Abstract
The most common variant of cutaneous T‐cell lymphomas (CTCL) is mycosis fungoides (MF). Patients with MF often experience a chronic course of disease. The spontaneous regression (SR) of MF is rare, and the factors that predict SR have not been recognized yet. Here, we are reporting a case of persistent MF who had prominent remission after COVID‐19. This case report supports the possible antineoplastic effect of SARS‐CoV‐2. Understanding the underlying etiology of such effect can result in development of new target therapies for MF.
Keywords: COVID‐19, cutaneous T‐cell lymphomas, mycosis fungoides
The purpose of this case report is to support the antineoplastic effect of SARS‐CoV‐2 in regression of lesions related to MF. The underlying mechanism of T‐cell depletion or oncolytic effects of COVID‐19 is not yet identified. Investigation of possible oncolytic role of COVID‐19 may help to develop novel target therapies for MF.
1. INTRODUCTION
Cutaneous T‐cell lymphomas (CTCL) that are characterized by accumulation of malignant T lymphocytes in the skin, are rare types of non‐Hodgkin lymphoma. 1 , 2 The most common variant is mycosis fungoides (MF) with characteristic patches, plaques, and tumors arising in the skin and an indolent behavior. 2 , 3 , 4 Patients with MF often experience a chronic course of disease, with waxing and waning skin lesions. 4 The spontaneous regression (SR) although rare, may occur; however, the factors that predict SR have not been identified yet. 5
Previously, complete remission (CR) of cutaneous T‐cell lymphoma was reported in an HIV‐infected patient associated with a falling CD4 count. 6 Moreover, a 61‐year‐old man with Hodgkin lymphoma had reduced lymphadenopathy after being infected by SARS‐CoV‐2 that it was probably because of cross‐reactivity of pathogen‐specific T cells with tumor antigens and activation of natural killer cells by inflammatory cytokines. 7
Since the beginning of the COVID‐19 pandemic studies have shown a clear decrease in peripheral lymphocytes and natural killer (NK) cells in COVID‐19 patients. 8 In fact, the lympho‐depletion induced by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has a crucial diagnostic role and represents a valid prognostic tool. 9 Here, we report an interesting case of MF who had a remission after COVID‐19.
2. CASE PRESENTATION
In August 2020, a 64‐year‐old male patient was presented with severe symptoms such as dry cough and dyspnea related to COVID‐19, confirmed by both chest tomography (CT) scan and oropharyngeal swab polymerase chain reaction (PCR). He was treated in outpatient setting by receiving supportive medications and maintaining home quarantine. No other specific drugs were administered.
His past medical history was positive for MF, diabetes, hyperlipidemia, and ischemic heart disease. His drug history was Metformin 1,000 mg three times a day, Rosuvastatin 10 mg daily, Aspirin 80 mg daily, and Nitroglycerin 2.6 mg daily. Laboratory test was only significant for a mild lymphopenia.
The patient had been diagnosed with early patch MF (stage 1B) after a five‐month history of pruritus and erythematous patches scattered over trunk and upper and lower extremities, back in November 2018. His condition was confirmed by skin biopsy and immuno‐histochemical studies.
The patient showed only partial remission with topical steroids and several courses of ultraviolet B (UVB) (79 sessions between December, 2018, and May, 2020), and all the patches were persistent after treatment.
Three weeks after the improvement of COVID‐19, our patient experienced complete remission of all of MF related lesions. We evaluated patient's life style, diet, and medication and no changes were seen. For six months, he was symptom free but after six months, some of his lesions recurred but with lesser severity and limited distribution on back. We made regular phone calls to evaluate this patient during two years, and now he has not any lesions. Patient's lesions before and after COVID‐19 are demonstrated in Figure 1.
FIGURE 1.
