Abstract
Aims:
To examine evidence for subtypes of opioid craving trajectories during medication for opioid use disorder (MOUD), and to (a) test whether these subtypes differed on MOUD-related outcomes, and (b) determine whether nonresponders could be identified before treatment initiation.
Design, Setting, and Participants:
Outpatients (n = 211) being treated with buprenorphine or methadone for up to 16 weeks. Growth mixture modeling was used to identify unobserved craving-trajectory subtypes. Support Vector Machines (SVM) were trained to predict subtype membership from pretreatment data.
Measurements:
Self-reported opioid craving (Ecological Momentary Assessment – EMA – three random moments per day). Participant-initiated EMA reports of drug use or higher-than-usual stress. Addiction Severity Index (ASI) pretreatment.
Findings:
Four craving trajectories were identified: Low (73%); High and Increasing (HIC) (10.9%); Increasing and Decreasing (8.5%); and Rapidly Declining (7.6%). The HIC subgroup reported the highest use of heroin, any opiate, and cannabis during treatment. The Low Craving subgroup reported the lowest use of heroin or any opiate use, and the lowest levels of stress and drug-cue exposure during treatment. SVM models predicting HIC membership before treatment initiation had a sensitivity of 0.70, specificity of 0.78, and accuracy of 0.77. Including 3 weeks of EMA reports increased sensitivity to 0.78, specificity to 0.84, and accuracy to 0.85.
Conclusions:
Subgroups of MOUD patients show distinct patterns of opioid craving during treatment. Subgroups differ on critical outcomes including drug-use lapse, stress, and exposure to drug cues. Data from enrollment and early in treatment may help focus clinical attention.
Keywords: opioid use disorder, craving, ecological momentary assessment, growth mixture models, MOUD, machine learning
1.0. Introduction
Medication for opioid use disorder (MOUD) reliably reduces, but often does not eliminate, craving for opioids (Fareed et al., 2011). Craving – the conscious, reportable urge to use drugs – is important to consider during MOUD for two main reasons. First, for participants in MOUD, craving is typically a distressing and intrusive experience, and thus inherently worth avoiding (Tiffany and Wray, 2012). Second, if and when craving leads to lapse during MOUD (Tiffany and Wray, 2012; Sayette, 2016), the risk of death from overdose is now much higher given that synthetic opioids such as fentanyl are increasingly found in illicit supplies of heroin, cocaine, and other drugs (Jones et al., 2018).
The present set of analyses were conducted in the context of two of our research group’s ongoing efforts using ambulatory assessment. First, with inferential statistics and clustering methodologies, we have sought to identify generalizable associations between mental states, environmental exposure, and lapse to drug use during treatment. Our findings have highlighted the heterogeneous nature of these relationships: namely that these associations vary across groups of people, making it difficult to draw conclusions that apply reliably to whole samples (Furnari et al., 2015; Panlilio et al., 2019; Burgess-Hull et al., 2021). Second, using machine-learning methods, we have developed personalized models to predict moments of risk for individual participants receiving MOUD. Regardless of the prediction target (e.g., craving, stress), we have found that how our models err (i.e., whether they are more likely to produce false positives or false negatives) varies according to the target’s trajectory of frequency within a person over time (Epstein et al., 2020). Furthermore, to reach optimal accuracy, our models require multiple weeks of data on an individual participant’s unique response patterns. One strategy for making our momentary-prediction models more accurate during the first few weeks of MOUD treatment – a time period conveying the highest risk for dropout and return to use (e.g., Hser et al., 2014; Soeffing, Martin, Fingerhood, Jasinski, & Rastegar, 2009) – is to identify the participant’s expected overall response trajectory as early in treatment as possible. Early identification of a participant’s expected response trajectory would have obvious clinical utility, opening the door to proactively personalized addiction treatment, enhanced risk stratification and resource allocation, and simplified communication within and across healthcare clinics.
For these reasons, we sought to detect subtypes of participants with similar trajectories of opioid craving over the course of treatment. Our participants were outpatients in the first 16 weeks of treatment with buprenorphine or methadone for OUD. Based on previous research examining changes in craving during treatment, either as a whole-sample (Bordnick and Schmitz, 1998; Tsui et al., 2014) or within subgroups identified with clustering methods (Oslin et al., 2009; Northrup et al., 2015), we expected to identify, at a minimum, high-craving and low-craving groups, and possibly groups with more complex patterns. To assess the criterion validity and clinical relevance of any groupings we identified, we examined the association between subtype membership and treatment-related outcomes, including intra-treatment drug use. Finally, we examined whether pre-treatment assessments and craving and stress reports early in treatment could be used to identify membership in high-risk subgroups before treatment initiation or during the first few weeks of treatment.
2.0. Materials and Methods
2.1. Participants
Participants (N=237) were opioid-dependent and being treated with methadone or buprenorphine in a 16-week study designed to develop and apply tools for the real-time assessment of exposure/response to drugs and psychosocial stress, and to examine how these exposures/responses vary by geographical location (National Clinical Trial Identifier: NCT00787423). The study took place at our treatment-research clinic in Baltimore, MD between July 2009 to April 2018. At enrollment, all participants were seeking treatment for opioid use disorder. During pre-enrollment screening, participants completed the Addiction Severity Index (ASI) (McLellan et al., 1985) and received physical/psychosocial assessments. Main eligibility criteria for enrollment were: aged 18 – 75, physical dependence on opioids, and residence in Baltimore city or a surrounding county. Exclusion criteria were: history of bipolar or any DSM-IV psychotic disorder; current DSM-IV alcohol or sedative-hypnotics dependence; cognitive impairment precluding informed consent or valid self-report; and medical conditions compromising study participation (e.g., cirrhosis, nephrotic syndrome, thyroid disease, ischemic heart disease, epilepsy, panhypopituitarism, adrenal insufficiency).
The study was approved by the IRB of the National Institute on Drug Abuse. Participants gave written informed consent before enrollment. All data were covered by a Federal Certificate of Confidentiality.
