Abstract
The pharmacokinetics and distribution in tissue of several novel triazole antifungal agents were studied in different animal species in order to select an appropriate lead compound. The purpose of the study was also to determine species differences in pharmacokinetics for SYN azoles to select the most appropriate species for secondary efficacy and toxicological evaluation of the selected compound. SYN-2836, SYN-2869, SYN-2903, and SYN-2921 were rapidly absorbed into the systemic circulation and reached maximum concentrations (Cmaxs) of 7.31 ± 2.53, 6.29 ± 0.85, 6.16 ± 0.39, and 3.41 ± 0.34 μg/ml, respectively, in BALB/c mice after administration of an oral dose of 50 mg/kg of body weight, with bioavailability being greater than 45% in all mice. The areas under the concentration-time curve from time zero to infinity (AUC0–∞s) after administration of a single intravenous dose of 20 mg/kg to mice varied between 25.0 and 63.6 μg · h/ml. The half-life was in the range of 4.5 to 6 h. In Sprague-Dawley rats there was no significant difference in AUC0–∞ after administration of a single intravenous dose of 20 mg/kg, but on oral administration, the bioavailability of SYN-2836 was extremely low, while that of SYN-2869 was only 14.7%. In New Zealand White rabbits the Cmax and the time to reach Cmax for SYN-2836 and SYN-2869 after administration of a single oral dose of 50 mg/kg were similar. There were significant differences in AUC0–∞ and half-life between SYN-2836 and SYN-2869. On the other hand, in beagle dogs the Cmax and AUC0–∞ of SYN-2836 after administration of a single oral dose of 30 mg/kg were 4.82 ± 1.54 μg/ml and 41.8 ± 15.7 μg · h/ml, respectively, which were threefold higher than those of SYN-2869. The concentrations of the SYN compounds in tissue indicated that the AUC0–∞s of SYN-2836, SYN-2869, SYN-2903, and SYN-2921 in mouse lungs were significantly different from each other. The ratios of the concentrations of the SYN azoles in lungs to those in plasma were also significantly different from those for itraconazole. Among the SYN azoles the highest concentration in the lungs was found for SYN-2869. The higher level of distribution of SYN-2869 into lung tissue was considered to contribute to the potent efficacy in respiratory tract infection models compared with the potency of itraconazole. Significant differences in the pharmacokinetics of these compounds were observed in different animal species, and selection of an animal model for further evaluation was based on results obtained from these studies.
The emergence of systemic and deep-seated mycoses in immunocompromised patients undergoing anticancer chemotherapy or organ transplantation or infected with human immunodeficiency virus has changed the universal pattern of infectious disease. Amphotericin B, the “gold standard” for antifungal therapy, is known to be the most efficacious drug in the treatment of deep-seated and systemic mycoses (9). Unfortunately, the severe nephrotoxicity, fever, nausea, vomiting, chills, and pain at the site of injection and other intrinsic side effects have limited its use, especially in kidney transplant patients (7). New liposomal formulations of amphotericin B have been used to reduce these side effects; however, their use has been restricted due to their high costs. To overcome these problems, pharmaceutical scientists have initiated efforts to introduce novel chemical entities with new mechanisms of action. The azole class of antifungal agents has been among the most well received compounds by clinicians in recent decades (1). Ketaconazole, fluconazole, and itraconazole are representatives of this class and have been used in the treatment of systemic and deep-seated mycoses (10). Flucanozole has been widely used in the treatment of candidiasis and cryptococcosis. Unfortunately, this compound does not show efficacy against systemic aspergillosis or invasive aspergillosis of the lung (2, 15). Itraconazole has been shown to have a wide spectrum of activity against yeasts and filamentous fungi, as well as dermatophytes (14). Although its efficacy against systemic candidiasis and cryptococcosis is less than that of fluconazole, it is reported to exhibit efficacy against systemic aspergillosis and invasive aspergillosis of the lung (8). This efficacy is considered by clinicians to be not significant due to the poor bioavailability of this compound (6). In the search for broad-spectrum antifungal agents that can be used to treat systemic fungal infection, a series of 2(2,4-difluorophenyl)-3-(4-substituted piperazin-1-yl)-1-(1,2,4-triazol-1-yl) butanols were synthesized. Systematic modifications of the piperazine moiety resulted in the discovery of novel triazoles. SYN-2869, (2R,3R)-2-(2,4-difluorophenyl)-1-(1H-1,2-triazole-1-yl)-3-[4-{4-[2-(4-trifluro methoxy-benzyl)-2H-1,2,4-triazol-3-one-4-yl]phenyl}piperazin-1- yl]butan-2-ol, is one such triazole. It is a broad-spectrum, orally active antifungal agent and is being evaluated for use in the treatment of Candida and Aspergillus infections, particularly invasive pulmonary aspergillosis (M. D. Abel, Y. Bathini, C. Ha, T. Furukawa, G. Kasitu, J. K. Khan, R. G. Micetich, D. Q. Nguyen, S. M. Salama, G. Samari, I. Sidhu, P. Spevak, N. Unemi, and M. Daneshtalab, Abstract of 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 148, p. 270, 1998; T. Furukawa, H. Saito, T. Uji, K. Nishida, F. Higashitani, N. Unemi, and H. Yamaguchi, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 149, p. 270, 1998). SYN-2869 exhibit a broad spectrum of antifungal activity in vitro, with its activity being comparable to that of itraconazole (S. M. Salama, A. Gandhi, H. Atwal, J. Simon, J. Khan, R. G. Micetich, and M. Daneshtalab, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 150, p. 270, 1998). Its derivatives SYN-2836 (which has a p-trifluoromethyl moiety at the benzylic moiety) and 3′-fluoro-substituted analogues of SYN-2836 and SYN-2869 (namely, SYN-2903 and SYN-2921, respectively) have shown activities comparable to those of other leading azoles (unpublished data). The purpose of the present study was to select a candidate compound among the SYN azoles as well as to evaluate differences in the pharmacokinetics of these novel triazole derivatives in mice, rats, rabbits, and dogs and to choose appropriate animal models for further efficacy and toxicological evaluations.