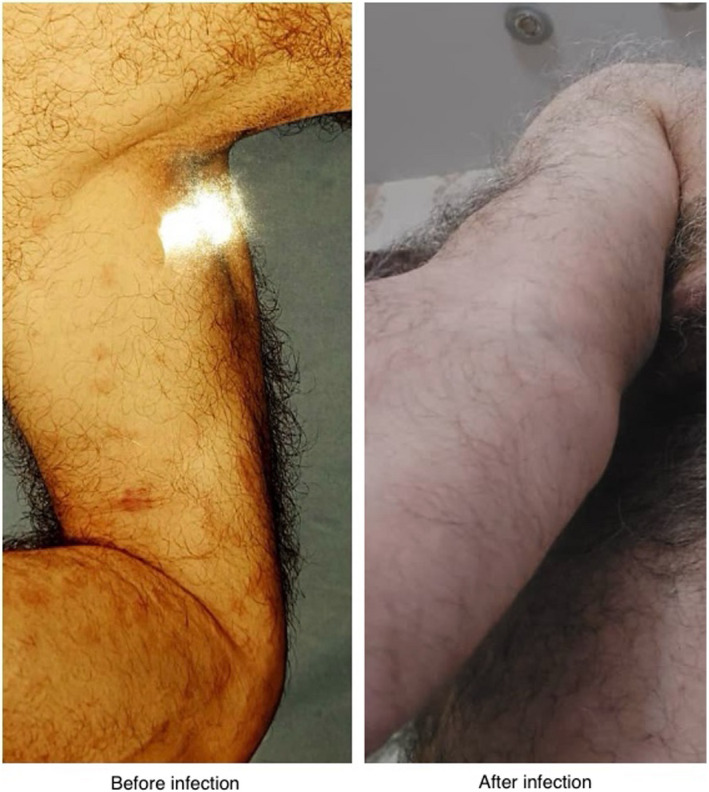
Patient's lesions before and after COVID‐19
3. DISCUSSION
The surprising clinical improvement of our case without any changes in his lifestyle may suggest an antineoplastic role for SARS‐CoV‐2 infection, as if the virus had acted as an oncolytic agent which previously seen in transient remission of natural killer cell and Hodgkin lymphoma. 7 , 10 , 11 In another study, Kandeel et al. 12 demonstrated remission of two cases of acute myeloid leukemia and acute lymphoid leukemia after COVID‐19 pneumonia due to evoking an anti‐tumor immune response through natural killer cells activation by cytokine releasing or cross‐reactivity of the virus‐specific T cells with tumor antigens.
This case experienced the remission of his condition 21 days after complete clearance of COVID‐19 infection confirmed by negative oropharyngeal swab PCR; however, recurrence of itching and skin patches was noticed after few months.
Several studies have illustrated a possible oncolytic role for a variety of viruses in patients with MF such as cutavirus and measles. 13 , 14 The antitumor immunomodulatory actions triggering lympho‐depletion are well established. In fact, oncolytic viruses are engineered to express some cytokines, including tumor necrosis factor alpha (TNF‐a) and interleukin‐2 (IL‐2), to deplete T cells, as a part of adoptive therapy. 15 , 16 Moreover, studies revealed smart agents with oncolytic influence such as herpes simplex virus (HSV) which can produce a harmless and effective antitumor drug in the treatment of other cancers, as well. 17
Furthermore, oncolytic viruses can exert antitumor activity that cause lymphocytic cells reduction identical to high dose chemotherapy. 18 The underlying mechanism for improvement of MF after COVID‐19 maybe related to the large amount of pro‐inflammatory cytokines, such as interleukin 6 (IL‐6), TNF‐a, and IL‐2, release during COVID‐19 infection that attract T and NK cells to the neoplastic T‐cells. 10 , 18
On the contrary, the SARS‐CoV‐2 could be a probable pivotal element in the apparent improvement of clinical features related to MF similar to human immunodeficiency virus (HIV). 6 , 10 Furthermore, in a previous report, an HIV seropositive patient developed MF that was surprisingly improved after development of apparent HIV disease. 6
4. CONCLUSION
This case report supports the possible antineoplastic effect of SARS‐CoV‐2, which has been previously suggested in a case of African 20‐years‐old male patient with a temporary remission of refractory NK/T‐cell lymphomas after COVID‐19 infection but relapse after recovery from SARS‐CoV‐2. 10 The underlying mechanism of T‐cell depletion or oncolytic effects of COVID‐19 in not known, and further studies in patients with MF who affected by SARS‐CoV‐2 are needed to analyze them and elucidate this possible role.
CONFLICT OF INTEREST
The authors declare that they have no competing or conflict of interests.
AUTHOR CONTRIBUTIONS
LO wrote the manuscript. SD and SN wrote and corrected the manuscript for its scientific basis. FH collected the data for the study. SD revised the manuscript for grammar and syntax mistakes. All authors read and approved the final manuscript.
CONSENT
Written informed consent was obtained from the patient for the publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor‐in‐Chief of this journal.
ACKNOWLEDGEMENT
We would like to thank Dr. Kevin Pehr, associate professor, dermatology, in McGill university for his help reviewing the writing.