2.2. Study Procedures
Upon enrollment, participants began daily treatment with methadone or buprenorphine under one of two study arms. Participants in the Methadone or Buprenorphine arm (n = 192) attended clinic 5 – 7 days a week and provided three weekly urine samples under observation. Participants in the office-based Buprenorphine treatment arm (n = 45) attended clinic and provided urine sample two days a week. Medication type was determined by the participant’s preference and clinical judgment of the study physician. Dosage was optimized for each participant to minimize withdrawal symptoms and side effects and reduce illicit opioid use. Average individualized medication dosage for each participant’s penultimate week of the study is displayed in Table 1. Urine was screened for opioids, cocaine, amphetamines, PCP, benzodiazepines, and cannabinoids.
Table 1.
Sample Characteristics: Pre-treatment and EMA Variables (n = 211)
| Pre-treatment Variables | In Treatment Variables | |||
|---|---|---|---|---|
| Variable | Mean (SD) | n (%) | Variable | Mean (SD) |
| Demographics | Medication | |||
| Age | 43.35 (9.57) | Methadone – n (%) | 99 (47) | |
| Male | 164 (78) | Medication Dosage (mg) | ||
| Non-white | 144 (69) | Methadone | 75.16 (34.78) | |
| Employed | 153 (73) | Buprenorphine/Naloxone | 11.97 (5.85) / 2.99 (1.46) | |
| Drug use | Time in Study | |||
| Alcohol use (past 30 days) | 4.84 (7.47) | Days in Study | 96.98 (23.14) | |
| Heroin use (past 30 days) | 19.75 (11.75) | Drug use During Study | ||
| Other opiate use (past 30 days) | 7.83 (10.40) | Any Drug Use | 0.27 (0.22) | |
| Cocaine use (past 30 days) | 4.18 (8.10) | Heroin Use | 0.14 (0.17) | |
| Cannabis use (past 30 days) | 3.27 (7.80) | Other Opiate Use | 0.02 (0.04) | |
| Polysubstance use (past 30 days) | 10.07 (10.51) | Any Opiate Use | 0.15 (0.17) | |
| Longest period of abstinence (months) | 12.74 (25.49) | Cocaine Use | 0.11 (0.16) | |
| Time since most recent abstinence (months) | 27.73 (43.41) | Cannabis Use | 0.07 (0.17) | |
| Times Tx for drug abuse (lifetime) | 2.47 (2.33) | Alcohol Use | 0.05 (0.10) | |
| Money on drugs (past 30 days) | 994.46 (1198.97) | Average interval between drug uses (hours) | 199.91 (239.30) | |
| Legal problems | Cue-Exposure, Stress & Social Contact | |||
| Arrested and charged (lifetime) | 7.56 (11.40) | Drug Cue Exposure | 0.89 (0.87) | |
| Months incarcerated (lifetime) | 38.04 (56.32) | Stress Events | 0.13 (0.23) | |
| Illegal Activities for money (past 30 days) | 4.69 (9.60) | Drug use with others | 0.10 (0.12) | |
| Physical, Psychological, & Social Health | Social contact | 1.87 (0.90) | ||
| Medical Problems (past 30 days) | 1.07 (4.80) | |||
| Bothered by psychological problems (past 30 days) | 0.21 (0.76) | Completion and Dropout Numbers | ||
| Mental health problems (lifetime) | 0.59 (0.98) | Dropout Category | n (%) | |
| Number of close friends | 2.67 (2.78) | Completed | 147 (70) | |
| Serious conflict with family (past 30 days) | 0.93 (4.05) | Dropout: Incarcerated | 5 (2) | |
| Dropout: Medical discharge | 2 (1) | |||
| Dropout: Transferred to another clinic | 15 (7) | |||
| Dropout: Non-compliant EMA | 8 (4) | |||
| Dropout: Other | 34 (16) | |||
Note. Medication dosages are individualized dosages derived from each participants penultimate week of treatment. “Dropout: Non-compliant EMA” indicates that a participant was noncompliant with the prespecified EMA procedures. “Dropout: Other” includes getting a job, expulsion due to diversion, moving to another state or region outside of study boundaries, or some other life event that disrupted their ability to participate in the study.
2.2.1. Ecological Momentary Assessment (EMA) Data Collection
After two weeks of treatment, participants received a smartphone programmed to emit three audible prompts per day at random times during each participant’s normal waking hours. For each prompt, participants were asked to rate their current opioid craving and stress (1 = “not at all” to 5 = “extremely”), who they were with, and whether they had seen, been offered, or seen others using opioids, cocaine, cannabis, methamphetamine, tobacco, or alcohol in the last 5 min or since they got to their present location. Participants were also asked to initiate event-contingent Ecological Momentary Assessment (EMA) entries when they used drugs or felt ‘more stressed, overwhelmed, or anxious than usual’ (1 = “not bad at all” to 10 = “the worst you’ve ever felt”). For each event-contingent entry, participants reported if they were with others, whether they had seen others using drugs, and their current level of opioid craving.
Participants carried their smartphone for up to 16 weeks and were paid $10–30 weekly for missing no more than 5 random prompts per week. They also received up to $300 for returning their smartphone at the end of the study. Participants were given a warning if they did not meet completion criteria; if they did not meet completion criteria for two consecutive weeks, they were not allowed to continue the study and were assisted with transfer to a community-based addiction treatment-center. To incentivize accurate reporting of drug use (without incentivizing drug use), participants received $5 each time they had a negative urine screen with no EMA-reported drug use, or $3 each time they had a positive urine screen that matched their EMA reported drug use.
2.3. Statistical Analyses
2.3.1. Growth Mixture Modeling
To create a variable reflecting opioid craving across the study, we averaged the random-prompt ratings of opioid craving within each day, generating a daily craving score. We then selected participants (n = 211) who completed at least 48 days of the study (~ 40% of study) and removed any participant days on which all opioid craving ratings were missing (185 out of 2089 days [0.9%]). We fit growth mixture models (GMMs) to identify longitudinal trajectories of craving during treatment using the lcmm package (Proust-Lima et al., 2017) in the statistical software R version 4.1.0 (R Core Team, 2021).
2.3.1.1. Model fitting and model selection
Following Ram and Grimm (2009) and Burgess-Hull (2020), we compared the fit of increasingly complex models of linear and nonlinear growth (e.g., quadratic and cubic polynomials, I-splines) for 1 – 7 trajectory subgroup models. We also examined variability within and across trajectory subgroups. The dependent variable for all models was average daily opioid craving, and the independent variables were day and a vector of dummy variables specifying the dropout category of the participant (Table 1).
