Graphical abstract
Keywords: Predictive modeling, Passive sampling, Biomonitoring, Disasters, Exposome
Abbreviations: CCCEH, Columbia Center for Children’s Environmental Health; CDC, Centers for Disease Control and Prevention; CLIA, Clinical Laboratory Improvement Amendments; CONC, concentration; FLU, fluorene; GC, gas chromatograph; IRB, Institutional Review Board; LDA, linear discriminant analysis; LOD, limit of detection; NAPH, naphthalene; NHANES, National Health and Nutrition Examination Survey; NIEHS, National Institute of Environmental Health Sciences; NIH, National Institute of Health; OH-PAH, hydroxy-polycyclic aromatic hydrocarbon; OSU, Oregon State University; PAH, polycyclic aromatic hydrocarbon; PHEN, phenanthrene; PTFE, polytetrafluoroethylene; PYR, pyrene; QC, quality control; SVOC, semi-volatile organic chemical; VOC, volatile organic chemical; WHO, World Health Organization
Abstract
During events like the COVID-19 pandemic or a disaster, researchers may need to switch from collecting biological samples to personal exposure samplers that are easy and safe to transport and wear, such as silicone wristbands. Previous studies have demonstrated significant correlations between urine biomarker concentrations and chemical levels in wristbands. We build upon those studies and use a novel combination of descriptive statistics and supervised statistical learning to evaluate the relationship between polycyclic aromatic hydrocarbon (PAH) concentrations in silicone wristbands and hydroxy-PAH (OH-PAH) concentrations in urine. In New York City, 109 participants in a longitudinal birth cohort wore one wristband for 48 h and provided a spot urine sample at the end of the 48-hour period during their third trimester of pregnancy. We compared four PAHs with the corresponding seven OH-PAHs using descriptive statistics, a linear regression model, and a linear discriminant analysis model. Five of the seven PAH and OH-PAH pairs had significant correlations (Pearson’s r = 0.35–0.64, p ≤ 0.003) and significant chi-square tests of independence for exposure categories (p ≤ 0.009). For these five comparisons, the observed PAH or OH-PAH concentration could predict the other concentration within a factor of 1.47 for 50–80% of the measurements (depending on the pair). Prediction accuracies for high exposure categories were at least 1.5 times higher compared to accuracies based on random chance. These results demonstrate that wristbands and urine provide similar PAH exposure assessment information, which is critical for environmental health researchers looking for the flexibility to switch between biological sample and wristband collection.
1. Introduction
1.1. Need for flexible alternatives for personal chemical exposure assessment
During events such as a pandemic, hurricane, or wildfire, it becomes difficult or impossible for researchers to safely collect personal chemical exposure information using traditional methods. Researchers evaluating personal chemical exposure often collect biological samples such as blood or urine (Aylward et al. 2014). Biomarkers in these biological samples serve as a proxy for personal chemical exposure and enable researchers to better understand the potential link between chemical exposure and health outcomes.
As seen with COVID-19, many environmental health researchers have not been able or allowed to collect biological samples due to government mandates and institutional policies, and many research projects were halted (NYT, 2020, Servick, 2020). For example, researchers at the Columbia Center for Children’s Environmental Health (CCCEH) have been collecting biological samples for birth cohort studies since 1998, but had to “ramp down” non-COVID-19 research in March 2020 to ensure the safety of research staff and study participants (Columbia 2020).
Despite such disruptions to normalcy, it is crucial that environmental health data collection continue. In June 2020, the National Institute for Environmental Health Sciences stated that environmental factors may play a part in how and where COVID-19 spreads (NIEHS 2020). The COVID-19 pandemic has highlighted the need for environmental health researchers to implement alternative personal chemical exposure assessment methodologies.
1.2. Silicone wearable characteristics
Silicone wearables are an additional exposure assessment tool that researchers use to measure chemical exposure. Study participants can wear these samplers as a wristband, lapel, dog tag, or other configuration (Dixon et al., 2020, O’Connell et al., 2014, Poutasse et al., 2020). Since silicone wearables were first reported on in 2014, they have been used in over 30 exposure assessment studies and have been worn by thousands of people on six continents (Bullock et al., 2020, Craig et al., 2019, De Vecchi et al., 2019, Dixon et al., 2020, Hammel et al., 2020, Hendryx et al., 2020, Reche et al., 2020, Reddam et al., 2020, Rohlman et al., 2019, Wang et al., 2020, Wang et al., 2019, Wise et al., 2020, Zuy et al., 2020). Silicone wearables are passive sampling devices, which sequester the fraction of chemicals that are available to transport across cellular membranes (Anderson and Hillwalker, 2008, O’Connell et al., 2014). Over 1530 different volatile and semi-volatile organic chemicals (VOCs and SVOCs), including flame retardants, pesticides, volatile organic chemicals, polycyclic aromatic hydrocarbons (PAHs), and phthalates, can be detected and quantified in silicone wearables (Bergmann et al. 2017). Depending on how the silicone wearables are worn, chemicals in silicone wearables can include contributions from several exposure routes, including inhalation, dermal contact, and potentially some ingestion exposure (Aerts et al., 2018, Dixon et al., 2020).
Study participants can easily and safely receive, wear, and return silicone wearables to researchers. Unlike biological sample collection, silicone wearables can be mailed and can be stored at ambient temperature when kept in airtight, impermeable containers, such as polytetrafluoroethylene (PTFE) bags (Anderson et al. 2017). Anderson et al. simulated transport and storage conditions and tested the recovery of a wide range of VOC and SVOCs in silicone wristbands (2017). Evidence from these studies suggest that researchers can transport silicone wearables through the mail without the need for ice or refrigeration as well as store the silicone wearables without losing valuable chemical data. For example, Anderson et al. demonstrated that under transport conditions (30 °C), VOCs were unchanged in the wristbands for 7 days (average 99% recovery) and SVOCs were stable up to one month (average 104% recovery; 2017). Long-term storage at −20 °C was tested with VOC concentrations stable for up to three months and with SVOC concentrations still stable at time of publishing storage stability data (six months; Anderson et al. 2017). This study has since been extended which indicates that SVOCs are stable at for at least 21 months at −20 °C (data unpublished).
Silicone wearable studies report high volunteer compliance (Bergmann et al., 2017, Donald et al., 2016, Harley et al., 2019, Kile et al., 2016, Vidi et al., 2017). For example, 100% of wristbands worn by rural farming families in Diender, Senegal (Donald et al. 2016) and 95% of wristbands worn by asthmatics in Eugene, OR, USA were returned to the laboratory and met study criteria (Rohlman et al. 2019). Volunteers in stressful environments have also worn silicone wearables with high compliance. There was 85% compliance for silicone wristbands mailed and worn by people in Houston, Texas, USA less than one month after Hurricane Harvey made landfall (Dixon et al. 2020) and 85% compliance for military-style silicone dog tags collected from on-duty firefighters in Kansas, USA (Poutasse et al. 2020). There has also been success integrating silicone wearables into studies requiring quick deployment (Dixon et al. 2020). Oregon State University developed a disaster Institutional Review Board (IRB) application in 2017 that has allowed researchers to respond quickly to unexpected events with silicone wearables (https://superfund.oregonstate.edu/disaster-irb), including Hurricane Harvey and Hurricane Florence (Rohlman et al. 2017).
To date, researchers have compared levels of chemicals in silicone wristbands (hereafter referred to as ‘wristbands’) to human biomarkers in seven studies. Each of these studies reported significant correlations between chemical concentrations in wristbands and the respective biological metabolite concentration (urine or serum), providing evidence that wristbands can capture the fraction of organic chemicals in the personal environment that are absorbed into the body (Dixon et al., 2018, Hammel et al., 2016, Hammel et al., 2018, Hammel et al., 2020, Hoffman et al., 2021, Levasseur et al., 2021, Quintana et al., 2019).
1.3. Study objective
Previously, our research group conducted a cohort study at CCCEH in New York City, USA where 22 pregnant women wore wristbands and provided spot urine samples (Dixon et al. 2018). Concentrations of PAHs in the wristbands strongly correlated with paired concentrations of OH-PAHs in urine (Spearman’s r = 0.44 to 0.76, p = 0.04 to <0.001), demonstrating that wristband data can align with urine OH-PAH data (Dixon et al. 2018). Here, we expanded our 2018 study to include over 100 paired sets of wristband and urine data. We used this larger sample size to capture wider ranges of PAH exposures and validate our previous findings based on the smaller cohort. The larger sample size also allowed for the use of supervised statistical learning. Our objective was to compare wristband PAH concentrations and urine OH-PAH concentrations as measures of personal PAH exposure. We hypothesized that descriptive statistics, a linear regression model, and a linear discriminant analysis model would reveal shared information between PAH and OH-PAH concentrations. To our knowledge, this is the first time that wristband data has been used to build supervised statistical models to predict human urinary biomarker concentrations. The seven OH-PAHs included in this study are regularly used as PAH biomarkers because they are excreted preferentially in the urine. This study provides critical data to environmental health researchers looking for the flexibility to use both urine samples and wristbands to assess personal exposure for select PAHs.
2. Materials and methods
2.1. Study cohort and design
This study leverages an ongoing longitudinal epidemiological birth cohort study at the CCCEH in New York City, USA. One hundred and nine women in their third trimester of pregnancy each wore one wristband for 48 h between 2013 and 2018. Wristbands were collected year-round (see Supplemental Material, Table S1). We also collected a spot urine sample from each woman at the end of the 48-hour period. Study participants could remove their wristband at night and place it near their bed, and were instructed not to place lotion or other personal care products directly on the wristbands. Participants ranged in age from 18 to 42, and 83% of the women were of Dominican origin (Table 1 ).
