Abstract
Stressors can undermine smokers’ attempts to quit smoking. Although contemporary theories and animal models support this idea, human research has struggled to demonstrate definitively the relationship between stressors and smoking. Researchers have employed more ecologically valid methods like ecological momentary assessment to address this question, but studies focusing explicitly on stressors remain sparse and findings inconsistent. The purpose of this study was to examine the effect of stressful event intensity on smoking and craving among cigarette smokers during a quit attempt. We conducted preregistered, complementary concurrent and prospective (i.e., 8-hour lag window between stressful event and outcomes) analyses to maximize statistical power and provide temporal ordering, respectively. We also conducted follow-up moderation (lag X stressful event intensity) analyses. We hypothesized that smokers would be more likely to report both smoking and craving as the intensity of stressful events increased. Cigarette smokers (N=125; 77 male) were randomly assigned to take nicotine replacement therapy (NRT) or placebo and provided 4X daily self-reports during the first 2 weeks of a quit attempt. Stressful events increased craving and the probability of smoking in concurrent analyses, and lag moderated the effect of stressful event intensity in follow-up prospective lagged analyses. NRT reduced the probability of smoking but not craving and did not moderate the effect of stressful events on smoking or craving. This study supports a prospective relationship between stressful events and smoking/craving in situ and demonstrates that NRT does not reduce the impact of stressors on smoking or craving.
Keywords: stress, craving, smoking, ecological momentary assessment, substance use
General Scientific Summary:
Stress has been hypothesized but not confirmed to cause drug use in humans. This study provides evidence that experiencing stressful events increases the probability of smoking and increases craving among cigarette smokers trying to quit.
INTRODUCTION
Clinicians, theorists, and individuals who smoke or use other drugs agree that stress, which we define as an individual’s dynamic, multisystemic adaptation to stressors (Sapolsky, 2015) affects the likelihood of a successful quit attempt. For example, at least three well-supported theoretical frameworks assign a causal role for stress in drug use. Negative reinforcement models posit that drug use reduces the stress response, promoting continued drug use (Baker et al., 2004). Stress neuroadaptation models state that central nervous system stress systems oppose the effects of drug use, and recurring drug use strengthens these stress systems to maintain homeostasis (Koob & Le Moal, 2008). Stress-impaired self-control models explain that stress disrupts prefrontal cortex network connections, which diminishes self-control and makes it difficult for individuals to resist using drugs during stress (Arnsten, 2009; Curtin et al., 2006). Animal models have provided strong evidence of stress-induced reinstatement of self-administration and drug-seeking behaviors in rodents, specifically when the stressor was acute and unpredictable (Mantsch et al., 2016).
The ongoing challenge has been developing valid methodologies to test hypotheses from these theoretical and animal models in humans. Despite strongly held beliefs, research examining the relationship between stress and drug use in humans has not yet convincingly corroborated these theories or animal models. Most research to date has been done in the laboratory to ensure precise manipulations, establish clear temporal ordering, and maintain control over participant environments. Unfortunately, these laboratory studies have often used small sample sizes that decrease confidence in both positive findings due to low positive predictive value and negative findings due to high false negative rates (for recent review of small-N laboratory studies, see Fronk et al., 2020). Even studies with large sample sizes, however, have failed to demonstrate that stressors consistently promote drug use: small effects in a meta-analysis of the effects of laboratory stressors on cigarette smoking were not robust to corrections for publication bias (Heckman et al., 2015), and Pratt and Davidson (2009) did not find evidence of stress-induced drinking.
Recently, psychology has made a concerted effort to use larger sample sizes. However, contemporary ethical considerations regarding promoting relapse among individuals in recovery make conducting “improved” laboratory studies of this phenomenon unlikely, although trial quit designs may be possible in some cases (for example, see McClure et al., 2013). Regardless, increasing statistical validity cannot overcome suboptimal ecological validity that arises from using laboratory stressors like shock administration, unpleasant films/pictures, or aversive noises and from measuring drug use in a contrived environment.
In situ research offers an attractive alternative to study the relationship between stress and drug use. In particular, self-report approaches to ecological momentary assessment (EMA) hold great promise for examining stressors and subsequent relapse because they allow individuals to report on their subjective experiences in the real world in real time (i.e., in situ). These methods give researchers access to ecologically valid stressors and naturalistic environments to observe use patterns. Unlike laboratory paradigms, self-report and other EMA approaches also permit continuous, longitudinal measurement over days to weeks. Given these advantages, it is unsurprising that there is a sizable body of in situ research that examines the relationship between momentary stress or negative affect and drug use (for recent examples, see Potter et al., 2021; Savoy et al., 2020, 2020; for review, see Sayette, 2017). Though common, conceptualizing stress as a momentary, affective state rather than a response to an explicit stressor has prevented researchers from drawing definitive conclusions regarding stressful events because reports of momentary stress or negative affect may instead capture stimulus-independent mood or psychiatric symptoms.
A much smaller but growing body of literature has examined explicit stressors in situ. Even among this small number of studies, there has been considerable variability in how stressors were assessed, ranging from the presence/absence of a stressor (Cronk & Piasecki, 2010; Preston, Kowalczyk, et al., 2018; Preston, Schroeder, et al., 2018; Shiffman & Waters, 2004) to individual stressor severity (Moran et al., 2018; Preston et al., 2017; Serre et al., 2018; Volz et al., 2014). Analysis strategies have also varied. Most studies have focused solely on concurrent data (i.e., stressor and drug use responses reported at the same time), making it difficult to demonstrate temporal ordering. Some prospective research has been conducted, but the variation in lag between stressor report and craving/drug use report is quite large, with measurement of craving/use occurring approximately 6 hours post-stressor (Cambron et al., 2019, 2020), the same evening (Armeli et al., 2007), the next day (Cronk & Piasecki, 2010; Neupert et al., 2017; Shiffman & Waters, 2004) or within 72 hours (Furnari et al., 2015). Methodological variation at this stage is both appropriate and beneficial to characterize fully the stressor-drug use relationship. However, when trying to aggregate across conflicting findings, it remains unclear whether inconsistencies reflect methodological variation or an unstable/non-existent effect.
We sought to make reasoned decisions based on the existing literature to address this variation. First, we studied the relationship between stressors and drug use in daily cigarette smokers. Although we expect that the stressor-drug use relationship exist for all substances, cigarette smokers are a particularly relevant population because there are currently 34 million smokers among U.S. adults alone (CDC, 2019). Additionally, ease of access to cigarettes enables dense behavior (i.e., frequent smoking), which may make it an excellent sample for temporally powerful tests. Participants in our sample were engaged in a real-world quit attempt and were randomly assigned active or placebo combination nicotine replacement therapy (NRT). This allowed us to examine stressor effects during a sensitive recovery period and to determine whether treatment would affect the magnitude of stressor effects.
Second, we chose to look explicitly at stressors rather than negative affect as described previously. This assessment strategy was guided by our conceptualization of stress, which specifically includes responding to an explicit stressor rather than reporting on momentary distress. Following existing in situ research that has examined stressor intensity (see above) as well as conclusions from basic stress research regarding the importance of stressor characteristics like intensity (Sapolsky, 2015), we measured stressor intensity on a scale that ranged from “no stressful event” through “extremely stressful event.”
Third, we focused on smoking as our primary outcome given the press for understanding the relationship between stressful events and drug use specifically, which is more difficult to study in the laboratory given ethical and burden constraints. However, we examined craving as a secondary outcome because craving is tightly connected to relapse, often precedes use, and can itself be very disruptive to individuals in recovery.
Fourth, we conducted concurrent and prospective analyses. These two analytic timeframes offer potential complementary strengths to characterize more fully the relationship between stressors and both smoking and craving. Concurrent analyses allowed us to use all valid EMA self-reports, whereas the prospective analyses allowed us to establish clear temporal ordering that is necessary (though not sufficient) to demonstrate a causal relationship. Fifth, for our prospective analyses, we focused a priori on a lag window of up to 8 hours for measuring smoking/craving following the stressful event. We chose a window that was shorter than most in the existing literature (most studies lag to next-day reports at least, as described above regarding methodological variation) to limit to when stressors retain strong influence on smoking and craving, yet long enough to allow for opportunities to smoke despite constraints (e.g., no-smoking policies at work). Our preregistered analyses involved a coarse, windowed approach (i.e., aggregating across all lag durations between stressors and outcomes up to 8 hours). However, we conducted additional analyses that included the specific lag associated with each outcome report as a predictor in the analysis model. This allowed us to test formally for moderation of the magnitude of the stressor-smoking/-craving relationship by the more precise prospective lag associated with each report.
