To the Editor:
Despite the efficacy of primary percutaneous coronary intervention (PCI), contractile dysfunction after ST-segment elevation myocardial infarction (STEMI) remains a persistent issue. Early detection of high-risk patients could help improve disease management during the initial time-period after infarction, when patients are most at risk for sudden cardiac death. The aim of this preliminary study was to investigate whether end-systolic (ES) global circumferential strain (GCS), measured with cardiac magnetic resonance (CMR) feature-tracking, would be a stronger predictor of functional recovery than peak GCS or late gadolinium enhancement (LGE).
Methods
Patient Population
This retrospective study was conducted at a single center. The study consisted of 31 patients with STEMI and baseline ejection fraction (EF) < 50%, who were enrolled between 2014 and 2019. A list of study exclusions is provided in the Methods section of the Supplement (Figure S1). The study protocol complies with the Declaration of Helsinki and was approved by our Institutional Review Board and Ethics Committees. Due to the retrospective nature of this study, CMR data was only available at baseline, while echocardiogram data was available at follow-up. Baseline CMR was performed within 1–2 days after primary PCI. Follow-up imaging via echocardiogram was conducted a median of 105 days (interquartile, 75–253 days) after initial onset of symptoms. The primary endpoint of this study was functional recovery at follow-up, as defined by an EF ≥ 50% quantified by echocardiogram.
CMR Imaging
CMR imaging was performed on a 1.5T MR scanner (Magnetom Aera, Siemens Medical, Erlangen, Germany) with 18-channel body coil and 12-channel spine coil. Patients underwent breath-held steady-state free precession cine imaging in the 2-, 3-, 4-chamber long axis view, and full left ventricular (LV) coverage with short axis stack from base to apex (scanner parameters provided in the Methods section of the Supplement). All patients received 0.2mmol/kg gadolinium-based contrast agent (GBCA). After 10 minutes, inversion time scout was performed to determine the inversion time needed to null the myocardium. Breath-held gradient echo inversion recovery LGE images were obtained in the same prescription as the cines.
Follow-up Echocardiogram Imaging
Echocardiogram images were acquired with Philips (EPIQ 7C or IE33, Netherland) as per standard clinical protocol (1). LV-EF was calculated by modified biplane method in the apical 4 and 2 chamber views. All images were interpreted by a board certified echocardiographer who was blinded to the clinical data.
CMR Image Processing
All CMR analyses were performed by a level-3 trained CMR reader on CMR42 v5.6.2 (Circle Cardiovascular Imaging, Inc., Calgary, Canada). The readers were blinded to the clinical data. The specific details related to the calculation of baseline EF, strain from feature-tracking, and infarct size are provided in the Methods section of the Supplement.
Statistical Analysis
Data analysis and visualization were performed using SAS (Version 9.4) and Excel (Version 2005). Continuous variables are expressed as mean ± standard deviation (SD) or median (Q1, Q3), depending on distribution. Details related to the logistic regression models, as well as the intraobserver and interobserver variability studies, are provided in the Methods section of the Supplement.
