Abstract
Objectives:
To describe the microscopic pulpal reactions resulting from orthodontically induced tooth movement associated with low-level laser therapy (LLLT) in rats.
Materials and Methods:
Forty-five young male Wistar rats were randomly assigned to three groups. In group I (n = 20), the maxillary right first molars were submitted to orthodontic movement with placement of a coil spring. In group II (n = 20), the teeth were submitted to orthodontic movement plus LLLT at 4 seconds per point (buccal, palatal, and mesial) with a GaAlAs diode laser source (830 nm, 100 mW, 18 J/cm2). Group III (n = 5) served as a control (no orthodontic movement or LLLT). Groups I and II were divided into four subgroups according to the time elapsed between the start of tooth movement and sacrifice (12 hours, 24 hours, 3 days, and 7 days).
Results:
Up until the 3-day period, the specimens in group I presented a thicker odontoblastic layer, no cell-free zone of Weil, pulp core with differentiated mesenchymal and defense cells, and a high concentration of blood vessels. In group II, at the 12- and 24-hour time points, the odontoblastic layer was disorganized and the cell-free zone of Weil was absent, presenting undifferentiated cells, intensive vascularization with congested capillaries, and scarce defense cells in the cell-rich zone. In groups I and II, pulpal responses to the stimuli were more intense in the area underneath the region of application of the force or force/laser.
Conclusions:
The orthodontic-induced tooth movement and LLLT association showed reversible hyperemia as a tissue response to the stimulus. LLLT leads to a faster repair of the pulpal tissue due to orthodontic movement.
Keywords: Orthodontics, Low-level laser therapy, Pulp, Rats
INTRODUCTION
Orthodontically induced tooth movement produces alterations in periodontal tissues and in the pulp-dentinal complex.1–3 While some authors1 have suggested that the mechanical stimulus is permanent and that the pulp loses its vitality, others2 have advocated that the orthodontic force has no long-term significant effect on the dental pulp. The vascular pulpal alterations caused by the orthodontic movement are related to the breathing rate, disturbances in the odontoblastic layer, pulpal obliteration, root resorption, and pulp necrosis.4
Stenvik and Mjör5 have reported that intrusive forces applied to human premolars result in vascular disturbances such as a disturbance in the odontoblastic layer and root resorption. Root resorption can be reversible or a degenerative process that could result in necrosis, depending on the orthodontic force application. Studies on tooth movement in rats have demonstrated early pulp hyperemia6 and tissue alterations consistent with inflammatory processes,7,8 which are, however, reversible if the aggression does not exceed the physiologic limit of tolerance of the pulp tissue.
Low-level laser therapy (LLLT) has shown positive effects on bone remodeling, optimizing the orthodontic treatment.9,10 LLLT has several biomodulating effects on cell functions, such as fibroblast proliferation, collagen synthesis, and organization of collagenous fibers.11–18 Furthermore, studies in vivo and in vitro have demonstrated that laser increases ATP levels15 and activates specific enzymes that accelerate tissue healing and repair, neovalcularization,11–15,18 and increases in leukocyte phagocytic activity.12,15 In addition, the laser effects are related to attenuation of painful symptoms in the diverse postoperative processes and mucosal lesions.11,18
Cruz et al9 demonstrated clinically that LLLT accelerates the orthodontic movement in humans. Kawasaki and Shimizu19 investigated the effects of low-power laser irradiation on bone remodeling during experimental tooth movement in rats and observed that the amount of bone formation and cell proliferation rate in the tension side as well as the number of osteoclasts in the pressure side were all significantly increased in the irradiated group when compared with the nonirradiated group. These findings suggest that LLLT can accelerate tooth movement accompanied by alveolar bone remodeling and thus reduce orthodontic treatment duration.
The purpose of this study was to describe microscopically the pulpal reactions resulting from orthodontically induced tooth movement associated with LLLT in rats.
MATERIALS AND METHODS
The research protocol was approved by the Institutional Ethics in Animal Experimentation and Use Committee (CEUA 05.1.666.53.6). Forty-five young male adult Wistar rats (Rattus norvegicus, albinus) weighing approximately 300 g were obtained from the Animal Care Facility of the School of Dentistry of Ribeirão Preto, University of São Paulo, Brazil.
