Abstract
Summary
Follow-up raloxifene therapy after denosumab discontinuation resulted in a decrease in bone mass to the pre-denosumab levels and a rebound increase of bone turnover markers. The decrease in lumbar bone mineral density was particularly evident when the body mass index was low, there were previous vertebral fractures, or lumbar bone mineral density before denosumab administration was low.
Introduction
Selective estrogen receptor modulators may be an alternative to bisphosphonates for treating rebound resorption after discontinuing denosumab. This study aimed to investigate the effects of follow-up raloxifene therapy after denosumab discontinuation in postmenopausal women.
Methods
This retrospective observational study included 61 patients who received 12-month follow-up raloxifene therapy after denosumab discontinuation. The primary endpoint was the bone mineral density change. The secondary endpoints were the changes in bone turnover markers and the incidence of new vertebral fractures.
Results
Raloxifene administration for 12 months after denosumab discontinuation resulted in a significantly lower bone mineral density at all sites compared to the level at 6 months after the last denosumab treatment (lumbar spine, − 5.48%; femoral neck, − 2.95%; total hip, − 3.52%; all, p < 0.001). The decrease in lumbar bone mineral density was particularly evident when the body mass index was low, there were previous vertebral fractures, or lumbar bone mineral density before denosumab administration was low. Marked increases in the bone turnover markers from baseline were noted after switching to raloxifene. However, no new vertebral fractures occurred during raloxifene treatment.
Conclusions
Follow-up raloxifene therapy after denosumab discontinuation resulted in a decrease in bone mass to the pre-denosumab levels and a rebound increase of bone turnover markers. Therefore, raloxifene administered sequentially after denosumab discontinuation was not effective in preventing rebound phenomenon.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00198-022-06388-w.
Keywords: Bone density, Denosumab, Raloxifene hydrochloride, Spinal fracture
Introduction
Denosumab is an osteoporosis treatment with a potent antiresorptive effect [1]. It has been widely prescribed in Korea for the treatment of various osteoporotic diseases, including postmenopausal osteoporosis, male osteoporosis, glucocorticoid-induced osteoporosis, and bone loss in patients with prostate cancer or those with breast cancer undergoing hormone ablation since 2017. In a large randomized controlled trial of denosumab, vertebral bone mineral density (BMD) increased by more than 5% when the drug was administered for 1 year [2, 3]. A similar increase in BMD and reduction in fracture risk was observed in several real-world practice studies [4–6]. Based on its efficacy in increasing bone density and reducing fractures, recent osteoporosis treatment guidelines have recommended denosumab as the alternative initial treatment for patients at high risk of osteoporotic fracture [7, 8].
Denosumab is an important therapeutic option for osteoporosis. However, there are concerns regarding the “rebound phenomenon” in which the beneficial skeletal effect of denosumab is rapidly reversed by a significant increase in osteoclast number and activity when discontinued, resulting in a profound increase in bone turnover compared to that at the pre-treatment level. When denosumab is discontinued, a rapid increase in bone resorption and a significant decrease in BMD have been reported, along with multiple vertebral fractures in some patients [9–15]. Therefore, another antiresorptive agent should be administered after discontinuing denosumab [16–18]. Among the currently available antiresorptive agents, most clinical data are available on the effect of zoledronic acid or alendronate after denosumab discontinuation [19–22]. Although raloxifene, a selective estrogen receptor modulator (SERM), has antiresorptive activity [7, 18], only a case report and a small study have evaluated its effect after denosumab discontinuation [23, 24].
According to the reimbursement criteria for osteoporosis medications of the Korea National Health Insurance System, denosumab is no longer reimbursed if the bone density increases to osteopenia level at all skeletal sites. Therefore, when bone density increases with denosumab, this costly treatment is discontinued and replaced by cheaper alternatives, such as generic oral bisphosphonates. In clinical situations where bisphosphonates cannot be used, raloxifene could be an alternative. There is a widespread belief that weak antiresorptive drugs, such as raloxifene, are insufficient as a follow-on therapy for denosumab; however, there is no evidence supporting this claim. Therefore, we aimed to analyze the changes in BMD, bone turnover markers (BTMs), and vertebral fracture incidence with raloxifene administration after discontinuation of denosumab.
Materials and methods
Study design and population
This was a retrospective observational study conducted at Seoul St. Mary’s Hospital. We screened 81 postmenopausal osteoporotic women who were switched to raloxifene therapy after denosumab was discontinued between January 2018 and June 2020. All patients were treated with denosumab (60 mg subcutaneously once every 6 months) 2 to 5 times. In some patients, bisphosphonate was administered for various durations prior to denosumab administration. After discontinuation, raloxifene (60 mg daily) was started, without delay, 6 months after the last denosumab administration and was administered for at least 12 months. Patients with underlying diseases or medications that could influence BMD or BTMs were excluded from the analysis. After exclusions, 61 patients were included in the final analysis (Supplementary Fig. 1).
