Abstract
The immune system has crucial roles in cancer development and treatment. Whereas adaptive immunity can prevent or constrain cancer through immunosurveillance, innate immunity and inflammation often promote tumorigenesis and malignant progression of nascent cancer. The past decade has witnessed the translation of knowledge derived from preclinical studies of antitumour immunity into clinically effective, approved immunotherapies for cancer. By contrast, the successful implementation of treatments that target cancer-associated inflammation is still awaited. Anti-inflammatory agents have the potential to not only prevent or delay cancer onset but also to improve the efficacy of conventional therapeutics and next-generation immunotherapies. Herein, we review the current clinical advances and experimental findings supporting the utility of an anti-inflammatory approach to the treatment of solid malignancies. Gaining a better mechanistic understanding of the mode of action of anti-inflammatory agents and designing more effective treatment combinations would advance the clinical application of this therapeutic approach.
Inflammation is part of the innate immune response to danger signals, tissue disruption and/or infection. Transient and properly terminated inflammation is beneficial yet chronic inflammation increases cancer risk1. Numerous environmental factors, including carcinogenic microbes, pollutants, tissue-damaging radiation, tobacco smoke, diesel exhaust fumes, particulate matter and dietary factors, can evoke chronic inflammation in multiple organ systems, especially those that are exposed to the external environment1. Left unresolved, chronic inflammatory responses can result in tumour promotion1. Tumour-associated inflammation, which entails intricate interactions between epithelial and stromal cells, can in some cases lead to epigenetic alterations that drive malignant progression and even initiate tumorigenesis. More generally, however, chronic inflammation results in the production of growth factors that support the development of newly emergent tumours and cause them to behave as “wounds that do not heal”2. Inflammation-reducing chemopreventive strategies that inhibit either the initiation or propagation of persistent inflammation might therefore prevent or delay cancer onset3. Anti-infective agents, nonsteroidal anti-inflammatory drugs (NSAIDs) and other commonly used drugs capable of reducing inflammation, such as statins and metformin, have been reported to decrease cancer risk and incidence4-8.
Cancer cell-intrinsic or therapy-elicited mechanisms, including metabolic changes, cell stress and cell death, also constitute important sources of tumour-associated inflammation1. The continuous production of various cytokines, chemokines and growth factors within the tumour microenvironment (TME) supports cancer cell proliferation, evolution and survival as well as tumour vascularization and immune dysregulation, all of which contribute to tumour progression, invasion, metastasis and therapy resistance. Thus, the use of anti-inflammatory agents, either alone or in combination with cytotoxic agents and targeted therapies, is an appealing strategy for the treatment of inflammation-driven cancers. This approach is effective in various animal models9-11, although the complexity and plasticity of human cancers and their ecosystem present major hurdles that need to be overcome for anti-inflammatory therapy to become truly successful. For example, the inhibition of inflammation mostly slows down tumour growth rather than killing cancer cells and therefore needs to be combined with cancer-specific cytotoxic drugs to fully eradicate the tumours. In addition, owing to the depletion of general survival factors, anti-inflammatory drugs can inflict bystander effects on non-cancerous tissues, which can in turn result not only in TME remodelling and therapy resistance but also in the increased susceptibility of non-malignant cells to non-specific cytotoxicity that can lead to toxicities12,13.
The past decade has witnessed a burgeoning of effective treatments based on the activation of anti-tumour immune responses, for example, using immune-checkpoint inhibitors (ICIs) or genetically engineered T cells14. Such immunotherapies induce durable responses in a subset of patients; however, primary or acquired therapy resistance occurs in the vast majority of patients. In many cases, immunotherapy resistance is attributable to the presence of a pro-inflammatory and immunosuppressive TME15 (BOX 1). In this context, anti-inflammatory drugs that target immunosuppressive cells or cytokines might render the cancer more susceptible to immune-mediated rejection. Moreover, akin to the treatment of autoimmune diseases, selective targeting of the key drivers of immunotherapy-induced inflammation might increase the response-to-toxicity ratio and thereby improve therapeutic outcomes. Consequently, the combination of anti-inflammatory therapy with immunotherapy might evolve into a successful approach for circumventing the obstacles associated with current treatment modalities.
Box 1∣. Antitumour immunity versus cancer-promoting inflammation.
External stimuli, host-specific endogenous factors and therapeutic interventions can promote the recruitment and activation of various types of inflammatory and/or immune cells that shape the tumour microenvironment18. Short-term inflammation, such as that induced by immune adjuvants used in vaccination approaches, can potentiate anticancer immunity through the enhancement of T cell priming; such effects can shape the course of early tumour development (through immune elimination and immunoediting) but can also enhance responses to immunotherapy257. By contrast, chronic inflammation is immunosuppressive and thus cancer promoting15. Accordingly, so-called ‘hot’ tumours that harbour extensive inflammatory infiltrates are not necessarily responsive to immunotherapy, unless immunosuppressive inflammation is attenuated. Not surprisingly, inducers of antitumour immunity, such as infectious or commensal microbes, as well as danger-associated molecular patterns can also trigger cancer-related inflammation and contribute to a refractory phenotype258-260.
The cancer-immunity cycle hypothesis261 posits that an optimal anticancer immune response relies on the iterative cooperation between immune cells, host factors and tumour antigens. This cycle is initiated by the release of tumour antigens and their presentation to T cells and is terminated upon the clearance of tumour cells by cytotoxic T lymphocytes. Unless efficiently initiated and precisely maintained, the cancer-immunity cycle can be broken and replaced by inflammation-promoted tumour progression2. By exploiting self-tolerance mechanisms and microenvironmental assistance, cancer cells can escape cytotoxic T lymphocyte-mediated killing, often by downregulating MHC class I-dependent antigen presentation262, but also through the production of immunosuppressive factors18. Thus, tumour cell-autonomous inflammatory traits alter the molecular circuits and cellular interactions that form the basis for antitumour immunity, such that they are highjacked to support malignant progression14.
When treating an immune ‘hot’ tumour, consideration of the common mechanisms underlying anticancer immunity and cancer-promoting inflammation is required. Hopefully, anti-inflammatory therapies will be found effective not only in abrogating tumour growth and progression but also in boosting anticancer immunity in synergy with other immunotherapies, such as immune-checkpoint inhibitors.
As a canonical cancer hallmark16, inflammation influences all stages of cancer development and treatment. The central inflammatory mediators governing cancer-autonomous intracellular modulation and intercellular communication within the TME have been covered in several reviews1,17-21. Herein, we draw on advances highlighting the use of anti-inflammatory agents for the prevention and/or treatment of solid malignancies, either in isolation or in combination with other therapeutic modalities. Rather than surveying all cancer-related inflammatory traits and mediators, we discuss what we believe are special opportunities and perils for anti-inflammatory approaches. Antiangiogenic agents and multi-kinase inhibitors, some of which exert anti-inflammatory effects, already occupy well-established places in the anticancer armamentarium and will not be included in this Review.
Anti-inflammatory treatments for cancer
Anti-infective agents
Several infectious diseases that result in chronic inflammation have been credibly linked to cancer initiation2. Accordingly, anti-infective agents have an important role in reducing the burden of inflammation-related cancers (FIG. 1).
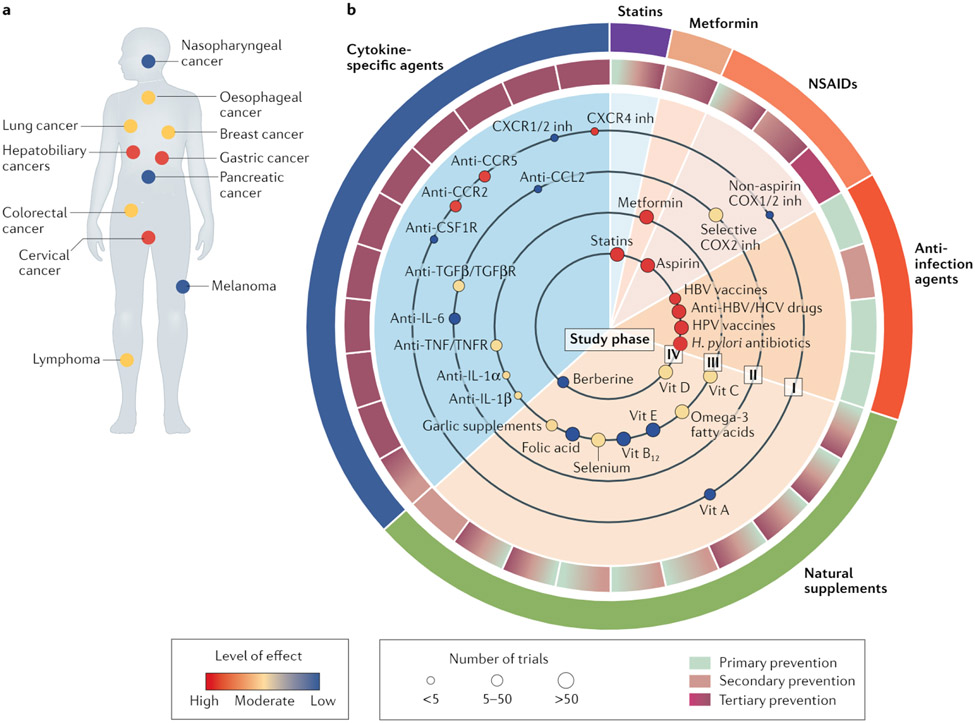
Fig. 1 ∣. Evidence grading for anti-inflammatory agents in cancer prevention.
a ∣ Statistics of anti-inflammatory treatments assigned to patients with different cancer types. Each small circle represents a cancer type for which anti-inflammatory treatments have been examined. The colour of the circles reflects the level of effect defined based on the number of positive versus negative prospective trials registered in the ClinicalTrials.gov database. b ∣ Anti-inflammatory treatments using various anti-infective agents or modalities, nonsteroidal anti-inflammatory drugs (NSAIDs), statins, metformin, cytokine-specific drugs, and natural supplements are illustrated according to levels of evidence and efficacy in the management of cancer. The arcs in the outer circle surrounding the central pie chart indicate the various categories of therapeutic agents. The shading in each portion of the inner circle surrounding the pie chart represents the preventive applications in which each respective agent (or class of agent) shown within the body of the pie chart has been evaluated: primary prevention to deduce the aetiological role of the therapeutic target, secondary prevention to mitigate development of the disease in at-risk populations or tertiary prevention for the treatment of cancer following diagnosis. Each small circle in the inner sectors of the pie chart reflects the clinical evidence for each agent, indicating the level of effect (circle colour), the number of clinical studies performed (circle size) and the phase of clinical testing (as indicated by the ‘study phase’ designation on each large circle within the pie chart). Ongoing trials are summarized in Supplementary Table 1. CCR, CC-chemokine receptor; COX, cyclooxygenase; CSF1R, colony stimulating factor 1 receptor; CXCR, CXC-chemokine receptor; HBV, hepatitis B virus; HCV, hepatitis C virus; HPV, human papillomavirus; inh, inhibitor; TGFβ, transforming growth factor-β; TGFβR, TGFβ receptor; TNF, tumour necrosis factor; TNFR, TNF receptor; Vit, vitamin.
Antiviral therapies.
Currently, hepatitis B virus (HBV) or hepatitis C virus (HCV) infections remain the leading causes of hepatocellular carcinoma (HCC)5. In addition to the direct oncogenic effects22, HBV or HCV infections can cause cancer-promoting inflammation. HBV vaccination has substantially reduced the global HCC burden and is continuing to do so; data from a population-based study indicate that the incidence of HCC in vaccinated birth cohorts is 75% lower than that in unvaccinated cohorts23. For infected individuals, interferon-based therapies, nucleoside or nucleotide analogues, and direct-acting antiviral agents, which inhibit the replication of HBV or HCV and/or promote their immune-mediated clearance, are estimated to decrease HCC risk by 50–80%5. Antiviral treatment is also effective in decreasing disease recurrence and improving postoperative survival outcomes in patients with HCC24-26. However, curative antiviral treatment of HBV or HCV infection alone is insufficient to entirely prevent HCC occurrence or recurrence, possibly owing to the presence of cirrhosis, diabetes, excess alcohol consumption, impaired liver function or other patient characteristics (such as age, sex, lifestyle and others)27-30.
Similarly, >90% of cervical cancers can be attributed to infection with human papillomavirus (HPV) types 16, 18, 31, 33, 45, 52 or 58 (REF.31). The results of international randomized controlled trials (RCTs) have demonstrated the substantial efficacy of HPV vaccines against cervical precancerous lesions (cervical intraepithelial neoplasia grade 2+). According to a large-scale meta-analysis reported in 2019 (REF.32), 5–9 years of population-based vaccination not only reduced the prevalence of HPV infection but also decreased the prevalence of cervical intraepithelial neoplasia grade 2+ by 51% in screened girls aged 15–19 years and by 31% in women aged 20–24 years. More recently, the results of a nationwide study in Sweden demonstrated that the cumulative incidence of cervical cancer was reduced from 94 cases per 100,000 in women who were unvaccinated to 47 cases per 100,000 in women vaccinated with the quadrivalent HPV vaccine (targeting HPV types 6, 11, 16 and 18) at 10–30 years of age33. Therefore, HPV vaccination has been implemented for cervical cancer prophylaxis in multiple age groups across different countries. High-level vaccination coverage in the population would likely result in cervical cancer elimination6, although specific antiviral drugs for treating established HPV infections are still lacking.
Epstein–Barr virus (EBV), the first tumour virus identified in humans, is associated with gastric cancer, nasopharyngeal cancer and lymphoma34. Currently, however, no approved therapies are available to prevent or treat EBV infection, despite intense research efforts.
Antibacterial therapies.
Helicobacter pylori, a bacteria that is carried by ~50% of the world population, is the strongest risk factor for gastric cancer and has therefore been designated by the WHO as a class I carcinogen35. Data from RCTs indicate that H. pylori eradication with broad-spectrum antibiotics not only prevents gastric cancer in individuals with asymptomatic infection or in those without precancerous lesions but also lowers the rates of metachronous gastric cancer development in patients with early stage gastric cancer or high-grade adenoma35-37. Among >2,250 residents of a high-risk region for gastric cancer in China, 2 weeks of H. pylori treatment resulted in early reductions in gastric cancer incidence and mortality that persisted beyond >22 years38.
Commensal bacteria, such as Fusobacterium nucleatum, have been found to increase the risk of colorectal cancer (CRC) development and to promote the progression of this disease39-42. Moreover, computational bioinformatics studies have identified microbial genetic signatures in blood or tumour tissues that distinguished patients with different cancer types from cancer-free individuals; these signatures provide a high-resolution landscape of cancer-associated microbes43. However, further studies are required to determine whether these bacteria induce cancer-promoting inflammation.
The antitumour effects of broad-spectrum antibiotics have been demonstrated in animal models, particularly in models of gastrointestinal cancers41,44,45; however, the current lack of species-specific antibiotics and the deleterious consequences of commensal dysbiosis have limited the therapeutic potential of microbial modulation in patients with cancer46. A future approach could entail the use of species-specific bacteriophages to selectively eliminate carcinogenic or tumour-promoting bacteria47. Vaccination against specific cancer-promoting microbes could also be considered, but antibacterial vaccines are rare and have mostly been ineffective.
Anti-fungal treatment.
Fungal infections have been found to be associated with oesophageal squamous cell carcinoma (ESCC) both in patients with autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy, who have increased susceptibility to chronic fungal infections, and in individuals without autoimmune disease48. These findings suggest that anti-fungal treatments could be used to reduce the incidence of ESCC; indeed, such therapy had anticancer effects in a mouse model of auto-immune polyendocrinopathy-candidiasis-ectodermal dystrophy-related ESCC48.
