Abstract
Purpose
To evaluate ocular surface disorders in students whose daily screen time increased due to distance learning during the COVID-19 pandemic.
Methods
Eighty-eight eyes of 44 cases were included in this cross-sectional study. The distance learning students with complaints of redness, stinging, and increased blinking were evaluated. Biomicroscopic examination findings, spherical equivalent, keratometry values, and average daily average screen time were recorded. Ocular Surface Disease Index (OSDI) survey and non-contact tear film breakup time (BUT) assessment (Topcon CA-800) were performed.
Results
Forty-four cases between 15 and 25 years old were evaluated; 25 were girls (56.8%), 19 were boys (43.2%), and the mean age was 19.2 ± 3.9 years (15–25). The mean daily screen time was 4.9 ± 0.9 h. The mean non-contact BUT was 3.18 ± 2.0 s (1.24–8.80 s), and the spherical equivalent was -1.39 ± 1.79. Punctate epitheliopathy was present in 33 eyes (37.5%) on biomicroscopic examination. The mean OSDI score was 37.12 ± 20.30 (10–75) points. A significant positive correlation was present between daily average screen time, punctate epitheliopathy (r = 0,341; p = 0,001), and OSDI score (r = 0,510; p < 0,001). There was also a significant positive correlation between the OSDI score and punctate epitheliopathy (r = 0.754; p < 0.001). There was no significant correlation between the non-contact BUT and punctate epitheliopathy, OSDI score, or daily screen time (p > 0.05).
Conclusion
Ocular surface disorders in students can be associated with increasing daily screen time due to distance learning.
Keywords: COVID-19 pandemic, Digital screen, Distance learning, Dry eye, OSDI, Tear breakup time
Introduction
Coronavirus disease 2019 (COVID-19) pandemic has been causing disruptions in daily life due to reasons such as lockdowns of countries and social distance measures for more than one year. According to UNESCO, schools were suspended from April 2020 in 188 countries. Efforts to open schools in autumn 2020 in our country and other countries remained inconclusive due to the increasing number of cases. Students have been continuing their education online using distance education for months. Students who have been at home for a long time due to lockdown access gaming, social media, and entertainment content via digital screens. They are exposed to digital screens for long hours since they also use digital screens to access distance education online. Previous studies report results demonstrating that the use of digital screens is associated with dry eye symptoms [1–3]. Digital eye strain associated with digital screen use has been described in former studies, which include dry eye, itching, foreign body sensation, watering, blurred vision, and headache symptoms [4].
Dry eye disease, as defined by the Tear Film and Ocular Surface Society (TFOS) DEWS II, is a multifactorial disease of the ocular surface accompanied by ocular symptoms, manifested by loss of homeostasis of the tear film. Tear film instability and hyperosmolarity, inflammation on the ocular surface, and neurosensory abnormalities are etiological factors that cause dry eye [5]. In our clinical practice, dry eye disease (DED) is mostly seen in adult patients, while its frequency in childhood is increasing due to environmental factors [6]. The difficulty of detection and verbalization of symptoms and subjective examination and evaluation may make the diagnosis of DED challenging in children and adolescents. Since it is known that allergic diseases are common in this age group, it is essential to consider that the differential diagnosis of DED vs allergic conjunctivitis may sometimes be difficult [7, 8].
In our study, we evaluated high school and university students presenting with ocular surface complaints who had increased daily use of digital screens during distance education performed due to the COVID-19 pandemic. We aimed to identify ocular surface diseases in our cases, considering the well-known association between long-term digital screen use and DED.
Methods
This observational descriptive non-comparative cross-sectional ocular surface function study evaluated the cases presenting to Elazığ Fethi Sekin City Hospital with hyperemia, stinging, and increased blinking frequency between October and December 2020. The study included 44 symptomatic high school and university students who had been taking distance education due to the COVID-19 pandemic and had increased daily digital screen use. Exclusion criteria included the previous diagnosis of dry eye, lagophthalmos, presence of any lid impairment (blepharitis, hordeolum, chalazion), history of ocular trauma or surgery, contact lens use, and oral or topical drug use. All cases and the parents of the adolescents were informed of the study, and their signed consents were obtained. The study protocol was approved by the Institutional Review Board of the Fırat University (approval no.: 2021/04–48), and the study was conducted in accordance with ethical standards set in the Declaration of Helsinki.