All patients were prescribed elemental calcium (500 mg) as calcium carbonate with cholecalciferol (1000 IU) daily (DicaMax 1000 Tab, Dalim Biotech, Wonju, Korea). Dual-energy X-ray absorptiometry (DXA) was performed at the start of denosumab administration (DXA1) and 6 months after the last administration of denosumab (DXA2). DXA was also performed 12 months after raloxifene administration (DXA3). BTMs and other biochemical markers were measured every 6 months. Lumbar spine anteroposterior and lateral radiography was conducted at DXA3. This study was approved by the Institutional Review Board of The Catholic University of Korea (KC20RISI0933).
Measurement of bone mineral density
The BMD (g/cm2) values of the lumbar spine, femoral neck, and total hip were measured using DXA (Horizon W, Hologic, Inc., Bedford, MA, USA) and analyzed by the same trained technician. The instruments were calibrated using a device-specific lumbar phantom in accordance with the manufacturer’s instructions. Changes in BMD are expressed as mean ± standard deviation (SD) with percentage changes. The least significant change was 0.030 g/cm2 for lumbar spine, 0.028 g/cm2 for femoral neck, and 0.027 g/cm2 for total hip. Normal BMD, osteopenia, and osteoporosis were defined by the lowest T-score at the lumbar spine, femoral neck, and total hip (normal BMD, T-score ≥ − 1.0; osteopenia, − 1.0 < T-score < − 2.5; and osteoporosis, T-score ≤ − 2.5) [25]. Our institution performed quality control of the bone density equipment according to the manufacturer’s protocol to control the quality of the bone density measurements.
Other measurements
Serum calcium, phosphorus, albumin, and creatinine levels were determined using an autoanalyzer (747 automatic analyzer, Hitachi, Tokyo, Japan). The serum cross-linked C-telopeptide of type I collagen (CTx) (Elecsys B-CrossLaps, Roche Diagnostics, Rotkreuz, Switzerland) and procollagen type 1 N-terminal propeptide (P1NP) (Elecsys Total P1NP, Roche Diagnostics) were determined in duplicate using an electrochemiluminescence immunoassay analyzer (Cobas e801, Roche Diagnostics). The 25-hydroxyvitamin D (25(OH)D) level (Access 25(OH) Vitamin D Total DXI reagent, Beckman Coulter, Inc., Brea, CA, USA) was measured using a UniCel DxI 800 Immunoassay Analyzer (Beckman Coulter, Inc.). All serum measurements were performed at each visit. When measuring the BMD, the patient’s height and weight were measured, and body mass index (BMI) was calculated as weight (kg)/height (m)2. The occurrence of fragility fractures, including vertebral fractures, was identified radiologically. The occurrence of vertebral fractures was identified radiologically. The presence of fractures was confirmed by two musculoskeletal radiologists.
Korea’s national health insurance reimbursement system
In Korea, almost all citizens subscribe to the government-run National Health Insurance System. Osteoporosis medication is no longer reimbursable once a patient’s BMD improves to the osteopenia range, that is, a T-score > − 2.5 at all skeletal sites. At this stage, the expense of denosumab increases significantly, and to contain costs, it must be replaced with a cheaper alternative.
Statistical analysis
All data were analyzed using SPSS version 20.0 for Windows (IBM Corp., Armonk, NY, USA). The data are presented as mean ± SD unless otherwise stated. Graphics were produced using GraphPad Prism (version 5.0; GraphPad Software, Inc., San Diego, CA, USA). For the clinical features, we used the chi-square test to analyze categorical variables and Student’s t-test to analyze continuous variables. The mean percentage changes from baseline in BMD and other biochemical markers were analyzed using the repeated measures analysis of variance and Dunnett’s method, where appropriate. Statistical significance was set at p < 0.05.
Results
Clinical characteristics at initiation of denosumab therapy
The patient clinical baseline characteristics at denosumab initiation are shown in Table 1. The mean age was 65.7 ± 6.0 years. Thirty-nine (63.9%) patients received bisphosphonate for a mean duration of 41.6 ± 4.2 months before denosumab treatment. A history of vertebral fracture was noted in 7 (11.5%) patients. The baseline BMD values were 0.778 ± 0.088 g/cm2, 0.605 ± 0.062 g/cm2, and 0.725 ± 0.076 g/cm2, for the lumbar spine, femoral neck, and total hip, respectively. The baseline T-scores were − 2.8 ± 0.6 for the lumbar spine, − 2.8 ± 0.6 for the femoral neck, and − 2.5 ± 0.7 for the total hip.
Table 1.