We hypothesized that smokers would display increased probability of smoking and increased craving as stressful event intensity increased. We expected this pattern of results in both concurrent and prospective (8-hour lag window) analyses. We also focused a priori on the stressful event intensity X treatment condition (combination NRT vs. placebo) interaction for both smoking and craving, but we did not offer any hypotheses about this interaction. In follow-up analyses, we formally tested for a stressful event intensity X prospective lag interaction and reported simple effects at lags of 1, 2, 4, 6, and 8 hours.
METHOD
Open Science and Preregistration
We value the principles of research transparency that are fundamental to the robustness and replicability of science and took several steps to follow emerging open science guidelines (Schönbrodt et al., 2015). We preregistered study-specific data exclusion criteria, operational definitions for predictors and outcomes, and our data analysis plan prior to the start of data analyses at the Open Science Framework (OSF; https://osf.io/y6d5k). We reported how we determined our sample size, all data exclusions, all manipulations, and all available measures in the study (Simmons et al., 2012). We completed a transparency checklist (see Supplement; Aczel et al., 2019). Finally, we made the data, analysis scripts and annotated results, questionnaires, and other study materials associated with this report publicly available (https://osf.io/8ycbn).
Participants
We used EMA self-reports from participants recruited from the Madison, WI (USA) community via online, television, and print advertisements as part of a grant-funded project from which these data were drawn1. As specified by the grant-funded project’s inclusion criteria, participants were required to:
smoke daily for ≥2 years
smoke ≥10 cigarettes per day
smoke within 30 minutes of waking up
display an expiratory carbon monoxide ≥6 parts per million (ppm) at the screening session
report motivation to quit smoking (moderate or higher on ordinal scale)
demonstrate successful, biologically confirmed (via expiratory carbon monoxide of <10 ppm) abstinence following their scheduled quit date
Furthermore, participants in that project were required to report no:
uncorrected auditory or visual problems
colorblindness
pregnancy
current or past month use of any psychiatric medication
severe or persistent mental illness
medical conditions that would contraindicate NRT treatment
medical or psychiatric conditions that would contraindicate exposure to electric shock administration (e.g., cardiac disorders, fibromyalgia, chronic pain).
Participants who did not meet these criteria were not enrolled in the grant-funded project. Additional data exclusions for the current study followed our preregistration (https://osf.io/y6d5k). Specifically, we excluded participants on a per-analysis basis who:
did not provide responses to ≥8 post-quit EMA self-reports.
did not report ≥2 (non-zero) stressful events across all their post-quit EMA self-reports.
were assigned to the placebo condition but reported that they purchased and used their own NRT during the two-week post-quit period (N=1)
This resulted in a full sample of 125 participants who met grant inclusion criteria and were included in at least one primary analysis (see Analysis Plan). In addition, we excluded individual EMA self-reports if they were incomplete (0 reports) or if they took >5 minutes to complete from the first to the final survey question (142 reports).
We compensated participants $20/hour for study visits, $0.50/EMA self-report they completed, and a $50 bonus for completing >90% of all reports. Participants also received a free 8-week supply of combination NRT (patches and lozenges) as part of their study compensation regardless of their treatment assignment. Participants assigned to placebo during the study received their active combination NRT at study completion.
General Procedure
During a screening visit, participants signed consent forms following a detailed description of all study requirements and procedures, and enrollment inclusion criteria were confirmed2. Participants completed a battery of self-report questionnaires on an iPad (Apple Inc.) using Qualtrics software (Provo, UT) to assess demographic information, smoking history and dependence, and other characteristics3. Participants then scheduled their quit date and received verbal and written instructions about the 3 weeks of EMA self-reports that began one week before their scheduled quit date.
Participants were randomly assigned between-subjects to one of two medication groups – active or placebo combination NRT (i.e., using nicotine patches and ad libitum lozenges) – using a single-blind procedure4. Participants in the active NRT condition (n=69) received combination NRT consisting of 21 mg nicotine patches and 2 mg nicotine lozenges. Participants in the placebo condition (n=66) received clear Polyethylene Terephthalate laminated patches and Altoids breath mints as placebos. All participants received the same instructions on how to use the patch and lozenges (consistent with the description on the package inserts). We instructed participants to report any side effects promptly so that steps could be taken to facilitate effective medication use.
All participants received two, 30-minute smoking cessation counseling sessions. This counseling emphasized identifying triggers for smoking, developing behavioral strategies to cope with urges, making plans to change one’s environment to reduce urges, and enhancing distress tolerance.
Self-Report Ecological Momentary Assessments
Participants provided 3 weeks (1 week pre-quit, 2 weeks post-quit) of brief 4x daily, real-time self-reports via web surveys in Qualtrics5. Only post-quit reports were used to test the present study hypotheses. This comprised 6,001 completed reports across 125 participants. These EMA self-reports were designed to capture participants’ experiences distributed throughout the day during the first 2 weeks of their quit attempt. Participants completed each report on a smartphone following a text message request that contained a link to a web survey. The four report requests came at the participant’s normal wake-up time, before midday, after midday, and at the participant’s normal bedtime. All requests were separated by at least one hour6.
During each report, participants reported the occurrence and intensity of any hassle or other stressful event since their last report on a five-point scale (anchors: “No Stressful Event” to “Extremely Stressful Event”). If more than one stressful event occurred, participants were asked to rate the most intense event. These reports of stressful event intensity served as the focal predictor in our analyses. During each report, participants also used five-point scales to report the current intensity of any craving (anchors: “Not At All” to “Extremely”) and the number of cigarettes smoked (anchors: “0” to “4 or more cigarettes”) since their last self-report. Smoking was dichotomized (Yes or No) because we were interested in whether a lapse occurred rather than the quantity smoked as per our pre-registration. Smoking and craving served as outcomes in our analyses.
Analysis Plan
Preregistered analyses.
For each outcome, we conducted concurrent and prospective analyses. In concurrent analyses, the measurements of stressful event intensity and associated smoking and craving were obtained from the same report. Applying the preregistered data exclusions yielded 6,001 pairs of responses to the stressful events, craving, and smoking questions across N=125 participants for concurrent analyses. As such, concurrent analyses potentially offered high power.
For prospective analyses, the measurements of smoking and craving were obtained from subsequent reports that were submitted within a preregistered, 8-hour lag window following the target (i.e., preceding) reports where stressful event intensity was reported. Smoking and craving were each aggregated across any reports after the target stressful event assessment up to 8 hours following that same target report.
Preregistered prospective analyses using the 8-hour lag window were limited to 3,684 pairs of responses to the stressful events, smoking, and craving questions across N=119 unique participants because no response regarding smoking and craving was available within 8 hours following responses to some stressful event questions (due to periods of sleep and missing reports). Despite this smaller sample size, temporal ordering of presumed cause and effect could be more clearly established because reports of stressful events preceded smoking and craving.
We evaluated our study hypotheses with linear mixed models and generalized mixed models implemented via the lme4 package in R. Separate models were estimated for concurrent and prospective analyses for smoking (within a generalized mixed model for binomial outcomes) and craving (within a linear mixed model). All models included fixed effects for stressful event intensity (level 1/event level; mean-centered within subjects following recommendations by Brauer and Curtin (2018)), treatment condition (level 2/person level; NRT coded as 0.5 and placebo coded as −0.5), and their interaction. All models included by-subject random intercepts and slopes for stressful event intensity. We report the model coefficients (Bs) and odds ratios (ORs) to quantify effect size of the fixed effects where appropriate. We tested fixed effects in linear mixed models using type III F tests with Kenward-Roger degrees of freedom approximation and in generalized mixed models using the Wald test.
All parameter estimates were derived using the bobyqa optimizer; however, we compared results across available optimizers and conducted alternative recommended analyses (e.g., alternative random effects structures, Bayesian approaches) to confirm that our results were robust across optimizers and to rule out any concerns regarding convergence or singular fits (see Supplement; Barr, 2013; Chung et al., 2013; McElreath, 2020). These additional analyses identified the same pattern of significant results as our primary analyses in all instances.
Post-hoc power analyses.
We quantified power across a range of meaningful effect sizes in post-hoc power analyses for preregistered concurrent and prospective (8-hour lag window) analyses. Power was greater than 94% in all instances. Details on these power analyses are reported in the Supplement.
Follow-up analyses.