Nonsteroidal anti-inflammatory drugs
NSAIDs are widely used as antipyretics, analgesics or anti-platelet agents (platelet aggregation inhibitors) for cardiovascular disease (CVD) prophylaxis, operating through the inhibition of cyclooxygenase (COX) activity. One such NSAID, aspirin, has been identified as a broad-spectrum cancer-preventive agent based on data from clinical and epidemiological studies24 (FIG. 1). Since 2015, the US Preventive Services Task Force has recommended the routine use of aspirin for the prevention of CRC among individuals aged 50–59 years who have a high risk of CVD and a low risk of bleeding49. A systematic review and meta-analysis of observational studies on aspirin use and digestive-tract cancers published up to March 2019 revealed that regular users had a 27% lower CRC risk (across 45 studies), a 33% lower ESCC risk (13 studies), a 39% lower risk of adenocarcinoma of the oesophagus and gastric cardia (10 studies), a 36% lower risk of stomach cancer (14 studies), a 38% lower risk of hepatobiliary cancer (5 studies) and a 22% lower risk of pancreatic cancer (15 studies) than non-users7. However, in other large-cohort prospective studies, regular aspirin use was not associated with a statistically significant reduction in pancreatic cancer risk, except in individuals with diabetes or higher baseline levels of systemic inflammation50. Nevertheless, the anti-inflammatory properties of aspirin make it a viable chemopreventive for those with an elevated risk of inflammation-related cancer. In two nationwide observational cohort studies of patients with chronic viral hepatitis in Sweden or Taiwan51,52, the long-term use of low-dose aspirin (≤160 or ≤100 mg daily, respectively, for ≥90 days) reduced the risk of HCC by 31% and 29%, respectively, without increasing the risk of gastrointestinal bleeding. Aspirin also seems to benefit patients with a hereditary cancer risk: in a double-blind RCT, patients with Lynch syndrome who were assigned to aspirin treatment had a 37% lower risk of developing CRC compared with those who received placebo53. An analysis of data from two large-cohort prospective observational studies performed in the USA revealed that regular aspirin use for at least 6 years decreased the overall incidence of gastrointestinal-tract cancers by 15% and of CRC by 19% but had no effect on breast, prostate or lung cancer incidence54. Furthermore, pooled results from RCTs in the setting of CVD prophylaxis (8 eligible trials encompassing 25,570 patients and 674 cancer deaths) demonstrated that daily aspirin use mitigated distant metastasis and deaths from certain cancers, specifically adenocarcinomas (particularly those that were non-metastatic at diagnosis)55.
Importantly, the post-diagnosis administration of aspirin has been shown to be sufficient to reduce overall gastrointestinal or oesophageal cancer mortality56. The survival benefit from post-diagnosis aspirin use specifically in patients with CRC was greater among those with PIK3CA-mutant and COX2-positive tumours57 or among those with low tumoural levels of PD-L1 (REF.58). The ongoing Add-Aspirin trial (ISRCTN74358648) is evaluating the effect of aspirin use after primary radical therapy for gastroesophageal, colorectal, breast or prostate cancer on disease recurrence and survival outcomes, with a predefined feasibility analysis revealing that this adjuvant therapy approach is well tolerated with a low incidence of toxicities (0.5% grade 3 and no upper gastrointestinal bleeding of any grade)59. However, a cautionary note comes from the ASPREE study; in this placebo-controlled RCT, low-dose aspirin (100 mg daily) was associated with increases in bleeding risk, in the incidence of cancers diagnosed with metastasis and, correspondingly, in cancer mortality in older adults (>65 years of age) without CVD, dementia or physical disability60-62.
Celecoxib and rofecoxib, two selective COX2 inhibitors, have demonstrated efficacy in the prophylaxis of colorectal adenomas (or adenomatous polyps) but are not routinely recommended for such indications because of their serious CVD risks63-65. A randomized phase II trial revealed that the addition of celecoxib to chemoradiotherapy did not provide an overall survival (OS) or progression-free survival (PFS) benefit in patients with unresectable stage III non-small-cell lung cancer66. In a trial involving patients with CRC, preoperative treatment with celecoxib did not improve responses to neoadjuvant immunotherapy with anti-PD-1 plus anti-CTLA4 antibodies67, although results of a preclinical study indicate that the inhibition of prostaglandin E2 (PGE2) synthesis (for example, through genetic ablation of COX expression) can overcome immune evasion in some mouse models of cancer68.
Of therapeutic significance, the intraoperative administration of ketorolac, an inhibitor of both COX1 and COX2, reduced the frequency of distant disease recurrence in patients with breast cancer, particularly in those with an elevated BMI (≥25 kg/m2)69. The use of other non-aspirin NSAIDs, such as ibuprofen, has also been associated with a decreased CRC risk, but further investigation of their overall therapeutic value in the prevention and/or treatment of cancer is warranted70.
Lipid-lowering drugs
High serum levels of LDL, a protein complex that is loaded with cholesterol, can lead to harmful inflammation. Statins are 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors that block cholesterol biosynthesis and are prophylactically used to treat CVD. Statins also have poorly understood anti-inflammatory properties, which are in fact important for their protective activity against CVD and have not been reported for other cholesterol-lowering treatments71. Early observations of the association between statin use and CRC risk came from RCTs in the setting of CVD71. In the population-based case–control Molecular Epidemiology of CRC study72, self-reported statin use for at least 5 years was significantly associated with a lower CRC risk (OR 0.50, 95% CI 0.40–0.63), even after adjustment for the presence or absence of hypercholesterolaemia as well as NSAID use, among other factors (OR 0.53,95% CI 0.38–0.74). A retrospective cohort study has revealed that prior statin use might reduce post-colonoscopy CRC incidence73. However, the results of meta-analyses of RCTs and other cohort studies indicate only modest protective effects of statins on CRC74-76.
In a cohort of 7,657 patients with newly diagnosed CRC, post-diagnosis statin use was associated with decreased cancer-specific mortality (fully adjusted HR 0.71, 95% CI 0.61–0.84)77. Nonetheless, when additional confounding variables were considered, neither population-based cohort studies nor the Surveillance, Epidemiology, and End Results (SEER)–Medicare database provided evidence supporting improved cancer-specific survival among statin users78,79.
Similar to other approaches to HCC chemoprevention, a profound beneficial effect of statin use has been observed in patients with viral hepatitis, diabetes or liver cirrhosis yet lower or no statistically significant effects have been observed in the general population80-87. Of note, the statin-related benefits were greater in Asian populations than in Western populations. Observational studies and clinical trials have also revealed that statins might be protective against H. pylori-related gastric cancer in both Asian and Western populations88. Well-designed, prospective, multicentre studies are needed to further validate the chemopreventive activity of statins.
Metformin
Type 2 diabetes mellitus (T2DM) has been linked to an increased incidence of and mortality from many types of cancers, including colorectal, pancreatic, hepatobiliary, breast and endometrial cancers89. Metformin is an oral biguanide used for the first-line treatment of T2DM. Data from epidemiological studies and meta-analyses have demonstrated an association between metformin use and a reduced incidence of pancreatic, hepatocellular, lung, colorectal and breast cancers in patients with T2DM89. Of note, a systematic review encompassing 10 studies involving a total of 334,307 patients with T2DM revealed that metformin use was associated with a 50% lower risk of HCC90-92. Specifically, this association was seen in a meta-analysis of observational studies (n = 8) after adjusting for potential confounding factors, such as the use of other antidiabetic agents; however, the evidence is still insufficiently strong to recommend chemoprevention using metformin in patients at high risk of HCC90-92. Therefore, additional prospective trials or observational studies evaluating the ability of metformin to reduce HCC risk are needed.
The results of a multicentre, double-blind, placebo-controlled phase III trial involving 151 patients without diabetes who had previously had single or multiple colorectal adenomas or polyps resected by endoscopy support a potential role for metformin in CRC prevention93. In this group, treatment with low-dose metformin (250 mg per day) resulted in a significantly lower incidence of metachronous polyps (RR 0.67, 95% CI 0.47–0.97) or adenomas (RR 0.60, 95% CI 0.40–0.92)93.
Given its low cost and good safety profile (the most serious complication is lactic acidosis, with an incidence of about 3–10 cases per 100,000 person-years)94, metformin has been extensively investigated as a possible therapeutic and chemopreventive agent in different cancer settings95 (FIG. 1). The anticancer activity of metformin is being evaluated in numerous ongoing phase II and III trials (Supplementary Table 1). For example, the combinational use of metformin and low-dose aspirin is being investigated for tertiary prevention (to avoid disease recurrence) after the resection of stage I–III CRC (NCT03047837)96. Encouragingly, in an open-label, phase II study involving patients with advanced-stage EGFR-mutant non-small-cell lung cancer97, the addition of metformin to EGFR tyrosine-kinase inhibitor therapy significantly prolonged PFS (median 13.1 months versus 9.9 months with EGFR tyrosine-kinase inhibitors alone; P = 0.03) and OS (median 31.7 months versus 17.5 months, respectively; P=0.02).
Targeted anti-inflammatory agents
IL-1 antagonists.
In the double-blind, placebo-controlled phase III CANTOS trial that had the primary aim of investigating the efficacy of the anti-IL-1β antibody canakinumab in preventing recurrent CVD, this anti-inflammatory therapy was found to have unanticipated activity in preventing lung cancer98. The CANTOS study included 10,061 patients who had atherosclerosis, a previous myocardial infarction and high serum levels of high-sensitivity C-reactive protein (CRP; ≥2 mg/l) but were free of previously diagnosed cancer. Of note, canakinumab therapy led to a dose-dependent reduction in circulating CRP and IL-6 levels. At a median follow-up duration of 3.7 years, canakinumab (300 mg subcutaneously every 3 months) was associated with a 67% reduction in lung cancer incidence (P < 0.0001), with a 39% reduction also seen with 150 mg dosing (P=0.034), as well as with a 77% reduction in lung cancer mortality (P = 0.0002), compared with placebo. However, fatal infections or sepsis occurred more frequently with canakinumab than with placebo, warranting caution. Further trials specifically designed to evaluate the efficacy of canakinumab in cancer prevention and treatment are ongoing (Supplementary Table 1).
The first-in-class anti-IL-1α monoclonal antibody MABp1 was developed to target systemic inflammation in cancer. Data from phase I–III trials demonstrate that MABp1 is well tolerated, with no dose-limiting toxicities observed, and can result in the stabilization of disease and symptoms (lean body mass and/or pain, fatigue or anorexia) in patients with various treatment-refractory advanced-stage solid tumours99,100. The investigators of the phase III trial of this agent99,100, which specifically involved patients with CRC refractory to oxaliplatin and irinotecan, concluded that MABp1 constitutes a new standard in the management of advanced-stage CRC.
Blockade of the TNF pathway.
Monoclonal antibody-based agents targeting TNF (infliximab) or its receptor (etanercept) have also been tested for tolerability and biological activity in patients with advanced-stage cancers; the observed therapeutic effects were modest, although the blockade of TNF signalling might contribute to disease stabilization101-105. ICIs are commonly associated with immune-related adverse events (irAEs) such as moderate-to-severe colitis106. Infliximab has been recommended for the management of irAEs that are refractory to glucocorticoids107. Furthermore, the treatment of advance-stage melanoma with either infliximab or certolizumab (another monoclonal antibody-based anti-TNF drug), each administered concomitantly with the anti-CTLA4 antibody ipilimumab and the anti-PD-1 antibody nivolumab, is currently being evaluated in a phase Ib trial (NCT03293784). Notably, anti-TNF therapy might not be feasible in patients with hepatitis given the indispensable role of TNF–TNFR1 signalling in liver regeneration108.
Anti-IL-6 agents.
IL-6 is one of the most crucial cytokines bridging cancer-promoting inflammation and immunosuppression109. Anti-IL-6 drugs, which are routinely used in the treatment of autoimmune conditions, have been tested in anticancer applications. In several phase I–II clinical trials, however, the clinical response to the anti-IL-6R antibody tocilizumab or the anti-IL-6 antibodies clazakizumab and siltuximab has been poor in patients with prostate, lung or breast cancers, multiple myeloma, or cancer-related cachexia (reviewed previously109,110). Thus, monotherapy with agents targeting IL-6 might have limited activity against solid tumours in non-stratified patients, although anti-IL-6 therapy is effective in reversing irAEs caused by immunotherapy111. In particular, tocilizumab has been approved for the treatment of cytokine-release syndrome (CRS) associated with chimeric antigen receptor (CAR) T cell therapy112.
Inhibition of TGFβ signalling.
Transforming growth factor-β (TGFβ)-targeted therapies have also been considered for the management of cancer. Galunisertib, a small-molecule inhibitor of the TGFβR1 kinase, has been demonstrated to be safe in patients with various cancers113. When administered in combination with gemcitabine to patients with unresectable pancreatic ductal adenocarcinoma (PDAC) or with sorafenib to patients with advanced-stage HCC, galunisertib had modest therapeutic activity114,115. Pending the outcome of trials using galunisertib, more-potent and more-specific small-molecule inhibitors of TGFβR1 have been developed and are being tested in combination with chemotherapy or emerging immunotherapy modalities (Supplementary Table 1). In addition, isoform-specific anti-TGFβ, pan-TGFβ or bi-functional anti-PD-L1-TGFβR2 antibodies are all being tested in phase I trials of different anticancer indications116.
Targeting cytokines mediating TAMs and MDSCs.
Antibodies or other antagonists targeting the colony-stimulating factor 1 receptor (CSF1R), the CC-chemokine receptor 2 (CCR2) or CCR5, which can deplete tumour-associated macrophages (TAMs) or otherwise abrogate their immunosuppressive inflammatory activities, have been found to be safe and tolerable in phase I trials117-120. Although anti-CSF1R antibodies did not have robust anticancer activity either alone or in combination with chemotherapy117,118, CCR2 (REF.119) and CCR5 (REF.120) antagonism led to objective clinical responses in patients with advanced-stage PDAC or CRC, respectively. Owing to their immunomodulatory potential, these TAM-targeted agents are also being explored for potential synergy with ICIs121.
The CXC-chemokine receptor 1 (CXCR1) and CXCR2 antagonist SX-682, which was designed to disrupt the trafficking of myeloid-derived suppressor cells (MDSCs) to tumours, is being tested in combination with pembrolizumab (an anti-PD-1 antibody) in a phase I/II trial involving patients with metastatic melanoma (NCT03161431). Additionally, data from the COMBAT trial (NCT02826486) indicate that the CXCR4 antagonist BL-8040 depletes MDSCs and increases the tumour infiltration of CD8+ T cells, with evidence also indicating that this agent might cooperate with pembrolizumab to improve antitumour immune responses and chemotherapy efficacy in patients with PDAC122.
Natural anti-inflammatory supplements
In addition to the drugs discussed above, some natural compounds might also help control inflammation and cancer. As an antioxidant and anti-inflammatory dietary supplement, vitamin C has been extensively explored for potential anticancer effects. However, contemporary data indicate that pharmacological vitamin C can enhance the cytotoxicity and therapeutic sensitivity of cancer cells only when administered intravenously at high doses and that it exerts anticancer effects primarily via pro-oxidant, rather than antioxidant, activity123.
Vitamin D can regulate the host immune system to potentiate immune responses as well as to attenuate harmful inflammatory reactions124. The results of several prospective observational studies indicate that plasma levels of the major circulating form of vitamin D, 25-hydroxyvitamin D, are inversely associated with the risk of CRC and prostate cancer125-127. Pre-treatment vitamin D levels have also been associated with PFS and OS in patients with advanced-stage CRC or Hodgkin lymphoma who received first-line chemotherapy128,129. However, a prophylactic benefit of vitamin D supplementation remains questionable. In 2,303 randomized postmenopausal women without a prior cancer diagnosis, nutritional supplementation with vitamin D and calcium did not reduce the risk of all-type cancer at 4 years compared with placebo130. In the VITAL (Vitamin D and Omega-3) trial, which involved a total of 25,871 cancer-free men and women (aged >50 years and >55 years, respectively), vitamin D supplementation (2,000 IU per day) for a median of 5.3 years was not associated with a reduced incidence of invasive cancer compared with placebo131. The findings of a meta-analysis of 52 RCTs involving 75,454 participants suggest that vitamin D supplementation could reduce the risk of cancer-related death by 16%132. Nonetheless, in the double-blind, phase II SUNSHINE trial involving patients with advanced-stage CRC, the addition of high-dose (8,000 IU per day for 14 days, followed by 4,000 IU per day) versus standard-dose (400 IU per day) vitamin D to standard chemotherapy resulted in no statistically significant difference in PFS (although a statistically significant difference in PFS was observed on multivariate analysis)133. Likewise, in the randomized single-centre AMATERASU trial in patients with digestive-tract cancers, postoperative vitamin D supplementation did not result in improved 5-year relapse-free survival or OS as compared with placebo134. In keeping with the anti-inflammatory properties of vitamin D, intake of this vitamin has been correlated with a reduced risk of ICI-related colitis135.