The animals were randomly assigned to three groups according to the treatment protocol. In group I (n = 20), the rats received a 5-mm-long closed nickel-titanium coil spring (Dental Morelli, São Paulo, SP, Brazil), which was placed from the maxillary right incisor to the maxillary right first molar to provide orthodontically induced mesial tipping of the molar (Figure 1). A force of approximately 0.4 N was applied. In group II (n = 20), the teeth were submitted to orthodontic movement associated with LLLT using a gallium aluminum arsenide (GaAlAs) diode laser source (830 nm, 100 mW, 18 J/cm2; Thera Laser, DMC, Sao Carlos, SP, Brazil). LLLT was standardized as related by Saito and Shimizu20 at 4-second exposures per point of the orthodontically moved tooth (buccal, palatal, and mesial) and perpendicular to the tooth axis. Group III (n = 5) served as a control (no orthodontic movement or LLLT). Groups I and II were divided into four subgroups according to the time elapsed between the start of tooth movement and euthanasia (12 hours, 24 hours, 3 days, and 7 days). The control animals were sacrificed at the beginning of the experiment.
Figure 1.
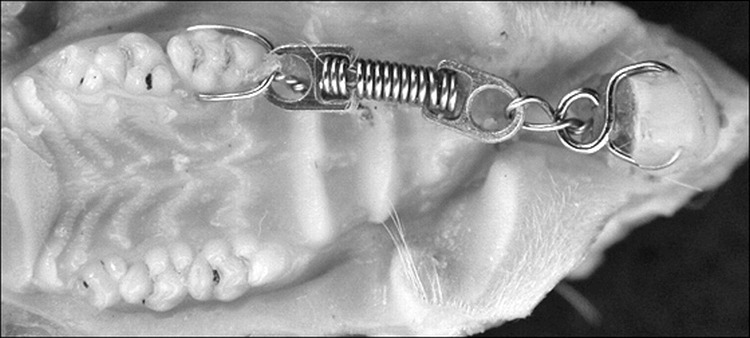
Coil spring used for orthodontic movement
For installation of the orthodontic appliance and daily LLLT applications, the animals were anesthetized by intramuscular injections of a combination of ketamine hydrochloride (Ketamina®, Agener–União Química Farmacêutica Nacional SA, São Paulo, SP, Brazil) and xylazine hydrochloride (Coopazine®, Coopers Brazil, São Paulo, SP, Brazil) at a ratio of 1:2 respectively (1 mL/kg body weight).
At the end of each period, the animals were sacrificed by an intraperitoneal injection of a lethal dose of sodium pentobarbital. After sacrifice, the anatomic pieces were maintained in individual and sterilized receptacles and were fixed by formaldehyde 10% for 48 hours. The anatomic pieces were embedded in paraffin, and serial longitudinal 5-μm-thick sections were obtained and stained with hematoxylin and eosin for histological analysis of tissue reactions. The specimens were examined with a light microscope (Axioplan, Zeiss, Oberoken, Germany) at 200× and 400×, and photomicrographs of representative areas were made for qualitative analysis of pulpal structures, cells, and blood vessels.
RESULTS
In group III (control), all specimens presented normal pulp tissue, with homogeneity of cell types, intercellular substance, fibers, nerves, and blood vessels. The pulp tissue exhibited all 4 structural layers identified at a histological level: odontoblastic layer, cell-free zone of Weil, cell-rich zone, and pulp core (Figure 2).
Figure 2.

Group III (control). Photomicrograph showing (a) the odontoblastic layer, (b) the cell-free zone of Weil, (c) the cell-rich zone, and (d) the pulp core (200×, HE)
The teeth that moved orthodontically in group I (tooth movement) showed pulp structure alterations consistent with an inflammatory process. In group II (tooth movement plus LLLT), the pulpal structures presented significant alterations in their components induced by laser irradiation, compared with groups I and III (control). Pulp responses were significantly more accentuated and seemed to be restricted to the area underneath the region submitted to orthodontic force and laser application.
In the earliest periods (up to 3 days), all specimens in group I presented odontoblasts juxtaposed to each other, presenting nuclei with extensive and diffused chromatin (hypertrophic) with appearance of an active cell and a more basophilic cytoplasm. Such characteristics conferred a greater volume to the odontoblastic layer and an apparently larger number of layers in comparison to the control group (Figure 3). The cell-free zone of Weil was absent, especially in the areas where the odontoblastic layer presented more accentuated alterations. In group II, the odontoblastic layer was completely disorganized in the earliest periods (12 hours and 24 hours; Figure 4). After 3 and 7 days, the cell-free zone of Weil could be seen again in the coronal pulp. The odontoblastic layer had a normal appearance.