All cases completed the Ocular Surface Disease Index (OSDI) questionnaire before the examination to evaluate ocular symptoms. The OSDI, developed by the Outcomes Research Group at Allergan (Irvine, CA), is a 12-item questionnaire created to provide a quick assessment of the symptoms of ocular discomfort consistent with dry eye and their consequence on vision-related functioning. The OSDI questionnaire included the following subscales: (1) ocular symptoms (OSDI symptoms), (2) vision-related activities in daily living (OSDI visual function), and (3) environmental triggers (OSDI triggers). Participants were grouped into four categories based on the OSDI score: normal (scores 0–12), mild dry eye symptoms (13–22), moderate dry eye symptoms (23–32), and severe dry eye symptoms (33–100) [9, 10]. Non-contact tear film breakup time (BUT) measurement (Topcon CA-800) was performed before the ophthalmologic examination.
All study participants underwent a full ophthalmic examination, including objective refraction, best-corrected visual acuity (BCVA-logMAR), slit-lamp biomicroscopy, intraocular pressure (IOP) measurement, and dilated fundus examination by indirect ophthalmoscopy with 90D lens. Spherical equivalent and keratometry measurements were performed using Topcon Auto Kerato-Refractometer KR-8800; we reported the mean value of three repeated measurements. Daily screen use durations and data on the most frequently used digital devices during the day were recorded. The staining pattern of the ocular surface with fluorescein was recorded. The status of the tear film was evaluated using fluorescein film breakup time (FBUT). The severity of punctate epitheliopathy was graded according to the scheme proposed by Bron et al. [11]. The presence of hyperemia and papillary and follicular reactions was evaluated during conjunctival examination.
Statistical analysis
Statistical analysis was performed using SPSS 20.0 software (IBM Corp., Armonk, NY, USA). Descriptive statistics were used to describe study population characteristics. Continuous data were expressed as means, standard deviations (SD), medians, and minimum–maximum values. The distribution normality of the continuous variables was tested with the Kolmogorov–Smirnov test. The Mann–Whitney U-test and independent-samples t-test were used to analyze the quantitative data. Spearman’s correlation test was used to evaluate the association between parameters. A p-value of < 0.05 was considered statistically significant.
Results
The study evaluated 88 eyes of 44 cases. Twenty-five patients (56.8%) were female, and 19 (43.2%) were male. The mean age was 19.2 ± 3.9 years (range, 15–25 years). The mean BCVA value was 0.03 ± 0.05, and the mean spherical equivalent value was -1.39 ± 1.79. The mean IOP was 16.2 ± 2.1 mmHg (11–19 mmHg). There were no pathological findings detected in the fundus examination of all cases. The demographics and refractive data of the subjects are shown in Table 1.
Table 1.
Basic characteristics
| Subjects | n = 44 |
|---|---|
| Age, y (mean ± SD) | 19.2 ± 3.9 |
| Gender, f/m | 25/19 |
| BCVA, logMAR (mean ± SD) | 0.03 ± 0.05 |
| K1 (mean ± SD) | 44.28 ± 2.07 |
| K2 (mean ± SD) | 45.92 ± 2.20 |
| Spherical equivalent (diopter, mean ± SD) | − 1.39 ± 1.79 |
BCVA, best-corrected visual acuity; SD, standard deviation
The mean non-contact BUT value was 3.18 ± 2.0 s (range, 1.24–8.80 s). The mean daily digital screen use time was 4.9 ± 0.9 h (range, 3–7 h). The digital devices used by the cases were smartphones in 60%, notebooks in 25%, and tablets in 15% of the cases. The mean FBUT value was 3.52 ± 1.74 s (range, 2–8 s). Biomicroscopic examination of the ocular surface with fluorescein revealed Oxford Scheme Grade I and II punctate epitheliopathy in 33 eyes (37.5%). No cases had a higher grade of punctate epitheliopathy. Conjunctival examination revealed hyperemia and papillary reaction in 34 eyes (38.6%).
