Abstract
The antibacterial activities of imipenem-cilastatin, meropenem-cilastatin, cefepime and ceftazidime against Enterobacter cloacae NOR-1, which produces the carbapenem-hydrolyzing β-lactamase NmcA and a cephalosporinase, and against one of its in vitro-obtained ceftazidime-resistant mutant were compared by using an experimental model of pneumonia with immunocompetent rats. The MICs of the β-lactams with an inoculum of 5 log10 CFU/ml were as follows for E. cloacae NOR-1 and its ceftazidime-resistant mutant, respectively: imipenem, 16 and 128 μg/ml, meropenem, 4 and 32 μg/ml, cefepime, <0.03 and 1 μg/ml, and ceftazidime, 1 and 512 μg/ml. The chromosomally located cephalosporinase and carbapenem-hydrolyzing β-lactamase NmcA were inducible by cefoxitin and meropenem in E. cloacae NOR-1, and both were stably overproduced in the ceftazidime-resistant mutant. Renal impairment was induced (uranyl nitrate, 1 mg/kg of body weight) in rats to simulate the human pharmacokinetic parameters for the β-lactams studied. Animals were intratracheally inoculated with 8.5 log10 CFU of E. cloacae, and therapy was initiated 3 h later. At that time, animal lungs showed bilateral pneumonia containing more than 6 log10 CFU of E. cloacae per g of tissue. Despite the relative low MIC of meropenem for E. cloacae NOR-1, the carbapenem-treated rats had no decrease in bacterial counts in their lungs 60 h after therapy onset compared to the counts for the controls, regardless of whether E. cloacae NOR-1 or its ceftazidime-resistant mutant was inoculated. A significant decrease in bacterial titers was observed for the ceftazidime-treated rats infected with E. cloacae NOR-1 only. Cefepime was the only β-lactam tested effective as treatment against infections due to E. cloacae NOR-1 or its ceftazidime-resistant mutant.
Although the carbapenems imipenem and meropenem have the broadest antimicrobial activity among the β-lactams, acquired resistance to these antibiotics has been reported in gram-negative rods (17, 28, 33). Mechanisms of resistance to carbapenems in members of the family Enterobacteriacae include modified penicillin-binding protein affinity, decreases in the levels of uptake of these β-lactams, or overproduction of naturally occurring β-lactamases (mostly cephalosporinases) with low levels of hydrolytic activity against carbapenems combined with a decrease in outer membrane permeability (3). However, carbapenem-hydrolyzing β-lactamases that hydrolyze several β-lactam classes including carbapenems have been reported recently in several strains of the family Enterobacteriacae (17). Since carbapenems are used more frequently, a larger number of these enzymes may be selected in vivo, as has already been observed (23). Thus, the impacts of such enzymes on the efficacy of β-lactam therapy may be of critical importance.
Enterobacter spp. are now among the five most common nosocomial pathogens isolated from patients in U.S. and European hospitals and account for about 10% of lower respiratory tract infections in intensive care units (31, 35). Since Enterobacter sp. strains are intrinsically resistant to aminopenicillins and narrow-spectrum cephalosporins due to their chromosomally encoded inducible cephalosporinase, they may acquire resistance to extended-spectrum cephalosporins during therapy by selecting mutants that constitutively overproduce cephalosporinases (5). These resistant strains, often referred to as “stably derepressed mutants,” produce enough β-lactamase to inactivate all currently available β-lactams except carbapenems and cefepime (14, 18, 30). Carbapenem-hydrolyzing β-lactamases have been detected in several enterobacterial species in Japan, Europe, and the United States, including Enterobacter cloacae and Serratia marcescens (19, 21, 30). The metalloenzyme IMP-1, which has a broad-spectrum hydrolytic substrate profile that includes extended-spectrum cephalosporinases and carbapenems, has been reported to be epidemic among Japanese isolates (1, 24). The IMP-1 gene is located on plasmids and integrons (1). An IMP-1-like producing strain has very recently been described in Italy, indicating that an IMP-1-like β-lactamase has reached Europe (6). Among the penicillinase group (Bush functional group 2f [4]), the carbapenem-hydrolyzing β-lactamases NmcA, IMI-1, and Sme-1 have been reported from several E. cloacae and S. marcescens isolates (19, 21, 23, 29). These enzymes significantly hydrolyze imipenem, hydrolyze meropenem less so, and do not hydrolyze extended-spectrum cephalosporins. Their activities are partially inhibited by clavulanic acid. Their genes are chromosomally located and are regulated by a regulatory Lys-R type protein, the gene for which is located immediately upstream of the β-lactamase gene. These divergently expressed β-lactamase and regulatory protein genes have common promoter regions, as found for the cephalosporinase ampC gene of E. cloacae, which is also regulated at least by an Lys-R type protein, AmpR (11). Both carbapenem-hydrolyzing β-lactamases and cephalosporinases are inducible upon the addition of strong inducers such as carbapenems or cefoxitin (28).
Taking into account the similar hydrolytic properties of the carbapenem-hydrolyzing β-lactamases of the penicillinase group, the aim of the present study was to compare the bactericidal efficacies in vivo of human regimens of imipenem, meropenem, cefepime, or ceftazidime against the NmcA-producing E. cloacae NOR-1 and one of its in vitro-obtained ceftazidime-resistant mutants by using a model of pneumonia in nonneutropenic rats developed previously (18).
MATERIALS AND METHODS
Organisms tested.
