Abstract
Purpose
High-risk lesions (HRLs) of the breast are an indication for chemoprevention, yet uptake is low, largely due to concerns about side effects. In 2019, low-dose (5 mg) tamoxifen was demonstrated to reduce breast cancer risk with improved tolerance. We describe chemoprevention uptake in an academic clinic before and after the introduction of low-dose tamoxifen.
Methods
Females age ≥ 35 with HRLs who established care from April 2017 through January 2020 and eligible for chemoprevention were included. Rates of chemoprevention initiation before and after the introduction of low-dose tamoxifen (pre-2019 vs. post-2019) were compared with chi-squared tests. Logistic regression identified demographic and clinical factors associated with chemoprevention initiation. Kaplan–Meier methods determined the rates of discontinuation.
Results
Among 660 eligible females with HRLs, 22.7% initiated chemoprevention. Median time from first visit to chemoprevention initiation was 54 days (interquartile range (IQR): 0–209); 31.0% (46/150) started chemoprevention > 6 months after their initial visit. Chemoprevention uptake was not significantly different pre-2019 vs. post-2019 (21.2% vs. 26.3%, p = 0.16); however, post-2019, low-dose tamoxifen became the most popular option (41.5%, 34/82). On multivariable analyses, age and breast cancer family history were significantly associated with chemoprevention initiation. Discontinuation rates at 1 year were lowest for low-dose tamoxifen (6.7%) vs. tamoxifen 20 mg (15.0%), raloxifene (20.4%), or an aromatase inhibitor (20.0%).
Conclusion
In this modern cohort, 22.7% of females with HRLs initiated chemoprevention with 31.0% initiating chemoprevention > 6 months after their first visit. Low-dose tamoxifen is now the most popular choice for chemoprevention, with low discontinuation rates at 1 year.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10549-022-06577-5.
Keywords: Chemoprevention, Low-dose tamoxifen, High-risk lesions, Lobular carcinoma in situ (LCIS), Atypical ductal hyperplasia (ADH), Atypical lobular hyperplasia (ALH)
Introduction
Women with high-risk lesions (HRLs) of the breast have an increased lifetime risk of developing breast cancer and are candidates for chemoprevention to modulate risk. HRLs include atypical ductal hyperplasia (ADH), atypical lobular hyperplasia (ALH), and lobular carcinoma in situ (LCIS). These HRLs are most often identified on biopsies in women undergoing mammographic screening. Data from randomized chemoprevention trials have demonstrated that 5 years of therapy will reduce the risk of invasive breast cancer by at least 50% among women with HRLs [1–6]. Four endocrine therapies (tamoxifen, raloxifene, exemestane, and anastrozole) are approved for breast cancer chemoprevention as they reduce the risk of estrogen-receptor-positive breast cancer [7], which is the type of breast cancer women with HRL are most likely to develop. The American Society of Clinical Oncology (ASCO) Clinical Practice Guidelines recommend considering these therapies for women with an increased risk of breast cancer [7].
Tamoxifen was the first FDA-approved medication for chemoprevention and introduced in 1998. Multiple retrospective studies [8–12] demonstrate that < 10% of women who are deemed to be at high-risk initiate chemoprevention and among those who start, only a fraction complete 5 years of recommended therapy. The most commonly cited reason for not taking tamoxifen is fear of side effects, especially endometrial cancer, blood clots, and menopausal symptoms [8, 13, 14]. One approach to address low rates of initiation is to identify and support patients at higher risk of breast cancer in a specialized clinic [15]. In prior work, this approach has demonstrated improved rates of chemoprevention uptake (24–37%), although high discontinuation rates remain a challenge [16, 17].
Chemoprevention strategies with fewer side effects and risks could also improve uptake and adherence. Data supporting a role for low-dose tamoxifen (5 mg compared to the standard dose of 20 mg) were presented at the San Antonio Breast Cancer Symposium in December 2018 and published shortly thereafter [6]. In a randomized clinical trial, which included patients with ADH, LCIS, and ductal carcinoma in situ (DCIS), low-dose tamoxifen offered similar efficacy for risk reduction after 3 years of therapy with a more favorable side effect profile than 20 mg of tamoxifen. After reviewing these data, our clinicians at Dana-Farber/Brigham and Women’s Cancer Center (DF/BWCC) began to offer this option to patients with HRL in January 2019.
Here, we sought to determine whether the introduction of low-dose tamoxifen in the DF/BWCC specialized breast cancer prevention clinic, known as the Breast Cancer Personalized Risk Assessment, Education and Prevention (B-PREP) program, was associated with higher rates of chemoprevention uptake overall and to identify the proportion of women choosing low-dose tamoxifen over other chemoprevention options. We also examined factors associated with chemoprevention uptake and discontinuation in our setting.
Methods
Study description
The study was approved by the Brigham and Women’s Hospital Institutional Review Board as a low-risk study and approved with waiver of consent. Women aged ≥ 35 with HRLs who were evaluated in the DF/BWCC B-PREP program from April 19, 2017 through January 1, 2020, and eligible for oral chemoprevention were identified from a prospectively maintained database. To minimize confounding factors in our cohort, women currently taking chemoprevention and those who had completed chemoprevention therapy prior to April 2017 were excluded. Patients with HRLs enrolled in specific intervention trials were also excluded and this included women who were participating in a trial examining the use of topical tamoxifen gel as an intervention that may modulate breast cancer risk [18].
All patients entering the B-PREP program complete a customized electronic intake survey to gather information on their demographics, hereditary, reproductive, lifestyle, and clinical risk factors for breast cancer. Details on this risk survey are included in the Supplemental Methods. Chart abstractions were performed for this effort to collect data on chemoprevention initiation, tolerance, and discontinuation, as well as any subsequent breast events, including benign and malignant diagnoses.
For patients referred to B-PREP for a HRL, the first visit involves a discussion of risk and risk-reducing strategies, and subsequently patients who do not initiate chemoprevention are seen annually or bi-annually, based on patient-provider discretion. Patients who choose to initiate chemoprevention are contacted within 2 to 4 weeks to assess whether they have started chemoprevention and to discuss whether they have experienced any side effects. Patients who start chemoprevention will typically return for an assessment within 4 to 6 months of starting therapy. Tamoxifen 20 mg, raloxifene, or an aromatase inhibitor (AI) was offered prior to January 2019, with low-dose tamoxifen introduced as an additional option after January 2019 for appropriate patients, yet the clinical discussion included the fact that low-dose tamoxifen was not supported by robust large-scale randomized control trial data.