Baseline characteristics of the study population at initiation of denosumab and raloxifene
| Clinical parameters | Study population (n = 61) |
|---|---|
| Age (years) | 65.7 ± 6.0 |
| Prior bisphosphonate exposure, n (%) | 39 (63.9) |
| Prior bisphosphonate treatment duration (months) | 41.6 ± 4.2 |
| Period between bisphosphonate cessation and denosumab initiation (months) | 2.5 ± 1.9 |
| Number of denosumab injection (times) | 2.6 (range: 2–5) |
| Height (cm) | 155.9 ± 5.2 |
| BMI (kg/m2) | 21.8 ± 2.9 |
| Prior vertebral fracture, n (%) | 7 (11.5) |
| Serum corrected calcium (mg/dl) | 9.0 ± 0.4 |
| Serum phosphorous (mg/dl) | 3.7 ± 0.4 |
| Serum 25-hydroxyvitamin D (ng/ml) | 34.8 ± 6.5 |
| BMD (g/cm2) at inition of denosumab | |
| Lumbar spine | 0.778 ± 0.088 |
| Femoral neck | 0.605 ± 0.062 |
| Total hip | 0.725 ± 0.076 |
| T-score at initiation of denosumab | |
| Lumbar spine | − 2.8 ± 0.6 |
| Femoral neck | − 2.8 ± 0.6 |
| Total hip | − 2.5 ± 0.7 |
| Bone turnover marker at initiation of denosumab | |
| CTx (ng/ml) | 0.32 ± 0.19 |
| P1NP (ng/ml) | 21.5 ± 8.6 |
| BMD (g/cm2) at inition of raloxifene | |
| Lumbar spine | 0.826 ± 0.082 |
| Femoral neck | 0.623 ± 0.076 |
| Total hip | 0.753 ± 0.089 |
| T-score at initiation of raloxifene | |
| Lumbar spine | − 2.5 ± 0.6 |
| Femoral neck | − 2.4 ± 0.6 |
| Total hip | − 2.1 ± 0.6 |
| Bone turnover marker at initiation of raloxifene | |
| CTx (ng/ml) | 0.13 ± 0.11 |
| P1NP (ng/ml) | 15.4 ± 5.3 |
| Diagnosis after denosumab treatment, n (%) | |
| Osteoporosis | 39 (63.9) |
| Osteopenia | 22 (36.1) |
| Reason for discontinuation of denosumab, n (%) | |
| Reimbursement issue due to improved T-score to osteopenia | 21 (34.4) |
| Planed dental procedure | 20 (32.8) |
| Patient’ preference | 16 (26.2) |
| Side effect | 4 (6.6) |
Continuous variables are presented as mean ± standard deviation; categorical variables are presented as number (percentage); BMI, body mass index; BMD, bone mineral density; CTx, c-terminal telopeptide of type 1 collagen; P1NP, procollagen type 1 N-terminal propeptide; T-score was based on the lowest value among the measurements of the different sites; Normal BMD, osteopenia, and osteoporosis were defined by the lowest T-score at the lumbar spine, femoral neck, and total hip (normal BMD, T-score ≥ − 1.0; osteopenia, − 1.0 < T-score < − 2.5; and osteoporosis: T-score ≤ − 2.5)
Clinical characteristics at initiation of raloxifene follow-up therapy
The clinical parameter changes at the initiation of raloxifene follow-up therapy are analyzed in Table 1. For the lumbar spine, BMD increased to 0.826 ± 0.082 g/cm2 (+ 6.17% from baseline) after a mean of 2.6 denosumab injections at 6-month intervals. Thirty-nine (63.9%) patients remained at osteoporosis levels after denosumab administration, and 22 (36.1%) patients improved to osteopenia level. The reasons for discontinuing denosumab are analyzed in Table 1. An improved T-score (to osteopenia) after denosumab treatment was the main reason in 21 (34.4%) patients. Dental procedures were the second most common reason for discontinuing denosumab (20 patients, 32.8%). Changes based on patient choice, such as refusal of injections or avoiding the clinic due to the COVID-19 pandemic, were the cause in 16 (26.2%) patients. For patients unable to visit the hospital due to the COVID-19 pandemic, an oral formulation was prescribed through telemedicine.
Percentage changes in bone mineral density
The longitudinal percentage change in BMD from the baseline value is shown in Fig. 1. Significant BMD increases at all measurement sites were observed during denosumab administration. The lumbar spine had the greatest increase from baseline after two consecutive denosumab administrations (+ 6.17 ± 1.98%, p < 0.001) (Fig. 1A). The BMD of the femoral neck and total hip increased significantly from the baseline value, + 2.98 ± 1.67%, and + 3.86 ± 1.82%, respectively, both p < 0.001) (Fig. 1B, C). After switching to raloxifene, there was a significant decrease in BMD at all measurement sites. The lumbar spine BMD decreased after the last administration of denosumab. The change was − 5.48 ± 1.87%, p < 0.001, compared to DXA2 (Fig. 1A). The femoral neck and total hip BMD also decreased compared to DXA2: − 2.95 ± 2.04%, p < 0.001, and − 3.52 ± 1.95%, p < 0.001), respectively (Fig. 1B, C). The gain in BMD after at least two consecutive doses of denosumab decreased after switching to raloxifene for 12 months. However, it did not fall below the baseline value at any measurement site (Fig. 1A, B, C).
Fig. 1.