The infecting organisms were either E. cloacae NOR-1 or its in vitro-obtained ceftazidime-resistant mutant. The original strain was isolated from a patient hospitalized in France and treated intravenously with 500 mg of imipenem; this strain produced an identified carbapenem-hydrolyzing β-lactamase, NmcA (23). An isogenic ceftazidime-resistant mutant was obtained by plating 9 log10 CFU of E. cloacae NOR-1 onto a Trypticase soy (TS) agar plate containing 32 μg of ceftazidime per ml. Resistant strains were obtained at a frequency of 5 × 10−7. One of them was retained for further analysis. The stability of the ceftazidime resistance phenotype of the mutant was checked by plating the strain onto either antibiotic-free or ceftazidime-containing plates. The same number of bacteria were obtained. To ensure pathogenicity, E. cloacae NOR-1 and its ceftazidime-resistant mutant were submitted to two subsequent passages in mice inoculated intraperitoneally and infected for 24 h. Then, the strains were stored at −70°C in Mueller-Hinton broth (bioMérieux, Marcy-l'Etoile, France) supplemented with 10% glycerol. Fresh inocula were prepared for each experiment from cultures grown for 24 h in 10 ml of TS broth (bioMérieux) and were then rinsed twice and suspended in normal saline prior to their use.
Antimicrobial agents.
Imipenem-cilastatin, cilastatin, and cefoxitin were from Merck Sharp & Dohme-Chibret (Paris, France), meropenem was from Zeneca Pharma (Cergy, France), cefepime was from Bristol-Myers Squibb (Paris, France), ceftazidime was from GlaxoWellcome (Evreux, France), and cephalothin was from Roche (Neuilly-sur-Seine, France). Antibiotic powders were freshly diluted with saline before each experiment with animals, according to the manufacturers' instructions.
Susceptibility testing.
MICs were determined in duplicate in Mueller-Hinton broth (bioMérieux) by means of a tube macrodilution method with geometric twofold serial dilutions and inocula of 5, 6, and 7 log10 CFU/ml. All plates were incubated at 37°C for 18 h prior to determination of the MICs of imipenem, meropenem, cefepime, and ceftazidime (22).
β-Lactamase assays.
β-Lactamase activities were determined in triplicate with or without cefoxitin (10 μg/ml) or meropenem (0.25 μg/ml) as the inducer. Overnight cultures of E. cloacae NOR-1 or its ceftazidime-resistant mutant were diluted 1:10 into 10 ml of TS broth. Then, the cultures were grown for an additional 2 h with or without inducer. Bacterial suspensions were centrifuged four times at 1,000 × g for 15 min each time. The pellets were suspended in 0.5 ml of phosphate buffer (pH 7.0) and were disrupted by sonication (twice for 30 s each time at 20 Hz) and centrifuged (30 min, 48,000 × g, 4°C). The supernatants containing the enzyme extracts were subjected to β-lactamase activity assays by UV spectrophotometry (Ultraspec 2000 spectrophotometer; Amersham Pharmacia Biotech, Orsay, France) at 30°C in 100 mM phosphate buffer (pH 7.0) with 100 μM cephalothin or 100 μM imipenem as the substrate (27). β-Lactamase activity was expressed as units of specific activity. One unit of specific activity was defined as the amount of enzyme that hydrolyzed 1 μmol of cephalothin or 1 μmol of imipenem per min per g of protein. The total protein content was determined by using a Bio-Rad assay kit (Bio-Rad, Ivry-sur-Seine, France) with bovine albumin as the standard.
Pharmacokinetic-pharmacodynamic studies.
Since rats eliminate antibiotics much more rapidly than humans, preliminary drug-dosing studies were run with noninfected rats to determine if the subcutaneous dose of 1 mg of uranyl nitrate (Merck, Darmstadt, Germany) per kg of body weight used previously (18) was optimal for impairing the renal function of the rats so as to simulate the pharmacokinetics of imipenem-cilastatin, meropenem, cefepime, and ceftazidime in healthy humans. Briefly, 4 days after the uranyl nitrate injection, each rat received a single 1-ml intraperitoneal injection of each antibiotic studied. Cilastatin (1:1) was given together with meropenem because rats produce in their lungs a dehydropeptidase that is able to hydrolyze meropenem (34). Ten blood samples (300 μl each) were collected via a catheter in the femoral vein during the 8 h following antibiotic administration and were placed into heparin-containing tubes (Microvacutainer system; Becton Dickinson, Rutherford, N.J.). Immediately after collection, each blood sample was gently reversed a few times to ensure complete mixing with the anticoagulant and was centrifuged at 1,000 × g for 10 min at 4°C to separate the plasma. Plasma samples were stored at −70°C and were assayed within 7 days. Saline (600 μl) was injected intra-arterially (via the catheter) after each blood sampling to restore the blood volume. Individual antibiotic pharmacokinetic parameters were determined by using a noncompartmental model (Siphar software package; Simed, Créteil, France).
The potential binding of the β-lactams studied to the plasma proteins of rats was assessed by exposing several concentrations of drugs to plasma. To obtain conditions comparable to those observed in our animals, pooled plasma obtained from renally impaired rats was used; antibiotic solutions were added to obtain final concentrations that corresponded to peak and middle-interval antibiotic concentrations observed in animals. A final antibiotic concentration of 4 μg/ml, corresponding to the French cutoff for determination of susceptibility to each β-lactam studied, was also obtained. The free antibiotic fractions in these preparations were determined in triplicate. Total and free antibiotic levels were determined after equilibration of the drug in plasma for 1 h at 37°C. The free drug concentrations were determined by ultrafiltration, using the Microsep 3 K Micropartition System (Filtron Technology Corporation, PolyLabo, Strasbourg, France).
