Abstract
The pharmacokinetic profiles of a traditional formulation of amphotericin B (Fungizone) and novel nanosphere and mixed micelle delivery systems developed for amphotericin B were compared and described. Six groups of male Wistar rats received intravenous injections of the different formulations. Plasma and tissue samples were obtained at 11 different times after dosing, with three animals used each time. The amphotericin B concentrations in plasma and tissues were analyzed by high-performance liquid chromatography. The plasma drug concentration-time profiles were best described by a two-compartment model. Models that described the observed single or double peak disposition kinetics in kidney, liver, and spleen were also developed. Parameter estimates from those models show that components of the formulation such as poloxamer 188, which is present in all new formulations, seem to play an important role in the rate of drug uptake by the tissues; in general, the levels of amphotericin B in tissues were increased after the administration of the new formulations compared with those after the administration of Fungizone. The increment in the baseline plasma creatinine level was used as an index of renal function. All formulations increased this baseline value, but the novel formulations exhibited fewer renal effects than Fungizone did. However, a direct relationship between drug exposure in the kidneys and development of renal damage could not be found.
Over the last 15 years there has been a dramatic increase in fungal infections. This increased incidence can be attributed to the lack of immunocompetence caused by chemotherapy, as well as the spread of AIDS. Amphotericin B (AmB) is the most effective and widely used antifungal agent for the treatment of systemic fungal diseases. However, therapy with AmB is hampered by a number of serious adverse effects such as fever, chills, nausea, hypokalemia, and nephrotoxicity (23). In general, all these side effects have been associated with undesirable increased and prolonged drug concentrations in tissues such as the kidneys (16); therefore, a substantial effort has been made toward the development of alternative formulations for AmB administration, with the aim of improving the therapeutic index of the drug by modifying the disposition in the tissues of the body.
Several lipid-based formulations of AmB such as Ambisome (liposomes), Amphocil (colloidal dispersion), and Abelcet (lipid complex) have appeared on the market over recent years (7, 15). The formulations have increased therapeutic indices with respect to that of Fungizone (the traditional formulation for AmB administration), but their higher economic costs are a major limitation in therapeutics (9). Another alternative could be the use of biodegradable polymeric carriers such as nanoparticles; these systems are thought to be more stable in biological fluids and during storage. Recently, different formulations, (i) nanospheres formed by a polymeric core of poly(ɛ-caprolactone) coated with poloxamer 188 and AmB and (ii) AmB-poloxamer 188 mixed micelles, have been developed in our laboratory by Espuelas et al. (11). The doses that produce 50% mortality in mice (LD50s) for these two new AmB formulations were higher than that of Fungizone. The LD50 was calculated as the number of deaths that occurred when different doses of the formulations were administered to mice by intravenous injection (11).
To date, most of the new formulations are usually characterized “in vivo” by means of the doses that elicit half of the maximum efficacy (ED50s) (19) and LD50s (1). Estimates of ED50 and LD50 include information on both the pharmacodynamic and the pharmacokinetic properties of a drug, which makes the interpretation of results a difficult task. In addition, when pharmacokinetics is the aim of the study, in general, values like the maximum concentration (Cmax), time at Cmax (Tmax), area under the concentration-versus-time curve (AUC) are reported. Although these values can be compared among formulations to address differences in the formulations, they cannot be used to describe and predict the time course of drug concentrations in plasma and tissues, which in fact can determine the therapeutic and toxic effects. Therefore, the aim of the current study was to characterize, by using pharmacokinetic models, the dispositions of new AmB formulations (11) in the plasma, livers, spleens, and kidneys of rats. We also include in the current study Fungizone as a reference formulation. In addition, the effects of those AmB formulations on renal function, measured as increasing levels of creatinine in plasma, were also explored and quantified.
MATERIALS AND METHODS
Chemicals.
AmB was kindly given by Bristol-Myers Squibb (Madrid, Spain), and piroxicam (internal standard) was supplied by Boral Química (Barcelona, Spain); desoxycholate-AmB (Fungizone) was purchased from Bristol-Myers Squibb (Madrid, Spain). Poly(ɛ-caprolactone; molecular weight, 42,500) and poloxamer 188 (Pluronic F68) were supplied by Sigma (Madrid, Spain). A creatinine standard (3 mg/100 ml) was obtained from Sigma. All other chemicals were of analytical grade and were purchased from standard commercial sources.
Preparation and characterization of AmB formulations. (i) Preparation of AmB formulations for intravenous administration. (a) Fungizone.
Fungizone (deoxycholate-AmB) was reconstituted with sterile water and was further diluted with a 5% glucose–water solution.
(b) AmB-Nsps.
AmB-nanospheres (AmB-Nsps) with 125 mg of poloxamer 188 (AmB-Nsp [125]) were prepared by the solvent displacement method described previously by Espuelas et al. (11). Briefly, in order to solubilize AmB (30 mg) and poly(ɛ-caprolactona) (125 mg), both were dissolved in 30 ml of a mixture of organic solvents (methanol-acetone, 1:2 [vol/vol]) acidified with 0.1 N HCl to pH 3. This organic phase was heated at 50°C for 10 min and was thereafter poured into 40 ml of distilled water by using poloxamer 188 (125 mg) as the surfactant with moderate stirring with a magnetic stirrer. Finally, the preparation was evaporated at 55 to 58°C under vacuum, and the final volume was adjusted to 10 ml. The formulations were neutralized with NaOH (0.1 N) to pH 5.5 before administration.
AmB-Nsps with 12.5 mg of poloxamer 188 (AmB-Nsps [12.5]) were prepared as described for AmB-Nsp [125], but with 12.5 mg of poloxamer 188.
