CONSPECTUS:
Represented by pacemakers, implantable electronic devices (CIEDs) are playing a vital life-saving role in modern society. Although the current CIEDs are evolving quickly in terms of performance, safety, and miniaturization, the bulky and rigid battery creates the largest hurdle toward further development of a soft system that can be attached and conform to tissues without causing undesirable physiologic changes. Over 50% of patients with pacemakers require additional surgery procedures to replace a drained battery. Abrupt battery malfunction and failure contributes up to 2.4% of implanted leadless pacemakers. The battery also has risks of lethal interference with diagnostic magnetic resonance imaging (MRI). Applying the implantable nanogenerators (i-NGs) technology to CIEDs is regarded as a promising solution to the battery challenge and enables self-powering capability. I-NGs based on the principle of either triboelectricity (TENG) or piezoelectricity (PENG) can convert biomechanical energy into electricity effectively. Meanwhile, a complete heartbeat cycle provides a biomechanical energy of ~0.7 J or an average power of 0.93 W, which is sufficient for the operation of CIEDs considering the power consumption of 5–10 μW for a pacemaker and 10–100 μW for a cardiac defibrillator. It is therefore practical to leverage the effective, soft, flexible, lightweight, and biocompatible i-NGs to eliminate the bulky battery component in CIEDs and achieve self-sustainable operation. In this rapidly evolving interdisciplinary field, materials innovation acts as a cornerstone that frames the technology development. Here we bring a few critical perspectives regarding materials design and engineering, which are essential in leading the NG-powered CIEDs toward clinical translations. This Account starts with a brief introduction of the cardiac electrophysiology, as well as its short history to interface the state-of-the-art cardiac NG technologies. Three key components of NG-powered CIEDs are discussed in detail, including the NG device itself, the packaging material, and the stimulation electrodes. Cardiac NG is the essential component that converts heartbeat energy into electricity. It demands high-performance electromechanical coupling materials with long-term dynamic stability. The packaging material is critical to ensure a long-term stable operation of the device on a beating heart. Given the unique operation environment, a few criteria need to be considered in its development, including flexibility, biocompatibility, antifouling, hemocompatibility, and bioadhesion. The stimulation electrodes are the only material interfacing the heart tissue electrically. They should provide capacitive charge injection and mimic the soft and wet intrinsic tissues for the sake of stable biointerfaces. Driven by the rapid materials and device advancement, we envision that the evolution of NG-based CIEDs will quickly move from epicardiac to intracardiac, from single-function to multifunction, and with a minimal-invasive implantation procedure. This trend of development will open many research opportunities in emerging materials science and engineering, which will eventually lead the NG technology to a prevailing strategy for powering future CIEDs.
Graphical Abstract
INTRODUCTION
The first clinical implantation of pacemakers was achieved in 1958, which paved the way for the rapid evolution of cardiac implantable electronic devices (CIEDs).1 CIEDs, including pacemakers, implantable cardioverter defibrillators (ICDs), and cardiac resynchronization therapy (CRT) devices, soon became a popular approach for treating cardiac diseases.2 Nowadays, benefiting from the rapid advancements of microelectronics and materials technology, CIEDs are showing an even more significant growth of implementations for patients with bradyarrhythmias, ventricular tachyarrhythmias, and advanced systolic heart failure. Despite their rapid application pace, the long-standing battery challenge still largely restricts the devices from further advancement. Batteries with bulky sizes contributed a large volume and weight in early pacemakers, limiting their wearability and implantability. This challenge was partially alleviated by the development of high-energy-density batteries, such as lithium carbon monofluoride, allowing smaller devices. However, the longevity of miniaturized batteries still remains a concern. Over 50% of all patients with pacemakers require additional surgery procedures to replace the battery due to energy depletion.3 Abrupt battery malfunction and failure contributes up to 2.4% of implanted leadless pacemakers.4 The downsides of battery also involve lethal interference with diagnostic magnetic resonance imaging (MRI) and compromised biocompatibility due to lack of flexibility.
To enable long-term operation of implantable medical devices, wireless in vivo charging is becoming an increasingly popular approach, represented by electromagnetic energy transfer, ultrasound energy transfer, and optical energy transfer.5–7 They are primarily used for health monitoring of non-life-threatening cases. Because battery charging largely relies on the patients, CIEDs used for critical health issues are still exclusively designed with primary batteries to eliminate human-related device failures. Therefore, the ultimate solution to the battery challenge of next generation CIEDs is to enable a care-free self-powering capability that operates life-long.
In recent years, the rise of human-powered electronics concept may provide an ideal solution to the battery challenge faced by CIEDs. Harvesting biomechanical energy and subsequently converting it to electricity is showing increasing promises for battery replacement. Indeed, there is abundant biomechanical energy in human bodies, particularly the continuous Watt-level biomechanical energy from heartbeats, which is sufficient for most CIEDs.8 Implantable nanogenerators (i-NGs) based on the principles of either piezoelectricity (PENG) or triboelectricity (TENG) have been developed to convert biomechanical energy from body motions into electricity.9–14 Compared to other energy harvesting technologies, i-NGs are highly efficient, flexible, and lightweight, exhibiting excellent compatibility with biological tissues. To date, i-NGs have been demonstrated with real-time cardiac monitoring capability;15–18 generating sufficient electricity to power pacemakers;18–22 and directly providing biphasic electricity beyond the threshold for heart stimulations.23 Therefore, adapting the i-NG technology to CIEDs is considered a promising solution to flexible self-powered cardiac devices.
So far, technologies for self-powered cardiac devices have been reviewed in a few articles, mostly from the device innovation standing point.11,24 Fundamentally, materials design and engineering draw the boundary where this technology may advance. Therefore, this Account tries to fill this gap by providing a few critical materials perspectives in the design strategies of NG-powered CIEDs. After a brief introduction of the cardiac electrophysiology, we discuss soft and flexible CIEDs based on state-of-the-art NG technology. Then, materials design and engineering of the key components of self-powered CIEDs, including the NG part, package, and stimulation electrodes, are discussed aiming toward clinical applications. Finally, this Account concludes with visions and outlooks for the next-generation self-powered CIEDs.
