Abstract
Vincristine has a wide spectrum of clinical activity and is currently used in the treatment of leukemia. Despite its high therapeutic properties, vincristine has common side effects. Accordingly, it is desirable to determine vincristine in plasma for the use of the drug with strict monitoring. In the present research, for the first time a hydrophobic deep eutectic solvent (DES) composed of methyltrioctylammonium chloride (MTOAC) and n-butanol in a molar ratio of 1 : 3 was used as the extractant in dispersive liquid–liquid microextraction (DLLME) for the extraction and determination of vincristine in the plasma of children with leukemia prior to its analysis by high-performance liquid chromatography-ultraviolet detection (HPLC-UV). Under optimal experimental conditions, the method showed good linearity with a correlation coefficient (R2) of 0.9986 in the linear range of 0.06–300 μg L−1, low limit of detection of 0.02 μg L−1 and acceptable extraction efficiency (EE) of 88.4%. In the final stage of the study, this proposed technique was successfully applied to determine vincristine in real plasma, and the obtained results demonstrated the ability of the synthesized DES to extract drugs from biological fluids.
Vincristine has a wide spectrum of clinical activity and is currently used in the treatment of leukemia.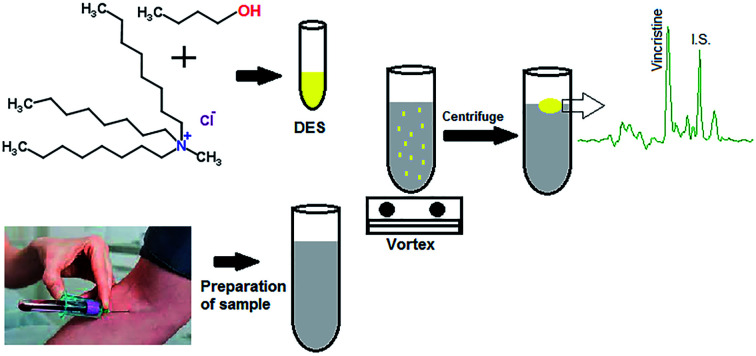
1. Introduction
The vinca alkaloids, vincristine, vinblastine, and vinorelbine, are structurally similar alkaloids composed of two multiring subunits, vindoline and catharanthine.1 The vinca alkaloids exert their cytotoxic effect by binding to tubulin, a dimeric protein that polymerizes to form microtubules.2 Vincristine is the most important alkaloid in the vinca group and is an anti-neoplastic drug.3 Vincristine has many applications in medicine and is currently used in the treatment of various diseases such as ewing sarcoma, wilms tumor, brain tumors, ALL, Hodgkin and non-Hodgkin lymphomas, rhabdomyosarcoma, soft tissue sarcomas and neuroblastoma.4 Vincristine is poorly absorbed if administered orally and is therefore administered intravenously as a bolus injection.5 The standard dose for vincristine is 1 to 2 mg m−2, administered every 1 to 3 weeks. For infants ≤1 year of age, vincristine dose is scaled to body weight (0.03 to 0.05 mg kg−1).6 After bolus administration, the vinca alkaloids manifest a rapid initial decline in plasma concentration (initial half-life of 5 to 10 minutes), followed by a prolonged terminal elimination phase with half-life of approximately 12 to 40 hours.7 The long terminal half-life and the large steady-state volume of distribution are consistent with avid and extensive tissue binding that is characteristic of these drugs. Vincristine clearance is more rapid in children than adults, and adults have a more than twofold longer terminal half-life. Vincristine disposition in children is highly variable, resulting in a wide interpatient range in drug exposure at a standard dose of 1.5 mg m−2.8 Despite its high therapeutic properties, vincristine has common side effects such as changes in feeling, constipation and difficulty walking, hair loss and headaches. In addition, other side effects such as lung damage, neuropathic pain, or low white blood cell count may occur when taking vincristine.9 Accordingly, it is favourable to assessment of vincristine in the plasma for the use of drug with strict monitoring.
