Abstract
This review systematically summarizes the C18-diterpenoid alkaloid (DA) compositions isolated from the genera Aconitum and Delphinium in the Delphineae tribe (Ranunculaceae). A total of 117 distinct C18-DA components have been reported, including 58 lappaconitine-type DAs, 54 ranaconitine-type DAs, and five rearranged-type DAs. These components mainly originated from plants from the subgenus Lycoctonum in the genus Aconitum or less frequently from plants within the genus Delphinium. Natural C18-DAs have exhibited a wide range of bioactivities, including analgesic, antiarrhythmic, anti-inflammatory, anti-tumor, and insecticidal activities, which are closely related to their chemical structures. The high chemical and biological diversities among the reported C18-DA constituents in Delphineae plants indicated their potential as a vast resource for drug discovery. Additionally, the Delphineae plant C18-DAs exhibited chemotaxonomic values and showed a high regularity of distribution at different taxonomic levels; therefore, the Delphineae plant C18-DAs can serve as good chemical molecular markers in the taxonomic treatment of plants within this tribe, especially in the infrageneric division.
This review systematically summarizes the C18-diterpenoid alkaloid (DA) compositions isolated from the genera Aconitum and Delphinium in the Delphineae tribe (Ranunculaceae).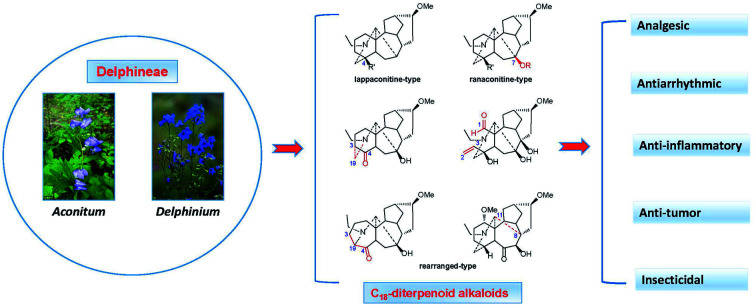
1. Introduction
The Delphineae tribe is morphologically characterized by zygomorphic flowers in the Ranunculaceae family, which consists of two species-rich genera, i.e., Aconitum L. and Delphinium L., while the latter also includes the genera Consolida Gray, Aconitella Spach, and Staphisagria J. Hill.1,2 This tribe comprises 700–800 species with approximately 350–400 species per genus, which amounts to nearly a quarter of all Ranunculaceae species.3–5Delphineae plants are distributed mainly in northern temperate regions, including in Asia, Europe, and North America, and occasionally in Africa.4,6 The center of diversity and speciation of this tribe is in the eastern Himalayas and southwestern China, as approximately 166 species of Aconitum and 150 species of Delphinium have been found in this region.4,5
Many species within the Delphineae tribe are highly valued as medicinal or ornamental plants and have been extensively utilized by various civilizations worldwide since antiquity. In many countries and regions, mainly in the Mediterranean and Asia, various Delphineae plants have been extensively employed as herbal medicines for thousands of years to treat multiple kinds of diseases, including rheumatism, traumatic injury, influenza, oedema, enteritis and stomach ache, fainting, various tumors, asthma, skin diseases such as ringworm and scabies, sciatica, migraine, arthralgia, toothache, neuralgia, and other kinds of pain.7–9 Especially in China, in addition to two Aconitum species officially listed in the Chinese Pharmacopoeia (A. carmichaelii Debeaux and A. kusnezoffii Reichb.), at least 76 species of Aconitum and 32 species of Delphinium are used as folk medicines due to their unique and proven therapeutic effects.10,11 On the other hand, Delphineae plants, especially plants from the genus Delphinium, feature various coloured flowers ranging from white, yellow, and red to blue, which have been widely cultivated for centuries as horticultural plants. Currently, some Delphinium species, such as D. elatum L., D. grandiflorum L., D. ajacis L. (C. ajacis Schur), have become one of the most famous and popular horticultural plants around the world, especially in Europe and America.
Delphineae plants have been phytochemically studied since the early 18th century. After hundreds of years of unremitting efforts on exploring their compositions, a large number of metabolites belonging to multiple types of natural products, including diterpenoid alkaloids (DAs), flavonoids, phenic and acids, steroids, and volatile components, have been reported.8,12 DAs have been acknowledged as the most characteristic and representative competents for the Delphinieae tribe, as it was reported that nearly 90% of naturally occurring DAs were found in this tribe.13 DAs could be further divided into four categories as C18-, C19-, C20-, and bis-types according to the number of carbons in their skeleton. Among them, C18-DAs are a highly specific group of compounds. Despite the small amount, they showed great research potential for their novel structures and broad bioactivities: previous studies have revealed a wide range of pharmacological actions for C18-DAs, including analgesic, antiarrhythmic, anti-inflammatory, anti-tumor, and insecticidal activities. In particular, the representative C18-DA lappaconitine (LA, 37) demonstrated prominent analgesic and antiarrhythmic effects and has been introduced as analgesic (China) and antiarrhythmic (Uzbekistan) drugs since the 1980s. These findings underscore the still large potential of C18-DAs in drug discovery and encourage further extensive investigation.
Additionally, the Delphinieae tribe is fame for its taxonomical complexity, as it possesses various complex morphological variations that lack clear relevance, and as a result, the taxonomy of this tribe is very challenging for botanists. Despite its long investigative history involving various systematic methodologies, the taxonomic treatment within this tribe, especially the infrageneric division and the species circumscription, is still frequently discussed and may remain unresolved for many years to come.14 Thus, using chemotaxonomy to assist and supplement the investigation is very important. Currently, in addition to zygomorphic flowers, the presence of DAs has been recognized as synapomorphy for this taxonomic group. Moreover, since Ichinohe proposed in 1978 that DAs could reflect the phylogenetic relation of Delphineae plants,15 DA chemotaxonomic values have been well-illustrated and widely accepted.16,17 Applying DAs to address corresponding taxonomic problems has been reported.18,19 However, most of these studies were focused on the more widespread DA C19- and C20-subtypes, and little attention has been given to the less common C18-subtypes. C18-DAs also possess great chemotaxonomic value, and could serve as a beneficial supplement to conventional systematic taxonomic approaches and provide useful information within this taxonomic phytogroup.
There are several previously published review articles and monographs involving C18-DAs,13,20,21 but they mainly focused on the progress of studies involving all types of DAs, and only a small portion of research has been devoted to C18-DAs. The research by Wang deserves more attention, as the work includes plentiful and varied descriptions of 78 C18-DAs with literature coverage to the end of July 2008.22 During the past decades, a number of new C18-DAs have been reported, and some of them possess previously undescribed C18-DA skeletons or impressive bioactivities. Hence, this review was prepared to summarize the research progress on phytochemistry, chemotaxonomy, and bioactivities of natural C18-DAs, which will facilitate further research and exploitation of these types of compounds and the utilization of Delphinieae plants.
