Abstract
A significant percentage of exogenous cholesterol was found in promastigotes and amastigotes of all studied species of Leishmania, suggesting a biological role for this molecule. Previous studies have shown that promastigotes of Leishmania uptake more low-density lipoprotein (LDL) particles under pharmacological pressure and are more susceptible to ergosterol inhibition in the absence of exogenous sources of cholesterol. This work shows that the host’s LDL is available to intracellular amastigotes and that the absence of exogenous cholesterol enhances the potency of sterol biosynthesis inhibitors in infected macrophages. A complete understanding of cholesterol transport to the parasitophorous vacuole can guide the development of a new drug class to be used in combination with sterol biosynthesis inhibitors for the treatment of leishmaniases.
Key words: leishmaniasis, low-density lipoprotein, cholesterol, sterol biosynthesis inhibitors
Leishmaniasis
Leishmaniases are caused by parasites of the genus Leishmania; they are unicellular eukaryotes that belong to the family Trypanosomatidae. The disease presents with several clinical manifestations, such as cutaneous, mucocutaneous, and visceral manifestations, and it occurs in the old and new worlds. More than 90% of visceral leishmaniasis cases worldwide have occurred in seven countries (Brazil, Ethiopia, India, Kenya, Somalia, South Sudan, and Sudan). In addition, Brazil is among the countries where more than 90% of cases of cutaneous leishmaniasis have been reported worldwide. 1 , 2 Since the 1940s, two commercially available formulations of pentavalent antimonials have been available for the treatment of leishmaniasis: N-methylglucamine antimoniate (meglumine antimoniate) (Glucantime®) and sodium stibogluconate (Pentostam®). Miltefosine, the only licensed oral drug for the treatment of leishmaniasis, has been employed for cutaneous and visceral leishmaniasis in some countries, such as India, Germany, and Colombia; 1 , 2 , 3 however, resistant cases have been reported, with increased failure in the treatment of visceral leishmaniasis. 3 Other drugs, such as pentamidine, amphotericin B, and paromomycin, are used in some circumstances, such as in cases of resistance to first-line treatment, even though they have significant toxic effects. 1 , 2
Sterol biosynthesis in Leishmania spp.
Sterols are structural lipids and are present in the membranes of most eukaryotic cells. Cholesterol is the essential mammalian cell sterol, but plants, fungi, and some protozoa synthesise other closely related sterols instead of cholesterol using the same synthetic pathway up to the 2,3-squalene epoxide step. 4 Leishmania spp. produce ergosterol and other 24-alkylated sterols (ergostane-based sterols) that are considered essential for both promastigote and amastigote forms. 5 , 6 , 7
The sterol biosynthetic pathway of Leishmania spp. and Trypanosoma cruzi has been deduced from studies with [14C]-acetate and [14C]-mevalonate and from the identification of intermediate sterols that accumulate after treatment with various sterol biosynthesis inhibitors. 5 , 8 The discovery of these intermediates suggested that sterol biosynthesis in Leishmania spp. occurs similarly to that in fungi, offering an opportunity for the development of targeted chemotherapy in the sterol biosynthetic pathway. 4 , 9 In fact, the inhibition of several enzymes of this pathway, such as HMG-CoA reductase, squalene epoxidase, lanosterol C-14 demethylase, and C-24 methyltransferase, by statins (simvastatin, lovastatin, and atorvastatin), allylamines (terbinafine), azoles (ketoconazole, miconazole, posaconazole, and itraconazole) and azasterols causes the death of Leishmania spp. 5 , 10 , 11 , 12 , 13 , 14 Therefore, the obvious conclusion is that some of these drugs, which have already been successfully used in clinics against fungal infections, could be alternatives for the treatment of leishmaniases. 5 , 9
Uptake of exogenous cholesterol
Although Leishmania spp. synthesise ergostane-derived sterols endogenously, they also obtain exogenous cholesterol, mainly through a low-density lipoprotein (LDL) receptor that is conserved in several trypanosomatids. This receptor is located in the flagellar pocket, and studies have shown that the cholesterol obtained by this parasite comes from the uptake and metabolism of LDL, which mainly contains cholesterol and cholesterol esters. 15