NG AS A SELF-SUSTAINABLE CARDIAC POWER SUPPLY
NG was recently developed as a new technology for converting biomechanical energy (e.g., muscle stretching, breathing, heart beating, etc.) into electrical energy. Compared to other energy-harvesting (e.g., biofuel cells, electromagnetics or thermo-electrics) and wireless charging technologies, the NG offers superior energy conversion efficiency and a much simpler design. With these advantages, it can be made small and flexible. The NG utilizes nanostructured functional materials as building blocks to generate electricity from physical displacement via the piezoelectric or triboelectric effect. Both principles are based on dielectric materials and use the surface-induced charge to output electricity.
The human body is a rich source of biomechanical energy. Of all organs and tissues, the heart is the most reliable and suitable site for biomechanical energy harvesting because of its continuous and consistent movement with relatively large displacements. The heartbeat is well regulated through an intrinsic electrical system, which coordinates the alternative contraction and relaxation of atria and ventricles for normal functions. As presented in Figure 1a, the system starts with the natural pacemaker cell (sinoatrial node, SA node) located in the right atrium producing an electrical pulse, which spreads through the walls of the atria and causes them to contract. This contraction happens a split second after the P wave in the ECG profile, providing an uneven strain (up to 50%) in atrial area.25 The electrical signal further travels through the atrioventricular node (AV node) to the His-Purkinje network of both ventricles and initiates their contractions later. These motions are marked by the QRS complex, which are mostly concentrated at ventricular area with a strain ranging from 20% (circumferential/longitudinal) to 70% (radial).26 All together, they form a complete heartbeat cycle, providing a biomechanical energy of ~0.7 J or an average power of 0.93 W.8,27 The excellent consistency and abundancy of the biomechanical energy from heartbeats is ideal for powering CIEDs.
Figure 1.
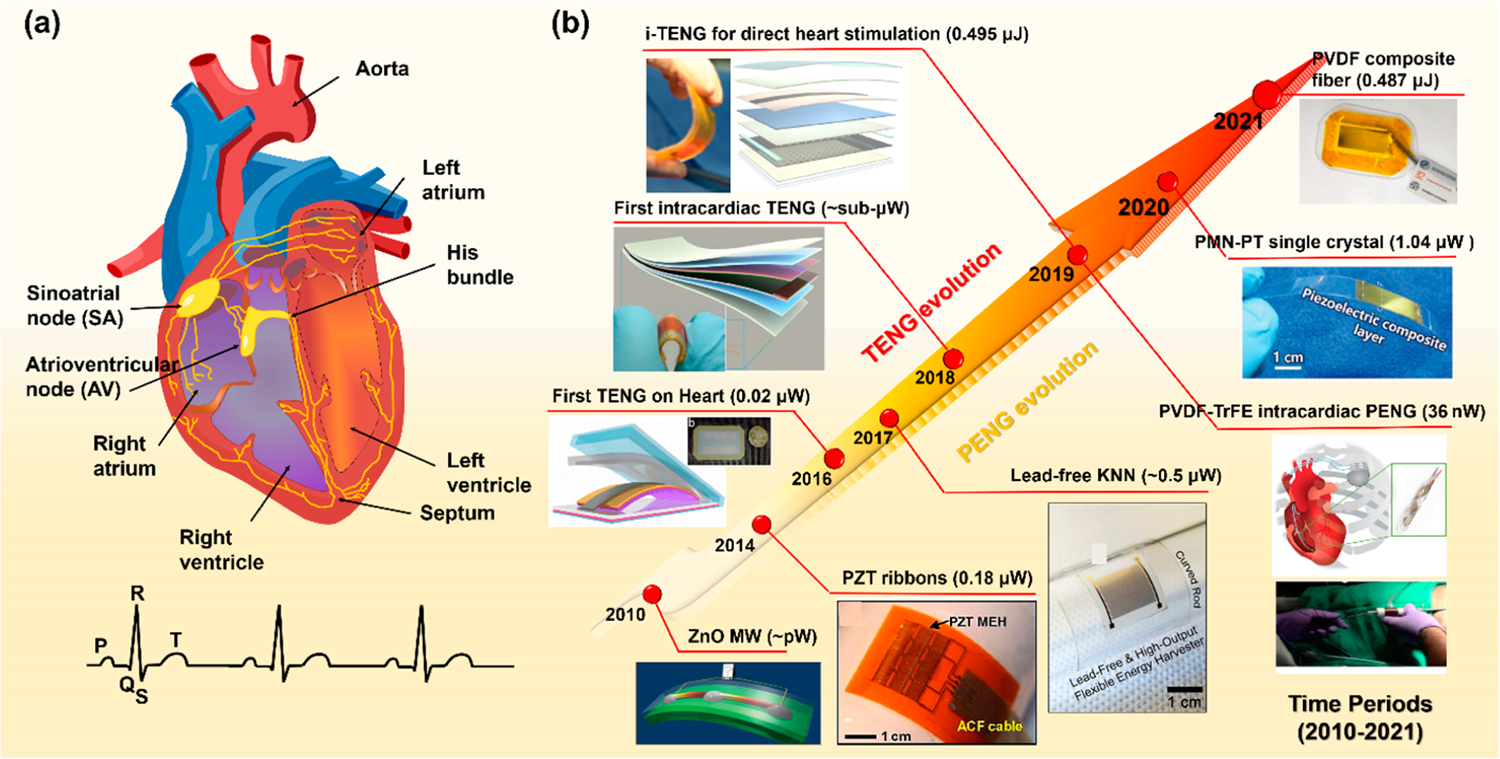
Cardiac conducction system and self-powered CIEDs. (a) The schematic of cardiac conduction system. (b) The evolution of cardiac NGs and self-powered CIEDs based on TENG (top left) and PENG (bottom right) technologies. The energy/power outputs are given as per cm2. Reproduced with permission from ref 29. Copyright 2009 Springer Nature. Reproduced with permission from ref 30. Copyright 2014 National Academy of Sciences. Reproduced with permission from ref 15. Copyright 2016 American Chemical Society. Reproduced with permission from ref 31. Copyright 2017 American Institute of Physics. Reproduced with permission from ref 17. Copyright 2019 Wiley-VCH. Reproduced with permission from ref 34. Copyright 2019 Elsevier. Reproduced with permission from ref 20. Copyright 2020 Wiley-VCH. Reproduced with permission from ref 22. Copyright 2021 Elsevier. Reproduced with permission from ref 19. Copyright 2019 Springer Nature.