Gas chromatography (GC) is not used for analysis of vincristine because it has a high boiling point and often requires derivatization. High performance liquid chromatography (HPLC) in combination with different detectors such as electrochemical,10 ultraviolet (UV),11 diode array detector (DAD)12 and mass spectrometry (MS)13–15 have been employed for the analysis of vincristine in various samples. HPLCMS is usually used for vincristine analysis because it is highly sensitive, but due to its high cost, the use of this technique is limited. On the other hand, HPLC-UV is better known for its simplicity and cheapness and is readily available in most laboratories. However, the use of microextraction techniques in sample preparation has led to acceptable results with HPLC-UV.16 Accordingly, a suitable extraction and preconcentration step prior to instrument detection is necessary. The two most widely used methods for extraction of vincristine in biological samples are solid-phase extraction (SPE)17 and liquid–liquid extraction (LLE).18 Advantages and disadvantages of these techniques have already been discussed.19
In recent years, researchers have shifted to extraction methods using less organic solvents, cheaper and more environmentally friendly. Dispersive liquid–liquid microextraction (DLLME) is extensively used in the current literature that first introduced by Assadi and co-workers in 2006.20 DLLME has attracted the attention of researchers due to its high enrichment factor, short extraction time, fast balance between organic and aqueous phases and very low consumption of organic solvents. For all its advantages, this technique had one major drawback: the use of toxic and volatile organic solvents as an extractant, which was not only very harmful to human health and the environment, but also reduced the reproducibility of experiments.21 Thus, the search for non-toxic and low-volatility green solvents is expanding the application of DLLME. Nowadays in addition to common organic solvents and ionic liquids, DESs have been employed in DLLME as the extractive media.22–27 Recently, DLLME combined with hydrophobic DES as extraction solvent has been developed for the preconcentration of organic and inorganic compounds in different matrices28–31 and several reviews have been written on this issue.32–35
In the present research, some hydrophobic DESs were synthesized and employed for the VA-DLLME method of vincristine from plasma of children with leukaemia followed by HPLC-UV. To synthesize DESs, various alcohols including n-nonanol, n-heptanol, n-butanol, glycerol and ethylene glycol as HBDs and methyltrioctylammonium chloride (MTOAC) as HBA were used. To completely eliminate the problems caused by the disperser solvent in the DLLME method, vortex-assisted was used to disperse the DES in the sample solution and improve the extraction efficiency. Under the optimized conditions, the VA-DLLME-DES method was validated and used for the analysis of vincristine in plasma of children with leukaemia.
2. Experimental
2.1. Reagents and solutions
Vincristine (purity >98%) and vinblastine (purity ≥95%; internal standard (IS)) were obtained from Aladdin Reagent Co., Ltd. (Shanghai, China). We initially made sure that none of the patients were treated with vinblastine. So, it was selected as the internal standard due to its structural similarity to vincristine. Stock standard solution of vincristine was prepared in methanol (5.0 mL; 1000 mg L−1), and kept at a −20 °C. Distilled water (6 times distilled) was purchased from Shaid Ghazi Pharmaceutical Company of Tabriz (Tabriz, Iran). HBA reagent (MTOAC; purity >97%) were obtained from Aladdin Biochemical Company (Shanghai, China). Methanol, acetonitrile, NaCl, KHCO3, phosphate salt and HBD reagents were purchased from Merck Company (Darmstadt, Germany).
2.2. Apparatus
Chromatographic analysis of vincristine was performed with a Knauer HPLC system equipped with a binary pumps Smartline-1000-1 and Smartline-1000-2, Knauer Smartline-UV-2500 detector (Berlin, Germany) and manual sample injector fitted with a 20 μL injection loop (model 7725i, Rheodyne, Cotati, CA, USA). Chromatographic data were recorded and analyzed using Chromgate software (version 3.1). An Anachem C18 column (15 cm × 4.6 mm, with 5 μm particle size) and an Anachem C18 guard column (4.6 mm × 1 cm) were applied to separation (Luton, UK). The mobile phase was isocratic at the flow rate of 1.0 mL min−1 and consisted of 35% NaH2PO4 buffer (0.02 M; pH 4.6) and 65% acetonitrile. The UV detection wavelength was set to 297 nm.
2.3. Sampling and sample preparation
Initially, to validate the proposed method, a blank plasma sample (drug-free) was obtained from a healthy volunteer who had not been exposed to any drug for at least one year. Four real plasma samples were taken from 4 children (2 girls and 2 boys aged from 10 to 16 years) with acute leukaemia who were treated with vincristine at Dr Mohammad Kermanshahi Hospital from Kermanshah, Iran. The samples were prepared as follows: to 200 μL of whole blood in the EDTA-contained test tube, 600 μL of a mixture of zinc sulphate (15%, w/v) and acetonitrile in a volume ratio of 3 to 2 is added and vortexed for 10 minutes. The mixture was then centrifuged and the supernatant was transferred to another clean test tube and made up to 5 mL with distilled water. The resulting solution was then subjected to the VA-DLLME procedure.