We conducted follow-up prospective analyses for both smoking and craving where we explicitly tested for moderation by the precise lag (rather than an 8-hour aggregated lag window) associated with each pair of stressful event intensity and outcome measurements. We paired each target report of stressful event intensity with the next report of smoking and craving, up to 12 hours following the stressful event report, and recorded the specific lag (time between measurements in hours). This resulted in a dataset with 5,216 pairs of observations across 125 participants that maintained the prospective temporal ordering. We tested the stressful event intensity X lag interaction by adding fixed effects for lag and the stressful event intensity X lag interaction to the fixed effects used in all previous analyses. We also added by-subject random slopes for lag and the stressful event intensity X lag interaction. Lag was mean-centered within subjects.
RESULTS
Key Sample Descriptive Statistics
Demographic and smoking history characteristics for the full sample (N=125) appear in Tables 1 and 2, respectively. Frequencies and percentages for reports of stressful event intensity, craving, and smoking appear in Table 3.
Table 1.
Participant Demographics
| Characteristic | Percentage(N) |
|---|---|
| Age* | 40.05(11.77) |
| Gender | |
| Male | 61.6%(77) |
| Female | 37.6%(47) |
| Other | 0.8%(1) |
| Race | |
| White | 68.8%(86) |
| Black or African American | 24.8%(31) |
| Multiracial | 0.8%(1) |
| American Indian | 2.4%(3) |
| Asian | 0.8%(1) |
| Unreported | 2.4%(3) |
| Ethnicity | |
| Non-Hispanic | 98.4%(123) |
| Hispanic or Latinx | 1.6%(2) |
| Treatment Group | |
| Active NRT | 52.0%(65) |
| Placebo | 48.0%(60) |
Values associated with age represent mean and standard deviation.
Table 2.
Smoking Individual Differences
| Characteristic | Mean(SD) |
|---|---|
| Current Cigarettes Per Day | 17.71(5.64) |
| Time to First Daily Cigarette(minutes) | 10.50(8.36) |
| Age of First Cigarette | 17.12(4.01) |
| Number of Years Smoking Daily | 18.90(11.00) |
| Number of Years Considering Quitting | 4.53(7.32) |
| Number of Past Quit Attempts | 4.90(6.35) |
| Current Motivation to Quit(0–10) | 9.28(1.02) |
| FTND Total Score | 5.60(1.31) |
| WISDM Total Score | 50.26(12.79) |
FTND=Fagerstrom Test of Nicotine Dependence; WISDM=Wisconsin Inventory of Smoking Dependence Motives
Table 3.
Dataset Characteristics
| Reported Value | Concurrent Analyses %(N) |
8-Hour Prospective Windowed Analyses %(N) |
Prospective Lag Moderation Analyses %(N) |
|---|---|---|---|
| Stressful Event Intensity | |||
| 0(No Stressful Event) | 64.2%(3851) | 63.1%(2326) | 64.3%(3776) |
| 1(Mildly Stressful) | 18.1%(1084) | 18.3%(676) | 18.0%(1057) |
| 2(Moderately Stressful) | 10.6%(634) | 10.8%(399) | 10.6%(620) |
| 3(Very Stressful) | 4.5%(271) | 4.9%(180) | 4.5%(264) |
| 4(Extremely Stressful) | 2.7%(161) | 2.8%(103) | 2.7%(159) |
| Craving | |||
| 0(Not At All) | 28.6%(1718) | 28.0%(1030) | 28.9%(1698) |
| 1(Mildly) | 30.4%(1822) | 30.3%(1115) | 30.4%(1784) |
| 2(Moderately) | 23.3%(1400) | 22.9%(842) | 23.3%(1368) |
| 3(Very) | 10.3%(618) | 10.7%(395) | 10.2%(600) |
| 4(Extremely) | 7.4%(443) | 8.2%(302) | 7.2%(426) |
| Smoking | |||
| 0(No) | 81.2%(4874) | 81.8%(3013) | 81.7%(4798) |
| 1(Yes) | 18.8%(1127) | 18.2%(671) | 18.3%(1078) |
Percentages and counts of reported values for stressful event intensity, craving, and smoking in datasets used for concurrent analyses (left), 8-hour windowed analyses (middle), and prospective lag moderation analyses (right).
Preregistered Concurrent and Prospective (8-hour lag window) Analyses
Concurrent smoking.
As predicted, the fixed effect of stressful event intensity was significant for concurrent smoking (OR=1.62, z=6.95, p<0.001). As participants reported greater intensity of stressful events, there was a higher probability that they would report smoking in the same EMA. The fixed effect of treatment condition was significant (OR=0.31, z=−2.93, p=0.003), with lower probability of smoking among participants receiving NRT than those in the placebo condition. There was not a significant treatment condition X stressful event intensity interaction (p=0.297).
Concurrent craving.
As predicted, there was a significant fixed effect of stressful event intensity for concurrent craving (B=0.22, F(1,114.86)=103.61, p<0.001). As participants reported greater intensity of stressful events, they also reported stronger craving in the same EMA. Neither the fixed effect of treatment condition (p=0.126) nor the treatment condition X stressful event intensity interaction (p=0.659) was significant.
Prospective smoking.
The fixed effect of stressful event intensity was marginal for prospective smoking across the 8-hour window (OR=1.24, z=1.79, p=0.073), with descriptively but not significantly higher probability of smoking over the subsequent 8 hours following report of greater stressful event intensity. The fixed effect of treatment condition was significant (OR=0.19, z=−3.13, p=0.002), with lower probability of smoking among participants receiving NRT vs. placebo. There was not a significant treatment condition X stressful event intensity interaction (p=0.631).
Prospective craving.
The fixed effect of stressful event intensity was not significant for prospective craving across the 8-hour window (p=0.179). Neither the fixed effect of treatment condition (p=0.153) nor the treatment condition X stressful event intensity interaction (p=0.843) was significant.
Follow-up Analyses of Lag Moderation Effects
The contrast of significant effects observed among concurrent analyses vs. marginal or non-significant effects during prospective window analyses could have resulted from the coarse aggregation of our outcomes across too long of a lag window between the target measurement of stressful event intensity and subsequent smoking/craving. Our follow-up moderation analyses used more precise, specific lags to address this concern.
Prospective smoking.
The stressful event intensity X prospective lag interaction fixed effect was significant for prospective smoking (OR=0.94, z=−2.06, p=0.040).7 The magnitude of the stressful event intensity fixed effect on smoking probability decreased with increasing lag between stressor and smoking measurements. To explore this interaction, we tested simple fixed effects of stressful event intensity at discrete lags (1, 2, 4, 6, and 8 hours; see Figure 1). The simple fixed effects of stressful event intensity on prospective smoking were significant for lags of 1 hour (OR=1.55, z= 2.87, p=0.004), 2 hours (OR=1.46, z=2.87, p=0.004), and 4 hours (OR=1.29, z=2.45, p=0.014). As participants reported greater intensity of stressful events, there was a higher probability that they would also report smoking when smoking was measured at these lags. In contrast, the simple fixed effects of stressful event intensity were not significant for 6-hour (p=0.246) and 8-hour (p=0.995) lags. Active NRT treatment reduced the likelihood of smoking (OR=0.25, z=−2.77, p=0.006), but the treatment X stressful event intensity interaction was not significant (p=0.334).
Figure 1. Prospective smoking by stressful event intensity and lag.