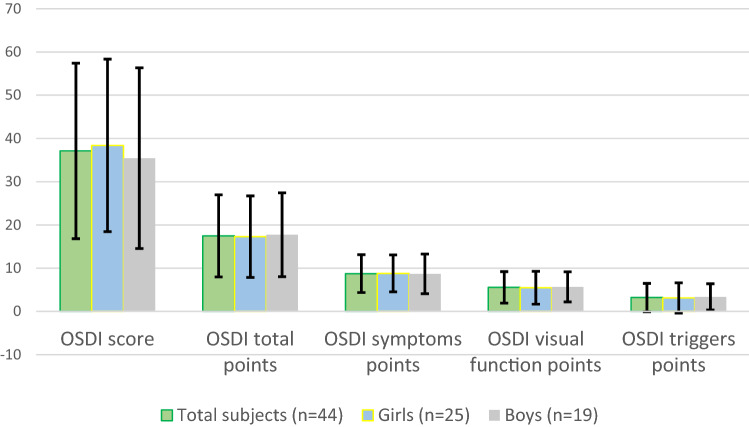
The mean score of the OSDI questionnaire was 37.12 ± 20.30 (10–75). This value is considered as a severe ocular surface disease (33–100) on the OSDI rating scale. The OSDI questionnaire results are shown in Table 2 and Fig. 1.
Table 2.
The OSDI questionnaire results
| Total Subjects (n = 44) |
Girls (n = 25) |
Boys (n = 19) |
p† | |
|---|---|---|---|---|
| OSDI score (mean ± SD) | 37.12 ± 20.30 | 38.40 ± 19.95 | 35.44 ± 20.90 | 0.502 |
| OSDI total points (mean ± SD) | 17.47 ± 9.49 | 17.28 ± 9.43 | 17.73 ± 9.69 | 0.825 |
| OSDI symptoms points (mean ± SD) | 8.75 ± 4.38 | 8.80 ± 4.27 | 8.68 ± 4.58 | 0.903 |
| OSDI visual function points (mean ± SD) | 5.56 ± 3.65 | 5.48 ± 3.80 | 5.68 ± 3.49 | 0.797 |
| OSDI triggers points (mean ± SD) | 3.22 ± 3.28 | 3.12 ± 3.49 | 3.36 ± 3.03 | 0.728 |
OSDI, Ocular Surface Disease Index; SD, standard deviation
p†: pairwise comparison between girls and boys
Fig. 1.
The OSDI questionnaire results. Abbreviations: OSDI, Ocular Surface Disease Index
Correlation analysis of the data showed significant positive correlation between daily average screen time and both OSDI score (rs = 0.510; p < 0.001) and punctate epitheliopathy (rs = 0.341; p = 0.001). OSDI score was also significantly positively correlated with punctate epitheliopathy (rs = 0.754; p < 0.001). A significant positive correlation was observed between FBUT value and non-contact BUT values (rs = 0.896; p < 0.001). Non-contact BUT value was not significantly correlated with daily average screen time and OSDI score (p > 0.05) (Table 3).
Table 3.
The correlation analysis of parameters
| OSDI score | Non-contact BUT | FBUT | Punctate Epitheliopathy | |
|---|---|---|---|---|
| Daily average screen time |
rs = 0.510** p < 0.001 |
rs = 0.003 p = 0.979 |
rs = 0.013 p = 0.901 |
rs = 0.341** p = 0.001 |
| OSDI score |
rs = 0.005 p = 0.966 |
rs = -0.003 p = 0.975 |
rs = 0.754** p < 0.001 |
BUT, breakup time; OSDI, Ocular Surface Disease Index
**Correlation is significant at the 0.01 level (two-tailed)
p values < 0.05 are shown in bold
Discussion
One of the consequences of the COVID-19 pandemic was that schools decided on distance education, albeit for a while. Increasing digital screen exposure in this process brings along many ocular surface problems as well as social and psychological effects. Studies that draw attention to the effects of distance education are increasing in the literature. In this study, we evaluated the ocular findings and OSDI score which is a reliable and frequently used questionnaire to evaluate ocular surface problems.