The relative fits for different models were compared by the AIC, BIC, posterior probabilities of trajectory membership (PPM), and differences between observed and model-predicted craving values. For more information on GMMs, model fitting, and selection, see the online Supplement.
2.3.2. Post-hoc comparisons of trajectory groups
Each participant was assigned to the craving-trajectory group corresponding to their highest PPM.1 We compared the groups on pre-treatment measures (e.g., demographics, drug-use history, health) by conducting pairwise chi-square and Mann-Whitney U tests (Mann and Whitney, 1947) adjusting for the false discovery rate (Benjamini and Hochberg, 1995).
To examine group differences in variables that were measured repeatedly over treatment, we computed the following variables:
Drug use during treatment.
We calculated drug use measures based on the number of days that any EMA entry (random prompt, event-contingent, or end-of-day) contained reports of: (a) “Any Drug Use” (heroin, cocaine, other opiates, cannabis, methamphetamine, benzodiazepines, unprescribed methadone or buprenorphine, alcohol, and/or other illicit drugs); (b) use of specific drug classes, including “Heroin Use,” “Other Opiate Use (Percocet, oxycodone, etc.),” “Any Opiate Use,” “Cocaine Use,” “Cannabis Use,” and “Alcohol Use”; and (c) “Drug Use with Others,” when a participant reported the presence of a companion when using drugs. We calculated an average number of drug-use reports per day by dividing the sum of each measure by the number of days a participant was in the study.
Drug cues and stress events.
“Drug Cue Exposure” was the average number of times a participant reported seeing any drugs (including alcohol/nicotine/tobacco), being offered any drugs, or seeing someone selling/using drugs. “Stress Events” was the mean of event-contingent stress reports per day.
Social exposure.
“Time with Others” was the average number of reports per day in which the participant reported the presence of a companion.
2.3.3. Machine-Learning Models
To determine whether variables collected before treatment initiation could predict craving-group membership, we trained a support vector machine (SVM) with a linear kernel using a subset of variables from the ASI (see supplemental material for details on variable selection). The primary prediction target was membership in a specific high-risk group G. We set the cost parameter to 5 and used inverse-frequency class weights to account for unbalanced class sizes. Accuracy metrics were calculated using a leave-one-out cross-validation procedure, in which the SVM is trained with all datapoints except one which is used as a test set to make predictions on. The procedure is repeated N times so that each datapoint serves as a test set once, where N is the number of datapoints.
We next tested whether the accuracy of the initial SVM model improved with the inclusion of two additional sets of predictors: (a) the cumulative sum of opioid craving and stress EMA reports from the first week, two weeks, or three weeks of the treatment study, and (b) estimated intercept and slope values from a linear regression fit to one week, two weeks, or three weeks of opioid craving EMA reports. We fit three separate models to determine whether more data on a patient’s craving and stress values early in treatment could improve prediction of craving-group membership: (1) a model with baseline ASI variables, 1 week of cumulative stress and craving values, and regression intercept and slope estimates from 1 week of craving data, (2) baseline ASI variables, 2 weeks of cumulative stress and craving values, and regression intercept and slope estimates from 2 weeks of craving data, and (3) baseline ASI variables, 3 weeks of cumulative stress and craving values, and regression intercept and slope estimates from 3 weeks of craving data.
3.0. Results
3.1. Sample
Table 1 shows pretreatment data on demographics, drug history, criminal history, and health characteristics, along with aggregated EMA variables for the whole study and completion/dropout rates. Mean years of age was 42.9, and most participants were male (78%), non-white (69%), and employed at least part time (73%). The distribution of treatment medication was 47% methadone and 53% buprenorphine. Most participants completed the study (70%) and the average time in the study was ~100 days. See Panlilio et al. (2019) for more information on associations among dropout, treatment type, craving, and other time-varying states/behaviors in this sample.
3.2. Identification of Opioid-Craving Trajectories
GMMs with quadratic I-splines with 4-equidistant-knots fit substantially better than the baseline, polynomial, and 3-knot I-spline GMMs. Supplemental Table 1 displays the model-fit statistics for the 1 – 6 trajectory 4-knot I-spline GMMs. The models with random intercepts and linear slopes had the best fits, with the Akaike information criteria (AIC) and Bayesian information criterion (BIC) both improving (i.e., decreasing) progressively as the number of trajectory subgroups increased from 1 to 4. There was disagreement between AIC and BIC about whether the 5-trajectory or the 4-trajectory models fit the best. However, comparisons of the model-predicted vs. observed values, sizes of the subgroups, and average posterior probabilities of membership (APPM) favored the 4-trajectory (random intercept + linear slope) model, and we selected this as the final model. More details about model selection can be found in the Supplement.
3.3. Description of 4-Trajectory Opioid-Craving Growth Mixture Models (GMMs)
Daily ratings of opioid craving for the four-trajectory model are displayed in Figure 1. The APPM and size of each trajectory subgroup are displayed in Table 2.
Figure 1.