Imipenem, meropenem, cefepime, and ceftazidime concentrations in rat plasma and ultrafiltrate were determined by a modified version of the high-pressure liquid chromatography assays described elsewhere (2, 8, 9, 12). The lower detection limits of the assays were 0.5, 0.5, 1, and 5 μg/ml for imipenem, meropenem, cefepime, and ceftazidime, respectively.
For each noninfected rat with uranyl nitrate-induced renal impairment, we determined the time that the free antibiotic concentration in plasma exceeded the MIC (T>MIC) for each strain, using MICs obtained with inoculum sizes of 5, 6, and 7 log10 CFU/ml.
Pneumonia model.
The animal model used was adapted from one previously developed in our laboratory (18). Briefly, male Wistar rats (weight, 280 to 300 g) were rendered renally insufficient by subcutaneous administration of 1 mg of uranyl nitrate per kg and were intraperitonally anesthetized 93 h later with phenobarbital (60 mg/kg), and each rat trachea was exposed by a vertical midline incision. A total of 0.5 ml of a bacterial suspension containing 8.5 log10 CFU of E. cloacae was injected intratracheally with a syringe with a 25-gauge needle. Following inoculation, the animals were gently shaken for 15 s to equally distribute the bacterial inoculum in the lungs. Previous studies had shown that 3 h after bacterial inoculation, all animals develop bilateral pneumonia with bacterial densities of >6 log10 CFU/g of tissue in both lungs and an intense inflammatory reaction.
Treatment regimens.
Each strain used to induce pneumonia was studied separately. Among the 200 animals included in this study, 92 and 83 of them infected with E. cloacae NOR-1 or its ceftazidime-resistant mutant, respectively, were still alive 3 h after bacterial inoculation. At this time, 10 rats from each study group were killed to document that pneumonia had been established. The remaining rats were randomly assigned to one control group (i.e., rats not treated with antibiotic) and four treatment groups. Treatment groups received intraperitoneal injections of imipenem-cilastatin (30 mg/kg/8 h each), meropenem-cilastatin (30 mg/kg/8 h each), cefepime (60 mg/kg/12 h), or ceftazidime (60 mg/kg/8 h). These dosages were retained to achieve concentrations in serum close to those observed in humans. Therapy began 3 h after bacterial inoculation and was continued for 2.5 days.
Evaluation of antibiotic treatments.
At 2.5 days, animals were killed approximately 5 to 7 h after administration of the last antibiotic dose. Blood was obtained by aortic puncture and placed in a heparin-containing tube, the tube was centrifuged, and the plasma was stored in two aliquots at −70°C for determination of antibiotic concentrations and creatinine levels. The imipenem-containing plasma was immediately mixed after sampling (1:1) with a stabilizing buffer containing equal volumes of 1 M morpholinoethane sulfonate and ethylene glycol before freezing. Creatinine levels in plasma were determined to document that renal impairment was established (18). The lungs were aseptically removed, gently blotted with sterile absorbent paper to remove blood, weighed, placed in 25 ml of ice-cold saline, and homogenized with an homogenizer (Ultraturax, Staufen, Germany). The homogenate was quantitatively cultured after serial dilution (up to 5 × 10−4) on Drigalski agar (bioMérieux) with a Spiral Système plater (Interscience, Saint-Nom-La-Bretèche, France). After overnight incubation at 37°C, the viable bacteria were counted and the counts were expressed as log10 CFU per gram of lungs. When no bacterial growth was noted, the value of the detection limit for the specific animal was entered for statistical analysis.
Statistical analysis.
Results are expressed as medians and their ranges. Bacterial counts in the lungs of the control and treatment groups were compared by one-way nonparametric analysis of variance (Kruskal-Wallis test); when the value of this test was statistically significant, the value for each treatment group was compared to those for the control group and each of the other treatment groups by using the Mann-Whitney U test. For all tests, a P value of <0.05 was considered significant.
RESULTS
Susceptibility testing.
The susceptibilities of E. cloacae NOR-1 and its ceftazidime-resistant mutant are given in Table 1 for various initial bacterial concentrations. E. cloacae NOR-1 was susceptible to cefepime and ceftazidime, was moderately susceptible to meropenem, and was resistant to imipenem. The ceftazidime-resistant mutant remained susceptible to cefepime but was resistant to imipenem, meropenem, and ceftazidime. As expected, an inoculum effect proportional to the bacterial titer was observed with the four β-lactams tested against the two strains but was less pronounced with cefepime against E. cloacae NOR-1.
TABLE 1.
In vitro susceptibilities of an E. cloacae NOR-1 strain and its in vitro-obtained ceftazidime-resistant mutant to the β-lactams studied for various inoculum sizes
| β-Lactam agent | MIC (μg/ml) with the indicated inoculum size (log10 CFU/ml):
|
|||||||
|---|---|---|---|---|---|---|---|---|
|
E. cloacae NOR-1
|
E. cloacae NOR-1 mutant
|
|||||||
| 4 | 5 | 6 | 7 | 4 | 5 | 6 | 7 | |
| Imipenem | 8 | 16 | 512 | >512 | 8 | 128 | >512 | >512 |
| Meropenem | 2 | 4 | 32 | 128 | 8 | 32 | 128 | 512 |
| Cefepime | <0.03 | <0.03 | <0.03 | 4 | 1 | 1 | 16 | 128 |
| Ceftazidime | 0.03 | 1 | 2 | 128 | 256 | 512 | >512 | >512 |
β-Lactamase biosynthesis.