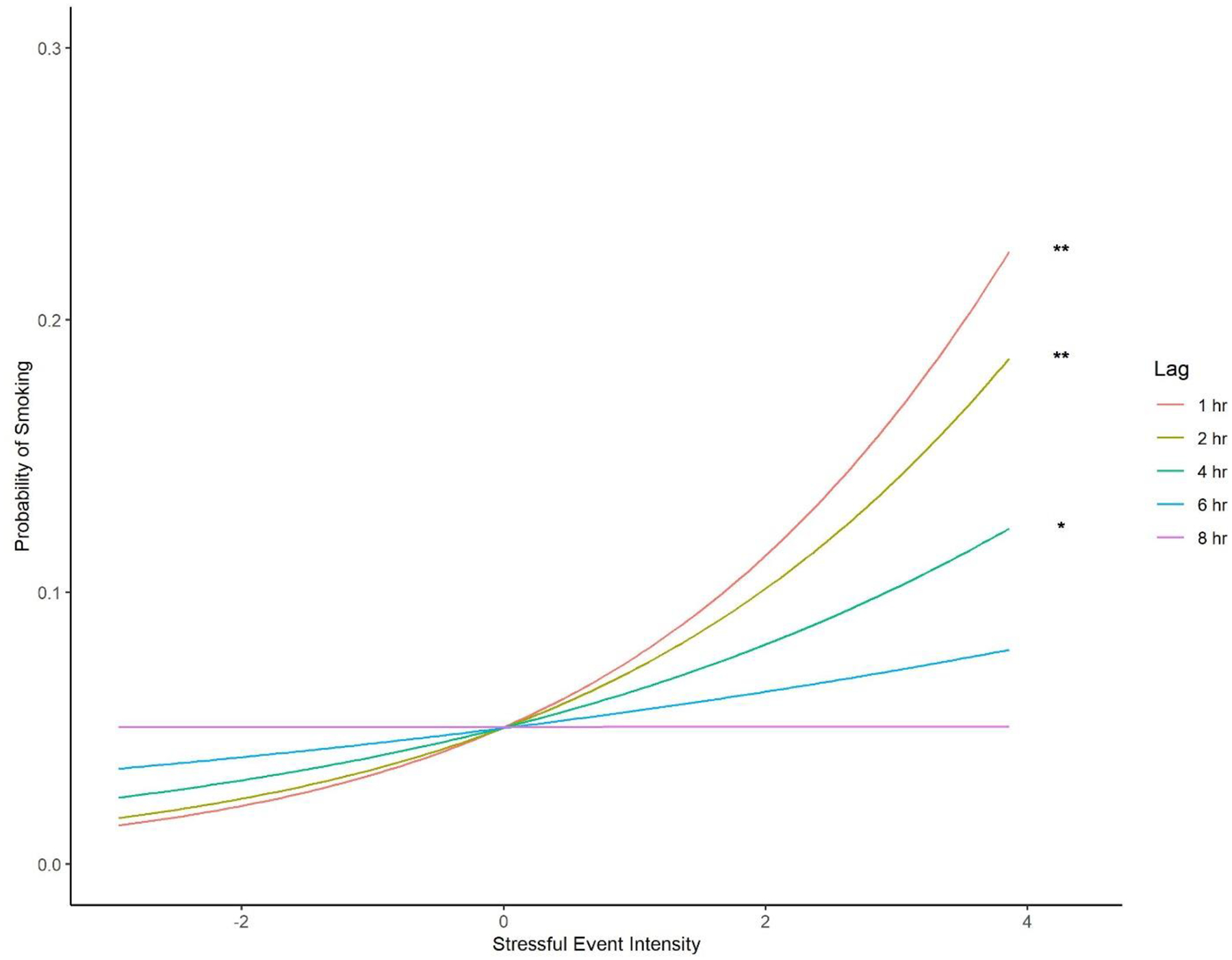
Simple fixed effects of stressful event intensity on smoking by lag from the lag moderation analyses. Y-axis indicates probability of smoking. X-axis depicts person-mean-centered stressful event intensity. Asterisks indicate significant simple fixed effects (*p<.05; **p<.01).
Prospective craving.
The stressful event intensity X lag interaction fixed effect was significant for prospective craving (B=−0.01, F(1,69.76)=4.81, p=0.032).8 The magnitude of the stressful event intensity fixed effect on craving decreased with increasing lag between stressor and craving measurements. To explore this interaction, we tested simple fixed effects of stressful event intensity at discrete lags (1, 2, 4, 6, and 8 hours; see Figure 2). The simple fixed effects of stressful event intensity on prospective craving were significant for lags of 1 hour (B=0.07, F(1,96.17)=4.74, p=0.032) and 2 hours (B=0.06, F(1,99.22)=4.36, p=0.039). As participants reported greater intensity of stressful events, they reported stronger craving when craving was measured at these lags. In contrast, the simple fixed effects of stressful event intensity were not significant for 4-hour (p=0.093), 6-hour (p=0.402), and 8-hour (p=0.805) lags. Neither the main effect of treatment (p=0.132) nor the treatment X stressful event intensity interaction (p=0.702) was significant.9
Figure 2. Prospective craving by stressful event intensity and lag.
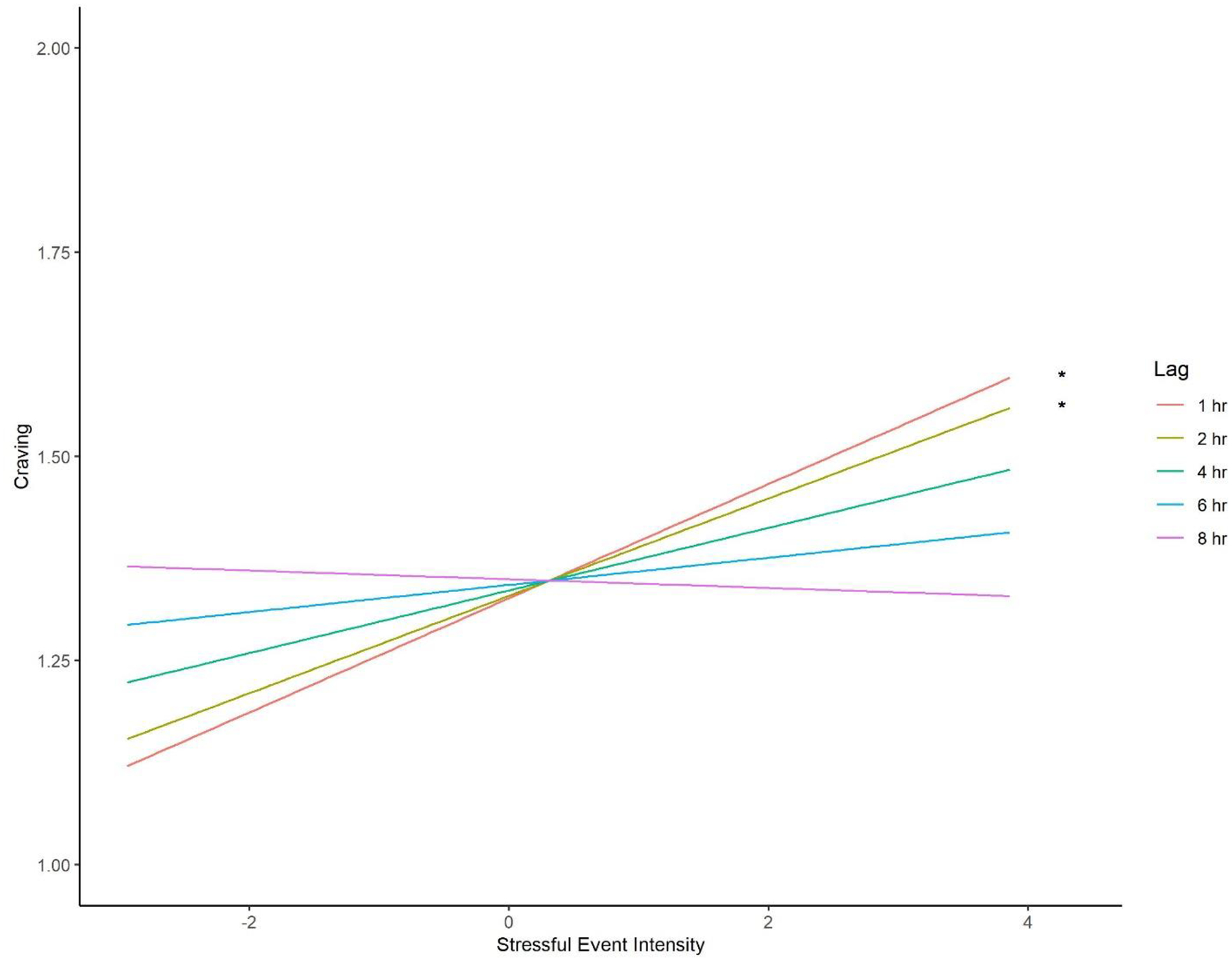
Simple fixed effects of stressful event intensity on craving by lag from the moderation analyses. Y-axis indicates craving, measured on an ordinal scale from no craving (0) to extreme craving (4). Visible anchors of 1 and 2 correspond to mild and moderate craving, respectively. X-axis depicts person-mean-centered stressful event intensity. Asterisks indicate significant simple fixed effects (*p<.05).
DISCUSSION
We found clear evidence that increases in stressful event intensity precede smoking and craving in situ. We delineated the temporal characteristics of these effects; specifically, the effects of stressful events on both smoking and craving were short-lived. We discuss our findings and their implications, and we review the strengths and limitations of the present study to inform and improve future research.