Figure 3.
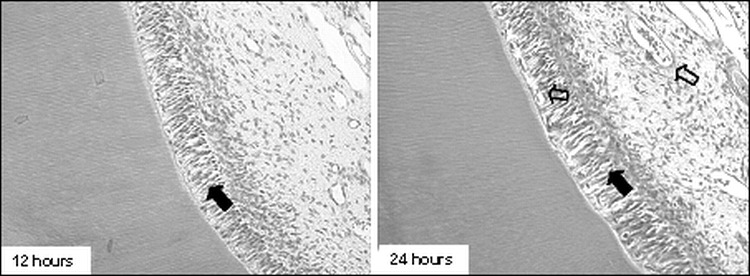
Group I. Photomicrographs showing the odontoblastic layer maintaining its characteristic organization, exhibiting nuclei with extensive and diffuse chromatin and a more basophilic cytoplasm, with more scattered cells (full arrows) and ramification of vessels full of erythrocytes, also in the odontoblastic layer (empty arrows; 200×, HE)
Figure 4.
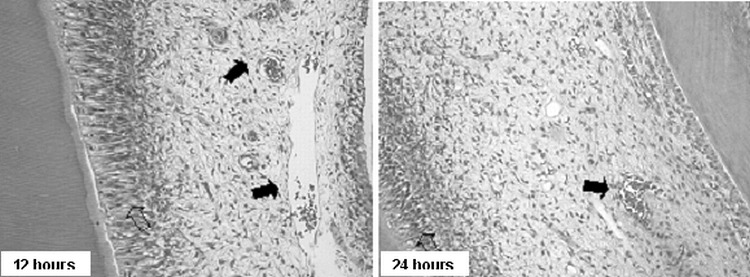
Group II. Photomicrographs showing the disorganization of the odontoblastic layer (empty arrows) and hyperemic capillaries (full arrows; 200×, HE)
The cell-rich zone was more pronounced at all periods in group I. It was possible to observe undifferentiated mesenchymal cells, fibroblasts, and defense cells. At the 12- and 24-hour periods, the fibroblast nuclei were more distinguished from each other due to the great amount of amorphous ground substance, suggesting an accumulation of interstitial liquid and edema, which made the pulp tissue less homogenous when compared with group III. Another interesting characteristic was the presence of small hemorrhagic areas in the pulp core, mainly in the root pulp, characterized by a large number of erythrocytes in this region (Figure 5). In group II, the fibroblast nuclei were intensely stained and presented loose chromatin with granules of different sizes. The presence of 1 or more nucleoli was markedly evident. There were only few defense cells.
Figure 5.
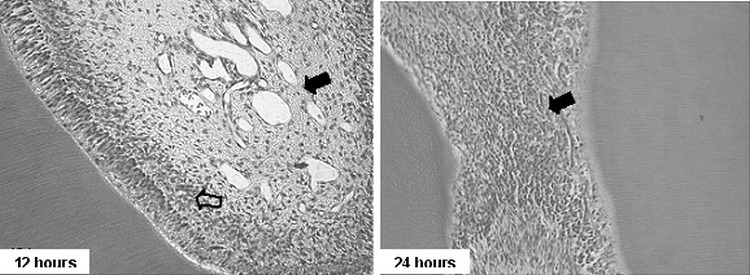
Group I. Photomicrographs showing the cell-rich layer (empty arrows) and blood vessel proliferation (full arrows; 200× HE at left, and 400× HE at right)
Vascularization was concentrated in the coronal pulp core in group I. A high concentration of blood vessels was found close to the odontoblastic layer, which were wider than those in group III. There were a large number of blood cells, erythrocytes, and leukocytes inside the vessels, greatest at the earliest periods. In the other groups, the number of erythrocytes inside the vessels diminished. Defense cells, such as neutrophils, eosinophils, and monocytes, were also identified at all experimental periods close to the odontoblastic layer. The initial periods (12 and 24 hours) of group II also exhibited a concentrated vascularization in the coronal and root pulp cores; however, it was characterized by blood vessels of greater diameter, containing a large number of blood cells. Despite the presence of hyperemic vessels in the pulp region underlying the odontoblastic layer, there was no evidence of large hemorrhagic areas.