MATERIALS AND METHODS
Chemicals.
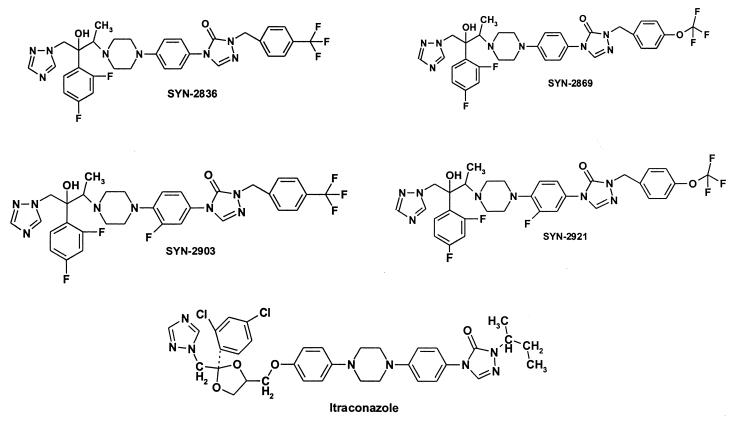
SYN-2869, its derivatives SYN-2836, SYN-2903, and SYN-2921, and the internal standard SYN-2506 were synthesized at SynPhar Laboratories Inc. (Edmonton, Alberta, Canada) (Fig. 1). The other chemicals were of reagent grade or high-performance liquid chromatography (HPLC) grade.
FIG. 1.
Structures of novel triazole antifungal agents SYN-2836, SYN-2869, SYN-2903, SYN-2921, and itraconazole.
Animals.
Male BALB/c mice (weight, 18 to 20 g) and Sprague-Dawley rats (weight, 250 to 300g) were supplied by Health Science Laboratory Animal Services, University of Alberta. New Zealand White rabbits were purchased from Charles River Company, Montreal, Canada, and beagle dogs were also supplied by Health Science Laboratory Animal Services, University of Alberta.
Preformulation.
SYN compounds are highly lipophilic and pH-dependent, aqueous soluble compounds with molecular masses of more than 600 Da. A microemulsion formulation was used with 125 mg of drug, 0.2 ml of 0.4 N HCl, 0.2 ml of 98% ethanol, 0.2 ml of cremophore, and 1.4 ml of saline to give a final concentration of 62.5 μg/ml. All species except beagle dogs were dosed with this formulation.
Pretreatment and dosing of the animals. (i) Mice.
The BALB/c mice were used after an acclimatization period of 7 days. The animals were housed in groups of five mice each in solid-bottom polypropylene mouse cages with clean wood-shaving bedding. Mouse chow feed and water were provided throughout the period. During the acclimatization period the temperature and humidity of the room with the mice were controlled. The photoperiod was 12 h of artificial light and 12 h of darkness. For intravenous administration the animals were anesthetized for 5 min by making them inhale methoxyflurane (Metofane). After the animal was properly secured, absolute alcohol was applied to the tail to dilate the vein and disinfect the injection site. After 1 min of application of alcohol, mild pressure was applied near the base of the tail over the vein with an index finger to secure it properly, and a needle was then gently introduced into the vein and solution was injected slowly. A 1-ml graduated hypodermic syringe with a 27.5-gauge disposable needle was used. For oral administration the animals were not anesthetized but were fasted overnight. At each time point (1, 2.5, 5, 15, 30, and 45 min and 1, 2, 4, 6, 8, 12, 16, and 24 h) three animals were euthanatized with carbon dioxide, and blood was immediately removed, as were other tissues including the lungs (13). These samples were stored at −20°C until they were analyzed. Single intravenous doses of SYN-2836 (10, 20, 35 and 50 mg/kg of body weight), SYN-2869 (20 mg/kg), SYN-2903 (20 mg/kg), SYN-2921 (20 mg/kg), and itraconazole (15 mg/kg) were given through the tail vein of each mouse (body weights ranged from 18 to 22 g). Single oral doses of SYN-2836, SYN-2869, SYN-2903, and SYN-2921 (each at 50 mg/kg) and itraconazole (at 30 mg/kg) were administered by gavage.