Longitudinal percentage changes of bone mineral density at the lumbar spine (A), femoral neck (B), and total hip (C). DXA1, at baseline (before denosumab initiation); DXA2, 6 months after the last administration of denosumab; DXA3, 12 months after raloxifene therapy; * p < 0.001 compared with DXA1; ** p < 0.001 compared with DXA2
The reduction in BMD after raloxifene treatment was significantly less at all skeletal sites in patients who, after denosumab administration, achieved osteopenia (T-score > − 2.5) than in those who remained at osteoporosis (T-score ≤ − 2.5) (Table 2). The reduction in BMD after raloxifene treatment was not significantly different in the subgroup analysis according to the duration of denosumab administration or bisphosphonate exposure before denosumab (data are not shown). The clinical feature differences according to the degree of BMD decrease after the administration of raloxifene are shown in Table 3. Compared to patients with a less than the median decrease in the lumbar spine BMD after raloxifene administration (− 5.48%), patients with a greater than median decrease had a lower baseline BMI (p = 0.033), more prevalent vertebral fractures (p = 0.037), lower baseline lumbar spine BMD (p = 0.041), and lower T-score after denosumab (p = 0.031) (Table 3).
Table 2.
Difference in the percentage changes of bone mineral density after 12 months’ administration of raloxifene according to the T-score achieved by denosumab treatment
| Osteopenia (T-score > − 2.5) (n = 22) * | Osteoporosis (T-score ≤ − 2.5) (n = 39) * | P value | |
|---|---|---|---|
| Lumbar spine | − 1.98 ± 1.76% | − 4.90 ± 2.11% | 0.002 |
| Femoral neck | − 0.95 ± 1.89% | − 4.01 ± 2.77% | 0.000 |
| Total hip | − 1.03 ± 2.09% | − 4.68 ± 2.84% | 0.000 |
Variables are presented as mean ± standard deviation. BMD, bone mineral density; * BMD achieved by denosumab treatment. Normal BMD, osteopenia, and osteoporosis were defined by the lowest T-score at the lumbar spine, femoral neck, and total hip (normal BMD, T-score ≥ − 1.0; osteopenia, − 1.0 < T-score < − 2.5; and osteoporosis, T-score ≤ − 2.5)
Table 3.
Differences in clinical parameters according to the decrease in the degree of lumbar spine bone mineral density after raloxifene administration
| Greater decrease than median (n = 30) * | Lesser decrease than median (n = 31) * | P value | |
|---|---|---|---|
| Age (years) | 65.3 ± 6.8 | 66.0 ± 5.9 | 0.672 |
| Prior bisphosphonate exposure, n (%) | 18 (60.0) | 21 (67.7) | 0.529 |
| Prior bisphosphonate duration (months) | 42.2 ± 4.9 | 45.4 ± 3.4 | 0.510 |
| Height (cm) | 157.0 ± 5.4 | 155.5 ± 5.1 | 0.325 |
| BMI (kg/m2) | 20.9 ± 2.4 | 22.6 ± 3.1 | 0.033 |
| Prior vertebral fracture, n (%) | 6 (20.0) | 1 (3.2) | 0.037 |
| Baseline lumbar spine BMD (g/cm2) | 0.751 ± 0.081 | 0.803 ± 0.097 | 0.041 |
| Baseline femoral neck BMD (g/cm2) | 0.619 ± 0.070 | 0.597 ± 0.049 | 0.209 |
| Baseline total hip BMD (g/cm2) | 0.729 ± 0.092 | 0.724 ± 0.066 | 0.834 |
| T-score after denosumab | − 2.9 ± 0.5 | − 2.4 ± 0.4 | 0.031 |
| Baseline CTx (ng/ml) | 0.32 ± 0.23 | 0.29 ± 0.15 | 0.714 |
| Baseline P1NP (ng/ml) | 27.3 ± 8.9 | 20.5 ± 9.9 | 0.565 |
| CTx after 12 months of raloxifene (ng/ml) | 0.42 ± 0.15 | 0.40 ± 0.19 | 0.910 |
| P1NP after 12 months of raloxifene (ng/ml) | 41.9 ± 15.9 | 42.3 ± 14.4 | 0.941 |
BMI, body mass index; BMD, bone mineral density; CTx; c-terminal telopeptide of type 1 collagen; P1NP, procollagen type 1 N-terminal propeptide
*Median, − 5.48%; continuous variables are presented as mean ± standard deviation; categorical variables are presented as number (percentage); p value by paired t-test; T-score was based on the lowest value among the measurements of the different sites
Percentage changes in bone turnover markers
The percentage changes in serum CTx and P1NP levels from baseline (before denosumab administration) to each time point are presented in Fig. 2. A significant reduction in serum CTx levels compared to the baseline value was observed during denosumab administration. At 6 months, the CTx level decreased by 71.7 ± 19.2% compared to the baseline value (p < 0.001). After switching from denosumab to raloxifene, the CTx level increased by 9.6 ± 8.6% within 6 months and had significantly increased by 27.9 ± 16.2% at 12 months (p < 0.001) (Fig. 2A). Significant reductions in the P1NP level were observed during denosumab treatment, − 38.7 ± 21.1% at 6 months after the first injection, p < 0.001, compared to the baseline value. Marked increases in the P1NP level compared to the baseline value were noted after switching to raloxifene (+ 86.7 ± 14.3% after 6 months and + 96.0 ± 23.7% after 12 months; both, p < 0.001 compared to the baseline) (Fig. 2B).