Follow-up was defined as last clinical contact, up to June 12, 2021 (this included both clinic visits and requests for a prescription renewal for a chemoprevention therapy). Patients were categorized into pre-2019 and post-2019 groups (using January 1, 2019, as a cutoff) to evaluate the chemoprevention initiation rates, based on the date of their initial visit. We examined chemoprevention regimens prescribed pre- vs. post-2019 by using each patient’s chemoprevention initiation date, and in the cases of those who discontinued therapy early, we collected all applicable reasons for discontinuation from the medical record.
Statistical analyses
Descriptive statistics summarized chemoprevention uptake over time as proportions at 3 and 6 months after their first B-PREP visit, and as medians and interquartile range (IQR). Rates of uptake were compared among patients whose initial visit was pre-2019 vs. post-2019, overall and stratified by menopausal status, using chi-squared tests and logistic regression.
We used univariable logistic regression to assess the association between patients’ baseline characteristics and initiation of any chemoprevention regimen. Variables with a p-value < 0.1 on univariable analysis were included in a multivariable logistic regression model.
We used Kaplan–Meier methods to evaluate the chemoprevention discontinuation rates, and the log rank test to compare discontinuation rates by regimen one year after initiation. Patients who tried multiple regimens were excluded from this analysis.
Finally, descriptive statistics were used to examine the reasons for discontinuation and outcomes as development of in situ or invasive breast cancer over the study period.
Results
Analytic cohort and chemoprevention initiation
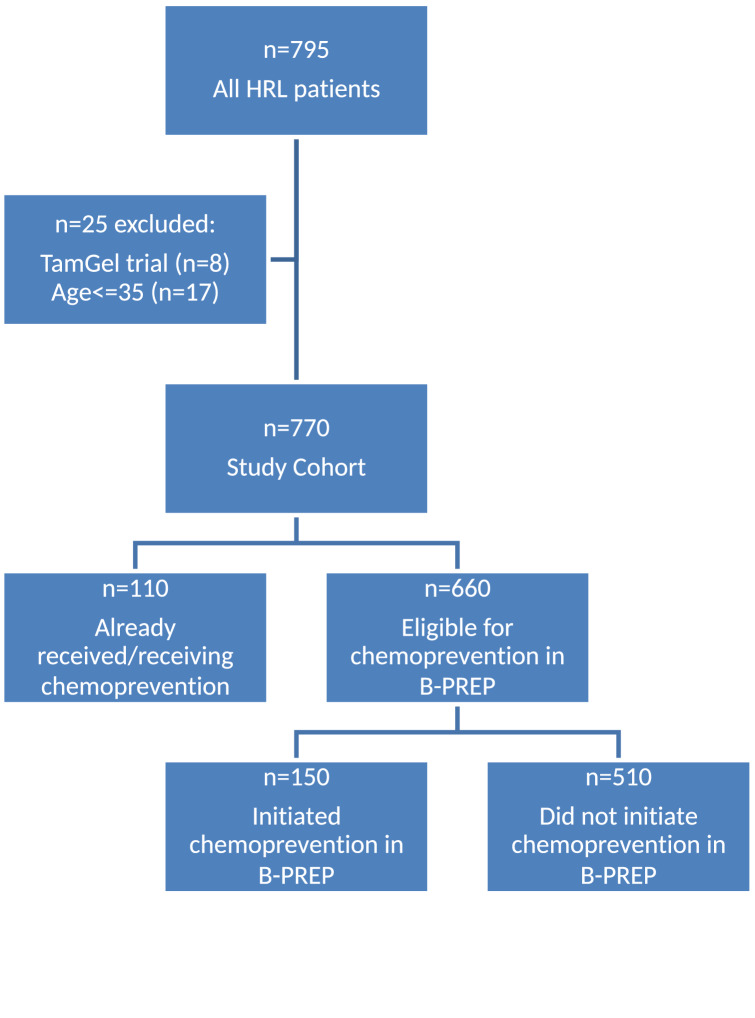
From April 2017 to January 2020, 795 patients with HRLs were evaluated in the B-PREP clinic and completed the risk assessment survey. We excluded 8 patients enrolled on intervention trials, 17 patients who were aged < 35 and 110 patients who had already received or were receiving chemoprevention at their first B-PREP encounter (Fig. 1).
Fig. 1.
Study flow diagram
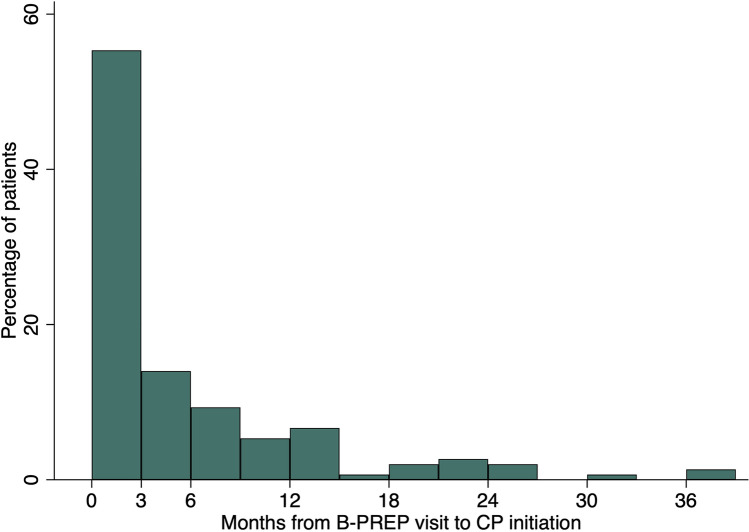
Among the 660 patients in the analytic cohort, 462 patients had their initial B-PREP visit between April 2017 and December 31, 2018, and 198 patients had their initial visit after January 1, 2019. The majority were White (81.5%) and postmenopausal (72.3%). 520 patients (78.8%) had atypical hyperplasia (ADH, ALH, or both) and 140 (21.2%) had LCIS with or without atypical hyperplasia. During the study period, 150 (22.7%) patients initiated chemoprevention. Median follow-up was 24 months (IQR 10–31) in the pre-2019 cohort and 10 months (IQR 1–15) in the post-2019 cohort. Median time from initial B-PREP visit to chemoprevention initiation was 54 days (IQR 0–209). 55.3% of patients (83/150) initiated chemoprevention within 3 months of their initial visit, 14.0% (21/150) in 3 to 6 months, and 30.7% (46/150) did not initiate chemoprevention until > 6 months from the initial visit (Fig. 2).
