinpeng Zheng: Methodology, Investigation, Validation, Formal analysis, Writing – original draft, Writing – review & editing. Caihong Lu: Investigation, Formal analysis, Data curation. Yaning Ding: Investigation, Data curation. Jinbang Zhang: Investigation, Data curation. Fangyun Tan: Data curation. Jingzhou Liu: Data curation. Guobao Yang: Data curation. Yuli Wang: Data curation. Zhiping Li: Data curation. Meiyan Yang: Data curation. Yang Yang: Data curation. Wei Gong: Conceptualization, Methodology, Writing – original draft, Writing – review & editing, Supervision, Project administration. Chunsheng Gao: Supervision, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was supported by the National Science and Technology Major Projects for “Major New Drugs Innovation and Development” (Grant No. 2018ZX09711003-008-001).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijpharm.2022.121719.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Acharya S., Sahoo S.K. PLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effect. Adv. Drug Deliv. Rev. 2011;63(3):170–183. doi: 10.1016/j.addr.2010.10.008. [DOI] [PubMed] [Google Scholar]
- Ahmed S., Karim M.M., Ross A.G., Hossain M.S., Clemens J.D., Sumiya M.K., Phru C.S., Rahman M., Zaman K., Somani J., Yasmin R., Hasnat M.A., Kabir A., Aziz A.B., Khan W.A. A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness. International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases. 2021;103:214–216. doi: 10.1016/j.ijid.2020.11.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed T.A., Aljaeid B.M. Preparation, characterization, and potential application of chitosan, chitosan derivatives, and chitosan metal nanoparticles in pharmaceutical drug delivery. Drug Des Devel Ther. 2016;10:483–507. doi: 10.2147/DDDT.S99651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anselmo A.C., Gupta V., Zern B.J., Pan D., Zakrewsky M., Muzykantov V., Mitragotri S. Delivering nanoparticles to lungs while avoiding liver and spleen through adsorption on red blood cells. ACS Nano. 2013;7(12):11129–11137. doi: 10.1021/nn404853z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anselmo A.C., Mitragotri S. Cell-mediated delivery of nanoparticles: taking advantage of circulatory cells to target nanoparticles. J Control Release. 2014;190:531–541. doi: 10.1016/j.jconrel.2014.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand N., Grenier P., Mahmoudi M., Lima E.M., Appel E.A., Dormont F., Lim J.M., Karnik R., Langer R., Farokhzad O.C. Mechanistic understanding of in vivo protein corona formation on polymeric nanoparticles and impact on pharmacokinetics. Nat. Commun. 2017;8:777. doi: 10.1038/s41467-017-00600-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner J.S., Mitragotri S., Muzykantov V.R. Red Blood Cell Hitchhiking: A Novel Approach for Vascular Delivery of Nanocarriers. Annu Rev Biomed Eng. 2021;23(1):225–248. doi: 10.1146/annurev-bioeng-121219-024239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner J.S., Pan D.C., Myerson J.W., Marcos-Contreras O.A., Villa C.H., Patel P., Hekierski H., Chatterjee S., Tao J.Q., Parhiz H., Bhamidipati K., Uhler T.G., Hood E.D., Kiseleva R.Y., Shuvaev V.S., Shuvaeva T., Khoshnejad M., Johnston I., Gregory J.V., Lahann J., Wang T., Cantu E., Armstead W.M., Mitragotri S., Muzykantov V. Red blood cell-hitchhiking boosts delivery of nanocarriers to chosen organs by orders of magnitude. Nat. Commun. 2018;9:2684. doi: 10.1038/s41467-018-05079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caly, L., Druce, J.D., Catton, M.G., Jans, D.A., Wagstaff, K.M., 2020. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral research 178, 104787. [DOI] [PMC free article] [PubMed]