Rapid advancements have been witnessed in both PENG and TENG for cardiac applications shortly after the NG technology was introduced (Figure 1b). The first demonstration of PENG on heart appeared in 2010 with a single ZnO microwire (MW) device.28,29 It was later followed by a series of developments based on ceramic perovskites, including PZT ribbons,30 lead-free potassium sodium niobate (KNN) films,31 and the relaxor PMN–PZT films.20,21 Over decades, by selecting and engineering higher performance ferroelectric material building blocks, the outputs of PENG have been improved over orders of magnitude from pW-level (ZnO) to μW-level (PMN–PT). This PMN–PT-based membranous NG conformed to the porcine heart and the power generated by harvesting the heartbeat was able to power up and operate a commercial pacemaker. Compared to materials evolution in PENG designs, the development of TENG has been more focused on structural configuration. It evolved from a simply packaged TENG in 201432 to a multilayered patch in 2019 with an output of up to ~65 V.19 It can be clearly seen that both NG technologies evolved differently toward in vivo high power generation as their different materials property requirements. Here, we discuss the development of both technologies toward potential practical clinical applications.
Materials for PENGs
Piezoelectric materials generate electricity from internal ionic displacements in response to mechanical stress. It is typically built on a single material component with a pair of electrodes to collect the charge along designed polarization directions (Figure 2a). Taking advantage of fruitful fundamental studies on the piezoelectricity and biocompatibility of ZnO nano-crystals, the first cardiac NG was made from a ZnO MW, supported by a soft polyimide substrate with package (Figure 2b).28 However, ZnO has a low electromechanical coupling (d33 < 10 pC/N) as well as a high rigidity. As such, only small electrical outputs (3 mV and 30 pA) were obtained from the heartbeat of rats. Those intrinsic limitations largely prohibited its further developments. Compared to ZnO, β-phase polyvinylidene fluoride (PVDF) has much better flexibility and piezoelectric property (d33 = 20–30 pC/N). When wrapped around the ascending aorta of the porcine heart, a PVDF film could produce an output voltage and current of 1.5 V and 300 nA, respectively (Figure 2c).33 This sub-μW level output power is a few orders of magnitude higher than those from ZnO, yet still cannot reach the required power level of most CIEDs (1–100 μW).22,34 As the mainstream of high-performance piezoelectric materials, ceramic perovskites (d33 up to 1000 pC/N) are still a preferred choice for many PENG developments. Despite their high brittleness, processing them to the nano/micro-level and compositing with a polymer matrix has been broadly used to bring desired flexibility to the devices. Thin film lead zirconate titanate (PZT)-based PENGs have been designed and applied epicardially to the cardiac system (Figure 2d–i).18 From such a flexible ceramic system, the normal porcine heartbeat could deliver an electric output up to 17.8 V and 1.75 μA (Figure 2d–ii), adequate for the operation of most CIEDs. Nevertheless, the biggest concern is the inclusion of toxic elements. This is a huge huddle for applying this group of materials inside the human body. Lead-free perovskite, such as KNN, has been exploited as an alternative. A lithium doped KNN film with interdigitated electrodes could generate 5 V and 0.7 μA electricity from the porcine heartbeats at ~80 bpm (Figure 2d–iii),31 slightly lower than PZT-based devices yet still high enough for most CIEDs. There are many other biocompatible perovskite-based materials such as BaTiO3 and LiNbO3, and a variety of doped materials can be potentially leveraged as building blocks for the futuristic cardiac PENGs.
Figure 2.
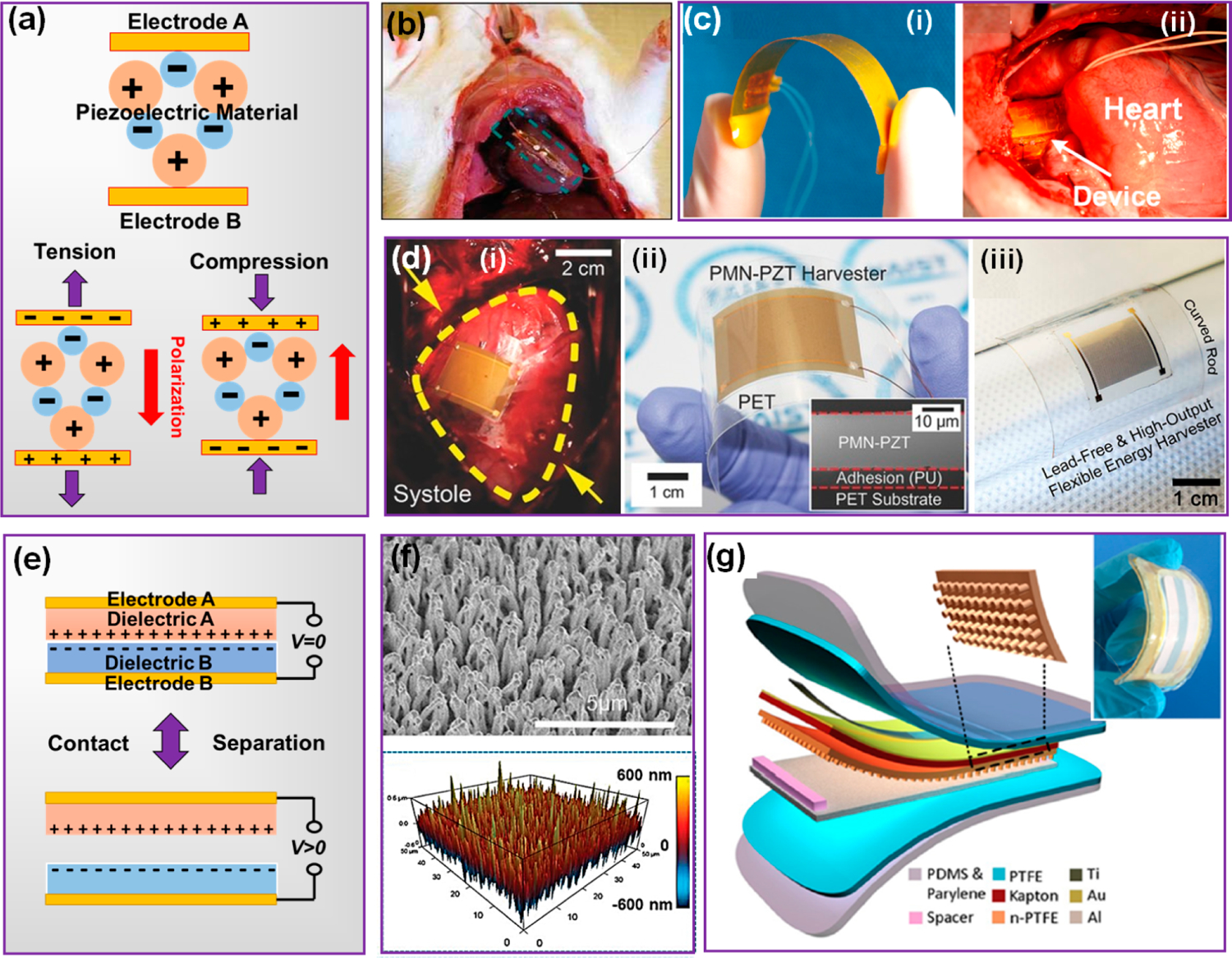