There were strong concurrent relationships between stressful events and smoking/craving. These findings align closely with existing evidence: concurrent in situ studies have consistently shown that stressors are related to both craving (Moran et al., 2018; Neupert et al., 2017; Preston et al., 2017; Serre et al., 2018; Volz et al., 2014) and drug use (Neupert et al., 2017; Preston, Kowalczyk, et al., 2018). We replicated these findings in cigarette smokers with well-powered, preregistered analyses.
Concurrent analyses are suboptimal, however, because they cannot establish temporal ordering. Although there may be theoretical benefit to showing that stressors, craving, and smoking or other drug use are meaningfully related, concurrent analyses in the laboratory or in situ can establish neither causality nor directionality. We need prospective analyses to demonstrate temporal ordering as a critical first step towards establishing causation.
Our prospective lag moderation analyses demonstrated this expected temporal ordering. Increases in stressful event intensity preceded increased probability of smoking and increased craving as would be expected if stressors caused these outcomes. However, the duration of the lag clearly moderated these effects. The strongest effects of stressful event intensity on smoking and craving occurred when the lag between the measurement of stressful event intensity and subsequent outcomes was shortest. Furthermore, the simple effects of stressful event intensity were not significant beyond 4 hours for smoking and 2 hours for craving. These prospective analyses establish stressors as a potential cause of smoking and craving but draw a clear temporal boundary on the duration of their effects.
These findings contribute meaningfully to prospective work examining the relationship between stressors and drug use/craving. Existing research attempting to establish temporal ordering has primarily been conducted in the laboratory. Well-powered laboratory research has generally demonstrated that stressors promote craving but has not yet convincingly or consistently demonstrated that stressors promote drug use (for recent review of laboratory literature, see Fronk et al., 2020). These inconsistent findings from laboratory research may have resulted from several limitations that are reduced or removed in our in situ study.
First, older laboratory studies were primarily conducted with small sample sizes, and contemporary ethical constraints surrounding the instigation of drug use in abstinent users make it unlikely that new, well-powered laboratory research will be conducted (but see McClure et al., 2013). By conducting our study in situ, we were able to follow a larger cohort of cigarette smokers during recovery without ethical concerns. Consequently, our study was extremely well-powered as measured in post-hoc power analyses for our preregistered analyses, thereby increasing its statistical validity.
In situ research provides additional benefits with respect to ecological validity. Laboratory research must not only administer stressors in a contrived environment but also use stressors that are titrated to be stressful for the average participant. Thus, some individuals may experience the laboratory manipulation as extremely stressful, whereas others may not find the manipulation stressful at all. In contrast, participants in the present study reported on events that they experienced in their natural environments and that were personally, subjectively stressful. Moreover, we were able to measure explicitly those stressors’ subjective intensity to capture that variation across individuals and stressors. Similar advantages with respect to ecological validity were present with respect to measuring smoking lapses in situ. Rather than smoking in a contrived, artificial laboratory setting, participants in the present study could smoke if, when, how, and where they wanted without the potential further influence of experimenter observation.
Ecological validity was further bolstered by measuring the effect of stressors on smoking and craving during a real-world quit attempt. This is important for two reasons. First, the impact of stressors may be stronger during the first few weeks after quitting smoking compared to before quitting (McCarthy et al., 2006). Thus, examining this relationship during the early weeks of a quit attempt offers a powerful window for observing the effect of stressors on smoking and craving. Second, anticipation of future smoking is sufficient to reduce responses to laboratory stressors among smokers (Bradford et al., 2015), suggesting that individuals in a brief (e.g., 24-hour) smoking deprivation study may not experience the same responses to stressors as individuals in a quit attempt, where there is no intention to smoke again.
Our in situ measurement strategy also allowed us to examine prospective effects with greater sampling density and over a longer timeframe than would be possible in the laboratory. We were able to assess the effect of stressful events on smoking and craving for prospective lags as short as 5 minutes but also up to 12 hours, with a relatively uniform distribution of lags through this broad window. We were also able to sample over up to 2 weeks of the quit attempt. Comparable measurement in the laboratory would have required significant participant burden (e.g., long laboratory sessions and many visits across 2 weeks).
Our in situ EMA approach addressed many of the above limitations associated with laboratory research. Thus, it is not surprising to us that research examining the effects of explicit stressors on smoking/craving has turned to these methods. In situ research that tests for prospective relationships between stressful events and smoking/craving holds considerable promise to balance trade-offs between statistical, ecological, and internal validity. Despite considerable in situ research examining momentary stress/negative affect and drug use/craving (Sayette, 2017), however, prospective in situ research examining explicit stressors remains nascent, and findings to date have been inconsistent. Our study adds to this emerging body of research and offers several explanations for previous inconsistent findings.
Most existing prospective research has considered long lags between reports of stressors and reports of smoking/craving. Many studies have lagged reports by at least a full day (Cronk & Piasecki, 2010; Neupert et al., 2017; Shiffman & Waters, 2004) and up to 72 hours (Furnari et al., 2015). Furthermore, prospective studies have generally used a windowed measurement approach (like our preregistered analysis strategy) where outcomes (e.g., smoking/craving) were aggregated across all lag durations up to these long upper limits. These broad measurement windows are inherently coarse and may mask short-lived effects of stressful events. In contrast, our moderation analyses allowed us to examine more precise, specific lags between reports of stressful events and reports of smoking/craving. Exploring precise lags rather than windowed, aggregate, or otherwise coarse measurement was key to delineate that the prospective effect of stressful events on both smoking and craving is short-lived.
We also chose to measure stressor intensity explicitly. Basic stress research has established the importance of characteristics such as intensity for determining an individual’s response to that stressor (Sapolsky, 2015). Additionally, evaluating intensity represents a key component of the stressor appraisal process, which has also been shown to affect responding (Lazarus, 1999). These stressor characteristics have been haphazardly considered in studies of the stressor-drug use relationship, and we have advocated recently for their more consistent inclusion (Fronk et al., 2020).
In summary, the current study offers several strengths that lend credence to our findings. Our study was well-powered with high statistical validity. It had high ecological validity as evidenced by focus on naturalistic stressors and smoking as well as measuring the stressor-smoking relationship during a true quit attempt. We established temporal ordering via prospective analyses. We clarified the duration of effects: measuring in situ allowed for longer and denser sampling than can happen in the laboratory, and our lag moderation analyses allowed for tighter, more precise measurement than most previous prospective in situ research. We also followed recommendations from basic stress research to tap stressor appraisal processes. Finally, we took many steps to maximize transparency and rigor of our research as described in the method.
Despite these strengths, we want to acknowledge several limitations, which we frame as opportunities for future research. Perhaps most apparent is that our lag moderation analyses, from which we draw primary conclusions regarding temporal ordering of stressful events and outcomes, were not preregistered. We believe these moderation analyses were the correct analytic approach and that the preregistered windowed analyses, which ignored lag duration, were imprecise and thus suboptimal; however, some caution is warranted.
Future research must measure precise lags not only to replicate the present findings but also to extend findings to other drugs. We need to examine how the timeframe for these effects may vary across drugs that differ with respect to their availability (e.g., licit vs. illicit drugs, cost, prescription vs. over-the-counter), pharmacological action (e.g., latency of psychoactive rise, duration of effect), and social/occupational acceptance (e.g., able to be used publicly, impairment during work). These differences may increase the duration of lag between the stressful event and drug use (e.g., an individual has to wait to drink until after work) or may eliminate the relationship entirely (e.g., by the time an individual can gain access to an illicit drug, they no longer feel subjective distress). Relatedly, asking participants about experiences since last report produces inherent noise in the lag duration that may be improved in future research with more frequent or precise assessment timing.
We also acknowledge the poor demographic representation in our sample. Although Black (27.4%) participants were relatively well-represented, we had poor representation from individuals of other minoritized backgrounds including bi- or multiracial individuals and individuals of Hispanic or Latinx descent. Using coarse racial and ethnic categories in demographic self-report data may also have obscured important variation in our sample, and our failure to capture other important aspects of identity (e.g., sexual orientation, limited options for gender identity) may have prevented us from exploring effects of intersectional identities. Consequently, our findings warrant caution as they may not generalize to individuals who identify with racial, ethnic, gender, and/or sexual orientation backgrounds that were not well-represented or well-characterized in our sample. We are committed to addressing and improving this ongoing issue in psychological science in future research from our laboratory.