- Casals E., Pfaller T., Duschl A., Oostingh G.J., Puntes V. Time evolution of the nanoparticle protein corona. ACS Nano. 2010;4(7):3623–3632. doi: 10.1021/nn901372t. [DOI] [PubMed] [Google Scholar]
- Chambers E., Mitragotri S. Prolonged circulation of large polymeric nanoparticles by non-covalent adsorption on erythrocytes. J Control Release. 2004;100(1):111–119. doi: 10.1016/j.jconrel.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420(6917):885–891. doi: 10.1038/nature01326. [DOI] [PubMed] [Google Scholar]
- Croci R., Bottaro E., Chan K.W.K., Watanabe S., Pezzullo M., Mastrangelo E., Nastruzzi C. Liposomal Systems as Nanocarriers for the Antiviral Agent Ivermectin. International journal of biomaterials. 2016;2016:1–15. doi: 10.1155/2016/8043983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y., Lv B., Zheng J., Lu C., Liu J., Lei Y., Yang M., Wang Y., Li Z., Yang Y., Gong W., Han J., Gao C. RBC-hitchhiking chitosan nanoparticles loading methylprednisolone for lung-targeting delivery. J Control Release. 2021;341:702–715. doi: 10.1016/j.jconrel.2021.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y., Gao F., Hao J., Liu Z. microRNA-1246 mediates lipopolysaccharide-induced pulmonary endothelial cell apoptosis and acute lung injury by targeting angiotensin-converting enzyme 2. American journal of translational research. 2017;9:1287–1296. [PMC free article] [PubMed] [Google Scholar]
- Formiga F.R., Leblanc R., de Souza Rebouças J., Farias L.P., de Oliveira R.N., Pena L. Ivermectin: an award-winning drug with expected antiviral activity against COVID-19. J Control Release. 2021;329:758–761. doi: 10.1016/j.jconrel.2020.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foroozesh M., Hamidi M., Zarrin A., Mohammadi-Samani S., Montaseri H. Preparation and in-vitro characterization of tramadol-loaded carrier erythrocytes for long-term intravenous delivery. The Journal of pharmacy and pharmacology. 2011;63:322–332. doi: 10.1111/j.2042-7158.2010.01207.x. [DOI] [PubMed] [Google Scholar]
- Glassman P.M., Villa C.H., Ukidve A., Zhao Z., Smith P., Mitragotri S., Russell A.J., Brenner J.S., Muzykantov V.R. Vascular Drug Delivery Using Carrier Red Blood Cells: Focus on RBC Surface Loading and Pharmacokinetics. Pharmaceutics. 2020;12:440. doi: 10.3390/pharmaceutics12050440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González Canga A., Sahagún Prieto A.M., Diez Liébana M.J., Fernández Martínez N., Sierra Vega M., García Vieitez J.J. The pharmacokinetics and interactions of ivermectin in humans–a mini-review. The AAPS journal. 2008;10(1):42–46. doi: 10.1208/s12248-007-9000-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson H.H., Holt-Casper D., Grainger D.W., Ghandehari H. Nanoparticle Uptake: The Phagocyte Problem. Nano Today. 2015;10(4):487–510. doi: 10.1016/j.nantod.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidary F., Gharebaghi R. Ivermectin: a systematic review from antiviral effects to COVID-19 complementary regimen. The Journal of antibiotics. 2020;73(9):593–602. doi: 10.1038/s41429-020-0336-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoemann C.D., Rodríguez González J., Guzmán-Morales J., Chen G., Jalali Dil E., Favis B.D. Chitosan coatings with distinct innate immune bioactivities differentially stimulate angiogenesis, osteogenesis and chondrogenesis in poly-caprolactone scaffolds with controlled interconnecting pore size. Bioact. Mater. 2022;10:430–442. doi: 10.1016/j.bioactmat.2021.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, C., Lei, T., Wang, Y., Cao, J., Yang, X., Qin, L., Liu, R., Zhou, Y., Tong, F., Umeshappa, C.S., Gao, H., 2020. Phagocyte-membrane-coated and laser-responsive nanoparticles control primary and metastatic cancer by inducing anti-tumor immunity. Biomaterials 255, 120159. [DOI] [PubMed]