2. Phytochemical studies of C18-DAs
Although the first C18-DA, LA (37), was isolated in 1895,23,24 with its structure confirmed in 1969,25 and several representative C18-DAs, e.g., lappaconidine (35),26 aconosine (4),27 and excelsine (29),28 were also discovered during the 1970s, the subtype of C18-DAs was defined much later. LA and its analogs have been structurally treated as C19-DAs for many years. However, in 1983, Wang et al. suggested to use C18-DAs for these alkaloids to distinguish them from C19-DAs,29 as they possess structural features that are distinctive compared to those of C19-DAs, namely, C18-DAs lack C-18, which generally appears as methyl or oxygenated methylene in C19-DAs. As the quantity of C18-DAs increased over decades, the term C18-DAs was gradually accepted. Currently, C18-DAs are mostly regarded as an independent group within DAs, which are also called “bisnorditerpenoid alkaloids”.20
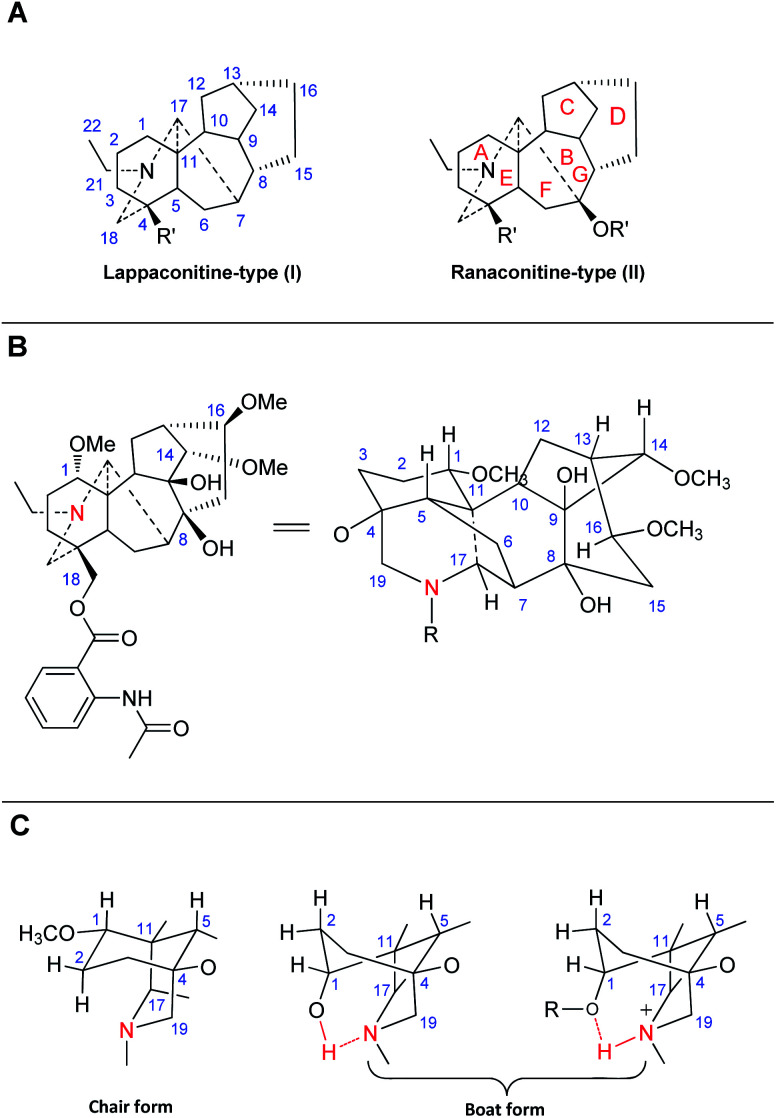
C18-DAs are usually classified into two typical subtypes based on whether an oxygen-containing functionality (e.g., OH, OMe, or OCH2O) is attached at C-7, namely, lappaconitine-type DAs (I), which do not possess an oxygen-containing functionality at C-7, and ranaconitine-type compounds (II), which do have an oxygen-containing functionality at this position (Fig. 1A).22 C18-DAs belonging to these two subtypes possess a heptacyclic framework, comprising four six-membered rings (A, B, D and E), including one six-membered N containing heterocyclic ring (E), and two five-membered rings (C and F). According to single crystal X-ray diffraction analysis of corresponding C18-DAs, e.g., LA (37, Fig. 1B), sepaconitine (46),30N-deacetyllappaconitine (41),31 and ranaconitine (102),32 the C18-DAs possess stable conformations for most of the rings except for rings A and D, as they only have one or two flexible atoms in the skeleta, which is identical to that of C19-DAs.33,34 Generally, rings C and F in C18-DAs exist in envelope form, rings E and G adopt chair form, and ring B is fixed in a boat conformation. The conformation of ring A in C18-DAs (as well as in C19-DAs) is mainly affected by the substituents at C-1 and the protonated N. These C18-DAs with OMe-1 or OAc-1 possess a chair conformation of ring A, while C18-DAs with OH-1 substituent have a ring A in boat conformation, which is stabilized by an intramolecular hydrogen bond between OH-1 and the lone electron pair at N (Fig. 1C).35,36 In addition, protonation of N also resulted in a boat conformation of ring A due to the intramolecular hydrogen.37 The conformation of ring D in C18-DAs is more complicated, and is associated with the substituents at C-13, C-15, and C-7. The fusion of several main rings is identical for all C18-DAs: A/B and E/F, trans; A/E and B/C, cis.
Fig. 1. (A) Two typical subtypes of C18-DAs; (B) the structure of LA (projection formula); (C) the conformations of ring A in C18-DAs.
In addition to lappaconitine- and ranaconitine-type C18-DAs, several compounds with unprecedented rearranged-type C18-DA skeletons (III) have also been discovered in recent years. To date, a total of 117 C18-DAs have been reported, and their trivial names, plant origins and references are listed in Table S1.† Herein, phytochemical studies of C18-DAs are summarized by category.
2.1. Lappaconitine-type
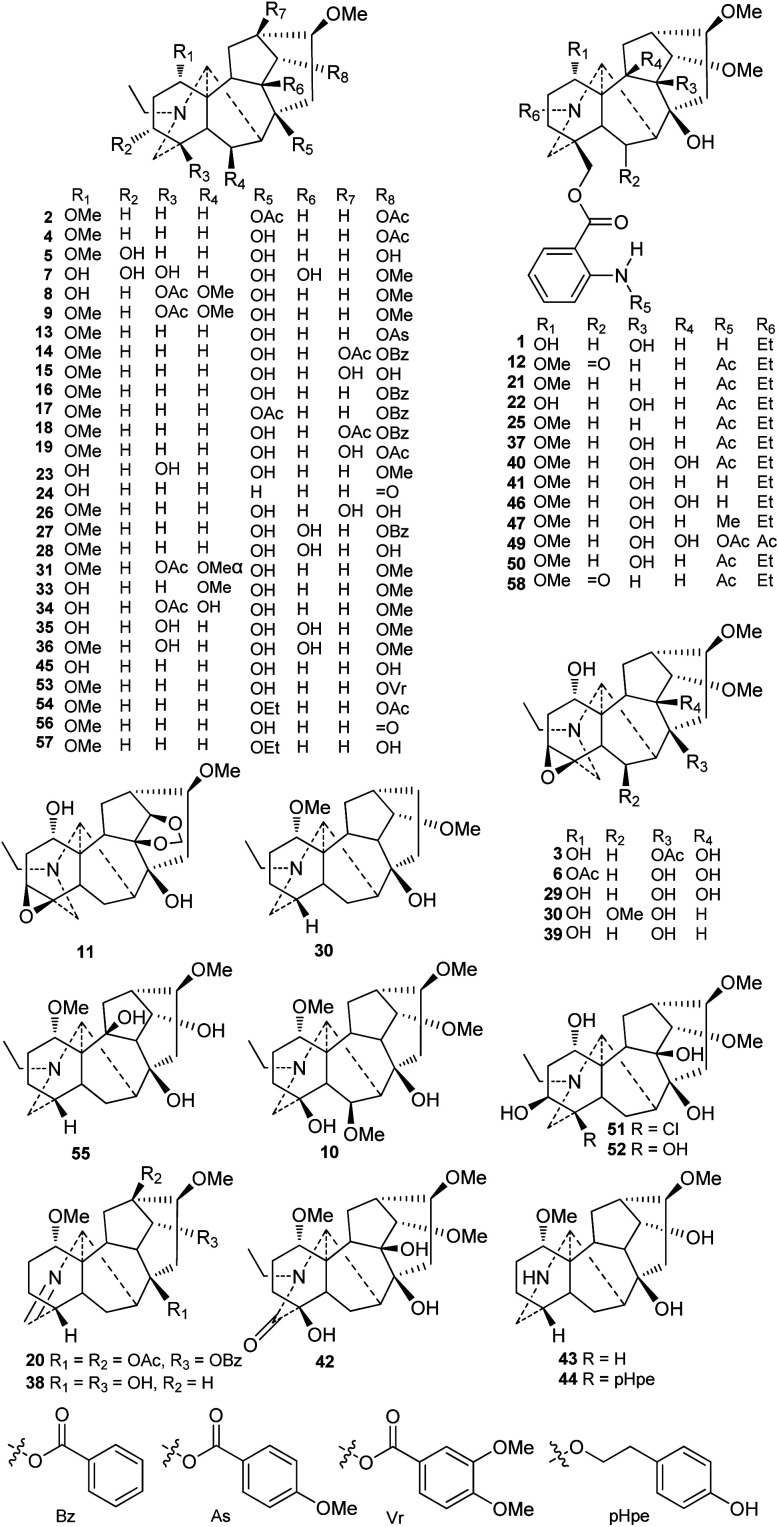
Currently, approximately 58 lappaconitine-type C18-DAs (type I) have been reported (Fig. 2). Most lappaconitine-type C18-DAs were found in the Aconitum species, while only a few exceptions have been reported, e.g., giraldine I (30) from D. giraldii Diels38 and 6-ketoartekorine (58) and artekorine (12) from Artemisia korshinskyi Krash. ex Poljakov in the family Compositae.39 In addition, four lappaconitines were found in both Aconitum and Delphinium plants, namely, delphicrispuline (21),40,41 lappaconidine (35),26,42,43 puberanidine (41),44–46 and sinomontanine A (50).47,48 Most of the lappaconitine-type C18-DAs are scattered in certain Aconitum species with a narrow distribution, and only a few of them have a relatively wide distribution, such as aconosine (4), dolaconine (26), lappaconidine (35), and puberanidine (41).
Fig. 2. Lappaconitine-type C18-DAs (1–58).
The structural diversity of C18-DAs is mainly determined by the state of the N atom and the oxygenated substituents that vary in their variety, quantity, position, and orientation. The N atom usually presents as a tertiary amine (NR3) with a diagnostic N-ethyl group. Among the reported lappaconitines, piepunendines A and B (43 and 44) from A. piepunense Hand.-Mazz. are characterized by the absence of the typical N-ethyl group,49 resulting in a secondary amine (NHR2), and sinaconitine B (49) from A. sinomontanum Nakai features a rare N–C(21) OMe acetamide group instead of the N-ethyl group.50 In addition, 19-oxolappaconine (42) from A. septentrionale Koelle possesses an N–C(19) O lactam group,51 which might be formed by the oxidization of OH-19. Delavaconitine G (20) from A. delavayi Franch.52 and liconosine A (38) from A. forrestii Stapf53 possess an uncommon N C(19) imine group.