(ii) Rats.
Sprague-Dawley rats were housed in groups of two rats each in solid-bottom polypropylene rat cages with clean wood-shaving bedding. Rat chow feed and water were provided throughout the period. During the acclimatization period the temperature and humidity of the room with the rats were controlled. The photoperiod was 12 h of artificial light and 12 h of darkness.
(a) Surgical procedure.
The rats were anesthetized with methoxyflurane or by an intraperitoneal injection of sodium pentobarbital. An incision anterior to the right clavicle was made, and the jugular vein was exposed. A small incision in the vein was made, and a catheter was introduced into the jugular vein and tied tightly. The catheter was subcutaneously exteriorized through an incision behind the right ear.
(b) Treatment procedure.
The selected doses were 20 and 50 mg/kg for the intravenous and oral routes of administration, respectively, for compounds SYN-2836 and SYN-2869. All animals were dosed intravenously through the jugular vein, and the same site was also used to withdraw blood samples after flushing the catheter to avoid contamination. For oral administration gavage was used to administer the drug, and samples were collected through the jugular vein at 5, 15, 30, and 45 min and 1, 2, 4, 6, 8, 12, 16, and 24 h postdosing. For intravenous administration, additional sampling times were 1 and 2.5 min.
(iii) Rabbits.
A single intravenous dose (20 mg/kg) of SYN-2836 was given through the marginal ear vein to each New Zealand White rabbit (body weight, 2.5 to 3.5 kg), and oral doses of 50 mg of SYN-2836 and SYN-2869 per kg were given via a feeding tube. Blood samples were withdrawn from the marginal ear vein for analysis starting at 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 6, 8, and 24 h postdosing.
(iv) Beagle dogs.
Single and multiple oral doses of 30 mg of SYN-2836 or SYN-2869 per kg and 136 mg of hydroxypropyl-β-cyclodextrin per kg were given to the dogs in gelatin capsules. Blood samples were collected from the saphenous vein at 5, 15, and 30 min and 1, 2, 4, 8, 12, 16, and 24 h after dosing.
Sample preparation and HPLC assay.
Ice-cold acetonitrile was added to each plasma sample at a ratio of 10:1 to allow the protein to precipitate, and the sample was then vortexed for 10 min. The samples were then centrifuged at 3,500 × g for 10 min and the supernatant was transferred to clean culture tubes. The clear solution was then dried under vacuum and was reconstituted by adding 200 μl of methanol. The interday precision and accuracy for a standard concentration of SYN-2869 were from 1.9 to 8.5% and from −1.4 to 4.4%, respectively. The precision and accuracy of intraday quality control samples were from 4.6 to 5.2% and from 4.6 to 12%, respectively. Similar results were obtained with the derivatives of SYN-2869 (12). Phosphate buffer (0.01 M; pH 7.8) was added (4× [wt/vol]) to the lung tissue samples, and the samples were ultrasonicated to homogenize the tissues. The samples were spiked with internal standard (SYN-2506), and the mixture was vortexed for 5 min to incorporate all components. Ice-cold acetonitrile (4× [vol/vol]) was added to the homogenate, and the mixture was vortexed for 5 min to precipitate the proteins. The samples were then centrifuged at 3,500 × g for 10 min, and the supernatant was transferred to clean culture tubes and then dried under vacuum and reconstituted by adding 200 μl of methanol (13).
Instrumentation.
The HPLC system comprised a Waters 717 autosampler, a Waters 996 Photo diode array detector, and a Waters 600 series solvent delivery system. Millenium Chromatography Manager, version 2.1, was used for data management. The injection volume was 50 μl, with the extraction wavelength for two-dimensional quantitation of the chromatogram being 263 nm. The flow rate was 1.0 ml/min. Mobile phase A was 100% acetonitrile, and mobile phase B was water. The initial condition of the elution program was 40% mobile phase A with stepwise gradients of 50% mobile phase A for 5 min, 70% mobile phase A for 10 min, 80% mobile phase A for 15 min, and maintenance of 80% mobile phase A for 20 min; finally, 40% mobile phase A was used for 25 min with equilibration for 5 min. The separation was performed with a Waters Symmetry C18 analytical column (5-μm particle size; 150 mm by 3.9 mm [inner diameter]) (12, 13).
Pharmacokinetic analysis.