Leishmania amazonensis promastigotes, under pharmacological pressure from sterol biosynthesis inhibitors (ketoconazole, miconazole, and simvastatin), endocytose more LDL from the culture medium. They are also more sensitive to these inhibitors when they are deprived of exogenous cholesterol sources, suggesting that cholesterol uptake may play a compensatory role when the ergosterol biosynthetic pathway is inhibited. 16 , 17 Disturbance of sterol biosynthesis by itraconazole and ketoconazole was also analysed in L. mexicana amastigotes, and this life cycle stage was shown to have greater sensitivity to these molecules. Treatment of amastigotes with itraconazole and ketoconazole was also shown to cause increased amounts of exogenous cholesterol, as seen in the untreated promastigote forms. 18
Leishmaniasis and cholesterol metabolism
Lipid alterations in plasma have been demonstrated in some infectious diseases, and cholesterol has been studied in many parasitic infections. 19 Specifically, in leishmaniasis, two study approaches are used globally: the correlation of systemic cholesterol homeostasis with the disease and the relationship of host cell cholesterol with the infection. The first approach is derived from observations that patients with visceral leishmaniasis have hypocholesterolemia. The second approach is derived from the importance of lipid rafts for the entry of the parasite into the macrophage and the presentation of antigens. Patients with visceral leishmaniasis have a decrease in serum LDL-cholesterol, leading to hypocholesterolemia, which is mainly evident in this form of the disease. 20 , 21 The two study approaches overlap in some respects but differ essentially in that macrophages per se are not responsible for cholesterol homeostasis, a role played mainly by hepatocytes.
Ghosh and colleagues 22 showed that hypercholesterolemia offers protection against infection caused by L. donovani, whereas hypocholesterolemia makes mice more susceptible to infection with the parasite. 22 Recent works have shown that the hypocholesterolemia observed in L. donovani infection is due to the release of metalloprotease GP63 by the parasites infecting Kupffer cells in the liver. GP63 cleaves dicer (endonuclease RNAse III) in hepatocytes, reducing the expression of miR122 and leading to reduced production of cholesterol. miR122 is a posttranscriptional regulator (miRNA) that is abundantly expressed in the liver and modulates various functions. It comprises more than 70% of the miRNA in the liver and is mainly responsible for homeostasis and lipid metabolism. The downstream events of GP63 release affect cholesterol homeostasis, leading to progression of the infection due to failure in antigen presentation. 23 , 24 However, the mechanisms of transport of GP63 from the parasitophorous vacuole of Kupffer cells to hepatocytes and the expression of GP63 by amastigotes remain to be elucidated. Corroborating these data, parallel studies have shown that lipoproteins can modulate the cellular immune response in leishmaniasis. 25 Other studies have shown that macrophages infected with L. donovani have significantly decreased membrane cholesterol. The administration of cholesterol in infected hamsters offers substantial protection, showing the importance of cholesterol in the macrophage membrane for correct antigen presentation and immune system activation. 26 , 27
On the other hand, the results published by Fernandes and colleagues 28 revealed that infection by L. major significantly increases the migration of inflammatory cells to atherosclerotic lesions and promotes atherogenesis. The process of cholesterolemia accompanies this increase. These effects of L. major primary infection are a consequence of the stimulation of the immune system, specifically stimulation of the inflammatory components of atherosclerosis, which are activated by parasites in macrophages. 28 There are some controversial results regarding the modulation of host cholesterol homeostasis, but there seems to be no doubt that Leishmania infection alters host cell cholesterol metabolism.