Similar to C19-DAs, hydroxyl (OH) and methoxyl (OMe) are the most common oxygenated substituents in lappaconitines. The methoxyl groups are mainly located at C-1, C-6, C-14, and C-16. Almost all of the reported lappaconitines contain OMe-16β with the exception of giraldine I (30), which lacks an oxygen-containing functionality at this position.38 In this type of DA, methoxyl groups at C-1 and C-14 are fixed in the α-orientation, while OMe-6 might have either an α- (e.g., compounds 31 and 32) or β-orientation (e.g., compounds 8–10). The OH groups are mainly distributed at C-1, C-4, C-8, and C-14 and are occasionally distributed at C-3, C-6, C-9, C-10, and C-13, and are easily esterified by various ester groups, including acetyl (Ac) and aroyl groups such as benzoyl (Bz), anisoyl (As), veratroyl (Vr), or anthranoyl.54 These aroyl groups have preferred substituent locations; for example, the Bz, As, and Vr groups are always substituted at C-14, and the anthranoyl group is exclusively located at C-18.
Six alkaloids featuring a 3,4-epoxy group were reported, including 8-acetylexcelsine (3),55 akirine (11),56 excelsine (29),28 kiritine (32),57 monticamine (39),58 and akiranine (10),59 which could be regarded as the characteristic substituents of C18-DAs that distinguish them from C19-DAs. There are two alkaloids, weisaconitines A and D (54 and 57), from A. weixiense W. T. Wang, which rarely have an oxyethyl at C-8.60 In addition, several lappaconitines that possess uncommon substituents have been reported; for example, sinomontanine N (51) from A. sinomontanum61 contains a chlorine (Cl) at C-4, which is rare in secondary metabolites produced by terrestrial plants, and piepunendine B (44) from A. piepunense has a unique 2-(p-hydroxyphenyl)ethoxy group at C-8.49
2.2. Ranaconitine-type
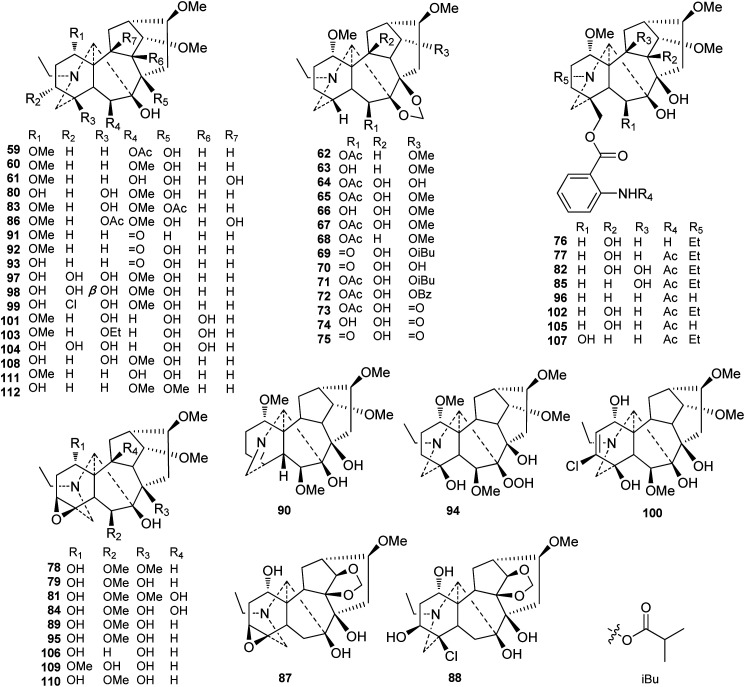
To our knowledge, 54 ranaconitine-type C18-DAs (type II) were reported from Delphineae plants (Fig. 3). Similarly, most of the ranaconitine-type C18-DAs were found in Aconitum plants. However, a certain number of ranaconitines are distributed in Delphinium plants. Most ranaconitines are tertiary amines, which is consistent with lappaconitine-type DAs, and only a few expectations have been reported, namely, imine lamarckinine (90)62 and secondary amines puberanine (96)44 and sinomontanine H (107).63 Although the variety of substituents in ranaconitines and lappaconitines is roughly identical, ranaconitines possess more oxygenated substituents. In addition to their oxygenated substituent at C-7, ranaconitines are also easier to be substituted by O, or OH, OAc, and OMe with a β-orientation at C-6. The dioxymethylene group (OCH2O) is more frequent in this type of compound and is mainly located at C-7/C-8. In rare cases, two alkaloids bear an OCH2O group between C-10 and C-14, namely, kirisines A and B (87 and 88) from A. barbatum Pers. (synonym A. kirinense).64 A series of ranaconitines possessing OH-10, including anthriscifolcines C-G (65–68), anthriscifolcones A and B (69 and 70), and anthriscifoltines C-G (71–75), were found in two varieties of D. anthriscifolium Hance.65–68 Among them, anthriscifolcines F and G (67 and 68) also feature an OH-16 substituent instead of the common OMe-16 substituent. There are also a series of ranaconitines bearing the characteristic 3,4-epoxy group that have been reported.69–72 Several ranaconitine-type compounds that feature rare substituents have also been reported; for example, puberumines C and D (99 and 100) from A. barbatum var. puberulum Ledeb. Fl. Ross. that possesses a chlorine at C-3 was reported.58 In addition, lineariline (94) from D. linearilobum (Trautv.) N. Busch contains a rare peroxyl group (OOH) at C-7.73
Fig. 3. Ranaconitine-type C18-DAs (59–112).
2.3. Rearranged-type
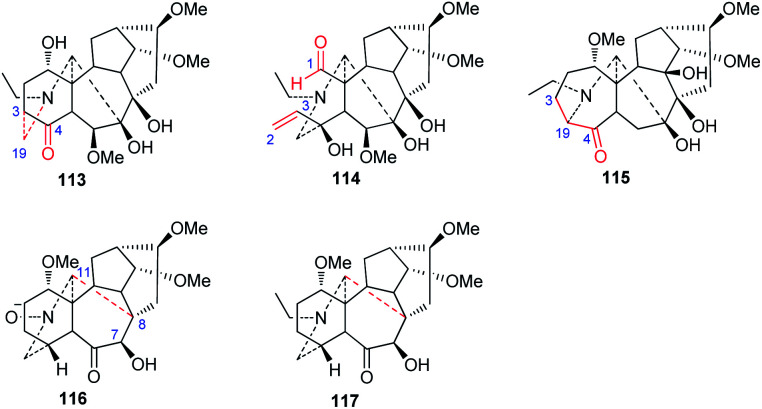
New C18-DA subtypes have seldom been reported—only four rearranged C18-DA skeletons have been discovered in recent decades (Fig. 4). Among them, puberudine (113) and puberunine (114) are two novel C18-DAs isolated from A. barbatum var. puberulum.58 The former alkaloid (113) possesses a novel seven-membered E ring, in which the typical C(19)–C(4) bond is rearranged to form a new C(19)–C(3) bond, and the latter (114) contains a seco A ring that is generated via C(1)–C(3) bond cleavage, forming an extra CHO-1 group and a Δ2,3 unit in the A ring. Sinomontadine (117) from A. sinomontanum features a rearranged seven-membered A ring, which might be formed by the incorporation of C-19 into the normal C(3)–C(4) bond.61 It is worth noting that the structure of sinomontadine (117) was confirmed by single-crystal X-ray diffraction experiments. Two C18-DAs with an unusual E ring similar to acoseptine-type C19-DA were also reported,74 namely, barpubenines A and B (114 and 115) from A. barbatum var. puberulum,75 in which the C(7)–C(17) bond was rearranged to a C(8)–C(17) bond, forming an additional ketone at C-7. In addition, barpubenine A is the only N-oxide in C18-DAs. In summary, these alkaloids represent the new subtypes of C18-DAs.
Fig. 4. Rearranged-type C18-DAs (113–117).
3. Chemotaxonomy
DAs have been acknowledged as good chemical molecular markers in the chemotaxonomy of Delphineae plants and have played a vital role in the taxonomy of the Delphineae tribe, especially the C19 and C20 subtypes of DAs, which have extensively clarified chemotaxonomic values and have been applied in many cases.18,76,77 However, in contrast to the unremitting efforts in exploring C18-DA components with novel structures in Delphineae plants, much less attention has been given to their chemotaxonomic values. The potential role that C18-DAs might play as chemical markers in the taxonomy of Delphineae plants has been largely ignored. Hence, the chemotaxonomic value of C18-DAs in the Delphineae tribe is discussed herein to facilitate the knowledge of Delphinieae plant taxonomy.