(c) AmB-MMs.
AmB-mixed micelles (AmB-MMs) with 125 mg of poloxamer 188 (AmB-MM [125]) were also prepared in the same way as AmB-Nsp [125] but without poly(ɛ-caprolactona).
AmB-MMs with 12.5 mg of poloxamer 188 (AmB-MM [12.5]) were prepared as described for AmB-MM [125], but with 12.5 mg of poloxamer 188.
Fungizone was reconstituted just before drug injection as explained above; AmB-Nsps and AmB-MMs were prepared on the day before the administration, and the doses were adjusted just before injection.
(ii) Physicochemical characterization of the formulations.
The physicochemical characterization of the different novel formulations was performed on the basis of two criteria, as published recently by Espuelas and coworkers (12, 13): (i) particle size and (ii) UV-visible spectroscopy. Since AmB-MMs are colloidal dispersions, for this particular case only UV-visible spectroscopy was used as a criterion for characterization.
(a) Particle size.
The mean particle size of the formulations was determined on a Zetamaster Instrument (Zeta sizer 4; Malvern Instruments, Malvern, United Kingdom). The measurements were performed after dilution (1:100) in distilled water. The mean ± standard deviation particle sizes of AmB-Nsp [125] and AmB-Nsp [12.5] obtained were 280 ± 30 and 350 ± 70 nm, respectively, with polydispersity indices of 0.1 for both formulations.
(b) UV-visible spectroscopy.
The UV absorption of the formulation was determined by diluting stock preparations (1 mg/ml) so that they contained a final AmB concentration of 10 μg/ml. Absorption spectra were registered with a Varian Cary 219 spectrophotometer.
In the absorption spectrum of Fungizone, a maximum of absorbance centered at approximately 350 nm was observed. Otherwise, the spectrum corresponding to AmB-Nsp [125] shows a new maximum of absorbance located at 375 nm. The maximum of absorbance for AmB-Nsp [12.5] was found to be displaced with respect to that for Fungizone. For AmB-MMs the results obtained were similar to those observed for AmB-Nsps. These results suggest that the AmB aggregation state is modified by its association with nanospheres. The drug concentrations in the dispersions were also calculated by measurement of the absorbance at 405 nm after appropriate dilution in methanol.
Animals.
With the approval of our Animal Care Committee, 231 male Wistar rats (weight, 225 to 250 g) were used throughout the study. During the experimental period the animals were housed individually in plastic cages at constant temperature (25°C) and humidity (70%), with a 12-h light and 12-h dark cycle (lights on from 8 a.m. to 8 p.m.). The rats were allowed free access to food and water, but from 12 h before drug administration to 6 h after drug administration food was withdrawn.
The animals were randomly divided into six groups (groups I to VI) of 33 rats each. The animals were slightly anesthetized with ether, and the drug was administered as a 5-min intravenous bolus injection through the vein tail. The maximum injected volume was 1 ml (range, 0.7 to 1 ml). All drug administrations were performed between 8 a.m. and 9 a.m. to minimize the potential influence of circadian rhythms in the disposition of the drug.
Group I received a single dose of 1 mg of Fungizone per kg of body weight. Groups II and III received single doses of 1 and 3 mg of AmB-MM [125] per kg, respectively, and groups IV, V, and VI received single doses of 3 mg of AmB-MM [12.5], AmB-Nsp [125], and AmB-Nsp [12.5] per kg, respectively. Just before cervical dislocation, the animals were slightly anesthetized with ether, and arterial blood (5 ml) was withdrawn from the abdominal aorta at the following times after drug injection: 5, 10, 15, and 30 min and 1, 3, 6, 10, 24, 48, and 72 h (three animals were used for each time point). At the time of killing, the kidneys, liver, and spleen were removed, weighed, and frozen at −20°C until analysis; blood samples were immediately centrifuged at 8,944 × g for 10 min to obtain the plasma. The plasma was also frozen until analysis of AmB and creatinine concentrations. Under these conditions the drug was found to be stable for at least 1 month.
HPLC assay.
The levels of AmB in plasma and tissues were quantified by high-performance liquid chromatography (HPLC). The method used for determination of the AmB concentrations in plasma and tissues was published recently by Echevarría et al. (10); briefly, an adequate amount of internal standard (piroxicam) and 200 μl of acetonitrile were added to a 100-μl sample of plasma. After vortex mixing (30 s), the sample was centrifuged at 8,944 × g for 10 min. The supernatant was evaporated to dryness in a vortex evaporator (60°C). The residue was finally dissolved in 250 μl of the chromatographic eluent and filtered, and 100 μl of this solution was injected into the HPLC system.
Liver, spleen, and kidney samples (0.5 g) were homogenized with an Ultraturrax-T25 dispersing apparatus (24,000 rpm, 3 min) with 2 ml of distilled water containing the internal standard. A total of 300 μl of the homogenate was added to 900 μl of methanol. The mixture was vortexed and centrifuged at 8,944 × g for 10 min. The supernatant was filtered, and 100 μl was injected into the chromatograph.
The chromatography system consisted of a pump (Waters 600E), an autosampler (Waters 700 Satellite WISP), an integrator (Waters 746), and a UV detector (Waters 486) set at 405 nm. Separation was achieved with an Ultrabase C18 reverse-phase column (250 by 4.6 mm [inner diameter]; 5-μm particle size; Scharlau, Barcelona, Spain), preceded by a guard column (45 by 4.6 mm [inner diameter]) filled with pellicular C18 (5-μm particle size; Perkin-Elmer, Norwalk, Conn.). The mobile phase was acetonitrile–acetic acid (10%)–water (41:43:16; vol/vol) at a flow rate of 1 ml/min. Chromatography was carried out at room temperature, and peak areas were measured for both AmB and the internal standard.