Table 1.
Study participant demographics.
| Continuous Characteristic | Range (n = 109) | Mean ± SD |
|---|---|---|
| Age (years) | 18–42 | 29.2 ± 6.1 |
| Categorical Characteristics | Number of Women (n = 106a) | Percent out of 106a |
| On Medicaid | 105 | 99.1% |
| Maternal Education | ||
| More than high school | 60 | 56.6% |
| High school graduate/GED | 24 | 22.6% |
| Did not complete high school | 22 | 20.8% |
| Maternal Ethnicity | ||
| African/African American | 4 | 3.8% |
| Asian | 2 | 1.9% |
| Caucasian | 0 | 0.0% |
| Dominican | 88 | 83.0% |
| Mexican/Mexican American | 3 | 2.8% |
| Other Hispanic | 2 | 1.9% |
| Other | 7 | 6.6% |
| Smoker in the home (environmental tobacco smoke) | 24 | 22.6% |
Categorical characteristic data is only available for 106 participants (data were unavailable for three participants).
2.2. Wristband methodology
2.2.1. Preparation and deployment
We purchased the wristbands from 24hourwristbands.com (Houston, Texas, US). To remove any chemicals in the silicone left over from the manufacturing process, we rinsed the wristbands with deionized water and placed them in a vacuum oven (300 °C for 12 h at 0.1 Torr; Blue M vacuum oven, no. POM18VC; Welch® DuoSeal pump, no. 1405, Mt. Prospect, Illinois, USA) as previously described (Dixon et al. 2018).
To ship the wristbands to the CCCEH, we packed the wristbands individually in airtight polytetrafluoroethylene (PTFE) bags (Welch Fluorocarbon, Dover, New Hampshire, US). After the 48-hour deployment period, wristbands were returned to the PTFE bags, sealed, and mailed back to Oregon State University (OSU).
2.2.2. Cleaning and extraction
We only extracted PAHs sequestered in the wristbands because this reflects the PAH fraction available to be absorbed into the body. Therefore, particles on the silicone surface were removed by cleaning the wristbands twice with 18 MΩ cm water and once with isopropanol (Dixon et al., 2018, O’Connell et al., 2014). To extract the PAHs of interest from the cleaned silicone, we followed the process described in O’Connell et al., 2014, Anderson et al., 2017, and Dixon et al. (2018). We added deuterated PAH surrogates to the wristbands after cleaning and before extraction, which were used to account for chemical recovery during the extraction process and to quantify the PAHs most similar in analytical behavior (Anderson et al. 2015). Briefly, we added two separate 50 mL volumes of ethyl acetate at room temperature to the wristbands and then concentrated the extracts to one mL under nitrogen (Turbo-Vap L, Biotage, Charlotte, North Carolina, US; RapidVap, LabConco, Kansas City, Missouri, US; N-EVAP 111, Organomation Associates, Berlin, Massachusetts, US). Additional details about laboratory equipment and chemicals are in the supplementary information document.
2.2.3. PAH quantification
We added the internal standard (perylene-d12) to each wristband extract prior to analysis. We analyzed wristband extracts for 61 PAHs with an Agilent 7890B gas chromatograph (GC) paired with a 7000C triple-quadrupole mass spectrometer (MS/MS, Santa Claire, California, US) (Anderson et al., 2015, Dixon et al., 2018). The instrument parameters are detailed in Anderson et al. (2015). Deuterated PAH surrogates were quantified relative to the internal standard and target PAHs were quantified relative to the most appropriate deuterated surrogate (Anderson et al. 2015). The limits of detection (LODs) for all 61 PAHs ranged from 7.36 to 11.9 log2 pmol/g wristband (calculated using a wristband mass of 5.71 g; Table S2). The PAH LODs are described in Anderson et al. and were calculated by taking repeated measurements of low concentration standards, calculating the standard deviation, and multiplying it by the Student’s t value for the 99% confidence interval (2015). The list of target PAHs and LODs are in Table S2 on the log2 pmol/g scale and in Table S3 on the pmol/g scale for all 61 PAHs, and in Table 2 for the four PAHs that correspond to OH-PAHs in this study. Hereafter, naphthalene, fluorene, phenanthrene, and pyrene are referred to as NAPH, FLU, PHEN, and PYR, respectively.
Table 2.
PAH method information and summary statistics for PAHs that correspond to OH-PAHs. Method information includes the Chemical Abstracts Service (CAS) number, molecular weight, and limit of detection (LOD).
| Target PAH | CAS Number | Molec-ular Weight (g/mol) | Instrument LODa (log2 pmol/g wristband) |
Number of Wristbands Without Matrix Interferenceb | Detection Frequency (%)c | Median (log2 pmol/g wristband) | Range (log2 pmol/g wristband) |
Interquartile Range (log2 pmol/g wristband) |
|---|---|---|---|---|---|---|---|---|
| Naphthalene | 91–20-3 | 128.2 | 10.5 | 109 | 100% | 16.4 | 11.0 – 21.5 | 1.48 |
| Fluorene | 86–73-7 | 166.2 | 9.70 | 80 | 96% | 16.2 | <LOD – 18.9 | 0.96 |
| Phenanthrene | 85–01-8 | 178.2 | 8.82 | 80 | 99% | 18.1 | <LOD – 20.8 | 0.83 |
| Pyrene | 129–00-0 | 202.3 | 8.51 | 100 | 76% | 15.3 | <LOD – 17.8 | 1.37 |
Wristband mass was used to convert instrument LODs into log2 pmol/g wristband units. We used the 5.71 g wristband mass value in the LOD unit conversion to present the lowest range of values. In subsequent data analysis, the appropriate corresponding LOD was compared to each PAH concentration.
In some cases, we were unable to detect a surrogate due to matrix interference and, therefore, were unable to quantify the target analytes related to the undetected surrogate. We report the number of wristbands for each target analyte that did not have matrix interference.
Detection frequency was calculated by dividing the number of wristbands with the target analyte detected by the number of wristbands in the study without matrix interference. Results rounded to the nearest percentage point.
Due to matrix interference in a minority of wristband samples, we were unable to detect the deuterated PAH surrogates. Consequently, we were unable to quantify the target analytes related to the undetected surrogate, so they were removed from further analyses. Matrix interference could be due to many factors such as compounds in personal care products or sweat. We report the number of wristbands for each target analyte that did not have matrix interference in Table 2 and Table S2.
2.2.4. Wristband quality control (QC) summary
Quality control and data quality objectives are described in Dixon et al. (2018). To identify and account for potential chemical contamination during preparation, transport, and laboratory processing, we created and analyzed several QC samples. Briefly, we collected blank wristbands from each group of prepared wristbands and we analyzed them using GC–MS. We collected and analyzed blank wristbands that traveled to and from study locations in the airtight PTFE bags. We collected and analyzed solvent extraction blanks by performing the extraction process without wristbands. We also collected and analyzed laboratory processing blank wristbands that went through all the laboratory processes (i.e., cleaning, extraction, and instrument analysis). During PAH quantification, we analyzed instrument blanks and calibration verifications (CVs) before and after approximately every ten samples to ensure our PAH method was meeting data quality objectives. Instrument blanks are GC vials filled with either hexane or ethyl acetate. We averaged and subtracted detected concentrations in the quality control samples from sample concentrations. Quality control samples were below the LODs for 51 of the 61 PAHs. All target chemicals were below the LODs for the instrument blanks. Surrogate recoveries averaged 84% across the study.
2.3. Urine sample methodology
2.3.1. Collection and storage
We froze spot urine samples (−80 °C) at CCCEH and then shipped the samples overnight on dry ice to the Centers for Disease Control and Prevention (CDC).
2.3.2. OH-PAH metabolite quantification
We added 100 μL of 13C-labeled OH-PAH internal standards, sodium acetate buffer containing β-glucuronidase/arylsulfatase enzyme, and ascorbic acid solution (Dixon et al., 2018, Wang et al., 2017). Overnight, enzymatic deconjugation yielded free OH-PAHs. Then we added ethanol, centrifuged the urine, and diluted the supernatant with deionized water.
To quantify the concentrations of OH-PAH metabolites in the urine, we used a Spark Holland Symbiosis online solid-phase extraction system (Emmen, Netherlands) paired with an AB Sciex 5500/6500 high-performance liquid chromatography isotope dilution tandem mass spectrometer (HPLC-MS/MS, Framingham, Massachusetts, USA) under negative electrospray ionization mode (Wang et al. 2017). We measured seven OH-PAH metabolites, which are the standard OH-PAHs currently included in the CDC’s U.S. National Health and Nutrition Examination Survey (NHANES): 1-OH-naphthalene (1-OH-NAPH), 2-OH-naphthalene (2-OH-NAPH), 2-OH-fluorene (2-OH-FLU), 3-OH-fluorene (3-OH-FLU), 1-OH-phenanthrene (1-OH-PHEN), 2-and-3-OH-phenanthrene (2-OH- & 3-OH-PHEN), and 1-OH-pyrene (1-OH-PYR). The OH-PAH LODs were estimated by repeated measurement of low concentration standards and by plotting the standard deviation of the measured concentration versus the standard concentration. The standard deviation at zero concentration, S0, was determined by the y-intercept of a linear regression analysis of the plot. The LODs, calculated as three times S0 (Taylor 1987), ranged from 0.0008 to 0.09 ng/mL (Wang et al. 2017), and are listed in Table 3 . We used an enzymatic reaction on a Roche chemistry analyzer (Basel, Switzerland) to measure creatinine.