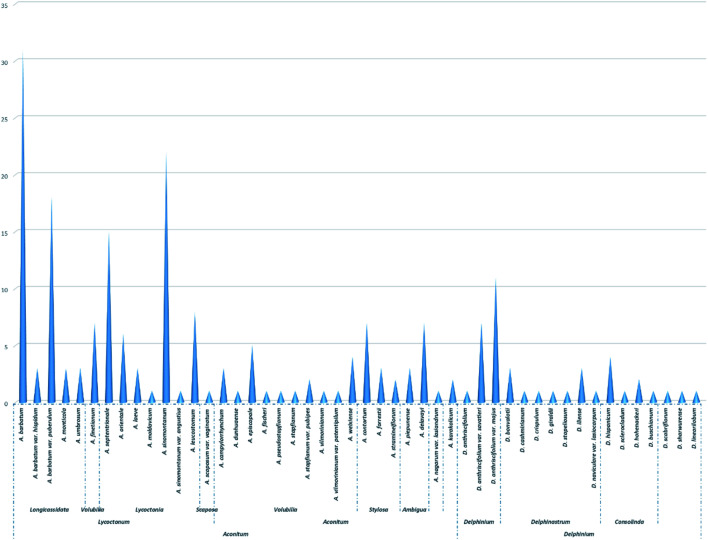
As shown in Fig. 5 and Table S2,† C18-DAs are mainly distributed in Aconitum plants—nearly 80% of C18-DAs for types I, II, and III have been found in Aconitum plants. Taxonomically, the genus Aconitum is usually divided into three distinct subgenera, i.e., Aconitum, Lycoctonum, and Gymnaconitum, based mainly on their morphological root differences.4 Most of the C18-DAs were found in plants belonging to the subgen. Lycoctonum, while much fewer C18-DAs were found in the subgen. Aconitum, and none were found in the subgen. Gymnaconitum, which contains only one species, i.e., A. gymnandrum Maxim. In the approximately 40 species in subgen. Lycoctonum worldwide, nearly 13 species or varieties have been found to contain C18-DA compositions. C18-DAs can be regarded as the predominant chemical constituents of subgen. Lycoctonum, which is distinguished from the subgen. Aconitum by the large number of aconitine-type C19-DAs and much fewer C18-DAs.7 From the perspective of chemotaxonomy,16,17 the evolution degree of DAs increases in the order of C20- < C19- < C18-DAs in terms of structural types, as C19-DAs possess more complex polycyclic structures derived biogenetically from C20-DAs, while C18-DAs are generated by the oxidative degradation of C19-DAs. In general, the more C20-DAs are distributed in plants, the more primitive the phytogroup; in contrast, the more C19- or C18-DAs are distributed in plants, the more evolved the phytogroup. Subgen. Lycoctonum has been accepted as a primitive group relative to the subgen. Aconitum. The fact that this subgenus is abundant in C18-DAs could be due to the parallel evolution that exists widely within the Delphineae tribe.16,78
Fig. 5. The distribution of C18-DAs in tribe Delphineae.
In addition, within the subgen. Lycoctonum, C18-DAs are rarely found in primordial species, such as A. apetalum (Huth) B. Fedtsch.79–81 and A. brevicalcaratum Diels82–84 in series Crassiflora, which has been certified by extensive phytochemical studies. Conversely, most of the C18-DAs are found in species belonging to ser. Longicassidata and Lycoctonia, which represent a relatively evolved position within this subgenus. In particular, several species have been reported to be abundant in C18-DAs, including A. barbatum (A. kirinense), A. barbatum var. puberulum, A. sinomontanum, and A. septentrionale. Thus, it can easily be concluded that within the subgen. Lycoctonum, an advanced species can possess more C18-DAs with abundant structural diversity. Thus, C18-DA can serve as an important indicator to reflect the degree of evolution of certain species within this subgenus.
According to collected data, C18-DA compositions could also be used to estimate genetic relationships or distinguish sibling species; for example, the occurrence of several C18-DAs (35, 36, 37, 41, 46, and 77) in both A. septentrionale and A. sinomontanum supports the morphological similarities of these taxa, and their apparent differences that may be useful to distinguish these taxa. It is worth noting that these conclusions should not be drawn until extensive phytochemical studies have been performed on the corresponding species.
Almost all of the C18-DAs found in subgen. Aconitum are lappaconitine-type DAs (Table S3†), and they are distributed mainly in plants belonging to the ser. Volubilia, followed by ser. Stylosa and Ambigua. There are ten species or varieties within ser. Volubilia that have been found to contain C18-DAs. It is commonly accepted that ser. Volubilia represents a relative evolutionary status in the subgen. Aconitum and is also considered an intermediate transitional phytogroup connecting the ser. Stylosa/Ambigua and ser. Inflata. The distributions of lappaconitine-type C18-DAs in these plants clearly revealed the close affiliation between these series within this subgenus. In addition, C18-DAs are rarely found in plants from these relatively primordial plants in the subgen, such as the A. tanguticum Stapf in ser. Tangutica,85–87 and A. rotundifolium Kar. et Kir. in ser. Rotundifolia.88,89 The component distribution was consistent with the degree of plant evolution within this phytogroup, which demonstrated the chemotaxonomic values of C18-DAs as chemotaxonomic markers.
When compared with that of Aconitum, much fewer C18-DAs have been found in the Delphinium genus, and most of the reported C18-DAs from Delphinium are ranaconitine-type compounds (Table S4†), which can be distinguished from the genus Aconitum by the existence of C18-DAs that belong to both types I, II, and III. It is also noted that a series of ranaconitine-type DAs were found in two varieties of D. anthriscifolium, namely, D. anthriscifolium var. savatieri and D. anthriscifolium var. majus,65–68 which belongs to the subgen. Delphinium. According to the species division of the genus Delphinium by Wang,5 the subgenus Delphinium contains only two species, namely, D. anthriscifolium and D. ludingense W. T. Wang, while another subgenus, Delphinastrum, comprises ca. 165 species. The abundance of C18-DAs in D. anthriscifolium distinguishes it from other Delphinium species, which supports the unique taxonomic position of this genus that was obtained by classical taxonomic methodologies. In addition, the occurrence of C18-DAs in Delphinium also supports the viewpoint that parallel evolution widely exists in plants within this tribe.
In addition, two C18-DAs (12 and 58) have been isolated from Artemisia korshinskyi in the family Compositae.39 However, the data are not sufficient enough to be useful in exploring their chemotaxonomic values.
In summary, C18-DAs exhibited some important distribution regularity within tribe Delphineae, which occurred as follows: (1) at the genus level, C18-DAs are distributed mainly in plants from the Aconitum genus and less so from Delphinium plants; (2) in terms of the subgenus, the subgen. Lycoctonum in the genus Aconitum is the richest source of C18-DAs, as a large number of compounds belonging to all types of C18-DAs have been found in this phytogroup. In contrast, the subgen. Aconitum possesses only lappaconitine-type C18-DAs in relatively low amounts. The genus Delphinium contains mainly ranaconitine-type C18-DAs and its subgen. Delphinium contributed much more C18-DAs than the subgen. Delphinastrum; (3) within a certain subgenus, C18-DAs are mainly distributed in relatively evolved phytogroups, such as ser. Longicassidata in subgen. Lycoctonum, and ser. Volubilia in subgen. Aconitum. Sibling spices with close genetic relationships might share some C18-DAs. Overall, these findings demonstrated the chemotaxonomic values of C18-DAs and support the potential of C18-DAs to serve as chemical molecular markers in the taxonomic treatment of plants from this tribe.
4. Bioactivities
4.1. Analgesic activity
Delphineae plants have long been employed for treatment of various kinds of pains in traditional medicines, which could be attributed to their characteristic DA compositions. The analgesic activities of DAs have been investigated since the 1980s, which have led to the development of three DAs as analgesic drugs, i.e., the C19-DAs 3-acetylaconitine and crassicauline A and the typical C18-DA LA (37). The hydrobromide salt of LA is used in commercial lappaconitine tablets and injection as the first non-addictive analgesic drug in China, and it is extensively employed for the clinical treatment of various types of mild or moderate pain, such as cancer pain, postoperative pain, and sciatica. Compared to these two C19-DA analgesic drugs (crassicauline A and 3-acetylaconitine), LA exhibited stronger antinociceptive efficacy and less toxicity. It has been observed in clinical practice that the analgesic effect of lappaconitine are generally approximately seven times greater than that of phenazone, a commonly used non-steroidal anti-inflammatory drug, and is almost equipotent to pethidine, with a longer maintenance time of approximately 2–22 h. Moreover, lappaconitine hydrobromide is non-narcotic, inducing neither morphine-like tolerance nor physical dependence. However, the analgesic effect of lappaconitine is slower than that of pethidine, and its clinical dosage range is relatively narrow; therefore, LA is not suitable for the treatment of severe pain.
In animal experiments, lappaconitine demonstrated unambiguous analgesic effects in various models, including writhing, tail-pinch, hot plate tests in mice, and electric stimulation tests in rats. It is generally accepted that the mechanism at supraspinal levels is responsible for LA analgesia, as it could block voltage-dependent Na+ channels in the central nervous system,90 affect calcium influx in the midbrain periaqueductal gray (PAG) and the cortex,91 stimulate descending pathways related to noradrenalin and serotonin,92 inhibit activities of hippocampal neurons,93 and decrease the transmission of nociceptive information via brain, substance P (SP) and/or somatostatin pathways.94 Another study indicated that the analgesic effect of LA might also be involved in the decrease in the expression and sensitization of P2X3 receptors in rat DRG neurons following chronic constriction injury.95
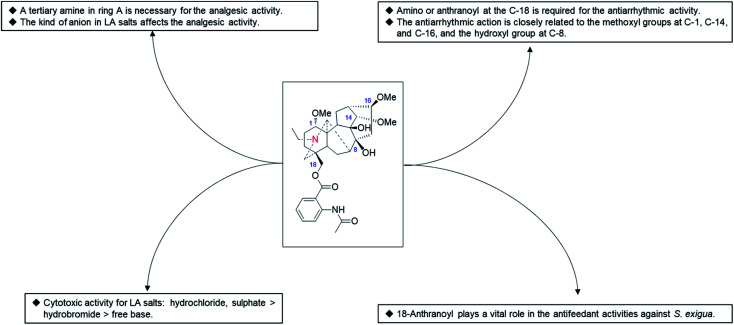
In addition to LA (37), several other natural C18-DAs, such as N-deacetyllappaconitine90 and 8-O-ethylaconosine,60 also exhibited certain analgesic activity, but little attention has been given to these alkaloids. A SAR study performed by Wang et al. revealed that the analgesic activity of LA analogues is closely related to the state of N in ring A and that a tertiary amine is an important structural feature necessary for the analgesic activity of the LA analogues (Fig. 6).96 In addition, the analgesic activity of LA salts is also affected by the kinds of ions, for example it was reported that the analgesic effect of LA hydrochloride is worse than that of its hydrobromide,97 while another study revealed that LA sulfate exhibited a more pronounced analgesic activity than other salt forms.98
Fig. 6. The SAR of LA analogues.