The administration of long-chain omega-3 fatty acids, an anti-inflammatory nutritional supplement that is often tested in parallel with vitamin D, has not been found to be effective in cancer prevention136,137. However, omega-3 supplementation has been associated with a reduced risk of colorectal adenomas among individuals with low plasma levels of such fatty acids at baseline and in the African-American population (OR 0.59, 95% CI 0.35–1.00)137. Similarly, the seAFOod Polyp Prevention trial did not meet its primary end point (an improved adenoma detection rate) but did suggest that the omega-3 polyunsaturated fatty acid eicosapentaenoic acid has a chemopreventive effect in reducing recurrent adenoma multiplicity138. Of note, contradictory results have been obtained in trials investigating other dietary supplements, including β-carotene, α-tocopherol (vitamin E), selenium, vitamin B12 and folic acid, some of which were even associated with an increased cancer risk or diminished chemotherapy responses38,137,139-143.
The antimicrobial, anti-platelet and lipid-lowering effects attributed to garlic supplements have earned these supplements a place in cancer prevention strategies. In a RCT conducted in a high-risk region for gastric cancer in Shandong, China, 3,365 participants were assigned to three different interventions (H. pylori treatment; vitamin C, vitamin E and selenium supplementation; or garlic extract and oil supplementation) or appropriate placebos; among these individuals, the use of garlic supplements for >7 years did not decrease the incidence of gastric cancer but did significantly reduce mortality from this disease (HR 0.66, 95% CI 0.43–1.00)38.
As a plant-derived natural alkaloid with antioxidant and antimicrobial properties, berberine might have a wide range of therapeutic benefits for patients with digestive system or metabolic diseases. In a double-blind, placebo-controlled RCT, berberine at 0.3 g twice daily significantly decreased the recurrence rate of colorectal adenoma and polypoid lesions after polypectomy (RR 0.77, 95% CI 0.66–0.91; P=0.001)144. Given the rather short follow-up duration of this study (2 years), the ability of berberine to prevent the occurrence of advanced colorectal adenomas or CRC remains to be determined.
Mechanisms of anti-inflammatory therapy
Suppression of oncogenic pathways
Targeting tumour cell-intrinsic pathways.
COX2-PGE2 signalling can directly confer epithelial cells with protumorigenic traits, thus facilitating the development of inflammation-driven cancer (FIG. 2). COX2 can activate the AKT, mTOR and NF-κB pathways to support cancer cell proliferation either directly or via PGE2 signalling. Consistently, aspirin and selective COX2 inhibitors have pro-apoptotic and antiproliferative effects on COX2-overexpressing cancer cells145. In addition, PGE2 silences tumour-suppressor genes by reinforcing their promoter methylation through a EP4–DNA methyltransferase pathway146. Correspondingly, combined treatment with celecoxib and decitabine effectively mitigates intestinal tumour development in ApcMin/+ mice146. Moreover, hepatic COX2 overexpression induces spontaneous HCC development in mice by reducing the expression of methylcytosine dioxygenase TET1 (a DNA demethylase), thereby resulting in increased DNA methylation, epigenetic silencing of tumour suppressor genes and in the activation of oncogenic pathways, which can be reversed by celecoxib treatment40. NSAID treatment can also neutralize a senescence-associated inflammatory response that promotes the growth and invasiveness of p53-deficient intestinal cells and thus prevents colorectal carcinogenesis147. Surprisingly, PGE2 also promotes colon regeneration in preclinical models of colitis through feedforward activation of the transcriptional regulator YAP1, which can trigger tumorigenesis; accordingly, administration of the NSAID indomethacin or of an EP4 antagonist exacerbates colitis but can prevent colon tumorigenesis in mice148.
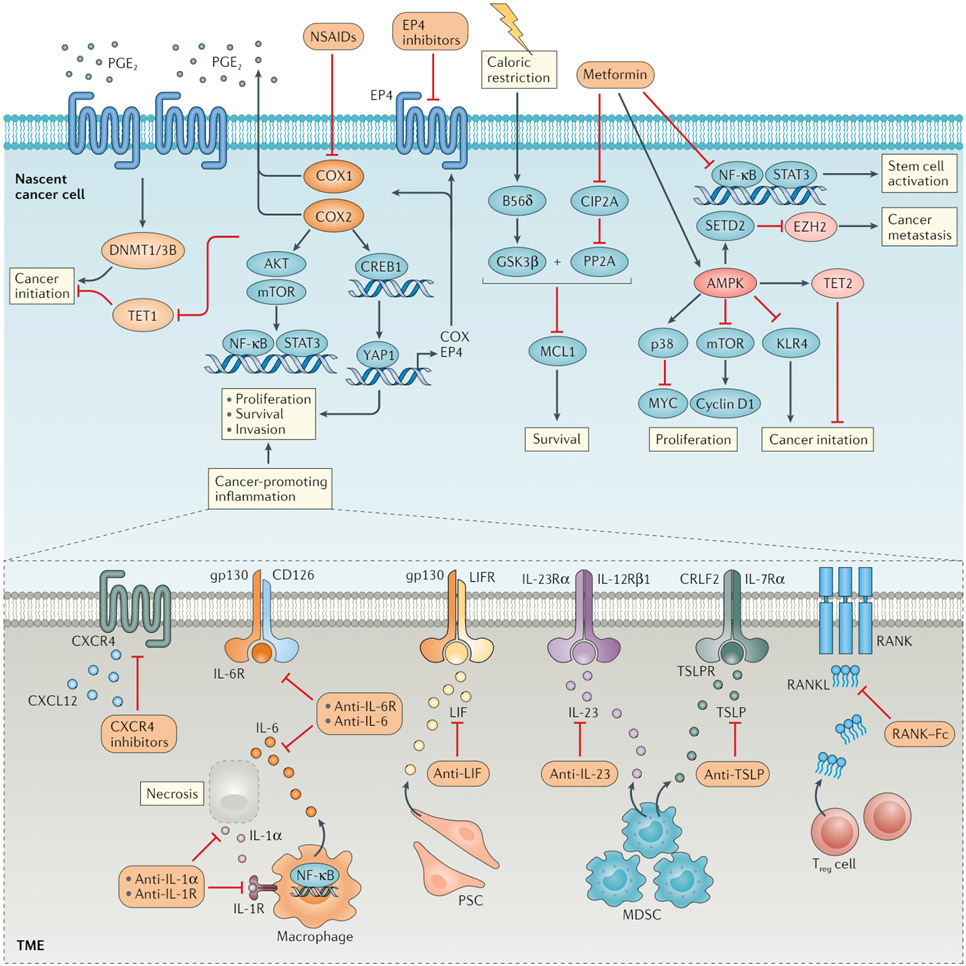
Fig. 2 ∣. Impact of anti-inflammatory agents on oncogenic pathways.
Cyclooxygenase 1 (COX1) and COX2, which are the targets of nonsteroidal anti-inflammatory drugs (NSAIDs), can activate AKT, mTOR and NF-κB to support cancer cell survival and proliferation, either directly or via the production of prostaglandin E2(PGE2). PGE2 produced in a COX2-dependent manner can bind to the PGE2 receptor EP4 and induce intracellular signal transduction. Whereas COX2 functions to downregulate the expression of DNA demethylase TET1, EP4 signalling upregulates the expression of DNA (cytosine 5)-methyltransferase 1 and/or 3B (DNMT1/3B). The altered expression of both of these epigenetic regulators results in silencing of tumour suppressor genes and thus promotes cancer initiation, which could potentially be prevented through treatment with the NSAID COX2 inhibitor celecoxib. PGE2-EP4 signalling can also activate a positive feedforward loop for tumour initiation and promotion involving upregulation of the transcriptional coactivator YAP1, which in turn upregulates the expression of COX enzymes and EP4. NSAIDs or EP4 antagonists might effectively disrupt this inflammation-driven process. The anticancer effects of metformin largely depend on the activation of AMPK, which negatively regulates downstream signalling cascades involved in cancer initiation. In addition, AMPK activation confers a tumour-suppressive epigenome via the stabilization of TET2 and/or degradation of the histone-lysine N-methyltransferase EZH2. In an AMPK-independent fashion, metformin attenuates an NF-κB-mediated inflammatory response required for stem cell function and can activate a protein phosphatase 2A (PP2A) pathway to exert control over cell survival via inhibition of the antiapoptotic protein MCL1. The latter mechanism is otherwise ineffective for cancer prevention in mice without GSK3β activation through caloric restriction. Various cytokines present in the inflammatory tumour microenvironment (TME), including IL-6, IL-23, leukaemia inhibitory factor (LIF), receptor activator of nuclear factor-κB ligand (RANKL), thymic stromal lymphopoietin (TSLP) and CXC-chemokine ligand 12 (CXCL12), can induce pro-tumorigenic signals via cognate receptors expressed by cancer cells themselves. Therefore, inhibitory antibodies or other antagonists targeting these cytokines or their receptors might have anticancer effects. These cytokines are derived from distinct immune cells, including macrophages, myeloid-derived suppressor cells (MDSCs) and regulatory T (Treg) cells or stromal cells, such as pancreatic stellate cells (PSCs), in a context-dependent manner. For instance, IL-6 can be produced abundantly by macrophages in response to necrotic tumour cells and IL-1α. Hence, the blockade of IL-1α signalling might prevent downstream tumorigenic events. B56δ, PP2A B subunit isoform B56δ; CIP2A, cancerous inhibitor of protein phosphatase 2A; CREB1, cAMP-responsive element-binding protein 1; CXCR4, CXC-chemokine receptor 4; STAT3, signal transducer and activator of transcription 3.
Via competitive inhibition of HMG-CoA reductase, the rate-limiting enzyme of the mevalonate pathway, statins can activate the AMPK and p38 MAPK pathways or suppress MYC phosphorylation to induce cell cycle arrest and apoptosis149-151. Similarly, metformin improves insulin sensitivity through the indirect activation of AMPK, which inhibits the activity of mTOR complex 1 (mTORC1), thereby leading to cell growth arrest and resulting in a broad cancer chemopreventive effect152. Metformin can also suppress cyclin D1 expression as well as the inflammatory responses associated with stem cell activation152-154. Consistent with the link between diabetes and cancer, hyperglycaemia impairs the AMPK-mediated phosphorylation and stabilization of TET2, leading to global DNA demethylation and the inhibition of tumour growth in mice; the anticancer effects of metformin might also depend on the reprogramming of a cancer-favourable epigenome via the activation of this AMPK-TET2 pathway155. Similarly, metformin has been shown to integrate metabolic and epigenetic signalling via an AMPK–SETD2–EZH2 axis, thereby suppressing prostate cancer metastasis156. Curiously, metformin impairs tumour growth only if administered during periods of fasting-induced hypoglycaemia in mice157. Mechanistically, the inhibition of CIP2A by metformin together with upregulation of the PP2A B subunit isoform B56δ under low glucose conditions activates GSK3β signalling, which leads to reduced levels of the pro-survival protein MCL1 and, ultimately, cancer cell death157 (FIG. 2).
Targeting tumour cell-extrinsic factors.
Early studies of diethylnitrosamine-induced HCC in mice have shown that IL-1α released by necrotic hepatocytes acts as an inflammatory switch that supports compensatory cell proliferation and HCC development, which could be counteracted using the IL-1R antagonist anakinra158. IL-6 is one of the central orchestrators of the inflammation-cancer interface, which directly enhances the proliferative and metastatic capacities of cancer cells159. Mouse models had revealed the therapeutic potential of drugs that target IL-6 or its receptor but, rather disappointingly, tocilizumab, clazakizumab and siltuximab had limited therapeutic activity in patients with cancer cachexia109. A novel IL-6 family member, leukaemia inhibitory factor (LIF), has been identified as a key pro-tumour paracrine factor secreted by activated pancreatic stellate cells; LIF blockade using a monoclonal antibody restricted the progression of PDAC in mice and augmented responses to chemotherapy by converting the cancer cells to a less aggressive and more drug-susceptible state9. In human and mouse prostate cancers, IL-23 produced by MDSCs can enhance androgen receptor (AR) activity in cancer cells, thus promoting a castration-resistant phenotype10. Accordingly, an anti-IL-23 antibody reversed resistance to androgen-deprivation therapy in a mouse model of prostate cancer10.
Receptor activator of nuclear factor-κB (RANK) signalling governs osteoclastogenesis and bone resorption and has been examined as a therapeutic target in patients with breast or prostate cancer bone metastases11. Interestingly, RANK ligand (RANKL)-producing regulatory T cells also promote the spread of RANK-expressing mammary carcinoma cells to the lung in mouse models160. Accordingly, the administration of an antagonistic RANK–Fc fusion protein substantially reduces pulmonary metastasis in these models160. Myeloid cell-derived thymic stromal lymphopoietin promotes the survival of tumour cells through induction of the antiapoptotic molecule BCL-2 and a neutralizing antibody to thymic stromal lymphopoietin inhibits the growth of both primary tumours and lung metastases in mice161.
CXCR4 is a chemokine receptor commonly expressed by multiple types of tumour cells; its ligand, CXCL12, is a component of the inflammatory TME and can enhance the survival and migration of tumour cells. Systemic administration of the CXCR4 antagonist plerixafor (AMD3100) inhibits the growth of intracranial glioblastoma and medulloblastoma xenografts in mice by reducing activation of the ERK and AKT signalling pathways162. Likewise, CXCR4 inhibition with motixafortide (BL-8040) restricts the growth of mouse neuroblastomas via the tumour-suppressive microRNAs miR-15a and miR-16-1, which silence BCL-2 and cyclin D1 expression163. Integrins, lectins and neuregulins are also known to contribute to the inflammatory TME and might therefore prove to be worthwhile therapeutic targets164-166 if their oncogenic capacities can be inhibited using the corresponding antagonists.
Dietary vitamin D3 and its analogue calcitriol regulate multiple genes by binding to the nuclear vitamin D receptor (VDR), a member of the steroid–thyroid–retinoid receptor superfamily of ligand-activated transcription factors. Theoretically, vitamin D and calcitriol could suppress inflammation, cancer cell proliferation, invasion and metastasis; such anticancer effects have been validated in mouse xenograft models but, unfortunately, have not consistently been demonstrated in humans125.
Disrupting the tumour-supporting stroma
Targeting inflammatory messengers.
Some soluble factors act as envoys by shuttling between the tumour and its stroma, thus constituting important targets for anti-inflammatory treatments (FIG. 3). In the aforementioned diethylnitrosamine-induced model of HCC, excessive production of reactive oxygen species (ROS) by inflammatory stromal cells results in oxidative DNA damage, hepatocyte death and compensatory cell proliferation167. Accordingly, oral administration of the chemical antioxidant butylated hydroxyanisole or vitamin E decreases ROS production and prevents diethylnitrosamine-induced HCC167. Similarly, butylated hydroxyanisole attenuates ROS-elicited TNF and IL-1β production by Kupffer cells and can prevent pre-malignant cholangiocellular lesions in mouse models of intrahepatic cholangiocarcinoma168. In addition, the antioxidant N-acetylcysteine prevents ROS-induced T cell death upon hepatic steatosis and delays HCC development in mice169 (FIG. 3a).