Fig. 2.
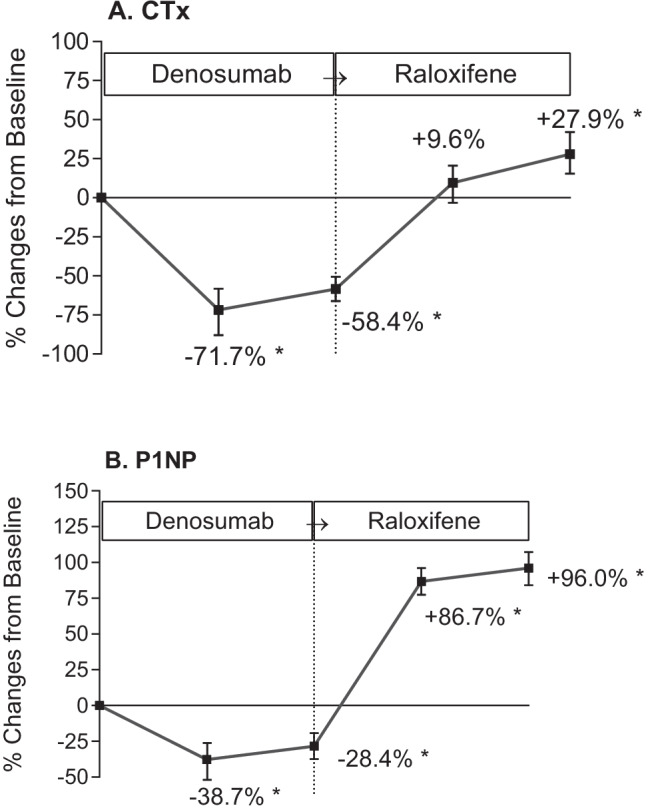
Percentage changes of bone turnover markers, CTx (A) and P1NP (B), during the study period
Other clinical parameters and safety
Serum-corrected calcium, serum phosphorus, serum 25(OH)D, height, and BMI were measured. No measured parameters changed significantly during the study period. Height did not change before or after denosumab administration or with raloxifene treatment after denosumab discontinuation (data are not shown). Four patients (6.6%) complained of injection-site pain during denosumab administration. During raloxifene administration, hot flashes (3 patients, 4.9%) and genital itchiness (2 patients, 3.2%) were reported. No serious adverse events that required discontinuation, such as severe hypocalcemia, osteonecrosis of the jaw, or atypical femoral fracture, were reported during denosumab or raloxifene administration. Finally, no new radiologic vertebral fractures were observed during the raloxifene treatment.
Discussion
In the present study, after 12 months’ administration of raloxifene, the BMD, which had increased after at least two doses of denosumab at 6-month intervals, decreased to the baseline level at all measurement sites. A significant increase in the CTx and P1NP levels was also observed. Patients with a low BMI, previous vertebral fractures, or low lumbar spine BMD before denosumab treatment showed a greater reduction in lumbar spine BMD after 12 months of follow-up raloxifene therapy.
Denosumab is a potent antiresorptive agent, but there are concerns about the “rebound phenomenon” that occurs when it is discontinued [26]. Bone density is lost rapidly [27], the suppressed BTMs rebound [28], and multiple vertebral fractures may eventually occur [12]. Therefore, several guidelines and position statements have recommended continuous administration of an antiresorptive agent to prevent the rebound phenomenon if denosumab is discontinued [7, 16–18, 29]. Among antiresorptive agents, intravenous infusion of zoledronic acid is primarily recommemded because of its high potency [16, 19–21, 30, 31]. The effects of alendronate [22] or risedronate [32] have also been reported. Although raloxifene has a weaker effect on increasing bone density than bisphosphonates, it is an antiresorptive agent-proved vertebral antifracture efficacy [7, 33]. Therapy with other antiresorptive agents is mandatory after discontinuation of denosumab. In such cases, bisphosphonates are generally recommended.
Discontinuation of denosumab may be necessary when compliance, because of potential side effects, is a concern, or if there are insurance reimbursement issues. In Korea, reimbursement is no longer provided if bone density increases to osteopenia levels, confirmed at all measurement sites, regardless of the treatment period. Therefore, when BMD increases with denosumab treatment, it must be discontinued and replaced with cheaper alternatives. However, it is specifically pertinent to Korea’s national health insurance reimbursement system and is not widely accepted by the international guidelines. In our study, 21 (34.4%) patients discontinued denosumab due to loss of reimbursement because their T-scores increased to osteopenia levels.
There is very little evidence on the effects on bone metabolism of follow-up therapy with raloxifene after discontinuation of denosumab. One case report suggested rapid bone loss after switching from denosumab to raloxifene, although the patients had also been exposed to multidisciplinary treatments for breast cancer [34]. Recently, Ebina et al. investigated the effects of raloxifene in patients previously treated with denosumab. The authors observed substantial bone loss after raloxifene treatment and reported that 23.1% of patients developed new vertebral fractures. However, this was a small study in 13 patients. Other study limitations were that vertebral fractures were confirmed only by the patients’subjective complaints, with no radiographic confirmation. In addition, the average age of the patients treated with raloxifene was 77.1 years [23]. The average age of the patients in our study was 65.7 years, and there may have been different responses to raloxifene.