The use of digital screens is increasing day by day. Students spend most of their time in front of the screen due to distance education and the reduced outdoor activities during the COVID-19 pandemic. Dry eye associated with digital screen use can be considered a multifactorial process. During the blink reflex, the lipid-containing secretion of the meibomian glands diffuses to the ocular surface and reduces evaporation. The decrease in the blink rate or incomplete blinking can be observed during digital screen use. This reduction increases interpalpebral ocular surface area, instability of the tear film layer, and evaporation [12]. Hirota et al. investigated the blinking behavior during digital screen use in their study. They suggested that increased incomplete blinking contributes to tear film imbalance more than the decrease in total blink rate [13]. Incomplete blinking causes a non-uniform lipid distribution because of reduced meibomian secretion. Thus, the evaporation of tears from the ocular surface increases and the tear breakup time decreases. Uchino et al. reported a similar result. They showed the association of short BUT and increased corneal staining with fluorescein—despite normal lacrimal function—with the use of digital display [14]. Since there is no contact to the ocular surface during non-contact BUT measurement, it is easier to obtain reproducible measurements without causing an imbalance in the tear film. There is a weak correlation between the results of BUT administered with fluorescein and non-contact BUT in healthy eyes, but non-contact BUT is tending to be higher [15]. However, the results of both measurements were generally similar in dry eye cases [15, 16]. In this study, the mean non-contact BUT value was found 3.18 ± 2.0 s. This value is less than 10 s and meets the criteria defined by TFOS DEWS II for the diagnosis of DED [5]. There was no significant correlation between daily screen time and OSDI score with non-contact BUT value (p = 0.979, p = 0.966, respectively). The longer BUT measurements and the lower OSDI scores expect in a stable tear film. Previous studies reported the existence of a significant negative correlation between tear breakup time and OSDI score. In addition, some studies reported no significant correlation similar to our results [17, 18].
Bartlett et al. reported a poor relationship between dry eye symptoms and signs [19]. In a study for dry eye tests in a healthy pediatric population, all participants had at least one abnormal result, and one-third of them were diagnosed with DED [20]. Results may vary, as not all epidemiological studies evaluate both dry eye symptoms and diagnostic tests together. In studies evaluating the relationship between screen use and DED specifically, a correlation was found between signs and symptoms [3, 20, 21]. In a study conducted in Korea, it was emphasized that there is a strong relationship between smartphone use and DED in the pediatric population. It suggested that outdoor activities are a protective factor against DED [3]. They observed that punctate epitheliopathy findings regressed and the OSDI score decreased when the smartphone use was restricted for 4 weeks. Important results have also been obtained in the pediatric population with the OSDI questionnaire, which is effective and recommended by the TFOS DEWS II for the diagnosis of DED in adults [3, 20]. We also observed the correlation between clinical findings and symptoms by including only the cases who applied to our clinic with dry eye symptoms. All of the cases in our study were students who used digital screens for at least 3 h a day due to distance education. There was a positive and significant correlation between the patients’ daily digital screen use time, OSDI scores, and the presence of punctate epitheliopathy (p < 0.001). In addition, a positive significant correlation was observed between OSDI score and punctate epitheliopathy (p < 0.001). The mean OSDI score of the cases in our study was found 37.12 ± 20.30. This value is accepted as a serious ocular surface disease (33–100). Although the OSDI scores of girls (38.40 ± 19.95) were slightly higher than those of boys (35.44 ± 20.90), there was no statistical difference (p = 0.502). In this study, the cases gave high-score answers to the OSDI questionnaire, especially about symptoms. Since it increases inflammation of the ocular surface, allergic conjunctivitis cases often have dry eye symptoms. Differential diagnosis of DED and allergic conjunctivitis is difficult because the symptoms and signs are similar.