The limit of quantitation (LOQ) for the quantification of AmB in plasma was 0.0066 μg/ml, with intraday variability, interday variability, and accuracy of 3.6, 6.8, and 4.5%, respectively.
For tissue samples, the LOQs were 0.2, 0.15, and 0.65 μg/g for the liver, spleen, and kidney, respectively. The intraday variabilities, interday variabilities, and accuracies were 4.2, 6.7, and 1.96%, respectively, for the liver, 6.8, 5.2, and 5.8%, respectively, for the spleen, and 3.9, 3.8, and 4.4%, respectively, for the kidney.
Pharmacokinetic analysis.
Pharmacokinetic analysis was carried out in two steps. First, the kinetics in plasma were modeled, and during the second step, drug profiles in the different tissues were described by using the predicted plasma AmB concentration-versus-time profiles as the input function for the tissues.
(i) Step 1. Pharmacokinetics in plasma.
The concentration-versus-time profiles for plasma were described by compartmental models, assuming first-order disposition and elimination kinetics. During the simultaneous analysis of the data obtained after the administration of doses of 1 and 3 mg of AmB-MM [125] per kg, a model describing the elimination process as a saturable (concentration-dependent) process was also evaluated.
The initial volume of distribution (V) and disposition and elimination rate constants were estimated during the fit. Pharmacokinetic parameters such as total plasma clearance (CL), apparent volume of distribution at steady-state (VSS), mean residence time (MRT), AUC, disposition half-life (t1/2α), and elimination half-life (t1/2β) were calculated from the estimated parameters by standard procedures (21).
(ii) Step 2. Pharmacokinetics in tissues.
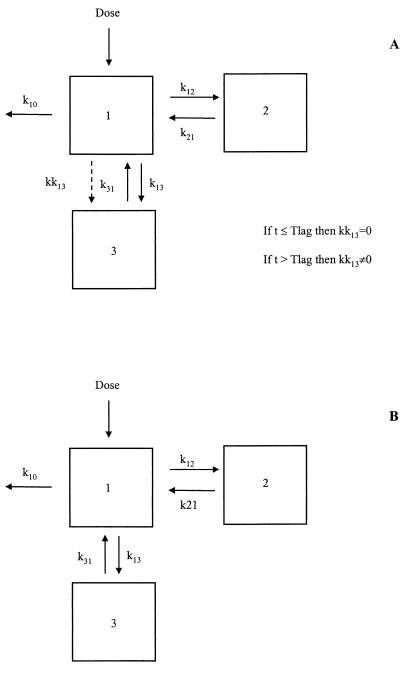
By exploration of the raw tissue AmB concentration-versus-time data for all the six groups, a double concentration peak was observed for liver and spleen. In order to describe this phenomenon properly, a model describing the distribution of AmB from plasma to the tissues by the use of two first-order distribution rate constants, as represented in Fig. 1A, was fitted to the data. One of the rate constants (kk13) acts only at times longer than the lag time (Tlag; a parameter also to be estimated by the model).
FIG. 1.
Scheme of the pharmacokinetic models proposed for the analysis of AmB concentrations in liver and spleen (A) and in kidney (B). k10, first-order elimination rate constant; k13 and k31, first-order distribution rate constants between plasma and liver, spleen, or kidney; kk31, first-order plasma to tissue (liver or spleen) distribution rate constant acting at times longer than Tlag; k12 and k21, first-order distribution rate constants between plasma and the peripheral compartment.
With respect to the kidneys, raw concentration-versus-time data revealed a unique peak of drug concentration; in this case, a simpler model was fitted to the data (Fig. 1B).
For each formulation and tissue, Cmax and Tmax were obtained by visual inspection of the data. In order to be related to renal toxicity, the area under the drug concentration in kidney-versus-time curve (AUCr) was calculated by the trapezoidal rule.
(iii) Model selection.
All the analyses were performed by the “naive pool data” approach (14). This method consists of fitting all the data together as if they had come from a single individual. In attempting to propose a model with the maximum number of parameters in common between the six groups, the data for all groups were fitted simultaneously. In the first stage, a reduced model was fitted to the data; in this model the six groups share all the parameters. During the following stage, the models fitted to the data have some but not all parameters in common for all groups, the last model fitted is the full model, which has a completely different set of parameters for each group. All the analyses were performed with the WinNonlin, version 1.5, computer program (Scientific Consulting, Inc.).
In order to select the best pharmacokinetic model, a number of criteria, including the Akaike information criterion (2), Schwartz criterion (22), and the F test (6) were used. In the last of these, the calculated value of F may be compared to the critical value derived from the distribution of F-Snédécor table in which the 5% level of significance is adopted.
In addition, the visual inspection of the residual plots and the calculated coefficients of variation of the pharmacokinetic parameter estimates were also used as a guide in selecting the best model. Coefficients of variation lower than 50% were considered acceptable.
Creatinine concentration determination.
Creatinine concentrations in plasma (CREA) were determined by the Taussky method (3), which is based on a color reaction. Plasma samples for CREA determination were obtained at the same time points and from the same animals used for AmB concentration determination. In addition, baseline CREAs were measured in a small group of animals (n = 7). A total of 4.5 ml of picric acid (3.9 M) was added to 0.5 ml of plasma, and the mixture was centrifuged at 880 × g for 5 min, and then 1 ml of NaOH (0.15 N) was added to 2.5 ml of the supernatant and the absorbances at 520 nm were measured with a UV 1203 UV-Visible spectrophotometer (Shimadzu). For each determination, 0.5 ml of a control solution and 0.5 ml of a blank solution were prepared with 0.5 ml of a 3-mg/100 ml creatinine solution and 0.5 ml of distilled water, respectively.