4.2. Antiarrhythmic activity
Since 1977, when Dzhakhangirov et al. first discovered the C20-DA napelline and the C19-DA heteratisine bearing considerable antiarrhythmic activity, the antiarrhythmic activity of various types of DAs has attracted much interest from scientists. In a large-scale screening for antiarrhythmic compounds from hundreds of DAs performed by Dzhakhangirov et al. using the models of aconitine-introduced arrhythmia in anesthetized rats and of irreversible cardiac fibrillation in alert mice,99 the representative C18-DA LA (37) and its major metabolite N-deacetyllappaconitine (41) exhibited pronounced antiarrhythmic and antifibrillatory action, with AAI (LD50/ED50) values of 118 and 146, respectively, which were more than 1000 times superior in antiarrhythmic activity and more than 50-fold superior in breadth of therapeutic action to the positive control novokainamid. Unlike novokainamid, these DAs also exerted powerful protective antifibrillatory action and prevented the death of animals poisoned with a lethal dose of aconitine, which highlights their potential as antiarrhythmic drugs. Subsequent studies demonstrated the negative inotropic effects of LA (37), which could increase the excitation threshold and eventually cause bradycardia and asystolia.100 This typical class-IC antiarrhythmic activity of LA (37) might be due to its electrophysiological properties on sodium channels, which could suppress the fast-incoming sodium current by long-term binding to the site 2 receptor, subsequently decreasing the depolarization rate, leading to a slowing of impulse propagation and a decrease of excitability in the conductive system of the heart.101 LA (37) also showed electrophysiological action on other ion currents, such as calcium and potassium, by modulating the expression of related genes, which might also be responsible for its antiarrhythmic activity.102 Finally, LA hydrobromide was introduced into medical practice as an antiarrhythmic agent under the name of allapinin in Uzbekistan, which was proven to be effective in the treatment of life-threatening forms of arrhythmias, namely, atrial fibrillation (AF) and ventricle rhythm disorders.103,104 Currently, this drug is included in the list of the most vitally important medicines approved by the Ministry of Health of the Russian Federation.
In addition to LA (37) and N-deacetyllappaconitine (41), other C18-DAs also showed antiarrhythmic activity, and several even exerted a superior antiarrhythmic action to LA (37) in some respects. For example, N-acetylsepaconitine (40) and ranaconitine (102) exhibited a stronger antiarrhythmic action in aconitine-introduced arrhythmia in rats, with AAI values of 214 and 124, respectively, which were greater than that of LA. Consistent results were obtained in a recent study which showed that N-acetylsepaconitine (40) could significantly prolong the ventricular premature (VP) action in aconitine-induced arrhythmia mice, with an effect better than that of lappaconitine, while their inhibition of ventricular tachycardia (VT) and ventricular flutter (VFL) was similar.75 From the data available, the presence of amino or anthranoyl groups at the C-18 position is required for the antiarrhythmic activity of C18-DAs, and their antiarrhythmic action is closely related to the methoxyl groups at C-1, C-14, and C-16, along with the hydroxyl group at C-8. Therefore, more efficient antiarrhythmic agents using available natural C18-DAs as lead compounds could be designed based on SAR analysis.
4.3. Anti-inflammatory activity
Traditionally, Aconitum and Delphinium plants have been widely used for the treatment of arthritis, which implies that their major constituents DAs possess certain anti-inflammatory activity. Although most of the newly discovered anti-inflammatory compounds from Delphineae plants are C19-DAs,105 there are also several reports that involve C18-DAs. For example, ranaconine-type C18-DA sinomontanine I (108) from A. sinomontanum showed a dose-dependent inhibitory effect on both lipopolysaccharide (LPS) and concanavalin A (ConA) induced splenic lymphocyte proliferation, with IC50 values of 8.909 and 3.661 μM, respectively.106 Four lappaconine-type C18-DAs from A. fischeri var. arcuatum (4, 9, 24, and 53) showed weak inhibitory activity against NO production in LPS-induced RAW 264.7 macrophages with an inhibition rate of approximately 30% at a concentration of 40 μM.107 In animal experiments, lappaconitine (8 and 16 mg kg−1, ig) effectively inhibited edema of the hind paw induced by injection of carrageenin, formaldehyde, and Freund's complete adjuvant (FCA) in rats, restrained swelling of ear induced by xylene in mice, and inhibited the proliferation of granulomas induced by injection of agar in rats.108 Another study also reported the anti-inflammatory effect of lappaconitine (37), which could inhibit the edema of the hind paw of rats induced by FCA and reduce the contents of TNF-α, IL-2, CIC and PGEa in their serum.109 In general, these studies preliminarily revealed the anti-inflammatory effects of C18-DAs in vitro and in vivo.
4.4. Anti-tumor activity
While most of the naturally occurring C18-DAs, including lappaconitine (37), which usually presented as freebase, showed only slightly active against human cancer cell lines during the primary screen,58,110 a certain degree of anti-cancer effect has also been discovered for several kinds of lappaconitine salts. It was reported that the hydrobromide salt of lappaconitine could suppress the growth of liver and S180 tumors of mice with inhibition rates of 11.20%∼53.08% and 29.81%∼53.96%, respectively111,112 and could inhibit the proliferation of human non-small cell lung cancer A549 cells dose dependently by arresting the cells in G1/G0 phase and downregulating the expression of Cyclin E1 (Table 1).113 Lappaconitine sulfate was also reported to possess antiproliferative activity against various human cancer cell lines: cerical neoplasm (HeLa),114 liver (HepG2),115 colon (HT-29),116 and lung (A549),117 which might be caused by activation of p38 MAPK-, mitochondrial-, and caspase-mediated apoptosis. In addition, the hydrochloride salt of lappaconitine has been observed for its ability to inhibit proliferation and to induce apoptosis in human colon cancer HCT-116 cells and human liver cancer HepG2 cells via mitochondrial and MAPK pathways.118,119 Generally, the sulfate and hydrochloride salts of lappaconitine exhibited a relatively higher cytotoxic effect than its hydrobromide salt, in combination with the fact that these two salts also possess better water solubility,98 indicating their higher potential in tumor therapy.
Cytotoxic effect of lappaconitine salts (IC50, mg mL−1, 48 h).
| Lappaconitine salts | A549 | HeLa | HepG2 | HCT-116 |
|---|---|---|---|---|
| Hydrobromide | 3.925 | — | — | — |
| Sulfate | 0.551 | 0.421 | 0.360 | — |
| Hydrochloride | — | — | 0.372 | 0.174 |
4.5. Insecticidal activity
Some species in the tribe Delphineae that are rich in C18-DA compositions have long been utilized as natural insecticides;4,73 therefore, C18-DAs might possess insecticidal activities, which has been preliminarily confirmed by several studies. Ulubelen et al. evaluated the repellent activity against the warehouse pest Tribolium casteneum (Herbst.) of 29 natural DAs isolated from Turkish Delphineae plants, including two common C18-DAs lappaconitine (37) and N-deacetyl lappaconitine (41).120 As a result, N-deacetyl lappaconitine (41) exerted a relatively high repellency class III effect (40.1–60%) with a repellency value of 50.00% at 3 mg mL−1, suggesting that it might be a promising candidate for insecticide development. However, lappaconitine (37) showed only a low-class II repellent effect (20.1–40%), with a repellency value of 34.37% at 3 mg mL−1.
In addition, three ranaconine-type C18-DAs (110, 79, 78) featuring a 3,4-epoxy group were screened for their insect antifeedant activity against the pests Colorado potato beetle (Leptinotarsa decemlineata) and Spodoptera littoralis.121 These three ranaconines showed a roughly similar effect against L. decemlineata with EC50 values lower than 5 μg cm−2 (Table 2). Alkaloids 110 and 79 also showed certain antifeedant activity against S. littoralis with EC50 values of 5.38 and 11.79 μg cm−2, respectively, while alkaloid 78 was completely invalid against this kind of pest. More recently, another study performed by Chen et al. revealed that C18-DA components in the Chinese Aconitum species A. leucostomum possess antifeedant activity against the larvae of Spodoptera exigua.122 Among the seven tested C18-DAs, N-acetylsepaconitine (40), N-deacetyl lappaconitine (41), and finaconitine (82) with an anthranoyl group at C-18 exerted strong antifeedant activities against S. exigua with EC50 values lower than 2 μg cm−2, followed by acosepticine (111), lappaconidine (35), and leucostonine (93) (EC50, 8–21 μg cm−2), while leucostine (92) was basically ineffective (EC50 > 30 μg cm−2). These results revealed that the 18-anthranoyl substituent plays a vital role in the antifeedant activities against S. exigua of C18-DAs, but more structure–activity relationship (SAR) research is required to confirm this.