Availability of LDL particles for intracellular amastigotes
LDL is the primary extracellular carrier of cholesterol, playing an essential physiological role in cell function and the regulation of metabolic pathways. Macrophages have LDL receptors that endocytose these particles under normal conditions. Under pathological conditions, such as hyperlipidemia, genetic disorders, and oxidative stress, specific components of LDL are oxidised, increasing its uptake. 29
Semine and colleagues 30 showed cholesterol trafficking in macrophages infected by L. mexicana. Infected macrophages increase LDL-cholesterol uptake and simultaneously decrease the level of NPC intracellular cholesterol transporter 1 (NPC1), which is responsible for late lysosomal cholesterol efflux, leading to retention of exogenous cholesterol in the parasitophorous vacuole (PV). Cholesterol concentrates around the parasites, forming a coating directly proportional to the number of parasites. 30
Furthermore, the parasites take up cholesterol from the host cell membrane. Cholesterol sequestration occurs soon after the onset of infection; this helps explain the cholesterol-dependent process in macrophages that influences the function of the immune response, such as antigen presentation. 30 This increase in cholesterol retention in the PV may be related to the decrease in cholesterol in the macrophage membrane, decreasing antigen presentation and contributing to disease progression.
Other works have demonstrated that infected macrophages alter their cholesterol metabolism by increasing expression of pathway enzymes, increasing LDL receptors, and decreasing ABC transporters that efflux cholesterol, thus leading to an accumulation of cholesterol within these cells. 31 , 32 The accumulated cholesterol may be used in some way by the parasite, since the presence of cholesterol in amastigotes has already been demonstrated. 18 , 33
Since the central cell affected in L. amazonensis infection is the macrophage, and it has LDL receptors, we studied whether amastigotes use LDL particles. To assess the availability of LDL to intracellular amastigotes, peritoneal macrophages were infected with L. amazonensis-GFP and incubated with LDL-Alexa 594 or infected with L. amazonensis and incubated with LDL-gold.
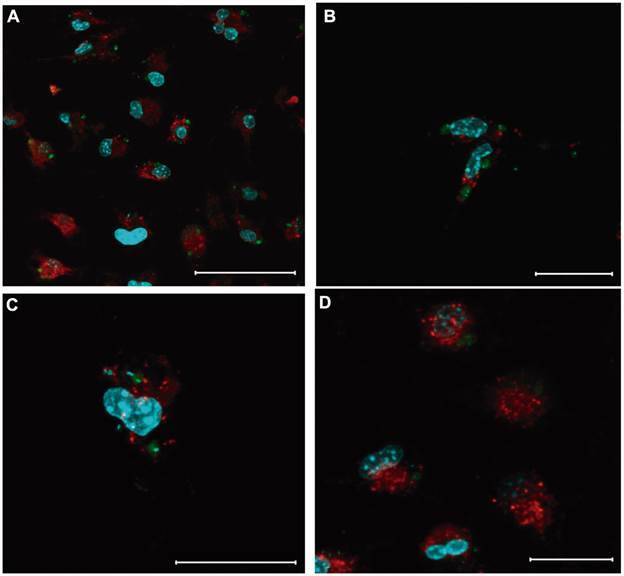
First, for the confocal microscopy experiments, peritoneal macrophages from Swiss mice (1.0 x 106/mL) were plated and adhered to Lab-teks (Thermo-Fisher) for 1 hour and infected with 3.0 x 106/mL metacyclic promastigotes of L. amazonensis-GFP (MHOM/BR/77/Josefa strain) for 72 h at 37ºC. Macrophages were washed and incubated with serum-free medium 24 h before incubation with LDL-labeled Alexa Fluor® 594 AcLDL (Invitrogen, Life Technologies Corporation). LDL particles (red) can be visualised very close to the intracellular amastigotes, probably inside the PV (Fig. 1). The PV has proteins that can transport molecules from or to the cytoplasm. In addition to this active transport, intracellular vesicles, such as those formed by LDL endocytosis, can fuse with PV, discharging its intravesicular content.