2.4. Preparation of DESs
To prepare DESs, MTOAC as HBA and different alcohols as HBD were mixed in a 1 : 1 molar ratio and the resulting mixture was stirred at 80 °C until a homogeneous, colorless liquid was obtained. A magnet and a magnetic stirrer were used for stirring. Other molar ratios were also formed in the same way. After cooling, the DESs were stored at room temperature for use in experiments. The volume of synthesized DES depends on the number of samples and their repetition. In this study, we synthesized 10 mL, which was enough until the end.
2.5. VA-DLLME procedure
A 5.0 mL of diluted and pre-treated plasma sample spiked or not with the vincristine containing 10.0 μg L−1 of vinblastine (IS) was poured into a test tube and 0.5 mL KHCO3 (5%, w/v) was added to adjust the pH. Then 80 μL of DES was added and vortexed for 20 min to obtain the cloudy solution. The DES is dispersed in very small droplets in the sample solution, creating a very large contact surface. At this time, the sample mixture was centrifuged at 5000 rpm for 5 min and the DES, which contains the analytes, was collected on the surface of the aqueous phase. The test tube was kept in freezer for few minutes to solidify DES. The solidified DES was transferred to another clean vial to melt at room temperature. Ultimately, 30 μL of collected phase was directly injected into HPLC for analysis.
2.6. Calculations of enrichment factor and extraction efficiency
The enrichment factor (EF) was defined as the ratio between the analytes concentration in the floated phase (Cflo) and initial concentration of analytes (C0) within the sample. The analyte concentration in the floated phase is obtained from the peak area and the calibration curve, and the initial concentration is known.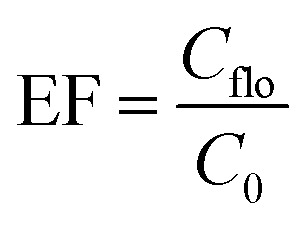
The extraction efficiency (%EE) was defined as the ratio between the amount of the analyte in the floating phase (nflo) and the initial amount of the analyte (n0) within the sample. 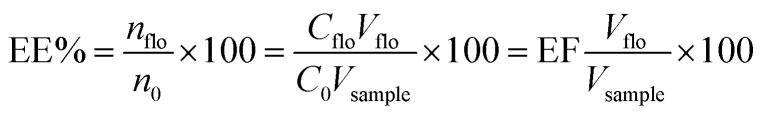 where Vflo and Vsample are the volumes of the floating phase and sample, respectively.
where Vflo and Vsample are the volumes of the floating phase and sample, respectively.
3. Results and discussion
3.1. Choice of DES type
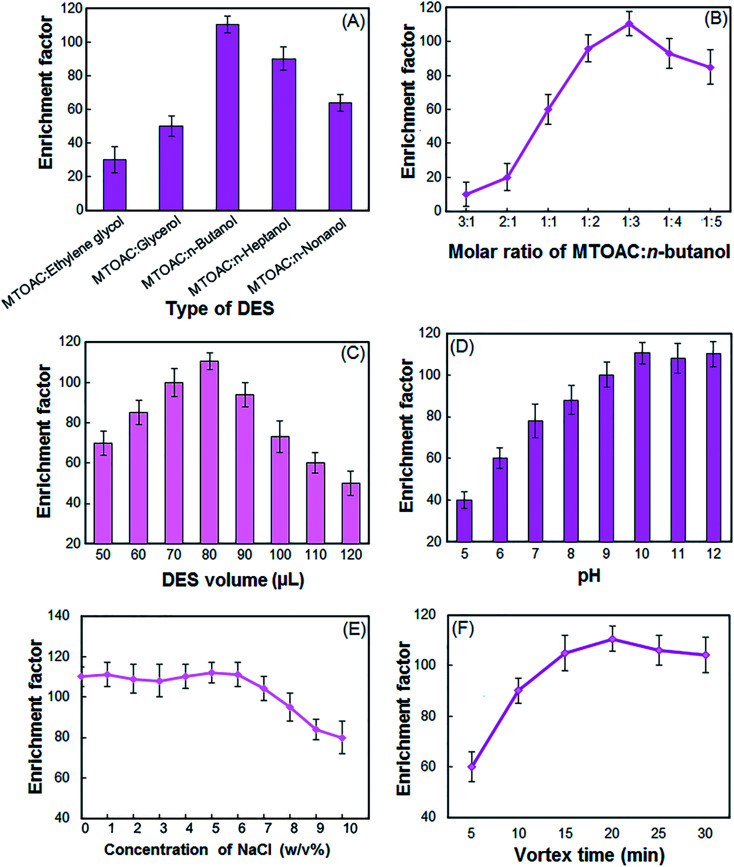
To evaluate the type of DES, MTOAC was combined with the five different alcohols listed and used as an extractant after the formation of the DES. The enrichment factor (EF) of the vincristine using different DES are shown in Fig. 1A. The results show that MTOAC:n-butanol has the highest EF among the examined different DESs. Therefore, MTOAC:n-butanol was selected as an optimum extraction solvent for further optimization studies.