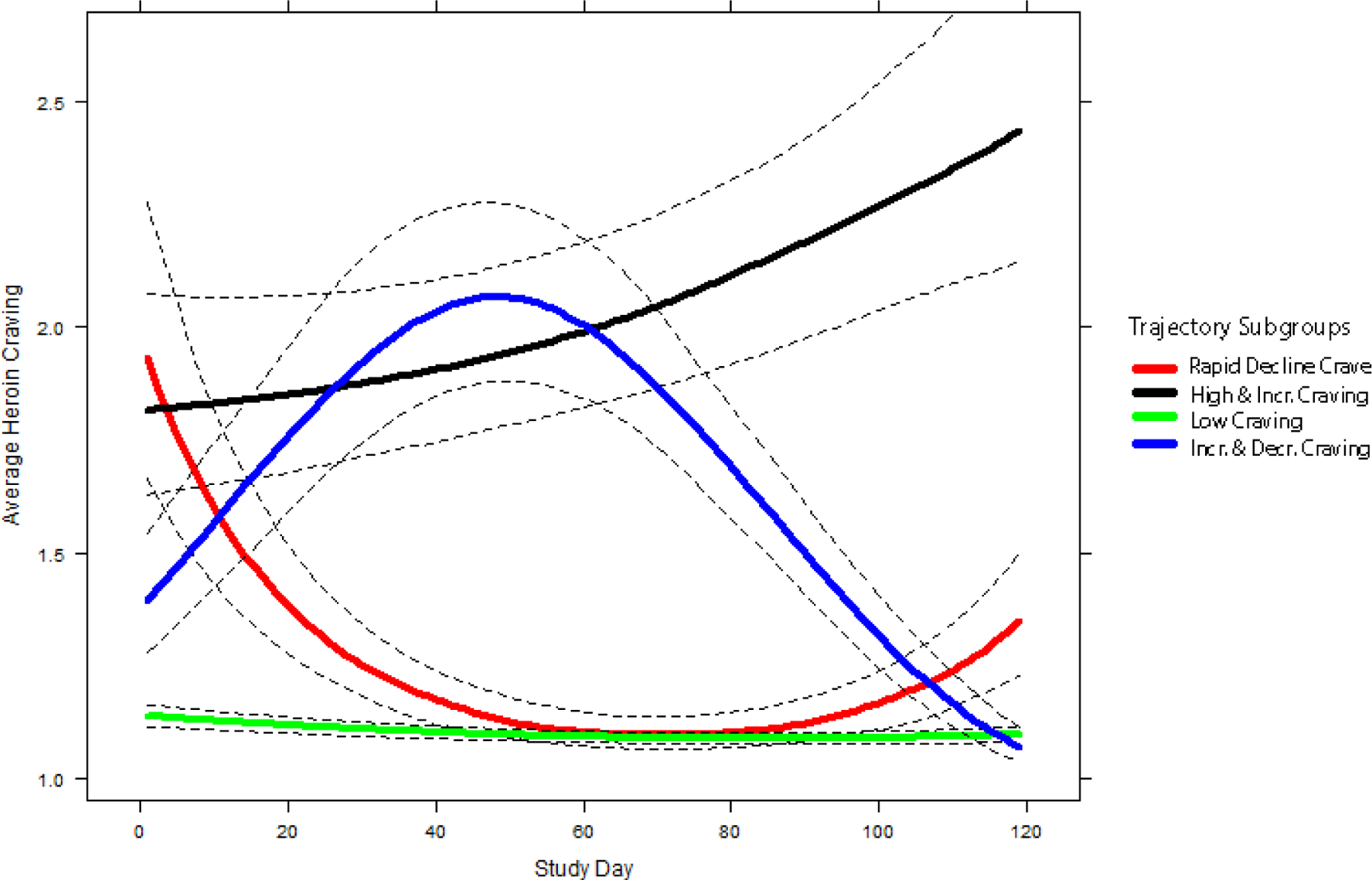
Mean trajectories (solid lines) and 95% pointwise CI’s (dashed lines) for self-reported opioid craving by study day. Average heroin craving for each trajectory subgroup was predicted by a 4-Trajectory growth mixture model with a random intercept and linear slope with I-splines (4-equidistant-knots).
Table 2.
Size, Posterior Probability of Membership, Demographic, and Treatment Characteristics of Heroin Craving Trajectory Subgroups
| Rapidly Declining Craving (subgroup 1) | High & Increasing Craving (subgroup 2) | Low Craving (subgroup 3) | Increasing & Decreasing Craving (subgroup 4) | |
|---|---|---|---|---|
| Size and Classification Accuracy | ||||
| N (% of sample) | 16 (7.6%) | 23 (10.9%) | 154 (73%) | 18 (8.5%) |
| Average Posterior Probability of Membership | 0.90 | 0.96 | 0.99 | 0.99 |
| Demographics, Treatment, and Days in Study | ||||
| Age (mean) | 42.71 | 43.09 | 43.57 | 42.38 |
| Male (%) | 88 | 87 | 77 | 61 |
| White (%) | 38 | 39 | 28 | 44 |
| Married (%) | 6 | 30a | 13a | 22 |
| Unemployed (%) | 25 | 30 | 25 | 39 |
| Monthly Income | 478.13 | 632.61 | 970.86 | 411.11 |
| Methadone (%) | 50 | 26a | 49a | 50 |
| Treatment Arm: | ||||
| Buprenorphine | 38 | 48 | 32 | 39 |
| Treatment Arm: OBOT | 13 | 26 | 19 | 11 |
| Methadone Dosage (mg) | 83.75 | 63.33a | 72.61b | 96.74ab |
| Buprenorphine Dosage (mg) | 12.25 | 11.08 | 12.16 | 11.69 |
| Days in Study (mean) | 84.75 | 96.87 | 98.06 | 98.72 |
Note. Medication dosages are individualized dosages derived from each participants penultimate week of treatment. Uppercase and bolded superscripts with the same letter indicate trajectory subgroups that differ from one another at p < 0.05. Lowercase non-bolded superscripts with the same letter indicate trajectory subgroups that differ from one another at p < 0.10.
The first trajectory group (n = 16, 7.6% of sample) was characterized by high initial levels of opioid craving that decreased during treatment. We labeled this the Rapidly Declining Craving trajectory. The second trajectory group (n = 23, 10.9% of sample) was characterized by high initial levels of opioid craving that increased further during treatment. We labeled this the High and Increasing Craving trajectory. The third group (n = 154, 73% of sample) was characterized by consistently low levels of opioid craving during treatment. We labeled this the Low Craving trajectory. The fourth group (n = 18, 8.5% of sample) was characterized by low initial levels of opioid craving that increased to a peak near the middle of treatment, then decreased. We labeled this the Increasing and Decreasing Craving trajectory.
3.4. Pretreatment Differences among Trajectory Groups
Pre- and in-treatment differences among the four trajectory groups are shown in Tables 2 and 3 and Figure 2. We also examined how each trajectory group differed from the rest of the sample.
Table 3.