Table 3.
OH-PAH method information and summary statistics on the log2 ng/g creatinine scale. Parent PAH and limit of detection (LOD) are provided for the seven OH-PAHs in the analytical method.
| Parent PAH | Target OH-PAH | OH-PAH LOD (ng/mL) |
Detection Frequency (% of 109 urine samples) |
Median (log2 ng/g creatinine) |
Range (log2 ng/g creatinine) |
Interquartile Range (log2 ng/g creatinine) |
|---|---|---|---|---|---|---|
| Naphthalene (NAPH) | 1-OH-NAPH | 0.06 | 100% | 9.73 | 7.84–12.5 | 1.19 |
| 2-OH-NAPH | 0.09 | 100% | 13.6 | 11.1–16.8 | 1.62 | |
| Fluorene (FLU) | 2-OH-FLU | 0.008 | 100% | 7.74 | 5.55–9.65 | 0.88 |
| 3-OH-FLU | 0.008 | 100% | 5.79 | 4.20–7.91 | 0.83 | |
| Phenanthrene (PHEN) | 1-OH-PHEN | 0.009 | 100% | 8.11 | 6.32–10.9 | 1.09 |
| 2-OH- & 3-OH-PHEN | 0.01 | 100% | 7.57 | 6.45–10.2 | 0.84 | |
| Pyrene (PYR) | 1-OH-PYR | 0.07 | 96% | 7.58 | <LOD–10.0 | 0.94 |
2.3.3. Urine QC summary
The QC process is described in Wang et al., 2017, Dixon et al., 2018. The CDC laboratory complies with the Clinical Laboratory Improvement Amendments of 1988 (CLIA) and is recertified every two years. The CDC laboratory follows strict quality control and assurance guidelines as mandated by CLIA. For example, each group of samples is analyzed alongside high- and low-concentration QC materials and reagent blanks to assure the accuracy and reliability of the data.
2.4. Data analysis
2.4.1. Wristband and urine data processing
We converted PAH concentrations to moles per gram wristband and log transformed the values (log2 pmol/g wristband). We did not include a concentration for a PAH if there was matrix interference.
We applied a creatinine correction to OH-PAH concentrations to adjust for urine dilution and log transformed the values (log2 ng/g creatinine). We excluded a urine sample if the creatinine concentration was greater than 300 mg/dL based on World Health Organization (WHO) guidelines (1996), which resulted in the exclusion of one urine sample (0.9% of total urine samples collected). We did not exclude creatinine values less than 30 mg/dL, even though this is also part of WHO’s guidelines, because analytical technology for measuring low levels of target analytes in dilute samples has vastly improved since the WHO guidelines were issued in 1996 (Barr et al. 2005).
We created low, medium, and high tertile categories for both the PAH and OH-PAH data as another way to compare wristbands and urine exposure assessment methodologies. The tertile categories were created by calculating the 33 and 66 percentile thresholds and the cutoffs are listed in Table S4. Researchers often use exposure tertiles to reveal associations between PAH exposures and health outcomes (Han et al., 2010, Jedrychowski et al., 2015, Rundle et al., 2019, Xia et al., 2009, Xu et al., 2010, Xu et al., 2013). Tertile comparisons have also been used to characterize how well a spot urine sample can predict urine levels collected in repeated samples (Mahalingaiah et al. 2008).
2.4.2. Statistical analysis
We conducted statistical analyses using the statistical software R, version 4.0.2 (R Development Core Team 2020). We excluded PAH and OH-PAH measurements with values below the LOD from statistical analyses in order to maintain the distributional properties of the datasets and ensure that statistical model assumptions were not violated. All statistical quantities and models were calculated and fitted to each pair of PAH and OH-PAH measurements (pairs are listed in Table 3).
We computed Pearson’s correlation coefficients to evaluate the linear relationship between PAH concentrations in the wristband and their associated OH-PAH concentrations in the urine. Student’s t-tests were used to test the null hypothesis that the correlation between wristband and urine measurements were equal to zero. In order to determine whether exposure categories assigned to each sample based on wristband PAH and urine OH-PAH tertile thresholds were associated with one another, Pearson’s chi-square test of independence was run to test the null hypothesis that PAH tertile categories were independent of OH-PAH tertile categories.
We fit two different types of supervised statistical models, a linear regression model and a linear discriminant analysis (LDA) model, to the data to further investigate the relationship and quantify the predictive relationship and efficacy between urine and wristband measurements. Unlike descriptive statistics, such as correlation, the results of supervised statistical models are conditional on which data are used as the response and which are used as the explanatory variables. Therefore, we ran each model twice, first, using the wristband data as the explanatory variable to predict urine data and, second, using the urine data as the explanatory variable to predict wristband data.
We fit linear regression models (Faraway 2014) using the log2 concentration values. We used the relative prediction error, on values on the original scales of pmol/g wristband and ng/g creatinine, as a measure of predictive performance of the linear regression models. We calculated the median relative prediction error as the median of ratios of the absolute difference between the observed and predicted concentration values and the observed concentration value, multiplied by 100.
We fit LDA models (Friedman et al. 2001) with the exposure category (low, med, high) from one data source as the response variable and the log2 concentration values of the other respective data source as the explanatory variable. For each sample, the model returned a predicted probability for each exposure group, and the predicted group was taken as the category with the highest predicted probability. We measured the predictive efficacy of each model using the prediction accuracy, defined as the ratio of the number of correct category predictions and the total number of predictions. We used the predicted probability values to calculate relative probabilities, defined as the predicted probability divided by the probability of a correct prediction by random chance (i.e., ⅓). We used leave-one-out cross-validation (Stone 1974) to calculate predictive performance metrics for all models.
We checked for potential seasonal effects, by fitting the supervised statistical models including an interaction between season of collection and log2 concentration. The season variable was defined as a categorical variable with four levels: winter (Dec - Feb), spring (Mar - May), summer (June - Aug), and fall (Sep - Nov).
3. Results and discussion
3.1. PAHs in the wristbands
We detected parent compounds NAPH, PHEN, FLU, and PYR in 100%, 99%, 96%, and 76% of wristbands without matrix interference, respectively (Table 2). We captured information on an additional 53 PAHs compared to the urinary OH-PAH method. Of the 61 PAHs tested in the wristbands, we detected 57 PAHs at least once. PAH detections ranged from three to 42 per wristband, with a median of 13. NAPH, 1-methylnaphthalene, and 2-methylnaphthalene were the most frequently detected PAHs (Table S2). The median PAH concentrations from all wristbands were highest for perylene (19.3 log2 pmol/g wristband), phenanthrene (18.1 log2 pmol/g wristband), and naphthalene (16.4 log2 pmol/g wristband; Table S2). This information on perylene, 1-methylnaphthalene, and 2-methylnaphthalene would not be captured with our OH-PAH method alone. Matrix interference numbers, detection frequency, median, and range information for all 61 PAHs is listed in Table S2 on the log2 pmol/g scale and in Table S3 on the pmol/g scale.
No two wristbands had identical PAH exposure profiles; concentrations were different for one or more PAHs between each pair of wristbands. Concentration profiles normalized (based on log2 concentrations to ensure normality) within each PAH are shown in Fig. S1.
3.2. OH-PAHs in the urine
We detected all seven OH-PAHs in 96% of urine samples. The four urine values below the LOD were 1-OH-PYR values (Table 3). Creatinine ranged from 16.7 to 295.6 mg/dL with a median value of 104.3 mg/dL after removing one urine sample with a creatinine value of 325.8 mg/dL. Concentration profiles normalized (based on log2 concentrations to ensure normality) within each OH-PAH are shown in Fig. S2.
Median OH-PAH concentrations were highest for 2-OH-NAPH (13.6 log2 ng/g creatinine), 1-OH-NAPH (9.73 log2 ng/g creatinine), and 1-OH-PHEN (8.11 log2 ng/g creatinine; Table 3). For NAPH, FLU, and PHEN, the related metabolites highest in concentration were 2-OH-NAPH, 2-OH-FLU, and 1-OH-PHEN, respectively, which is consistent with the results reported in Dixon et al. (2018). Detection frequency, median, and range information for each OH-PAH is listed in Table 3 in the log2 ng/g creatinine scale and in Table S5 in the ng/g creatinine scale.
3.3. Quantifying associations between PAHs and OH-PAHs
3.3.1. Correlations
A statistically significant linear relationship was observed for six out of the seven PAH and OH-PAH comparisons (Table 4 ). We saw no evidence of a linear association between NAPH and 2-OH-NAPH (r = 0.09, p = 0.38). Although the p-value for the association between FLU and 3-OH-FLU was significant (p = 0.02), the correlation was weaker (r = 0.26) relative to the other significant correlations.
Table 4.