- Ibrahim M.M., Abd-Elgawad A.E., Soliman O.A., Jablonski M.M. Nanoparticle-based topical ophthalmic formulations for sustained celecoxib release. J. Pharm. Sci. 2013;102:1036–1053. doi: 10.1002/jps.23417. [DOI] [PubMed] [Google Scholar]
- Jermain B., Hanafin P.O., Cao Y., Lifschitz A., Lanusse C., Rao G.G. Development of a Minimal Physiologically-Based Pharmacokinetic Model to Simulate Lung Exposure in Humans Following Oral Administration of Ivermectin for COVID-19 Drug Repurposing. J. Pharm. Sci. 2020;109(12):3574–3578. doi: 10.1016/j.xphs.2020.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju M., Liu B., He H., Gu Z., Liu Y., Su Y., Zhu D., Cang J., Luo Z. MicroRNA-27a alleviates LPS-induced acute lung injury in mice via inhibiting inflammation and apoptosis through modulating TLR4/MyD88/NF-κB pathway. Cell cycle (Georgetown. Tex.) 2018;17:2001–2018. doi: 10.1080/15384101.2018.1509635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur H., Shekhar N., Sharma S., Sarma P., Prakash A., Medhi B. Ivermectin as a potential drug for treatment of COVID-19: an in-sync review with clinical and computational attributes. Pharmacological reports : PR. 2021;73(3):736–749. doi: 10.1007/s43440-020-00195-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krolewiecki, A., Lifschitz, A., Moragas, M., Travacio, M., Valentini, R., Alonso, D.F., Solari, R., Tinelli, M.A., Cimino, R.O., Álvarez, L., Fleitas, P.E., Ceballos, L., Golemba, M., Fernández, F., Fernández de Oliveira, D., Astudillo, G., Baeck, I., Farina, J., Cardama, G.A., Mangano, A., Spitzer, E., Gold, S., Lanusse, C., 2021. Antiviral effect of high-dose ivermectin in adults with COVID-19: A proof-of-concept randomized trial. EClinicalMedicine 37, 100959. [DOI] [PMC free article] [PubMed]
- Lee S.J., Park S.Y., Jung M.Y., Bae S.M., Kim I.S. Mechanism for phosphatidylserine-dependent erythrophagocytosis in mouse liver. Blood. 2011;117:5215–5223. doi: 10.1182/blood-2010-10-313239. [DOI] [PubMed] [Google Scholar]
- Li H., Jin K., Luo M., Wang X., Zhu X., Liu X., Jiang T., Zhang Q., Wang S., Pang Z. Size Dependency of Circulation and Biodistribution of Biomimetic Nanoparticles: Red Blood Cell Membrane-Coated Nanoparticles. Cells. 2019;8(8):881. doi: 10.3390/cells8080881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N.a., Zhao L., Zhan X. Quantitative proteomics reveals a broad-spectrum antiviral property of ivermectin, benefiting for COVID-19 treatment. J. Cell. Physiol. 2021;236(4):2959–2975. doi: 10.1002/jcp.30055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R., An Y., Jia W., Wang Y., Wu Y., Zhen Y., Cao J., Gao H. Macrophage-mimic shape changeable nanomedicine retained in tumor for multimodal therapy of breast cancer. J Control Release. 2020;321:589–601. doi: 10.1016/j.jconrel.2020.02.043. [DOI] [PubMed] [Google Scholar]
- López-Medina E., López P., Hurtado I.C., Dávalos D.M., Ramirez O., Martínez E., Díazgranados J.A., Oñate J.M., Chavarriaga H., Herrera S., Parra B., Libreros G., Jaramillo R., Avendaño A.C., Toro D.F., Torres M., Lesmes M.C., Rios C.A., Caicedo I. Effect of Ivermectin on Time to Resolution of Symptoms Among Adults With Mild COVID-19: A Randomized Clinical Trial. JAMA. 2021;325:1426–1435. doi: 10.1001/jama.2021.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmudpour, M., Roozbeh, J., Keshavarz, M., Farrokhi, S., Nabipour, I., 2020. COVID-19 cytokine storm: The anger of inflammation. Cytokine 133, 155151. [DOI] [PMC free article] [PubMed]
- Malek A.M., Alper S.L., Izumo S. Hemodynamic shear stress and its role in atherosclerosis. JAMA. 1999;282:2035–2042. doi: 10.1001/jama.282.21.2035. [DOI] [PubMed] [Google Scholar]