NG as a self-sustainable power supply. (a) Working mechanism of PENG. (b) ZnO-MW based i-PENG harvesting energy from a rat heart. Reproduced with permission from ref 28. Copyright 2010 Wiley-VCH. (c) Digital image of a PVDF-based NG (i) and its cardiac energy harvesting by wrapping around the aorta (ii). Reproduced with permission from ref 33. Copyright 2016 Elsevier. (d) Perovskite ceramic-based i-PENGs: (i) a PZT-based PENG operating on a porcine heart and (ii) a PENG built on PMN–PT thin films (Reproduced with permission from ref 18. Copyright 2017 Wiley-VCH); (iii) a PENG built on lead-free KNN thin films. (Reproduced with permission from ref 31. Copyright 2017 American Institute of Physics). (e)Working mechanism of CS-mode TENG. (f) Dense nanowire array created by ICP on a polymer surface. Reproduced with permission from ref 17. Copyright 2019 Wiley-VCH. (g) Multilayered TENG designed for cardiac applications. Reproduced with permission from ref 16. Copyright 2016 American Chemical Society.
Materials for TENGs
Compared to PENG, TENG typically has a much broader material selection. The triboelectric principle requires two dissimilar dielectric materials that have different electron affinities (Figure 2e). Contact of these two materials induces surface charges with opposite polarities. Once separated, the electric potential will be induced between the two back electrodes. The triboelectric activities of different materials have been well documented. Rather than materials engineering, the development of TENG for cardiac applications are primarily focused on surface engineering of strong tribo-active films to improve the induced charge densities, as well as device configurations to achieve high responsivity to heartbeats. Two surface engineering strategies are generally adopted in cardiac TENG designs. One way is to increase the contact surface area by creating nano/microscale surface structures to improve the effective surface charge density. Inductively coupled plasma (ICP) etching is a commonly used method to turn flat polymer surface into nanowire arrays (Figure 2f).17 A 3–4-fold boost in both voltage output and current output could typically be achieved by this approach. This strategy uses high-energy ion bombardment to randomly remove polymer molecules from the surface and to fuse remaining molecules into fine wire bundles. As this process does not have strong materials correlation, it can be effectively applied to many different tribo-active polymer materials, including polytetrafluoroethylene (PTFE), fluorinated ethylene propylene (FEP), and polyimide (Kapton). It becomes a nearly standard approach for high-performance TENG fabrication.
Another strategy is to directly inject charges into the triboelectric materials, such as by corona poling.17 This approach does not change any surface features but only drives high-energy electrons or ions deep into the polymer layer. Since the surface charge densities of polymers are usually far below their maximum surface charge densities (determined by the breakdown electrical field of air), this approach is effective for most polymers to improve their triboelectric charge density. Typically, a 3–4-fold output improvement can be expected by this approach as well, and the enhancement would remain effective for millions of cycles up to years-long. Furthermore, these two approaches could be applied simultaneously to double the enhancement effect for TENG output. In a design of a symbiotic cardiac TENG, the triboactive PTFE layer was first treated with ICP to create dense nanowire arrays on the surface and further injected charges by a corona poling under a high voltage (5 kV).19 As a result, when attached to left ventricle of a porcine heart (at ~82 bpm), the implanted TENG generated a remarkably high voltage of 65.2 V and a current of 0.5 μA, together yielding 0.495 μJ of energy. It exceeded the endocardial pacing threshold energy (0.377 μJ), indicating the capability of direct pacing without any addition energy storage components.