Correlation and chi-square test of independence test statistics for PAH concentrations in wristbands and OH-PAH concentrations in urine.
| PAH in Wristbands | OH-PAH in Urine | Pearson Coefficient (p-value) | Chi-Square Test of Independence p-value |
|---|---|---|---|
| Phenanthrene (PHEN) | 2-OH- & 3-OH-PHEN | 0.64 (<0.001) | 0.009 |
| PHEN | 1-OH-PHEN | 0.60 (<0.001) | 0.002 |
| Fluorene (FLU) | 2-OH-FLU | 0.56 (<0.001) | <0.001 |
| Naphthalene (NAPH) | 1-OH-NAPH | 0.41 (<0.001) | 0.003 |
| Pyrene (PYR) | 1-OH-PYR | 0.35 (0.003) | 0.001 |
| FLU | 3-OH-FLU | 0.26 (0.02) | 0.73 |
| NAPH | 2-OH-NAPH | 0.09 (0.38) | 0.003 |
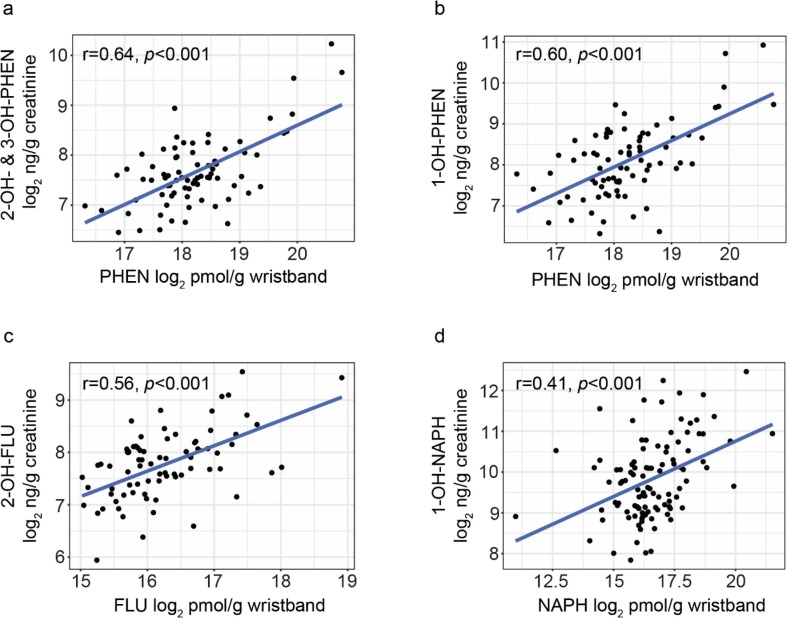
In Fig. 1 , the scatterplots corresponding to the four highest correlation coefficients are displayed; these four correlation coefficients ranged from 0.41 (NAPH and 1-OH-NAPH) to 0.64 (PHEN and 2-OH- & 3-OH-PHEN). The remaining three scatterplots are in Fig. S3.
Fig. 1.
Scatterplots of PAH concentrations in the wristbands plotted against OH-PAH concentrations in the urine for the four comparisons with the highest observed Pearson’s correlation coefficients: (a) phenanthrene (PHEN) and 2-OH– & 3-OH-PHEN, (b) PHEN and 1-OH-PHEN, (c) fluorene (FLU) and 2-OH-FLU, and (d) naphthalene (NAPH) and 1-OH-NAPH.
No evidence of seasonal effects in overall concentration or trend was found, as all marginal and interaction model terms related to season had p-values > 0.16. We also explored summing the OH-PAHs from the same parent PAH; however, we did not observe improvement in the associations between PAHs and OH-PAHs.
3.3.2. Comparison of correlations to prior pilot study (Dixon et al. 2018)
Here, we observed similar correlation trends as in 2018. However, a notable difference is that we observed statistically significant linear relationships between PHEN and 2-OH- & 3-OH-PHEN (p < 0.001), FLU and 2-OH-FLU (p < 0.001), and FLU and 3-OH-FLU (p = 0.02) in this study and that was not observed in the Dixon et al. study (2018), which could be due to the larger sample size (22 participants in the 2018 study compared to 109 participants in this study).
Another difference is that there was a significant correlation for the relationship between NAPH in the wristbands and 2-OH-NAPH in the urine (p = 0.04) in Dixon et al., although the correlation was weaker (Spearman’s r = 0.44) relative to the other significant correlations (2018). In our larger study with 109 participants, we did not see a correlation for the relationship between NAPH and 2-OH-NAPH (Pearson’s r = 0.09, p = 0.38). This shift in results could be due to having a larger range in observed PAH concentrations (14.4–21.5 log2 pmol/g wristband in the 2018 study vs. 11.0–21.5 log2 pmol/g wristband in this study) from a larger sample size.
3.3.3. Chi-square test of independence
The tertiles for six of the seven comparisons showed statistically significant associations, with p-values < 0.05 (Table 4). For those six, there was significantly more agreement in the assigned exposure level categories (low, medium, and high) between the wristbands and urine datasets than expected by agreement if assignment was done randomly.
Five of the seven PAH and OH-PAH comparisons had both significant correlations and associations between exposure categories. The weak relationship observed between FLU and 3-OH-FLU translates into very little agreement between exposure category assignments (p = 0.73). Despite no evidence of a linear correlation between NAPH and 2-OH-NAPH, the chi-square test of independence was significant (p = 0.003), driven primarily by several samples which fell very close to the lower tertile threshold for either the urine or wristband datasets (Fig. S4).
3.4. Evaluating predictions between PAHs and OH-PAHs
3.4.1. Linear regression prediction model
The distribution of relative prediction error percentages was skewed; thus, a median summary value was used to describe the error central tendencies. The median relative predictive error percentage describes the typical distance of predictions from the respective observed values. For example, when using an observed PHEN concentration, 2-OH- & 3-OH-PHEN could be predicted within 20.8%, or less, of the observed concentration for 50% of observations when leave-one-out cross-validation was used (Table 5 ).
Table 5.
Median relative error percentages and prediction accuracy of supervised statistical model predictions for OH-PAH concentrations (conc.) in urine and PAH conc. in wristbands.
| PAH in Wristbands | OH-PAH in Urine | Median Relative Error Percentage for Linear Model Predictions |
Prediction Accuracy of Linear Discriminant Analysis Model |
||
|---|---|---|---|---|---|
| Using PAH Conc. to Predict OH-PAH Conc. | Using OH-PAH Conc. to Predict PAH Conc. | Using PAH Conc. to Predict OH-PAH Tertiles | Using OH-PAH Conc. to Predict PAH Tertiles | ||
| Phenanthrene (PHEN) | 2-OH- & 3-OH-PHEN | 20.8 | 27.7 | 0.47 | 0.49 |
| PHEN | 1-OH-PHEN | 33.1 | 28.4 | 0.49 | 0.49 |
| Fluorene (FLU) | 2-OH-FLU | 23.9 | 27.3 | 0.53 | 0.49 |
| Naphthalene (NAPH) | 1-OH-NAPH | 42.7 | 46.7 | 0.49 | 0.43 |
| Pyrene (PYR) | 1-OH-PYR | 33.8 | 36.7 | 0.45 | 0.48 |
| FLU | 3-OH-FLU | 31.8 | 36.3 | 0.37 | 0.36 |
| NAPH | 2-OH-NAPH | 49.2 | 50.4 | 0.35 | 0.30 |
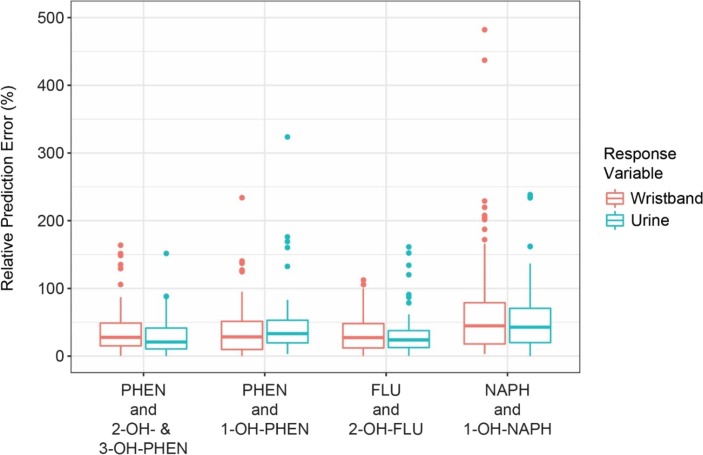
The median relative prediction error percentages were within 36.7% of observed values for all pairs of PAH and OH-PAH measurements (Table 5) with the exception of pairs involving NAPH. The full distributions of relative prediction error percentages for the pairs of PAH and OH-PAH measurements that had the four highest correlation coefficients are displayed in Fig. 2 , and the distributions of the remaining pairs are visualized in Fig. S5. For the five PAH, OH-PAH comparisons with significant observed Pearson’s correlation coefficients and significant chi-square tests, the observed PAH or OH-PAH concentration could predict the other concentration within a factor of 1.47 for 50–85% of the measurements, depending on the pair.
Fig. 2.