Bone loss was consistently observed after discontinuation of denosumab in our study, in line with the above reports [23, 34]. The lumbar spine had the greatest bone loss after 12 months of raloxifene treatment. In previous literature, the bone mass gained during denosumab treatment was lost, and the baseline value was reached within 12 months of the last denosumab injection if no treatment was given [27, 28]. The CTx level also increased to above the baseline values after denosumab discontinuation [28]. In our study, the administration of raloxifene did not prevent an abrupt increase in BTMs after discontinuation of denosumab. Reports of multiple vertebral fractures at 9–16 months after the last denosumab injection were associated with sudden bone loss and elevation of BTMs [9, 24, 35]. In our study, administration of raloxifene after discontinuation of denosumab did not effectively suppress the decrease in BMD or increase in BTMs. Interestingly, Bone et al. reported that femoral BMD decreased lower than the baseline value with discontinuation of denosumab [3]. In our study, with subsequent administration of raloxifene, the femoral neck BMD remained higher than the baseline value.
In the present study, the decrease in lumbar spine BMD was substantial after discontinuation of denosumab despite administering raloxifene. Bone loss in the lumbar spine was greater in patients with a low baseline BMI, prior vertebral fracture, or low lumbar BMD measured before denosumab treatment. A history of previous vertebral fractures is likely to be associated with a greater decrease in lumbar spine BMD after administration of raloxifene. Previous fractures were accompanied by overall microarchitectural deterioration as well as reduced bone quality [36]; hence, the discontinuation of denosumab may have more effects in patients with previous fractures. Therefore, caution may be required when switching denosumab to raloxifene in patients with prevalent vertebral fractures.
Moreover, BMD decreased significantly after raloxifene in patients who remained osteoporotic (T-score ≤ − 2.5) after denosumab administration. Therefore, raloxifene should be administered with caution when there is a high risk of fracture. If osteopenia is achieved with denosumab, the BMD decrease will be marginal after subsequent administration of raloxifene. Thus, the use of raloxifene is more appropriate in such cases. However, Kendler et al. reported that the greater the BMD percentage change after denosumab administration, the greater the BMD loss after subsequent alendronate administration [22]. A direct comparison is difficult because the participants in the study, the administered drug, and the administration period of denosumab are different; nevertheless, this result should be confirmed in future studies.
Our study has some limitations. First, this was a retrospective study with a lower level of evidence than that of a prospective study. Second, our study did not include a control group that had no treatment after denosumab was discontinued. However, not taking any follow-up treatment after discontinuing denosumab may raise ethical issues given the known problems. Therefore, we indirectly compared the data after discontinuation of denosumab to data in the existing literature, although the analysis groups were different. A comparison of SERMs with various active comparators after discontinuation of denosumab will also be an interesting research topic. Third, patient compliance was not assessed in this study. All patients included in the analysis had timely follow-up without loss; however, the lack of confirmation of drug compliance is a limitation. Fourth, lumbar spine anteroposterior and lateral radiography were conducted to confirm vertebral fractures, but there were limitations in confirming vertebral fractures in all areas, including the thoracic spine. Lastly, the relatively short study period and the small number of patients with fractures are also limitations. Additionally, it is necessary to consider the rebound phenomenon according to the duration of denosumab administration. These shortcomings can be resolved through future long-term prospective studies.
Administration of raloxifene for 12 months after denosumab discontinuation did not maintain the increased bone mass, and BTMs rebounded. Moreover, the reduction in lumbar spine BMD was greater in the patients who had vertebral fractures before denosumab administration or those who had a lower lumbar spine BMD or lower BMI. In conclusion, raloxifene sequentially administered after denosumab discontinuation is not effective in preventing rebound phenomenon. We believe that this is a novel finding. These findings need to be validated by a prospective study to draw clear conclusions regarding the sequential treatment with denosumab-SERMs.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This study was selected as a Young Investigator Award by the Korean Society for Bone and Mineral Research in 2020.
Data availability
The datasets used and analyzed during the study are available from the corresponding author on reasonable request.
Declarations
Ethics approval
The study was approved by the Institutional Review Board of the Catholic Medical Center (No. KC20RISI0933).
Consent to participate/publication
Not applicable.