Antifeedant effects of C18-DAs (EC50, μg cm−2, and 95% confidence limits).
| C18-DAs | L. decemlineata | S. littoralis | S. exigua |
|---|---|---|---|
| 78 | 1.92 (0.66, 5.54) | ≈ 50 | — |
| 79 | 2.36 (0.47, 11.80) | 5.38 (1.43, 20.37) | — |
| 110 | 3.31 (1.10, 9.94) | 11.79 (11.70, 11.89) | — |
| 35 | — | — | 17.65 (11.10, 28.07) |
| 40 | — | — | <2 (0.85, 1.73) |
| 41 | — | — | 1.88 (1.12, 3.18) |
| 82 | — | — | 1.45 (0.75, 2.81) |
| 92 | — | — | >30 |
| 93 | — | — | 20.75 (14.09, 30.54) |
| 111 | — | — | 8.59 (5.98, 12.36) |
5. Conclusions
This review systematically summarizes the C18-DA compositions isolated from the Delphineae tribe in the Ranunculaceae family in recent decades. A total of 117 distinct C18-DA components, including 58 lappaconitines, 54 ranaconitines, and five rearranged-types, with identified structures have been reported, and these components are mainly from plants from the subgen. Lycoctonum in the genus Aconitum and less so from the genus Delphinium. Natural C18-DAs have exhibited a wide range of bioactivities, including analgesic, antiarrhythmic, anti-inflammatory, anti-tumor, and insecticidal activities, which are closely related to their chemical structures. The high chemical diversity among the reported C18-DA constituents in Delphineae plants indicated their potential as a vast resource for drug discovery. Furthermore, C18-DAs in Delphineae plants showed chemotaxonomic values and a high regularity of distribution at different taxonomic levels, which could be utilized to serve as good chemical molecular markers in the taxonomic treatment of plants within this tribe especially with infrageneric division.
Although C18-DAs in Delphineae plants have attracted considerable interest, there is still potential for more research. First, pharmacological investigations on C18-DAs are restricted to widespread compounds, especially LA, while most less-common C18-DAs are still largely unexplored. The potential of other C18-DAs constituents in drug discovery remains ignored, as well as their SAR. More extensive pharmacological studies of other C18-DAs are necessary. Furthermore, there are few reported data focused on the toxicity, side effects, and clinical efficiency of C18-DAs, which hinders its application and promotion in therapy.
Second, in chemotaxonomic studies, most of the current studies implemented by phytochemists are still aiming at discovering compounds with new structures and are not aiming at illuminating or characterizing the chemical constituent profiles of certain plants. Phytochemists usually prefer to publish new compounds, and known or common C18-DAs are largely ignored and are often not reported. Thus, only a few C18-DAs have been reported in a certain species, and fewer than five C18-DAs have been reported for most of these investigated species. The data on C18-DAs in Delphineae plants are still insufficient and fragmentary, and more complete reports based on extensive investigations are needed. In addition, in the reported phytochemical studies on C18-DAs in Delphineae, the content of certain C18-DA in plants is lacking, which has also been reported as a key reference for taxonomy that reveals evolutionary degrees. Chemotaxonomic studies on C18-DA composition in the Delphineae tribe should also consider the content in addition to the chemical structural diversity of metabolites. While little information can be obtained during the conventional process of studying phytochemicals, incorporating conventional analysis methods such as HPLC, UV, or MS to acquire the contents of these important chemical markers is encouraged in further research.
Conflicts of interest
The authors declare no conflict of interest.
Supplementary Material
Acknowledgments
This work was financially supported by a grant from the National Natural Science Foundation of China (No. 31860095), a grant from the Excellent Young Talents Fund Program of Zunyi Medical University (No. 18zy-005, to X.-Y. Y.), a grant from New Academic Talents Training Program of Zunyi Medical University (No. [2017]5733–038), and a grant from Science and Technology Project of Zunyi (No. [2018]21).
Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra08132b
References
- Jabbour F. Renner S. S. Taxon. 2011;60:1029–1040. doi: 10.1002/tax.604007. [DOI] [Google Scholar]
- DuPasquier P. E. Andro-Durand V. Batory L. Wang W. Jabbour F. PhytoKeys. 2021;180:81–110. doi: 10.3897/phytokeys.180.67126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbour F. Renner S. S. Mol. Phylogenet. Evol. 2012;62:928–942. doi: 10.1016/j.ympev.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Wang W. C. and Michael J. W., Flora of China, 2001 [Google Scholar]
- Wang W. C. Guihaia. 2019;39:1425–1469. [Google Scholar]
- Benn M. H. Okanga I. F. Manavu R. M. Phytochemistry. 1989;28:919–922. doi: 10.1016/0031-9422(89)80143-8. [DOI] [Google Scholar]
- Ali S. Chouhan R. Sultan P. Hassan Q. P. Gandhi S. G. Adv. Tradit. Med. 2021 doi: 10.1007/s13596-021-00565-8. [DOI] [Google Scholar]
- Yin T. P. Cai L. Ding Z. T. RSC Adv. 2020;10:13669–13686. doi: 10.1039/D0RA00813C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin T. P. Cai L. Ding Z. T. RSC Adv. 2020;10:35072–35089. doi: 10.1039/D0RA06811J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G. Tang L. Zhou X. Wang T. Kou Z. Wang Z. J. Ethnopharmacol. 2015;160:173–193. doi: 10.1016/j.jep.2014.11.043. [DOI] [PubMed] [Google Scholar]
- Nyirimigabo E. Xu Y. Li Y. Wang Y. Agyemang K. Zhang Y. J. Pharm. Pharmacol. 2015;67:1–19. doi: 10.1111/jphp.12310. [DOI] [PubMed] [Google Scholar]
- Yin T. P. Yan Y. F. Yang X. Y. Li W. Biochem. Syst. Ecol. 2021;97:104300. doi: 10.1016/j.bse.2021.104300. [DOI] [Google Scholar]
- Wang F. P. Chen Q. H. Liu X. Y. Nat. Prod. Rep. 2010;27:529–570. doi: 10.1039/B916679C. [DOI] [PubMed] [Google Scholar]
- Luo Y. Acta Phytotaxon. Sin. 2005;43:289–386. doi: 10.1360/aps040102. [DOI] [Google Scholar]
- Ichinohe Y. J. Japan. Chem. 1978;32:111–126. [Google Scholar]
- Xiao P. G. Wang F. P. Gao F. Yan L. P. Chen D. L. Liu Y. J. Syst. Evol. 2006;44:1–46. doi: 10.1360/aps050046. [DOI] [Google Scholar]
- Hao X. J. Yang C. R. Chen S. Y. Zhou J. J. Syst. Evol. 1985;23:321–335. [Google Scholar]
- Gao F. Zhu S. A. Wu W. Wang X. G. Song L. Biochem. Syst. Ecol. 2010;38:1052–1055. doi: 10.1016/j.bse.2010.09.003. [DOI] [Google Scholar]
- Cook D. Manson J. S. Gardner D. R. Welch K. D. Irwin R. E. Biochem. Syst. Ecol. 2013;48:123–131. doi: 10.1016/j.bse.2012.11.015. [DOI] [Google Scholar]
- Shen Y. Liang W. J. Shi Y. N. Kennelly E. J. Zhao D. K. Nat. Prod. Rep. 2020;37:763–796. doi: 10.1039/D0NP00002G. [DOI] [PubMed] [Google Scholar]
- Yunusov M. S. Nat. Prod. Rep. 1991;8:499–526. doi: 10.1039/NP9910800499. [DOI] [PubMed] [Google Scholar]
- Wang F. P., Chen Q. H. and Liang X. T., The C18-diterpenoid alkaloids, 2009, pp. 1–78 [DOI] [PubMed] [Google Scholar]