Relative prediction error percentages based on linear regression models for PAH concentrations in wristbands predicting OH-PAH concentration in urine (blue) and for OH-PAH concentrations in urine predicting PAH concentrations in wristbands (red) for the four comparisons with the highest observed Pearson’s correlation coefficients: phenanthrene (PHEN) and 2-OH– & 3-OH-PHEN, PHEN and 1-OH-PHEN, fluorene (FLU) and 2-OH-FLU, and naphthalene (NAPH) and 1-OH-NAPH. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.4.2. Tertile predictions
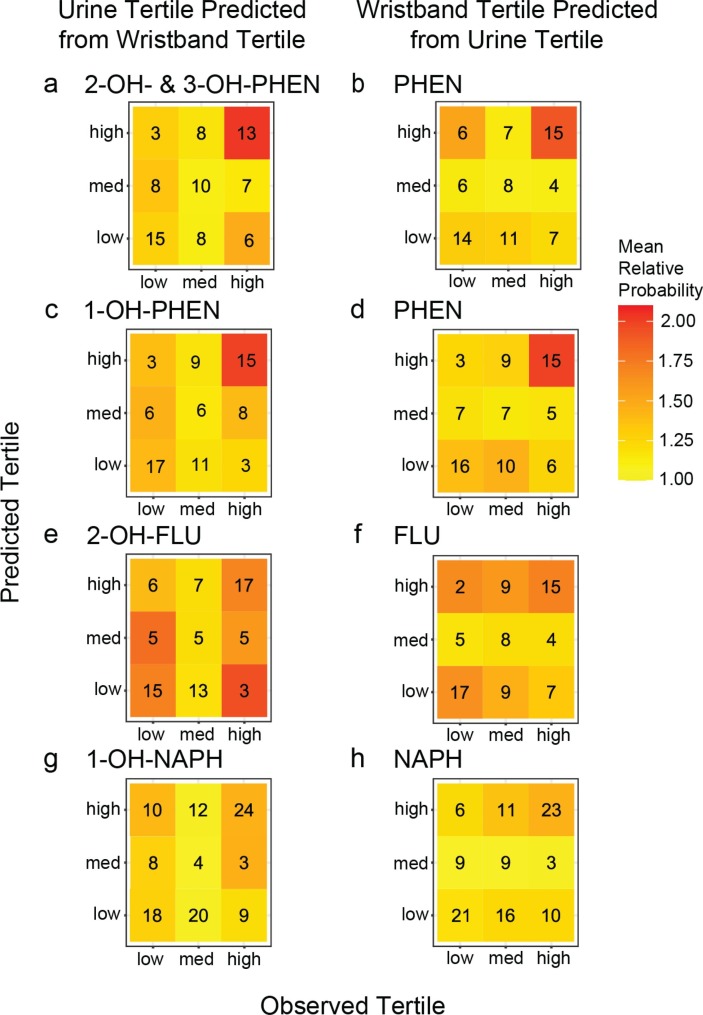
The cross-validation prediction accuracies are in Table 5. For the five PAH and OH-PAH pairs with both significant correlations and chi-squared tests, prediction accuracies were significantly better (p < 0.05) than expected compared to random chance (0.33) by at least 10% (Table 5). The number of correct and incorrect classifications by exposure category for each model are shown in Fig. 3 and Fig. S6, and each cell is colored by the mean relative prediction probability. For example, in Fig. 3a, of the 26 individuals with observed 2-OH– & 3-OH-PHEN in the high tertile, 13 were correctly predicted to be in the high tertile and seven were incorrectly predicted to be in the medium tertile based on wristband PHEN data. Fig. S7 shows an example of what predictions look like when a model provides perfect classifications and what average predictions look like under a model making random predictions.
Fig. 3.
Classification matrices for tertile category agreement between observed and predicted exposure categories in the urine for the four comparisons with the highest observed Pearson’s correlation coefficients: (a-b) 2-OH- & 3-OH-PHEN and PHEN, (c-d) 1-OH-PHEN and PHEN, (e-f) 2-OH-FLU and FLU, and (g-h) 1-OH-NAPH and NAPH. Color indicates relative mean prediction probability. Observed exposure tertile is given on the x-axis and predicted exposure tertile based on associated compound data, on the y-axis.
Although NAPH and 2-OH-NAPH were significantly associated according to the chi-squared test, the prediction accuracy for this pair was commensurate with the expected classification accuracy by random chance, due to difficulty in predicting categories close to tertile thresholds.
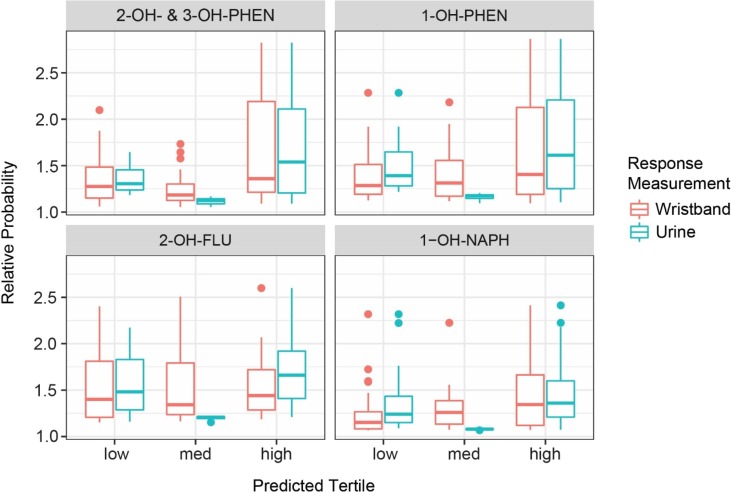
For all PAH and OH-PAH pairs, the large majority of correct classifications were samples from the high and low exposure categories, while most misclassifications were from the medium exposure category. The classification accuracies based on the high exposure category are given for each pair in Table 6 . Additionally, the distributions of relative prediction probabilities for each exposure category are given in Fig. 4 and Fig. S8. Prediction accuracies for the high exposure category were 1.5 times higher or more compared to accuracies based on random chance for the five PAH and OH-PAH pairs with both significant correlations and chi-squared tests, and these predictions had relative prediction probabilities significantly higher than other categories.
Table 6.
Prediction accuracy of linear discriminant analysis model predictions for the highest exposure category for OH-PAH concentrations (conc.) in urine and PAH conc. in wristbands.
| PAH in Wristbands | OH-PAH in Urine | Prediction Accuracy of Linear Discriminant Analysis Model |
|
|---|---|---|---|
| Using PAH Conc. to Predict Highest OH-PAH Tertile | Using OH-PAH Conc. to Predict Highest PAH Tertile | ||
| Phenanthrene (PHEN) | 2-OH- & 3-OH-PHEN | 0.58 | 0.50 |
| PHEN | 1-OH-PHEN | 0.58 | 0.58 |
| Fluorene (FLU) | 2-OH-FLU | 0.57 | 0.68 |
| Naphthalene (NAPH) | 1-OH-NAPH | 0.64 | 0.67 |
| Pyrene (PYR) | 1-OH-PYR | 0.68 | 0.71 |
| FLU | 3-OH-FLU | 0.50 | 0.44 |
| NAPH | 2-OH-NAPH | 0.06 | 0.28 |
Fig. 4.
Relative probability boxplots by observed exposure category based on prediction for PAH concentrations in wristbands (red) or OH-PAH concentrations in urine (blue) for the four comparisons with the highest observed Pearson’s correlation coefficients: phenanthrene (PHEN) and 2-OH– & 3-OH-PHEN, PHEN and 1-OH-PHEN, fluorene (FLU) and 2-OH-FLU, and naphthalene (NAPH) and 1-OH-NAPH. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.5. Potential sources of variation when comparing PAHs in wristbands and OH-PAHs in urine
While we saw evidence of significant relationships between PAHs and OH-PAHs, we also observed differences between the data for some pairs of PAHs and OH-PAHs. For example, the linear discriminant analysis models identified exposure category misclassifications, especially with the medium exposure category (Fig. 3). We also observed evidence of a weak correlation for FLU and 3-OH-FLU and no evidence of a linear association between NAPH and 2-OH-NAPH (Table 4). However, we did not expect exposure data collected from wristband and urine to match perfectly. Even concentrations of the same metabolite in different biological matrices do not always correlate well (Genuis et al., 2012, Kuwayama et al., 2013).
The following factors described in subsections 3.5.1 to 3.5.3 (i.e., exposure routes, types of PAHs, PAH metabolism, OH-PAHs, and spot urine samples) could be part of the reason why we observed differences between PAHs and OH-PAHs.
3.5.1. Exposure routes and types of PAHs
Chemical concentrations in wristbands and metabolite concentrations in urine are both used as proxies for personal chemical exposure; however, they reflect different types of exposure and PAHs. Chemicals absorbed by wristbands include contributions from inhalation, dermal contact, and potentially some ingestion exposure (Aerts et al., 2018, Dixon et al., 2020). Aerts et al. hypothesizes that some chemicals are excreted through sweat and absorbed by the wristband (2017). There is a lack of information on what proportion of chemical exposure is excreted through sweat, both for PAHs (Li et al. 2021) and for other types of chemicals (Genuis et al. 2011). Although there are unknowns about the sweat excretion route, wristband concentrations largely reflect chemical exposure in a person’s external environment.
In contrast, metabolite concentrations in urine reflect chemical exposure in a person’s internal environment from all exposure routes (Needham et al. 2005). Further, urine metabolites represent the proportion of those chemicals that were metabolized within the body and excreted in the urine. For instance, PAH metabolites with lower molecular weights (such as two and three rings) are excreted preferentially in the urine, while metabolites with relatively higher molecular weights are excreted preferentially in the feces (Ramesh et al. 2004). Urinary metabolite concentrations reflect both inter- and intra-variability as well (Aylward et al., 2014, Grimmer et al., 1997, Li et al., 2010). For example, OH-PAH concentrations can be influenced by variation in physiological characteristics (e.g., urinary flow rate) and inter-individual variation in chemical toxicokinetics (e.g., health conditions, age, and medications can impact metabolic capacity) (Grimmer et al. 1997).