Conflicts of interest
None.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Khosla S, Hofbauer LC. Osteoporosis treatment: recent developments and ongoing challenges. Lancet Diabetes Endocrinol. 2017;5:898–907. doi: 10.1016/s2213-8587(17)30188-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756–765. doi: 10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]
- 3.Bone HG, Wagman RB, Brandi ML, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017;5:513–523. doi: 10.1016/s2213-8587(17)30138-9. [DOI] [PubMed] [Google Scholar]
- 4.Yusuf AA, Cummings SR, Watts NB, Feudjo MT, Sprafka JM, Zhou J, Guo H, Balasubramanian A, Cooper C. Real-world effectiveness of osteoporosis therapies for fracture reduction in post-menopausal women. Arch Osteoporos. 2018;13:33. doi: 10.1007/s11657-018-0439-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi NK, Solomon DH, Tsacogianis TN, Landon JE, Song HJ, Kim SC. Comparative safety and effectiveness of denosumab versus zoledronic acid in patients with osteoporosis: a cohort study. J Bone Miner Res. 2017;32:611–617. doi: 10.1002/jbmr.3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fraser TR, Flogaitis I, Moore AE, Hampson G. The effect of previous treatment with bisphosphonate and renal impairment on the response to denosumab in osteoporosis: a ‘real-life’ study. J Endocrinol Invest. 2020;43:469–475. doi: 10.1007/s40618-019-01131-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eastell R, Rosen CJ, Black DM, Cheung AM, Murad MH, Shoback D. Pharmacological management of osteoporosis in postmenopausal women: an endocrine society* clinical practice guideline. J Clin Endocrinol Metab. 2019;104:1595–1622. doi: 10.1210/jc.2019-00221. [DOI] [PubMed] [Google Scholar]
- 8.Shoback D, Rosen CJ, Black DM, Cheung AM, Murad MH, Eastell R (2020) Pharmacological management of osteoporosis in postmenopausal women: an endocrine society guideline update. J Clin Endocrinol Metab 105 10.1210/clinem/dgaa048 [DOI] [PubMed]
- 9.Anastasilakis AD, Makras P. Multiple clinical vertebral fractures following denosumab discontinuation. Osteoporos Int. 2016;27:1929–1930. doi: 10.1007/s00198-015-3459-5. [DOI] [PubMed] [Google Scholar]
- 10.Aubry-Rozier B, Gonzalez-Rodriguez E, Stoll D, Lamy O. Severe spontaneous vertebral fractures after denosumab discontinuation: three case reports. Osteoporos Int. 2016;27:1923–1925. doi: 10.1007/s00198-015-3380-y. [DOI] [PubMed] [Google Scholar]
- 11.Anastasilakis AD, Yavropoulou MP, Makras P, Sakellariou GT, Papadopoulou F, Gerou S, Papapoulos SE. Increased osteoclastogenesis in patients with vertebral fractures following discontinuation of denosumab treatment. Eur J Endocrinol. 2017;176:677–683. doi: 10.1530/eje-16-1027. [DOI] [PubMed] [Google Scholar]
- 12.Anastasilakis AD, Polyzos SA, Makras P, Aubry-Rozier B, Kaouri S, Lamy O. Clinical features of 24 patients with rebound-associated vertebral fractures after denosumab discontinuation: systematic review and additional cases. J Bone Miner Res. 2017;32:1291–1296. doi: 10.1002/jbmr.3110. [DOI] [PubMed] [Google Scholar]
- 13.Popp AW, Varathan N, Buffat H, Senn C, Perrelet R, Lippuner K. Bone mineral density changes after 1 year of denosumab discontinuation in postmenopausal women with long-term denosumab treatment for osteoporosis. Calcif Tissue Int. 2018;103:50–54. doi: 10.1007/s00223-018-0394-4. [DOI] [PubMed] [Google Scholar]
- 14.Cummings SR, Ferrari S, Eastell R, et al. Vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo-controlled FREEDOM trial and its extension. J Bone Miner Res. 2018;33:190–198. doi: 10.1002/jbmr.3337. [DOI] [PubMed] [Google Scholar]
- 15.Burckhardt P, Faouzi M, Buclin T, Lamy O. Fractures after denosumab discontinuation: a retrospective study of 797 cases. J Bone Miner Res. 2021 doi: 10.1002/jbmr.4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsourdi E, Zillikens MC, Meier C, et al. Fracture risk and management of discontinuation of denosumab therapy: a systematic review and position statement by ECTS. J Clin Endocrinol Metab. 2020 doi: 10.1210/clinem/dgaa756. [DOI] [PubMed] [Google Scholar]
- 17.Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists/American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis-2020 update. Endocr Pract. 2020;26:1–46. doi: 10.4158/gl-2020-0524suppl. [DOI] [PubMed] [Google Scholar]
- 18.Kim BK, Kim CH, Min YK. Preventing rebound-associated fractures after discontinuation of denosumab therapy: a position statement from the Health Insurance Committee of the Korean Endocrine Society. Endocrinol Metab (Seoul) 2021;36:909–911. doi: 10.3803/EnM.2021.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Everts-Graber J, Reichenbach S, Ziswiler HR, Studer U, Lehmann T. A Single Infusion of Zoledronate in Postmenopausal Women Following Denosumab Discontinuation Results in Partial Conservation of Bone Mass Gains. J Bone Miner Res. 2020;35:1207–1215. doi: 10.1002/jbmr.3962. [DOI] [PubMed] [Google Scholar]