Fig. 1: distribution of low-density lipoprotein (LDL) particles in macrophages infected with Leishmania amazonensis-GFP. Peritoneal macrophages infected with L. amazonensis-GFP were incubated with LDL-Alexa 594 particles for 30 (A and B) or 150 min (C - D) at concentrations of 0.125 (A and C) and 0.5 µg/mL (B and D). After this period, the cells were washed three times in phosphate buffered saline (PBS), fixed with 4% formaldehyde for 10 min and incubated with DAPI for 20 min. Slides were mounted using Prolong® Gold Antifade Mounting Fluid (Invitrogen, Life Technologies Corporation) and analysed under a Zeiss LSM 510 META confocal microscope. Green: L. amazonensis amastigotes; red: LDL particles; blue: nuclei/kinetoplasts (DAPI). Bars: A = 50 µm; B-D = 20 µm.
For the transmission electron microscopy experiments, peritoneal macrophages from Swiss mice (1.0 x 106/mL) were plated, adhered to small Petri dishes for 1 hour, and infected with 3.0 x 106/mL metacyclic promastigotes of L. amazonensis (strain MHOM/BR/77/LTB0016) for 3 h. After this period, the plates were washed with serum-free medium and incubated for 72 h at 37ºC. Four hours before the end of the final incubation period, the plates were incubated with colloidal gold-labeled LDL 34 at a 1:10 dilution. After this period, infected and uninfected macrophages were washed with phosphate buffered saline (PBS) and fixed for 40 min in a 2.5% glutaraldehyde solution diluted in 0.1 M sodium cacodylate buffer, pH 7.4. 35 Ultrathin sections were collected, stained with uranyl acetate and lead citrate, and examined in a Jeol 1011 transmission electron microscope (Tokyo, Japan) at Plataforma de Microscopia Eletrônica at Fiocruz. LDL purification was performed as described by Poumay and Ronveaux, 36 with some modifications.
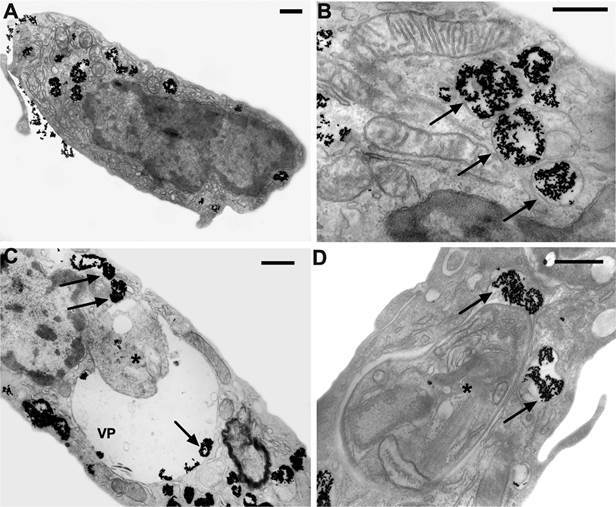
The confocal microscopy results were corroborated by transmission electron microscopy. Fig. 2A-B shows the distribution of LDL particles in uninfected macrophages. LDL-gold particles (dark spots) were endocytosed and stored in endocytic vacuoles to be processed and used by the host cell. In Fig. 2C-D, macrophages infected with L. amazonensis were incubated with LDL-gold particles. The endocytic vacuoles containing the LDL particles fused with the PV. We also observed the presence of free LDL particles inside the PV, even at very close distances to the amastigotes, suggesting adhesion to the parasite membrane. These images demonstrate that intracellular amastigotes have access to LDL and, therefore, may be obtaining their content, mainly cholesterol. The parasite may use the cholesterol of the LDL particles inside the PV, since the presence of cholesterol in amastigotes has already been demonstrated. 18 , 33
Fig. 2: availability of low-density lipoprotein (LDL)-gold to intracellular amastigotes. Peritoneal macrophages infected or not with Leishmania amazonensis were incubated with LDL-gold (10 nm) for 4 h and analysed by transmission electron microscopy. A and B: uninfected macrophages; C and D: infected macrophages. Arrows: LDL particles; asterisks: L. amazonensis amastigotes; VP: parasitophorous vacuole. The asterisk represents the amastigotes within the parasitophorous vacuole. Bars = 0.5 µm.