The total area under the plasma or tissue concentration-time curve (AUC) from time zero to time infinity (AUC0–∞) was calculated by linear trapezoidal rule extrapolation by the equation AUClast + Cn/λz, where AUClast is the last recorded AUC, Cn denotes either the observed or predicted concentration at the last sample time, and λz is the first-order rate constant associated with the terminal (log-linear) portion of the curve and is estimated via nonlinear regression of the concentration-versus-time curve. The terminal half-life was calculated as −ln(2)/λz. Clearance (CL) from plasma was determined by dose/AUC0–∞, and the volume of distribution (V) was based on the terminal phase and was determined by dose/λz× AUC0–∞; for the extravascular model CL and V are calculated as V/F and CL/F, where F is the fraction of the dose absorbed. Mean residence time extrapolated to infinity (MRTinf) and the volume of distribution at steady state (VSS) for bolus intravenous administration were calculated as AUMCinf/AUCinf (where AUMCinf is the area under the first moment of the concentration-time curve extrapolated to infinity and AUCinf is AUC extrapolated to infinity) and dose/AUC0–∞ × MRTinf. The absolute bioavailability was calculated from AUC0–∞ after oral administration and AUC0–∞ after intravenous administration with appropriate dose correction. Pharmacokinetic parameters were calculated by noncompartmental analysis with the Winnonln computer program.
Statistical analysis.
All results are reported as means ± standard deviations. Simple linear regression was used to relate AUC for plasma and lung versus dose. The paired t test was used to compare the AUCs for plasma and those for other tissues at the corresponding doses. For the other kinetic parameters, a one-way analysis of variance was used to evaluate the difference at different doses and with different compounds. When a difference was detected, Duncan's multiple comparison test was used to evaluate the differences among groups. The level of significance was set at P equal to 0.05. SPSS computer software was used for all statistical calculations.
RESULTS
Pharmacokinetics of SYN azoles after intravenous and oral administration to mice.
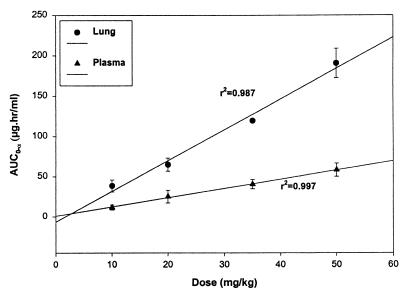
The mean CL of SYN-2836 given at doses of 10, 20, 35, and 50 mg/kg were similar (Table 1). The increase in the mean AUC0–∞ was linear, with an r2 value of 0.997 (Fig. 2). The half-life of SYN-2836 given at 10 mg/kg was 1.1 ± 0.28 h and increased twofold when SYN-2836 was at 20 mg/kg but with no further significant increase when the drug was given at 35 and 50 mg/kg. The VSS values for doses of 20, 35, and 50 mg/kg were significantly different from that for a dose of 10 mg/kg (Table 1). Mean AUCs after administration of a single intravenous dose of 20 mg of SYN-2869 and SYN-2903 per kg were significantly different from those for the rest of the compounds tested. The CL values of SYN-2836, which has a p-trifluoromethyl group at the terminal benzylic moiety, and SYN-2921, the 3′-fluoro-substituted analog of SYN-2869, were about twofold higher than that of SYN-2869 (Table 2). The mean maximum concentrations (Cmaxs) of SYN-2836, SYN-2869, SYN-2903, and SYN-2921 in the same microemulsion were 7.31 ± 2.53, 6.29 ± 0.85, 6.16 ± 0.39, and 3.41 ± 0.34, respectively, after administration of an oral dose of 50 mg/kg.
TABLE 1.
Mean values of pharmacokinetic parameters for SYN-2836 in mouse plasma after administration of single intravenous doses ranging from 10 to 50 mg/kg
| Parameter | 10 mg/kg | 20 mg/kg | 35 mg/kg | 50 mg/kg |
|---|---|---|---|---|
| AUC (μg · h/ml) | 11.69 ± 3.07a | 25.20 ± 7.9 | 40.62 ± 6.95 | 58.3 ± 8.33 |
| CL (ml/min/kg) | 15 ± 3.5 | 14 ± 4 | 14.5 ± 2.5 | 14.5 ± 2 |
| Half-life (h) | 1.11 ± 0.28b | 2.75 ± 0.19 | 3.14 ± 0.46 | 2.14 ± 0.15 |
| MRT (h) | 0.82 ± 0.33b | 2.61 ± 0.78 | 3.07 ± 0.44 | 2.18 ± 0.39 |
| VSS (liter/kg) | 0.77 ± 0.4b | 2.3 ± 1.13 | 2.71 ± 0.77 | 1.92 ± 0.55 |
P < 0.05 versus 50 mg/kg.
P < 0.05 versus 20, 35, and 50 mg/kg.
FIG. 2.
Mean AUC for SYN-2836 in plasma and lung versus dose after administration of a single intravenous dose to mice. AUC0–α refers to AUC0–∞.
TABLE 2.