Fig. 2.
Distribution of time from initial B-PREP visit to chemoprevention initiation
Rates of chemoprevention uptake after the introduction of low-dose tamoxifen
The rate of chemoprevention uptake did not significantly differ based on whether patients had their first B-PREP visit pre- vs. post-2019. For women seen for their initial visit pre-2019, chemoprevention uptake was 21.2% (98/462) as compared to 26.3% (52/198) for those who established care post-2019 (p = 0.16). In premenopausal women, chemoprevention uptake was not significantly different in the pre- and post-2019 cohorts (25.4%, 30/118 vs. 30.8%, 20/65, p = 0.44). In postmenopausal women, chemoprevention was also not statistically different pre- and post-2019 (19.8%, 68/344 vs. 24.1%, 32/133, p = 0.30).
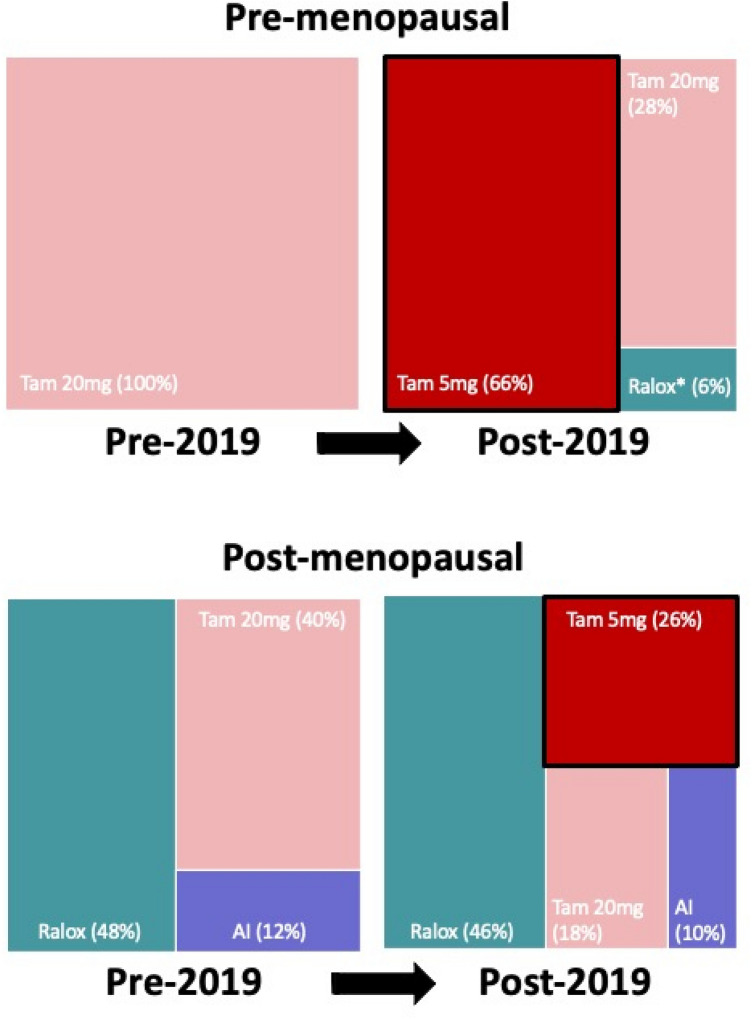
When we examined chemoprevention regimens based on the actual initiation date of therapy, the regimens initiated pre-2019 differed compared to those initiated post-2019 (p < 0.001, Fig. 3). Among patients who initiated chemoprevention pre-2019, the most commonly prescribed regimen was tamoxifen 20 mg in premenopausal women (100%, 18/18) and raloxifene in postmenopausal women (48.0%, 24/50, Supplemental Table 1). Among patients who initiated chemoprevention post-2019, low-dose tamoxifen was used in 65.6% (21/32) of premenopausal patients and 26.0% (13/50) of postmenopausal patients.
Fig. 3.
Chemoprevention regimen pre-2019 vs. post-2019 by menopausal status
Factors associated with chemoprevention initiation
By age category, women aged 41–50 had the highest rate of chemoprevention uptake (30.1%, 53/176) than other age categories: aged 35–40 (10.5%, 2/19), 51–60 (25.7%, 70/272), 61–70 (14.2%, 20/141), and aged > 70 (9.6%, 5/52, Table 1). Older women (aged 61–70 and aged > 70) were less likely to initiate chemoprevention compared to women aged 51–60 and this finding was statistically significant on univariable analyses, p = 0.008 and p = 0.02, respectively. In univariable analyses, age category (p < 0.001) and a family history of breast cancer (p = 0.04) were significantly associated with chemoprevention initiation (Table 1). Black or African American women were less likely than White women to initiate chemoprevention, and this was a borderline association (p = 0.08). Postmenopausal women were also less likely than pre- or perimenopausal women to begin chemoprevention (p = 0.08). Current and former smokers had a lower odds of chemoprevention than non-smokers and smoking status showed borderline associations on univariable analysis (p = 0.07, Table 1).
Table 1.