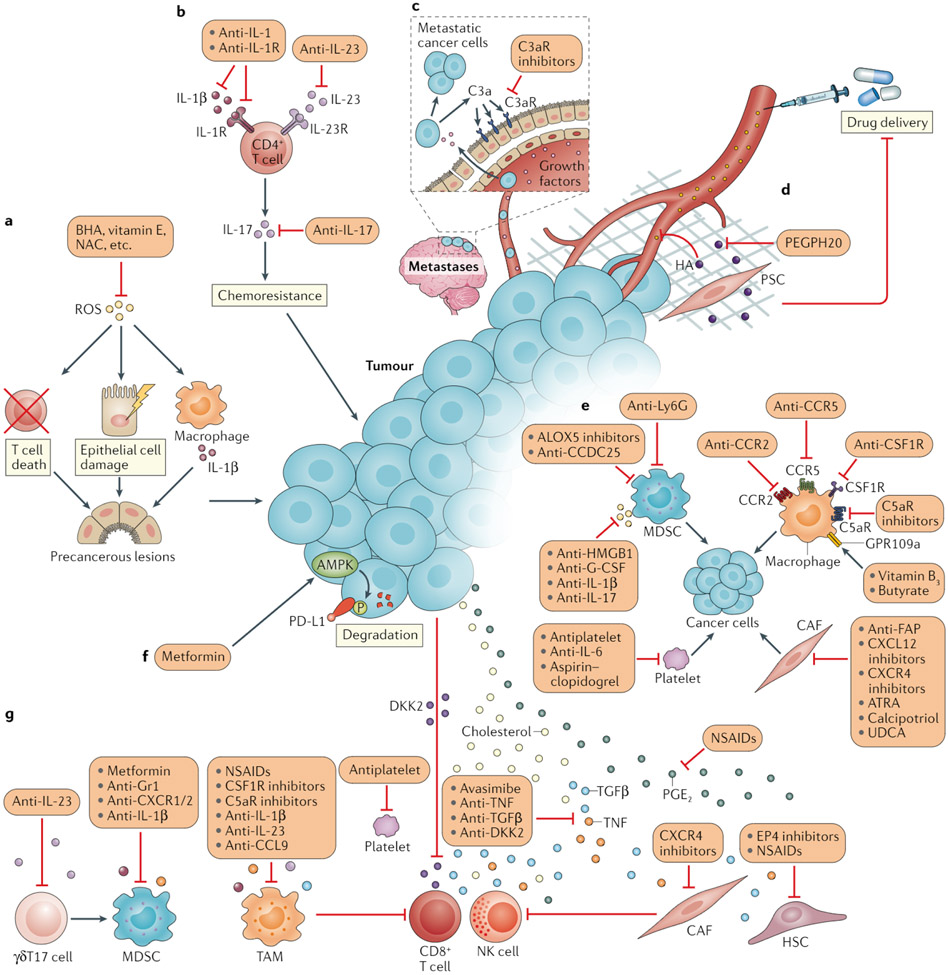
Fig. 3 ∣. Approaches to resolving cancer-associated inflammation and normalizing antitumour immunity.
a ∣ Reactive oxygen species (ROS) can cause epithelial cell damage, immune cell death, unresolved inflammation and subsequent precancerous lesions. ROS and their effects can be counteracted by antioxidants, such as butylated hydroxyanisole (BHA), vitamin E and N-acetylcysteine (NAC). b ∣ IL-17 has a pivotal role in inflammation-driven cancer initiation as well as in angiogenesis and chemotherapy resistance. IL-17 is generally produced by CD4+ T cells in response to IL-23 or IL-1β. Accordingly, antagonistic antibodies targeting IL-17, IL-23 or IL-1β receptor (IL-1R) have substantial therapeutic anticancer effects in mice170-172. c ∣ Leptomeningeal metastatic cells can secret complement component 3 (C3a), which activates the C3a receptor (C3aR) expressed on the choroid plexus epithelium and thereby enables circulating growth factors to enter the leptomeningeal space via disruption of the blood–brain barrier. C3aR antagonism might therefore interrupt the nutrition supply to metastatic cells in the cerebrospinal fluid. d ∣ The glycosaminoglycan hyaluronan (HA) is a central component of the extracellular matrix, fostering tissue stiffness and restricting drug perfusion. A PEGylated form of PH20 hyaluronidase (PEGPH20), which can degrade HA in the tumour microenvironment, is capable of enhancing chemotherapeutic efficacy174. e ∣ Tumour-associated macrophages (TAMs), neutrophils or myeloid-derived suppressor cells (MDSCs), cancer-associated fibroblasts (CAFs) and platelets directly communicate with cancer cells and supply them with various pro-tumorigenic signals, which can be interrupted by targeting the corresponding inflammatory stimulants or their receptors. f ∣ Tumour cells often express the inhibitory immune-checkpoint protein PD-L1 to avoid elimination by CD8+ T cells; AMPK can catalyse the phosphorylation and thus promote the degradation of PD-L1, and this process can be activated by metformin. g ∣ Moreover, cholesterol, prostaglandin E2 (PGE2), tumour necrosis factor (TNF), transforming growth factor-β (TGFβ) and oncoproteins such as Dickkopf-related protein 2 (DKK2) present in the tumour-associated inflammatory milieu can directly suppress the function of CD8+ T cells and natural killer (NK) cells; therefore, the cholesterol acyltransferase inhibitor avasimibe, nonsteroidal anti-inflammatory drugs (NSAIDs), PGE2 receptor EP4 antagonists, and cytokine-specific antibodies or antagonists might reinforce the antitumour effects of these cytotoxic lymphocytes. TAMs, MDSCs, CAFs (or hepatic stellate cells (HSCs)), platelets and other cell types that coordinate the inflammatory responses within the tumour microenvironment also frequently produce factors that are suppressive to effector lymphocytes. Other pro-inflammatory cells, such as IL-17-producting γδT (γδT17) cells, can further strengthen the immunosuppressive phenotype of MDSCs. Anti-inflammatory strategies for depleting or reprogramming these cells might restore the cancer–immunity cycle. ALOX5, arachidonate 5-lipoxygenase; ATRA, all-trans retinoic acid; CCDC25, coiled-coil domain containing protein 25; CCL, CC-chemokine ligand; CCR, CC-chemokine receptor; CSF1R, colony-stimulating factor 1 receptor; CXCL, CXC-chemokine ligand; CXCR, CXC-chemokine receptor; C5aR, complement component 5a receptor; FAR fibroblast activation protein; G-CSF, granulocyte colony-stimulating factor; GPR109A, G protein-coupled receptor 109A; HMGB1, high mobility group box1; Ly6G, lymphocyte antigen 6G (also known as Gr1); UDCA, ursodeoxycholic acid.
IL-17 is implicated in the pathogenesis of both non-alcoholic steatohepatitis (NASH) and alcoholic steatohepatitis, including links with liver inflammation, fibrogenesis and carcinogenesis. In mice, targeting IL-17 or its upstream inducer IL-23 markedly suppressed the development of NASH-associated or alcoholic steatohepatitis-associated HCC170,171. Intriguingly, CD4+ T cell-derived IL-17 was found to blunt the anticancer efficacy of chemotherapeutic agents in mice and this effect could be averted by blocking the IL-1β-IL-1R pathway172 (FIG. 3b).
Complement component 3 (C3) is upregulated in mouse and human leptomeningeal metastatic cells and can activate C3a receptor (C3aR) signalling in the choroid plexus epithelium, which in turn can alter the composition of the cerebrospinal fluid in a manner that promotes tumour growth173. Correspondingly, a C3aR antagonist is effective in reducing breast and lung cancer leptomeningeal metastases in mice173 (FIG. 3c).
The glycosaminoglycan hyaluronan (HA), a major component of the extracellular matrix, is abundant in the microenvironment of chronic inflammatory diseases as well as of several malignancies. In a genetically engineered mouse model (GEMM) of PDAC, HA has been identified as a crucial modifier of tumour vascular function and enzymatic depletion of HA using PEGylated human recombinant PH20 hyaluronidase (PEGPH20) substantially enhanced drug delivery and therapeutic efficacy with diminished tumour growth174 (FIG. 3d). Unfortunately, however, the addition of PEGPH20 to standard chemotherapy was found to increase drug toxicity and thus to decrease treatment durations in patients with this disease175.
Targeting inflammatory cells.
Macrophages are the dominant orchestrators of cancer-promoting inflammatory signals and an abundance of TAMs is associated with high-grade tumours and a poor prognosis176. The cytokine CSF1 has important roles in regulating macrophage recruitment and function through CSF1R signalling (FIG. 3e). CSF1R inhibition can specifically deplete TAMs and suppresses glioma progression177 and lung cancer brain metastasis178 in mouse models. Increased CSF1 expression has been observed in patients with prostate cancers treated with radiotherapy or androgen-deprivation therapy; in mouse models, this upregulation of CSF1 culminates in acquired treatment resistance, which can be reversed through CSF1R inhibition179,180. In a different model, CSF1R inhibition does not affect mammary tumour growth or metastasis but rather sensitizes the tumours to chemotherapy181. Chemokines and chemokine receptors have been implicated in tumour infiltration by macrophages. Accordingly, antibodies targeting CCL2 or CCL5 impede tumour growth and dissemination in multiple preclinical models176. In a mouse model with excessive complement activation, the administration of a complement component C5a anaphylatoxin chemotactic receptor (C5aR) antagonist attenuates macrophage-mediated inflammation and tumorigenesis182. The G protein-coupled receptor 109A (GPR109A) has anti-inflammatory effects on colonic macrophages and dendritic cells (DCs) and is essential for the induction of IL-18 expression in colonic epithelium; the GPR109A agonists niacin (vitamin B3) and butyrate efficiently suppress inflammation-induced and ApcMin/+ intestinal tumours in mice183.
Neutrophils often accumulate in both primary tumours and pre-metastatic niches in response to diverse inflammatory milieus184. Moreover, the neutrophil-depleting antibody anti-Ly6G effectively prevents tumour cell dissemination to distant organs in mice185 (FIG. 3e). Pro-inflammatory molecules, such as high mobility group box 1 (HMGB1), IL-1β, IL-17 and G-CSF, help shape the tumour-promoting phenotypes of neutrophils; thus, the neutralization or inhibition of these factors is effective in reducing metastasis in preclinical models186,187. Additionally, pharmacological inhibition of the leukotriene-generating enzyme arachidonate 5-lipoxygenase or neutrophil extracellular trap-associated signals (the latter using anti-CCDC25 antibodies) interferes with the pro-metastatic functions of neutrophils in many settings188-190.
Fibroblasts are typically the most abundant stromal component within solid tumours and these cells can become activated during tissue inflammation and fibrogenesis. As such, cancer-associated fibroblasts (CAFs) are integrally involved in cancer-promoting inflammation. CAF-targeting therapies, including fibroblast activation protein-neutralizing antibodies, CXCL12–CXCR4 pathway antagonists, all-trans retinoic acid, the VDR ligand calcipotriol, and the immunomodulatory and anti-inflammatory agent ursodeoxycholic acid, have demonstrated anticancer activities in preclinical models191,192 (FIG. 3e).
Platelets are hypothesized to promote tumour cell dissemination. For example, platelet accumulation and tumour angiogenesis is markedly inhibited using antiplatelet or anti-IL-6 antibodies, which enhances the therapeutic efficacy of paclitaxel in a mouse model of ovarian cancer193. Moreover, suppression of platelet activation and aggregation using aspirin and clopidogrel can abrogate HBV-associated or NASH-associated inflammation and carcinogenesis194,195 (FIG. 3e).
Promotion of antitumour immunity
Using conventional anti-inflammatory drugs.
Mounting evidence from preclinical studies indicates that conventional anti-inflammatory drugs exert immunomodulatory functions (FIG. 3f,g). Notably, COX inhibition synergizes with PD-1 inhibition in eradicating mouse tumours68,196. Mechanistically, tumour-derived PGE2 impairs the natural killer cell-mediated recruitment of conventional type 1 DCs, thus culminating in tumour immune evasion, which could be reversed by treatment with aspirin or celecoxib68,196 (FIG. 3g). Consistent with its immunosuppressive properties, PGE2 produced by senescent hepatic stellate cells directly compromises T cell function in mice with HCC induced by a high-fat diet197. Accordingly, an EP4 antagonist restores antitumour immunity and attenuates HCC development in this model197. PGE2 has also been implicated in the M2 polarization of macrophages198.
Metformin triggers AMPK activation and consequently silences hypoxia-inducible factor-α, which is a transcription factor crucial in inducing CD39 and CD73 expression on MDSCs; CD39 and CD73 mediate production of the immunosuppressive factor adenosine; therefore, suppression of the expression of these proteins by metformin reinvigorated the antitumour activity of CD8+ T cells in patients with ovarian cancer199 (FIG. 3g). Interestingly, metformin-induced AMPK activation can directly cause PD-L1 phosphorylation, which results in abnormal glycosylation, endoplasmic reticulum accumulation and endoplasmic reticulum-associated protein degradation, thereby manifesting a potential mechanism for increased T cell activity and, thus, immunotherapy efficacy200 (FIG. 3f). Furthermore, increased infiltration of CD8+ T cells and other immune cells into the TME has been observed in patients with ESCC receiving low-dose metformin201.
The modulation of cholesterol metabolism can also potentiate T cell receptor signalling and prevent T cell exhaustion. Specifically, the disruption of cholesterol esterification using the acyl-CoA cholesterol acyltransferase 1 inhibitor avasimibe potentiates the antitumour effect of PD-1 inhibition in preclinical models202,203. Moreover, conventional anti-platelet agents can improve T cell-based therapy in mouse models in which platelets constrain T cell-mediated anticancer immunity through a glycoprotein A repetitions predominant (GARP)–TGFβ axis204 (FIG. 3g).
Using targeted agents.
Targeted anti-inflammatory agents might also harness the host immune system to fight cancer and many of these agents depend on the manipulation of myeloid cell plasticity (FIG. 3). CSF1R inhibition directly depletes or reprogrammes immunosuppressive TAMs and consequently improves antitumour immune responses in many preclinical models205-208. TAMs can also have an important role in antibody-based cancer therapy through antibody-dependent cellular phagocytosis. Surprisingly, when phagocytosing tumour DNA, TAMs impart immunosuppression through the upregulation of PD-L1 and indoleamine 2,3-dioxygenase (IDO) expression, which is dependent on inflammasome activation and IL-1β production; therefore, treatment with an anti-IL-1β antibody substantially improves the efficacy of anti-HER2 therapy in immunocompetent mice bearing HER2+ breast cancer cells209. In another model of breast cancer210, genetic deficiency of IL-1β expression increases the intratumoural DC to macrophage ratio and anti-IL-1β antibodies synergize with anti-PD-1 treatment in tumour elimination. In response to inflammatory stimulation or chemoattraction, CD11b+Gr1+ myeloid cells frequently infiltrate tumour sites and exert an immunosuppressive effect in various mouse models; the pharmacological depletion or segregation of these cells (which are typically referred to as MDSCs) from tumours using an anti-Gr1 antibody or a CXCR1/2 antagonist, respectively, restored antitumour immunity211-214. Notably, the overexpression of IL-1β led to MDSC mobilization and activation in the early stage of gastric carcinogenesis and, therefore, anti-IL-1β therapy might prevent cancer development by stimulating turnover of the immunosuppressive environment of neoplastic tissues215.
Remarkably, studies using human CRC samples have revealed that inflammatory DCs can induce IL-17-producing γδT (γδT17) cells in an IL-23-dependent manner216. In addition to IL-17, these γδT17 cells also secrete TNF, IL-8 and GM-CSF, all of which can attract MDSCs and sustain their immunosuppressive activity, which is reversible with IL-23 neutralization216 (FIG. 3g). Furthermore, in a GEMM of inflammatory lung cancer, co-blockade of IL-23 and CCL9 abrogated MYC-induced immune exclusion and tumour progression217.
The C5a–C5aR axis contributes to the immunosuppressive effects of myeloid cells. For example, C5a generated locally in the TME can recruit MDSCs and promote their production of ROS and reactive nitrogen species, which hamper the antitumour activity of CD8+ T cells. Preclinically, the pharmacological inhibition or genetic ablation of C5aR impairs tumour growth via increases in the abundance and cytotoxicity of CD8+ T cells218. Furthermore, the C5aR antagonist PMX-53 improves the antitumour efficacy of immunotherapy or chemotherapy in various mouse models219,220. A phase I trial of the C5aR1 monoclonal antibody IPH5401 in combination with the anti-PD-L1 antibody durvalumab in patients with advanced-stage solid tumours is ongoing221 (STELLAR-001; NCT03665129). Phagocytosis checkpoint proteins, such as CD47, are additional novel targets for cancer immunotherapy222 and might provide more opportunities for combinations with anti-inflammatory agents.