Ohadi L, Hosseinzadeh F, Dadkhahfar S, Nasiri S. Oncolytic effect of SARS‐CoV‐2 in a patient with mycosis fungoides: A case report. Clin Case Rep. 2022;10:e05682. doi: 10.1002/ccr3.5682
Funding information
No additional funding for the execution of the present study was received
DATA AVAILABILITY STATEMENT
The data and materials used in the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Kashani‐Sabet M, McMillan A, Zackheim HS. A modified staging classification for cutaneous T‐cell lymphoma. J Am Acad Dermatol. 2001;45(5):700‐706. [DOI] [PubMed] [Google Scholar]
- 2. Willemze R, Jaffe ES, Burg G, et al. WHO‐EORTC classification for cutaneous lymphomas. Blood. 2005;105(10):3768‐3785. [DOI] [PubMed] [Google Scholar]
- 3. Wood DE. National Comprehensive Cancer Network (NCCN) clinical practice guidelines for lung cancer screening. Thorac Surg Clin. 2015;25(2):185‐197. [DOI] [PubMed] [Google Scholar]
- 4. Prince HM, Duvic M, Martin A, Sterry W, Assaf C, Straus DJ. Incidence of spontaneous remission in patients with CD25‐positive mycosis fungoides/Sezary syndrome receiving placebo. J Am Acad Dermatol. 2012;67(5):867‐875. [DOI] [PubMed] [Google Scholar]
- 5. Talpur R, Bassett R, Duvic M. Prevalence and treatment of Staphylococcus aureus colonization in patients with mycosis fungoides and Sézary syndrome. Br J Dermatol. 2008;159(1):105‐112. [DOI] [PubMed] [Google Scholar]
- 6. Sorrells T, Pratt L, Newton J, Graham S, Ryan M. Spontaneous regression of granulomatous mycosis fungoides in an HIV positive patient. J Am Acad Dermatol. 1997;37(5):876‐880. [DOI] [PubMed] [Google Scholar]
- 7. Challenor S, Tucker D. SARS‐CoV‐2‐induced remission of Hodgkin lymphoma. Br J Haematol. 2021;192(3):415. [DOI] [PubMed] [Google Scholar]
- 8. Pasin F, Mascalchi Calveri M, Calabrese A, et al. Oncolytic effect of SARS‐CoV2 in a patient with NK lymphoma. Acta Biomed. 2020;91(3):e2020047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang F, Nie J, Wang H, et al. Characteristics of peripheral lymphocyte subset alteration in COVID‐19 pneumonia. J Infect Dis. 2020;221(11):1762‐1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pasin F, Calveri MM, Pizzarelli G, et al. Oncolytic effect of SARS‐CoV2 in a patient with NK lymphoma. Acta Bio Medica: Atenei Parmensis. 2020;91(3):e2020047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Donia A, Shahid R, Nawaz M, Yaqub T, Bokhari H. Can we develop oncolytic SARS‐CoV‐2 to specifically target cancer cells? Ther Adv Med Oncol. 2021;13:1‐2. doi: 10.1177/17588359211061988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kandeel EZ, Refaat L, Abdel‐Fatah R, et al. Could COVID‐19 induce remission of acute leukemia? Hematology. 2021;26(1):870‐873. [DOI] [PubMed] [Google Scholar]
- 13. Phan TG, Dreno B, da Costa AC, et al. A new protoparvovirus in human fecal samples and cutaneous T cell lymphomas (mycosis fungoides). Virology. 2016;496:299‐305. [DOI] [PubMed] [Google Scholar]
- 14. Heinzerling L, Künzi V, Oberholzer PA, Kündig T, Naim H, Dummer R. Oncolytic measles virus in cutaneous T‐cell lymphomas mounts antitumor immune responses in vivo and targets interferon‐resistant tumor cells. Blood. 2005;106(7):2287‐2294. [DOI] [PubMed] [Google Scholar]
- 15. Snijder J, Mihyawi N, Frolov A, Ewton A, Rivero G. Spontaneous remission in diffuse large cell lymphoma: a case report. J Med Case Rep. 2019;13(1):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Musto P, D'Arena G, Meillo L, Cascavilla N, Ladogana S, Carotenuto M. Spontaneous remission in acute myeloid leukaemia: a role for endogenous productionof tumour necrosis factor and interleukin‐2? Br J Haematol. 1994;87(4):879‐880. [DOI] [PubMed] [Google Scholar]
- 17. Shen Y, Nemunaitis J. Herpes Simplex Virus 1 (HSV‐1) for cancer treatment. Cancer Gene Ther. 2006;13(11):975‐992. [DOI] [PubMed] [Google Scholar]
- 18. Santos JM, Cervera‐Carrascon V, Havunen R, et al. Adenovirus coding for interleukin‐2 and tumor necrosis factor alpha replaces lymphodepleting chemotherapy in adoptive T cell therapy. Mol Ther. 2018;26(9):2243‐2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data and materials used in the current study are available from the corresponding author on reasonable request.