CREA was obtained by the following expression: CREA = (Absproblem/Abscontrol) · 3, where Absproblem and Abscontrol are the absorbances measured for each plasma sample and for the control solution, respectively.
For each formulation all CREA-versus-time datum points were pooled, and the area under the CREA-versus-time curve (AUCc) was calculated by the trapezoidal rule and was normalized by dose; maximum CREA (Ccmax) and time to Ccmax (Tcmax) were obtained by visual inspection of the data.
RESULTS
Pharmacokinetics of AmB in plasma.
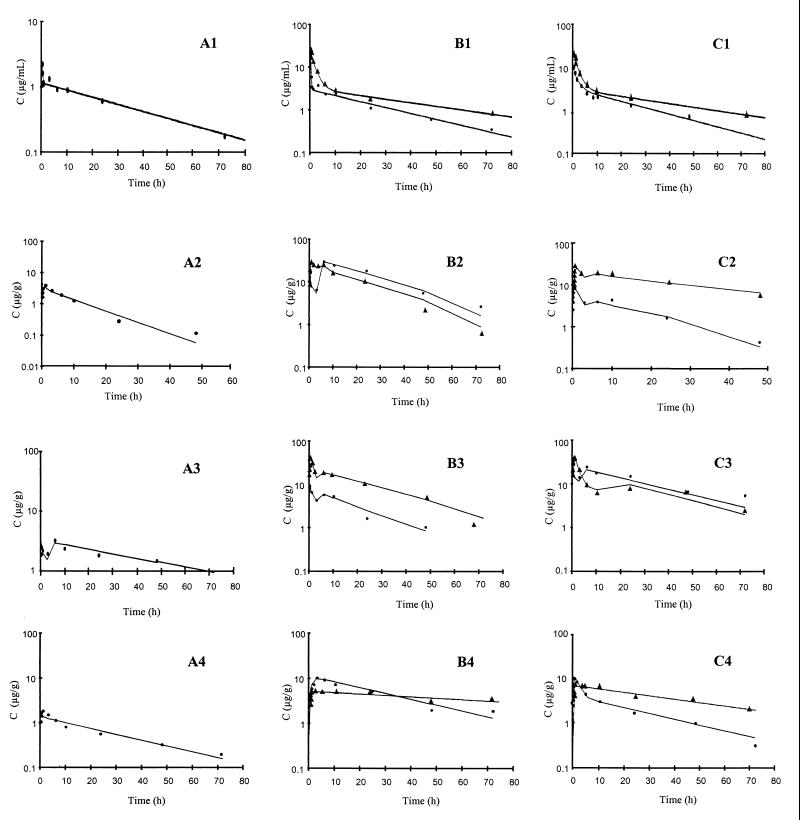
For all groups, a two-compartment model adequately describes the kinetics of AmB in plasma. Fig. 2A1, B1, and C1 show the adequacy of the model describing the disposition of AmB in plasma for all formulations tested during the study.
FIG. 2.
(A) Mean observed AmB concentrations (C) in plasma (A1), liver (A2), spleen (A3), and kidney (A4) for group I. (B) Mean observed AmB concentrations in plasma (B1), liver (B2), spleen (B3), and kidney (B4) for groups III (●) and IV (▴). (C) mean observed AmB concentrations in plasma (C1), liver (C2), spleen (C3), and kidney (C4) for groups V (●) and VI (▴). Solid lines, model predictions.
Although the same pharmacokinetic model was used for all formulations, the values of the pharmacokinetic parameters estimated differ among the formulations (P < 0.05). Table 1 lists the most representative pharmacokinetic parameters and their coefficients of variation obtained from those estimated during the fit. It can be observed that the parameters that reflect distribution processes (t1/2α, V, VSS) vary the most among the formulations. However, the calculated value of CL is similar for all formulations.
TABLE 1.
Estimates of values for pharmacokinetic parameters in plasma and their coefficients of variation after an intravenous bolus injection of AmB
| Pharmacokinetic parameter | Formulation (dose)
|
|||||
|---|---|---|---|---|---|---|
| Fungizone (1 mg/kg) | AmB-Nsp [125] (3 mg/kg) | AmB-Nsp [12.5] (3 mg/kg) | AmB-MM [125] (1 mg/kg) | AmB-MM [125] (3 mg/kg) | AmB-MM [12.5] (3 mg/kg) | |
| t1/2α (h) | 0.125 (40)a | 0.95 (21) | 0.65 (28) | 0.18 (32) | 0.10 (24) | 0.09 (26) |
| t1/2β (h) | 19.6 (5) | 23.5 (8) | 18.2 (10) | 23.1 (21) | 23.1 (12) | 21.8 (14) |
| AUC (μg · h/ml) | 37.4 (4) | 100 (3) | 141 (8) | 47.4 (14) | 102 (10) | 96.8 (8) |
| CL (ml/h/kg) | 27 (4) | 30 (3) | 21 (8) | 21 (14) | 29 (10) | 30 (8) |
| V (ml/kg) | 253.6 (24) | 253.6 (5) | 108.4 (17) | 103 (24) | 121 (63) | 121.2 (16) |
| VSS (ml/kg) | 744 (6) | 892 (6) | 472 (11) | 672 (13) | 949 (13) | 812 (13) |
| MRT (h) | 27.9 (5) | 27.2 (6) | 23.4 (11) | 31.9 (21) | 32.4 (14) | 26.9 (14) |
Values in parentheses are coefficients of variation (in percent).