Fig. 1. Effect of the different types of DES (A), molar ratio of MTOAC:n-butanol (B), DES volume (C), sample solution pH (D), NaCl concentration (E) and vortex time (F) on the enrichment factor of the vincristine. Final extraction conditions: types of DES, MTOAC:n-butanol; proportion of MTOAC:n-butanol, 1 : 3; volume of the sample solution, 5 mL; sample solution pH, 10.5; volume of the DES, 80 μL; vortex time, 20 min; room temperature. All experiments were repeated 3 times and the standard deviation of 3 times was shown as an error bar.
3.2. Choice of HBA : HBD molar ratio
The molar ratio of HBA and HBD should be selected so that the best DES is formed and it is most effective in the extraction of target analytes. The molar ratio between the HBA and the HBD greatly affects the physicochemical properties of DES. To study the effect of this parameter, selected HBA and HBD were mixed with different ratios of 1 : 1, 1 : 2, 1 : 3, 1 : 4, 1 : 5, 2 : 1, and 3 : 1. The results showed that the above compounds in molar ratios of 3 : 1 and 2 : 1 cannot form a DES. Based on the results in Fig. 1B, the combination of the HBA and HBD in other ratios has a positive effect on the EF of the vincristine. However, when HBA and HBD are combined in a 1 : 3 ratio, the resulting DES has a better extraction efficiency. Therefore, 1 : 3 molar ratio was chosen in the experiments.
3.3. Choice of DES volume
In liquid phase microextraction, the volume of extractant is a significant parameter that affects the EF and EE. It is clear that the low volume of the extraction solvent cannot completely extract the analytes and surplus volume of extraction solvent will reduce the EF. In order to study the effect of volume of DES on the performance of the proposed procedure, different volumes of DES (50–120 μL at 10 μL intervals) were tested. According to Fig. 1C, the EF of the vincristine increased when the DES volume increased from 50 to 80 μL. With further increase in DES volume, the EF presented a strongly downtrend. It is clear that by increasing the volume of DES, the volume of the floated phase increases. By increasing the volume of floated phase, the enrichment factor decreases because of dilution effect. Thus 80 μL of DES was used as the optimal volume.
3.4. Choice of sample solution pH
Vincristine with pKa = 8.66, 10.85 has a weakly basic property and so the sample solution pH should be adjusted in alkaline range to ensure that vincristine molecule present in their charge less form which leads to an increase in their transfer from the aqueous solution into DES.11 The effect of pH on the EF of the vincristine was studied within the pH range of 5–12. The pH adjustment was carried out in the presence of phosphate buffer, ammonium buffer and carbonate buffer. As can be seen in Fig. 1D, the maximum EF was observed when the sample solution pH was higher than 10. Therefore, pH of 10.5 was selected.
3.5. Salt effect
The effect of salt addition on the extraction efficiency of vincristine was studied in the NaCl solution with different concentrations ranged from 0–10% (w/v). As shown in the Fig. 1E, for concentrations of 0 to 6% sodium chloride, the EF is almost unchanged, Because saling-out both extracts more vincristine and increases the EF (positive effect), and by reducing the solubility of DES in the aqueous phase increases the final volume of DES and ultimately the amount of EF (negative effect). These two contrasting effects make vincristine's efficiency almost constant. For higher amounts of salt, the negative effect prevails on positive effect and the EF decreases. As a result, the experiments were performed without salt addition.
3.6. Effect of vortex time
To determine the best duration for the extraction efficiency of vincristine, vortex times ranging from 0 to 30 min were examined. In the 20 min, the maximum EF was achieved and after that, little change was observed (Fig. 1F). At times longer than 20 min, a small decrease in EF is observed. Because of the prolonged DES dispersion time in solution, complete separation of the two phases by centrifugation is not performed. Therefore, the vortex time of 20 min was chosen as the optimum time.
3.7. Analytical figures of merit
Under the optimized conditions, the analytical performance characteristics of the VA-DLLME method including linearity (LR), limit of detection (LOD), limit of quantification (LOQ), accuracy, precision (intra-day and inter-day), EF and EE are listed in Table 1.