The present study had several assessment-related limitations. We failed to measure stressor characteristics beyond intensity. Future research should incorporate characteristics known to affect stressor responses such as stressor duration and chronicity (Segerstrom & Miller, 2004), predictability (Kaye et al., 2017; Weiss, 1972), the specific type of stressful event (Cohen et al., 2019), and controllability (Weiss, 1972). Parsing boundary conditions of the effect of stressors on craving and drug use will be critical not only for understanding these relationships but also for identifying targets in psychosocial treatments for substance use disorders.
We also measured stressful event intensity via participants’ subjective reports. Individual differences in personality (e.g., neuroticism) or psychiatric disorders may affect participants’ appraisal of stressor intensity, including whether an event was considered stressful at all. We attempted to minimize between-person variation by centering stressful event intensity around each person’s mean intensity but this cannot fully account for individual differences. Furthermore, individual difference may influence the degree to which participants focused on internal experiences (e.g., rumination about past events) rather than discrete, external stressors (e.g., Peeters et al., 2003). Future research could explicitly examine the role of key individual difference moderators of stressor appraisal.
We also assessed discrete smoking lapses rather than full relapse to smoking. Future research will be needed to operationalize when smoking behavior transitions from lapse to relapse, clarify how to make that distinction within in situ research, and examine the effect of stressful events on the probability of relapse rather than lapse.
Finally, we relied solely on self-report EMA in the present study to capture subjective stressor appraisal. Self-report measures have been the most common way that psychological scientists have ventured into EMA. However, we are poised as a field to explore a wider array of EMA in situ approaches. Advances in wearable technologies may allow passive measurement of stress (e.g., via autonomic nervous system and other physiologicla responses; for recent example, see Nakajima et al., 2020) and drug use (e.g., via transdermal sensing of alcohol excretion or gait analysis to indicate impairment). These assessment strategies may help to capture the other systems (e.g., autonomic nervous system) beyond subjective appraisal that make up a multisystemic stress response. We may also be able to take advantage of text content in SMS messages, social media posts, or phone calls with natural language processing approaches that can discern stress- or drug-related words. These passive approaches increase sampling density while lowering participant burden, which could permit more precise assessment of the temporal characteristics of key causal effects (e.g., lags measured in minutes rather than hours). They would also support longer assessment periods to monitor drug use and recovery (e.g., fluctuations in stressor-induced drug use across periods of use, abstinence, and relapse).
The statistical significance of our concurrent and prospective (lag moderation) findings establish that explicit stressors, smoking, and craving are meaningfully related and thus support the core thesis shared by the theories of stress and addiction that motivated this work. However, statistical significance does not necessarily imply practical importance. Consequently, we dedicate our final observations to the clinical significance and implications of our findings.
The effects of stressful events on smoking in the first few hours following stressor report were small but likely still clinically meaningful (Chen et al., 2010). These effects hovered at odds ratios of about 1.5, meaning that the odds of smoking (vs. not smoking) increased by 50% for each unit-increase in stressful event intensity (e.g., from “No stressful event” to “Mildly stressful event”). In contrast, the effect sizes for craving at lags of 1 and 2 hours indicate only a quarter-point increase in craving on our five-point ordinal scale if a participant experienced an extremely stressful event vs. “No stressful event”. It is possible, however, that these effects were diminished because all participants received smoking cessation counseling that was not designed to be stress-specific but did contain stress-relevant components. The stressor-smoking/-craving relationship may be stronger still in real-world contexts where people rarely receive counseling.
Beyond issues of absolute effect size magnitude, the pattern of effect size change over the duration of the lag depicted in Figure 3 is also clinically meaningful. The consistent decrease in effect size magnitude from concurrent smoking analyses through each of the specific prospective lags suggests that the magnitude of the concurrent effect likely reflects the same processes present in the prospective analysis, only at an even shorter lag. In contrast, the large, discontinuous reduction in effect size magnitude between concurrent and prospective analyses for craving suggests that the strong concurrent effect reflects both causal and reverse causal relationships between stressors and craving. In other words, in addition to stressors causing craving, it may be that the concurrent analyses also detected craving’s effect on subsequent reports of stress; that is, participants reported the experience of a stressful event because they craved a cigarette. This might represent post-hoc rationalization on the part of the participant (e.g., “I’m really craving a cigarette – I must be stressed!”), or it might reflect that craving itself is a stressful event (e.g., aversive craving experience, uncertainty about ability to fight urges).
Figure 3. Effect size for stressful event intensity on smoking and craving by lag.
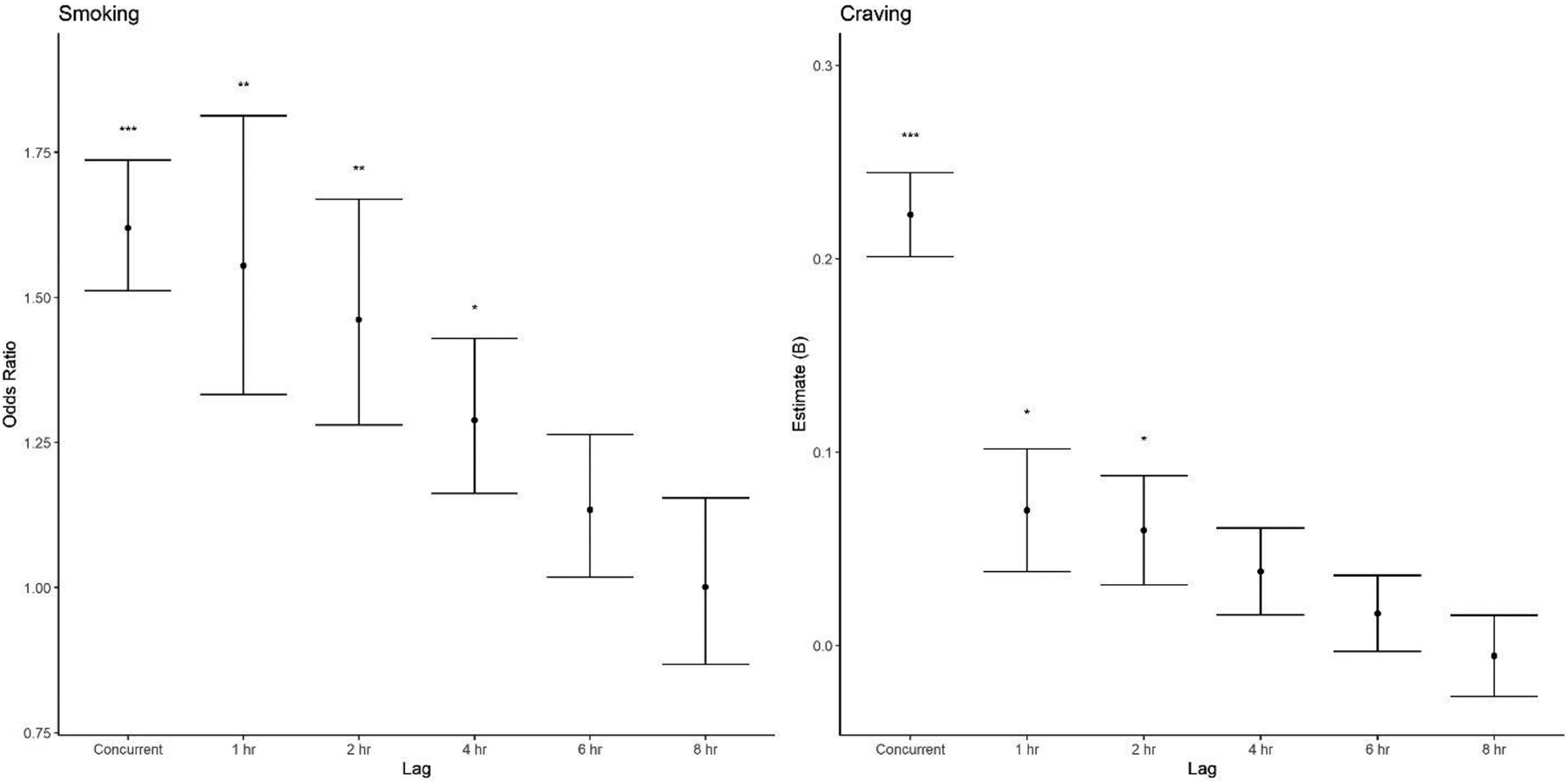
Effect sizes for the fixed effect of stressful event intensity on smoking (left) and craving (right). Odds ratios are depicted for the dichotomous outcome (smoking), and raw model coefficients (Bs) are depicted for the quantitative outcome (craving). Points represent effect sizes for simple fixed effects at different lags between the report that measured stressful event intensity vs. the report that measured these outcomes. Error bars indicate +/−1 standard error around the effect size. Significant fixed effects are indicated by asterisks above each line (*p<.05; **p<.01; ***p<.001).