Influence of exogenous cholesterol on the leishmanicidal activity on intracellular amastigotes
After observing that intracellular amastigotes access LDL endocytosed by macrophages, we evaluated the effect of the absence of exogenous cholesterol sources on the leishmanicidal activity of ketoconazole and miconazole. Murine peritoneal macrophages (1.0 x 106 cells/mL) were infected with 3 x 106 promastigotes/mL of L. amazonensis for 3 hours and incubated in RPMI medium with cholesterol-free Nutridoma® (Roche) or foetal bovine serum (FBS) in the absence or presence of miconazole or ketoconazole (0-16 µM) for 72 h. After incubation, the slides were stained with fast panoptic, and the infection index was determined by counting under an optical microscope. The infection index was calculated using the formula: % infected macrophages x nº of amastigotes/nº total of macrophages.
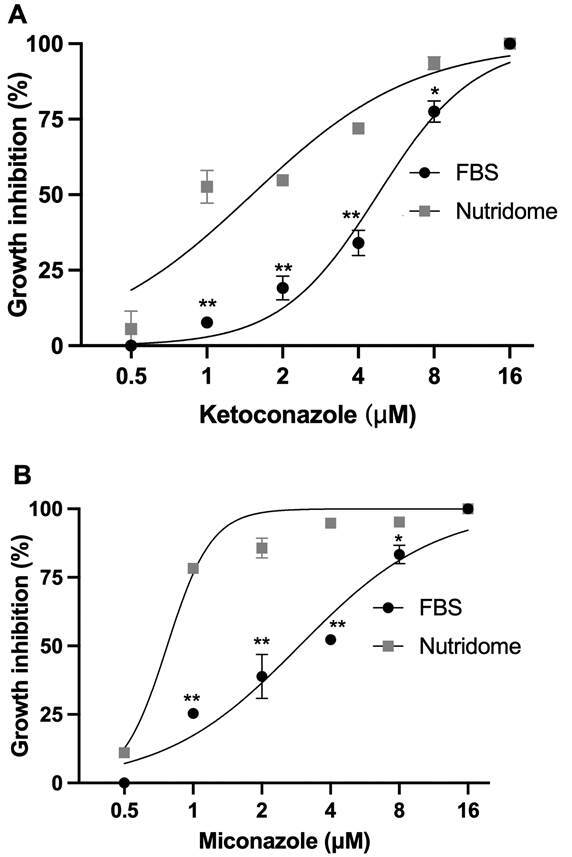
The parasites became more sensitive to the leishmanicidal activity of ketoconazole (Fig. 3A) and miconazole (Fig. 3B) when infected macrophages were cultured in Nutridoma compared to FBS. Ketoconazole had an EC50 of 4.7 µM (95% CI: 4.2-5.4 µM) in the presence of FBS, while Nutridoma had an EC50 of 1.5 µM (95% CI: 1.1-2.0 µM). Miconazole had an EC50 of 2.9 µM (95% CI: 2.3-3.6 µM) with FBS and 0.77 µM (95% CI: 0.68-0.85 µM) with Nutridoma. The results suggest that the absence of exogenous cholesterol can influence the activity of ergosterol biosynthesis inhibitors. Intracellular amastigotes were more sensitive to ketoconazole and miconazole when incubated in cholesterol-free medium, suggesting that even in the intracellular environment, L. amazonensis uses cholesterol to replace ergosterol in an attempt to maintain its membrane properties.