The estimates of the pharmacokinetic parameters obtained during the analysis of data for doses of 1 and 3 mg of AmB-MM [125] per kg indicated the existence of differences in the distribution process (P < 0.05) after the administration of the two different doses, but the elimination of the drug was not affected by the dose (P > 0.05).
Pharmacokinetics of AmB in tissue. (i) Liver.
Figure 2A2, B2, and C2 show the raw concentration-versus-time profiles (depicted with symbols) in which the double peak can be observed. Model predictions show the adequacy of the model in describing the kinetics of AmB in liver. In addition, the pharmacokinetic parameters were estimated with high precision (coefficient of variation, <25%) (Table 2). When estimating kk13, the value of such an estimate was almost equal to the one obtained for k13 for all the groups; for that reason we used a simpler model in which kk13 was constrained to be equal to k13. As for plasma, the model used was common for all formulations, but the estimates of the parameters were not (P < 0.05); k13 (kk13) ranged from 2.3 to 23.0 h−1, and k31 ranged from 0.5 to 5.9 h−1. No differences in Tlag were found (P > 0.05).
TABLE 2.
Estimates of values for pharmacokinetic parameters and their coefficients of variation and observed Cmax and Tmax values for different tissues after an intravenous bolus injection of AmB
| Tissue and pharmacokinetic parameter | Formulation (dose)
|
|||||
|---|---|---|---|---|---|---|
| Fungizone (1 mg/kg) | AmB-Nsp [125] (3 mg/kg) | AmB-Nsp [12.5] (3 mg/kg) | AmB-MM [125] (1 mg/kg) | AmB-MM [125] (3 mg/kg) | AmB-MM [12.5] (3 mg/kg) | |
| Liver | ||||||
| k13 = kk13 (h−1) | 4.4 (15)a | 5.5 (18) | 2.45 (15) | 9.7 (9) | 23.0 (10) | 2.3 (17) |
| k31 (h−1) | 4.13 (20) | 5.9 (22) | 1.13 (22) | 2.8 (14) | 4.2 (17) | 0.5 (24) |
| Tlag (h) | 3 | 3 | 3 | 3 | 3 | 3 |
| Cmax (μg/g) | 4.23 ± 1.20b | 9.23 ± 1.39 | 30.3 ± 2.29 | 12.5 ± 2.82 | 49.3 ± 6.33 | 42.5 ± 4.34 |
| Tmax (h) | 0.083 | 0.25 | 0.5 | 0.25 | 0.167 | 0.5 |
| Spleen | ||||||
| k13 = kk13 (h−1) | 6.6 (17) | 13.7 (18) | 6.9 (9) | 28 (37) | 15.3 (17) | 4.17 (9) |
| k31 (h−1) | 5.23 (20) | 5.58 (21) | 2.78 (13) | 12.2 (14) | 3.7 (20) | 0.99 (15) |
| Tlag (h) | 6 | 3 | 3 | 10 | 10 | 10 |
| Cmax (μg/g) | 4.30 ± 0.18 | 31.3 ± 1.52 | 50.6 ± 13.4 | 19.9 ± 4.71 | 53.9 ± 7.29 | 37.7 ± 3.90 |
| Tmax (h) | 0.25 | 0.5 | 0.167 | 0.083 | 0.25 | 1 |
| Kidney | ||||||
| k13 (h−1) | 4.61 (13) | 1.99 (15) | 0.6 (13) | 1.95 (15) | 1.5 (16) | 0.3 (17) |
| k31 (h−1) | 4.42 (17) | 1.35 (22) | 0.3 (23) | 0.76 (22) | 0.42 (20) | 0.08 (27) |
| AUC1 (μg · h/g) | 20.58 (11) | 96.32 (21) | 163.9 (8) | 162.6 (11) | 207.8 (13) | 206.3 (6) |
| Cmax (μg/g) | 2.4 ± 0.34 | 9.2 ± 0.07 | 9.11 ± 0.95 | 5.71 ± 0.59 | 9.25 ± 0.29 | 6.5 ± 0.50 |
| Tmax (h) | 0.25 | 0.5 | 1 | 3 | 6 | 0.167 |
Values in parentheses are coefficients of variation (in percent).
Mean ± standard deviation.
(ii) Spleen.
The results obtained from the data on drug disposition in the spleen were very similar to those obtained from the data on drug disposition in the liver. The model performed very well in predicting the concentration-versus-time profiles (Fig. 2A3, B3, and C3). The pharmacokinetic parameters (Table 2) varied significantly (P < 0.05) between formulations, and all of them were estimated with enough precision (coefficient of variation, <45%). Tlag estimates were not different among the new formulations (P > 0.05) but differed significantly from that for Fungizone (P < 0.05).
(iii) Kidney.
Figure 2A4, B4, and C4 show the raw and model prediction concentration data for some particular formulations. Table 2 also lists the estimates of the parameters of the disposition of AmB in the kidney. Parameter estimates also show considerable differences between formulations (P < 0.05), a pattern seen for the liver and spleen, as described above.
Creatinine concentration results.
Table 3 lists the values of the measurements used to evaluate renal function across formulations. Ccmaxs tended, for all formulations, to be higher than the baseline values and occurred within the first hour after drug injection. On the basis of Ccmax values, Fungizone and AmB-MM [125] could be considered the formulations with the highest potential for nephrotoxicity because they had the greatest effect on renal function.