- 20.Makras P, Appelman-Dijkstra NM, Papapoulos SE, van Wissen S, Winter EM, Polyzos SA, Yavropoulou MP, Anastasilakis AD. The duration of denosumab treatment and the efficacy of zoledronate to preserve bone mineral density after its discontinuation. J Clin Endocrinol Metab. 2021 doi: 10.1210/clinem/dgab321. [DOI] [PubMed] [Google Scholar]
- 21.Sølling AS, Harsløf T, Langdahl B. Treatment with zoledronate subsequent to denosumab in osteoporosis: a randomized trial. J Bone Miner Res. 2020;35:1858–1870. doi: 10.1002/jbmr.4098. [DOI] [PubMed] [Google Scholar]
- 22.Kendler D, Chines A, Clark P, Ebeling PR, McClung M, Rhee Y, Huang S, Stad RK. Bone mineral density after transitioning from denosumab to alendronate. J Clin Endocrinol Metab. 2020;105:e255–264. doi: 10.1210/clinem/dgz095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ebina K, Hashimoto J, Kashii M, et al. Effects of follow-on therapy after denosumab discontinuation in patients with postmenopausal osteoporosis. Mod Rheumatol. 2021;31:485–492. doi: 10.1080/14397595.2020.1769895. [DOI] [PubMed] [Google Scholar]
- 24.Lamy O, Gonzalez-Rodriguez E, Stoll D, Hans D, Aubry-Rozier B. Severe rebound-associated vertebral fractures after denosumab discontinuation: 9 clinical cases report. J Clin Endocrinol Metab. 2017;102:354–358. doi: 10.1210/jc.2016-3170. [DOI] [PubMed] [Google Scholar]
- 25.Kanis JA, Melton LJ, 3rd, Christiansen C, Johnston CC, Khaltaev N. The diagnosis of osteoporosis. J Bone Miner Res. 1994;9:1137–1141. doi: 10.1002/jbmr.5650090802. [DOI] [PubMed] [Google Scholar]
- 26.Anastasilakis AD, Makras P, Yavropoulou MP, Tabacco G, Naciu AM, Palermo A (2021) Denosumab discontinuation and the rebound phenomenon: a narrative review. J Clin Med10.3390/jcm10010152 [DOI] [PMC free article] [PubMed]
- 27.Bone HG, Bolognese MA, Yuen CK, Kendler DL, Miller PD, Yang YC, Grazette L, San Martin J, Gallagher JC. Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J Clin Endocrinol Metab. 2011;96:972–980. doi: 10.1210/jc.2010-1502. [DOI] [PubMed] [Google Scholar]
- 28.Miller PD, Bolognese MA, Lewiecki EM, McClung MR, Ding B, Austin M, Liu Y, San Martin J. Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy: a randomized blinded phase 2 clinical trial. Bone. 2008;43:222–229. doi: 10.1016/j.bone.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 29.Tsourdi E, Langdahl B, Cohen-Solal M, Aubry-Rozier B, Eriksen EF, Guañabens N, Obermayer-Pietsch B, Ralston SH, Eastell R, Zillikens MC. Discontinuation of denosumab therapy for osteoporosis: a systematic review and position statement by ECTS. Bone. 2017;105:11–17. doi: 10.1016/j.bone.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Anastasilakis AD, Papapoulos SE, Polyzos SA, Appelman-Dijkstra NM, Makras P. Zoledronate for the prevention of bone loss in women discontinuing denosumab treatment. a prospective 2-year clinical trial. J Bone Miner Res. 2019;34:2220–2228. doi: 10.1002/jbmr.3853. [DOI] [PubMed] [Google Scholar]
- 31.Everts-Graber J, Reichenbach S, Gahl B, Ziswiler HR, Studer U, Lehmann T. Risk factors for vertebral fractures and bone loss after denosumab discontinuation: a real-world observational study. Bone. 2021;144:115830. doi: 10.1016/j.bone.2020.115830. [DOI] [PubMed] [Google Scholar]
- 32.Horne AM, Mihov B, Reid IR. Bone loss after romosozumab/denosumab: effects of bisphosphonates. Calcif Tissue Int. 2018;103:55–61. doi: 10.1007/s00223-018-0404-6. [DOI] [PubMed] [Google Scholar]
- 33.Qaseem A, Forciea MA, McLean RM, et al. Treatment of low bone density or osteoporosis to prevent fractures in men and women: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166:818–839. doi: 10.7326/m15-1361. [DOI] [PubMed] [Google Scholar]
- 34.Gonzalez-Rodriguez E, Stoll D, Lamy O. Raloxifene has no efficacy in reducing the high bone turnover and the risk of spontaneous vertebral fractures after denosumab discontinuation. Case Rep Rheumatol. 2018;2018:5432751. doi: 10.1155/2018/5432751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McClung MR, Wagman RB, Miller PD, Wang A, Lewiecki EM. Observations following discontinuation of long-term denosumab therapy. Osteoporos Int. 2017;28:1723–1732. doi: 10.1007/s00198-017-3919-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Genant HK, Delmas PD, Chen P, Jiang Y, Eriksen EF, Dalsky GP, Marcus R, San Martin J. Severity of vertebral fracture reflects deterioration of bone microarchitecture. Osteoporos Int. 2007;18:69–76. doi: 10.1007/s00198-006-0199-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analyzed during the study are available from the corresponding author on reasonable request.