Baseline characteristics and univariable and multivariable logistic regression analyses evaluating factors associated with initiation of chemoprevention
| Characteristic | Total (n) | Chemoprevention initiated, n (column %) |
Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|---|---|---|
| No n = 510 |
Yes n = 150 |
OR (95% CI) | p-value | OR (95% CI) | p-value | ||
| Age | < 0.001 | ||||||
| 35–40 | 19 | 17 (3) | 2 (1) | 0.3 (0.1–1.5) | 0.16 | 0.4 (0.1–1.9) | 0.24 |
| 41–50 | 176 | 123 (24) | 53 (36) | 1.2 (0.8–1.9) | 0.31 | 1.4 (0.7–2.9) | 0.32 |
| 51–60 | 272 | 202 (40) | 70 (47) | Ref | – | Ref | – |
| 61–70 | 141 | 121 (24) | 20 (13) | 0.5 (0.3–0.8) | 0.008 | 0.5 (0.3–0.8) | 0.008 |
| > 70 | 52 | 47 (9) | 5 (3) | 0.3 (0.1–0.8) | 0.02 | 0.3 (0.1–0.8) | 0.01 |
| Race | 0.08 | ||||||
| White | 538 | 412 (81) | 126 (84) | Ref | – | Ref | – |
| Black/African American | 26 | 24 (5) | 2 (1) | 0.3 (0.1–1.2) | 0.08 | 0.3 (0.1–1.2) | 0.08 |
| Other | 81 | 59 (12) | 22 (15) | 1.2 (0.7–2.1) | 0.46 | 1.2 (0.7–2.1) | 0.47 |
| Unknown | 15 | 15 (3) | 0 (0) | No events | – | No events | – |
| Hispanic ethnicity | 0.61 | ||||||
| No | 580 | 445 (87) | 135 (90) | Ref | – | ||
| Yes | 57 | 42 (8) | 15 (10) | 1.2 (0.6–2.2) | 0.61 | ||
| Unknown | 23 | 23 (5) | 0 (0) | No events | – | ||
| Ashkenazi Jewish | 0.15 | ||||||
| No | 538 | 410 (80) | 128 (85) | Ref | – | ||
| Yes | 62 | 48 (9) | 14 (9) | 0.9 (0.5–1.7) | 0.83 | ||
| Unknown | 60 | 52 (10) | 8 (5) | 0.5 (0.2–1.0) | 0.07 | ||
| Menopausal status at first visit | 0.09 | ||||||
| Pre/Peri | 183 | 133 (26) | 50 (33) | Ref | – | Ref | – |
| Post | 477 | 377 (74) | 100 (67) | 0.7 (0.5–1.0) | 0.08 | 1.2 (0.6–2.5) | 0.56 |
| Alcohol use | 0.71 | ||||||
| Never/rarely | 356 | 278 (55) | 78 (52) | Ref | – | ||
| 1–4 drinks/week | 173 | 130 (25) | 43 (28) | 1.2 (0.8–1.8) | 0.45 | ||
| 5–9 drinks/week | 94 | 75 (15) | 19 (13) | 0.9 (0.5–1.6) | 0.72 | ||
| > 10 drinks/week | 14 | 9 (2) | 5 (3) | 2.0 (0.6–6.1) | 0.23 | ||
| Unknown | 23 | 18 (4) | 5 (3) | 1.0 (0.4–2.8) | 0.99 | ||
| BMI | 0.34 | ||||||
| Under/Normal (< 25) | 247 | 188 (37) | 59 (39) | Ref | – | ||
| Overweight (25–29.9) | 220 | 165 (32) | 55 (37) | 1.1 (0.7–1.6) | 0.78 | ||
| Obese (> 30.0) | 187 | 151 (30) | 36 (24) | 0.8 (0.5–1.2) | 0.25 | ||
| Unknown | 6 | 6 (1) | 0 (0) | No events | – | ||
| Smoking | 0.07 | ||||||
| Never | 431 | 324 (64) | 107 (71) | Ref | – | Ref | |
| Former | 185 | 147 (29) | 38 (25) | 0.8 (0.5–1.2) | 0.25 | 0.9 (0.6–1.4) | 0.67 |
| Current | 20 | 19 (4) | 1 (1) | 0.2 (0.0–1.2) | 0.08 | 0.1 (0.0–1.1) | 0.07 |
| Unknown | 24 | 20 (4) | 4 (3) | 0.6 (0.2–1.8) | 0.37 | 1.3 (0.4–4.3) | 0.72 |
| Breast density on mammogram | 0.28 | ||||||
| 1 or 2 | 208 | 166 (32) | 42 (28) | Ref | – | ||
| 3 or 4 | 391 | 294 (58) | 97 (65) | 1.3 (0.9–2.0) | 0.20 | ||
| Unknown | 61 | 51 (10) | 11 (7) | 0.9 (0.4–1.8) | 0.71 | ||
| Family history of breast cancer | 0.04 | ||||||
| No | 286 | 232 (45) | 54 (36) | Ref | – | Ref | |
| Yes | 374 | 278 (55) | 96 (64) | 1.5 (1.0–2.2) | 0.04 | 1.6 (1.1–2.3) | 0.03 |
| Genetic risk (defined as BRCAPRO or Myriad score > 5%) | 0.74 | ||||||
| No | 560 | 434 (85) | 126 (84) | Ref | – | ||
| Yes | 100 | 76 (15) | 24 (16) | 1.1 (0.7–1.8) | 0.74 | ||
| HRL type | 0.17 | ||||||
| Atypical hyperplasia | 520 | 408 (80) | 112 (75) | Ref | – | ||
| LCIS | 140 | 102 (20) | 38 (25) | 1.4 (0.9–2.1) | 0.16 | ||
P-values are in bold represent association between baseline characteristic and initiation of chemoprevention for the univarable analysis. (this is distinct from p-values representing OR for a given row)
BMI body mass index; CI confidence interval; OR odds ratio; LCIS lobular carcinoma in situ
In the multivariable model, only age and family history of breast cancer remained significantly associated with chemoprevention uptake. Notably, the association between menopausal status and chemoprevention uptake was attenuated in this model. Women aged 61–70 years (odds ratio (OR) 0.5, 95% confidence interval (CI) 0.3–0.8, p = 0.007) or aged > 70 years (OR 0.3, 95% CI 0.1–0.8, p = 0.01) were less likely to initiate chemoprevention compared to the reference age category of 51–60 years. Women aged ≤ 40 years were less likely to initiate chemoprevention, but this was not statistically significant (OR 0.4, 95% CI 0.1–1.9, p = 0.24). Chemoprevention use was lower in Black or African American women compared to White women (OR 0.3, 95% CI 0.1–1.2, p = 0.08) although this did not reach statistical significance. Odds of chemoprevention uptake were also nonsignificantly lower among smokers (OR 0.15, 95% CI 0.02–1.1, p = 0.07).
Rates of chemoprevention discontinuation by therapy
Among the 150 patients who initiated chemoprevention in B-PREP, median follow-up from chemoprevention initiation was 15 months (IQR 8–24). 17 (11.2%) patients were excluded from the analysis of discontinuation due to loss of follow-up (n = 8) or because they tried multiple chemoprevention therapies (n = 9). Discontinuation rates among the remaining 133 patients were calculated (Table 2 and Supplemental Fig. 1). At 1 year, 20.0% (95% CI 3.1–79.6%) of patients who had started an AI, 20.4% (95% CI 10.7–36.8%) who had started raloxifene, and 15.0% (95% CI 7.4–29.0%) who had started tamoxifen 20 mg had discontinued therapy, compared to 6.7% (95% CI 1.7–24.1%) of women who started low-dose tamoxifen (p = 0.55, Table 2).