Results
The clinical characteristics of the included patients (Table S1), as well as the predicted probability curves (Figures S3–S8), are provided in the Supplement. A representative example of the circumferential strain vs. time curves is shown for a single patient in Figure 1.
Figure 1:
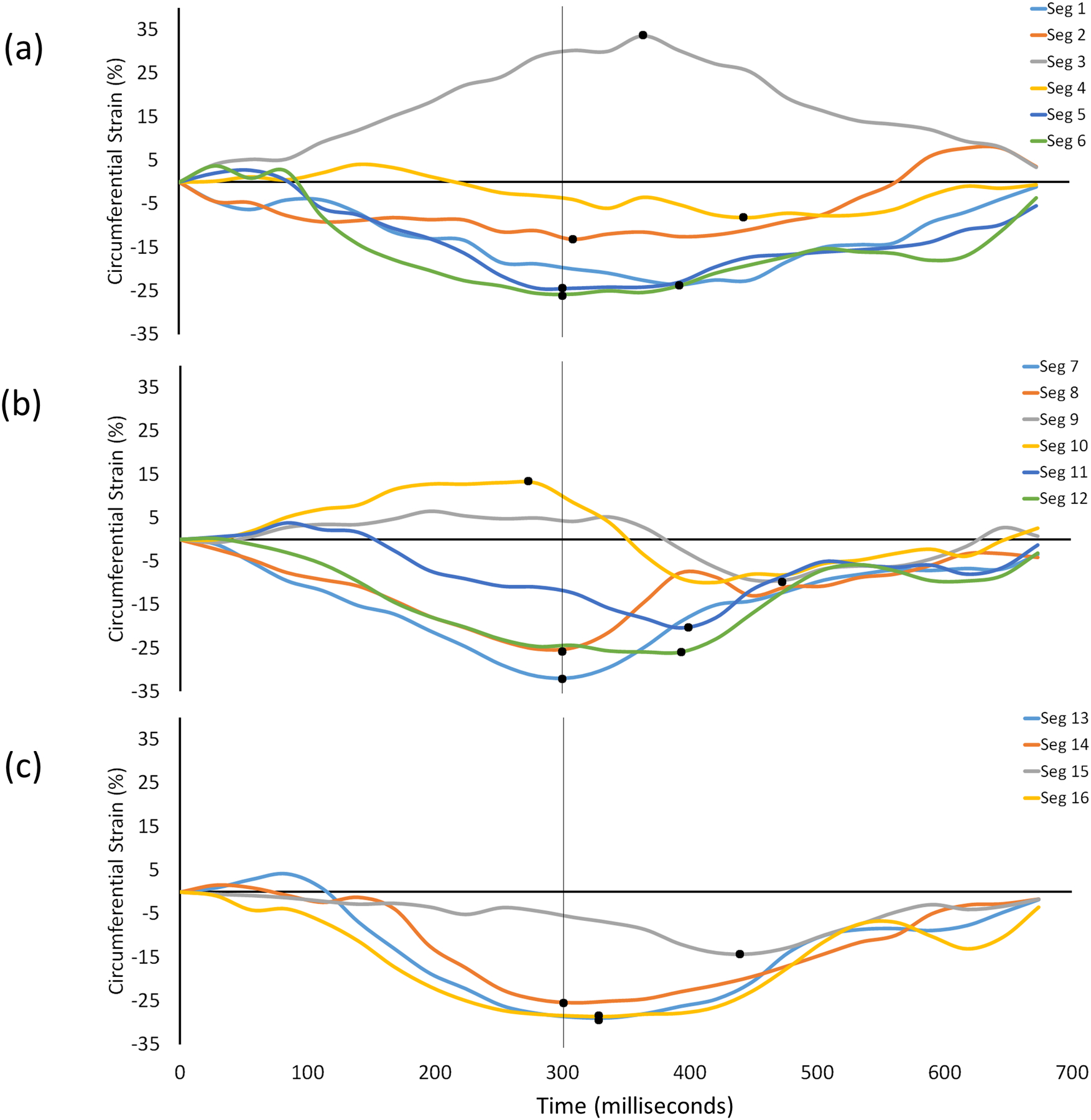
Example of circumferential strain vs. time curves measured in a single patient. Each curve represents the strain in one of the 16 AHA segments. The solid black dot indicates the peak value of strain in that segment. The vertical line at 300 ms indicates the ES time point where the ES value of strain was measured. Panel (a) represents the basal segments, (b) represents the mid-ventricular segments, and (c) represents the apical segments. Note that due to the dysfunctional MI region, the peak value of strain in each myocardial segment can occur at a different time point during the cardiac cycle. Thus, global averages of peak strain are based on inconsistent time points.
Receiver Operating Characteristic Analysis
A summary of the analysis is given in Table 1. Briefly, the ROC analysis determined that ES GCS (AUC=0.910, 95% CI=0.786–1.0, p=0.008) and peak GCS (AUC=0.803, 95% CI=0.640–0.967, p=0.012) were predictive of a follow-up EF ≥ 50%, whereas LGE (AUC=0.667, 95% CI=0.465–0.868, p=0.131) was not predictive. Comparing the ROC curves revealed that ES GCS was a superior predictor to peak GCS (p=0.040) and LGE (p=0.018). The difference between peak GCS and LGE in predicting functional recovery was not significant (p = 0.261).
Table 1.
Overview of receiver operating characteristic analyses of individual parameters for predicting follow-up ejection fraction ≥ 50%.
| Variable | Cut-off value | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | AUC | 95% CI | P value |
|---|---|---|---|---|---|---|---|---|
| LGE | 19.2% | 69.2% | 61.1% | 56.2% | 73.3% | 0.667 | 0.465–0.868 | 0.131 |
| Peak GCS | −12.4% | 69.2% | 83.3% | 75.0% | 78.9% | 0.803 | 0.640–0.967 | 0.012 |
| ES GCS | −10.3% | 84.6% | 77.8% | 73.3% | 87.5% | 0.910 | 0.786–1.0 | 0.008 |
LGE: Late Gadolinium Enhancement, GCS: Global Circumferential Strain, ES: End-Systolic, PPV: Positive Predictive Value, NPV: Negative Predictive Value, AUC: Area Under the Curve, CI: Confidence Interval
An additional analysis was conducted where pain to balloon time (PTBT) was added to each model as a second predictor. Details of this analysis can be found in the Results section of the Supplement (Figure S2).
Univariate and Multivariate Analysis
The estimated odds ratio (OR) was computed relative to a one SD increase in the variable of interest (Table 2). The ES GCS was found to be highly predictive of functional recovery, where the estimated OR for a 2.6 unit increase in strain was observed to be 0.046 (95% CI=0.005–0.453, p=0.008). The peak GCS (OR=0.252, 95% CI=0.086–0.742, p=0.012) was found to be somewhat predictive of functional recovery. However, the LGE (OR=0.532, 95% CI=0.234–1.209, p=0.131) was not found to be a useful predictor of recovery.
Table 2.