Mean values of pharmacokinetic parameters for SYN-2836, SYN-2869, SYN-2903, SYN-2921, and Itraconazole in mouse plasma after administration of a single intravenous dose of 20 mg/kg
| Parameters | SYN-2836 | SYN-2869 | SYN-2903 | SYN-2921 | Itraconazolea |
|---|---|---|---|---|---|
| AUC (μg · h/ml) | 25.20 ± 7.9 | 52.33 ± 5.26b | 63.62 ± 26.83b | 25.01 ± 1.68 | 24.73 ± 1.86 |
| CL (ml/min/kg) | 14 ± 4c | 8.5 ± 3.5 | 6 ± 3 | 13.5 ± 1c | 9.5 ± 0.5 |
| Half-life (h) | 2.75 ± 0.19d | 5.56 ± 3.03 | 4.59 ± 1.68 | 6.15 ± 1.47 | 1.59 ± 0.23d |
| MRT (h) | 2.61 ± 0.78 | 4.17 ± 1.45 | 4.53 ± 1.67 | 4.34 ± 0.58 | 1.47 ± 0.26 |
| VSS (liters/kg) | 2.3 ± 1.13 | 2.73 ± 0.74 | 2.0 ± 0.28 | 4.68 ± 0.38e | 0.99 ± 0.12 |
Intravenous dose of 15 mg/kg.
P < 0.05 versus SYN-2836 and SYN-2921.
P < 0.05 versus SYN-2869 and SYN-2903.
P < 0.05 versus SYN-2869, SYN-2903, and SYN-2921.
P < 0.05 versus SYN-2836, SYN-2869, and SYN-2903.
Pharmacokinetics of SYN azoles after intravenous and oral administration to rats.
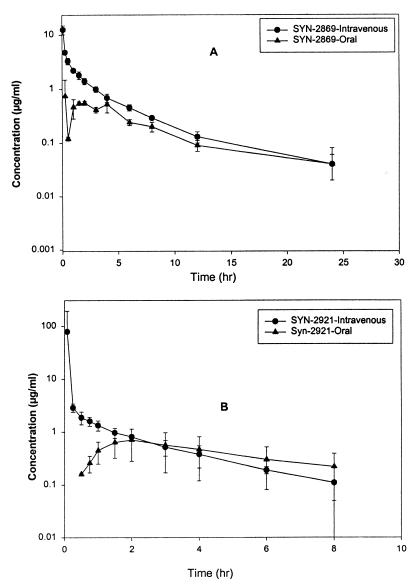
The mean AUC0–∞ after administration of single intravenous doses of 20 mg of SYN-2836, SYN-2869, SYN-2903, and SYN-2921 per kg were 12.22 ± 2.1, 12.92 ± 0.73, 32.55 ± 7.11, and 7.86 ± 4.16 μg · h/ml, respectively. The mean CL for SYN 2921 was significantly different from the mean CLs for SYN-2836, SYN-2869, and SYN-2903. No significant difference in half-life or V was found among these compounds. The oral bioavailabilities of SYN-2836 and SYN-2903 were extremely low, while SYN-2869 and SYN-2921 had absolute bioavailabilities of 14.7 and 23.6%, respectively (Fig. 3).
FIG. 3.
Mean plasma concentration-time profiles of SYN-2869 (A) and SYN-2921 (B) after administration of single intravenous (20 mg/kg) and oral (50 mg/kg) doses to rats.
Pharmacokinetics of SYN-2836 and SYN-2869 in New Zealand White rabbits and beagle dogs.
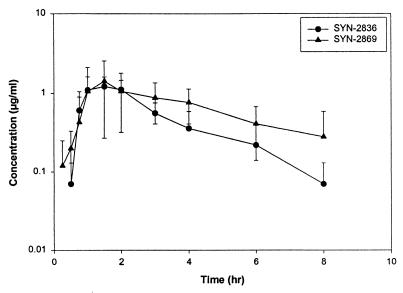
In New Zealand White rabbits the mean AUC0–∞ observed for SYN-2869 was three times higher than that for SYN-2836, while in beagle dogs the AUC0–∞ for SYN-2836 was double that for SYN-2869. The Cmax and the time to reach Cmax (Tmax) were similar for both of these compounds (Fig. 4), but the half-life of SYN-2836 was fourfold lower than that of SYN-2869 in New Zealand White rabbits. On the other hand, there was no difference in the half-lives of SYN-2836 and SYN-2869 in beagle dogs (Table 3).
FIG. 4.
Concentration-versus-time profiles for SYN-2836 and SYN-2869 in New Zealand White rabbits after administration of a single oral dose of 50 mg/kg.
TABLE 3.
Mean values of pharmacokinetic parameters for SYN-2836 and SYN-2869 in plasma of New Zealand White rabbits and beagle dogs
| Parameter | New Zealand White rabbits (n = 3)a
|
Beagle dogs (n = 3)b
|
||
|---|---|---|---|---|
| SYN-2836 | SYN-2869 | SYN-2836 | SYN-2869 | |
| Cmax (μg/ml) | 1.26 ± 0.41 | 1.30 ± 0.28 | 4.82 ± 1.54 | 2.19 ± 1.22 |
| Tmax (h) | 1.33 ± 0.28 | 1.66 ± 0.28 | 1.3 ± 0.3 | 2.5 ± 0.8 |
| AUC (μg · h/ml) | 3.74 ± 1.79 | 10.59 ± 2.7 | 41.83 ± 15.79 | 21.85 ± 7.61 |
| CL/F (ml/min/kg) | 277.1 ± 173.5 | 87.7 ± 23.3 | 15.78 ± 5.7 | 28.8 ± 6.6 |
| Half-life (h) | 1.24 ± 0.67 | 5.73 ± 2.7 | 9.0 ± 0.8 | 8.87 ± 2.05 |
| V/F (liters/kg) | 23.48 ± 2.11 | 37.58 ± 9.9 | 9.56 ± 2.31 | 19.74 ± 10.81 |
Single oral dose of 50 mg/kg.