- Matthay M.A., Ware L.B., Zimmerman G.A. The acute respiratory distress syndrome. J Clin Invest. 2012;122(8):2731–2740. doi: 10.1172/JCI60331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mock D.M., Matthews N.I., Strauss R.G., Burmeister L.F., Schmidt R., Widness J.A. Red blood cell volume can be independently determined in vitro using sheep and human red blood cells labeled at different densities of biotin. Transfusion. 2009;49:1178–1185. doi: 10.1111/j.1537-2995.2009.02095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty S.S., Sahoo C.R., Padhy R.N. Targeting Some Enzymes with Repurposing Approved Pharmaceutical Drugs for Expeditious Antiviral Approaches Against Newer Strains of COVID-19. AAPS PharmSciTech. 2021;22:214. doi: 10.1208/s12249-021-02089-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokra D., Kosutova P. Biomarkers in acute lung injury. Respir. Physiol. Neurobiol. 2015;209:52–58. doi: 10.1016/j.resp.2014.10.006. [DOI] [PubMed] [Google Scholar]
- Öcal H., Arıca-Yegin B., Vural İ., Goracinova K., Çalış S. 5-Fluorouracil-loaded PLA/PLGA PEG-PPG-PEG polymeric nanoparticles: formulation, in vitro characterization and cell culture studies. Drug Dev. Ind. Pharm. 2014;40(4):560–567. doi: 10.3109/03639045.2013.775581. [DOI] [PubMed] [Google Scholar]
- Onoue S., Yamada S., Chan H.K. Nanodrugs: pharmacokinetics and safety. Int. J. Nanomed. 2014;9:1025–1037. doi: 10.2147/IJN.S38378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, D., Vargas-Morales, O., Zern, B., Anselmo, A.C., Gupta, V., Zakrewsky, M., Mitragotri, S., Muzykantov, V., 2016. The Effect of Polymeric Nanoparticles on Biocompatibility of Carrier Red Blood Cells. PloS one 11, e0152074. [DOI] [PMC free article] [PubMed]
- Peña‐Silva R., Duffull S.B., Steer A.C., Jaramillo‐Rincon S.X., Gwee A., Zhu X. Pharmacokinetic considerations on the repurposing of ivermectin for treatment of COVID-19. Br. J. Clin. Pharmacol. 2021;87(3):1589–1590. doi: 10.1111/bcp.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry J.L., Reuter K.G., Kai M.P., Herlihy K.P., Jones S.W., Luft J.C., Napier M., Bear J.E., DeSimone J.M. PEGylated PRINT nanoparticles: the impact of PEG density on protein binding, macrophage association, biodistribution, and pharmacokinetics. Nano Lett. 2012;12(10):5304–5310. doi: 10.1021/nl302638g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmith V.D., Zhou J.(., Lohmer L.R.L. The Approved Dose of Ivermectin Alone is not the Ideal Dose for the Treatment of COVID-19. Clin. Pharmacol. Ther. 2020;108(4):762–765. doi: 10.1002/cpt.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surnar B., Kamran M.Z., Shah A.S., Basu U., Kolishetti N., Deo S., Jayaweera D.T., Daunert S., Dhar S. Orally Administrable Therapeutic Synthetic Nanoparticle for Zika Virus. ACS Nano. 2019;13(10):11034–11048. doi: 10.1021/acsnano.9b02807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenzer S., Docter D., Kuharev J., Musyanovych A., Fetz V., Hecht R., Schlenk F., Fischer D., Kiouptsi K., Reinhardt C., Landfester K., Schild H., Maskos M., Knauer S.K., Stauber R.H. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nat. Nanotechnol. 2013;8(10):772–781. doi: 10.1038/nnano.2013.181. [DOI] [PubMed] [Google Scholar]
- Trailovic S.M., Ivanovic S.R., Varagić V.M. Ivermectin effects on motor coordination and contractions of isolated rat diaphragm. Res. Vet. Sci. 2011;91(3):426–433. doi: 10.1016/j.rvsc.2010.09.016. [DOI] [PubMed] [Google Scholar]
- Trailovic S.M., Nedeljkovic J.T. Central and peripheral neurotoxic effects of ivermectin in rats. The Journal of veterinary medical science. 2011;73(5):591–599. doi: 10.1292/jvms.10-0424. [DOI] [PubMed] [Google Scholar]