- Rosendahl H. V. J. Pharm. 1896;4:262–266. [Google Scholar]
- Schulze H. Ulfert F. Arch. Pharm. 1922;260:230–243. doi: 10.1002/ardp.19222600113. [DOI] [Google Scholar]
- Mollov N. Tada M. Marion L. Tetrahedron Lett. 1969;10:2189–2192. doi: 10.1016/S0040-4039(01)88118-1. [DOI] [Google Scholar]
- Tel'nov V. A. Yunusov M. S. Rashkes Y. V. Yunusov S. Y. Chem. Nat. Comp. 1971;7:601–604. doi: 10.1007/BF00568417. [DOI] [Google Scholar]
- Murav'eva D. A. Plekhanova T. I. Yunusov M. S. Chem. Nat. Comp. 1972;8:132–133. doi: 10.1007/BF00564471. [DOI] [Google Scholar]
- Tel'nov V. A. Yunusov M. S. Yunusov S. Y. Chem. Nat. Comp. 1973;9:132–133. doi: 10.1007/BF00580928. [DOI] [Google Scholar]
- Wang F. P. Fang Q. C. Acta Pharm. Sin. 1983;7:514–521. [PubMed] [Google Scholar]
- Shi X. W. Lu Q. Q. Zhou J. H. Cui X. A. Acta Crystallogr. E. 2015;71:o550–o551. doi: 10.1107/S205698901501258X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X. W. Lu Q. Q. Zhou J. H. Cui X. A. Acta Crystallogr. E. 2015;71:o576–577. doi: 10.1107/S2056989015012335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J. H. Li Y. Zhang L. Wang D. Q. Nat. Prod. Res. 2012;26:1451–1453. doi: 10.1080/14786419.2011.603319. [DOI] [PubMed] [Google Scholar]
- Zeng Z. Qasem A. M. A. Kociok-Köhn G. Rowan M. G. Blagbrough I. S. RSC Adv. 2020;10:18797–18805. doi: 10.1039/D0RA03811C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Z. Kociok-Köhn G. Woodman T. J. Rowan M. G. Blagbrough I. S. Eur. J. Org. Chem. 2021;2021:2169–2179. doi: 10.1002/ejoc.202100179. [DOI] [Google Scholar]
- Deng H. Y. Chen Q. H. Wang F. P. Nat. Prod. Commun. 2014;9:785–786. [PubMed] [Google Scholar]
- Zhang Z. T. Wang L. Chen Q. F. Chen Q. H. Chen D. L. Liu X. Y. Wang F. P. Tetrahedron. 2013;69:5859–5866. doi: 10.1016/j.tet.2013.05.029. [DOI] [Google Scholar]
- Wang F. P. Chen D. L. Deng H. Y. Chen Q. H. Liu X. Y. Jian X. X. Tetrahedron. 2014;70:2582–2590. doi: 10.1016/j.tet.2014.01.066. [DOI] [Google Scholar]
- Zhou X. L. Chen Q. H. Wang F. P. Chem. Pharm. Bull. 2004;52:456–458. doi: 10.1248/cpb.52.456. [DOI] [PubMed] [Google Scholar]
- Sham'yanov I. D. Tashkhodzhaev B. Mukhamatkhanova R. F. Sultankhodzhaev M. N. Levkovich M. G. Abdullaev N. D. Antipin M. Y. Chem. Nat. Comp. 2012;48:616–621. doi: 10.1007/s10600-012-0326-x. [DOI] [Google Scholar]
- Jiang S. H. Song B. Z. Zhou B. N. Acta Chim. Sin. 1988;46:26–29. [Google Scholar]
- Ulubelen A. Mericli A. H. Mericli F. Kolak U. Ilarslan R. Voelter W. Phytochemistry. 1998;50:513–516. doi: 10.1016/S0031-9422(98)00547-0. [DOI] [Google Scholar]
- Mericli F. Mericli A. H. Seyhan G. V. Bahar M. Desai H. K. Ozcelik H. Ulubelen A. Pharmazie. 2002;57:761–762. [PubMed] [Google Scholar]
- Bitis L. Suzgec S. Sozer U. Ozcelik H. Zapp J. Kiemer A. K. Mericli F. Mericli A. H. Helv. Chim. Acta. 2007;90:2217–2221. doi: 10.1002/hlca.200790229. [DOI] [Google Scholar]
- Yu D. Q. Das B. C. Planta Med. 1983;49:85–89. doi: 10.1055/s-2007-969821. [DOI] [PubMed] [Google Scholar]
- Xue W. J. Zhao B. Zhao J. Y. Sagdullaev S. S. Akber Aisa H. Phytochem. Lett. 2019;33:12–16. doi: 10.1016/j.phytol.2019.06.009. [DOI] [Google Scholar]
- Shamma M. Chinnasamy P. Miana G. A. Khan A. Bashir M. Salazar M. Beal J. L. J. Nat. Prod. 1979;42:615–623. doi: 10.1021/np50006a006. [DOI] [PubMed] [Google Scholar]
- Wang F. P. Peng C. S. Jian X. X. Chen D. L. J. Asian Nat. Prod. Res. 2001;3:15–22. doi: 10.1080/10286020108042834. [DOI] [PubMed] [Google Scholar]
- Xue W. J. Zhao B. Kodirova D. R. Zhao J. Y. Aisa H. A. Chem. Nat. Comp. 2020;56:771–774. doi: 10.1007/s10600-020-03146-4. [DOI] [Google Scholar]
- Cai L. Chen D. L. Wang F. P. Nat. Prod. Commun. 2006;1:191–194. doi: 10.1177/1934578X0600100303. [DOI] [Google Scholar]
- Tan J. J. Tan C. H. Ruan B. Q. Jiang S. H. Zhu D. Y. J. Asian Nat. Prod. Res. 2006;8:535–539. doi: 10.1080/10286020500175643. [DOI] [PubMed] [Google Scholar]
- Srivastava S. K. Fitoterapia. 1990;61:189. [Google Scholar]
- Jiang S. H. Wang H. Q. Li Y. M. Lin S. J. Tan J. J. Zhu D. Y. Chinese Chem. Lett. 2007;18:409–411. doi: 10.1016/j.cclet.2007.01.031. [DOI] [Google Scholar]
- Chen S. Y. Qiu L. G. Acta Botanica Yunnanica. 1989;11:267–270. [Google Scholar]
- Yin T. P. Cai L. Fang H. X. Fang Y. S. Li Z. J. Ding Z. T. Phytochemistry. 2015;116:314–319. doi: 10.1016/j.phytochem.2015.05.002. [DOI] [PubMed] [Google Scholar]
- Nishanov A. A. Sultankhodzhaev M. N. Yunusov M. S. Kondrat'ev B. G. Chem. Nat. Comp. 1991;27:222–225. doi: 10.1007/BF00629765. [DOI] [Google Scholar]
- Nishanov A. A. Tashkhodzhaev B. Usupova I. M. Sultankhodzhaev M. N. Chem. Nat. Comp. 1992;28:466–469. doi: 10.1007/BF00630652. [DOI] [Google Scholar]
- Feng F. Liu W. Y. Chen Y. S. Ye W. C. Liu J. H. Zhao S. X. J. Chin. Pharm. Univ. 2003;34:17–20. [Google Scholar]
- Mu Z. Q. Gao H. Huang Z. Y. Feng X. L. Yao X. S. Org. Lett. 2012;14:2758–2761. doi: 10.1021/ol3008217. [DOI] [PubMed] [Google Scholar]
- Sultankhodzhaev M. N. Boronova Z. S. Nishanov A. A. Chem. Nat. Comp. 1997;33:700–701. doi: 10.1007/BF02249649. [DOI] [Google Scholar]
- Zhao D. K. Ai H. L. Zi S. H. Zhang L. M. Yang S. C. Guo H. C. Shen Y. Chen Y. P. Chen J. J. Fitoterapia. 2013;91:280–283. doi: 10.1016/j.fitote.2013.09.013. [DOI] [PubMed] [Google Scholar]
- Zhang Q. Tan J. J. Chen X. Q. Gao Z. B. Jia Q. Chen K. X. Li Y. M. Tetrahedron Lett. 2017;58:1717–1720. doi: 10.1016/j.tetlet.2017.03.013. [DOI] [Google Scholar]
- De la Fuente G. Orribo T. Gavin J. A. Acosta R. D. Heterocycles. 1993;36:1455–1458. doi: 10.3987/COM-92-6245. [DOI] [Google Scholar]
- Peng C. S. Chen D. L. Chen Q. H. Wang F. P. Chinese J. Org. Chem. 2005;25:1235–1239. [Google Scholar]
- Jiang G. Y. Qin L. L. Gao F. Huang S. Zhou X. L. Fitoterapia. 2020;141:104477. doi: 10.1016/j.fitote.2020.104477. [DOI] [PubMed] [Google Scholar]
- Shan L. H. Zhang J. F. Chen L. Wang J. X. Huang S. Zhou X. L. Nat. Prod. Commun. 2015;10:2067–2068. doi: 10.1177/1934578X1501001213. [DOI] [PubMed] [Google Scholar]
- Song L. Liang X. X. Chen D. L. Jian X. X. Wang F. P. Chem. Pharm. Bull. 2007;55:918–921. doi: 10.1248/cpb.55.918. [DOI] [PubMed] [Google Scholar]
- Shan L. H. Zhang J. F. Gao F. Huang S. Zhou X. L. J. Asian Nat. Prod. Res. 2018;20:423–430. doi: 10.1080/10286020.2017.1335309. [DOI] [PubMed] [Google Scholar]
- Wang S. Zhou X. L. Gong X. M. Fan X. Y. Lan M. S. J. Asian Nat. Prod. Res. 2016;18:141–146. doi: 10.1080/10286020.2015.1056522. [DOI] [PubMed] [Google Scholar]
- Hohmann J. Forgo P. Hajdú Z. Varga E. Máthé I. J. Nat. Prod. 2002;65:1069–1072. doi: 10.1021/np020026z. [DOI] [PubMed] [Google Scholar]
- Alva A. Grandez M. Madinaveitia A. De la Fuente G. Gavin J. A. Helv. Chim. Acta. 2004;87:2110–2119. doi: 10.1002/hlca.200490190. [DOI] [Google Scholar]
- Jiang Q. P. Sung W. L. Heterocycles. 1985;23:11–15. doi: 10.3987/R-1985-01-0011. [DOI] [Google Scholar]
- Almanza G. Bastida J. Codina C. De la Fuente G. Phytochemistry. 1997;45:1079–1085. doi: 10.1016/S0031-9422(97)00085-X. [DOI] [Google Scholar]