Table 2.
Rates of chemoprevention discontinuation by regimen at 1 year among patients who tried a single regimen
| 1 year | ||
|---|---|---|
| Rate (%) | 95% CI (%) | |
| Tamoxifen 20 mg | 15.0 | 7.4–29.0 |
| Tamoxifen 5 mg | 6.7 | 1.7–24.1 |
| Raloxifene | 20.4 | 10.7–36.8 |
| Aromatase Inhibitor | 20.0 | 3.1–79.6 |
CI confidence interval
Among the 9 patients who changed chemoprevention regimens during the follow-up period, 7 were initially on tamoxifen 20 mg and 2 were on raloxifene. All 7 patients on tamoxifen 20 mg changed to low-dose tamoxifen and one ultimately discontinued therapy. One of the 2 patients on raloxifene changed to tamoxifen 20 mg and one changed to low-dose tamoxifen; the patient who tried low-dose tamoxifen ultimately discontinued therapy due to side effects. In summary, 6 of 9 patients who started a standard chemoprevention regimen changed to low-dose tamoxifen and have continued this therapy. An additional patient changed to tamoxifen 20 mg and has continued treatment.
Reasons for discontinuation
Overall, 32 patients (21.3%) discontinued their first chemoprevention regimen and 23 did not try a new chemoprevention regimen. Among the 9 patients who discontinued tamoxifen 20 mg, reasons for cessation included vasomotor symptoms (2), abnormal liver function tests (2), skin dryness (1), vaginal dryness (1), mood changes (1), insomnia (1), “decided to stop” (1), Crohn’s flare (1), and hirsutism (1). Among 3 patients who discontinued low-dose tamoxifen, 2 reported vasomotor symptoms and one reported “better off tamoxifen” as reasons for discontinuation. For the one female who stopped AI therapy, vasomotor symptoms were the reason for discontinuation. Among 10 patients who stopped raloxifene, reasons for discontinuation included vasomotor (6), thrombus (1), small intestinal bacterial overgrowth (1), “did not tolerate, unspecified” (1) and a diagnosis of pancreatic cancer (1).
Outcomes
Overall, 10/660 (1.7%) patients developed in situ (n = 3) or invasive (n = 7) breast cancer during a median follow-up of 15.2 m (IQR 6.0–27.1 m); an additional 1 patient developed an angiosarcoma of the breast and was censored at this time. The 3-year rate of breast cancer development was 3.9% (95% CI 1.7–8.7%). Among those who developed breast cancer, 8 had never initiated chemoprevention (6 invasive, 2 DCIS) and 2 had taken chemoprevention only briefly (5 months and < 2 weeks) and were not taking chemoprevention at the time of their cancer diagnosis.
Discussion
In a cohort of women with HRLs evaluated in our specialized high-risk breast clinic who were eligible for chemoprevention, 22.7% (150/660) initiated a chemoprevention regimen. Chemoprevention uptake after the introduction of low-dose tamoxifen was ~ 20% higher (pre-2019: 21.2% vs post-2019: 26.3%), but this difference did not reach statistical significance in our study. After low-dose tamoxifen became available for chemoprevention, low-dose tamoxifen was the most commonly prescribed option with 65.6% of premenopausal and 26.0% of postmenopausal women selecting this therapy. To the best of our knowledge, this is the first report on the uptake of low-dose tamoxifen in real-world patients with HRLs. These findings suggests that low-dose option may have facilitated chemoprevention initiation in a small group of women who otherwise would not have initiated chemoprevention at all.
In women at high-risk for breast cancer, chemoprevention reduces the risk of hormone receptor-positive breast cancer by ~ 50%. Despite excellent clinical trial data [1–6] and national guidelines encouraging chemoprevention among women with an increased risk of breast cancer [19, 20], uptake has been low [21]. Two meta-analyses reported low uptake of chemoprevention in non-trial settings: 5.8% in one (after excluding an outlying study [12]) and 8.7% in a second meta-analysis [11]. Academic centers that offer high-risk breast cancer clinics may improve upon the low chemoprevention rates. For example, both our DFCI/BWCC high-risk program experience and a single-institution study from Memorial Sloan Kettering Cancer Center demonstrated chemoprevention uptake greater than 20% compared to average uptake of < 10% [16]. In the MSKCC study, 41.8% had LCIS and 50.3% had ADH or ALH, and these characteristics were statistically associated with chemoprevention use compared to patients without a HRL diagnosis.
In this context, the B-PREP clinic was designed to provide personalized care for women at high-risk of developing breast cancer with a focus on women with HRLs. In B-PREP, most patients did not initiate chemoprevention at their first visit, but among those who did initiate chemoprevention, most did so within 55 days. In fact, 30.7% of those who initiated chemoprevention began > 6 months after their first visit to B-PREP. This may demonstrate that ongoing discussions and education about chemoprevention among women with HRLs can increase uptake and longer follow-up of this cohort may demonstrate higher chemoprevention use. We hope to investigate this observation in future by investigating whether the number of visits to B-PREP influences chemoprevention uptake.
One notable aspect of our study is that we investigated patient characteristics that may be predictors of chemoprevention initiation in this HRL cohort. Among 11 established breast cancer risk factors, only age and a family history of breast cancer were predictive of chemoprevention uptake in the multivariable model. In a similar study from New York [16], women with confirmed genetic mutations (including ATM, BRCA1, BRCA2, CDH1, CHEK2, MSH2/MLH1, TP53, and MUTYH) were more likely to initiate chemoprevention. We did not examine this factor specifically in our cohort since patients who have undergone germline genetic testing and who have been identified as having a pathogenic variant in a hereditary cancer gene are not followed in B-PREP. Among patients who have not undergone germline genetic testing, we did examine estimated genetic risk defined as BRCAPRO or Myriad score > 5% as a proxy for hereditary cancer syndrome, and this was not significant on univariable analyses to be associated with chemoprevention uptake. These findings compared to prior work may represent that patients with a confirmed germline pathogenic variant in a cancer gene have a greater awareness of their cancer risk than patients who may be at risk for a cancer gene and await cancer genetic testing.