- Villa C.H., Anselmo A.C., Mitragotri S., Muzykantov V. Red blood cells: Supercarriers for drugs, biologicals, and nanoparticles and inspiration for advanced delivery systems. Adv. Drug Deliv. Rev. 2016;106:88–103. doi: 10.1016/j.addr.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa C.H., Pan D.C., Zaitsev S., Cines D.B., Siegel D.L., Muzykantov V.R. Delivery of drugs bound to erythrocytes: new avenues for an old intravascular carrier. Therapeutic delivery. 2015;6(7):795–826. doi: 10.4155/tde.15.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa C.H., Seghatchian J., Muzykantov V. Drug delivery by erythrocytes: “Primum non nocere”. Transfus Apher Sci. 2016;55(3):275–280. doi: 10.1016/j.transci.2016.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagstaff K.M., Rawlinson S.M., Hearps A.C., Jans D.A. An AlphaScreen®-based assay for high-throughput screening for specific inhibitors of nuclear import. J. Biomol. Screen. 2011;16(2):192–200. doi: 10.1177/1087057110390360. [DOI] [PubMed] [Google Scholar]
- Wang, Y., Zhou, C., Ding, Y., Liu, M., Tai, Z., Jin, Q., Yang, Y., Li, Z., Yang, M., Gong, W., Gao, C., 2021. Red blood cell-hitchhiking chitosan nanoparticles for prolonged blood circulation time of vitamin K(1). International journal of pharmaceutics 592, 120084. [DOI] [PubMed]
- Weiss L., Zeigel R. Cell surface negativity and the binding of positively charged particles. J. Cell. Physiol. 1971;77(2):179–185. doi: 10.1002/jcp.1040770208. [DOI] [PubMed] [Google Scholar]
- Xia T., Kovochich M., Liong M., Zink J.I., Nel A.E. Cationic polystyrene nanosphere toxicity depends on cell-specific endocytic and mitochondrial injury pathways. ACS Nano. 2008;2(1):85–96. doi: 10.1021/nn700256c. [DOI] [PubMed] [Google Scholar]
- Yan S., Ci X., Chen N.a., Chen C., Li X., Chu X., Li J., Deng X. Anti-inflammatory effects of ivermectin in mouse model of allergic asthma. Inflamm Res. 2011;60(6):589–596. doi: 10.1007/s00011-011-0307-8. [DOI] [PubMed] [Google Scholar]
- Yang M., Li J., Gu P., Fan X. The application of nanoparticles in cancer immunotherapy: Targeting tumor microenvironment. Bioact. Mater. 2021;6(7):1973–1987. doi: 10.1016/j.bioactmat.2020.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, S.N.Y., Atkinson, S.C., Wang, C., Lee, A., Bogoyevitch, M.A., Borg, N.A., Jans, D.A., 2020. The broad spectrum antiviral ivermectin targets the host nuclear transport importin α/β1 heterodimer. Antiviral research 177, 104760. [DOI] [PubMed]
- Yao Q., Yang G., Wang H., Liu J., Zheng J., Lv B., Yang M., Yang Y., Gao C., Guo Y. Aging erythrocyte membranes as biomimetic nanometer carriers of liver-targeting chromium poisoning treatment. Drug Deliv. 2021;28(1):1455–1465. doi: 10.1080/10717544.2021.1949075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T., Malugin A., Ghandehari H. Impact of silica nanoparticle design on cellular toxicity and hemolytic activity. ACS Nano. 2011;5(7):5717–5728. doi: 10.1021/nn2013904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelepukin I.V., Yaremenko A.V., Shipunova V.O., Babenyshev A.V., Balalaeva I.V., Nikitin P.I., Deyev S.M., Nikitin M.P. Nanoparticle-based drug delivery via RBC-hitchhiking for the inhibition of lung metastases growth. Nanoscale. 2019;11(4):1636–1646. doi: 10.1039/c8nr07730d. [DOI] [PubMed] [Google Scholar]
- Zhang H., Wu T., Yu W., Ruan S., He Q., Gao H. Ligand Size and Conformation Affect the Behavior of Nanoparticles Coated with in Vitro and in Vivo Protein Corona. ACS Appl. Mater. Interfaces. 2018;10(10):9094–9103. doi: 10.1021/acsami.7b16096. [DOI] [PubMed] [Google Scholar]
- Zhang X., Qiu M., Guo P., Lian Y., Xu E., Su J. Autologous Red Blood Cell Delivery of Betamethasone Phosphate Sodium for Long Anti-Inflammation. Pharmaceutics. 2018;10:286. doi: 10.3390/pharmaceutics10040286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Song Y., Ci X., An N., Ju Y., Li H., Wang X., Han C., Cui J., Deng X. Ivermectin inhibits LPS-induced production of inflammatory cytokines and improves LPS-induced survival in mice. Inflamm. Res. 2008;57(11):524–529. doi: 10.1007/s00011-008-8007-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.