As heart has a nonflat geometry and a unique moving pattern, it is important for TENG to be designed with a configuration that can sensitively respond to heartbeats. While the TENG configurations include various modes, the contact-separation (C–S) mode is the most common one in an implanted system due to its simple structure and minimal space requirement. Most TENGs harvesting cardiac energy are designated as a patch which could be either attached on the atrium/ventricle surface or inserted below the apex. Early development of cardiac TENGs adopted planar triboelectric films with a single-layer package.32 The planar sheet-like geometry, however, was not able to conformally attach to the soft and curvy heart surface. Meanwhile, the 50 μm single-layered PDMS package was not effective for preventing biofluid infiltration. Therefore, this design only yielded moderate outputs of 2–3 V and ~0.1 μA, with a short unspecified lifetime. A triboelectric layer fixed by a titanium strip was later introduced to the cardiac TENG design, which significantly raised the heart-driven electric outputs to 10 V and 4 μA (Figure 2g).16 This improvement was a result of the resilient titanium strip that enabled better adherence to the heart surface and more responsive internal motions of the device. A multilayer design of PTFE and PDMS polymers was applied to the package, which considerably improved the mechanically robust and insulation against biofluids.
Dynamic Stability
A healthy heart beats >3 billion times in lifetime. Cardiac tissues consistently experience dynamic mechanical stimuli. The stability of the NG materials under dynamic strain is rather critical for practical applications for CIEDs. Therefore, it is necessary to show the dynamic stability of any cardiac NGs to be developed. At a minimum, the dynamic stability should be evaluated ex vitro, such as in a simulated liquid environment. Targeting for a 10-year lifetime of a cardiac i-NG, it will subject to 3.15 × 108 times straining given an average heartbeat rate of 60 bpm. Testing such a large number is not practical for typical lab-based research. Increasing the frequency of strain may compromise the time constraint and provide a reasonable estimation. A continuous ultrasonic bath filled with biological solution, such as saline or PBS may serve this purpose. It typically operates at a frequency from 40 to 50 kHz. One hour of ultrasound bath ideally corresponds to the number of straining actions equivalent to heartbeats in 5 years and may provide the first-principle experimental evaluation.35 Nevertheless, it should be noted that the displacements from ultrasonic waves and human heartbeat are significantly different from each other. The heart tissue experiences much higher strain (50–60%)26 during each cardiac cycle. A more accurate test needs to be conducted at the same level of strain, while a higher frequency is still helpful to reduce the testing time. For example, 10 million continuous straining cycles of a NG at a frequency of 50 Hz could provide ~1-year operation lifespan at a heartbeat rate of 60 bpm. It should also be noted that the higher frequency provides a higher straining rate, providing more intensive testing conditions to the NGs. As thus, the equivalent lifetime should be much longer than the testing period, though there is no quantitative scaling factor being established between the straining rate and lifetime so far. We noticed that most current developments of i-NG are still mostly focused on the outputs. More attention needs to be placed on the dynamic stability of the devices, and more quantified testing criteria need to be established. The stability evaluation should also cover both the electromechanical functional materials and the packaging materials.
PACKAGING MATERIALS FOR NG-POWERED CIEDS
Due to the special implantation environment, CIEDs have high requirements on packaging materials for protecting the electronics from the biological surroundings and protecting the surrounding tissue from electrical or chemical influences. A titanium shell is often used in current CIEDs, providing a biocompatible and inert interface at the implantation sites. Obviously, this type of package is rigid, and mechanically incompatible to the soft tissue. It is intrinsically unsuitable to systems that need to be flexible and/or mobile, such as i-NGs. Therefore, in addition to good biocompatibility, the packaging materials for NG-powered CIEDs need to provide long-term hermetic protection together with tissue-matching flexibility and stable biointerfaces.
Flexible Biomaterials Package with Desired Biocompatibility
A good understanding of materials–tissue interaction is necessary for selecting and designing package materials. Foreign materials that do not belong to the body may trigger a foreign-body reaction (FBR) (Figure 3a), which is usually accompanied by inflammation and capsulations.36 Specifically, when a foreign material is interfaced with a biological system, it would be recognized as invasive substances and quickly be covered by a layer of proteins (e.g., collagens, laminins, and fibronectin), and may trigger the immune system to phagocytize and digest the implants (inflammation). The adjacent environment of the implantation then becomes harsh and acidic because of the released reactive oxygen species (ROS) and degradative enzymes. If the foreign material is too big to digest, the macrophages fuse to giant cells to secrete cytokines and induce fibroblasts to deposit a dense collagen layer forming a capsule.