Age was predictive of chemoprevention uptake, with women aged 41–60 more likely to initiate chemoprevention compared to women aged > 61 or aged < 40. Overall, uptake among women ages ≤ 50 was higher than in prior work [16, 22]. In our study, 28.2% (55/195) of women aged ≤ 50 initiated chemoprevention compared to 10.1% (37/337) at a single institute in New York and 10.6% (136/1279) in the United Kingdom. Our higher uptake may be due to the B-PREP model and how providers counsel patients with HRLs on chemoprevention as there was not an increase in overall uptake among premenopausal women after the introduction of low-dose tamoxifen in January 2019. There are ongoing efforts exploring how certain clinical interventions like decision aids may enhance chemoprevention among women who are high-risk for developing breast cancer (NCT03069742) [23].
Although not statistically significant, our findings show that White women were more likely to initiate chemoprevention (23.4%) compared to Black women (7.7%). Disparities in breast cancer prevention, screening, and care between Black and White women are well established in the literature [24–28]. We plan to explore barriers to chemoprevention in this population and design interventions so that we may better counsel women of color about their breast cancer risk and engage them in risk-reducing strategies.
This manuscript is the first to report on the uptake of low-dose tamoxifen in the real world. Our study demonstrates that low-dose tamoxifen is a widely accepted option for chemoprevention among patients in the B-PREP clinic. Among premenopausal women, 65.6% elected tamoxifen 5 mg/day after January 2019. Among postmenopausal women, low-dose tamoxifen also became a popular choice. Due to the limited follow-up, we cannot make conclusions on whether low-dose tamoxifen will remain a popular option in future. Our clinical use and counseling around low-dose tamoxifen have evolved since we introduced it into our practice, as recent data suggest that postmenopausal women or women with low estradiol levels (< 15.8 pg/mL) may derive the most benefit [29], and therefore in premenopausal women who start and tolerate low-dose tamoxifen, we are gradually titrating the dose up to 20 mg as a potential strategy to overcome any intolerable side effects that occur with starting 20 mg of tamoxifen upfront. Additionally, we counsel our patients with HRLs and increased breast density that low-dose tamoxifen may reduce their mammographic density which could aid in early detection and reduce the excess risk associated with high mammographic density [30].
When low-dose tamoxifen was introduced into our clinic in 2019, there was significant media coverage both locally and nationally about this therapy. This momentum continued from January 2019 to May 2019 when ASCO Clinical Practice Guidelines updated their recommendations to support low-dose tamoxifen as an option for chemoprevention [7]. In September 2019, the United States Preventative Services Task Force updated their 2013 guidelines [31] and advocated for clinicians to consider chemoprevention among women high-risk of breast cancer. Increased awareness of low-dose tamoxifen due to media coverage and guideline changes regarding this option may have influenced patients’ willingness to try a chemoprevention therapy.
Notably, our findings showed discontinuation rates at 1 year were lower among women who began low-dose tamoxifen (6.7%) compared to tamoxifen 20 mg (15.0%), AI (20.0%), or raloxifene (20.4%). Although this finding was not statistically significant, possibly due to small sample sizes, this trend suggests that low-dose tamoxifen may be more tolerable than other chemoprevention agents (raloxifene and AI) and the 20 mg tamoxifen dosing. Conclusions about discontinuation rates beyond 1 year are limited since sample sizes were small and confidence intervals were wide; we hope in future to report on this endpoint with longer follow-up and a larger cohort. Additionally, low-dose tamoxifen seems to be a good option if a patient does not tolerate their first chemoprevention as 66% of patients who discontinued a standard chemoprevention were able to start and tolerate low-dose tamoxifen. With longer follow-up, we hope to further explore discontinuation trends and to investigate if duration of chemoprevention changes with the introduction of low-dose tamoxifen from 5 to 3 years.
Limitations of this study include that the results may not be generalizable to other settings since B-PREP is a specialized clinic at an academic center. However, aspects of the B-PREP program can likely be replicated in other centers to optimize high-risk breast cancer care. Our assessment of low-dose tamoxifen uptake pre- and post-2019 was performed considering date of first visit in the B-PREP clinic and by date of chemoprevention uptake, and we presumed that all providers in B-PREP began to offer low-dose tamoxifen starting in January 2019. However, it is possible that not all patients in the post-2019 cohort were offered low-dose tamoxifen since some providers may have been slower to incorporate the low-dose tamoxifen option into their clinical practice, and we did not explore chemoprevention uptake by provider. We do know that eight of the patients among 462 who established care in B-PREP pre-2019 started low-dose tamoxifen post-2019, and therefore, our estimates of uptake in the low-dose tamoxifen era may be underestimates. Our findings are also limited by the available data on chemoprevention uptake, adherence, and discontinuation in the electronic medical record and missing data due to a lack of provider documentation, which may have influenced our multivariate analyses. Long-term follow-up on adherence and discontinuation of chemoprevention is also limited and may have influenced the findings, especially as this effort overlapped with the COVID-19 pandemic.
Conclusion
Our findings demonstrate that the introduction of low-dose tamoxifen in 2019 influenced patterns of care for women with HRLs in our program. Although low-dose tamoxifen did not increase overall chemoprevention uptake to a statistically significant level, low-dose tamoxifen quickly became the most popular option among premenopausal women and a preferred option among postmenopausal women compared to tamoxifen 20 mg. Patients with HRLs who decline chemoprevention after an initial discussion may reconsider and initiate chemoprevention at a later date if offered low-dose tamoxifen as an option. In our experience, low-dose tamoxifen has a favorable side effect profile and lower discontinuation rate compared to other chemoprevention regimens. We hope these findings describing the use of low-dose tamoxifen in the real world may prompt future studies to better characterize the long-term benefits of low-dose tamoxifen in premenopausal and postmenopausal females who are at high-risk of developing breast cancer.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
BB, AL, and TK contributed to conceptualization and design, data collection/curation, data analysis, visualization, writing—original draft, and review & editing. FK and LEP contributed to data collection/curation, writing—original draft, and review & editing. MH contributed to conceptualization and design, data collection/curation, and writing—review & editing. MKG, RS, and JG contributed to data collection/curation and writing—review & editing.
Funding
This research was funded in part by a Leadership Development Grant from the Susan G. Komen Foundation.
Data availability
All data generated or analyzed during this study are included in this published article.