Analytical characteristics of VA-DLLME-DES followed by HPLC-UV for determination of vincristine.
| Parameter | Analytical feature |
|---|---|
| Linear range (μg L−1) | 0.06–300 |
| RSD% (intra-day, n = 7) | 3.7 |
| RSD% (inter-day, n = 7) | 5.9 |
| Accuracy% (intra-day, n = 7) | 92.0–108.6 |
| Accuracy% (inter-day, n = 7) | 91.4–105.3 |
| r 2 | 0.9986 |
| Limit of detection (μg L−1) (S/N = 3, n = 7) | 0.02 |
| Limit of quantification (μg L−1) (S/N = 10, n = 7) | 0.06 |
| Extraction efficiency (%) | 88.4 |
| Enrichment factor | 110.5 |
According to the results, linearity was ranged from 0.06–300 μg L−1 with r2 of 0.9986. The precision and accuracy of the proposed method was tested by analysing plasma samples at different concentration levels (low, medium and high) in the linear range of the calibration curve. For intra-day and inter-day, preparation and analysis of blood samples were repeated 7 times on the same day and 3 consecutive days, respectively. For this purpose, a certain amount of vincristine was added to blood samples and extracted by the presented method and then analysed. The results showed RSDs for intra-day and inter-day were 3.7% and 5.9%, respectively. The accuracy of inter-day and intra-day for above experiments ranged from 92.0–108.6% and 91.4–105.3, respectively.
The LOD (signal-to-noise ratio of 3) and LOQ (signal-to-noise ratio of 10) for the vincristine were 0.02 μg L−1 and 0.06 μg L−1 respectively. The LLOQ for vincristine was 0.06 μg L−1 with accuracy 95.3%. The EF and EE% were 110.5 and 88.4%, respectively.
3.8. Analysis of real samples
To demonstrate the capability of the VA-DLLME-DES, the procedure was applied to the analysis of vincristine in different brands of drug and plasma samples. Since vincristine is one of the main drugs in the treatment of leukaemia and unfortunately the main brands of the drug are not available recently, we decided to first examine the active ingredient of the drug in the brands used. The results are then compared to provide documented information about these drugs to oncologists. For this purpose, Richter and Cipla vincristine, which are the most consumed in Iran, were analysed. The results showed that the active ingredient of the drug in both brands are almost comparable.
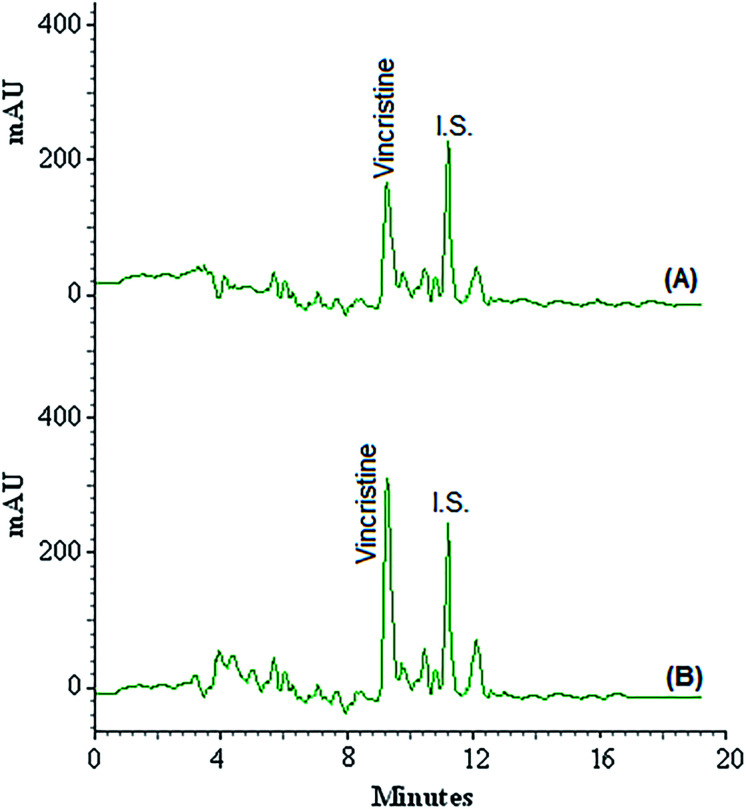
Four plasma samples were taken from 4 children (2 girls and 2 boys aged from 10 to 16 years) with leukaemia who were treated with vincristine at Dr Mohammad Kermanshahi Hospital from Kermanshah, Iran. The results are collected in Table 2 and show that all plasma samples contained vincristine in the range of 2.5 ± 0.3 to 13.2 ± 1.1 mg L−1. Due to the high concentration of vincristine in the plasma samples and the departure of the linear range of the calibration curve, all samples were diluted 200 times before using the VA-DLLME-DES method. The aim of this work was to develop a microextraction method for extracting very small amounts of drugs in biological samples. The patients in the study due to receiving high doses of the vincristine, the concentration of the vincristine in their blood was high and out of linear range of the method calibration curve. So we had to dilute the samples. Fig. 2 shows the chromatograms of plasma sample taken from 10 year-old girl (A) and the corresponding spiked ones at concentration of 5.0 mg L−1 for vincristine (B). Accuracy was calculated as the relative errors for the analysis of known amounts of vincristine added to actual plasma samples using the presented procedure. Relative recovery (RR%) of vincristine in these samples (spiked with different amounts of vincristine) is summarized in Table 2, ranging from 92.0 to 108.6%. The results revealed that the matrices of plasma had little effect on the VA-DLLME-DES for determination of vincristine.