Figure 3.
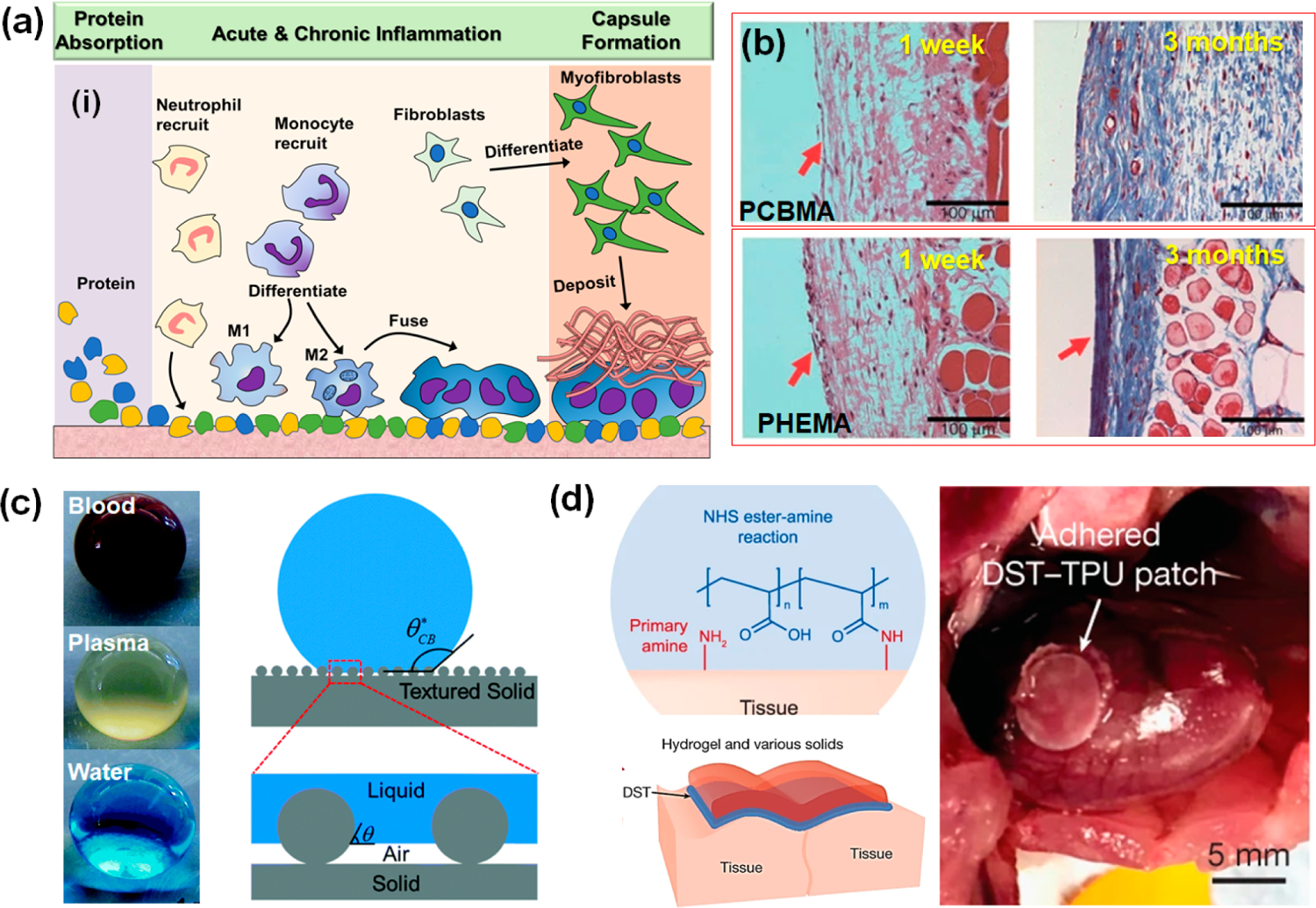
Packaging materials for i-NG and CIEDs. (a) Foreign-body reaction schematics. (b) H&E staining images of PCBMA zwitterionic hydrogel and PHEMA hydrogel implanted for 1 week and 3 months in rats. Reproduced with permission from ref 42. Copyright 2013 Springer Nature. (c) Super-repellent surface bringing hemocompatibility by creating nanoscale textures with trapped air pockets. Reproduced with permission from ref 46. Copyright 2019 Royal Society of Chemistry. (d) Bioadhesive made of hydrogel material and its stable adhesion on a beating rat heart in vivo. Reproduced with permission from ref 49. Copyright 2019 Springer Nature.
A biomaterials package could significantly reduce the inflammation. A group of polymeric biomaterials, such as polydimethylsiloxane (PDMS), Parylene C, polyimide (PI), and PTFE have been proved generally compatible to the in vivo tissue environment. Their dielectric nature could provide good insulation to the systems preventing charge leakage, meanwhile offering significantly enhanced softness and flexibility compared to a metal package. Therefore, these polymeric materials have all been used for i-NG packaging with confirmed biocompatibility. Some new package materials have also been used currently on implantable devices. However, they are either less stable with higher water/air permeability (such as Ecoflex and Silbione) or have a short protection period far less than the lifetime of typical CIEDs (such as dimethacrylate-functionalized perfluoropolyether).37,38 Usually, basic biocompatibility assessment starts with cytotoxicity test by cell culture assays prior to in vivo tests. Although a variety of biocompatibility tests have been used for the evaluation of biomedical materials and devices, the in vitro cytotoxicity test, and the in vivo implantation test are the most representative and effective tests for the NG-based devices. In a typical study, the adherence, growth, and viability of rat smooth muscle cells (SMCs) were examined on a PI packaged PZT PENG.30 Although PZT contains toxic elements, the PI film coating allowed the SMCs to well adhere to the device surface with evident spreading and intact cytoskeletal structures. A high cell viability after 9 days of culture confirmed the in vitro biocompatibility. Similar cellular biocompatibility has also been reported in other common polymeric NG package materials.
The in vivo biocompatibility test is necessary to confirm the implantation potential of polymer-packaged NGs. Multiple techniques need to be implemented together to reveal the compatibility from different perspectives. In a representative case, PDMS and PDMS/Parylene-C packaged PVDF NGs were implanted inside ICR mice for up to six months.39 A whole-body 3D projection CT first revealed the packaged NGs stayed at the original place while the surrounding tissue remained normal (no apparent density changes) over the implantation period. Hemotoxylin and Eosin (H&E) staining was used to examine the tissue inflammation, where a good biocompatible package should not show any obvious infiltrations of lymphocytes in the adjacent tissues including skin, tissue, and muscle layers. Periodic blood and serum tests are also important to evaluate the physiological and biochemical states.
Antifouling Property
Although biopolymer encapsulation can effectively lower inflammation, a fibrous capsule from macrophages, fibroblasts, and capillaries would still likely build up. It could severely affect the operation of the i-NG, as the capsule attenuates mechanical stimuli and limits the device sensitivity and energy harvesting capability. Therefore, antifouling is an important property for NG-powered CIEDs in order to keep a long-term steady operation and provide a reliable performance.
Blocking nonspecific protein absorption prevents all following FBR processes, including the fibrous capsule. One material strategy that can potentially suppress fouling is an antifouling coating. For instance, poly(ethylene glycol) (PEG) and poly(ethylene oxide) (PEO) coating can increase surface hydrophilicity and thus reduce binding with proteins.40 A hydrogel is another type of soft material that can achieve antifouling, primarily due to its hydrophilic nature that resists protein absorption.41 Due to their high flexibility, hydrogels are particularly suitable for i-NG development. Among all kinds of hydrogels, zwitterionic hydrogels have been found capable of completely eliminating nonspecific protein adsorption. A study of poly(carboxybetaine methacrylate) (PCBMA) zwitterionic hydrogel revealed its excellent nonfouling quality.42 When implanted in C57BL/6 mice, the PCBMA hydrogel induced almost zero inflammatory response in the first week and did not elicit any capsule formation even after three months (Figure 3b). This is due to its electrostatically induced surface hydration that repels protein from adsorption. It is also worth mentioning that the PCBMA hydrogel has a tissue-matching modulus down to ~0.16 MPa, favorably interfacing the supporting tissues. Other hydrogel coatings such as the PEG-based hydrogel, poly(N-vinylpyrrolidone)-based (PVP-based) hydrogel, and enzyme-based hydrogel have revealed antifouling performances as well.43 However, while their performances are inferior to that of the nonfouling zwitterionic ones, their practicality was limited by the less inherent stability and biocompatibility.