Declarations
Conflict of interest
J.E.G. reports institutional research funding from Myriad Genetics, Ambry Genetics, and Invitae Genetics; consulting for Helix Genetics (compensation) and Earli (no compensation); leading two clinical trials for Astra-Zeneca; serving on the scientific advisory board of Konica Minolta (no compensation); conducting a sponsored lecture for Clinical Care Options, LLC; Editorial Services publications, President, Fellows of the AACR Academy, and member, Foundation Board of the American Association for Cancer Research; co-scientific director, Breast Cancer Research Foundation; board of directors, Facing Our Risk of Cancer Empowered; spousal consulting fees from Novartis Oncology, GTx Pharmaceuticals, Aleta BioTherapeutics and H3 Biomedicine, Inc.; a spousal advisory board membership at Oric Pharmaceuticals; and spousal scientific advisory board memberships at Kronos Bio, Susan G. Komen for the Cure, James P. Wilmot Foundation, Inc., Diane Helis Henry Medical Research Foundation, Adrienne Helis Melvin Medical Research Foundation, and Global Biological Standards Institute. T.K. reports speakers honoraria and advisory board for Exact Sciences (formerly Genomic Health); Faculty – PrecisCa Cancer information service; and serving on the Global advisory board for Besins Healthcare . All remaining authors have declared no conflict of interest.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the IRB of Brigham and Women’s Hospital.
Waiver of consent
The study was approved by the Brigham and Women’s Institutional Review Board as a low-risk study and approved with a waiver of consent.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, Vogel V, Robidoux A, Dimitrov N, Atkins J, Daly M, Wieand S, Tan-Chiu E, Ford L, Wolmark N. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90(18):1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 2.Goss PE, Ingle JN, Ales-Martinez JE, Cheung AM, Chlebowski RT, Wactawski-Wende J, McTiernan A, Robbins J, Johnson KC, Martin LW, Winquist E, Sarto GE, Garber JE, Fabian CJ, Pujol P, Maunsell E, Farmer P, Gelmon KA, Tu D, Richardson H, Investigators NCMS Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364(25):2381–2391. doi: 10.1056/NEJMoa1103507. [DOI] [PubMed] [Google Scholar]
- 3.Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, Bevers TB, Fehrenbacher L, Pajon ER, Wade JL, 3rd, Robidoux A, Margolese RG, James J, Runowicz CD, Ganz PA, Reis SE, McCaskill-Stevens W, Ford LG, Jordan VC, Wolmark N, National Surgical Adjuvant B, Bowel P. Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 Trial: preventing breast cancer. Cancer Prev Res (Phila) 2010;3(6):696–706. doi: 10.1158/1940-6207.CAPR-10-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cuzick J, Sestak I, Forbes JF, Dowsett M, Knox J, Cawthorn S, Saunders C, Roche N, Mansel RE, von Minckwitz G, Bonanni B, Palva T, Howell A, Investigators I-I Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial. Lancet. 2014;383(9922):1041–1048. doi: 10.1016/S0140-6736(13)62292-8. [DOI] [PubMed] [Google Scholar]
- 5.Cuzick J, Sestak I, Cawthorn S, Hamed H, Holli K, Howell A, Forbes JF, Investigators I-I. Tamoxifen for prevention of breast cancer: extended long-term follow-up of the IBIS-I breast cancer prevention trial. Lancet Oncol. 2015;16(1):67–75. doi: 10.1016/S1470-2045(14)71171-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeCensi A, Puntoni M, Guerrieri-Gonzaga A, Caviglia S, Avino F, Cortesi L, Taverniti C, Pacquola MG, Falcini F, Gulisano M, Digennaro M, Cariello A, Cagossi K, Pinotti G, Lazzeroni M, Serrano D, Branchi D, Campora S, Petrera M, Buttiron Webber T, Boni L, Bonanni B. Randomized placebo controlled trial of low-dose tamoxifen to prevent local and contralateral recurrence in breast intraepithelial neoplasia. J Clin Oncol. 2019;37(19):1629–1637. doi: 10.1200/JCO.18.01779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Visvanathan K, Fabian CJ, Bantug E, Brewster AM, Davidson NE, DeCensi A, Floyd JD, Garber JE, Hofstatter EW, Khan SA, Katapodi MC, Pruthi S, Raab R, Runowicz CD, Somerfield MR. Use of endocrine therapy for breast cancer risk reduction: ASCO clinical practice guideline update. J Clin Oncol. 2019;37(33):3152–3165. doi: 10.1200/JCO.19.01472. [DOI] [PubMed] [Google Scholar]
- 8.Port ER, Montgomery LL, Heerdt AS, Borgen PI. Patient reluctance toward tamoxifen use for breast cancer primary prevention. Ann Surg Oncol. 2001;8(7):580–585. doi: 10.1007/s10434-001-0580-9. [DOI] [PubMed] [Google Scholar]
- 9.Fagerlin A, Zikmund-Fisher BJ, Nair V, Derry HA, McClure JB, Greene S, Stark A, Hensley Alford S, Lantz P, Hayes DF, Wiese C, Claud Zweig S, Pitsch R, Jankovic A, Ubel PA. Women's decisions regarding tamoxifen for breast cancer prevention: responses to a tailored decision aid. Breast Cancer Res Treat. 2010;119(3):613–620. doi: 10.1007/s10549-009-0618-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor R, Taguchi K. Tamoxifen for breast cancer chemoprevention: low uptake by high-risk women after evaluation of a breast lump. Ann Fam Med. 2005;3(3):242–247. doi: 10.1370/afm.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith SG, Sestak I, Forster A, Partridge A, Side L, Wolf MS, Horne R, Wardle J, Cuzick J. Factors affecting uptake and adherence to breast cancer chemoprevention: a systematic review and meta-analysis. Ann Oncol. 2016;27(4):575–590. doi: 10.1093/annonc/mdv590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ropka ME, Keim J, Philbrick JT. Patient decisions about breast cancer chemoprevention : a systematic review and meta-analysis. J Clin Oncol. 2010;28(18):3090–3095. doi: 10.1200/JCO.2009.27.8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bober SL, Hoke LA, Duda RB, Regan MM, Tung NM. Decision-making about tamoxifen in women at high risk for breast cancer: clinical and psychological factors. J Clin Oncol. 2004;22(24):4951–4957. doi: 10.1200/JCO.2004.05.192. [DOI] [PubMed] [Google Scholar]