One of the main differences between the lased and nonlased groups was the presence of an intense vascularization. In group II at 3 days, mild hemorrhage was observed close to the odontoblastic layer, and some erythrocytes were seen invading this layer (Figure 6). Inside the vessels, there were a smaller number of blood cells compared with the earlier evaluation periods, but they were still congested with liquid substance.
Figure 6.
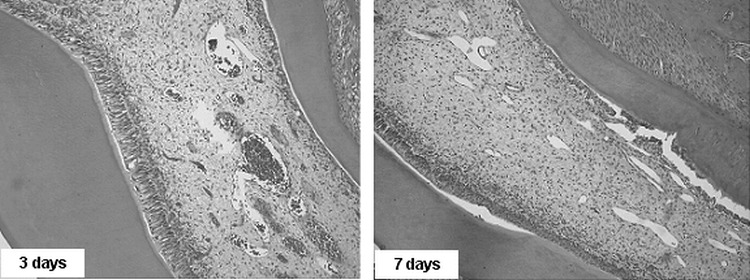
Group II. Photomicrographs after being subjected to orthodontic force and laser application, showing vessel proliferation with pulp hyperemia (3 days) and vessel proliferation in the 7-day period (200×, HE)
Groups I and II exhibited characteristics of normality at 7 days. All 4 structural layers were evident, with the same arrangement as that observed in the control animals (group III). The blood vessels of the pulp core contained fewer blood cells as compared with the initial periods of tooth movement.
DISCUSSION
Since the introduction of low-power lasers to the market, several studies have been conducted13,18,20,21 to evaluate their therapeutic effects. These effects include pain relief, acceleration of tissue healing and repair, acceleration of new vascularization, wound closure, greater formation of granulation tissue, fibroblast and collagen fiber proliferation, increase of ATP synthesis, release of preformed histamine, decrease of intracellular pH and changes in cell proliferation and motility, phagocytosis, and immune response.12,15–17
LLLT has yielded important outcomes in orthodontics, with positive effects on bone remodeling. The findings of an in vivo19 study in which an orthodontic force was applied to rat molars to cause experimental tooth movement demonstrated a greater amount of newly formed bone, cell proliferation on the tension side, and a large number of osteoclasts on the pressure side. This indicates that low-power laser irradiation can accelerate tooth movement. In humans, this tissue response seems to be similar.9
The alterations occurring in the pulp tissue during experimentally induced tooth movement have been extensively investigated.1,2,5–8 However, the association of orthodontic tooth movement with LLLT might produce different effects on the pulp tissue, as the LLLT has advantageous effects for orthodontics, especially those related to the relief of the painful symptoms triggered by force application to a tooth.13,14,18,20,21
The literature has shown controversial results with respect to pulpal alterations caused by orthodontic tooth movement, mainly those regarding revascularization, which may be explained by differences in the methodological designs.1,5–8
The analysis of the results of group II was complex because of the small number of studies addressing the pulpal responses resulting from LLLT application. Most studies refer to high-power laser irradiation, temperature changes,11,22 and the presence of free radicals in the tooth pulp.22 It is acknowledged that laser wavelength, total delivered energy, frequency, and dose, as well as the optical properties of the irradiated tissues, are all directly related to cell response to laser therapy.21 The histological findings of group II demonstrate significant pulp alterations resulting from LLLT compared with groups I and III. In orthodontic practice, LLLT has been used to potentiate tooth movement9,19 and to relieve the pain associated with the application of the orthodontic force.14,20
The alterations in the odontoblastic layer observed in group I are consistent with the consensual outcomes reported in previous studies.1,7,8 In the earliest periods of group II, this layer was completely disorganized, similar to group I. The pulp region that presented the greatest alteration was the mesial surface, accompanying the pressure side of the orthodontically induced tooth movement.7,8
The cell-free zone of Weil was absent in the initial periods of group I, which is in agreement with the results of previous studies,1,7,8 especially where there were more accentuated alterations of the odontoblastic layer. The cell-free zone of Weil was restored at the 7-day period with normalization of the odontoblastic layer. In group II, this layer could be observed after the third day.