Rather than just being protected, the biphasic electrical output of NGs may actively eliminate biofouling directly.44 The mechanism was suggested to be the electrical disturbance from the NG signals to the electrical double layer. Disturbing the surface charges at the liquid–solid interface can effectively suppress the initial formation of the conditioning layer and then prevent a later stage of protein and microbe attachments. Although no study has been conducted in a biological environment, this study opened a new possibility for preventing biofouling without introducing additional coating toward the NG device. This strategy may be particularly promising for NG-powered CIEDs, which have high requirements on materials surfaces, interfaces, and device architecture.
Hemocompatibility
Current implantable cardiac devices are primarily positioned outside of the heart, or epicardially. Accordingly, most reported cardiac NGs were also epicardial devices conforming to the surface of heart muscles. Typically, implantation of this kind of cardiac device requires a complex and very invasive surgery, which may come with severe complications such as infections, air leaks, and respiration failures. Development of next generation cardiac devices is moving toward endocardiac, and thereby the implantation can be achieved through a less-invasive catheterization. For NG applications, the internal mechanical motions and blood pressure fluctuations inside the heart also provide substantial mechanical energy. While the application potential is promising, as the device is in direct contact with blood, the hemocompatibility of the packaging material is critical in order to avoid disrupting the blood cells (hemolysis) or activating the coagulation pathways (thrombo-genicity).
In an early attempt of transcatheter NG, hemolytic and clotting reactions were evaluated on its PDMS encapsulation in vitro for up to 4 h.17 The average hemolysis rate of the PDMS package (1.08%) was below the standard (5%) set by international standards organization (ISO). Imaging analysis showed that red blood cells (RBCs) retained a good cell morphology on the package surface. Very few adhered platelets retaining the round morphology were observed and they had no abnormalities. These initial results demonstrated a good hemocompatibility of the PDMS package in a short period of time. Nevertheless, so far, there is no long-term in vivo study on the hemocompability of flexible devices.
Surface engineering to increase the hydrophobicity and oleophobicity is an effective approach to improve the hemocompatibility. Super-repellent (SR) surfaces have recently been demonstrated with excellent hemocompatibility.45,46 The SR surface was typically designed by creating nanoscale textures with a high number of trapped air pockets between the liquid–solid interface (Figure 3c). It can significantly reduce the blood cell or platelet adhesion by minimizing the blood–solid interfacial contact area. SR surfaces can also induce a slip effect to RBCs and platelets, which alters the shear stresses at the blood–solid interface, leading to less damage to both cells. Take the PDMS material as an example, super-repellent surfaces were fabricated by femtosecond laser ablation combined with soft lithography, atomic layer deposition assisted sacrificial etching, and CO2-pulsed-laser ablation. Compared to pristine PDMS surfaces, such SR PDMS surfaces exhibited an 87% reduction in the number of adhered platelets together with no platelet activation. Therefore, creating SR surfaces may be a promising solution for designing appropriate package materials for blood-contacting intracardiac CIEDs.
Bioadhesion
A stable and uniform attachment to the supporting tissue is another important practical tissue for implantable devices. Currently, a stable attachment is mostly achieved by sutures. For commercial pacemakers, the pulse generator is accommodated in a subcutaneous pocket, while the leads are inserted through subclavian vein puncture. Both leads and pulse generator in the underlying tissue are secured by non-absorbable sutures to prevent migration or twiddler’s syndrome. Inevitably, sutures would create punctures through the device. Though just in the packaging material, these punctures could act as the weak spots, where damages initiate. This is particularly problematic for soft or polymer-based materials due to their low mechanical strength. In some cases, a fibrous capsule from tissue regrowth is considered favorable to further stabilized the implants over a long period. Nevertheless, as operation of an i-NG needs to effectively translate the motions of supporting tissue to its own strain, it places a higher requirement on the attachment. As mentioned earlier, a fibrous capsule needs to be avoided. The constant straining action of the i-NG repeatedly imposes highly localized stress at the stitch spots and jeopardizes the structure integrity. Therefore, it is essential to implement a suture-free procedure for NG-powered CIEDs in order to sustain a stable and reliable in vivo performance.
State-of-the-art bioadhesives built on hydrogels could offer strong interfacial bonding as well as excellent flexibility and may potentially address the attachment challenge of i-NGs. The bioadhesive hydrogels also have intrinsic properties that are very close to the soft and wet tissues which may improve the biocompatibility at biointerfaces.47 Yuk et al. exploited a design strategy to chemically anchor the long-chain polymer network of a tough hydrogel onto a solid surface by covalent cross-linking the hydrogel to a silane-modified surface.48 Through this approach, a series of polyacrylamide (PAAm)-based hydrogels exhibited exceptional bonding energy (~1000 J m−2) with various materials including glass, silicon, aluminum, titanium, and ceramics. Further, a hydrogel adhesive was recently created from networks of biopolymer (gelatin or chitosan) and cross-linked poly(acrylic acid) (grafted with N-hydrosuccinimide ester), showing excellent adhesion stability to various biological tissues such as skin and muscle (Figure 3d).49 The strong adhesion was attributed to the covalent cross-linking between the N-hydroxysuccinimide ester group in the hydrogel and the amine groups on the tissue surface. An ex vivo porcine heart experiment showed that the hydrogel can bond devices stably onto the constantly moving heart surface, while allowing the device to perform its full function (drug delivery) over 12 h. Long-term in vivo experiments on rat hearts confirmed its high stability, with mild inflammations. This pioneer investigation suggested that adding a thin layer of bioadhesive hydrogel to the insulating package material could be a beneficial feature for packaging NG-powered CIEDs. It can avoid potential suture damage, while enabling a quick and stable implantation.