This observation reestablishes craving as an important, stressful component of recovery. Although the prospective effects of stressful events on craving are short-lived, there is an opportunity for a vicious cycle: if craving itself is perceived as a stressful event, then craving may become the next stressor that subsequently leads to smoking. This is perhaps unsurprising: cravings are unpredictable, uncontrollable, and often quite intense – in other words, they encapsulate many established stressor characteristics. These similarities also speak to the considerable overlap among concepts of stress, affect, and craving (Sayette, 2016). Although cravings are conceptualized and assessed separately from stressors, recognizing their similarities may have important treatment implications. For example, considering cravings as a stressor may suggest that craving leads to smoking in a different way (i.e., via a stress mechanism) and should be addressed differently with more active coping strategies rather than passive distraction techniques.
Finally, treatment with NRT reduced the probability of smoking but did not reduce craving. Moreover, NRT did not moderate the effect of stressful event intensity on smoking or craving, and supplemental analyses confirmed that these null effects did not simply reflect noncompliance. In other words, treatment with combination NRT, an FDA-approved first-line medication for smoking cessation, is not addressing the impact of stressors.
The present study is a robust demonstration that stressors precede and likely promote both smoking and craving. Although there are many causes of drug use and relapse to drug use, the experience of stressors is frequent and ubiquitous. Moreover, individuals with substance use disorders may experience a disproportionate number of stressors (e.g., financial or housing uncertainty, legal issues, medical problems). Stressors may be more frequent during early recovery, particularly if unpredictable aversive withdrawal symptoms, unexpected exposure to drug cues, and changes in social networks and support are perceived as stressors. Additionally, craving itself may be experienced as stressful, creating a vicious cycle among stressful events, craving, and smoking that may lead to even more smoking if not addressed. Thus, it will be worth devoting time and research effort towards developing and/or refining treatments that can reduce the effect of stressors on both craving and drug use. These treatment development efforts should focus on both novel medications and promising psychosocial interventions (e.g., mindfulness-based relapse prevention; for a recent review of avenues for stress-focused treatments, see Fronk et al., 2020).
Supplementary Material
Funding:
Research reported in this publication was supported by the National Institute on Drug Abuse (NIDA) of the National Institutes of Health (NIH) under award number R01 DA033809 (J Curtin)
The authors would like to acknowledge Susan E. Schneck for her assistance with data collection and manuscript compilation.
This research was performed using the compute resources and assistance of the UW-Madison Center for High Throughput Computing (CHTC) in the Department of Computer Sciences. The CHTC is supported by UW-Madison, the Advanced Computing Initiative, the Wisconsin Alumni Research Foundation, the Wisconsin Institutes for Discovery, and the National Science Foundation, and is an active member of the Open Science Grid, which is supported by the National Science Foundation and the U.S. Department of Energy’s Office of Science.
This study was approved by the University of Wisconsin-Madison Health Sciences Institutional Review Board (HS IRB #2012-0326, “Smoking, Stress, and Treatment”).
Footnotes
Participants were originally recruited as part of a grant-funded project (R01 DA033809). See Supplement for additional details relevant to grant-funded project’s specific aims.
Biologically-confirmed abstinence was evaluated at a later visit 12–36 hours post-quit.
See Supplement for all battery measures.
Experimenters and data analysts were aware of participants’ assigned treatment conditions. However, bias was removed from data analysis by explicitly preregistering the analysis plan. Furthermore, this study does not focus on main effects of treatment condition.
Complete list of all EMA questions appears in the supplement.
Due to software limitations, participants could submit additional responses at other times. EMA responses submitted close in time (<5 minutes between responses) were merged into one response. The merged response included the maximum reported stressor intensity and the mean craving across responses consistent with the focus of those questions (i.e., intensity of the most severe stressor vs. current craving). The merged response also summed all reported smoking. These responses were time-stamped based on the first of the merged responses.
Additional analyses (see Supplement for full results) showed that the effect of stressful event intensity on smoking was not moderated by baseline nicotine dependence (FTND: p=0.776, WISDM: p=0.249), gender (p=0.632), or time since quit date (p=0.106) and that the precise lag X stressful event intensity interaction remained significant when controlling for prior report smoking (OR=0.91, z=−2.66, p=0.008).
Additional analyses (see Supplement for full results) showed that the effect of stressful event intensity on craving was not moderated by baseline nicotine dependence (FTND: p=0.346, WISDM: p=0.732), gender (p=0.663), or time since quit date (p=0.628) and that the precise lag X stressful event intensity interaction remained significant when controlling for prior report craving (B=−0.011, F(1,69.328)=5.316, p=0.024).
Additional analyses (see Supplement for full results) clarified that the null effects of treatment were not due to noncompliance. Compliance did not moderate the main effect of treatment on smoking (p=0.464) or craving (p=0.323), and compliance did not moderate the treatment X stressful event interaction for smoking (p=0.734) or craving (p=0.537). Compliance did not differ (p=0.515) between participants assigned to placebo (M=70.52%, SD=27.01%) vs. active NRT (M=73.53%, SD=24.58%).
REFERENCES
- Aczel B, Szaszi B, Sarafoglou A, Kekecs Z, Kucharský Š, Benjamin D, Chambers C, Fisher A, Gelman A, Gernsbacher M, Ioannidis J, Johnson E, Jonas K, Kousta S, Lilienfeld S, Lindsay D, Morey C, Monafò M, Newell B, … Wagenmakers E. (2019). A consensus-based transparency checklist. Nature Human Behaviour, 1–3. 10.1038/s41562-019-0772-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armeli S, Dehart T, Tennen H, Todd M, & Affleck G (2007). Daily Interpersonal Stress and the Stressor–Vulnerability Model of Alcohol Use. Journal of Social and Clinical Psychology, 26(8), 896–921. 10.1521/jscp.2007.26.8.896 [DOI] [Google Scholar]
- Arnsten A (2009). Stress signalling pathways that impair prefrontal cortex structure and function. Nature Reviews. Neuroscience, 10(6), 410–422. 10.1038/nrn2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker T, Piper M, McCarthy D, Majeskie M, & Fiore M (2004). Addiction motivation reformulated: An affective processing model of negative reinforcement. Psychological Review, 111(1), 33–51. [DOI] [PubMed] [Google Scholar]
- Barr D (2013). Random effects structure for testing interactions in linear mixed-effects models. Frontiers in Psychology, 4. 10.3389/fpsyg.2013.00328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford D, Curtin J, & Piper M (2015). Anticipation of smoking sufficiently dampens stress reactivity in nicotine-deprived smokers. Journal of Abnormal Psychology, 124(1), 128–136. 10.1037/abn0000007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauer M, & Curtin J (2018). Linear mixed-effects models and the analysis of nonindependent data: A unified framework to analyze categorical and continuous independent variables that vary within-subjects and/or within-items. Psychological Methods, 23(3), 389–411. 10.1037/met0000159 [DOI] [PubMed] [Google Scholar]
- Cambron C, Haslam A, Baucom B, Lam C, Vinci C, Cinciripini P, Li L, & Wetter D (2019). Momentary precipitants connecting stress and smoking lapse during a quit attempt. Health Psychology, 38(12), 1049–1058. https://doi.org/10/ggdj6w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambron C, Hopkins P, Burningham C, Lam C, Cinciripini P, & Wetter D (2020). Socioeconomic status, mindfulness, and momentary associations between stress and smoking lapse during a quit attempt. Drug and Alcohol Dependence, 209. 10.1016/j.drugalcdep.2020.107840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. (2019, November 18). Current Cigarette Smoking Among Adults in the United States. Centers for Disease Control and Prevention. https://www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking/index.htm [Google Scholar]