Fig. 3: effect of the absence of exogenous cholesterol on the leishmanicidal activity of ketoconazole and miconazole. Peritoneal macrophages infected with Leishmania amazonensis were incubated in RPMI culture medium supplemented with foetal bovine serum (FBS) or cholesterol-free Nutridoma. Cultures remained untreated or were treated with ketoconazole (A) or miconazole (B) for 72 h. The experiments were carried out in triplicate, n = 3. The graphs are representative of an experiment with the standard deviation value. The graphs and IC50 values were obtained using the GraphPad Prism 7 program. Student’s t test, *p < 0.5, **p > 0.001.
Concluding remarks
In conclusion, the data presented here suggest that intracellular amastigotes access the host’s LDL and that the availability of this pool of cholesterol can be used by the parasite to overcome the effect of sterol biosynthesis inhibitors. A complete understanding of this phenomenon can guide the development of a new drug class to be used in combination with sterol biosynthesis inhibitors for the treatment of leishmaniases.
ACKNOWLEDGEMENTS
To the Blood Bank of the Hemotherapy Service of the Clementino Fraga Filho University Hospital of the Federal University of Rio de Janeiro (Rio de Janeiro, RJ, Brazil) through agreement No. 171/07 signed by the Hospital Research Ethics Committee and the Lipids Biochemistry Laboratory, Institute of Medical Biochemistry, UFRJ. The authors thank Rede de Plataformas Tecnológicas, FIOCRUZ for providing the confocal microscope.
REFERENCES
- 1.Burza S, Croft SL, Boelaert M. Leishmaniasis. Lancet. 2018;392:951–970. doi: 10.1016/S0140-6736(18)31204-2. [DOI] [PubMed] [Google Scholar]
- 2.Mann S, Frasca K, Scherrer S, Henao-Martínez AF, Newman S, Ramanan P, et al. A review of leishmaniasis: current knowledge and future directions. Curr Trop Med Rep. 2021 doi: 10.1007/s40475-021-00232-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sasidharan S, Saudagar P. Leishmaniasis where are we and where are we heading? Parasitol Res. 2021;120:1541–1554. doi: 10.1007/s00436-021-07139-2. [DOI] [PubMed] [Google Scholar]
- 4.Nes WD. Biosynthesis of cholesterol and other sterols. Chem Rev. 2011;111:6423–6451. doi: 10.1021/cr200021m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberts CW, McLeod R, Rice DW, Ginger M, Chance ML, Goad LJ. Fatty acid and sterol metabolism potential antimicrobial targets in apicomplexan and trypanosomatid parasitic protozoa. Mol Biochem Parasitol. 2003;126:129–142. doi: 10.1016/s0166-6851(02)00280-3. [DOI] [PubMed] [Google Scholar]
- 6.Darnet S, Blary A, Chevalier Q, Schaller H. Phytosterol profiles, genomes and enzymes - An overview. Front Plant Sci. 2021;12:665206–665206. doi: 10.3389/fpls.2021.665206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goad LJ, Holz GG, Beach DH. Sterols of Leishmania species Implications for biosynthesis. Mol Biochem Parasitol. 1984;10:161–170. doi: 10.1016/0166-6851(84)90004-5. [DOI] [PubMed] [Google Scholar]
- 8.Ginger ML, Chance ML, Goad LJ. Elucidation of carbon sources used for the biosynthesis of fatty acids and sterols in the trypanosomatid Leishmania mexicana. Biochem J. 1999;342(2):397–405. [PMC free article] [PubMed] [Google Scholar]
- 9.Jordá T, Puig S. Regulation of ergosterol biosynthesis in Saccharomyces cerevisiae. Genes. 2020;11:E795. doi: 10.3390/genes11070795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goad LJ, Holz GG, Beach DH. Sterols of ketoconazole-inhibited Leishmania mexicana mexicana promastigotes. Mol Biochem Parasitol. 1985;15:257–279. doi: 10.1016/0166-6851(85)90089-1. [DOI] [PubMed] [Google Scholar]