For all periods of group I, the cell-rich zone toward the pulp core was more intensive when compared with that of group III, presenting fibroblasts, undifferentiated mesenchymal cells, and defense cells. These findings are consistent with those of earlier studies.7,8 Alterations consistent with inflammatory process events were found in all evaluation periods. The increase in the number of blood cells, erythrocytes, and leukocytes in the blood vessels and in the connective pulp tissue7,8 reveals a chemotactic reaction and the presence of tissue-irritating agents in this area.23 Cell migration and liquid accumulation in the pulp tissue are part of the inflammatory process, whose main triggering event is the alteration of pulpal blood flow.6–8 The initial hyperemia (12 hours and 24 hours) decreased gradually up to the 3-day period. This increase in pulp vascularity and blood flow during orthodontic movement are alterations ascribed to the start of the inflammatory process6–8 as defensive reactions due to the alterations in tissue histophysiology arising from the mechanical stimuli.8
In group II, the cell-rich layer was abundant and thicker in comparison to groups I and III, presenting fibroblasts and undifferentiated mesenchymal cells with characteristics of high cellular activity. These findings corroborate the reports on LLLT effects on fibroblast and collagen fiber proliferation.12,15–17 No defense cells or edema were found in any specimen of group II. The findings of group II may be explained by LLLT's capacity of promoting pain relief, faster tissue healing and repair and new vascularization, wound closure, greater formation of granulation tissue, and proliferation of fibroblasts and collagen fibers.15–17 During the 3-day period, the presence of some erythrocytes indicated a mild hemorrhage, with congested vessels containing plasmatic proteins. Hyperemia is the pulp response to an irritating stimulus and is associated with an attempt to avoid the establishment an inflammatory process and edema.23 The main difference between the laser-irradiated and nonirradiated groups was the intense vascularization, which may be justified by the acceleration of new vascularization induced by LLLT.17
The results of this study show an optimization of the orthodontic treatment when associated with the use of LLLT. The results of the qualitative analysis suggest that from a clinical standpoint, the pain usually associated with the orthodontic treatment may be attenuated by the LLLT's biomodulating effects. In addition, no inflammatory process was observed, and only an initial hyperemia developed as a tissue reaction to the mechanical stimulus, which indicates that the vital pulp has defense capacity. Stereological studies are required to analyze these data quantitatively.
Although there have been several studies that have addressed the action of LLLT therapy on bone repair and osteogenesis, there are few reports on its effects on the pulp tissue. Further research is required to develop more solid scientific bases for the clinical use of LLLT and to describe the mechanism action of low-power lasers as there are only a few studies in this field and different methodologies have been employed.
CONCLUSIONS
Orthodontically induced tooth movement associated with LLLT produced an increase in the vascularization, and this factor could accelerate pulp tissue repair.
Laser therapy is beneficial to orthodontic movement.
Acknowledgment
This study was supported by a grant-in-aid from FAPESP.
REFERENCES
- 1.Anstendig H. S, Kronman J. H. A histological study of pulpal reaction to orthodontic tooth movement in dogs. Angle Orthod. 1972;42:50–55. doi: 10.1043/0003-3219(1972)042<0050:AHSOPR>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 2.Kvinnsland S, Heyeraas K, Flord E. S. Effect of experimental tooth movement on periodontal and pulpal blood flow. Eur J Orthod. 1989;11:200–205. doi: 10.1093/oxfordjournals.ejo.a035986. [DOI] [PubMed] [Google Scholar]
- 3.Reitan K. Clinical and histological observation on tooth movement during and after orthodontic treatment. Am J Orthod. 1967;53:721–745. doi: 10.1016/0002-9416(67)90118-2. [DOI] [PubMed] [Google Scholar]
- 4.Baume L. J. The biology of pulp and dentine. Monogr Oral Sci. 1980;8:1–246. [PubMed] [Google Scholar]
- 5.Stenvik A, Mjör A. A pulp and dentine reactions to experimental tooth intrusion. Am J Orthod. 1970;57:370–385. doi: 10.1016/s0002-9416(70)90219-8. [DOI] [PubMed] [Google Scholar]