Determination of vincristine in plasma samples and relative recovery of spiked vincristine in these samplesa.
| Plasma samples | Added (mg L−1) | Found, mean ± SDb (n = 3) (mg L−1) | Relative recovery (%) |
|---|---|---|---|
| Taken from a 10 year-old girl | 0 | 5.2 ± 0.4 | — |
| 5 | 10.6 ± 1.2 | 107.7 | |
| Taken from a 12 year-old boy | 0 | 9.3 ± 0.8 | — |
| 10 | 20.1 ± 1.6 | 108.6 | |
| Taken from a 13 year-old girl | 0 | 2.5 ± 0.3 | — |
| 15 | 17.3 ± 1.4 | 92.0 | |
| Taken from a 16 year-old boy | 0 | 13.2 ± 1.1 | — |
| 20 | 34.1 ± 2.4 | 106.8 |
These data are based on the diluted volumes of plasma samples and dilution effect was considered for calculation of them.
Standard deviation.
Fig. 2. Chromatograms of plasma sample taken from 10 year-old girl (A) and the corresponding spiked ones at concentration of 5.0 mg L−1 (B) obtained by using VA-DLLME combined HPLC-UV.
3.9. Comparison of VA-DLLME-DES with those previous techniques
The VA-DLLME-DES followed by HPLC-UV is compared with other microextraction techniques for preconcentration and analysis of vincristine in different samples. As shown in Table 3, one of the important advantages of this method is the lower detection limit and better linear range than other previous methods. Extensive reduction of toxic and expensive organic solvents is one of the important advantages of this method compared to other methods. The relative standard deviation of this method is less than other methods, except for one case which is equal to it. Another important advantage of this method is that, unlike the conventional DLLME, it does not require a disperser solvent. These features prove that VA-DLLME-DES is an easy, cheap and reproducible method that can be employed for the extraction and preconcentration of vincristine in biological fluids.
Comparison of VA-DLLME-DES with other extraction methods for determination of vincristine in biological samples.
| Methods | LODa (μg L−1) | LRb (μg L−1) | RSDc% | Matrix | Extractant volume (μL) | Reference |
|---|---|---|---|---|---|---|
| SPE-LC-MSd | 0.09 | 0.18–180 | <20 | Infant plasma | 1000 | 1 |
| CA-HPLC-DADe | 18.9 | 236–1180 | 3.7 | Human serum | 3000 | 2 |
| SPE-UHPLC-MS/MSf | — | 0.2–400 | <9.8 | Human serum | 1000 | 3 |
| SBME-HPLC-UVg | 15 | 50–5000 | <5.8 | Plasma and urine | — | 11 |
| SPE-HPLC-ECDh | 0.4 | 1–50 | 4.8 | Mononuclear cells | — | 10 |
| VA-DLLME-HPLC-UV | 0.02 | 0.06–300 | 3.7 | Plasms of children | 80 | This work |
LOD, limit of detection.
LR, linear range.
RSD, relative standard deviation.
Solid phase extraction-liquid chromatography-mass spectroscopy.
Chemometrics-assisted high performance liquid chromatography coupled with diode array detector.
Solid phase extraction-ultra-high performance liquid chromatography-tandem mass spectrometry.
Solvent bar microextraction-high performance liquid chromatography-ultraviolet detection.
Solid phase extraction-high performance liquid chromatography-electrochemical detection.