In addition to myeloid cells, CAFs, B cells, γδT cells, type 1 innate lymphoid cells (ILC1) and mucosal-associated invariant T cells can also curtail antitumour immune responses under certain inflammatory conditions191,223-227 Nonetheless, specific anti-inflammatory strategies for targeting these cell types are lacking, with the possible exception of CAFs. For instance, CXCR4 inhibition can render tumours responsive to immunotherapy by overcoming the fibrotic and immunosuppressive TME in both HCC and PDAC models228,229.
TNF can directly trigger activation-induced death of T cells, whereas TGFβ hampers cytotoxic immune cell function (FIG. 3g). Hence, targeting either of these cytokines using etanercept or galunisertib, respectively, enhances the antitumour effects of ICIs108,116. Oncogenic pathways also impart unconventional inflammatory signals to directly suppress cytotoxic lymphocytes. For example, loss of the tumour suppressors APC in intestinal tumour cells or of PTEN in melanoma cells causes them to secrete Dickkopf-related protein 2 (DKK2), which impedes signal transducer and activator of transcription 5 (STAT5) activation within immune cells via binding to LDL receptor-related protein 5 (LRP5)230. Accordingly, an anti-DKK2 antibody reactivates tumour-infiltrating natural killer cells and CD8+ T cells and potentiates responses to PD-1 inhibition in mouse models of these cancers230.
Mitigating irAEs.
Anti-inflammatory agents also have indispensable roles in attenuating irAEs. As discussed, judicious use of an anti-IL-6R antibody can attenuate CAR T cell-induced CRS, which can otherwise limit the therapeutic value of this innovative immunotherapy231. IL-1R antagonism via anakinra or CAR T cell engineering is also capable of abrogating CRS-related mortality in mouse models231. Likewise, prophylactic use of TNF antagonists can ameliorate immune-related colitis associated with dual anti-CTLA4 and anti-PD-1 inhibition in a mouse model of colon cancer108. Of importance, these anti-inflammatory therapies can further enhance immunotherapy efficacy and prolong survival in preclinical models, which are desirable characteristics for clinical use.
Pro-tumour perils of anti-inflammatories
Increasing clinical and preclinical evidence supports the broad therapeutic activity of metformin across a wide range of cancer types. However, BRAF-mutant melanoma has been demonstrated to escape the growth-inhibitory stress imposed by metformin232. The dual-specificity phosphatase DUSP6 negatively regulates ERK activity downstream of oncogenic BRAF, and AMPK hyperactivation by metformin results in the targeting of DUSP6 for degradation and thereby potentiates the ERK-driven expression of VEGFA, which bypasses the inhibitory effects of metformin and AMPK on mTORC1 signalling; thus, metformin counterproductively stimulates angiogenesis and accelerates tumour growth232. Inhibitors of mTOR (the kinase component of mTORC1) have long been considered as potential cancer treatments. As an immunosuppressive drug, however, the mTOR inhibitor rapamycin was found to activate the pro-tumorigenic factor STAT3 in a mouse model of steatotic HCC. Mechanistically, hepatocyte-specific loss of mTORC1 activity promoted hepatocarcinogenesis through the hyperactivation of AKT owing to the disruption of a negative feedback loop233. Another potential peril associated with metformin use relates to the consequent increases in circulating levels of growth/differentiation factor 15 (GDF15), which induces weight loss and correlates with cachexia and poor survival outcomes in patients with cancer234,235.
With statin treatment for PDAC, the disruption of cholesterol biosynthesis can induce the sterol response element-binding protein (SREBP)-dependent expression of TGFβ and the epithehal-to-mesenchymal transition in cancer cells236, which might promote disease progression. Glucocorticoids are generally used as anti-inflammatory and immunosuppressive agents for treating chemotherapy-related or immunotherapy-induced adverse events in patients with advanced-stage cancer. Multiomics data from patient-derived xenograft models indicate that these agents can activate glucocorticoid receptor signalling at distant metastatic sites, which in turn increases metastatic colonization and reduces mouse survival via upregulation of the tyrosine-protein kinase transmembrane receptor ROR1 (REF.237). The intravasation of tumour cells is a key process involved in metastatic dissemination to distant organs. Accordingly, the anticoagulant warfarin has been shown to increase vascular leakiness in mammary tumours, which was accompanied by substantial increases in the numbers of circulating tumour cells and lung metastases238.
Cytokine-specific or cytokine receptor-specific therapeutic agents have been tested in proof-of-concept trials across many cancer types; however, paradoxical findings underscore the importance of careful clinical application and further interrogation into their molecular mechanism. Despite the reported clinical benefits of anti-IL-1β therapy98, IL-1β is essential for cancer immunosurveillance in various contexts (FIG. 4). First, immunogenic cell death in established tumours is associated with the activation of DCs with an intact inflammasome machinery to secrete IL-1β, which primes tumour-specific T cell responses239. Conversely, the anticancer effects of chemotherapeutic agents are dampened with the coadministration of an IL-1β-neutralizing antibody239. Second, the IL-1β-STAT1-interferon regulatory factor 1 (IRF1) axis is fundamental for IL-9 and IL-21 production by T helper 9 (TH9) cells, as demonstrated by the downregulation of Irf1, Il9 and Il21 expression in tumour-infiltrating TH9 cells following treatment with an IL-1R antagonist; this pathway was found to be crucial for the anticancer functions of TH9 cells in mice240. Third, in an Apc-based mouse model of CRC, IL-1R signalling in epithelial cells and T cells has pro-tumorigenic effects, whereas myeloid cell-specific IL-1R signalling counteracts tumour-promoting dysbiosis and inflammation241. Fourth, in mouse models of breast cancer, a systemic IL-1β-mediated inflammatory response has been shown to prevent metastasis-initiating cell differentiation and colonization of distant tissues, and the inhibition of IL-1R signalling at the primary tumour site results in metastatic progression242. In keeping with these preclinical findings, patients with breast cancer expressing high levels of IL-1β have been found to have better survival outcomes than those with low IL-1β expression242. Similarly, several different counterintuitive effects might explain the disappointing clinical outcomes achieved to date with agents targeting CSF1R. For example, in a GEMM of glioblastoma, macrophages that persist following CSF1R inhibition produce insulin-like growth factor 1 (IGF1), which can drive tumour recurrence through PI3K activation12. Surprisingly, CSF1R inhibition can also affect CAFs and, in particular, causes these cells to express the granulocyte-specific chemokine CXCL1, which initiates an immune inhibitory circuit243. Compelling evidence from single cell-based analyses of CRCs from mice indicates that antagonism of CSF1R depletes inflammatory F4/80hi myeloid cell populations, while sparing those with pro-angiogenic and immunosuppressive properties13. In mouse models of breast cancer, treatment with a CCL2 antagonist reduces the abundance of TAMs and metastases by retaining inflammatory monocytes in the bone marrow, but cessation of such treatment results in a lethal rebound effect mediated by IL-6 and VEGF secretion244.
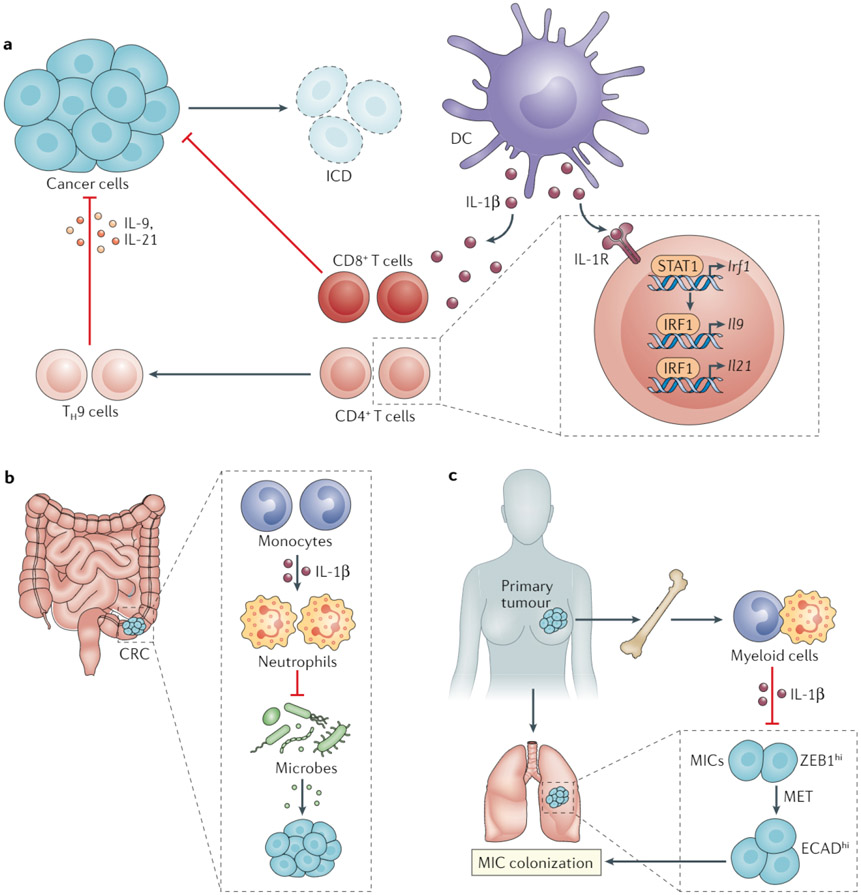
Fig. 4 ∣. Potential perils of anti-IL-1β therapy for cancer.
The discovery of important roles for IL-1 in promoting antitumour immunity and suppressing cancer-promoting inflammation warrants careful consideration in approaches to targeting this cytokine for cancer therapy. a ∣ Chemotherapy-induced immunogenic cell death (ICD) of cancer cells activates dendritic cells (DCs) and thereby results in the production of IL-1β, which is mandatory for the cross-priming of antitumour CD8+ T cells. IL-1β also dictates the anticancer effect of T helper 9 (TH9) cells by upregulating IL-9 and IL-21 expression in these CD4+ T cells via signal transducer and activator of transcription 1 (STAT1) and interferon regulatory factor 1 (IRF1)240. b ∣ In a mouse model of colorectal cancer (CRC), monocyte-derived IL-1β imparts an anti-inflammatory phenotype in neutrophils, which attenuate intestinal dysbiosis and cancer progression241. c ∣ In models of breast cancer, primary tumours elicit a systemic inflammatory response that includes the expansion of bone marrow and circulating myeloid cells and the production of IL-1β by these myeloid cells suppresses metastatic colonization by preventing mesenchymal-to-epithelial transition (MET) of metastasis-initiating cells (MICs)242. ECAD, E-cadherin; ZEB1, zinc finger E-box binding homeobox 1.
Conceivably, neutrophil-targeting strategies might also have poor anticancer efficacy because neutrophils have immunostimulatory activities in certain scenarios245,246. Likewise, the complement system might enhance the clinical responses to various cancer treatments, including monoclonal antibodies, vaccines and radiotherapy, reminiscent of the risks associated with complement-targeted therapeutics221,247. The depletion of CAFs might also induce immune evasion, for example, by increasing the abundance of regulatory T cells in the TME of PDAC248.
Antioxidants are believed to exert anticancer effects owing primarily to interference with pro-tumorigenic redox signalling. Paradoxically, in some preclinical models, antioxidant treatments accelerate tumour progression and metastasis through the inactivation of tumour suppressors or metabolic reprogramming249-252, mirroring outcomes observed in the clinical setting143.
Given the extensive molecular intersections and crosstalk, cellular adaptability, and organ-specific contexture, anti-inflammatory therapy targeting a single immunomodulatory factor might lead to tumour evolution or TME remodelling. Together, the paradoxical findings discussed above should prompt special caution when translating anti-inflammatory therapies into clinical use.
Conclusions
To date, many drugs and drug candidates have been used both preclinically and clinically to curtail the inflammatory conditions that fuel cancer development and progression. Numerous preclinical studies have provided insights into the mechanisms underlying the intricate interactions between cancer, inflammation and immunity, which should eventually lead to more innovative anti-inflammatory cancer therapies reaching the clinic. Considering the advances outlined herein, researchers and oncologists working together should be capable of developing successful strategies to inhibit cancer-related inflammation and of making such an approach a main-stay of modern cancer therapy. So far, anti-inflammatory strategies have proven rather effective in cancer prevention and conventional drugs such as aspirin have led to a much larger reduction in cancer mortality than novel and far more sophisticated targeted therapies. Given our improved understanding of the TME, research tools and animal models, we are hopeful that, in the next decade, several anti-inflammatory therapies will advance to the clinic and prove effective in preventing or treating cancer.
The numerous and diverse links between cancer and inflammation all present therapeutic opportunities, especially when the concept of ‘inflammation’ is broadened to include viral, bacterial and fungal infections. However, numerous hurdles and uncertainties remain in every step of translating an anti-inflammatory agent into clinical use. First, whether the inflammatory redundancies identified in preclinical models are targetable and druggable remains unclear. Second, the heterogeneity and plasticity of the TME present problems in targeting a single cytokine or even a single cell type. The effects of disrupted negative feedback loops and the activation of compensatory pathways is also hard to predict. Third, given that patients with cancer are typically assigned to conventional treatments, a need exists to identify more specific inflammatory targets that are responsible for therapy resistance or adverse events. Fourth, contrary to other targeted therapies, clinically applicable biomarkers for the selection of anti-inflammatory agents and assessment of their anticancer effects are lacking. Fifth, the effects of endogenous factors, such as the patients age and microbiota, on the magnitude of inflammatory responses and on the outcomes of anti-inflammatory treatments remain to be determined253,254. Notably, the composition of the microbiota has been shown to affect immunotherapy efficacy, suggesting that microbial interventions could potentially be leveraged to improve cancer prevention or treatment255,256. By necessity, defining a therapeutic paradigm for anti-inflammatory treatments would require optimized pharmaceutical programmes as well as appropriate animal models and clinical trial designs. In addition, integrative high-resolution analyses using multiomics, single-cell and/or spatial-based technologies should provide deeper insights into local therapeutic responses and the exact cellular and molecular consequences of anti-inflammatory treatments. Finally, the deployment of personalized, multi-agent, anti-inflammatory regimens in the era of immunotherapy is possibly another key to treating cancer.
Supplementary Material
Key points.
Inflammation-related biological processes influence all stages of cancer development and treatment; environmental risk factors and both tumour-extrinsic and tumour-intrinsic inflammatory processes have been linked to tumour initiation, promotion and progression.
Several conventional drugs with anti-inflammatory properties have demonstrated protective effects against cancer but are yet to be deployed in at-risk populations and properly evaluated for therapeutic applicability.
Cytokine-specific agents with anti-inflammatory activities have antitumour efficacy in preclinical studies but evidence demonstrating activity against solid tumours in clinical trials is scarce.
Preclinical studies have revealed that anti-inflammatory drugs can suppress cancer development through multiple mechanisms.
Monotherapy with anti-inflammatory agents can elicit cell adaptability and/or affect tumour evolution in heterogeneous cancer types, leading to therapy resistance or even accelerated disease progression.
Overcoming current obstacles to the clinical introduction of anti-inflammatory therapy will require the development of effective combination regimens and the identification of reliable response biomarkers.
Acknowledgements
The work of the authors is supported by grants from the National Key Research and Development Program of China (2016YFC0905900 to B.S.), the State Key Program of the National Natural Science Foundation (81930086 to B.S.; 81871970 and 81672801 to J.H.) and the US NIH (U01AA027681, R01CA211794, R01CA234128, P01CA128814, R01CA198103 and Tower Cancer Research Grant to M.K.). The work of J.H. is also supported by the Hundred Talent Program of Sun Yat-sen University. Figures in this review were drafted with the assistance of Dr Haiyan Zhang (Sun Yat-sen University Cancer Center).
Footnotes
Competing interests
M.K. has received research support from Merck and Aduro Pharmaceuticals. J.H. and B.S. declare no competing interests.