- 11.Haughan PA, Chance ML, Goad LJ. Synergism in vitro of lovastatin and miconazole as anti-leishmanial agents. Biochem Pharmacol. 1992;44:2199–2206. doi: 10.1016/0006-2952(92)90347-l. [DOI] [PubMed] [Google Scholar]
- 12.Dinesh N, Pallerla DSR, Kaur PK, Kishore Babu N, Singh S. Exploring Leishmania donovani 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGR) as a potential drug target by biochemical, biophysical, and inhibition studies. Microb Pathog. 2014;66:14–23. doi: 10.1016/j.micpath.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Zakai HA, Zimmo SK. Effects of itraconazole and terbinafine on Leishmania major lesions in BALB/c mice. Ann Trop Med Parasitol. 2000;94:787–791. doi: 10.1080/00034980020027979. [DOI] [PubMed] [Google Scholar]
- 14.Andrade-Neto VV, Rebello KM, Pereira TM, Torres-Santos EC. Effect of itraconazole-ezetimibe-miltefosine ternary therapy in murine visceral leishmaniasis. Antimicrob Agents Chemother. 2021;65(5):e02676–e02620. doi: 10.1128/AAC.02676-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bastin P, Stephan A, Raper J, Saint-Remy JM, Opperdoes FR, Courtoy PJ. An M(r) 145,000 low-density lipoprotein (LDL)-binding protein is conserved throughout the Kinetoplastida order. Mol Biochem Parasitol. 1996;76:43–56. doi: 10.1016/0166-6851(95)02537-5. [DOI] [PubMed] [Google Scholar]
- 16.De Cicco NNT, Pereira MG, Corrêa JR, Andrade-Neto VV, Saraiva FB, Chagas-Lima AC. LDL uptake by Leishmania amazonensis involvement of membrane lipid microdomains. Exp Parasitol. 2012;130:330–340. doi: 10.1016/j.exppara.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 17.Andrade-Neto VV, Cicco NNT, Cunha-Junior EF, Canto-Cavalheiro MM, Atella GC, Torres-Santos EC. The pharmacological inhibition of sterol biosynthesis in Leishmania is counteracted by enhancing LDL endocytosis. Acta Trop. 2011;119:194–198. doi: 10.1016/j.actatropica.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Hart DT, Lauwers WJ, Willemsens G, Bossche HV, Opperdoes FR. Perturbation of sterol biosynthesis by itraconazole and ketoconazole in Leishmania mexicana mexicana infected macrophages. Mol Biochem Parasitol. 1989;33:123–134. doi: 10.1016/0166-6851(89)90026-1. [DOI] [PubMed] [Google Scholar]
- 19.Portugal LR, Fernandes LR, Cesar GC, Santiago HC, Oliveira DR, Silva NM. Infection with Toxoplasma gondii increases atherosclerotic lesion in ApoE-deficient mice. Infect Immun. 2004;72:3571–3576. doi: 10.1128/IAI.72.6.3571-3576.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lal CS, Kumar A, Kumar S, Pandey K, Kumar N, Bimal S. Hypocholesterolemia and increased triglyceride in pediatric visceral leishmaniasis. Clin Chim Acta. 2007;382(1-2):151–153. doi: 10.1016/j.cca.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 21.Liberopoulos E, Alexandridis G, Bairaktari E, Elisaf M. Severe hypocholesterolemia with reduced serum lipoprotein(a) in a patient with visceral leishmaniasis. Ann Clin Lab Sci. 2002;32(3):305–308. [PubMed] [Google Scholar]