In all cultures, when β-lactamase activity against imipenem was measured, only carbapenem-hydrolyzing β-lactamase activity was indicated since the E. cloacae cephalosporinase did not hydrolyze imipenem. However, when β-lactamase activity was measured with cephalothin as the substrate, activity resulted from the activities of both the cephalosporinase and the carbapenem-hydrolyzing β-lactamase, since E. cloacae NOR-1 produces both enzymes. The β-lactamase activity of the ceftazidime-resistant E. cloacae NOR-1 mutant was 250-fold higher than that determined with the original strain when imipenem was used as the substrate, indicating an overproduction of the carbapenem-hydrolyzing β-lactamase (Table 2). Since the carbapenem-hydrolyzing β-lactamase does not significantly increase the ceftazidime MIC, even when its gene is located on a multicopy recombinant plasmid (23), its overproduction in ceftazidime-resistant E. cloacae NOR-1 could not account for the observed resistance to ceftazidime. As expected, the β-lactamase activity of the ceftazidime-resistant E. cloacae NOR-1 mutant was about 1,000-fold higher than that determined for E. cloacae NOR-1 with cephalothin as the substrate, indicating an overproduction of the cephalosporinase as the molecular mechanism for the acquired resistance to ceftazidime.
TABLE 2.
β-Lactamase activities of an E. cloacae NOR-1 strain and its in vitro-obtained ceftazidime-resistant mutant with or without inducers
| Strain and growth condition | β-Lactamase activitya
|
|
|---|---|---|
| Imipenem substrate | Cephalothin substrate | |
| E. cloacae NOR-1 | 5 | 25 |
| E. cloacae NOR-1 mutant | 1,250 | 20,850 |
| E. cloacae NOR-1 + cefoxitinb | 30 | 800 |
| E. cloacae NOR-1 + meropenemb | 33 | 780 |
| E. cloacae NOR-1 mutant + cefoxitin | 1,370 | 20,880 |
| E. cloacae NOR-1 mutant + meropenem | 1,280 | 19,750 |
β-Lactamase activity is expressed as units of specific activity. One unit of specific activity was defined as the enzymatic activity which hydrolyzed 1 μmol of cephalothin or imipenem per min per g of protein. Results are geometric means of three independent measures. Standard deviations were within 10% of the geometric mean in all cases.
The final concentrations added to the cultures were 10 and 0.25 μg/ml for cefoxitin and meropenem, respectively.
Carbapenem-hydrolyzing β-lactamase activity in E. cloacae NOR-1 was similarly induced sixfold by both cefoxitin and meropenem (Table 2). The β-lactamase activity of E. cloacae NOR-1 was also induced similarly by cefoxitin and meropenem when cephalothin was used as the substrate and was induced to a greater extent (32-fold) when cephalothin was used as the substrate than when imipenem was used as the substrate. These results show that although when cephalothin is used as the substrate the β-lactamase activity resulted from both the carbapenem-hydrolyzing β-lactamase and cephalosporinase activities, the later was clearly inducible in E. cloacae NOR-1, as expected for an E. cloacae cephalosporinase. Finally, β-lactamase activities against cephalothin and imipenem in ceftazidime-resistant E. cloacae NOR-1 were no longer inducible by cefoxitin or meropenem. This indicated that both the cephalosporinase and the carbapenem-hydrolyzing β-lactamase activities were overproduced (or stably derepressed) in the E. cloacae NOR-1 mutant.
Pharmacokinetic-pharmacodynamic analyses.
The values of the pharmacokinetic parameters for each antibiotic given to renally insufficient rats were similar to those observed when a 1-g imipenem or meropenem dose or a 2-g cefepime or ceftazidime dose is given intravenously to healthy humans (Table 3). In particular, the level of binding of each β-lactam to the plasma proteins of rats was relatively low and was linear over the range of concentrations tested; we secondarily calculated the free concentrations of each antibiotic given to renally impaired rats as the product of multiplying its free fraction by the concentration of total antibiotic. The percentages of the dosing interval that the free drug concentrations exceeded the MICs for the two strains, by using the MIC obtained for various inoculum sizes, are given in Table 4.
TABLE 3.
Pharmacokinetics for antibiotics given intraperitonally to noninfected rats with uranyl nitrate-induced renal impairment
| β-Lactam agent | No. of animals | Dose (mg/kg) | Median (range) antibiotic level (μg/ml) in plasma
|
Protein binding (%)a | Half-life (h)a | Area under curve (μg · h/ml)a | |
|---|---|---|---|---|---|---|---|
| Peak (30 min after dosing) | Trough (8 h after dosing) | ||||||
| Imipenem | 5 | 30 | 83 (66–104) | <0.5 (<0.5–1) | 37 (30–53) | 1.0 (0.7–1.3) | 112 (95–145) |
| Meropenem | 6 | 30 | 76 (61–96) | <0.5 (<0.5–<0.5) | 48 (37–57) | 0.9 (0.6–1.4) | 102 (94–136) |
| Cefepime | 6 | 60 | 160 (125–220) | 12 (6–28) | 17 (12–24) | 2.0 (1.4–2.8) | 422 (188–615) |
| Ceftazidime | 4 | 60 | 197 (139–214) | 15 (5–24) | 20 (9–22) | 2.2 (1.9–2.6) | 346 (266–382) |
Median, with range in parentheses.
TABLE 4.