Supplementary information
Supplementary information is available for this paper at https://doi.org/10.1038/s41571-020-00459-9.
References
- 1.Greten FR & Grivennikov SI Inflammation and cancer: triggers, mechanisms, and consequences. Immunity 51, 27–41 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grivennikov SI, Greten FR & Karin M Immunity, inflammation, and cancer. Cell 140, 883–899 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balkwill F & Mantovani A Inflammation and cancer: back to Virchow? Lancet 357, 539–545 (2001). [DOI] [PubMed] [Google Scholar]
- 4.Diakos CI, Charles KA, McMillan DC & Clarke SJ Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 15, e493–e503 (2014). [DOI] [PubMed] [Google Scholar]
- 5.Yang JD et al. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol 16, 589–604 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brisson M et al. Impact of HPV vaccination and cervical screening on cervical cancer elimination: a comparative modelling analysis in 78 low-income and lower-middle-income countries. Lancet 395, 575–590 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bosetti C, Santucci C, Gallus S, Martinetti M & La Vecchia C Aspirin and the risk of colorectal and other digestive tract cancers: an updated meta-analysis through 2019. Ann. Oncol 31, 558–568 (2020). [DOI] [PubMed] [Google Scholar]
- 8.Chapelle N, Martel M, Toes-Zoutendijk E, Barkun AN & Bardou M Recent advances in clinical practice: colorectal cancer chemoprevention in the average-risk population. Gut 69, 2244–2255 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi Y et al. Targeting LIF-mediated paracrine interaction for pancreatic cancer therapy and monitoring. Nature 569, 131–135 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calcinotto A et al. IL-23 secreted by myeloid cells drives castration-resistant prostate cancer. Nature 559, 363–369 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahern E, Smyth MJ, Dougall WC & Teng MWL Roles of the RANKL-RANK axis in antitumour immunity - implications for therapy. Nat. Rev. Clin. Oncol 15, 676–693 (2018). [DOI] [PubMed] [Google Scholar]
- 12.Quail DF et al. The tumor microenvironment underlies acquired resistance to CSF-1R inhibition in gliomas. Science 352, aad3018 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang L et al. Single-cell analyses inform mechanisms of myeloid-targeted therapies in colon cancer. Cell 181, 442–459.e29 (2020). [DOI] [PubMed] [Google Scholar]
- 14.Galon J & Bruni D Tumor immunology and tumor evolution: intertwined histories. Immunity 52, 55–81 (2020). [DOI] [PubMed] [Google Scholar]
- 15.Shalapour S & Karin M Pas de deux: control of anti-tumor immunity by cancer-associated inflammation. Immunity 51, 15–26 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanahan D & Weinberg RA Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011). [DOI] [PubMed] [Google Scholar]
- 17.Elinav E et al. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat. Rev. Cancer 13, 759–771 (2013). [DOI] [PubMed] [Google Scholar]
- 18.Fridman WH, Zitvogel L, Sautes-Fridman C & Kroemer G The immune contexture in cancer prognosis and treatment. Nat. Rev. Clin. Oncol 14, 717–734 (2017). [DOI] [PubMed] [Google Scholar]
- 19.Ho WJ, Jaffee EM & Zheng L The tumour microenvironment in pancreatic cancer - clinical challenges and opportunities. Nat. Rev. Clin. Oncol 17, 527–540 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhong Z, Sanchez-Lopez E & Karin M Autophagy, inflammation, and immunity: a troika governing cancer and its treatment. Cell 166, 288–298 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bruni D, Angell HK & Galon J The immune contexture and Immunoscore in cancer prognosis and therapeutic efficacy. Nat. Rev. Cancer 20, 662–680 (2020). [DOI] [PubMed] [Google Scholar]
- 22.Cancer Genome Atlas Research Network. Comprehensive and integrative genomic characterization of hepatocellular carcinoma. Cell 169, 1327–1341.e23 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang MH et al. Long-term effects of hepatitis B immunization of infants in preventing liver cancer. Gastroenterology 151, 472–480.e1 (2016). [DOI] [PubMed] [Google Scholar]
- 24.Yin J et al. Effect of antiviral treatment with nucleotide/nucleoside analogs on postoperative prognosis of hepatitis B virus-related hepatocellular carcinoma: a two-stage longitudinal clinical study. J. Clin. Oncol 131, 3647–3655 (2013). [DOI] [PubMed] [Google Scholar]
- 25.Huang G et al. Antiviral therapy improves postoperative survival in patients with hepatocellular carcinoma: a randomized controlled trial. Ann. Surg 261, 56–66 (2015). [DOI] [PubMed] [Google Scholar]
- 26.Okamura Y et al. The achievement of a sustained virological response either before or after hepatectomy improves the prognosis of patients with primary hepatitis C virus-related hepatocellular carcinoma. Ann. Surg. Oncol 26, 4566–4575 (2019). [DOI] [PubMed] [Google Scholar]
- 27.Kanwal F et al. Risk of hepatocellular cancer in HCV patients treated with direct-acting antiviral agents. Gastroenterology 153, 996–1005.e1 (2017). [DOI] [PubMed] [Google Scholar]
- 28.Yip TC et al. Impact of age and gender on risk of hepatocellular carcinoma after hepatitis B surface antigen seroclearance. J. Hepatol 67, 902–908 (2017). [DOI] [PubMed] [Google Scholar]
- 29.Nahon P et al. Incidence of hepatocellular carcinoma after direct antiviral therapy for HCV in patients with cirrhosis included in surveillance programs. Gastroenterology 155, 1436–1450.e6 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Papatheodoridis GV et al. Hepatocellular carcinoma prediction beyond year 5 of oral therapy in a large cohort of Caucasian patients with chronic hepatitis B. J. Hepatol 72, 1088–1096 (2020). [DOI] [PubMed] [Google Scholar]
- 31.de Sanjose S et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 11, 1048–1056 (2010). [DOI] [PubMed] [Google Scholar]
- 32.Drolet M, Benard E, Perez N, Brisson M & HPV Vaccination Impact Study Group. Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: updated systematic review and meta-analysis. Lancet 394, 497–509 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lei J et al. HPV vaccination and the risk of invasive cervical cancer. N. Engl. J. Med 383, 1340–1348 (2020). [DOI] [PubMed] [Google Scholar]
- 34.Munz C Latency and lytic replication in Epstein-Barr virus-associated oncogenesis. Nat. Rev. Microbiol 17, 691–700 (2019). [DOI] [PubMed] [Google Scholar]
- 35.Choi IJ et al. Helicobacter pylori therapy for the prevention of metachronous gastric cancer. N. Engl. J. Med 378, 1085–1095 (2018). [DOI] [PubMed] [Google Scholar]
- 36.Wong BC et al. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA 291, 187–194 (2004). [DOI] [PubMed] [Google Scholar]
- 37.Ford AC, Forman D, Hunt RH, Yuan Y & Moayyedi P Helicobacter pylori eradication therapy to prevent gastric cancer in healthy asymptomatic infected individuals: systematic review and meta-analysis of randomised controlled trials. BMJ 348, g3174 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li WQ et al. Effects of Helicobacter pylori treatment and vitamin and garlic supplementation on gastric cancer incidence and mortality: follow-up of a randomized intervention trial. BMJ 366, I5016 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mima K et al. Fusobacterium nucleatum and T cells in colorectal carcinoma. JAMA Oncol. 1, 653–661 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu T et al. Fusobacterium nucleatum promotes chemoresistance to colorectal cancer by modulating autophagy. Cell 170, 548–563.e16 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bullman S et al. Analysis of fusobacterium persistence and antibiotic response in colorectal cancer. Science 358, 1443–1448 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mima K et al. Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis. Gut 65, 1973–1980 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poore GD et al. Microbiome analyses of blood and tissues suggest cancer diagnostic approach. Nature 579, 567–574 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44.Schulz MD et al. High-fat-diet-mediated dysbiosis promotes intestinal carcinogenesis independently of obesity. Nature 514, 508–512 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ma C et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science 360, eaan5931 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Helmink BA, Khan MAW, Hermann A, Gopalakrishnan V & Wargo JA The microbiome, cancer, and cancer therapy. Nat. Med 25, 377–388 (2019). [DOI] [PubMed] [Google Scholar]
- 47.Dong X et al. Bioinorganic hybrid bacteriophage for modulation of intestinal microbiota to remodel tumor-immune microenvironment against colorectal cancer. Sci. Adv 6, eaba1590 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu F et al. Autoreactive T cells and chronic fungal infection drive esophageal carcinogenesis. Cell Host Microbe 21, 478–493.e7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bibbins-Domingo K & U.S. Preventive Services Task Force. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: U.S. preventive services task force recommendation statement. Ann. Intern. Med 164, 836–845 (2016). [DOI] [PubMed] [Google Scholar]
- 50.Khalaf N et al. Regular use of aspirin or non-aspirin nonsteroidal anti-inflammatory drugs is not associated with risk of incident pancreatic cancer in two large cohort studies. Gastroenterology 154, 1380–1390.e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simon TG et al. Association of aspirin with hepatocellular carcinoma and liver-related mortality. N. Engl. J. Med 382, 1018–1028 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee TY et al. Association of daily aspirin therapy with risk of hepatocellular carcinoma in patients with chronic hepatitis B. JAMA Intern. Med 179, 633–640 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burn J et al. Cancer prevention with aspirin in hereditary colorectal cancer (Lynch syndrome), 10-year follow-up and registry-based 20-year data in the CAPP2 study: a double-blind, randomised, placebo-controlled trial. Lancet 395, 1855–1863 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cao Y et al. Population-wide Impact of long-term use of aspirin and the risk for cancer. JAMA Oncol. 2, 762–769 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rothwell PM et al. Effect of daily aspirin on risk of cancer metastasis: a study of incident cancers during randomised controlled trials. Lancet 379, 1591–1601 (2012). [DOI] [PubMed] [Google Scholar]
- 56.Frouws MA et al. Effect of low-dose aspirin use on survival of patients with gastrointestinal malignancies; an observational study. Br. J. Cancer 116, 405–413 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liao X et al. Aspirin use, tumor PIK3CA mutation, and colorectal-cancer survival. N. Engl. J. Med 367, 1596–1606 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hamada T et al. Aspirin use and colorectal cancer survival according to tumor CD274 (programmed cell death 1 ligand 1) expression status. J. Clin. Oncol 35, 1836–1844 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Joharatnam-Hogan N et al. Aspirin as an adjuvant treatment for cancer: feasibility results from the Add-Aspirin randomised trial. Lancet Gastroenterol. Hepatol 4, 854–862 (2019). [DOI] [PubMed] [Google Scholar]
- 60.McNeil JJ et al. Effect of aspirin on cancer incidence and mortality in older adults. J Natl Cancer Inst. 10.1093/jnci/djaa114 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mahady SE et al. Major GI bleeding in older persons using aspirin: incidence and risk factors in the ASPREE randomised controlled trial. Gut 10.1136/gutjnl-2020-321585 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McNeil JJ et al. Effect of aspirin on all-cause mortality in the healthy elderly. N. Engl. J. Med 379, 1519–1528 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bertagnolli MM et al. Celecoxib for the prevention of sporadic colorectal adenomas. N. Engl. J. Med 355, 873–884 (2006). [DOI] [PubMed] [Google Scholar]
- 64.Arber N et al. Celecoxib for the prevention of colorectal adenomatous polyps. N. Engl. J. Med 355, 885–895 (2006). [DOI] [PubMed] [Google Scholar]
- 65.Baron JA et al. A randomized trial of rofecoxib for the chemoprevention of colorectal adenomas. Gastroenterology 131, 1674–1682 (2006). [DOI] [PubMed] [Google Scholar]
- 66.Bi N et al. Effect of concurrent chemoradiation with celecoxib vs concurrent chemoradiation alone on survival among patients with non-small cell lung cancer with and without cyclooxygenase 2 genetic variants: a phase 2 randomized clinical trial. JAMA Netw. Open 2, e1918070 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chalabi M et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat. Med 26, 566–576 (2020). [DOI] [PubMed] [Google Scholar]
- 68.Zelenay S et al. Cyclooxygenase-dependent tumor growth through evasion of immunity. Cell 162, 1257–1270 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Desmedt C et al. Potential benefit of intra-operative administration of ketorolac on breast cancer recurrence according to the patient’s body mass index. J. Natl Cancer Inst 110, 1115–1122 (2018). [DOI] [PubMed] [Google Scholar]
- 70.Felix AS et al. Relationships of tubal ligation to endometrial carcinoma stage and mortality in the NRG oncology/gynecologic oncology group 210 trial. J. Natl Cancer Inst 107, djv158 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chan AO et al. Prevalence of colorectal neoplasm among patients with newly diagnosed coronary artery disease. JAMA 298, 1412–1419 (2007). [DOI] [PubMed] [Google Scholar]
- 72.Poynter JN et al. Statins and the risk of colorectal cancer. N. Engl. J. Med 352, 2184–2192 (2005). [DOI] [PubMed] [Google Scholar]
- 73.Cheung KS et al. Statins reduce the progression of non-advanced adenomas to colorectal cancer: a postcolonoscopy study in 187 897 patients. Gut 68, 1979–1985 (2019). [DOI] [PubMed] [Google Scholar]
- 74.Bonovas S, Filioussi K, Tsavaris N & Sitaras NM Statins and cancer risk: a literature-based meta-analysis and meta-regression analysis of 35 randomized controlled trials. J. Clin. Oncol 24, 4808–4817 (2006). [DOI] [PubMed] [Google Scholar]
- 75.Dale KM, Coleman CI, Henyan NN, Kluger J & White CM Statins and cancer risk: a meta-analysis. JAMA 295, 74–80 (2006). [DOI] [PubMed] [Google Scholar]
- 76.Bardou M, Barkun A & Martel M Effect of statin therapy on colorectal cancer. Gut 59, 1572–1585 (2010). [DOI] [PubMed] [Google Scholar]
- 77.Cardwell CR, Hicks BM, Hughes C & Murray LJ Statin use after colorectal cancer diagnosis and survival: a population-based cohort study. J. Clin. Oncol 32, 3177–3183 (2014). [DOI] [PubMed] [Google Scholar]
- 78.Hoffmeister M et al. Statin use and survival after colorectal cancer: the importance of comprehensive confounder adjustment. J. Natl Cancer Inst 107, djv045 (2015). [DOI] [PubMed] [Google Scholar]
- 79.Emilsson L et al. Examining bias in studies of statin treatment and survival in patients with cancer. JAMA Oncol. 4, 63–70 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.El-Serag HB, Johnson ML, Hachem C & Morgana RO Statins are associated with a reduced risk of hepatocellular carcinoma in a large cohort of patients with diabetes. Gastroenterology 136, 1601–1608 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tsan YT, Lee CH, Wang JD & Chen PC Statins and the risk of hepatocellular carcinoma in patients with hepatitis B virus infection. J. Clin. Oncol 30, 623–630 (2012). [DOI] [PubMed] [Google Scholar]
- 82.Singh S, Singh PP, Singh AG, Murad MH & Sanchez W Statins are associated with a reduced risk of hepatocellular cancer: a systematic review and meta-analysis. Gastroenterology 144, 323–332 (2013). [DOI] [PubMed] [Google Scholar]
- 83.Tsan YT et al. Statins and the risk of hepatocellular carcinoma in patients with hepatitis C virus infection. J. Clin. Oncol 31, 1514–1521 (2013). [DOI] [PubMed] [Google Scholar]
- 84.Bonovas S, Nikolopoulos G & Sitaras NM Statins and reduced risk of hepatocellular carcinoma in patients with hepatitis C virus infection: further evidence is warranted. J. Clin. Oncol 31, 4160 (2013). [DOI] [PubMed] [Google Scholar]
- 85.Simon TG, Bonilla H, Yan P, Chung RT & Butt AA Atorvastatin and fluvastatin are associated with dose-dependent reductions in cirrhosis and hepatocellular carcinoma, among patients with hepatitis C virus: Results from ERCHIVES. Hepatology 64, 47–57 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim G, Jang SY, Nam CM & Kang ES Statin use and the risk of hepatocellular carcinoma in patients at high risk: a nationwide nested case-control study. J. Hepatol 68, 476–484 (2018). [DOI] [PubMed] [Google Scholar]