- 22.Ghosh J, Das S, Guha R, Ghosh D, Naskar K, Das A. Hyperlipidemia offers protection against Leishmania donovani infection role of membrane cholesterol. J Lipid Res. 2012;53:2560–2572. doi: 10.1194/jlr.M026914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghosh J, Bose M, Roy S, Bhattacharyya SN. Leishmania donovani targets Dicer1 to downregulate miR-122, lower serum cholesterol, and facilitate murine liver infection. Cell Host Microbe. 2013;13:277–288. doi: 10.1016/j.chom.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Descoteaux A, Moradin N, Duque GA. Leishmania dices away cholesterol for survival. Cell Host Microbe. 2013;13(3):245–247. doi: 10.1016/j.chom.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 25.Soares NM, Leal TF, Fiúza MC, Reis EAG, Souza MAL, Dos-Santos WL. Plasma lipoproteins in visceral leishmaniasis and their effect on Leishmania-infected macrophages. Parasit Immunol. 2010;32:259–266. doi: 10.1111/j.1365-3024.2009.01187.x. [DOI] [PubMed] [Google Scholar]
- 26.Sen S, Roy K, Mukherjee S, Mukhopadhyay R, Roy S. Restoration of IFN R subunit assembly, IFN? signaling and parasite clearance in Leishmania donovani infected macrophages: role of membrane cholesterol. PLoS Pathog. 2011;7:e1002229. doi: 10.1371/journal.ppat.1002229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Banerjee S, Ghosh J, Sen S, Guha R, Dhar R, Ghosh M. Designing therapies against experimental visceral leishmaniasis by modulating the membrane fluidity of antigen-presenting cells. Infect Immun. 2009;77:2330–2342. doi: 10.1128/IAI.00057-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernandes LR, Ribeiro ACC, Segatto M, Santos LFFF, Amaral J, Portugal LR. Leishmania major self-limited infection increases blood cholesterol and promotes atherosclerosis Development. Cholesterol. 2013;2013:754580–754580. doi: 10.1155/2013/754580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsimikas S, Miller YI. Oxidative modification of lipoproteins mechanisms, role in inflammation and potential clinical applications in cardiovascular disease. Curr Pharm Des. 2011;17:27–37. doi: 10.2174/138161211795049831. [DOI] [PubMed] [Google Scholar]
- 30.Semini G, Paape D, Paterou A, Schroeder J, Barrios-Llerena M, Aebischer T. Changes to cholesterol trafficking in macrophages by Leishmania parasites infection. Microbiologyopen. 2017;6(4):e00469. doi: 10.1002/mbo3.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rabhi I, Rabhi S, Ben-Othman R, Rasche A, Daskalaki A, Trentin B. Sysco consortium Transcriptomic signature of Leishmania infected mice macrophages: a metabolic point of view. PLoS Negl Trop Dis. 2012;6:e1763. doi: 10.1371/journal.pntd.0001763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Osorio y Fortéa J.La Llave E.Regnault B.Coppée J-Y.Milon G.Lang T Transcriptional signatures of BALB/c mouse macrophages housing multiplying Leishmania amazonensis amastigotes. BMC Genomics. 2009;10:119–119. doi: 10.1186/1471-2164-10-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berman JD, Goad LJ, Beach DH, Holz GG. Effects of ketoconazole on sterol biosynthesis by Leishmania mexicana mexicana amastigotes in murine macrophage tumor cells. Mol Biochem Parasitol. 1986;20:85–92. doi: 10.1016/0166-6851(86)90145-3. [DOI] [PubMed] [Google Scholar]
- 34.Slot JW, Geuze HJ. A new method of preparing gold probes for multiple-labeling cytochemistry. Eur J Cell Biol. 1985;38:87–93. [PubMed] [Google Scholar]
- 35.Ennes-Vidal V, Menna-Barreto RFS, Santos ALS, Branquinha MH. d'Avila-Levy CM MDL28170, a calpain inhibitor, affects Trypanosoma cruzi metacyclogenesis, ultrastructure and attachment to Rhodnius prolixus midgut. PLoS One. 2011;6(4):e18371. doi: 10.1371/journal.pone.0018371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poumay Y, Ronveaux-Dupal MF. Rapid preparative isolation of concentrated low-density lipoproteins and of lipoprotein-deficient serum using vertical rotor gradient ultracentrifugation. J Lipid Res. 1985;26:1476–1480. [PubMed] [Google Scholar]