TABLE 3.
Renal toxicity-related measurements obtained from creatinine levels in plasma
| Parameter | Formulation (dose)
|
|||||
|---|---|---|---|---|---|---|
| Fungizone (1 mg/kg) | AmB-Nsp [125] (3 mg/kg) | AmB-Nsp [12.5] (3 mg/kg) | AmB-MM [125] (1 mg/kg) | AmB-MM [125] (3 mg/kg) | AmB-MM [12.5] (3 mg/kg) | |
| AUCc (mg · h/100 ml) | 134 | 26.3 | 47 | 118 | 48 | 178 |
| Ccmax (mg/100 ml) | 5.0 ± 2.6a | 1.8 ± 0.4 | 1.7 ± 0.6 | 2.1 ± 0.7 | 1.9 ± 0.4 | 7.7 ± 2.6 |
| Tcmax (h) | 0.25 | 0.5 | 0.25 | 0.167 | 0.5 | 0.25 |
Mean ± standard deviation.
DISCUSSION
At present AmB can be considered the first choice for the treatment of disseminated mycoses, with nephrotoxicity being the major limitation in therapeutics. In the current study the disposition pharmacokinetics in plasma and tissue of novel and traditional formulations of AmB after injection of an intravenous bolus of AmB to rats were investigated.
The study has focused mainly on modeling of the time course of the drug in several tissues. The liver, spleen, and kidney were chosen since the liver and spleen are the most frequent organs where fungal infections appear, and the kidney is the organ where AmB produces toxicity (5). We also evaluated the effects of the different AmB formulations on renal function in terms of the increment of creatinine levels in plasma.
The studied formulations (nanospheres and mixed micelles) have already been explored from an efficacy-versus-toxicity perspective (11). In addition, their physicochemical characteristics have previously been published by Espuelas et al. (12, 13). During the present study the procedure for developing such formulations was similar to the one used by Espuelas et al. (11), as were the results obtained during their characterization.
The pharmacokinetics of AmB in plasma for all formulations were best described by a two-compartment model. The pharmacokinetic parameters obtained for Fungizone were similar to those obtained by other investigators when the kinetics of this formulation were compared with those of Abelcet after administration of single or repeated doses in rats (4, 20).
The disposition of the AmB-MM [125] formulation at two different doses (1 and 3 mg/kg) was evaluated in plasma; pharmacokinetic distribution parameters changed significantly with dose, suggesting that for a particular formulation distribution mechanisms are dose dependent at this range of doses. After the administration of three different doses (0.28, 0.45, and 0.84 mg/kg, respectively) of Fungizone by intravenous infusion to rats, Chow et al. (8) did not observe the dose dependence, but recently, several investigators observed this phenomenon after the administration of liposomal AmB to rabbits (17) and neutropenic patients (25).
On the basis of our results it seems that the different components of the formulations such as poloxamer 188 and poly(ɛ-caprolactona) do not modify the elimination of the drug, as no differences in CL were observed between formulations. On the other hand, the distribution process reflected by t1/2α, V, and VSS seems to differ among formulations to a high degree, which could be considered a priori an initial goal in the development of new formulations. In general, the Cmaxs in the tissues studied were higher for all new formulations than for the reference formulation (Table 2).
Exploring the raw concentration-versus-time profiles, a double peak of concentration was found in the liver and spleen for all formulations. Our analytical technique could not distinguish between free or complexed AmB, in fact, the total AmB concentration (the AmB concentration in plasma plus the AmB bound to the component of the formulation) in plasma and tissues was determined. The physiological explanation for this phenomenon could be connected to the fact that the entrance of free AmB and AmB-poloxamer complex into the liver and spleen takes place in different ways, depending on whether AmB is free. Our hypothesis is that, first, the AmB-poloxamer 188 complex gains access to the tissues (first concentration peak). AmB returns to the plasma once the complex has been degraded into the tissue, and then there is a redistribution of free AmB into the tissue (second peak). This is the first time that such a behavior has been observed and was not observed previously probably owing to the need for an intensive sampling over a short period of time. Tissue AmB concentrations were measured six times in the first 3 h of the study. The parameter called Tlag may represent both the degradation of the poloxamer 188 complex in tissue and the return of free AmB to the plasma compartment. In principle, one can think that the particular size or liposolubility of formulations could influence Tlag. In fact, there were differences in this parameter for the spleen, depending on the type of formulation; Tlags were estimated to be 3, 6, and 10 h for AmB-Nsps, Fungizone, and AmB-MMs, respectively. However, no differences were seen between the Tlag values for the new formulations for the liver.
The estimated values of k13 and kk13 were similar, probably indicating that the distribution of complexed AmB and free AmB is a perfusion rather than a permeation limitation process. By exploring the rate constants for the drug entering the liver and spleen and comparing these constants for the various formulations, it was found that components of the polymeric formulations such as poloxamer 188 are important determinants in the rate of drug uptake in those organs. As shown in Table 4, an increase in the polymer concentration can result in higher values for k13 in AmB-Nsps and AmB-MMs for the liver and spleen. This finding is in agreement with those of other investigators who have presented results that suggest that, in general, with the exception of poloxamer 188, surfactants can decrease the rate of penetration into tissue (18).
TABLE 4.