Fraction of dosing interval that free antibiotic concentrations in plasma exceeded MIC for E. cloacae NOR-1 or its mutant
| β-Lactam agent | % of dosing intervala
|
|||||
|---|---|---|---|---|---|---|
|
E. cloacae NOR-1 at inoculum size (log10 CFU/ml) of:
|
E. cloacae NOR-1 mutant at inoculum size (log10 CFU/ml) of:
|
|||||
| 5 | 6 | 7 | 5 | 6 | 7 | |
| Imipenem | 23 (7–29) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| Meropenem | 31 (23–39) | 12 (9–16) | 0 (0–0) | 12 (9–15) | 0 (0–0) | 0 (0–0) |
| Cefepime | 100 (100–100) | 100 (100–100) | 80 (69–100) | 100 (96–100) | 56 (46–61) | 12 (0–17) |
| Ceftazidime | 100 (100–100) | 100 (100–100) | 19 (13–22) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
Median, with range in parentheses.
Efficacy of therapy.
The 10 animals from each study group killed at the start of therapy presented with bilateral pneumonia, with median counts of 7.0 log10 CFU/g of lung (range, 6.1 to 7.6 log10 CFU/g of lung) and 6.3 log10 CFU/g of lung (range, 6.0 to 7.2 CFU/g of lung) for E. cloacae NOR-1 and its ceftazidime-resistant mutant, respectively. Twenty-one of 155 infected animals died during the antibiotic treatment period; these animals received no antibiotic (n = 3), imipenem-cilastatin (n = 4), meropenem-cilastatin (n = 6), cefepime (n = 4), or ceftazidime (n = 4).
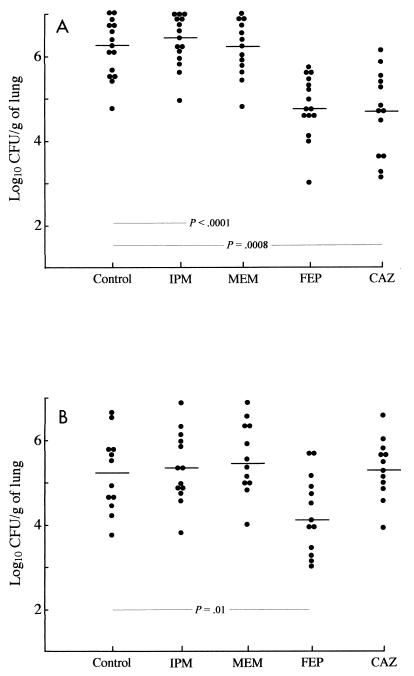
At the time of killing (i.e., 60 h after starting therapy and 5 to 7 h after administration of the last antibiotic dose), creatinine levels in plasma were not statistically different between the study groups, indicating that renal impairment was identical regardless of the treatment received (Table 5). At that time, β-lactam concentrations in plasma did not differ significantly when β-lactams were administered to animals infected with either E. cloacae NOR-1 or its ceftazidime-resistant mutant and were then pooled to simplify the presentation. As indicated in Table 5, these β-lactam levels were broadly similar to those usually reported in human plasma. At the end of the period of study (2.5 days), the bacterial counts in untreated animals were 6.2 log10 CFU/g of lung (range, 4.9 to 7.0 log10 CFU/g of lung) and 5.2 log10 CFU/g of lung (range, 3.9 to 6.6 CFU/g of lung) for E. cloacae NOR-1 and its ceftazidime-resistant mutant, respectively. At 60 h after the onset of therapy the carbapenem-treated rats had bacterial counts in their lungs similar to those in the lungs of untreated animals, regardless of whether E. cloacae NOR-1 or its ceftazidime-resistant mutant was inoculated (Fig. 1). Ceftazidime treatment led to a significant decrease in the bacterial titers in the lungs only in rats inoculated with E. cloacae NOR-1, while cefepime decreased significantly the bacterial titers in the lungs of rats inoculated with E. cloacae NOR-1 or its ceftazidime-resistant mutant.
TABLE 5.
Creatinine and antibiotic levels in rat plasma observed at the time of killinga
| β-Lactam agent | No. of animals | Creatinine level in plasma (μmol/liter)b | Plasma antibiotic concn (μg/ml)b |
|---|---|---|---|
| None | 27 | 217 (83–469) | |
| Imipenem | 28 | 218 (74–489) | 1 (<0.5–10) |
| Meropenem | 25 | 193 (81–477) | 1 (<0.5–8) |
| Cefepime | 28 | 235 (84–517) | 15 (6–107) |
| Ceftazidime | 26 | 211 (98–451) | 28 (3–121) |
The rats were killed 60 h after the initiation of therapy.
Median, with range in parentheses.
FIG. 1.
Number of CFU of E. cloacae NOR-1 (A) and its ceftazidime-resistant mutant (B) per gram of lung in rats treated with either imipenem (IPM), meropenem (MEM), cefepime (FEP), or ceftazidime (CAZ). Each symbol represents a single animal. The horizontal bar indicates the median for each group. Statistical differences between groups are indicated for each strain.
DISCUSSION
The aim of our work was to study the therapeutic potential of β-lactam antibiotics for the treatment of infections due to an E. cloacae strain that produces a cephalosporinase and a carbapenem-hydrolyzing β-lactamase. The initial mortality rate was relatively low (25 of 200 animals) and was mainly the result of trauma from the operation or overwhelming sepsis. Since the mortality rate during the treatment period was near zero and the rate of spontaneous clearance of bacteria was low (∼1 log10 CFU of E. cloacae/g of tissue), the level of clearance of bacteria from the lungs was used to compare treatment groups.