The most preferred digital device among our cases for distance education was smartphones (60%). Previous studies reported that smartphones are associated with DED more than other digital devices [1] [21]. Choi et al. reported the use of smartphones increased inflammatory markers and reactive oxygen derivatives on the ocular surface. Also, they observed increased subjective symptoms such as asthenopia, fatigue, and headache [21]. The smartphones have a smaller screen compared to other devices, are kept at a closer reading distance, and are more ergonomically uncomfortable. Therefore, smartphone use may increase these subjective symptoms. As it is known, focusing on the smartphone screen for a long time at a close reading distance increases the accommodation effort [22]. Considering the relationship between accommodation and myopia, we may be faced with the reality of increased myopia throughout the pandemic. The first publications on cases with myopic progression have already begun to be published. Wang et al. reported that the COVID-19 pandemic causes myopia progression in school-aged children [23]. In this study, the mean spherical equivalent was measured as -1.39 ± 1.79 D. However, since we do not know the previous refractive errors of our cases, we cannot associate this myopic result with distance education, screen use, and accommodation.
The small number of cases, absence of a control group with restricted screen use, and inability to exclude the accompanying allergic conjunctivitis findings (such as conjunctival hyperemia and papillary reactions) in some cases are the limitations of our study. Although it has been reported at different rates in the literature, MGD is one of the leading causes of dry eye disease [24]. However, since we aimed to evaluate the relationship between dry eye and screen use, we excluded other etiological causes of dry eye as much as possible. Cases with blepharitis in the slit-lamp examination were excluded from the study. Unfortunately, meibography, which is a more objective evaluation method, could not be applied to the cases.
As long as restrictions and distance learning continue, we believe there will be studies to support our findings in future. We hope that similar reports will contribute to finding a more optimized formula for distance learning and more direct students toward outdoor activities. These results will facilitate the early diagnosis and treatment of DED during the routine ophthalmologic examination of pediatric and adolescent patients. We hope that our results will draw attention to the routine ophthalmologic examinations of the young population should not be interrupted.
Authors Contribution
All authors contributed to the study conception and design. All authors commented on previous versions of the manuscript, read, and approved the final manuscript.
Funding
No funding was received for this study.
Declarations
Conflict of interest:
The authors declare that there is no conflict of interest regarding the publication of this article.
Availability of data and material
All data can be accessed transparently.
Ethical approval
The study protocol was approved by the Clinical Studies Ethics Committee of the Fırat University (Approval No.: 2021/04–48).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Seda Liman Uzun, Email: sedalimanuzun@gmail.com.
Husna Topcu, Email: husnaozturk@gmail.com.
References
- 1.Moon JH, Lee MY, Moon NJ. Association between video display terminal use and dry eye disease in school children. J Pediatr Ophthalmol Strabismus. 2014;51(2):87–92. doi: 10.3928/01913913-20140128-01. [DOI] [PubMed] [Google Scholar]
- 2.Köksoy Vayısoğlu S, Öncü E, Dursun Ö, et al. Investigation of dry eye symptoms in lecturers by ocular surface disease index. Turk J Ophthalmol. 2019;49(3):142–148. doi: 10.4274/tjo.galenos.2018.67915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moon JH, Kim KW, Moon NJ. Smartphone use is a risk factor for pediatric dry eye disease according to region and age: a case control study. BMC Ophthalmol. 2016;16(1):188. doi: 10.1186/s12886-016-0364-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mohan A, Sen P, Shah C, et al. Prevalence and risk factor assessment of digital eye strain among children using online e-learning during the COVID-19 pandemic: Digital eye strain among kids (DESK study-1) Indian J Ophthalmol. 2021;69(1):140–144. doi: 10.4103/ijo.IJO_2535_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II Definition and classification report. Ocul Surf. 2017;15(3):276–283. doi: 10.1016/j.jtos.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Uchino M, Dogru M, Uchino Y, et al. Japan ministry of health study on prevalence of dry eye disease among Japanese high school students. Am J Ophthalmol. 2008;146(6):925–9.e2. doi: 10.1016/j.ajo.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 7.Alves M, Dias AC, Rocha EM. Dry eye in childhood: epidemiological and clinical aspects. Ocul Surf. 2008;6(1):44–51. doi: 10.1016/s1542-0124(12)70104-0. [DOI] [PubMed] [Google Scholar]