Drug use and Drug use History by Heroin Craving Trajectory Subgroups
| Rapidly Declining Craving (subgroup 1) | High & Increasing Craving (subgroup 2) | Low Craving (subgroup 3) | Increasing & Decreasing Craving (subgroup 4) | |
|---|---|---|---|---|
| Drug Use History | ||||
| Alcohol use (past 30 days) | 7.00 | 4.70 | 4.72 | 4.11 |
| Alcohol use (lifetime) | 5.56 | 6.43 | 5.89 | 5.17 |
| Heroin use (past 30 days) | 23.81 | 23.09 | 19.36 | 15.22 |
| Heroin use (lifetime) | 16.47a | 16.87b | 14.59c | 9.22abc |
| Other opiate use (past 30 days) | 9.69a | 5.52ab | 7.28c | 13.33bc |
| Other opiate use (lifetime) | 2.19 | 2.35 | 1.18a | 2.67a |
| Cocaine use (past 30 days) | 4.69 | 2.17 | 4.90 | 2.33 |
| Cocaine use (lifetime) | 7.06 | 5.04 | 6.10 | 3.72 |
| More than one substance (past 30 days) | 0.81 | 0.26 | 0.47 | 1.28 |
| More than one substance (lifetime) | 1.19a | 0.61bC | 0.22 | 1.06abC |
| Longest period of abstinence (months) | 4.69AB | 2.17Ac | 4.90cD | 2.33BD |
| Time since most recent abstinence (months) | 7.06a | 5.04 | 6.10a | 3.72 |
| # of Times Overdosed | 0.94 | 0.57 | 0.38 | 0.11 |
| Times Treated for drug abuse | 3.50aBc | 2.09a | 2.38B | 2.22c |
| Money spent on drugs (past 30 days) | 1040.00A | 986.52b | 1026.31 | 561.94Ab |
| Family history of drug problems | 1.56 | 1.83 | 1.49 | 1.94 |
Note. Data were collected from the Addiction Severity Index structured interview conducted prior to enrollment. Uppercase and bolded superscripts with the same letter indicate trajectory subgroups that differ from one another at p < 0.05. Lowercase non-bolded superscripts with the same letter indicate trajectory subgroups that differ from one another at p < 0.10.
Figure 2.
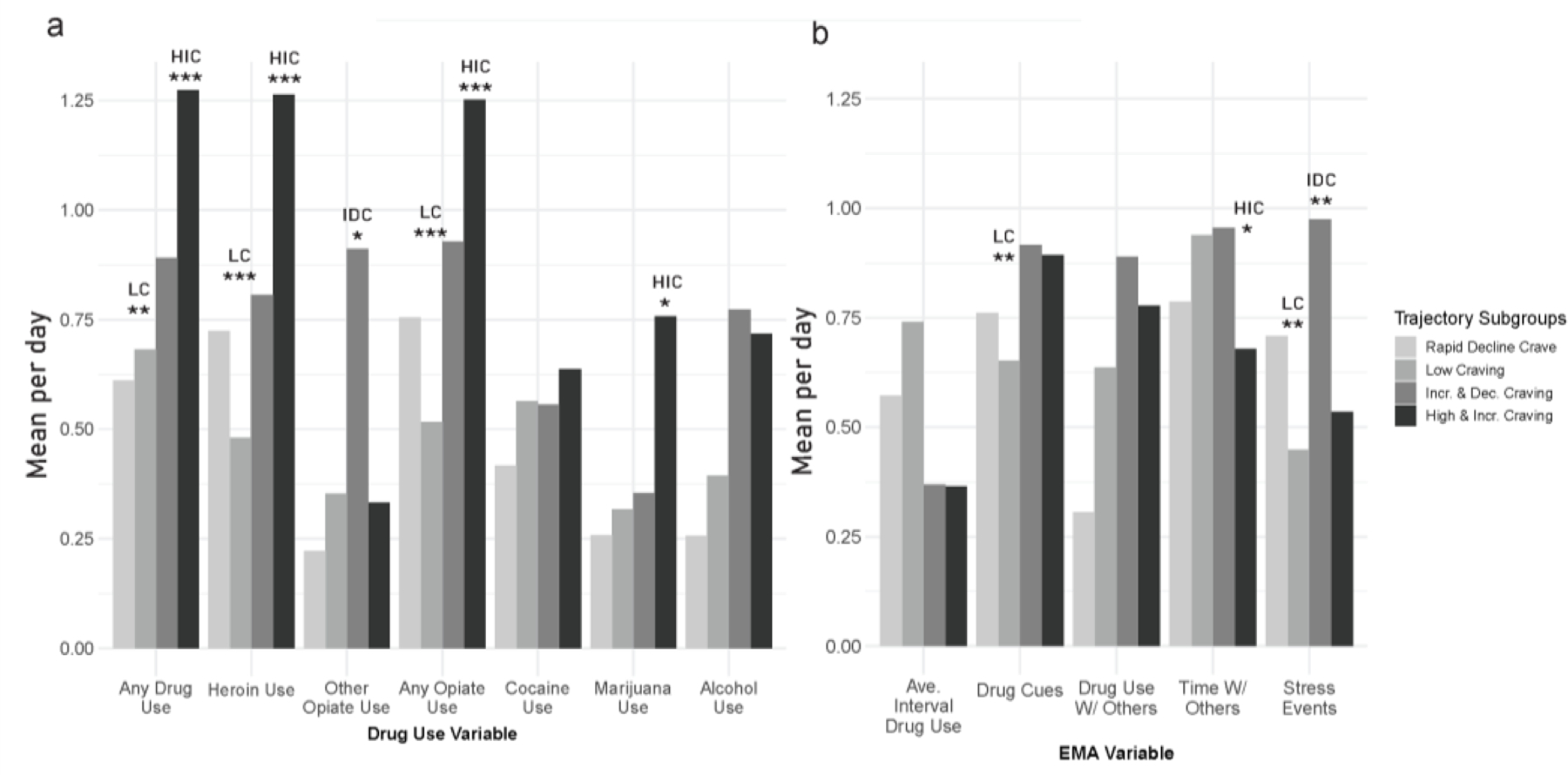
Trajectory group differences for drug use (panel a) and average interval between drug use, cue exposure, social contact, and stress during treatment (panel b). All variables have been standardized by dividing by the root-mean square. Non-standardized values for each variable by trajectory subgroup are displayed in supplementary Table 3. HIC = High and Increasing Craving trajectory, LC = Low Craving trajectory. * = subgroup comparison (vs. all other subgroups) significant at p < 0.05, ** = subgroup comparison (vs. all other subgroups) significant at p < 0.01, *** = subgroup comparison (vs. all other subgroups) significant at p < 0.001.