Comparison between concentration of poloxamer 188 used for each of the polymeric formulations on the basis of k13 (kk13) and k31 estimates
| Tissue and pharmacokinetic parameter | Formulation (dose)
|
|||
|---|---|---|---|---|
| AmB-Nsp [125] (3 mg/kg) | AmB-Nsp [12.5] (3 mg/kg) | AmB-MM [125] (3 mg/kg) | AmB-MM [12.5] (3 mg/kg) | |
| Liver | ||||
| k13 = kk13 (h−1) | 5.5 (18)a | 2.45 (15) | 23.0 (10) | 2.3 (17) |
| k31 (h−1) | 5.9 (22) | 1.13 (22) | 4.2 (17) | 0.5 (24) |
| Spleen | ||||
| k13 = kk13 (h−1) | 13.7 (18) | 6.9 (9) | 15.3 (17) | 4.17 (9) |
| k31 (h−1) | 5.58 (21) | 2.78 (13) | 3.7 (20) | 0.99 (15) |
Values in parentheses are coefficients of variation (in percent).
The physicochemical characteristics of the novel formulations such as the size, the liposolubility, and the proportion of the different components of the formulations may play an important role in the pharmacokinetics of the formulation. When we tried to correlate these characteristics with the pharmacokinetic estimates obtained, the only relationship that we could establish was the one discussed above regarding the impact of poloxamer 188 on the rate constants for input into the liver and spleen. On the basis of these results, if quick, high levels of drug in those tissues are desired, the use of more poloxamer 188 in the formulation is recommended.
Particularly for AmB-Nsps, the drug is adsorbed into the particle surface. When these nanospheres are prepared with poloxamer 188, this polymer is also adsorbed into the surface and AmB forms mixed micelles with the nonadsorbed polymer (80%). The mixed system is formed by the AmB adsorbed into the particle and mixed micelles of AmB-poloxamer 188 (12, 13). The size of the AmB-poloxamer 188 complex is recognized by the macrophages present in the liver and spleen, with this phenomenon being responsible for the differences in pharmacokinetics seen between formulations.
For the case of the kidney, no double peak was found and all formulations showed similar Cmax values (when normalized by dose), supporting the hypothesis that only free AmB is able to access the kidney. The AUCr value was lower for Fungizone than for the rest of the formulations, but surprisingly, renal toxicity (measured as increasing levels of creatinine in plasma) was found to be higher for Fungizone. Among the new formulations, AmB-MM [12.5] exhibited the greatest effect on the kidney and could be considered the most nephrotoxic. A similar result had previously been reported by Espuelas et al. (11), as determined by using LD50s for mice. Increasing levels of creatinine in plasma have also been used by other investigators, resulting in a good index that can be used to explore the effect of AmB on renal function (17, 26). The possibility exists that the polymeric components of the formulation, poloxamer 188 and poly(ɛ caprolactone), may produce renal dysfuction. However, they are nontoxic polymers, and no increases in creatinine levels were observed. In addition, the LD50 of poloxamer 188 when administered to rats is 7 g/kg, and 0.5 mg/kg was the dose administered in this study (24).
Our results indicate that there is no direct relationship between drug disposition in the kidney (reflected as Tmax, Cmax, or AUCr) and a renal effect. Such a relationship suggests instead a need for a complex analysis like pharmacodynamic modeling to relate drug exposure to the effect on renal function.
To summarize the results of the current study, the dispositions of new formulations of AmB in plasma, liver, spleen, and kidney have been characterized with pharmacokinetic models. The profiles for plasma were best characterized by a two-compartment model, with distribution being the process that varied the most between formulations. To describe the disposition of AmB in liver and spleen, a model with two first-order rate constants that account for the transfer from plasma to the tissues was fitted to the data. Our results suggest that poloxamer 188 plays an important role in the disposition of AmB in tissue. The effects of the formulations on renal function were also evaluated, but no relationship between drug disposition in kidney and increasing levels of creatinine in plasma was found. All new formulations tested show fewer renal effects than the reference formulation Fungizone.
ACKNOWLEDGMENT
This work was partially supported by Proyectos de Investigación Universidad de Navarra (grant PIUNA-96 240501), Pamplona, Navarra, Spain.