3.5.2. PAH metabolism and OH-PAHs
Additionally, wristband results may not exactly match urine results because of PAH metabolism. For instance, there is not always a direct link between parent PAH exposure (e.g., naphthalene) and the related OH-PAH [e.g., 1-OH-naphthalene; (CDC 2017)]. In particular, the evaluation of 1-OH-naphthalene and 2-OH-naphthalene does not account for metabolites derived through the glutathione pathway, potentially leading to underestimations of true naphthalene exposure (Ayala et al. 2015). OH-PAHs can also be generated by other parent compounds. For example, in addition to naphthalene, the insecticide carbaryl can also generate 1-OH-naphthalene in the body (Meeker et al., 2007, Wheeler et al., 2014). Lastly, OH-PAHs are not only formed from human metabolism. OH-PAHs can also be formed in ice, water, and the atmosphere, and have been reported in the environment (Barrado et al., 2012, Ge et al., 2016, Wang et al., 2007).
3.5.3. Spot urine sample limitations
The comparison between wristbands and urine may be further complicated because we analyzed one spot urine sample. Spot urine samples reflect a shorter window of PAH exposure than the wristbands. Forty-eight-hour urine voids or pooling spot urine samples could potentially correlate better with the wristbands in this study compared to spot urine samples. We collected a spot urine sample because they are regularly collected to research PAH exposure in epidemiologic studies and it is less of a burden for participants than a 48-hour urine void or collecting multiple spot urine samples. Despite the limitations of spot urine samples, we observed significant results when quantifying associations between PAH and OH-PAH concentrations and we were able to predict data from observed data using spot urine.
3.6. Limitations and additional considerations
Study participants may not be representative of the larger U.S. adult population. Participants were in their third-trimester of pregnancy in the largest U.S. city and had volunteered to participate in a longitudinal birth cohort study. Future research with random or probability-based sampling may reflect a stronger relationship between wristbands and urine due to potentially increased metabolic variability in pregnant women. However, there are visually similar concentration distributions between creatinine concentrations in this study and the 2013–2016 NHANES (data accessed 8/24/2020 from cdc.gov/nchs/nhanes; Fig. S9). In addition, despite the potentially increased variability in data from our convenience sample, we still observed evidence of significant relationships between PAH and OH-PAH concentrations in our study.
We looked for 61 PAHs in wristbands and seven OH-PAHs in urine. The additional PAHs in the wristbands provide additional insight into personal PAH exposure. For instance, we detected an additional 53 PAHs that we would not capture if we only focused on the four PAHs corresponding to the seven OH-PAHs. Our wristband PAH method was able to include more target analytes than the urine OH-PAH method since higher molecular weight PAH metabolites are excreted preferentially in the feces rather than the urine (Ramesh et al. 2004). The additional PAH information captured by the wristbands can be helpful for future research needs, such as evaluating the toxicity of the mixture of chemicals that might affect this study population’s health and providing additional wristband data that can be compared to other wristband studies.
3.7. Conclusions
Wristbands can provide particular advantages in situations when it may be difficult or impossible to collect urine. These situations may include studies in the wake of pandemics, hurricanes, fires, earthquakes, tornados, civil/political unrest, and power outages. Previously, wristbands have been used to collect personal chemical exposure data less than one month after a category four hurricane made landfall in Texas (Hurricane Harvey), which led to widespread flooding (Dixon et al. 2019). Study participants wore a wristband for seven days and then mailed it to Oregon State University for analysis.
To our knowledge, this is the first application of supervised statistical learning to evaluate the relationship between chemical concentrations in wristbands and metabolite concentrations in biological samples. Previously, other studies have relied on evaluating correlation coefficients and significance to compare data from wristbands and other personal exposure assessment methods (Dixon et al., 2018, Hammel et al., 2016, Hammel et al., 2018, Hammel et al., 2020, Quintana et al., 2019). However, metrics such as correlation are limited in capturing the uncertainty with making real-world inference and predictions on unseen test cases. Therefore, we ran supervised statistical models, with cross-validation, to further examine and quantify the full relationship between different exposure assessment methods and predictive efficacy for unseen observations.
Due to the inherent differences in the exposure assessment methods and collection of spot urine samples, we did not expect complete agreement between all PAH concentrations in wristbands and OH-PAH concentrations. However, our significant results from quantifying the associations between PAHs and OH-PAHs, paired with our ability to predict data from observed data using linear regression models and LDA models, demonstrates that urine and wristbands can be used to gather complementary information for PAH personal exposure assessment.
4. Ethics approval
We obtained informed consent from study participants in accordance with the Columbia University Institutional Review Board (IRB: AAAK6753). The involvement of the CDC laboratory did not constitute engagement in human subject research. All participants included in this study provided informed written consent to participate. All participants included in this study provided informed written consent for publication of the aggregate data.
CRediT authorship contribution statement
Holly M. Dixon: Methodology, Investigation, Writing – review & editing, Writing – original draft, Visualization, Project administration. Lisa M. Bramer: Methodology, Investigation, Formal analysis, Writing – review & editing, Data curation, Writing – original draft, Visualization, Software, Validation. Richard P. Scott: Investigation, Writing – review & editing, Resources. Lehyla Calero: Investigation, Resources. Darrell Holmes: Investigation, Resources. Elizabeth A. Gibson: Writing – review & editing. Haleigh M. Cavalier: Writing – review & editing. Diana Rohlman: Writing – review & editing. Rachel L. Miller: Writing – review & editing, Supervision. Antonia M. Calafat: Writing – review & editing, Supervision. Laurel Kincl: Conceptualization, Writing – review & editing, Funding acquisition. Katrina M. Waters: Conceptualization, Methodology, Writing – review & editing, Supervision, Funding acquisition. Julie B. Herbstman: Conceptualization, Methodology, Writing – review & editing, Supervision, Funding acquisition. Kim A. Anderson: Conceptualization, Methodology, Supervision, Project administration, Funding acquisition.
Declaration of Competing Interest
Kim A. Anderson and Diana Rohlman, authors of this research, disclose a financial interest in MyExposome, Inc., which is marketing products related to the research being reported. The terms of this arrangement have been reviewed and approved by Oregon State University in accordance with its policy on research conflicts of interest. The authors have no other relevant financial or non-financial interests to disclose.
Acknowledgments
Acknowledgements
We thank the participants in this study. At Oregon State University, we thank Michael Barton, Carolyn Poutasse, Kaley Adams, Peter Hoffman, Kyle Messier, Clarisa Caballero-Ignacio, Samantha Samon, Lane Tidwell, Richard Evoy, Caoilinn Haggerty, Ty Bryde, Teresa Valdez, Kaci Graber, Jessica Scotten, Ian Moran, Christine Ghetu, and other members of the Food Safety and Environmental Stewardship Laboratory. At Columbia, we thank the Fair Start study team, including the laboratory and data cores who supported this research. Pacific Northwest National Laboratory is a multi-program laboratory operated by Battelle for the U.S. Department of Energy under contract DEAC05-76RL01830. We also thank Yuesong Wang, Lei Meng, Erin Pittman, Debra Trinidad, Tao Jia, and the late Xiaoyun Ye for the measures of the PAH urinary biomarkers.
Funding Sources
Research reported in this publication was supported by the National Institute of Health (NIH) under award number UH3OD023290 and the National Institute of Environmental Health Sciences (NIEHS) under award numbers 1R21ES024718, 4R33ES024718, P30ES030287, and P42ES016465. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or NIEHS. Holly M. Dixon was supported in part by NIEHS Fellowship T32ES007060 and the ARCS Foundation®. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control (CDC). Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the U.S. Department of Health and Human Sciences.
Handling Editor: Adrian Covaci
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envint.2022.107226.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Aerts R., Joly L., Szternfeld P., Tsilikas K., De Cremer K., Castelain P., Aerts J.-M., Van Orshoven J., Somers B., Hendrickx M., Andjelkovic M., Van Nieuwenhuyse A.n. Silicone wristband passive samplers yield highly individualized pesticide residue exposure profiles. Environ. Sci. Technol. 2018;52(1):298–307. doi: 10.1021/acs.est.7b05039. [DOI] [PubMed] [Google Scholar]
- Anderson K.A., Hillwalker W.E. Encyclopedia of Ecology. Elsevier Publishing Inc.; Oxford: 2008. Bioavailability; pp. 348–357. [Google Scholar]
- Anderson K.A., Szelewski M.J., Wilson G., Quimby B.D., Hoffman P.D. Modified ion source triple quadrupole mass spectrometer gas chromatograph for polycyclic aromatic hydrocarbon analyses. J. Chromatogr. A. 2015;1419:89–98. doi: 10.1016/j.chroma.2015.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson K.A., Points G.L., Donald C.E., Dixon H.M., Scott R.P., Wilson G., Tidwell L.G., Hoffman P.D., Herbstman J.B., O'Connell S.G. Preparation and performance features of wristband samplers and considerations for chemical exposure assessment. J. Expo Sci. Environ. Epidemiol. 2017;27(6):551–559. doi: 10.1038/jes.2017.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala D.C., Morin D., Buckpitt A.R., Coulombe R.A. Simultaneous quantification of multiple urinary naphthalene metabolites by liquid chromatography tandem mass spectrometry. PLoS ONE. 2015;10(4):e0121937. doi: 10.1371/journal.pone.0121937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylward L.L., Hays S.M., Smolders R., Koch H.M., Cocker J., Jones K., Warren N., Levy L., Bevan R. Sources of variability in biomarker concentrations. J. Toxicol. Environ. Health. 2014;17(1):45–61. doi: 10.1080/10937404.2013.864250. [DOI] [PubMed] [Google Scholar]
- Barr D.B., Wilder L.C., Caudill S.P., Gonzalez A.J., Needham L.L., Pirkle J.L. Urinary creatinine concentrations in the U.S. population: Implications for urinary biologic monitoring measurements. Environ. Health Perspect. 2005;113:192–200. doi: 10.1289/ehp.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrado A.I., Garcia S., Castrillejo Y., Perez R.M. Hydroxy–PAH levels in atmospheric PM 10 aerosol samples correlated with season, physical factors and chemical indicators of pollution. Atmos. Pollut. Res. 2012;3(1):81–87. [Google Scholar]
- Bergmann A.J., North P.E., Vasquez L., Bello H., del Carmen Gastañaga Ruiz M., Anderson K.A. Multi-class chemical exposure in rural Peru using silicone wristbands. J. Expo Sci. Environ. Epidemiol. 2017;27(6):560–568. doi: 10.1038/jes.2017.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock E.J., Schafsnitz A.M., Wang C.H., Broadrup R.L., Macherone A., Mayack C., White H.K. Silicone wristbands as passive samplers in honey bee hives. Vet. Sci. 2020;7:86. doi: 10.3390/vetsci7030086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC, 2017. Biomonitoring summary: Naphthalene. https://www.cdc.gov/biomonitoring/Naphthalene_BiomonitoringSummary.html. Accessed May 3, 2021.