Live subject statement
The authors state that all experiments were performed in compliance with the relevant laws and institutional guidelines. The research ethics committee of Kermanshah University of Medical Sciences has approved the experiments about live subjects (Code of Ethics: IR.KUMS-REC. 1399.1125). The authors also state that informed consent was obtained for any experimentation with human subjects and Kermanshah University of Medical Sciences is committed to the protection and safety of human subjects involved in research.
4. Conclusions
In this work, a hydrophobic DES as extractant for VA-DLLME followed by liquid chromatography has been used for the extraction and analysis of vincristine in plasms samples. In this method, a DES consisting of TOMAC and n-butanol with 1 : 3 molar ratio, was very effective for preconcentration of the vincristine. In this method, with a very small volume of extraction solvent that is non-toxic, extraction efficiency is higher than previous methods. The application of presented method in the analysis of vincristine in plasms indicated that this method was reputable and appropriate for the extraction of drugs in trace levels. In addition, the method can be used to the determination of different drugs in other intricate samples.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the Research Council of Kermanshah University of Medical Sciences (Grant Number: 990969) for the financial. Also, the authors appreciate the Research Center for Environmental Determinants of Health (RCEDH) for their cooperation in the analysis of real samples.
References
- Schmidt M. S. Huang R. Classon R. J. Murry D. J. J. Pharm. Biomed. Anal. 2006;41:540–543. doi: 10.1016/j.jpba.2005.11.039. [DOI] [PubMed] [Google Scholar]
- Liu Z. Wu H.-L. Li Y. Gu H.-W. Yin X.-L. Xie L.-X. Yu R.-Q. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2016;1026:114–123. doi: 10.1016/j.jchromb.2015.08.008. [DOI] [PubMed] [Google Scholar]
- Yang F. Wang H. Liu M. Hu P. Jiang J. J. Chromatogr. A. 2013;1275:61–69. doi: 10.1016/j.chroma.2012.12.026. [DOI] [PubMed] [Google Scholar]
- Conter V. Valsecchi M. G. Silvestri D. et al. . Lancet. 2007;369:123–131. doi: 10.1016/S0140-6736(07)60073-7. [DOI] [PubMed] [Google Scholar]
- Rahmani R. Zhou X.-J. Cancer Surv. 1993;17:269–281. [PubMed] [Google Scholar]
- Crom W. R. de Graaf S. S. N. Synold T. et al. . J. Pediatr. 1994;125:642–649. doi: 10.1016/S0022-3476(94)70027-3. [DOI] [PubMed] [Google Scholar]
- Teuffel O. Kuster S. P. Hunger S. P. Conter V. Hitzler J. Ethier M.-C. Shah P. S. Beyene J. Sung L. Leukemia. 2011;25:1232–1238. doi: 10.1038/leu.2011.84. [DOI] [PubMed] [Google Scholar]
- Sethi V. S. Jackson Jr D. V. White D. R. Richards II F. Stuart J. J. Muss H. B. Cooper M. R. Spurr C. L. Cancer Res. 1981;41:3551–3555. [PubMed] [Google Scholar]
- Sulphate V., The American Society of Health-System Pharmacists, 2015, retrieved January 2, 2015 [Google Scholar]
- Groninger E. Koopmans P. Kamps W. de Graaf S. Uges D. Ther. Drug Monit. 2003;25:441–446. doi: 10.1097/00007691-200308000-00004. [DOI] [PubMed] [Google Scholar]
- Kiani M. Qomi M. Hashemian F. Rajabi M. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2018;1072:397–404. doi: 10.1016/j.jchromb.2017.10.054. [DOI] [PubMed] [Google Scholar]
- Sun Y.-M. Wu H.-L. Wang J.-Y. Liu Z. Zhai M. Yu R.-Q. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2014;962:59–67. doi: 10.1016/j.jchromb.2014.05.027. [DOI] [PubMed] [Google Scholar]