- 8.Toda I, Shimazaki J, Tsubota K. Dry eye with only decreased tear break-up time is sometimes associated with allergic conjunctivitis. Ophthalmology. 1995;102(2):302–309. doi: 10.1016/s0161-6420(95)31024-x. [DOI] [PubMed] [Google Scholar]
- 9.Denoyer A, Rabut G, Baudouin C. Tear film aberration dynamics and vision-related quality of life in patients with dry eye disease. Ophthalmology. 2012;119(9):1811–1818. doi: 10.1016/j.ophtha.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Schiffman RM, Christianson MD, Jacobsen G, et al. Reliability and validity of the ocular surface disease index. Arch Ophthalmol. 2000;118(5):615–621. doi: 10.1001/archopht.118.5.615. [DOI] [PubMed] [Google Scholar]
- 11.Bron AJ, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea. 2003;22(7):640–650. doi: 10.1097/00003226-200310000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Schlote T, Kadner G, Freudenthaler N. Marked reduction and distinct patterns of eye blinking in patients with moderately dry eyes during video display terminal use. Graefes Arch Clin Exp Ophthalmol. 2004;242(4):306–312. doi: 10.1007/s00417-003-0845-z. [DOI] [PubMed] [Google Scholar]
- 13.Hirota M, Uozato H, Kawamorita T, et al. Effect of incomplete blinking on tear film stability. Optom Vis Sci. 2013;90(7):650–657. doi: 10.1097/OPX.0b013e31829962ec. [DOI] [PubMed] [Google Scholar]
- 14.Uchino M, Yokoi N, Uchino Y, et al. Prevalence of dry eye disease and its risk factors in visual display terminal users: the Osaka study. Am J Ophthalmol. 2013;156(4):759–766. doi: 10.1016/j.ajo.2013.05.040. [DOI] [PubMed] [Google Scholar]
- 15.Nichols JJ, Nichols KK, Puent B, et al. Evaluation of tear film interference patterns and measures of tear break-up time. Optom Vis Sci. 2002;79(6):363–369. doi: 10.1097/00006324-200206000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Lan W, Lin L, Yang X, et al. Automatic noninvasive tear breakup time (TBUT) and conventional fluorescent TBUT. Optom Vis Sci. 2014;91(12):1412–1418. doi: 10.1097/opx.0000000000000418. [DOI] [PubMed] [Google Scholar]
- 17.Kyei S, Dzasimatu SK, Asiedu K, et al. Association between dry eye symptoms and signs. J Curr Ophthalmol. 2018;30(4):321–325. doi: 10.1016/j.joco.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fenga C, Aragona P, Di Nola C, et al. Comparison of ocular surface disease index and tear osmolarity as markers of ocular surface dysfunction in video terminal display workers. Am J Ophthalmol. 2014;158(1):41–48.e2. doi: 10.1016/j.ajo.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 19.Bartlett JD, Keith MS, Sudharshan L, et al. Associations between signs and symptoms of dry eye disease: a systematic review. Clin Ophthalmol. 2015;9:1719–1730. doi: 10.2147/opth.S89700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rojas-Carabali W, Uribe-Reina P, Muñoz-Ortiz J, et al. High prevalence of abnormal ocular surface tests in a healthy pediatric population. Clin Ophthalmol. 2020;14:3427–3438. doi: 10.2147/opth.S266261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi JH, Li Y, Kim SH, et al. The influences of smartphone use on the status of the tear film and ocular surface. PLoS ONE. 2018;13(10):e0206541. doi: 10.1371/journal.pone.0206541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yazici A, Sari ES, Sahin G, et al. Change in tear film characteristics in visual display terminal users. Eur J Ophthalmol. 2015;25(2):85–89. doi: 10.5301/ejo.5000525. [DOI] [PubMed] [Google Scholar]
- 23.Wang J, Li Y, Musch DC, et al. Progression of Myopia in school-aged children after COVID-19 home confinement. JAMA Ophthalmol. 2021 doi: 10.1001/jamaophthalmol.2020.6239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan TCY, Chow SSW, Wan KHN, et al. Update on the association between dry eye disease and meibomian gland dysfunction. Hong Kong Med J. 2019;25(1):38–47. doi: 10.12809/hkmj187331. [DOI] [PubMed] [Google Scholar]