- Columbia, 2020. Columbia research: Research ramp-down. https://research.columbia.edu/COVID-19_Research/Research-Ramp-down. Accessed July 8 2020. Last Updated March 18 2020.
- Craig J.A., Ceballos D.M., Fruh V., Petropoulos Z.E., Allen J.G., Calafat A.M., Ospina M., Stapleton H.M., Hammel S., Gray R., Webster T.F. Exposure of nail salon workers to phthalates, di (2-ethylhexyl) terephthalate, and organophosphate esters: A pilot study. Environ. Sci. Technol. 2019;53(24):14630–14637. doi: 10.1021/acs.est.9b02474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vecchi R., Ripper J.S.C., Roy D., Breton L., Marciano A.G., de Souza P.M.B., de Paula C.M. Using wearable devices for assessing the impacts of hair exposome in Brazil. Sci. Rep. 2019;9:1–10. doi: 10.1038/s41598-019-49902-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon H.M., Scott R.P., Holmes D., Calero L., Kincl L.D., Waters K.M., Camann D.E., Calafat A.M., Herbstman J.B., Anderson K.A. Silicone wristbands compared with traditional polycyclic aromatic hydrocarbon exposure assessment methods. Anal. Bioanal. Chem. 2018;410:3059–3071. doi: 10.1007/s00216-018-0992-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon H.M., Armstrong G., Barton M., Bergmann A.J., Bondy M., Halbleib M.L., Hamilton W., Haynes E., Herbstman J., Hoffman P., Jepson P., Kile M.L., Kincl L., Laurienti P.J., North P., Paulik L.B., Petrosino J., Points G.L., Poutasse C.M., Rohlman D., Scott R.P., Smith B., Tidwell L.G., Walker C., Waters K.M., Anderson K.A. Discovery of common chemical exposures across three continents using silicone wristbands. R. Soc. Open Sci. 2019;6(2):181836. doi: 10.1098/rsos.181836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon H.M., Poutasse C.M., Anderson K.A. In: Total exposure health: An introduction. first ed. Phillips K., Yamamoto D., Racz L., editors. CRC Press; 2020. Silicone wristbands and wearables to assess chemical exposures; pp. 139–160. [Google Scholar]
- Donald C.E., Scott R.P., Blaustein K.L., Halbleib M.L., Sarr M., Jepson P.C., Anderson K.A. Silicone wristbands detect individuals' pesticide exposures in West Africa. R. Soc. Open Sci. 2016;3(8):160433. doi: 10.1098/rsos.160433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraway J.J. CRC Press; Boca Raton, FL: 2014. Linear models with R. [Google Scholar]
- Friedman, J., Hastie, T., Tibshirani, R., 2001. The elements of statistical learning. vol 1. vol 10. Springer series in statistics, New York.
- Ge L., Na G., Chen C.-E., Li J., Ju M., Wang Y., Li K., Zhang P., Yao Z. Aqueous photochemical degradation of hydroxylated PAHs: Kinetics, pathways, and multivariate effects of main water constituents. Sci. Total Environ. 2016;547:166–172. doi: 10.1016/j.scitotenv.2015.12.143. [DOI] [PubMed] [Google Scholar]
- Genuis S.J., Birkholz D., Rodushkin I., Beesoon S. Blood, urine, and sweat (BUS) study: Monitoring and elimination of bioaccumulated toxic elements. Arch. Environ. Contam. Toxicol. 2011;61:344–357. doi: 10.1007/s00244-010-9611-5. [DOI] [PubMed] [Google Scholar]
- Genuis S.J., Beesoon S., Lobo R.A., Birkholz D. Human elimination of phthalate compounds: blood, urine, and sweat (BUS) study. Sci. World J. 2012 doi: 10.1100/2012/615068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimmer G., Jacob J., Dettbarn G., Naujack K.-W. Determination of urinary metabolites of polycyclic aromatic hydrocarbons (PAH) for the risk assessment of PAH-exposed workers. Int. Arch. Occup. Environ. Health. 1997;69(4):231–239. doi: 10.1007/s004200050141. [DOI] [PubMed] [Google Scholar]
- Hammel S.C., Hoffman K., Webster T.F., Anderson K.A., Stapleton H.M. Measuring personal exposure to organophosphate flame retardants using silicone wristbands and hand wipes. Environ. Sci. Technol. 2016;50(8):4483–4491. doi: 10.1021/acs.est.6b00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammel S.C., Phillips A., Hoffman K., Stapleton H.M. Evaluating the use of silicone wristbands to measure personal exposure to brominated flame retardants. Environ. Sci. Technol. 2018 doi: 10.1021/acs.est.8b03755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammel S.C., Hoffman K., Phillips A.L., Levasseur J.L., Lorenzo A.M., Webster T.F., Stapleton H.M. Comparing the use of silicone wristbands, hand wipes, and dust to evaluate children’s exposure to flame retardants and plasticizers. Environ. Sci. Technol. 2020;54(7):4484–4494. doi: 10.1021/acs.est.9b07909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y., Xia Y., Zhu P., Qiao S., Zhao R., Jin N., Wang S., Song L., Fu G., Wang X. Reproductive hormones in relation to polycyclic aromatic hydrocarbon (PAH) metabolites among non-occupational exposure of males. Sci. Total Environ. 2010;408(4):768–773. doi: 10.1016/j.scitotenv.2009.11.021. [DOI] [PubMed] [Google Scholar]
- Harley K.G., Parra K.L., Camacho J., Bradman A., Nolan J.E.S., Lessard C., Anderson K.A., Poutasse C.M., Scott R.P., Lazaro G., Cardoso E., Gallardo D., Gunier R.B. Determinants of pesticide concentrations in silicone wristbands worn by Latina adolescent girls in a California farmworker community: The COSECHA youth participatory action study. Sci. Total Environ. 2019;652:1022–1029. doi: 10.1016/j.scitotenv.2018.10.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendryx M., Wang S., Romanak K.A., Salamova A., Venier M. Personal exposure to polycyclic aromatic hydrocarbons in Appalachian mining communities. Environ. Pollut. 2020;257:113501. doi: 10.1016/j.envpol.2019.113501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K., Levasseur J.L., Zhang S., Hay D., Herkert N.J., Stapleton H.M. Monitoring human exposure to organophosphate esters: comparing silicone wristbands with spot urine samples as predictors of internal dose. Environ. Sci. Technol. Lett. 2021;8:805–810. doi: 10.1021/acs.estlett.1c00629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedrychowski W.A., Perera F.P., Maugeri U., Majewska R., Mroz E., Flak E., Camann D., Sowa A., Jacek R. Long term effects of prenatal and postnatal airborne PAH exposures on ventilatory lung function of non-asthmatic preadolescent children. Prospective birth cohort study in Krakow. Sci. Total Environ. 2015;502:502–509. doi: 10.1016/j.scitotenv.2014.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kile M.L., Scott R.P., O’Connell S.G., Lipscomb S., MacDonald M., McClelland M., Anderson K.A. Using silicone wristbands to evaluate preschool children's exposure to flame retardants. Environ. Res. 2016;147:365–372. doi: 10.1016/j.envres.2016.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwayama K., Tsujikawa K., Miyaguchi H., Kanamori T., Iwata Y.T., Inoue H. Time-course measurements of caffeine and its metabolites extracted from fingertips after coffee intake: a preliminary study for the detection of drugs from fingerprints. Anal. Bioanal. Chem. 2013;405(12):3945–3952. doi: 10.1007/s00216-012-6569-3. [DOI] [PubMed] [Google Scholar]
- Levasseur J.L., Hammel S.C., Hoffman K., Phillips A.L., Zhang S., Ye X., Calafat A.M., Webster T.F., Stapleton H.M. Young children’s exposure to phenols in the home: Associations between house dust, hand wipes, silicone wristbands, and urinary biomarkers. Environ. Int. 2021;147 doi: 10.1016/j.envint.2020.106317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Romanoff L.C., Lewin M.D., Porter E.N., Trinidad D.A., Needham L.L., Patterson D.G., Sjödin A. Variability of urinary concentrations of polycyclic aromatic hydrocarbon metabolite in general population and comparison of spot, first-morning, and 24-h void sampling. J. Expo Sci. Environ. Epidemiol. 2010;20(6):526–535. doi: 10.1038/jes.2009.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Zhang X., Fu Y., Xu Y., Chen J., Lu S. Backward modeling of urinary test reliability for assessing PAH health risks: An approximation solution for naphthalene. Environ. Pollut. 2021;273 doi: 10.1016/j.envpol.2021.116522. [DOI] [PubMed] [Google Scholar]
- Mahalingaiah S., Meeker J.D., Pearson K.R., Calafat A.M., Ye X., Petrozza J., Hauser R. Temporal variability and predictors of urinary bisphenol A concentrations in men and women. Environ. Health Perspect. 2008;116(2):173–178. doi: 10.1289/ehp.10605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker J.D., Barr D.B., Serdar B., Rappaport S.M., Hauser R. Utility of urinary 1-naphthol and 2-naphthol levels to assess environmental carbaryl and naphthalene exposure in an epidemiology study. J. Expo Sci. Environ. Epidemiol. 2007;17(4):314–320. doi: 10.1038/sj.jes.7500502. [DOI] [PubMed] [Google Scholar]
- NIEHS, 2020. The COVID-19 pandemic from a global environmental health perspective. National Institute of Environmental Health Sciences: Global Environmental Health Newsletter. https://www.niehs.nih.gov/research/programs/geh/geh_newsletter/2020/6/articles/the_covid19_pandemic_from_a_global_environmental_health_perspective.cfm. Issue June 22.