- 87.Simon TG et al. Lipophilic Statins and risk for hepatocellular carcinoma and death in patients with chronic viral hepatitis: results from a nationwide Swedish population. Ann. Intern. Med 171, 318–327 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Singh PP & Singh S Statins are associated with reduced risk of gastric cancer: a systematic review and meta-analysis. Ann. Oncol 24, 1721–1730 (2013). [DOI] [PubMed] [Google Scholar]
- 89.Shlomai G, Neel B, LeRoith D & Gallagher EJ Type 2 diabetes mellitus and cancer: the role of pharmacotherapy. J. Clin. Oncol 34, 4264–4269 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Singh S, Singh PP, Singh AG, Murad MH & Sanchez W Anti-diabetic medications and the risk of hepatocellular cancer: a systematic review and meta-analysis. Am. J. Gastroenterol 108, 881–891 (2013). [DOI] [PubMed] [Google Scholar]
- 91.Singh S, Singh PP, Roberts LR & Sanchez W Chemopreventive strategies in hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol 11, 45–54 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Llovet JM et al. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2, 16018 (2016). [DOI] [PubMed] [Google Scholar]
- 93.Higurashi T et al. Metformin for chemoprevention of metachronous colorectal adenoma or polyps in post-polypectomy patients without diabetes: a multicentre double-blind, placebo-controlled, randomised phase 3 trial. Lancet Oncol. 17, 475–483 (2016). [DOI] [PubMed] [Google Scholar]
- 94.Foretz M, Guigas B & Viollet B Understanding the glucoregulatory mechanisms of metformin in type 2 diabetes mellitus. Nat. Rev. Endocrinol 15, 569–589 (2019). [DOI] [PubMed] [Google Scholar]
- 95.Vancura A, Bu P, Bhagwat M, Zeng J & Vancurova I Metformin as an anticancer agent. Trends Pharmacol. Sci 39, 867–878 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Petrera M et al. The ASAMET trial: a randomized, phase II, double-blind, placebo-controlled, multicenter, 2×2 factorial biomarker study of tertiary prevention with low-dose aspirin and metformin in stage I-III colorectal cancer patients. BMC Cancer 18, 1210 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Arrieta O et al. Effect of metformin plus tyrosine kinase inhibitors compared with tyrosine kinase inhibitors alone in patients with epidermal growth factor receptor-mutated lung adenocarcinoma: a phase 2 randomized clinical trial. JAMA Oncol. 5, e192553 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ridker PM et al. Effect of interleukin-1 beta inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet 390, 1833–1842 (2017). [DOI] [PubMed] [Google Scholar]
- 99.Hong DS et al. MABp1, a first-in-class true human antibody targeting interleukin-1 alpha in refractory cancers: an open-label, phase 1 dose-escalation and expansion study. Lancet Oncol. 15, 656–666 (2014). [DOI] [PubMed] [Google Scholar]
- 100.Hickish T et al. MABp1 as a novel antibody treatment for advanced colorectal cancer: a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 18, 192–201 (2017). [DOI] [PubMed] [Google Scholar]
- 101.Madhusudan S et al. Study of etanercept, a tumor necrosis factor-alpha inhibitor, in recurrent ovarian cancer. J. Clin. Oncol 23, 5950–5959 (2005). [DOI] [PubMed] [Google Scholar]
- 102.Monk JP et al. Assessment of tumor necrosis factor alpha blockade as an intervention to improve tolerability of dose-intensive chemotherapy in cancer patients. J. Clin. Oncol 24, 1852–1859 (2006). [DOI] [PubMed] [Google Scholar]
- 103.Harrison ML et al. Tumor necrosis factor alpha as a new target for renal cell carcinoma: two sequential phase II trials of infliximab at standard and high dose. J. Clin. Oncol 25, 4542–4549 (2007). [DOI] [PubMed] [Google Scholar]
- 104.Larkin JM et al. A phase I/II trial of sorafenib and infliximab in advanced renal cell carcinoma. Br. J. Cancer 103, 1149–1153 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Brown ER et al. A clinical study assessing the tolerability and biological effects of infliximab, a TNF-alpha inhibitor, in patients with advanced cancer. Ann. Oncol 19, 1340–1346 (2008). [DOI] [PubMed] [Google Scholar]
- 106.Postow MA, Sidlow R & Hellmann MD Immune-related adverse events associated with immune checkpoint blockade. N. Engl. J. Med 378, 158–168 (2018). [DOI] [PubMed] [Google Scholar]
- 107.Brahmer JR et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: american society of clinical oncology clinical practice guideline. J. Clin. Oncol 36, 1714–1768 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Perez-Ruiz E et al. Prophylactic TNF blockade uncouples efficacy and toxicity in dual CTLA-4 and PD-1 immunotherapy. Nature 569, 428–432 (2019). [DOI] [PubMed] [Google Scholar]
- 109.Jones SA & Jenkins BJ Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer. Nat. Rev. Immunol 18, 773–789 (2018). [DOI] [PubMed] [Google Scholar]
- 110.Johnson DE, O’Keefe RA & Grandis JR Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol 15, 234–248 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kang S, Tanaka T, Narazaki M & Kishimoto T Targeting interleukin-6 signaling in clinic. Immunity 50, 1007–1023 (2019). [DOI] [PubMed] [Google Scholar]
- 112.Shah NN & Fry TJ Mechanisms of resistance to CAR T cell therapy. Nat. Rev. Clin. Oncol 16, 372–385 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rodon J et al. First-in-human dose study of the novel transforming growth factor-beta receptor I kinase inhibitor LY2157299 monohydrate in patients with advanced cancer and glioma. Clin. Cancer Res 21, 553–560 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Melisi D et al. Galunisertib plus gemcitabine vs. gemcitabine for first-line treatment of patients with unresectable pancreatic cancer. Br. J. Cancer 119, 1208–1214 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kelley RK et al. A phase 2 study of galunisertib (TGF-beta 1 receptor type i inhibitor) and sorafenib in patients with advanced hepatocellular carcinoma. Clin. Transl. Gastroenterol 10, e00056 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Batlle E & Massaguē J Transforming growth factor-beta signaling in immunity and cancer. Immunity 50, 924–940 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Papadopoulos KP et al. First-in-human study of AMG 820, a monoclonal anti-colony-stimulating factor 1 receptor antibody, in patients with advanced solid tumors. Clin. Cancer Res 23, 5703–5710 (2017). [DOI] [PubMed] [Google Scholar]
- 118.Gomez-Roca CA et al. Phase I study of emactuzumab single agent or in combination with paclitaxel in patients with advanced/metastatic solid tumors reveals depletion of immunosuppressive M2-like macrophages. Ann. Onco 130, 1381–1392 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nywening TM et al. Targeting tumour-associated macrophages with CCR2 inhibition in combination with FOLFIRINOX in patients with borderline resectable and locally advanced pancreatic cancer: a single-centre, open-label, dose-finding, non-randomised, phase 1b trial. Lancet Oncol. 17, 651–662 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Halama N et al. Tumoral immune cell exploitation in colorectal cancer metastases can be targeted effectively by Anti-CCR5 therapy in cancer patients. Cancer Cell 29, 587–601 (2016). [DOI] [PubMed] [Google Scholar]
- 121.DeNardo DG & Ruffell B Macrophages as regulators of tumour immunity and immunotherapy. Nat. Rev. Immunol 19, 369–382 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bockorny B et al. BL-8040, a CXCR4 antagonist, in combination with pembrolizumab and chemotherapy for pancreatic cancer: the COMBAT trial. Nat. Med 26, 878–885 (2020). [DOI] [PubMed] [Google Scholar]
- 123.Ngo B, Van Riper JM, Cantley LC & Yun J Targeting cancer vulnerabilities with high-dose vitamin C. Nat. Rev. Cancer 19, 271–282 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zitvogel L, Pietrocola F & Kroemer G Nutrition, inflammation and cancer. Nat. Immunol 18, 843–850 (2017). [DOI] [PubMed] [Google Scholar]
- 125.Feldman D, Krishnan AV, Swami S, Giovannucci E & Feldman BJ The role of vitamin D in reducing cancer risk and progression. Nat. Rev. Cancer 14, 342–357 (2014). [DOI] [PubMed] [Google Scholar]
- 126.Shui IM et al. Vitamin D-related genetic variation, plasma vitamin D, and risk of lethal prostate cancer:a prospective nested case-control study. J. Natl Cancer Inst 104, 690–699 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ma Y et al. Association between vitamin D and risk of colorectal cancer: a systematic review of prospective studies. J. Clin. Oncol 29, 3775–3782 (2011). [DOI] [PubMed] [Google Scholar]
- 128.Yuan C et al. Plasma 25-hydroxyvitamin D levels and survival in patients with advanced or metastatic colorectal cancer: findings from CALGB/SWOG 80405 (Alliance). Clin. Cancer Res 25, 7497–7505 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Borchmann S et al. Pretreatment vitamin D deficiency is associated with impaired progression-free and overall survival in Hodgkin lymphoma. J. Clin. Oncol 37, 3528–3537 (2019). [DOI] [PubMed] [Google Scholar]
- 130.Lappe J et al. Effect of vitamin D and calcium supplementation on cancer incidence in older women: a randomized clinical trial. JAMA 317, 1234–1243 (2017). [DOI] [PubMed] [Google Scholar]
- 131.Manson JE et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N. Engl. J. Med 380, 33–44 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang Y et al. Association between vitamin D supplementation and mortality: systematic review and meta-analysis. BMJ 366, 14673 (2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ng K et al. Effect of high-dose vs standard-dose vitamin D3 supplementation on progression-free survival among patients with advanced or metastatic colorectal cancer: the SUNSHINE randomized clinical trial. JAMA 321, 1370–1379 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Urashima M et al. Effect of vitamin D supplementation on relapse-free survival among patients with digestive tract cancers: the AMATERASU randomized clinical trial. JAMA 321, 1361–1369 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Grover S et al. Vitamin D intake is associated with decreased risk of immune checkpoint inhibitor-induced colitis. Cancer 126, 3758–3767 (2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Manson JE et al. Marine n-3 fatty acids and prevention of cardiovascular disease and cancer. N. Engl. J. Med 380, 23–32 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Song M et al. Effect of supplementation with marine omega-3 fatty acid on risk of colorectal adenomas and serrated polyps in the US general population: a prespecified ancillary study of a randomized clinical trial. JAMA Oncol. 6, 108–115 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hull MA et al. Eicosapentaenoic acid and aspirin, alone and in combination, for the prevention of colorectal adenomas (seAFOod Polyp Prevention trial): a multicentre, randomised, double-blind, placebo-controlled, 2×2 factorial trial. Lancet 392, 2583–2594 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ebbing M et al. Cancer incidence and mortality after treatment with folic acid and vitamin B12. JAMA 302, 2119–2126 (2009). [DOI] [PubMed] [Google Scholar]
- 140.Martinez ME, Jacobs ET, Baron JA, Marshall JR & Byers T Dietary supplements and cancer prevention: balancing potential benefits against proven harms. J. Natl Cancer Inst 104, 732–739 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Vollset SE et al. Effects of folic acid supplementation on overall and site-specific cancer incidence during the randomised trials: meta-analyses of data on 50,000 individuals. Lancet 381, 1029–1036 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fortmann SP, Burda BU, Senger CA, Lin JS & Whitlock EP Vitamin and mineral supplements in the primary prevention of cardiovascular disease and cancer: an updated systematic evidence review for the U.S. Preventive Services Task Force. Ann. Intern. Med 159, 824–834 (2013). [DOI] [PubMed] [Google Scholar]
- 143.Ambrosone CB et al. Dietary supplement use during chemotherapy and survival outcomes of patients with breast cancer enrolled in a cooperative group clinical trial (SWOG S0221). J. Clin. Oncol 38, 804–814 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chen YX et al. Berberine versus placebo for the prevention of recurrence of colorectal adenoma: a multicentre, double-blinded, randomised controlled study. Lancet Gastroenterol. Hepatol 5, 267–275 (2020). [DOI] [PubMed] [Google Scholar]
- 145.Kern MA et al. Proapoptotic and antiproliferative potential of selective cyclooxygenase-2 inhibitors in human liver tumor cells. Hepatology 36, 885–894 (2002). [DOI] [PubMed] [Google Scholar]
- 146.Xia D, Wang D, Kim SH, Katoh H & DuBois RN Prostaglandin E2 promotes intestinal tumor growth via DNA methylation. Nat. Med 18, 224–226 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pribluda A et al. A senescence-inflammatory switch from cancer-inhibitory to cancer-promoting mechanism. Cancer Cell 24, 242–256 (2013). [DOI] [PubMed] [Google Scholar]
- 148.Kim HB et al. Prostaglandin E2 Activates YAP and a positive-signaling loop to promote colon regeneration after colitis but also carcinogenesis in mice. Gastroenterology 152, 616–630 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sutter AP et al. Cell cycle arrest and apoptosis induction in hepatocellular carcinoma cells by HMG-CoA reductase inhibitors. Synergistic antiproliferative action with ligands of the peripheral benzodiazepine receptor. J. Hepatol 43, 808–816 (2005). [DOI] [PubMed] [Google Scholar]
- 150.Yang PM et al. Inhibition of autophagy enhances anticancer effects of atorvastatin in digestive malignancies. Cancer Res. 70, 7699–7709 (2010). [DOI] [PubMed] [Google Scholar]
- 151.Cao Z et al. MYC phosphorylation, activation, and tumorigenic potential in hepatocellular carcinoma are regulated by HMG-CoA reductase. Cancer Res. 71, 2286–2297 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Miao ZF et al. A metformin-responsive metabolic pathway controls distinct steps in gastric progenitor fate decisions and maturation. Cell Stem Cell 26, 910–925.e6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ben Sahra I et al. The antidiabetic drug metformin exerts an antitumoral effect in vitro and in vivo through a decrease of cyclin D1 level. Oncogene 27, 3576–3586 (2008). [DOI] [PubMed] [Google Scholar]
- 154.Hirsch HA, Iliopoulos D & Struhl K Metformin inhibits the inflammatory response associated with cellular transformation and cancer stem cell growth. Proc. Natl Acad. Sci. USA 110, 972–977 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wu D et al. Glucose-regulated phosphorylation of TET2 by AMPK reveals a pathway linking diabetes to cancer. Nature 559, 637–641 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yuan H et al. SETD2 restricts prostate cancer metastasis by integrating EZH2 and AMPK signaling pathways. Cancer Cell 38, 350–365.e7 (2020). [DOI] [PubMed] [Google Scholar]
- 157.Elgendy M et al. Combination of hypoglycemia and metformin impairs tumor metabolic plasticity and growth by modulating the PP2A-GSK3beta-MCL-1 axis. Cancer Cell 35, 798–815.e5 (2019). [DOI] [PubMed] [Google Scholar]
- 158.Sakurai T et al. Hepatocyte necrosis induced by oxidative stress and IL-1 alpha release mediate carcinogen-induced compensatory proliferation and liver tumorigenesis. Cancer Cell 14, 156–165 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Naugler WE & Karin M The wolf in sheep’s clothing: the role of interleukin-6 in immunity, inflammation and cancer. Trends Mol. Med 14, 109–119 (2008). [DOI] [PubMed] [Google Scholar]
- 160.Tan W et al. Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL-RANK signalling. Nature 470, 548–553 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kuan EL & Ziegler SF A tumor-myeloid cell axis, mediated via the cytokines IL-1alpha and TSLP, promotes the progression of breast cancer. Nat. Immunol 19, 366–374 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Rubin JB et al. A small-molecule antagonist of CXCR4 inhibits intracranial growth of primary brain tumors. Proc. Natl Acad. Sci. USA 100, 13513–13518 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Klein S et al. CXCR4 promotes neuroblastoma growth and therapeutic resistance through miR-15a/16-1-mediated ERK and BCL2/cyclin D1 pathways. Cancer Res. 78, 1471–1483 (2018). [DOI] [PubMed] [Google Scholar]
- 164.Tong M et al. Efficacy of annexin A3 blockade in sensitizing hepatocellular carcinoma to sorafenib and regorafenib. J. Hepatol 69, 826–839 (2018). [DOI] [PubMed] [Google Scholar]
- 165.Cooper J & Giancotti FG Integrin signaling in cancer: mechanotransduction, stemness, epithelial plasticity, and therapeutic resistance. Cancer Cell 35, 347–367 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhang Z et al. Tumor microenvironment-derived NRG1 promotes antiandrogen resistance in prostate cancer. Cancer Cell 38, 279–296.e9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Maeda S, Kamata H, Luo JL, Leffert H & Karin M IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell 121, 977–990 (2005). [DOI] [PubMed] [Google Scholar]