REFERENCES
- 1.Adler-Moore J P, Fuji G, Lee M A. In vitro and in vivo interactions of Ambisome with pathogenic fungi. J Liposome Res. 1993;3:151–156. [Google Scholar]
- 2.Akaike H. A new look at the statistical model identification. IEEE Trans Automat Control. 1974;19:716–723. [Google Scholar]
- 3.Bartels H, Böhmer M, Heierli C. Serum Kreatinibestimmung ohne Enteiweissen. Clin Chim Acta. 1972;37:193–197. doi: 10.1016/0009-8981(72)90432-9. [DOI] [PubMed] [Google Scholar]
- 4.Bhamra R, Sa'ad A, Bolcsak L E, Janoff A S, Swenson C E. Behaviour of amphotericin B lipid complex in plasma in vitro and in the circulation of rats. Antimicrob Agents Chemother. 1997;41:886–892. doi: 10.1128/aac.41.5.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boswell G W, Bekersky I, Buell D, Hiles R, Walsh T J. Toxicological profile and pharmacokinetics of a unilamellar liposomal vesicle formulation of amphotericin B in rats. Antimicrob Agents Chemother. 1998;42:263–268. doi: 10.1128/aac.42.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boxenbaum H G, Riegelman S, Elashoff R M. Statistical estimations in pharmacokinetics. J Pharmacokinet Biopharm. 1974;2:123–148. doi: 10.1007/BF01061504. [DOI] [PubMed] [Google Scholar]
- 7.Brajtburg J, Bolard J. Carrier effects on biological activity of amphotericin B. Clin Microbiol Rev. 1996;9:512–531. doi: 10.1128/cmr.9.4.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chow H H, Wu Y, Mayersohn M. Pharmacokinetics of amphotericin B in rats as a function of dose following constant-rate intravenous infusion. Biopharm Drug Dispos. 1995;16:461–473. doi: 10.1002/bdd.2510160604. [DOI] [PubMed] [Google Scholar]
- 9.Cooke J. Pharmacoeconomics of antifungal therapy. Bone Marrow Transplant. 1994;14(Suppl. 5):S18–S20. [PubMed] [Google Scholar]
- 10.Echevarría I, Barturen C, Renedo M J, Dios-Viéitez M C. High-performance liquid chromatographic determination of amphotericin B in plasma and tissue. Application to pharmacokinetic and tissue distribution studies in rats. J Chromatogr A. 1998;819:171–176. doi: 10.1016/s0021-9673(98)00425-7. [DOI] [PubMed] [Google Scholar]
- 11.Espuelas M S, Legrand P, Irache J M, Gamazo C, Orecchioni A M, Devissaguet J-P, Ygartua P. Poly(ɛ-caprolactone) nanospheres as an alternative way to reduce amphotericin B toxicity. Int J Pharm. 1997;158:19–27. [Google Scholar]
- 12.Espuelas M S, Legrand P, Chéron M, Barratt G, Puisieux F, Devissaguet J-P, Irache J M. Interaction of amphotericin B with polymeric colloids. 1. A spectroscopic study. Colloid Surf B Biointerf. 1998;11:141–151. [Google Scholar]
- 13.Espuelas M S, Legrand P, Chéron M, Barratt G, Puisieux F, Renedo M J, Devissaguet J-P, Irache J M. Interaction of amphotericin B with polymeric colloids. 2. Effect of poloxamer on the adsorption of amphotericin B onto poly(ɛ caprolactone) nanospheres. Colloid Surf B Biointerf. 1998;11:203–212. [Google Scholar]
- 14.Garrido M J, Valle M, Calvo R, Trocóniz I F. Altered plasma and brain disposition and pharmacodynamics of methadone in abstinent rats. J Pharmacol Exp Ther. 1999;288:179–187. [PubMed] [Google Scholar]
- 15.Hillery A M. Supramolecular lipidic drug delivery systems: from laboratory to clinic. A review of the recently introduced commercial liposomal and lipid-based formulations of amphotericin B. Adv Drug Deliv Rev. 1997;24:345–363. [Google Scholar]
- 16.Kreft B, De Wit C, Marre R, Sack K. Experimental studies on the nephrotoxicity of amphotericin B in rats. J Antimicrob Chemother. 1991;28:271–281. doi: 10.1093/jac/28.2.271. [DOI] [PubMed] [Google Scholar]
- 17.Lee J W, Amantea M A, Francis P A, Navarro E E, Bacher J, Pizzo P A, Walsh T J. Pharmacokinetics and safety of a unilamellar liposomal formulation of amphotericin B (Ambisome) in rabbits. Antimicrob Agents Chemother. 1994;38:713–718. doi: 10.1128/aac.38.4.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moghimi S M. Mechanisms regulating body distribution of nanospheres conditioned with pluronic and tetronic block co-polymers. Adv Drug Deliv Rev. 1995;16:183–193. [Google Scholar]
- 19.Müllen A B, Carter K C, Baillie A J. Comparison of the efficacies of various formulations of amphotericin B against murine viceral leishmaniasis. Antimicrob Agents Chemother. 1997;41:2089–2092. doi: 10.1128/aac.41.10.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olsen S J, Swerdel M R, Blue B, Clark J M, Bonner D P. Tissue distribution of amphotericin B lipid complex in laboratory animals. J Pharm Pharmacol. 1991;43:831–835. doi: 10.1111/j.2042-7158.1991.tb03189.x. [DOI] [PubMed] [Google Scholar]
- 21.Rowland M, Tozer T N. Clinical pharmacokinetics. Concepts and applications. 3rd ed. Media, Pa: The William & Wilkins Co.; 1995. [Google Scholar]
- 22.Schwarz G. Estimating the dimension of a model. Ann Stat. 1978;6:461–468. [Google Scholar]
- 23.Tasset C, Preat V, Bernard A, Roland M. Comparison of nephrotoxicities of different polyoxyethyleneglycol formulations of amphotericin B in rats. Antimicrob Agents Chemother. 1992;36:1525–1531. doi: 10.1128/aac.36.7.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wade A, Weller P J. Handbook of pharmaceutical excipients. Washington, D.C.: American Pharmaceutical Association; 1994. p. 352. [Google Scholar]
- 25.Walsh T J, Yeldandi V, Mcevoy M, Gonzalez C, Chanock S, Freifeld A, Seibel N I, Withcomb P O, Jarosinsky P, Boswell G, Bekersky I, Alak A, Buell D, Barret J, Wilson W. Safety, tolerance, and pharmacokinetics of a small unilamellar formulation of amphotericin B (Ambisome) in neutropenic patients. Antimicrob Agents Chemother. 1998;42:2391–2398. doi: 10.1128/aac.42.9.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wasan K M, Kennedy A L, Cassidy S M, Ramaswamy M, Holtorf L, Wen-Lin Chou J, Pritchard P H. Pharmacokinetics, distribution in serum lipoproteins and tissues, and renal toxicities of amphotericin B and amphotericin B lipid complex in a hypercholesterolemic rabbit model: single-dose studies. Antimicrob Agents Chemother. 1998;42:3146–3152. doi: 10.1128/aac.42.12.3146. [DOI] [PMC free article] [PubMed] [Google Scholar]