- Guilhaumou R. Solas C. Rome A. Giocanti M. Andre N. Lacarelle B. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2010;878:423–427. doi: 10.1016/j.jchromb.2009.12.015. [DOI] [PubMed] [Google Scholar]
- Schmidt M. S. Huang R. Classon R. J. Murry D. J. J. Pharm. Biomed. Anal. 2006;41:540–543. doi: 10.1016/j.jpba.2005.11.039. [DOI] [PubMed] [Google Scholar]
- Guilhaumou R. Solas C. Rome A. Giocanti M. Andre N. Lacarelle B. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2010;878:423–427. doi: 10.1016/j.jchromb.2009.12.015. [DOI] [PubMed] [Google Scholar]
- Pirsaheb M. Fattahi N. Shamsipur M. Khodadadi T. J. Sep. Sci. 2013;36:684–689. doi: 10.1002/jssc.201200872. [DOI] [PubMed] [Google Scholar]
- Gómez-Canela C. Ventura F. Caixach J. Lacorte S. Anal. Bioanal. Chem. 2014;406:3801–3814. doi: 10.1007/s00216-014-7805-9. [DOI] [PubMed] [Google Scholar]
- Ramírez J. Ogan K. Ratain M. J. Cancer Chemother. Pharmacol. 1997;39:286–290. doi: 10.1007/s002800050574. [DOI] [PubMed] [Google Scholar]
- Ahmadi-Jouibari T. Fattahi N. Food Addit. Contam., Part A. 2015;32:1140–1147. doi: 10.1080/19440049.2015.1049565. [DOI] [PubMed] [Google Scholar]
- Rezaee M. Assadi Y. Milani Hosseini M.-R. Aghaee E. Ahmadi F. Berijani S. J. Chromatogr. A. 2006;1116:1–9. doi: 10.1016/j.chroma.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Ahmadi-Jouibari T. Fattahi N. Shamsipur M. J. Pharm. Biomed. Anal. 2014;94:145–151. doi: 10.1016/j.jpba.2014.01.044. [DOI] [PubMed] [Google Scholar]
- Ataee M. Ahmadi-Jouibari T. Fattahi N. Int. J. Environ. Anal. Chem. 2016;96:271–283. doi: 10.1080/03067319.2016.1150464. [DOI] [Google Scholar]
- Sadeghi M. Nematifar Z. Irandoust M. Fattahi N. Hamzei P. Barati A. Ramezani M. Shamsipur M. RSC Adv. 2015;5:100511–100521. doi: 10.1039/C5RA15311E. [DOI] [Google Scholar]
- Karimaei M. Sharafi K. Moradi M. Ghaffari H. R. Biglari H. Arfaeinia H. Fattahi N. Anal. Methods. 2017;9:2865–2872. doi: 10.1039/C7AY00530J. [DOI] [Google Scholar]
- Taheri S. Jalali F. Fattahi N. Jalili R. Bahrami G. J. Sep. Sci. 2015;38:3545–3551. doi: 10.1002/jssc.201500636. [DOI] [PubMed] [Google Scholar]
- Ahmadi-Jouibari T. Fattahi N. Shamsipur M. Pirsaheb M. J. Pharm. Biomed. Anal. 2013;85:14–20. doi: 10.1016/j.jpba.2013.06.030. [DOI] [PubMed] [Google Scholar]
- Pirsaheb M. Fattahi N. Shamsipur M. Food Control. 2013;34:378–385. doi: 10.1016/j.foodcont.2013.05.013. [DOI] [Google Scholar]
- Cunha S. C. Fernandes J. TrAC, Trends Anal. Chem. 2018;105:225–239. doi: 10.1016/j.trac.2018.05.001. [DOI] [Google Scholar]
- Akramipour R. Golpayegani M. R. Gheini S. Fattahi N. Talanta. 2018;186:17–23. doi: 10.1016/j.talanta.2018.04.042. [DOI] [PubMed] [Google Scholar]
- Pirsaheb M. Fattahi N. RSC Adv. 2018;8:11412–11418. doi: 10.1039/C8RA00912K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golpayegani M. R. Akramipour R. Fattahi N. J. Pharm. Biomed. Anal. 2021;193:113735. doi: 10.1016/j.jpba.2020.113735. [DOI] [PubMed] [Google Scholar]
- Li G. Row K. H. TrAC, Trends Anal. Chem. 2019;120:115651. doi: 10.1016/j.trac.2019.115651. [DOI] [Google Scholar]
- Santana-Mayor A. Rodríguez-Ramos R. Herrera-Herrera A. V. Socas-Rodríguez B. Rodríguez-Delgado M. A. TrAC, Trends Anal. Chem. 2021;134:116108. doi: 10.1016/j.trac.2020.116108. [DOI] [Google Scholar]
- Makoś P. Słupek E. Gębicki J. Microchem. J. 2020;152:104384. doi: 10.1016/j.microc.2019.104384. [DOI] [Google Scholar]
- Grau J. Azorín C. Benedé J. L. Chisvert A. Salvador A. J. Sep. Sci. 2022;45:210–222. doi: 10.1002/jssc.202100609. [DOI] [PubMed] [Google Scholar]