3.4.1. Demographics, medication type, and retention in treatment
The trajectory groups did not reliably differ on sex, income, MOUD medication type (methadone vs. buprenorphine), or days in the study. However, the High and Increasing Craving trajectory were the most likely to be married (χ2 = 4.62, p = .032, φc = 0.15) and the Increasing and Decreasing Craving trajectory received the highest methadone dosage near the end of treatment (χ2 = 5.83, p = .016, d = −0.69) compared to participants in other trajectory groups.
3.4.2. Drug history
The Increasing and Decreasing Craving trajectory reported the highest levels of non-heroin opioid use during their lifetime (χ2 = 4.29, p = .038, d = −0.46) and in the 30 days (χ2 = 5.14, p = .023, d = −0.59) prior to treatment enrollment. However, the Increasing and Decreasing Craving trajectory also reported the lowest lifetime use of heroin (χ2 = 5.52, p = .019, d = 0.57) and had spent the least amount of money on drugs during the past 30 days (χ2 = 3.50, p = .061, d = 0.37). The Rapidly Declining Craving trajectory reported the longest time since their most recent abstinence (χ2 = 4.03, p = .045, d = −0.31) and the most lifetime episodes of treatment for addiction (χ2 = 6.28, p = .012, d = −0.50) prior to enrollment. Finally, the Low Craving trajectory group reported the lowest lifetime use of non-heroin opioids (χ2 = 3.96, p = .047, d = 0.45).
3.5. Differences among Trajectory Groups During Treatment
Figure 2 displays trajectory-group differences in drug use, cue exposure, stress, and social contact during treatment. Because most differences were between the High and Increasing Craving group and the Low Craving group, we examined how each of those two groups differed from the other groups combined. Pairwise comparisons for all subgroups are in supplemental Table 2.
Compared to all other groups, the High and Increasing Craving trajectory had the highest frequency of overall drug use during treatment (χ2 = 11.4, p = < .001, d = −0.92). This trajectory also had the highest frequencies of heroin use (χ2 = 13.97, p < .001, d = −0.96), any opioid use (χ2 = 13.29, p < .001, d = −0.92), and cannabis use (χ2 = 5.67, p = 0.017, d = −0.48). In contrast, the Low Craving trajectory had the lowest frequency of heroin use (χ2 = 16.63, p < .001, d = 0.64) and any opioid use (χ2 = 18.91, p < .001, d = 0.68). The Increasing and Decreasing Craving trajectory had the highest frequency of non-heroin opioid use (χ2 = 5.43, p = .020, d = −0.63).
The Low Craving trajectory also reported the lowest frequency of exposure to drug cues (χ2 = 7.45, p = .006, d = 0.30), the highest level of social contact (χ2 = 4.50, p = .034, d = −0.33), and the lowest rate of event-contingent entries of stress (χ2 = 6.61, p = .010, d = 0.32). The High and Increasing Craving trajectory had the lowest frequency of social contact (χ2 = 6.49, p = .011, d = 0.58) and the Increasing and Decreasing Craving trajectory had the highest rate of event-contingent stress entries (χ2 = 9.16, p = .002, d = −0.59).
3.6. Prediction of High-risk Trajectory-Group Membership from Pre-treatment Data
Because the highest levels of craving and in-treatment drug use were observed in the High and Increasing Craving (HIC) subgroup, we selected membership in the HIC as the primary prediction target.
The confusion matrix for the SVM model predicting HIC membership using a subset of ASI baseline variables is shown in Supplemental Table 3. The model predicted 16/23 (sensitivity = 0.70) of HIC participants and achieved an overall accuracy of 0.77, a specificity of 0.78, and a negative predictive value (NPV) of 0.95, but a positive predictive value (PPV) of only 0.28. Including 1 week of participants’ EMA ratings of opioid craving and stress and regression intercept and slope values from 1 week of craving data did not improve sensitivity (0.65) but did improve specificity (0.87), PPV (0.39), and overall accuracy (0.85). Including 2 weeks of data improved all accuracy metrics, resulting in a sensitivity of 0.74, a specificity of 0.91, a PPV of 0.50, an NPV of 0.97, and an accuracy of 0.89. Adding in 3 weeks of data improved only sensitivity (0.78) and resulted in slightly lower specificity (0.86), PPV (0.41), and accuracy (0.85). The confusion matrices and accuracy metrics for the 1– 2- and 3-week SVM models are shown in the Supplemental Material.
4.0. Discussion
In these analyses, we used growth mixture modeling and EMA data to characterize longitudinal patterns of opioid craving in outpatients enrolled in MOUD. The results suggest substantial heterogeneity in craving patterns, producing discernible “types,” or groups, with clinically significant differences in outcomes such as drug use during treatment.
The largest group had consistently low and stable levels of opioid craving during treatment (Low Craving; 73%). The other participants fell almost equally into three groups with more complex patterns. One group had initially high levels of craving that increased during treatment (High and Increasing Craving; 10.9%). Another group had an inverted U-shaped pattern of craving (Increasing and Decreasing Craving; 8.5%). Another group started with high levels of craving, but their craving rapidly decreased during the first few weeks of treatment (Rapidly Declining Craving; 7.6%).
The trajectory groups also differed on intra-treatment variables typically associated with treatment success. The main differences were observed for the High and Increasing Craving and Low Craving trajectory groups, who had, respectively, the highest and lowest rates of heroin and any opiate use during treatment. The High and Increasing Craving group also had the highest rates of cannabis use. These findings support previous research linking high levels of opioid craving and more frequent substance use during treatment (Blanken et al., 2012; McHugh et al., 2014; Preston et al., 2018). However, self-reported past 30-day heroin and other opiate use from the ASI was not associated with membership in the High and Increasing Craving or Low Craving subgroups. Thus, in this sample, self-reported intensity of recent opioid use was not reliably able to differentiate participants with the lowest and highest rates of any opiate use during treatment.