- Kolak U. Ozturk M. Ozgokce F. Ulubelen A. Phytochemistry. 2006;67:2170–2175. doi: 10.1016/j.phytochem.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Usmanova S. K. Bessonova I. A. Abdullaev N. D. Levkovich M. G. Chem. Nat. Comp. 1999;35:91–93. doi: 10.1007/BF02238219. [DOI] [Google Scholar]
- Ablajan N. Zhao B. Zhao J. Y. Wang B. L. Sagdullaev S. S. Aisa H. A. Phytochemistry. 2021;181:112567. doi: 10.1016/j.phytochem.2020.112567. [DOI] [PubMed] [Google Scholar]
- Yin T. P. Shu Y. Mei R. F. Wang J. P. Cai L. Ding Z. T. Biochem. Syst. Ecol. 2018;81:99–101. doi: 10.1016/j.bse.2018.10.004. [DOI] [Google Scholar]
- He Y. Q. Ma Z. Y. Yang Q. Du B. Z. Jing Z. X. Yao B. H. Hamann M. T. Biochem. Syst. Ecol. 2010;38:554–556. doi: 10.1016/j.bse.2010.06.004. [DOI] [Google Scholar]
- Mucher W. Phyton. 1993;33:51–76. [Google Scholar]
- Zhang J. F. Chen L. Huang S. Shan L. H. Gao F. Zhou X. L. J. Nat. Prod. 2017;80:3136–3142. doi: 10.1021/acs.jnatprod.7b00380. [DOI] [PubMed] [Google Scholar]
- Wan L. X. Zhang J. F. Zhen Y. Q. Zhang L. Li X. Gao F. Zhou X. L. J. Nat. Prod. 2021;84:1067–1077. doi: 10.1021/acs.jnatprod.0c01111. [DOI] [PubMed] [Google Scholar]
- Hu Z. X. An Q. Tang H. Y. Chen Z. H. Aisa H. A. Zhang Y. Hao X. J. Phytochemistry. 2019;167:112111. doi: 10.1016/j.phytochem.2019.112111. [DOI] [PubMed] [Google Scholar]
- Shu Y. Yin T. P. Wang J. P. Gan D. Zhang Q. Y. Cai L. Ding Z. T. Chinese J. Nat. Med. 2018;16:866–870. doi: 10.1016/S1875-5364(18)30128-6. [DOI] [PubMed] [Google Scholar]
- Jiang H. Huang S. Gao F. Zhen Y. Li C. Zhou X. Nat. Prod. Res. 2019;33:1741–1746. doi: 10.1080/14786419.2018.1437435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. H. Chen D. H. J. Integr. Plant Biol. 1994;36:148–152. [Google Scholar]
- Fan X. Yang L. Liu Z. Lin L. Li C. Guo S. Wang Z. Wang Z. Sui F. Phytochemistry. 2019;160:71–77. doi: 10.1016/j.phytochem.2018.11.008. [DOI] [PubMed] [Google Scholar]
- Zhang Z. T. Chen D. L. Chen Q. H. Wang F. P. Helv. Chim. Acta. 2013;96:710–718. doi: 10.1002/hlca.201200256. [DOI] [Google Scholar]
- Li H. Y. Yan B. C. Wei L. X. Sun H. D. Puno P. T. Nat. Prod. Bioprospect. 2021;11:459–464. doi: 10.1007/s13659-021-00310-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. F. Li Y. Gao F. Shan L. H. Zhou X. L. J. Asian Nat. Prod. Res. 2019;21:716–724. doi: 10.1080/10286020.2018.1473384. [DOI] [PubMed] [Google Scholar]
- Zhou X. Obaid Arhema Frejat F. Xu W. Shan L. Heterocycles. 2017;94:1903–1908. doi: 10.3987/COM-17-13768. [DOI] [Google Scholar]
- Li Y. F. Zheng Y. M. Yu Y. Gan Y. Gao Z. B. Acta Pharmacol. Sin. 2019;40:451–459. doi: 10.1038/s41401-018-0067-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X. Tang X. C. Acta Pharmacol. Sin. 1989;10:504–507. [Google Scholar]
- Ono M. Satoh T. Jpn. J. Pharmacol. 1992;58:251–257. doi: 10.1016/S0021-5198(19)39736-7. [DOI] [PubMed] [Google Scholar]
- Ameri A. Metzmeier P. Peters T. Br. J. Pharmacol. 1996;118:577. doi: 10.1111/j.1476-5381.1996.tb15440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M. Satoh T. Jpn. J. Pharmacol. 1991;55:523–530. doi: 10.1016/S0021-5198(19)39922-6. [DOI] [PubMed] [Google Scholar]
- Ou S. Zhao Y. D. Xiao Z. Wen H. Z. Cui J. Ruan H. Z. Neurochem. Int. 2011;58:564–573. doi: 10.1016/j.neuint.2011.01.016. [DOI] [PubMed] [Google Scholar]
- Wang J. L. Shen X. L. Chen Q. H. Qi G. Wang W. Wang F. P. Chem. Pharm. Bull. 2009;57:801–807. doi: 10.1248/cpb.57.801. [DOI] [PubMed] [Google Scholar]
- Sun W. X. Dong T. G. Ding C. M. Adv. Mater. Res. 2012;343:1049–1052. [Google Scholar]
- Sun W. Zhang S. Wang H. Wang Y. Med. Chem. Res. 2015;24:3474–3482. doi: 10.1007/s00044-015-1402-0. [DOI] [Google Scholar]
- Dzhakhangirov F. N. Sultankhodzhaev M. N. Tashkhodzhaev B. Salimov B. T. Chem. Nat. Comp. 1997;33:190–202. doi: 10.1007/BF02291540. [DOI] [Google Scholar]
- Heubach J. F. Schüle A. Planta Med. 1998;64:22–26. doi: 10.1055/s-2006-957359. [DOI] [PubMed] [Google Scholar]
- Wright S. N. Mol. Pharmacol. 2001;59:183–192. doi: 10.1124/mol.59.2.183. [DOI] [PubMed] [Google Scholar]
- Vakhitova Iu V. Farafontova E. I. Khisamutdinova R. Iunusov V. M. Cypasheva I. P. Iunusov M. S. Bioorg. Khim. 2013;39:105–116. doi: 10.1134/s1068162013010111. [DOI] [PubMed] [Google Scholar]
- Yunusov M. S. Russ. Chem. Bull. 2011;60:633–638. doi: 10.1007/s11172-011-0098-7. [DOI] [Google Scholar]
- Akhiyarov A. A. Lobov A. N. Ivanov S. P. Spirikhin L. V. Gabbasov T. M. Tsyrlina E. M. Yunusov M. S. Russ. Chem. Bull. 2020;69:567–571. doi: 10.1007/s11172-020-2800-0. [DOI] [Google Scholar]
- Yin T. P. Hu X. F. Mei R. F. Shu Y. Gan D. Cai L. Ding Z. T. Phytochem. Lett. 2018;25:152–155. doi: 10.1016/j.phytol.2018.04.001. [DOI] [Google Scholar]
- Zhang J. Li Y. Z. Cui Y. W. Jia P. Yue Z. G. Song B. Song X. M. Rec. Nat. Prod. 2018;13:114–120. doi: 10.25135/rnp.89.18.05.296. [DOI] [Google Scholar]
- Chen L. Zhou X. Qin L. Xing F. Heterocycles. 2021;102:1330–1336. doi: 10.3987/COM-21-14460. [DOI] [Google Scholar]
- Liu M. P. Ju Y. Dang Y. L. Pharmacol. Clin. Chinese Mate. Med. 2004;20:13–14. [Google Scholar]
- Hu J. M. Xiao L. Y. Pan J. Q. Li J. J. Chinese Med. Mater. 2009;32:420–422. [Google Scholar]
- Li Y. Zeng J. Tian Y. H. Hou Y. Da H. Fang J. Gao K. Phytochemistry. 2021;190:112880. doi: 10.1016/j.phytochem.2021.112880. [DOI] [PubMed] [Google Scholar]
- Lin N. Xiao L. Y. Lin P. Y. Zhang D. Chen Q. W. TCM Res. 2005;18:16–18. [Google Scholar]
- Hu J. M. Chen F. Y. China Pharm. 2008;19:2343–2344. [Google Scholar]
- Sheng L. H. Xu M. Xu L. Q. Xiong F. J. Chin. Med. Mater. 2014;37:840–843. [PubMed] [Google Scholar]
- Ma J. Y. Chen X. l. Hou C. J. Zhu J. Z. Han X. F. Zhang J. Guo H. Y. Chin. Pharm. J. 2017;52:1038–1043. [Google Scholar]
- Zhang X. Ma J. Song N. Guo Y. Hui L. Sang C. Pharmacology. 2020;105:705–714. doi: 10.1159/000506081. [DOI] [PubMed] [Google Scholar]
- Qu D. N. Zhang X. M. Sang C. Y. Zhou Y. Q. Ma J. Y. Hui L. Med. Chem. Res. 2019;28:907–916. doi: 10.1007/s00044-019-02346-0. [DOI] [Google Scholar]
- Qu D. Ma J. Song N. Hui L. Yang L. Guo Y. Sang C. Acta Histochem. 2020;122:151557. doi: 10.1016/j.acthis.2020.151557. [DOI] [PubMed] [Google Scholar]
- Hui L. Ma J. Song N. Zhang X. Qu D. Sang C. Li H. Pharmacogn. Mag. 2021;17:334–341. doi: 10.4103/pm.pm_251_20. [DOI] [Google Scholar]
- Song N. Ma J. Hu W. Guo Y. Hui L. Aamer M. Ma J. Acta Histochem. 2021;123:151736. doi: 10.1016/j.acthis.2021.151736. [DOI] [PubMed] [Google Scholar]
- Ulubelen A. Mericli A. H. Mericli F. Kilincer N. Ferizli A. G. Emekci M. Pelletier S. W. Phytother. Res. 2001;15:170–171. doi: 10.1002/ptr.688. [DOI] [PubMed] [Google Scholar]
- Reina M. González-Coloma A. Phytochem. Rev. 2007;6:81–95. doi: 10.1007/s11101-006-9013-5. [DOI] [Google Scholar]
- Chen L. Wang Q. Huang S. Shan L. H. Gao F. Zhou X. L. Chinese J. Org. Chem. 2017;37:1839–1843. doi: 10.6023/cjoc201702021. [DOI] [Google Scholar]