- 168.Yuan D et al. Kupffer cell-derived Tnf triggers cholangiocellular tumorigenesis through JNK due to chronic mitochondrial dysfunction and ROS. Cancer Cell 31, 771–789.e6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ma C et al. NAFLD causes selective CD4+ T lymphocyte loss and promotes hepatocarcinogenesis. Nature 531, 253–257 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Gomes AL et al. Metabolic inflammation-associated IL-17A causes non-alcoholic steatohepatitis and hepatocellular carcinoma. Cancer Cell 30, 161–175 (2016). [DOI] [PubMed] [Google Scholar]
- 171.Ma HY et al. IL-17 signaling in steatotic hepatocytes and macrophages promotes hepatocellular carcinoma in alcohol-related liver disease. J. Hepatol 72, 946–959 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bruchard M et al. Chemotherapy-triggered cathepsin B release in myeloid-derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth. Nat. Med 19, 57–64 (2013). [DOI] [PubMed] [Google Scholar]
- 173.Boire A et al. Complement component 3 adapts the cerebrospinal fluid for leptomeningeal metastasis. Cell 168, 1101–1113.e13 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Jacobetz MA et al. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut 62, 112–120 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ramanathan RK et al. Phase IB/II randomized study of FOLFIRINOX plus pegylated recombinant human hyaluronidase versus FOLFIRINOX alone in patients with metastatic pancreatic adenocarcinoma: SWOG S1313. J. Clin. Oncol 37, 1062–1069 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mantovani A, Marchesi F, Malesci A, Laghi L & Allavena P Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol 14, 399–416 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Pyonteck SM et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat. Med 19, 1264–1272 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wu SY et al. Nicotine promotes brain metastasis by polarizing microglia and suppressing innate immune function. J. Exp. Med 217, e20191131 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Xu J et al. CSF1R signaling blockade stanches tumor-infiltrating myeloid cells and improves the efficacy of radiotherapy in prostate cancer. Cancer Res. 73, 2782–2794 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Escamilla J et al. CSF1 receptor targeting in prostate cancer reverses macrophage-mediated resistance to androgen blockade therapy. Cancer Res. 75, 950–962 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Salvagno C et al. Therapeutic targeting of macrophages enhances chemotherapy efficacy by unleashing type I interferon response. Nat. Cell Biol 21, 511–521 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Bonavita E et al. PTX3 is an extrinsic oncosuppressor regulating complement-dependent inflammation in cancer. Cell 160, 700–714 (2015). [DOI] [PubMed] [Google Scholar]
- 183.Singh N et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 40, 128–139 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Tuting T & de Visser KE Cancer. How neutrophils promote metastasis. Science 352, 145–146 (2016). [DOI] [PubMed] [Google Scholar]
- 185.Jaillon S et al. Neutrophil diversity and plasticity in tumour progression and therapy. Nat. Rev. Cancer 20, 485–503 (2020). [DOI] [PubMed] [Google Scholar]
- 186.Bald T et al. Ultraviolet-radiation-induced inflammation promotes angiotropism and metastasis in melanoma. Nature 507, 109–113 (2014). [DOI] [PubMed] [Google Scholar]
- 187.Coffelt SB et al. IL-17-producing gammadelta T cells and neutrophils conspire to promote breast cancer metastasis. Nature 522, 345–348 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wculek SK & Malanchi I Neutrophils support lung colonization of metastasis-initiating breast cancer cells. Nature 528, 413–417 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Albrengues J et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science 361, eaao4227 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Yang L et al. DNA of neutrophil extracellular traps promotes cancer metastasis via CCDC25. Nature 583, 133–138 (2020). [DOI] [PubMed] [Google Scholar]
- 191.Chen X & Song E Turning foes to friends: targeting cancer-associated fibroblasts. Nat. Rev. Drug Discov 18, 99–115 (2019). [DOI] [PubMed] [Google Scholar]
- 192.Yoshimoto S et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature 499, 97–101 (2013). [DOI] [PubMed] [Google Scholar]
- 193.Stone RL et al. Paraneoplastic thrombocytosis in ovarian cancer. N. Engl. J. Med 366, 610–618 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sitia G et al. Antiplatelet therapy prevents hepatocellular carcinoma and improves survival in a mouse model of chronic hepatitis B. Proc. Natl Acad. Sci. USA 109, E2165–E2172 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Malehmir M et al. Platelet GPIbα is a mediator and potential interventional target for NASH and subsequent liver cancer. Nat. Med 25, 641–655 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Bottcher JP et al. NK cells stimulate recruitment of cdc1 into the tumor microenvironment promoting cancer immune control. Cell 172, 1022–1037.e14 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Loo TM et al. Gut microbiota promotes obesity-associated liver cancer through PGE2-mediated suppression of antitumor immunity. Cancer Discov. 7, 522–538 (2017). [DOI] [PubMed] [Google Scholar]
- 198.Larsson K et al. COX/mPGES-1/PGE2 pathway depicts an inflammatory-dependent high-risk neuroblastoma subset. Proc. Natl Acad. Sci. USA 112, 8070–8075 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Li L et al. Metformin-induced reduction of CD39 and CD73 blocks myeloid-derived suppressor cell activity in patients with ovarian cancer. Cancer Res. 78, 1779–1791 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Cha JH et al. Metformin promotes antitumor immunity via endoplasmic-reticulum-associated degradation of PD-L1. Mol. Cell 71, 606–620.e7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Wang S et al. Low-dose metformin reprograms the tumor immune microenvironment in human esophageal cancer: results of a phase II clinical trial. Clin Cancer Res. 26, 4921–4932 (2020). [DOI] [PubMed] [Google Scholar]
- 202.Yang W et al. Potentiating the antitumour response of CD8+ T cells by modulating cholesterol metabolism. Nature 531, 651–655 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ma X et al. Cholesterol induces CD8+ T cell exhaustion in the tumor microenvironment. Cell Metab. 30, 143–156.e5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Rachidi S et al. Platelets subvert T cell immunity against cancer via GARP-TGFβ axis. Sci. Immunol 2, eaai791 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ries CH et al. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell 25, 846–859 (2014). [DOI] [PubMed] [Google Scholar]
- 206.Zhu Y et al. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res. 74, 5057–5069 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Marigo I et al. T cell cancer therapy requires CD40-CD40L activation of tumor necrosis factor and inducible nitric-oxide-synthase-producing dendritic cells. Cancer Cell 30, 377–390 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Zhu Y et al. Disruption of tumour-associated macrophage trafficking by the osteopontin-induced colony-stimulating factor-1 signalling sensitises hepatocellular carcinoma to anti-PD-L1 blockade. Gut 68, 1653–1666 (2019). [DOI] [PubMed] [Google Scholar]
- 209.Su S et al. Immune checkpoint inhibition overcomes ADCP-induced immunosuppression by macrophages. Cell 175, 442–457.e23 (2018). [DOI] [PubMed] [Google Scholar]
- 210.Kaplanov I et al. Blocking IL-1 beta reverses the immunosuppression in mouse breast cancer and synergizes with anti-PD-1 for tumor abrogation. Proc. Natl Acad. Sci. USA 116, 1361–1369 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Lu X et al. Effective combinatorial immunotherapy for castration-resistant prostate cancer. Nature 543, 728–732 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Liao W et al. KRAS-IRF2 axis drives immune suppression and immune therapy resistance in colorectal cancer. Cancer Cell 35, 559–572.e7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Highfill SL et al. Disruption of CXCR2-mediated MDSC tumor trafficking enhances anti-PD1 efficacy. Sci. Transl. Med 6, 237ra267 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Greene S et al. Inhibition of MDSC Trafficking with SX-682, a CXCR1/2 inhibitor, enhances NK-cell immunotherapy in head and neck cancer models. Clin. Cancer Res 26, 1420–1431 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Tu S et al. Overexpression of interleukin-1beta induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer Cell 14, 408–419 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Wu P et al. γδT17 cells promote the accumulation and expansion of myeloid-derived suppressor cells in human colorectal cancer. Immunity 40, 785–800 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Kortlever RM et al. Myc cooperates with ras by programming inflammation and immune suppression. Cell 171, 1301–1315.e14 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Markiewski MM et al. Modulation of the antitumor immune response by complement. Nat. Immunol 9, 1225–1235 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Medler TR et al. Complement C5a fosters squamous carcinogenesis and limits T cell response to chemotherapy. Cancer Cell 34, 561–578.e6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Ajona D et al. A combined PD-1/C5a blockade synergistically protects against lung cancer growth and metastasis. Cancer Discov. 7, 694–703 (2017). [DOI] [PubMed] [Google Scholar]
- 221.Reis ES, Mastellos DC, Ricklin D, Mantovani A & Lambris JD Complement in cancer: untangling an intricate relationship. Nat. Rev. Immunol 18, 5–18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Feng M et al. Phagocytosis checkpoints as new targets for cancer immunotherapy. Nat. Rev. Cancer 19, 568–586 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Shalapour S et al. Immunosuppressive plasma cells impede T-cell-dependent immunogenic chemotherapy. Nature 521, 94–98 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Shalapour S et al. Inflammation-induced IgA+ cells dismantle anti-liver cancer immunity. Nature 551, 340–345 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Daley D et al. γδ T cells support pancreatic oncogenesis by restraining alphabets T cell activation. Cell 166, 1485–1499.e15 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Gao Y et al. Tumor immunoevasion by the conversion of effector NK cells into type 1 innate lymphoid cells. Nat. Immunol 18, 1004–1015 (2017). [DOI] [PubMed] [Google Scholar]
- 227.Yan J et al. MAIT cells promote tumor initiation, growth, and metastases via tumor MR1. Cancer Discov. 10, 124–141 (2020). [DOI] [PubMed] [Google Scholar]
- 228.Chen Y et al. CXCR4 inhibition in tumor microenvironment facilitates anti-programmed death receptor-1 immunotherapy in sorafenib-treated hepatocellular carcinoma in mice. Hepatology 61, 1591–1602 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Jiang H et al. Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat. Med 22, 851–860 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Xiao Q et al. DKK2 imparts tumor immunity evasion through beta-catenin-independent suppression of cytotoxic immune-cell activation. Nat. Med 24, 262–270 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Giavridis T et al. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat. Med 24, 731–738 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Martin MJ, Hayward R, Viros A & Marais R Metformin accelerates the growth of BRAF V600E-driven melanoma by upregulating VEGF-A. Cancer Discov. 2, 344–355 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Umemura A et al. Liver damage, inflammation, and enhanced tumorigenesis after persistent mTORC1 inhibition. Cell Metab. 20, 133–144 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Coll AP et al. GDF15 mediates the effects of metformin on body weight and energy balance. Nature 578, 444–448 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Suriben R et al. Antibody-mediated inhibition of GDF15-GFRAL activity reverses cancer cachexia in mice. Nat. Med 26, 1264–1270 (2020). [DOI] [PubMed] [Google Scholar]
- 236.Gabitova-Cornell L et al. Cholesterol pathway inhibition induces TGF-beta signaling to promote basal differentiation in pancreatic cancer. Cancer Cell 38, 567–583.e11 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Obradovic MMS et al. Glucocorticoids promote breast cancer metastasis. Nature 567, 540–544 (2019). [DOI] [PubMed] [Google Scholar]
- 238.Wagenblast E et al. A model of breast cancer heterogeneity reveals vascular mimicry as a driver of metastasis. Nature 520, 358–362 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Ghiringhelli F et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat. Med 15, 1170–1178 (2009). [DOI] [PubMed] [Google Scholar]
- 240.Vegran F et al. The transcription factor IRF1 dictates the IL-21-dependent anticancer functions of TH9 cells. Nat. Immunol 15, 758–766 (2014). [DOI] [PubMed] [Google Scholar]
- 241.Dmitrieva-Posocco O et al. Cell-type-specific responses to interleukin-1 control microbial invasion and tumor-elicited inflammation in colorectal cancer. Immunity 50, 166–180.e7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Castano Z et al. IL-1beta inflammatory response driven by primary breast cancer prevents metastasis-initiating cell colonization. Nat. Cell Biol 20, 1084–1097 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Kumar V et al. Cancer-associated fibroblasts neutralize the anti-tumor effect of CSF1 receptor blockade by inducing PMN-MDSC infiltration of tumors. Cancer Cell 32, 654–668.e5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Bonapace L et al. Cessation of CCL2 inhibition accelerates breast cancer metastasis by promoting angiogenesis. Nature 515, 130–133 (2014). [DOI] [PubMed] [Google Scholar]
- 245.Singhal S et al. Origin and role of a subset of tumor-associated neutrophils with antigen-presenting cell features in early-stage human lung cancer. Cancer Cell 30, 120–135 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Ponzetta A et al. Neutrophils driving unconventional T cells mediate resistance against murine sarcomas and selected human tumors. Cell 178, 346–360.e24 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Roumenina LT, Daugan MV, Petitprez F, Sautes-Fridman C & Fridman WH Context-dependent roles of complement in cancer. Nat. Rev. Cancer 19, 698–715 (2019). [DOI] [PubMed] [Google Scholar]
- 248.Ozdemir BC et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 25, 719–734 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Sayin VI et al. Antioxidants accelerate lung cancer progression in mice. Sci. Transl. Med 6, 221ra215 (2014). [DOI] [PubMed] [Google Scholar]
- 250.Le Gal K et al. Antioxidants can increase melanoma metastasis in mice. Sci. Transl. Med 7, 308re8 (2015). [DOI] [PubMed] [Google Scholar]
- 251.Wiel C et al. BACH1 stabilization by antioxidants stimulates lung cancer metastasis. Cell 178, 330–345.e22 (2019). [DOI] [PubMed] [Google Scholar]
- 252.Piskounova E et al. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature 527, 186–191 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Lopez-Otin C, Blasco MA, Partridge L, Serrano M & Kroemer C The hallmarks of aging. Cell 153, 1194–1217 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Biragyn A & Ferrucci L Gut dysbiosis: a potential link between increased cancer risk in ageing and inflammaging. Lancet Oncol. 19, e295–e304 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Finlay BB et al. Can we harness the microbiota to enhance the efficacy of cancer immunotherapy? Nat. Rev. Immunol 20, 522–528 (2020). [DOI] [PubMed] [Google Scholar]
- 256.Wargo JA Modulating gut microbes. Science 369, 1302–1303 (2020). [DOI] [PubMed] [Google Scholar]
- 257.Chen DS & Mellman I Elements of cancer immunity and the cancer-immune set point. Nature 541, 321–330 (2017). [DOI] [PubMed] [Google Scholar]
- 258.Elinav E, Garrett WS, Trinchieri G & Wargo J The cancer microbiome. Nat. Rev. Cancer 19, 371–376 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Gong T, Liu L, Jiang W & Zhou R DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat. Rev. Immunol 20, 95–112 (2020). [DOI] [PubMed] [Google Scholar]
- 260.McLaughlin M et al. Inflammatory microenvironment remodelling by tumour cells after radiotherapy. Nat. Rev. Cancer 20, 203–217 (2020). [DOI] [PubMed] [Google Scholar]
- 261.Chen DS & Mellman I Oncology meets immunology: the cancer-immunity cycle. Immunity 39, 1–10 (2013). [DOI] [PubMed] [Google Scholar]
- 262.Palucka AK & Coussens LM The basis of oncoimmunology. Cell 164, 1233–1247 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.