Single oral dose of 30 mg/kg.
Distribution of new antifungal azoles in lungs and comparison with that of itraconazole in mice after intravenous administration.
The mean AUC0–∞ values for the lung after the administration of SYN-2836 at doses of 10, 20, 35, and 50 mg/kg were between 38.54 and 190.67 μg · h/ml (Table 4), having a linear increase with dose (Fig. 2). The AUC0–∞ for the lung after the administration of a 20-mg/kg dose of SYN-2869 was significantly different from those obtained after the administration of the other azoles tested (Table 4). The ratios of the concentrations of the SYN azoles in the lungs to those in plasma were higher than that for itraconazole (Table 4).
TABLE 4.
Mean values of pharmacokinetic parameters for SYN-2836, SYN-2869, SYN-2903, SYN-2921, and itraconazole in mouse lung after intravenous administration
| Drug (dose [mg/kg]) | AUC (μg · h/ml) | L/P ratioa |
|---|---|---|
| SYN-2836 | 2.73 ± 0.86 | |
| 10 | 38.54 ± 7.4b | |
| 20 | 64.98 ± 8.26b | |
| 35 | 119.44 ± 2.21b | |
| 50 | 190.67 ± 18.21b | |
| SYN-2869 (20) | 128.5 ± 15.5c | 2.32 ± 0.12 |
| SYN-2903 (20) | 100.3 ± 10.78 | 1.79 ± 0.75 |
| SYN-2921 (20) | 66.3 ± 7.31 | 2.65 ± 0.28 |
| Itraconazole | 1.60 ± 0.16 |
L/P ratio, ratio of concentration in lungs to concentration in plasma after intravenous administration.
Data are significantly different from each other.
P < 0.05 versus SYN-2836, SYN-2903, and SYN-2921.
DISCUSSION
Although the mean CL of SYN-2836 in mice after the administration of doses of 10, 20, 35, and 50 mg/kg did not show any difference, the half-life, MRT, AUC0–∞, and VSS after the administration of a dose of 10 mg/kg were significantly different from those achieved after the administration of the rest of the doses. The increase in half-life and the higher V observed between the dose of 10 mg/kg and the rest of the doses may be attributed to the high ratio of the concentration in tissue to the concentration in plasma for this compound. The AUC0–∞-versus-dose plot for SYN-2836 in plasma was found to be linear (r2 = 0.997), indicating no dose dependency of SYN-2836 in plasma in the mouse model over the dose range studied (10 to 50 mg/kg).
In a comparison of the four SYN azoles (namely, SYN-2836, SYN-2869, SYN-2903, and SYN-2921) after the administration of a single intravenous dose of 20 mg/kg to BALB/c mice, significant differences in half-life, CL, AUC, and V were found (Table 2). The significant differences in AUC0–∞ found between SYN-2836 and SYN-2869 on the one hand and SYN 2903 and SYN 2921 on the other may be due to the addition of oxygen at the C-4 position. The effect of oxygen at the C-4 position on CL was different when fluorine was present on the phenyl ring adjacent to the piperazine moiety. In the case of SYN-2836 and SYN-2903, which do not have oxygen at the C-4 position, the addition of fluorine reduced the CL, while the addition of fluorine on the phenyl ring, as in SYN-2869 and SYN-2921, had an opposite effect on CL. In vitro studies on the identification and kinetic characterization of the cytochrome P450 isoforms responsible for the biotransformation of SYN-2836 and SYN-2869 in human liver microsomes found no prominent difference in intrinsic CL (Vmax/Km) between these two compounds from these microsomes (3, 11). Species differences in terms of the intrinsic CL of these compounds and V contributed to the differences in the pharmacokinetic profiles. The significant difference in V between SYN-2921 and the other azoles tested was indicative of a higher ratio of the concentration in tissue to the concentration in plasma, with SYN 2921 being the most lipophilic compound among the four azoles tested.