Previous research has examined evidence for trajectories of drug craving in non-opioid-using populations. Two studies of patients enrolled in inpatient/outpatient treatment for cocaine use disorder (Bordnick and Schmitz, 1998) and alcohol use disorder (Oslin et al., 2009) found evidence for distinct craving trajectories. Furthermore, participants with the highest craving levels had the poorest treatment outcomes. The study most similar to ours used GMM to characterize trajectories of opioid craving across four weeks in outpatients treated with buprenorphine for OUD. However, we cannot directly compare those results to ours, because weeks three and four in that study were a dose taper (Northrup et al., 2015). That study did find four groups that differed in baseline severity and trajectories during identical treatment. These differences were also associated with propensity to lapse. Thus, to the extent these findings can be combined, they appear to support the existence of patterns of craving during treatment for SUDs, and the prognostic importance of those patterns.
The persistently low levels of craving reported by our Low Craving group may reflect a bidirectional relationship between low craving and low mean levels of exposure to drug-related stimuli and stressful events (Carter and Tiffany, 1999; McCarthy et al., 2006; Epstein et al., 2009; Serre et al., 2015; Preston et al., 2018), and high exposure to protective factors such as social support (Wasserman, Stewart, & Delucchi; 2001; Zhu et al., 2018). Similarly, causation may be bidirectional between craving and ongoing drug use in the High and Increasing Craving group, because drug use can prime craving for more drug use (Jaffe et al., 1989; Marks et al., 2015). The Rapidly Declining Craving group seems to represent treatment response (though we cannot conclude that without a placebo control), and, more speculatively, the Increasing and Decreasing Craving group might represent incubation of craving (Bedi et al., 2011) followed by treatment response. Examination of factors that coincide with changes in craving over time should provide greater insight into the patterns we observed, including possibly heterogenous mechanisms within specific subgroups (e.g., reliable links between medication dosage increases/decreases and important trend changes in craving for specific participants in a subgroup [see e.g., the Increasing and Decreasing Craving subgroup]).
We found that by training an SVM with only a subset of items from the ASI – one of the most commonly used assessment measures in addiction research – we were able to correctly identify a majority (70%) of participants in the High and Increasing Craving group before they started MOUD. Furthermore, including cumulative EMA ratings of craving and stress and regression intercept and slope estimates of craving ratings at 1-week increments, systematically improved most of our classification metrics: the 2-week model had an overall accuracy of 0.89, sensitivity of 0.74, specificity of 0.91, and a negative predictive value of 0.91. Interestingly, adding 3 weeks of EMA data only improved the sensitivity of the model but slightly decreased specificity and PPV. Examination of the mean trajectories (Figure 1) for the four trajectory subgroups may provide some explanation for this counterintuitive finding: at around 3 weeks, the craving levels of the Increasing and Deceasing Craving group almost overlap with those of the High and Increasing Craving group. Thus, the cumulative sum of craving reports and regression slope estimates from these two groups might be very similar and could lead to increased misclassifications.
To our knowledge, this is the first use of pretreatment assessments to predict both drug use and the course of craving during MOUD. It is important to point out that only a single variable from the ASI – marital status – reliably differentiated the High and Increasing Craving group from the three other subgroups. Furthermore, only a handful of drug-use-history variables – including prior opioid use, money spent on drugs, and number of previous addiction treatment episodes – reliably differentiated the other three craving-trajectory subgroups. This suggests that the use of most person-level items provided by the ASI, such as prior drug-use/addiction history, are not sufficient to discriminate high-risk patterns of craving and drug use during treatment from lower-risk patterns in real-world clinical settings. However, future research should directly compare whether risk estimates derived from our SVM model outperform prognoses generated by experienced addiction clinicians; accumulating evidence suggests that this very well may be the case (see e.g., Symons et al., 2019; Symons et al., 2020; Taylor, Moore, Cheung, & Brandt, 2018). Although our ability to rule out negative cases was excellent (NPV: 0.97), our false positive rate was relatively high (PPV: 0.50 for the 2-week model); any clinical use of our method would need to be done with attention to these prediction properties.
These findings should be interpreted in the context of some limitations. First, the EMA data used to characterize the craving trajectories were collected from a nonrandom sample of participants seeking treatment at our treatment-research clinic in Baltimore, MD. Replication is necessary to determine whether these findings generalize to samples from other regions. Second, the length of data collection may have affected survey responses, leading to participant fatigue. Examination of the EMA responses over time did not reveal evidence for problematic changes in variability or increases in problematic responses (e.g., providing the same response for all questions). Finally, GMM can be sensitive to sample characteristics and distributional assumptions. Future studies should examine whether these findings are robust to sample and alternative distributional characteristics.
These limitations notwithstanding, our findings provide empirical support for the presence of longitudinal groups of participants with OUD who report different patterns of craving during MOUD. These group differences in craving have important implications for participants’ subjective experiences and well-being during treatment and are predictive of treatment success. We plan to examine whether the addition of other baseline questionnaires or time-varying data early in treatment (e.g., urine drug tests) can improve the pretreatment identification of risk for craving. In addition, we plan to examine whether similar trajectory models generalize to larger and more regionally/ethnically diverse samples by beta-testing our framework in a multi-clinic addiction practice in the Maryland region. It would be worthwhile to further examine how early identification of longitudinal response patterns could be used to improve personalized prediction models/algorithms (e.g., by better specifying which cases to include in the training data), and whether adjunctive treatments might reduce in-treatment craving levels and improve treatment outcomes.
Supplementary Material
Highlights.
People in treatment for opioid-use disorder differ in patterns of momentary craving
Longitudinal patterns of craving are associated with drug use, stress, and cue-exposure during treatment
Data collected at treatment enrollment can help identify higher risk patient subtypes
Role of Funding Source:
This study was supported by the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health. The funding source had no role in the writing of this report.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of Interest: none
Because the final GMM had average posterior probabilities of membership in each subgroup of ≥ 0.90, we determined that it was appropriate to use a classify-analyze approach to subgroup assignment.