- NYT, 2020. When coronavirus closes your lab, can science go on? The New York Times. https://www.nytimes.com/2020/03/23/science/coronavirus-closed-labs.html. March 23.
- O’Connell S.G., Kincl L.D., Anderson K.A. Silicone wristbands as personal passive samplers. Environ. Sci. Technol. 2014;48(6):3327–3335. doi: 10.1021/es405022f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poutasse C.M., Poston W.S.C., Jahnke S.A., Haddock C.K., Tidwell L.G., Hoffman P.D., Anderson K.A. Discovery of firefighter chemical exposures using military-style silicone dog tags. Environ. Int. 2020;142:105818. doi: 10.1016/j.envint.2020.105818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana P.J.E., Hoh E., Dodder N.G., Matt G.E., Zakarian J.M., Anderson K.A., Akins B., Chu L., Hovell M.F. Nicotine levels in silicone wristband samplers worn by children exposed to secondhand smoke and electronic cigarette vapor are highly correlated with child’s urinary cotinine. J. Expo Sci. Environ. Epidemiol. 2019;29(6):733–741. doi: 10.1038/s41370-019-0116-7. [DOI] [PubMed] [Google Scholar]
- R Development Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2020. R: A Language and Environment for Statistical Computing. [Google Scholar]
- Ramesh A., Walker S.A., Hood D.B., Guillén M.D., Schneider K., Weyand E.H. Bioavailability and risk assessment of orally ingested polycyclic aromatic hydrocarbons. Int. J. Toxicol. 2004;23(5):301–333. doi: 10.1080/10915810490517063. [DOI] [PubMed] [Google Scholar]
- Reche C., Viana M., van Drooge B.L., Fernández F.J., Escribano M., Castaño-Vinyals G., Nieuwenhuijsen M., Adami P.E., Bermon S. Athletes' exposure to air pollution during World Athletics Relays: A pilot study. Sci. Total Environ. 2020;717:137161. doi: 10.1016/j.scitotenv.2020.137161. [DOI] [PubMed] [Google Scholar]
- Reddam A., Tait G., Herkert N., Hammel S.C., Stapleton H.M., Volz D.C. Longer commutes are associated with increased human exposure to tris (1, 3-dichloro-2-propyl) phosphate. Environ. Int. 2020;136:105499. doi: 10.1016/j.envint.2020.105499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlman, D., Bethel, J., Hoffman, P., Tidwell, L., Anderson, K., 2017. Oregon State University chemical exposure disaster study protocol. Superfund Research Program, Pacific Northwest Center for Translational Environmental Health Research, Oregon State University. https://disasterinfo.nlm.nih.gov/search/id:24238. Accessed June 2 2021 Last Updated May 11 2021.
- Rohlman D., Dixon H.M., Kincl L., Larkin A., Evoy R., Barton M., Phillips A., Peterson E., Scaffidi C., Herbstman J.B., Waters K.M., Anderson K.A. Development of an environmental health tool linking chemical exposures, physical location and lung function. BMC Public Health. 2019;19(1) doi: 10.1186/s12889-019-7217-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundle A.G., Gallagher D., Herbstman J.B., Goldsmith J., Holmes D., Hassoun A., Oberfield S., Miller R.L., Andrews H., Widen E.M. Prenatal exposure to airborne polycyclic aromatic hydrocarbons and childhood growth trajectories from age 5–14 years. Environ. Res. 2019;177 doi: 10.1016/j.envres.2019.108595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servick K. Updated: Labs go quiet as researchers brace for long-term coronavirus disruptions. Science. 2020 [Google Scholar]
- Stone M. Cross-validatory choice and assessment of statistical predictions. J. Roy. Stat. Soc.: Ser. B (Methodol.) 1974;36:111–133. [Google Scholar]
- Taylor J.K. Quality assurance of chemical measurements. Routledge. 1987 doi: 10.1201/9780203741610. [DOI] [Google Scholar]
- Vidi P.-A., Anderson K.A., Chen H., Anderson R., Salvador-Moreno N., Mora D.C., Poutasse C., Laurienti P.J., Daniel S.S., Arcury T.A. Personal samplers of bioavailable pesticides integrated with a hair follicle assay of DNA damage to assess environmental exposures and their associated risks in children. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2017;822:27–33. doi: 10.1016/j.mrgentox.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Kawamura K., Zhao X., Li Q., Dai Z., Niu H. Identification, abundance and seasonal variation of anthropogenic organic aerosols from a mega-city in China. Atmos. Environ. 2007;41(2):407–416. [Google Scholar]
- Wang S., Romanak K.A., Hendryx M., Salamova A., Venier M. Association between thyroid function and exposures to brominated and organophosphate flame retardants in rural central Appalachia. Environ. Sci. Technol. 2020;54(1):325–334. doi: 10.1021/acs.est.9b04892. [DOI] [PubMed] [Google Scholar]
- Wang S., Romanak K.A., Stubbings W.A., Arrandale V.H., Hendryx M., Diamond M.L., Salamova A., Venier M. Silicone wristbands integrate dermal and inhalation exposures to semi-volatile organic compounds (SVOCs) Environ. Int. 2019;132:105104. doi: 10.1016/j.envint.2019.105104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Meng L., Pittman E.N., Etheredge A., Hubbard K., Trinidad D.A., Kato K., Ye X., Calafat A.M. Quantification of urinary mono-hydroxylated metabolites of polycyclic aromatic hydrocarbons by on-line solid phase extraction-high performance liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2017;409(4):931–937. doi: 10.1007/s00216-016-9933-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler A.J., Dobbin N.A., Héroux M.-E., Fisher M., Sun L., Khoury C.F., Hauser R., Walker M., Ramsay T., Bienvenu J.-F., LeBlanc A., Daigle É., Gaudreau E., Belanger P., Feeley M., Ayotte P., Arbuckle T.E. Urinary and breast milk biomarkers to assess exposure to naphthalene in pregnant women: an investigation of personal and indoor air sources. Environ. Health. 2014;13(1) doi: 10.1186/1476-069X-13-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, 1996. Biological monitoring of chemical exposure in the workplace: guidelines. vol WHO/HPR/OCH/96.1. World Health Organization, Geneva, Switzerland.
- Wise C.F., Hammel S.C., Herkert N.J., Ma J., Motsinger-Reif A., Stapleton H.M., Breen M. Comparative exposure assessment using silicone passive samplers indicates domestic dogs are sentinels to support human health research. Environ. Sci. Technol. 2020;54:7409–7419. doi: 10.1021/acs.est.9b06605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y., Zhu P., Han Y., Lu C., Wang S., Gu A., Fu G., Zhao R., Song L., Wang X. Urinary metabolites of polycyclic aromatic hydrocarbons in relation to idiopathic male infertility. Hum. Reprod. 2009;24:1067–1074. doi: 10.1093/humrep/dep006. [DOI] [PubMed] [Google Scholar]
- Xu X., Cook R.L., Ilacqua V.A., Kan H., Talbott E.O., Kearney G. Studying associations between urinary metabolites of polycyclic aromatic hydrocarbons (PAHs) and cardiovascular diseases in the United States. Sci. Total Environ. 2010;408(21):4943–4948. doi: 10.1016/j.scitotenv.2010.07.034. [DOI] [PubMed] [Google Scholar]
- Xu X., Hu H., Kearney G.D., Kan H., Sheps D.S. Studying the effects of polycyclic aromatic hydrocarbons on peripheral arterial disease in the United States. Sci. Total Environ. 2013;461:341–347. doi: 10.1016/j.scitotenv.2013.04.089. [DOI] [PubMed] [Google Scholar]
- Zuy Y., Sweck S.O., Dockery C.R., Potts G.E. HPLC detection of organic gunshot residues collected with silicone wristbands. Anal. Methods. 2020;12(1):85–90. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.