The Cmaxs of the SYN azoles after the administration of an oral dose of 50 mg/kg to mice varied between 3.41 and 7.31 μg/ml. The observed mean absolute bioavailabilities of the four SYN compounds are well correlated with their relative calculated log P (partition coefficient) values and their ability to interact with surrounding water molecules. Namely, SYN-2836, which has a p-trifluoromethyl group at the terminal benzylic moiety, had a mean absolute bioavailability of 75.8% ± 16.45%, whereas SYN-2869, which has a p-trifluoromethoxy group at the terminal benzylic moiety, had a mean absolute bioavailability of 60.26% ± 22.68%. On the other hand, the mean absolute bioavailabilities of SYN-2903 and SYN-2921, the 3′-fluoro-substituted analogues of SYN-2836 and SYN-2869, respectively, were 45.89% ± 89% and 83.92% ± 6.67%, respectively. The respective calculated log P values for SYN-2836, SYN-2869, SYN-2903, and SYN-2921 are 3.04, 2.88, 3.26, and 3.10. From the data presented above it is evident that replacement of a trifluoromethyl group by a trifluoromethoxy group lowered the lipophilicity and improved the bioavailability in the presence of fluorine, while, on the other hand, the presence of a trifluromethoxy group in the absence of fluorine did not improve bioavailability, with a lowering of the lipophilicity (compare the log P values for SYN-2903 and SYN-2921 with those for SYN-2836 and SYN-2869). It is well known that the addition of fluorine to organic molecules has a dual effect on the physicochemical properties of these compounds. First, it increases the lipophilicity of the molecule, and second, due to the availability of its three lone pairs of electrons, it participates in hydrogen binding with water and attracts more water molecules from the surrounding area. As a result, a significant improvement in the absorption and distribution characteristics of the drug would be expected. This dual effect is not so evident in the case of SYN-2836 and SYN-2903, for which the increase in lipophilicity has lowered the bioavailability, while the effect of fluorine was negligible. Although in the presence of trifluromethoxy the increase in lipophilicity is similar to that in the presence of fluorine, the increase in bioavailability is much higher for SYN-2921 than for SYN-2869 in the presence of fluorine. For these series of compounds, from the CL data obtained, and from a comparison of lipophilicity and absolute bioavailability, the role of the C-4 oxygen seems to be more significant than substitution of a fluorine on the aromatic ring on pharmacokinetics. SYN azoles, especially SYN-2836, showed differences in their pharmacokinetics characteristics in different species, especially in Sprague-Dawley rats (Table 5). Although no significant difference in AUC0–∞ was observed for SYN-2836 and SYN-2869 after the administration of a single intravenous dose of 20 mg/kg to rats, the oral bioavailability of SYN-2836 was extremely low compared to the 14% bioavailability of SYN-2869. The main reason for much the lower bioavailability of SYN-2836 was extensive first-pass biliary excretion of this compound in rats (unpublished data). The Cmaxs and Tmaxs after the administration of a single oral dose of 50 mg/kg to rabbits were similar for SYN-2836 and SYN-2869. A threefold difference in CL is a reflection of the half-life of SYN-2869 in rabbits. The oral bioavailability of SYN-2836 in rabbits was 58%. Significant species differences in the pharmacokinetics has been observed for SYN-2836 and SYN-2869, which were the two compounds evaluated with nonrodent species. For instance, a higher AUC was achieved with SYN-2869 in rabbits, whereas this was reversed in beagle dogs. The CL of SYN-2836 was higher in rabbits, while the CL of SYN-2869 was higher in beagle dogs. In beagle dogs both drugs had the same half-lives. The higher CL from beagle dogs was due to the higher V of SYN-2869. Although these two compounds exhibit the same metabolic pathways in dogs (4, 5) due to their similar structures, the species differences in total body CL had a significant effect on the overall pharmacokinetic parameters, which in turn would affect the efficacy outcome in models consisting of higher animals.
TABLE 5.
Mean values of pharmacokinetic parameters for SYN-2836 in plasma of mice, rats, and rabbits after administration of a single intravenous dose of 20 mg/kg
| Parameters | Mice | Rat | Rabbit |
|---|---|---|---|
| AUC (μg · h/ml) | 25.20 ± 7.9 | 12.01 ± 2.08 | 25.6 ± 15.35 |
| CL (ml/min/kg) | 14 ± 4 | 27.4 ± 4.66 | 9.75 ± 1.36 |
| Half-life (h) | 2.75 ± 0.19 | 4.4 ± 4.4 | 1.9 ± 0.8 |
| MRT (h) | 2.61 ± 0.78 | 2.0 ± 0.76 | 3.2 ± 1.8 |
| VSS (liters/kg) | 2.3 ± 1.13 | 4.05 ± 1.75 | 3.38 ± 0.42 |
The ratios of the concentration in the lungs to the concentration plasma, which were calculated as the ratios of the AUC0–∞ for the lung and to that for plasma, were very similar between SYN-2836, SYN-2869, and SYN-2921, while similar results were obtained with SYN-2903 and itraconazole (Table 4). The higher ratios of the AUC0–∞ for the lungs to that for plasma for the SYN compounds compared with that for itraconazole are indicative of the improved distribution of SYN compounds in the lungs. SYN-2869, on the other hand, for which the AUC for the lung was higher than those for SYN-2836, SYN-2903, and SYN-2921 after administration of the same dose of 20 mg/kg, contributed to the potent efficacy of the compound in a mouse model of Aspergillus fumigatus infection of the respiratory tract (Furukawa et al., 38th ICAAC). In vitro the levels of binding of the SYN compounds to plasma proteins of both rats and mice are >99% but are not different, indicating that differences in pharmacokinetic characteristic are not just due to differences in plasma protein binding but are due to actual changes in free drug CL (unpublished data).