As expected, E. cloacae NOR-1 produces basal levels of cephalosporinase. Since ceftazidime is a weak cephalosporinase inducer, it was logical that it was active for the treatment of rats infected with E. cloacae NOR-1. In the case of infection with the stably derepressed E. cloacae NOR-1 mutant, only cefepime was active since ceftazidime is hydrolyzed by overproduction of the chromosomally mediated cephalosporinase. On the contrary, cefepime retained good activity against infection due to the ceftazidime-resistant mutant. It is known that cefepime is active against such strains because of a combination of factors, including faster penetration through the outer membranes of gram-negative bacteria and a low affinity for enterobacterial cephalosporinases (13, 14, 26). The cefepime activity in the present study agrees with the results obtained in studies of infections due to a ceftazidime-resistant E. cloacae type strain (18, 25). Moreover, the results obtained with experimental models in the present study were recently supported by a clinical study in which 15 of 17 infections due to ceftazidime-resistant and cefepime-susceptible Enterobacter sp. strains were successfully treated with cefepime. In particular, cefepime was successful as treatment for chronic infections that had responded poorly to repeated therapy (30).
Since the carbapenem-hydrolyzing β-lactamase NmcA does not hydrolyze ceftazidime or cefepime, even when it is produced at a high level, it was logical that NmcA, whatever its in vivo level, did not play any role in the results obtained for cefepime and ceftazidime when they were used as treatments for infections due to E. cloacae NOR-1 or its ceftazidime-resistant mutant. Our results indicated that imipenem and meropenem are equally ineffective for the treatment of infections due to E. cloacae NOR-1. Although these results could have been predicted by the high MIC of imipenem, they are more surprising for meropenem, which has a relatively low MIC. At least two hypotheses may explain the inefficacy of meropenem. An inoculum effect may provide large amounts of the carbapenem-hydrolyzing β-lactamase NmcA in the lungs of animals and may lead to in vivo resistance to meropenem, as indicated by our in vitro studies. Interestingly, meropenem (as imipenem [23]) or cefoxitin significantly induced the cephalosporinase activity and the carbapenem-hydrolyzing β-lactamase activity, both of which are found in E. cloacae NOR-1. The cephalosporinase induction by carbapenems is not of clinical relevance since carbapenems are not hydrolyzed significantly by enterobacterial cephalosporinases. On the contrary, the induction of the carbapenem-hydrolyzing β-lactamase NmcA of E. cloacae NOR-1 by meropenem may also explain the in vivo inefficacy of meropenem. In this regard, it has recently been shown that clavulanate, a potent inducer of cephalosporinase from Pseudomonas aeruginosa, may antagonize the antibacterial activity of ticarcillin in a ticarcillin-clavulanate combination even when MICs of ticarcillin-clavulanate are below the breakpoint for resistance (16). The inefficacy of meropenem for the treatment of E. cloacae NOR-1 infection was not due to meropenem hydrolysis by rat lung dehydropeptidase since cilastatin addition permitted the retrieval of levels in plasma close to those obtained with the regimens used for humans.
Interestingly, and for reasons that are not yet known, the ceftazidime-resistant E. cloacae NOR-1 mutant produced not only high levels of cephalosporinase but also high levels of the carbapenem-hydrolyzing β-lactamase NmcA, which increased significantly the MICs of imipenem and meropenem. These high levels of both β-lactamases were no longer inducible. This result implies that the ceftazidime-resistant E. cloacae NOR-1 mutant is a stably derepressed mutant not only for cephalosporinase biosynthesis but also for NmcA biosynthesis. Therefore, meropenem and imipenem were not active as treatments for infections due to the ceftazidime-resistant E. cloacae NOR-1 mutant.
According to the current model for cephalosporinase regulation (11), it may be hypothesized that a mutated ampD gene in the ceftazidime-resistant E. cloacae NOR-1 mutant produced an inactive AmpD protein, thus explaining the high levels of both cephalosporinase and the carbapenem-hydrolyzing β-lactamase (15). AmpD is an amidase that cleaves peptidoglycan precursors, thus preventing their accumulation in the cytoplasm (10). In cases of an inactive AmpD, these precursors displace AmpR from its repressor binding site in the ampR-ampC promoter regions, thus explaining the stable overproduction of cephalosporinase. In this regard, it should be remembered that the cephalosporinase and the carbapenem-hydrolyzing β-lactamase NmcA are at least regulated by the structurally related LysR-type proteins, AmpR and NmcR, respectively.
For β-lactam antibiotics, T>MIC is the better pharmacokinetic parameter for influencing the outcome of infection (7). Maximal killing is approached when concentrations are one to four times the MIC 60 to 70% of the time, provided that the levels of unbound drug are used to assess the efficacy of highly protein-bound drugs. However, since the efficacies of β-lactams are affected by the inoculum size (7), T>MIC correlates better with drug efficacy when the MIC is determined with the corresponding inoculum size, as observed in our study.
In conclusion, cefepime, which is more stable than narrow-spectrum cephalosporins against the activities of the cephalosporinases and the carbapenem-hydrolyzing β-lactamase NmcA of E. cloacae NOR-1, even in cases of overproduction, was the best β-lactam for the treatment of experimental infections due to such isolates. It may also decrease in vivo the likelihood of selection of carbapenem-hydrolyzing β-lactamase overproducers, as is known for the selection of cephalosporinase overproducers. Since the other carbapenem-hydrolyzing β-lactamases of the penicillinase group, Sme-1 and IMI-1, have similar hydrolytic properties and are regulated similarly to NmcA (28), it is likely that cefepime may cure infections due to Sme-1 or IMI-1-producing strains. On the contrary, the efficacy of cefepime for the treatment of infections due to enterobacteria that produce carbapenem-hydrolyzing β-lactamases of other types such as the metalloenzyme IMP-1 cannot be deduced from our experimental data. Actually, IMP-1 has a much larger β-lactam substrate profile than NmcA. Finally, further work shall be directed toward assessment of the efficacy of combined antibiotic therapy, including therapy with aminoglycosides or fluoroquinolones, which are often used for the treatment of pneumonia due to Enterobacter sp. strains.