- Chen H, Cohen P, & Chen S (2010). How Big is a Big Odds Ratio? Interpreting the Magnitudes of Odds Ratios in Epidemiological Studies. Communications in Statistics - Simulation and Computation, 39(4), 860–864. 10.1080/03610911003650383 [DOI] [Google Scholar]
- Chung Y, Rabe-Hesketh S, Dorie V, Gelman A, & Liu J (2013). A nondegenerate penalized likelihood estimator for variance parameters in multilevel models. Psychometrika, 78(4), 685–709. 10.1007/s11336-013-9328-2 [DOI] [PubMed] [Google Scholar]
- Cohen S, Murphy M, & Prather A (2019). Ten Surprising Facts About Stressful Life Events and Disease Risk. Annual Review of Psychology, 70, 577–597. 10.1146/annurev-psych-010418-102857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronk N, & Piasecki T (2010). Contextual and subjective antecedents of smoking in a college student sample. Nicotine & Tobacco Research, 12(10), 997–1004. 10.1093/ntr/ntq136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin J, McCarthy D, Piper M, & Baker T (2006). Implicit and explicit drug motivational processes: A model of boundary conditions. In Wiers R & Stacy A (Eds.), Handbook of Implicit Cognition And Addiction (pp. 233–250). Sage Publications Inc. [Google Scholar]
- Fronk G, Sant’Ana S, Kaye J, & Curtin J (2020). Stress Allostasis in Substance Use Disorders: Promise, Progress, and Emerging Priorities in Clinical Research. Annual Review of Clinical Psychology, 16(1), null. 10.1146/annurev-clinpsy-102419-125016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furnari M, Epstein D, Phillips K, Jobes M, Kowalczyk W, Vahabzadeh M, Lin J, & Preston K (2015). Some of the people, some of the time: Field evidence for associations and dissociations between stress and drug use. Psychopharmacology, 232(19), 3529–3537. 10.1007/s00213-015-3998-7 [DOI] [PubMed] [Google Scholar]
- Heckman B, Carpenter M, Correa J, Wray J, Saladin M, Froeliger B, Drobes D, & Brandon T (2015). Effects of experimental negative affect manipulations on ad libitum smoking: A meta-analysis. Addiction, 110(5), 751–760. 10.1111/add.12866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye J, Bradford D, Magruder K, & Curtin J (2017). Probing for Neuroadaptations to Unpredictable Stressors in Addiction: Translational Methods and Emerging Evidence. Journal of Studies on Alcohol and Drugs, 78(3), 353–371. 10.15288/jsad.2017.78.353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G, & Le Moal M (2008). Addiction and the Brain Antireward System. Annual Review of Psychology, 59, 29–53. https://doi.org/10/1146/annurev.psych.59.103006.093548 [DOI] [PubMed] [Google Scholar]
- Lazarus R (1999). Stress and emotion: A new synthesis. Springer Publishing Co. [Google Scholar]
- Mantsch J, Baker D, Funk D, Lê A, & Shaham Y (2016). Stress-induced reinstatement of drug seeking: 20 years of progress. Neuropsychopharmacology, 41(1), 335–356. 10.1038/npp.2015.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy D, Piasecki T, Fiore M, & Baker T (2006). Life Before and After Quitting Smoking: An Electronic Diary Study. Journal of Abnormal Psychology, 115(3), 454–466. [DOI] [PubMed] [Google Scholar]
- McClure E, Vandrey R, Johnson M, & Stitzer M (2013). Effects of Varenicline on Abstinence and Smoking Reward Following a Programmed Lapse. Nicotine & Tobacco Research, 15(1), 139–148. 10.1093/ntr/nts101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElreath R (2020). Statistical Rethinking: A Bayesian Course with Examples in R and STAN. CRC Press. [Google Scholar]
- Moran L, Kowalczyk W, Phillips K, Vahabzadeh M, Lin J, Mezghanni M, Epstein D, & Preston K (2018). Sex differences in daily life stress and craving in opioid-dependent patients. The American Journal of Drug and Alcohol Abuse, 44(5), 512–523. 10.1080/00952990.2018.1454934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima M, Lemieux A, Fiecas M, Chatterjee S, Sarker H, Saleheen N, Ertin E, Kumar S, & al’Absi M (2020). Using novel mobile sensors to assess stress and smoking lapse. International Journal of Psychophysiology, 158, 411–418. 10.1016/j.ijpsycho.2020.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neupert S, Desmarais S, Gray J, Cohn A, Doherty S, & Knight K (2017). Daily stressors as antecedents, correlates, and consequences of alcohol and drug use and cravings in community-based offenders. Psychology of Addictive Behaviors: Journal of the Society of Psychologists in Addictive Behaviors, 31(3), 315–325. 10.1037/adb0000276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters F, Nicolson N, Berkhof J, Delespaul P, & deVries M (2003). Effects of daily events on mood states in major depressive disorder. Journal of Abnormal Psychology, 112(2), 203–211. 10.1037/0021-843X.112.2.203 [DOI] [PubMed] [Google Scholar]
- Potter L, Haaland B, Lam C, Cambron C, Schlechter C, Cinciripini P, & Wetter D (2021). A time-varying model of the dynamics of smoking lapse. Health Psychology, 40(1), 40–50. 10.1037/hea0001036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt W, & Davidson D (2009). Role of the HPA axis and the A118G polymorphism of the mu-opioid receptor in stress-induced drinking behavior. Alcohol and Alcoholism (Oxford, Oxfordshire), 44(4), 358–365. 10.1093/alcalc/agp007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston K, Kowalczyk W, Phillips K, Jobes M, Vahabzadeh M, Lin J, Mezghanni M, & Epstein D (2017). Context and craving during stressful events in the daily lives of drug-dependent patients. Psychopharmacology, 234(17), 2631–2642. 10.1007/s00213-017-4663-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston K, Kowalczyk W, Phillips K, Jobes M, Vahabzadeh M, Lin J, Mezghanni M, & Epstein D (2018). Before and after: Craving, mood, and background stress in the hours surrounding drug use and stressful events in patients with opioid-use disorder. Psychopharmacology, 235(9), 2713–2723. 10.1007/s00213-018-4966-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston K, Schroeder J, Kowalczyk W, Phillips K, Jobes M, Dwyer M, Vahabzadeh M, Lin J, Mezghanni M, & Epstein D (2018). End-of-day reports of daily hassles and stress in men and women with opioid-use disorder: Relationship to momentary reports of opioid and cocaine use and stress. Drug and Alcohol Dependence, 193, 21–28. 10.1016/j.drugalcdep.2018.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky R (2015). Stress and the brain: Individual variability and the inverted-U. Nature Neuroscience, 18(10), 1344–1346. 10.1038/nn.4109 [DOI] [PubMed] [Google Scholar]
- Savoy E, Businelle M, Nguyen N, Chen T, Neighbors C, Norton P, Taing M, & Reitzel L (2020). Examining moment to moment affective determinants of smoking rate following a quit attempt among homeless daily smokers. Addictive Behaviors, 115, 106788. 10.1016/j.addbeh.2020.106788 [DOI] [PubMed] [Google Scholar]
- Sayette M (2016). The Role of Craving in Substance Use Disorders: Theoretical and Methodological Issues. Annual Review of Clinical Psychology, 12, 407–433. 10.1146/annurev-clinpsy-021815-093351 [DOI] [PubMed] [Google Scholar]
- Sayette M (2017). The effects of alcohol on emotion in social drinkers. Behaviour Research and Therapy, 88, 76–89. 10.1016/j.brat.2016.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönbrodt F, Maier M, Heene M, & Zehetleitner M (2015). Voluntary commitment to research transparency. http://www.researchtransparency.org
- Segerstrom S, & Miller G (2004). Psychological stress and the human immune system: A meta-analytic study of 30 years of inquiry. Psychological Bulletin, 130(4), 601–630. 10.1037/0033-2909.130.4.601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serre F, Fatseas M, Denis C, Swendsen J, & Auriacombe M (2018). Predictors of craving and substance use among patients with alcohol, tobacco, cannabis or opiate addictions: Commonalities and specificities across substances. Addictive Behaviors, 83, 123–129. 10.1016/j.addbeh.2018.01.041 [DOI] [PubMed] [Google Scholar]
- Shiffman S, & Waters A (2004). Negative affect and smoking lapses: A prospective analysis. Journal of Consulting and Clinical Psychology, 72(2), 192–201. 10.1037/0022-006X.72.2.192 [DOI] [PubMed] [Google Scholar]
- Simmons J, Nelson L, & Simonsohn U (2012). A 21 Word Solution [SSRN Scholarly Paper]. 10.2139/ssrn.2160588 [DOI]
- Volz A, Dennis P, Dennis M, Calhoun P, Wilson S, & Beckham J (2014). The Role of Daily Hassles and Distress Tolerance in Predicting Cigarette Craving During a Quit Attempt. Nicotine & Tobacco Research, 16(6), 872–875. 10.1093/ntr/ntt286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J (1972). Psychological factors in stress and disease. Scientific American, 226(6), 104–113. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