- 14.Heisey R, Pimlott N, Clemons M, Cummings S, Drummond N. Women's views on chemoprevention of breast cancer: qualitative study. Can Fam Phys. 2006;52:624–625. [PMC free article] [PubMed] [Google Scholar]
- 15.Weiss A, Grossmith S, Cutts D, Mikami SA, Suskin JA, Graichen MK, Rojas NA, Pace LE, Joyce E, Rhei E, Scheib R, Bychkovsky B, Garber JE, Morganstern D, King TA. Customized breast cancer risk assessment in an ambulatory clinic: a portal for identifying women at risk. Breast Cancer Res Treat. 2019;175(1):229–237. doi: 10.1007/s10549-018-05116-5. [DOI] [PubMed] [Google Scholar]
- 16.Flanagan MR, Zabor EC, Stempel M, Mangino DA, Morrow M, Pilewskie ML. Chemoprevention uptake for breast cancer risk reduction varies by risk factor. Ann Surg Oncol. 2019;26(7):2127–2135. doi: 10.1245/s10434-019-07236-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roche CA, Tang R, Coopey SB, Hughes KS. Chemoprevention acceptance and adherence in women with high-risk breast lesions. Breast J. 2019;25(2):190–195. doi: 10.1111/tbj.13064. [DOI] [PubMed] [Google Scholar]
- 18.Afimoxifene in reducing the risk of breast cancer in women with mammographically dense breast. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03063619. Accessed 15 Sept 2020
- 19.Visvanathan K, Hurley P, Bantug E, Brown P, Col NF, Cuzick J, Davidson NE, Decensi A, Fabian C, Ford L, Garber J, Katapodi M, Kramer B, Morrow M, Parker B, Runowicz C, Vogel VG, 3rd, Wade JL, Lippman SM. Use of pharmacologic interventions for breast cancer risk reduction: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2013;31(23):2942–2962. doi: 10.1200/jco.2013.49.3122. [DOI] [PubMed] [Google Scholar]
- 20.Moyer VA, USPST Force Medications to decrease the risk for breast cancer in women: recommendations from the US Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159(10):698–708. doi: 10.7326/0003-4819-159-10-201311190-00717. [DOI] [PubMed] [Google Scholar]
- 21.Bevers TB, Ward JH, Ahrendt G, Arun BK, Cohen JG, Colditz GA, al. e NCCN Clinical Practice Guidelines in Oncology. Breast Cancer Risk Reduction. Version 1.2021. https://www.nccn.org/professionals/physician_gls/pdf/breast_risk.pdf. Accessed 13 July 2021.
- 22.Donnelly LS, Evans DG, Wiseman J, Fox J, Greenhalgh R, Affen J, Juraskova I, Stavrinos P, Dawe S, Cuzick J, Howell A. Uptake of tamoxifen in consecutive premenopausal women under surveillance in a high-risk breast cancer clinic. Br J Cancer. 2014;110(7):1681–1687. doi: 10.1038/bjc.2014.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Study of Web-based Decision Aids for Increasing Breast Cancer Chemoprevention in the Primary Care Setting. https://clinicaltrials.gov/ct2/show/NCT03069742. Accessed 5 Jan 2022
- 24.Hunt BR, Hurlbert MS. Black:white disparities in breast cancer mortality in the 50 largest cities in the United States, 2005–2014. Cancer Epidemiol. 2016;45:169–173. doi: 10.1016/j.canep.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 25.Jemal A, Ward EM, Johnson CJ, Cronin KA, Ma J, Ryerson B, Mariotto A, Lake AJ, Wilson R, Sherman RL, Anderson RN, Henley SJ, Kohler BA, Penberthy L, Feuer EJ, Weir HK. Annual Report to the Nation on the Status of Cancer, 1975–2014 featuring survival. J Natl Cancer Inst. 2017 doi: 10.1093/jnci/djx030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCarthy AM, Bristol M, Domchek SM, Groeneveld PW, Kim Y, Motanya UN, Shea JA, Armstrong K. Health care segregation, physician recommendation, and racial disparities in BRCA1/2 testing among women with breast cancer. J Clin Oncol. 2016;34(22):2610–2618. doi: 10.1200/JCO.2015.66.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee CI, Zhu W, Onega T, Henderson LM, Kerlikowske K, Sprague BL, Rauscher GH, O'Meara ES, Tosteson ANA, Haas JS, diFlorio-Alexander R, Kaplan C, Miglioretti DL. Comparative access to and use of digital breast tomosynthesis screening by women's race/ethnicity and socioeconomic status. JAMA Netw Open. 2021;4(2):e2037546. doi: 10.1001/jamanetworkopen.2020.37546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haas JS, Hill DA, Wellman RD, Hubbard RA, Lee CI, Wernli KJ, Stout NK, Tosteson AN, Henderson LM, Alford-Teaster JA, Onega TL. Disparities in the use of screening magnetic resonance imaging of the breast in community practice by race, ethnicity, and socioeconomic status. Cancer. 2016;122(4):611–617. doi: 10.1002/cncr.29805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DeCensi A, Puntoni M, Johansson H, Guerrieri-Gonzaga A, Caviglia S, Avino F, Cortesi L, Ponti A, Pacquola MG, Falcini F, Gulisano M, Digennaro M, Cariello A, Cagossi K, Pinotti G, Lazzeroni M, Serrano D, Briata IM, Buttiron Webber T, Boni L, Bonanni B. Effect modifiers of low-dose tamoxifen in a randomized trial in breast noninvasive disease. Clin Cancer Res. 2021;27(13):3576–3583. doi: 10.1158/1078-0432.CCR-20-4213. [DOI] [PubMed] [Google Scholar]
- 30.Eriksson M, Czene K, Conant EF, Hall P. Use of low-dose tamoxifen to increase mammographic screening sensitivity in premenopausal women. Cancers (Basel) 2021;13:2. doi: 10.3390/cancers13020302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Force USPST, Owens DK, Davidson KW, Krist AH, Barry MJ, Cabana M, Caughey AB, Doubeni CA, Epling JW, Jr, Kubik M, Landefeld CS, Mangione CM, Pbert L, Silverstein M, Tseng CW, Wong JB. Medication use to reduce risk of breast cancer: US preventive services task force recommendation statement. JAMA. 2019;322(9):857–867. doi: 10.1001/jama.2019.11885. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article.