Univariate logistic regression analyses for predicting follow-up ejection fraction ≥ 50%.
| Variable | OR per + 1 SD | 95% CI | P value |
|---|---|---|---|
| LGE | 0.532 per + 15.5 | 0.234–1.209 | 0.131 |
| Peak GCS | 0.252 per + 3.3 | 0.086–0.742 | 0.012 |
| ES GCS | 0.046 per + 2.6 | 0.005–0.453 | 0.008 |
OR: Odds Ratio, SD: Standard Deviation, CI: Confidence Interval, LGE: Late Gadolinium Enhancement, GCS: Global Circumferential Strain, ES: End-Systolic
An additional analysis was conducted where PTBT was added to each model as a second variable. Details of this multivariate analysis can be found in the Supplement (Table S2).
Intra- and Interobserver Variability of Strain
The strain calculations were highly reproducible. Details of this analysis are in the Results section of the Supplement.
Discussion
The key finding of this study is that ES GCS, which is measured 1–2 days after primary PCI via CMR feature-tracking, can be used as a predictor of functional recovery in STEMI patients. Moreover, ES GCS was found to be a superior predictor to peak GCS and LGE, even after adjusting for PTBT. The results of the current study support the increasing accumulation of evidence that GCS is a valuable predictor of functional recovery and clinical outcomes in MI patients (2–9). However, a key difference is that all of these prior studies focused on the use of peak GCS rather than ES GCS, which has been shown here to be a superior predictor. Another benefit of utilizing ES GCS is that it does not require the administration of contrast agents to make a predictive measurement. GBCA is contraindicated in patients with renal failure, due to increased risk of nephrogenic systemic fibrosis (10).
Limitations of this study include the retrospective design, imbalance of gender ratio, echo for follow-up EF, and sample size. However, the patient cohort was representative of the typical STEMI population in terms of culprit vessels and comorbidities.
Supplementary Material
Acknowledgments:
Grant Support:
This research was supported by National Institutes of Health grants U01 HL133359 and R56 HL124266.
References
- 1.Mitchell C, Rahko PS, Blauwet LA, et al. Guidelines for Performing a Comprehensive Transthoracic Echocardiographic Examination in Adults: Recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr 2019;32(1):1–64. [DOI] [PubMed] [Google Scholar]
- 2.Buss SJ, Krautz B, Hofmann N, et al. Prediction of functional recovery by cardiac magnetic resonance feature tracking imaging in first time ST-elevation myocardial infarction. Comparison to infarct size and transmurality by late gadolinium enhancement. Int J Cardiol 2015;183:162–170. [DOI] [PubMed] [Google Scholar]
- 3.Holmes AA, Romero J, Levsky JM, et al. Circumferential strain acquired by CMR early after acute myocardial infarction adds incremental predictive value to late gadolinium enhancement imaging to predict late myocardial remodeling and subsequent risk of sudden cardiac death. J Interv Card Electrophysiol 2017;50(3):211–218. [DOI] [PubMed] [Google Scholar]
- 4.Khan JN, Nazir SA, Singh A, et al. Relationship of Myocardial Strain and Markers of Myocardial Injury to Predict Segmental Recovery After Acute ST-Segment-Elevation Myocardial Infarction. Circ Cardiovasc Imaging 2016;9(6). [DOI] [PubMed] [Google Scholar]
- 5.Mangion K, Carrick D, Clerfond G, et al. Predictors of segmental myocardial functional recovery in patients after an acute ST-Elevation myocardial infarction. Eur J Radiol 2019;112:121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mordi I, Bezerra H, Carrick D, Tzemos N. The Combined Incremental Prognostic Value of LVEF, Late Gadolinium Enhancement, and Global Circumferential Strain Assessed by CMR. JACC Cardiovasc Imaging 2015;8(5):540–549. [DOI] [PubMed] [Google Scholar]
- 7.Nucifora G, Muser D, Tioni C, Shah R, Selvanayagam JB. Prognostic value of myocardial deformation imaging by cardiac magnetic resonance feature-tracking in patients with a first ST-segment elevation myocardial infarction. Int J Cardiol 2018;271:387–391. [DOI] [PubMed] [Google Scholar]
- 8.Wong DT, Leong DP, Weightman MJ, et al. Magnetic resonance-derived circumferential strain provides a superior and incremental assessment of improvement in contractile function in patients early after ST-segment elevation myocardial infarction. Eur Radiol 2014;24(6):1219–1228. [DOI] [PubMed] [Google Scholar]
- 9.Cha MJ, Lee JH, Jung HN, Kim Y, Choe YH, Kim SM. Cardiac magnetic resonance-tissue tracking for the early prediction of adverse left ventricular remodeling after ST-segment elevation myocardial infarction. Int J Cardiovasc Imaging 2019;35(11):2095–2102. [DOI] [PubMed] [Google Scholar]
- 10.Perez-Rodriguez J, Lai S, Ehst BD, Fine DM, Bluemke DA. Nephrogenic systemic fibrosis: incidence, associations, and effect of risk factor assessment--report of 33 cases. Radiology 2009;250(2):371–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