ACKNOWLEDGMENTS
This work was funded by the Ministère de l'Education Nationale et de la Recherche (UPRES, JE 2227) and a grant-in-aid from Merck Sharp & Dohme Chibret, Paris, France.
We are in debt to Nadia Hidri for help in preliminary experiments.
REFERENCES
- 1.Arakawa Y, Murakami M, Suzyki K, Ito H, Wacharotayankun R, Ohsuka S, Kato N, Ohta M. A novel integron-like element carrying the metallo-beta-lactamase gene blaIMP. Antimicrob Agents Chemother. 1995;39:1612–1615. doi: 10.1128/aac.39.7.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbhaiya R H, Forgue S T, Shyu W C, Papp E A, Pittman K A. High-pressure liquid chromatographic analysis of BMY 28142 in plasma and urine. Antimicrob Agents Chemother. 1987;31:55–59. doi: 10.1128/aac.31.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellido F, Péchère J C, Hancock R E W. Reevaluation of the factors involved in the efficacy of new β-lactams against Enterobacter clocae. Antimicrob Agents Chemother. 1991;35:73–78. doi: 10.1128/aac.35.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bush K, Jacoby G A, Medeiros A A. A functional classification scheme for beta-lactamases and its correlation with molecular structure. Antimicrob Agents Chemother. 1995;39:1211–1233. doi: 10.1128/aac.39.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chow J W, Fine M J, Shlaes D M, Quinn J P, Hooper D C, Johnson M P, Ramphal R, Wagener M M, Miyashiro D K, Yu V L. Enterobacter bacteremia: clinical features and emergence of antibiotic resistance during therapy. Ann Intern Med. 1991;115:585–590. doi: 10.7326/0003-4819-115-8-585. [DOI] [PubMed] [Google Scholar]
- 6.Cornaglia G, Riccio M L, Mazzariol A, Lauretti L, Fontana R, Rossolini G M. Appearance of IMP-1 metallo-β-lactamase in Europe. Lancet. 1999;353:899–900. doi: 10.1016/s0140-6736(98)05954-6. [DOI] [PubMed] [Google Scholar]
- 7.Craig W A. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis. 1998;26:1–12. doi: 10.1086/516284. [DOI] [PubMed] [Google Scholar]
- 8.Elkhaïli H, Nierdergang S, Pompei D, Linger L, Lévèque D, Jehl F. High-performance liquid chromatographic assay for meropenem in serum. J Chromatogr. 1996;686:19–26. doi: 10.1016/s0378-4347(96)00205-8. [DOI] [PubMed] [Google Scholar]
- 9.Gravallese D A, Musson D G, Pauliukonis L T, Bayne W F. Determination of imipenem (N-formimidoyl thienamycin) in human plasma and urine by high-performance liquid chromatography, comparison with microbiological methodology and stability. J Chromatogr. 1984;310:71–84. doi: 10.1016/0378-4347(84)80069-9. [DOI] [PubMed] [Google Scholar]
- 10.Holtje J E, Kopp U, Ursinus A, Wiedemann B. The negative regulator of beta-lactamase induction AmpD is a N-acetyl-anhydromuramyl-l-alanine amidase. FEMS Microbiol Lett. 1994;122:159–164. doi: 10.1111/j.1574-6968.1994.tb07159.x. [DOI] [PubMed] [Google Scholar]
- 11.Jacobs C, Frère J M, Normark S. Cytosolic intermediates for cell wall biosynthesis and degradation control inducible beta-lactam resistance in gram negative bacteria. Cell. 1997;88:823–832. doi: 10.1016/s0092-8674(00)81928-5. [DOI] [PubMed] [Google Scholar]
- 12.Jehl F, Gaillon C, Monteil H. High-performance liquid chromatography of antibiotics. J Chromatogr. 1990;531:509–548. doi: 10.1016/s0378-4347(00)82293-8. [DOI] [PubMed] [Google Scholar]
- 13.Jones R N, Flushs P C. Activity of cefepime (BMY-28142) and cefpirome (HR 810) against gram negative bacilli resistant to cefotaxime or ceftazidime. J Antimicrob Chemother. 1989;23:163–165. doi: 10.1093/jac/23.1.163. [DOI] [PubMed] [Google Scholar]
- 14.Knapp C C, Washington J A. Activity of cefepime, ceftazidime, and ceftizoxime against mutants of Enterobacteriaceae and Pseudomonas aeruginosa derepressed for class I β-lactamase. J Antimicrob Chemother. 1989;24:1011–1012. doi: 10.1093/jac/24.6.1011. [DOI] [PubMed] [Google Scholar]
- 15.Kopp U, Wiedemann B, Lindquist S, Normark S. Sequences of wild-type and mutant ampD genes of Citrobacter freundii and Enterobacter cloacae. Antimicrob Agents Chemother. 1993;37:224–228. doi: 10.1128/aac.37.2.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lister P D, Gardner V M, Sanders C C. Clavulanate induces expression of the Pseudomonas aeruginosa AmpC cephalosporinase at physiologically relevant concentrations and antagonizes the antibacterial activity of ticarcillin. Antimicrob Agents Chemother. 1991;43:882–889. doi: 10.1128/aac.43.4.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Livermore D M. Acquired carbapenemases. J Antimicrob Chemother. 1997;39:673–676. doi: 10.1093/jac/39.6.673. [DOI] [PubMed] [Google Scholar]