SYN-2869 has a broad spectrum of activity against pathogenic yeasts, molds and dermatophytes (Salama et al., 38th ICAAC) and excellent in vivo efficacy against systemic candidiasis, aspergillosis, cryptococcosis, and invasive pulmonary aspergillosis in relevant murine models (Furukawa et al., 38th ICAAC). Due to the favorable pharmacokinetic profile of SYN-2869, including the excellent distribution pattern in lungs and efficacy in murine models, SYN-2869 was identified from among the four lead compounds as a candidate compound for further efficacy and toxicological evaluation in an appropriate animal model based on the pharmacokinetic profiles generated with different animal species. SYN-2869 is considered a promising new triazole antifungal agent for the treatment of fungal infections, especially invasive aspergillosis.
REFERENCES
- 1.Blachford N R. Treatment of oral candidosis with itraconazole: a review. J Am Acad Dermatol. 1990;23:567–570. doi: 10.1016/0190-9622(90)70256-h. [DOI] [PubMed] [Google Scholar]
- 2.Brammer K W, Farrow P R, Faulkner J K. Pharmacokinetics and tissue penetration of Fluconazole in humans. Rev Infect Dis. 1990;12(Suppl. 3):S318–S326. doi: 10.1093/clinids/12.supplement_3.s318. [DOI] [PubMed] [Google Scholar]
- 3.Bu H Z, Khan J K, Montaseri H, Micetich R G, Daneshtalab M. Identification of the phase I metabolites of novel antifungal agents SYN-2836, SYN-2869, SYN-2903 and SYN-2921 in liver microsomes using electrospray HPLC/MS/MS: interspecies comparison and role of CYP3A4. Pharm Sci. 1998;1(Suppl. 1):S-40. [Google Scholar]
- 4.Bu H Z, Poglod M, Micetich R G, Khan J K. Structure elucidation of three isomeric metabolites of SYN-2836, a novel antifungal agent, in dogs via LC/MS and LC/MS/MS methodologies. J Mass Spectrom. 1999;34:1185–1194. doi: 10.1002/(SICI)1096-9888(199911)34:11<1185::AID-JMS879>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 5.Bu, H. Z., M. Poglod, R. G. Micetich, and J. K. Khan. Novel sample preparation method facilitating identification of urinary drug metabolites by liquid chromatography-tandem mass spectrometry. J. Chromatogr. B, in press. [DOI] [PubMed]
- 6.Cauwenbergh G. Pharmacokinetics of itraconazole. Mycoses. 1994;37(Suppl.):27–33. [PubMed] [Google Scholar]
- 7.Clements J S, Jr, Peacock J E., Jr Amphotericin B revisited: reassessment of toxicity. Am J Med. 1990;88:5-22N–5-27N. [PubMed] [Google Scholar]
- 8.Denning D W, Tucker R M, Hanson L H. Treatment of invasive aspergillosis with itraconazole. Am J Med. 1990;86:791–800. doi: 10.1016/0002-9343(89)90475-0. [DOI] [PubMed] [Google Scholar]
- 9.Gallis H A, Drew R H, Pickard W W. Amphotericin B: 30 years of clinical experience. Rev Infect Dis. 1990;12:308. doi: 10.1093/clinids/12.2.308. [DOI] [PubMed] [Google Scholar]
- 10.Graybill J R. New antifungal agents. Eur J Clin Microbiol Infect Dis. 1989;5:402–412. doi: 10.1007/BF01964056. [DOI] [PubMed] [Google Scholar]
- 11.Khan J K, Bu H Z, Micetich R G, Daneshtalab M. Identification and kinetic characterization of cytochrome P450 isoforms responsible for biotransformation of SYN-2836 and SYN-2869 in human liver microsomes. Pharm Sci. 1998;1(Suppl. 1):S-45. [Google Scholar]
- 12.Khan J K, Montaseri H, Poglod M, Bu H Z, Daneshtalab M, Micetich R G. High performance liquid chromatographic analysis of new triazole antifungal agent SYN-2869 and its derivatives in plasma. J Pharm Biomed Anal. 1999;20:791–797. doi: 10.1016/s0731-7085(99)00080-1. [DOI] [PubMed] [Google Scholar]
- 13.Khan, J. K., H. Montaseri, M. Poglod, H. Z. Bu, M. Daneshtalab, and R. G. Micetich. High-performance liquid chromatographic assay for the determination of novel triazole antifungal agents in tissue. Application to tissue distribution studies. Biomed. Chromatogr., in press. [DOI] [PubMed]
- 14.Le Conte P, Joly V, Saint-Julien L, Gillardin J M, Carbon C, Yeni P. Tissue distribution and antifungal effect of liposomal itraconazole in experimental cryptococcosis and pulmonary aspergillosis. Am Rev Respir Dis. 1992;145:424–429. doi: 10.1164/ajrccm/145.2_Pt_1.424. [DOI] [PubMed] [Google Scholar]
- 15.Saag M S, Dismukes W E. Azole antifungal agents: emphasis on new triazoles. Antimicrob Agents Chemother. 1988;32:1–8. doi: 10.1128/aac.32.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]