- 6.Nixon C. E, Saviano J. A, King G. J, Keeling S. D. Histomorphometric study of dental pulp during orthodontic tooth movement. J Endod. 1993;19:13–16. doi: 10.1016/S0099-2399(06)81034-4. [DOI] [PubMed] [Google Scholar]
- 7.Santamaria M, Jr, Milagres D, Stuani A. S, Stuani M. B, Ruellas A. C. Initial changes in pulpal microvasculature during orthodontic tooth movement: a stereological study. Eur J Orthod. 2006;28:217–220. doi: 10.1093/ejo/cji117. [DOI] [PubMed] [Google Scholar]
- 8.Santamaria M, Jr, Milagres D, Iyomasa M. M, Stuani M. B. S, Ruellas A. C. O. Initial pulp changes during orthodontic movement: histomorphological evaluation. Braz Dent J. 2007;18:34–39. doi: 10.1590/s0103-64402007000100008. [DOI] [PubMed] [Google Scholar]
- 9.Cruz D. R, Kohara E. K, Ribeiro M. S, Wetter N. U. Effects of low-intensity laser therapy on the orthodontic movement velocity of human teeth: a preliminary study. Laser Surg Med. 2004;35:117–120. doi: 10.1002/lsm.20076. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi Y, Takagi H, Sakay H, Hashimoto F, Mataki S, Kobayashi K, Kato Y. Effects of local administration of osteocalcin on experimental tooth movement. Angle Orthod. 1998;68:259–266. doi: 10.1043/0003-3219(1998)068<0259:EOLAOO>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 11.Coluzzi D. J. An overview of laser wavelengths used in Dentistry. Dent Clin N Am. 2000;44:753–765. [PubMed] [Google Scholar]
- 12.Demir H, Yaray S, Kirnap M, Yaray K. Comparison of the effects of laser and ultrasound treatments on experimental wound healing in rats. J Rehabil Res Dev. 2004;41:721–728. doi: 10.1682/jrrd.2003.08.0131. [DOI] [PubMed] [Google Scholar]
- 13.Koichiro K, Shimizu N. Effect of low-energy laser irradiation on bone remodeling during experimental tooth movement in rats. Laser Surg Med. 2000;26:282–291. doi: 10.1002/(sici)1096-9101(2000)26:3<282::aid-lsm6>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 14.Lim H. M, Lew K. K, Tay D. K. A clinical investigation of the efficacy of low level laser therapy in reducing orthodontic postadjustment pain. Am J Orthod Dentofacial Orthop. 1995;108:614–622. doi: 10.1016/s0889-5406(95)70007-2. [DOI] [PubMed] [Google Scholar]
- 15.Lirani-Galvão A. P, Jorgetti V, Silva O. L. Comparative study of low-level laser therapy and low-intensity pulsed ultrasound effect on bone in rats. Photomed Laser Surg. 2006;24:735–740. doi: 10.1089/pho.2006.24.735. [DOI] [PubMed] [Google Scholar]
- 16.De Oliveira R. F, Oliveira D. A, Monteiro W, Zangaro R. A, Magini M, Soares C. P. Comparison between the effect of low-level laser therapy and low-intensity pulsed ultrasonic irradiation in vitro. Photomed Laser Surg. 2008;26:6–9. doi: 10.1089/pho.2007.2112. [DOI] [PubMed] [Google Scholar]
- 17.Sulewski J. G. Historical survey of laser dentistry (lasers and light amplification in dentistry) Dent Clin North Am. 2000;44:717–765. [PubMed] [Google Scholar]
- 18.Ueda Y, Shimizu N. Effects of pulse frequency of low-level laser therapy (LLLT) on bone nodule formation in rat calvarial cells. J Clin Laser Med Surg. 2003;21:271–277. doi: 10.1089/104454703322564479. [DOI] [PubMed] [Google Scholar]
- 19.Kawasaki K, Shimizu N. Effects of low-energy laser irradiation on bone remodeling during experimental tooth movement in rats. Lasers Surg Med. 2000;26:282–291. doi: 10.1002/(sici)1096-9101(2000)26:3<282::aid-lsm6>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 20.Saito S, Shimizu N. Stimulatory effects of low-power laser irradiation on bone regeneration in midpalatal surface during expansion in the rat. Am J Orthod Dentofacial Orthop. 1997;111:525–532. doi: 10.1016/s0889-5406(97)70152-5. [DOI] [PubMed] [Google Scholar]
- 21.Takeda Y. Irradiation effect of low-energy laser on alveolar bone after tooth extraction: experimental study in rats. Int J Oral Maxillofac Surg. 1988;17:388–391. doi: 10.1016/s0901-5027(88)80070-5. [DOI] [PubMed] [Google Scholar]
- 22.Ma T, Marangoni R. D, Flint W. In vitro comparison of debonding force and intrapulpal temperature changes during ceramic orthodontic bracket removal using a carbon dioxide laser. Am J Orthod Dentofacial Orthop. 1997;111:203–210. doi: 10.1016/s0889-5406(97)70217-8. [DOI] [PubMed] [Google Scholar]
- 23.Ten Cate A. R. St Louis, Mo: CV Mosby; 1991. Oral Histology: Development, Structure and Function. [Google Scholar]