- 18.Mimoz O, Jacolot A, Léotard S, Hidri N, Samii K, Nordmann P, Petitjean O. Efficacies of cefepime, ceftazidime, and imipenem alone or in combination with amikacin in rats with experimental pneumonia due to ceftazidime-susceptible or -resistant Enterobacter cloacae strains. Antimicrob Agents Chemother. 1998;42:3304–3308. doi: 10.1128/aac.42.12.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naas T, Nordmann P. Analysis of a carbapenem-hydrolyzing class A β-lactamase from Enterobacter cloacae and of its LysR-type regulatory protein. Proc Natl Acad Sci USA. 1994;91:7693–7697. doi: 10.1073/pnas.91.16.7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naas T, Livermore D M, Nordmann P. Characterization of an LysR family protein, SmeR from Serratia marcescens S6, its effect on expression of the carbapenem-hydrolyzing β-lactamase Sme-1, and comparison of this regulator with other beta-lactamase regulators. Antimicrob Agents Chemother. 1995;39:629–637. doi: 10.1128/AAC.39.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naas T, Vandel L, Sougakoff W, Livermore D M, Nordmann P. Cloning and sequence analysis of the gene for a carbapenem-hydrolyzing class A β-lactamase, Sme-1, from Serratia marcescens S6. Antimicrob Agents Chemother. 1994;38:1262–1270. doi: 10.1128/aac.38.6.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A3. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1993. [Google Scholar]
- 23.Nordmann P, Mariotte S, Naas T, Labia R, Nicolas M H. Biochemical properties of a carbapenem-hydrolyzing β-lactamase from Enterobacter cloacae and cloning of the gene into Escherichia coli. Antimicrob Agents Chemother. 1993;37:939–946. doi: 10.1128/aac.37.5.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Osano E, Arakawa Y, Wacharotayankun R, Ohta M, Horii T, Ito H, Yoshimura F, Kato N. Molecular characterization of an enterobacterial metallo-beta-lactamase found in a clinical isolate of Serratia marcescens that shows imipenem resistance. Antimicrob Agents Chemother. 1994;38:71–78. doi: 10.1128/aac.38.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palmer S M, Kang S L, Cappeletty D M, Rybak M J. Bactericidal killing of cefepime, ceftazidime, cefotaxine, and ceftriaxone against Staphylococcus aureus and β-lactamase-producing strains of Enterobacter aerogenes and Klebsiella pneumoniae in an in vitro infection model. Antimicrob Agents Chemother. 1995;39:1764–1771. doi: 10.1128/aac.39.8.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phelps D J, Carlton D D, Farrell C A, Kessler R E. Affinity of cephalosporins for β-lactamases as a factor in antibacterial efficacy. Antimicrob Agents Chemother. 1986;29:845–848. doi: 10.1128/aac.29.5.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Philippon L N, Naas T, Bouthors A T, Barakett V, Nordmann P. OXA-18, a class D clavulanic acid-inhibited extended-spectrum β-lactamase from Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1997;41:2188–2195. doi: 10.1128/aac.41.10.2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rasmussen B A, Bush K. Carbapenem-hydrolyzing β-lactamases. Antimicrob Agents Chemother. 1997;41:223–232. doi: 10.1128/aac.41.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rasmussen B A, Bush K, Keeney D, Yang Y, Hare R, O'Gara C, Medeiros A A. Characterization of IMI-1 β-lactamase, a class A carbapenem-hydrolyzing enzyme from Enterobacter cloacae. Antimicrob Agents Chemother. 1996;40:2080–2086. doi: 10.1128/aac.40.9.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanders W E, Tenney J H, Kessler R E. Efficacy of cefepime in the treatment of infections due to multiply resistant Enterobacter species. Clin Infect Dis. 1996;23:454–461. doi: 10.1093/clinids/23.3.454. [DOI] [PubMed] [Google Scholar]
- 31.Schaberg D R, Culver D H, Gaynes R P. Major trends in the microbial etiology of nosocomial infection. Am J Med. 1991;91(Suppl. 3B):72–75. doi: 10.1016/0002-9343(91)90346-y. [DOI] [PubMed] [Google Scholar]
- 32.Senda K, Arakawa Y, Ichiyama S, Nakashima K, Ito H, Ohsuka S, Shimokata K, Kato N, Ohta M. PCR detection of metallo-beta-lactamase gene (blaIMP) in gram-negative rods resistant to broad-spectrum beta-lactams. J Clin Microbiol. 1996;34:2909–2913. doi: 10.1128/jcm.34.12.2909-2913.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Towner K J. Clinical importance and antibiotic resistance of Acinetobacter spp. J Med Microbiol. 1997;46:721–726. doi: 10.1099/00222615-46-9-721. [DOI] [PubMed] [Google Scholar]
- 34.Tsuji M, Ishii Y, Ohno A, Miyazaki S, Yamaguchi K. In vitro and in vivo antibacterial activities of S-4661, a new carbapenem. Antimicrob Agents Chemother. 1998;42:94–99. doi: 10.1128/aac.42.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vincent J L, Bihari D J, Suter P M, Bruining H A, White J, Nicolas-Chanoine M H, Wolff M, Spencer R C, Hemmer M for the EPIC International Advisory Committee. The prevalence of nosocomial infection in intensive care units in Europe. JAMA. 1995;274:639–644. [PubMed] [Google Scholar]