Conflict of Interest:Nothing Declared
References
- Bedi G, Preston KL, Epstein DH, Heishman SJ, Marrone GF, Shaham Y, De Wit H, 2011. Incubation of cue-induced cigarette craving during abstinence in human smokers. Biological Psychiatry 69, 708–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y, 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological) 57, 289–300. [Google Scholar]
- Blanken P, Hendriks VM, Koeter MW, van Ree JM, van den Brink W, 2012. Craving and illicit heroin use among patients in heroin-assisted treatment. Drug Alcohol Depend 120, 74–80. [DOI] [PubMed] [Google Scholar]
- Bordnick PS, Schmitz JM, 1998. Cocaine craving: an evaluation across treatment phases. J Subst Abuse 10, 9–17. [DOI] [PubMed] [Google Scholar]
- Burgess-Hull AJ, 2020. Finite mixture models with student t distributions: an applied example. Prevention Science, 1–12. [DOI] [PMC free article] [PubMed]
- Burgess-Hull AJ, Smith KE, Schriefer D, Panlilio LV, Epstein DH, Preston KL, 2021. Longitudinal patterns of momentary stress during outpatient opioid agonist treatment: A growth-mixture-model approach to classifying patients. Drug Alcohol Depend 226, 108884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST, 1999. Meta-analysis of cue-reactivity in addiction research. Addiction 94, 327–340. [PubMed] [Google Scholar]
- Epstein DH, Willner-Reid J, Vahabzadeh M, Mezghanni M, Lin JL, Preston KL, 2009. Real-time electronic diary reports of cue exposure and mood in the hours before cocaine and heroin craving and use. Arch Gen Psychiatry 66, 88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DH, Tyburski M, Kowalczyk WJ, Burgess-Hull AJ, Phillips KA, Curtis BL, Preston KL, 2020. Prediction of stress and drug craving ninety minutes in the future with passively collected GPS data. NPJ Digit Med 3, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareed A, Vayalapalli S, Stout S, Casarella J, Drexler K, Bailey SP, 2011. Effect of methadone maintenance treatment on heroin craving, a literature review. J Addict Dis 30, 27–38. [DOI] [PubMed] [Google Scholar]
- Furnari M, Epstein DH, Phillips KA, Jobes ML, Kowalczyk WJ, Vahabzadeh M, Lin JL, Preston KL, 2015. Some of the people, some of the time: field evidence for associations and dissociations between stress and drug use. Psychopharmacology 232, 3529–3537. [DOI] [PubMed] [Google Scholar]
- Jaffe JH, Cascella NG, Kumor KM, Sherer MA, 1989. Cocaine-induced cocaine craving. Psychopharmacology 97, 59–64. [DOI] [PubMed] [Google Scholar]
- Jones CM, Einstein EB, Compton WM, 2018. Changes in synthetic opioid involvement in drug overdose deaths in the United States, 2010–2016. JAMA 319, 1819–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann HB, Whitney DR, 1947. On a test of whether one of two random variables is stochastically larger than the other. Annals of Mathematical Statistics 18, 50–60. [Google Scholar]
- Marks KR, Pike E, Stoops WW, Rush CR, 2015. Alcohol administration increases cocaine craving but not cocaine cue attentional bias. Alcholism: Clinical and Experimental Research 39, 1823–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy DE, Piasecki TM, Fiore MC, Baker TB, 2006. Life before and after quitting smoking: an electronic diary study. J Abnorm Psychol 115, 454–466. [DOI] [PubMed] [Google Scholar]
- McHugh RK, Fitzmaurice GM, Carroll KM, Griffin ML, Hill KP, Wasan AD, Weiss RD, 2014. Assessing craving and its relationship to subsequent prescription opioid use among treatment-seeking prescription opioid dependent patients. Drug Alcohol Depend 145, 121–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Cacciola J, Griffith J, Evans F, Barr HL, O’Brien CP, 1985. New data from the Addiction Severity Index. Reliability and validity in three centers. J Nerv Ment Dis 173, 412–423. [DOI] [PubMed] [Google Scholar]
- Northrup TF, Stotts AL, Green C, Potter JS, Marino EN, Walker R, Weiss RD, Trivedi M, 2015. Opioid withdrawal, craving, and use during and after outpatient buprenorphine stabilization and taper: a discrete survival and growth mixture model. Addictive Behaviors 41, 20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oslin DW, Cary M, Slaymaker V, Colleran C, Blow FC, 2009. Daily ratings measures of alcohol craving during an inpatient stay define subtypes of alcohol addiction that predict subsequent risk for resumption of drinking. Drug Alcohol Depend 103, 131–136. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Stull SW, Kowalczyk WJ, Phillips KA, Schroeder JR, Bertz JW, Vahabzadeh M, Lin JL, Mezghanni M, Nunes EV, Epstein DH, Preston KL, 2019. Stress, craving and mood as predictors of early dropout from opioid agonist therapy. Drug Alcohol Depend 202, 200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston KL, Kowalczyk WJ, Phillips KA, Jobes ML, Vahabzadeh M, Lin JL, Mezghanni M, Epstein DH, 2018. Before and after: craving, mood, and background stress in the hours surrounding drug use and stressful events in patients with opioid-use disorder. Psychopharmacology 235, 2713–2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proust-Lima C, Philipps V, Liquet B, 2017. Estimation of extended mixed models using latent classes and latent processes: the R package lcmm. Journal of Statistical Software 78, 1–56. [Google Scholar]
- Ram N, Grimm KJ, 2009. Growth mixture modeling: a method for identifying differences in longitudinal change among unobserved groups. International Journal of Behavioral Development 33, 565–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/. [Google Scholar]
- Sayette MA, 2016. The role of craving in substance use disorders: theoretical and methodological issues. Annu Rev Clin Psychol 12, 407–433. [DOI] [PubMed] [Google Scholar]
- Serre F, Fatseas M, Swendsen J, Auriacombe M, 2015. Ecological momentary assessment in the investigation of craving and substance use in daily life: a systematic review. Drug Alcohol Depend 148, 1–20. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Wray JM, 2012. The clinical significance of drug craving. Ann N Y Acad Sci 1248, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui JI, Anderson BJ, Strong DR, Stein MD, 2014. Craving predicts opioid use in opioid-dependent patients initiating buprenorphine treatment: a longitudinal study. Am J Drug Alcohol Abuse 40, 163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman DA, Stewart AL, & Delucchi KL (2001). Social support and abstinence from opiates and cocaine during opioid maintenance treatment. Drug and Alcohol Dependence, 65(1), 65–75. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Evans EA, Mooney LJ, Saxon AJ, Kelleghan A, Yoo C, & Hser YI (2018). Correlates of long-term opioid abstinence after randomization to methadone versus buprenorphine/naloxone in a multi-site trial. J Neuroimmune Pharmacol, 13(4), 488–497. 10.1007/s11481-018-9801-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