ELECTRODE MATERIALS DESIGN FOR DELIVERING STIMULATION
Electrodes interfacing tissues to deliver electricity (charge) are indispensable in CIEDs. There are two primary mechanisms of charge delivery/injection at the electrode–tissue interface,50 as illustrated in Figure. 4a. The first one is the Faradaic charge injection, where charges are transferred between the electrode and tissue by Faradaic reduction or oxidation. Faradaic injection is associated with forming new products in the tissue/biofluids. Another mechanism is the capacitive charge injection which involves the charging and discharging at the biointerface. The charging/discharging behavior was largely determined by the capacitance of electrical double layer at the interface. As the Faradaic reaction generates toxic species, particularly ROS that causes tissue damages, it should be avoided, and the capacitive manner is more desired in tissue stimulations.
Figure 4.
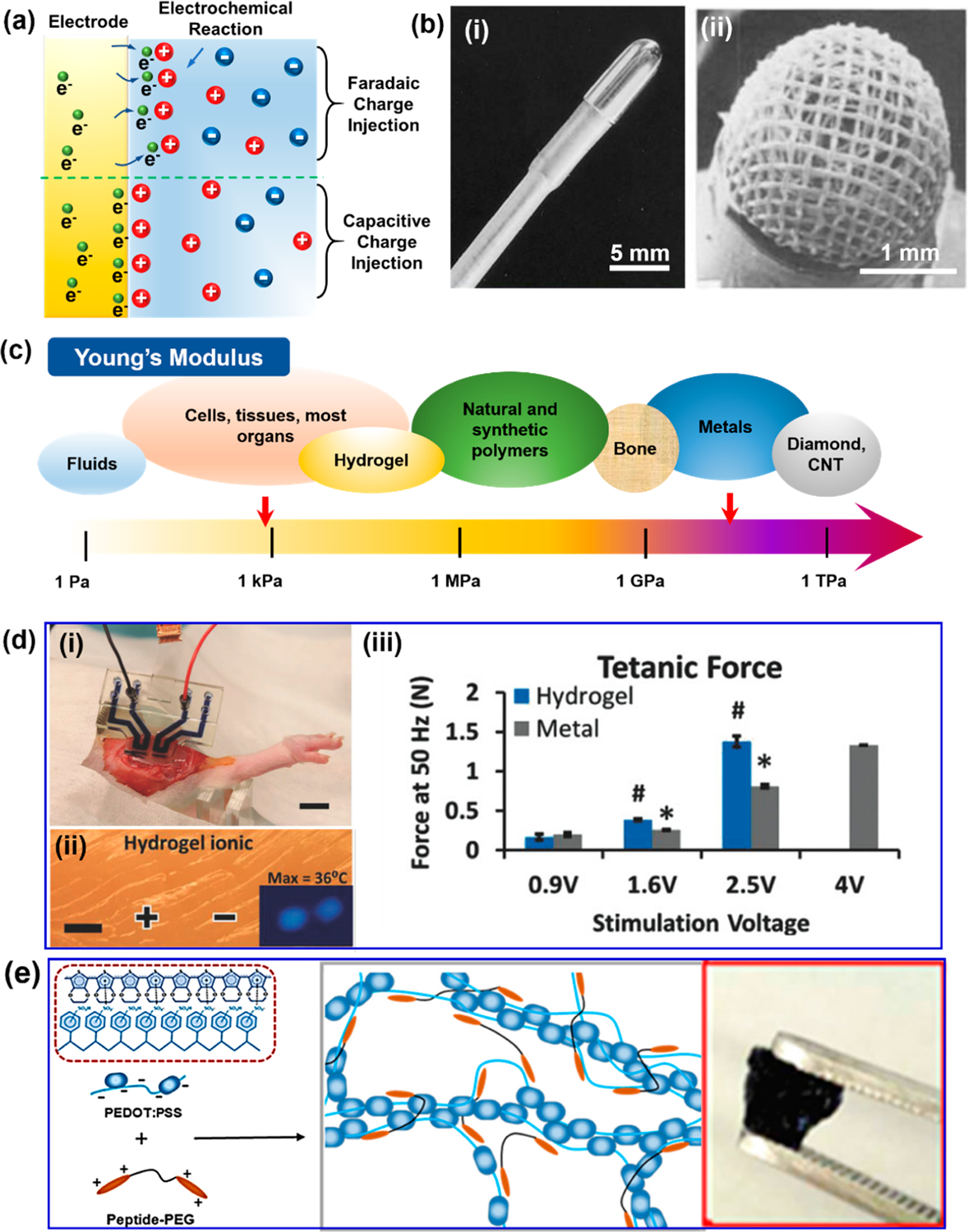
Electrode materials design for delivering stimulation. (a) Two mechanisms of charge delivery/injection at the electrode–tissue interface. (b) Different designs of stimulation electrode facing biotissues. (i) Electrode with a small contact area. (ii) Porous electrode. Reproduced with permission from ref 51. Copyright 2014 Wiley-VCH. (c) Young’s modulus comparison of common materials. (d) Ionic conductive hydrogel as stimulation electrode. (i) Hydrogel electrodes stimulating rat tissue. (ii) Ionic hydrogel electrodes reduce local heating damage. (iii) Tetanic forces of rat tibialis anterior muscle generated by the hydrogel electrodes and standard gold electrodes (control). Reproduced with permission from ref 53. Copyright 2018 Wiley-VCH. (e) Schematic showing the electronic conductive hydrogel made of peptide and PEDOT:PSS. The right inset is a photo of this hydrogel. Reproduced with permission from ref 54. Copyright 2018 American Chemical Society.
Noble Metal Electrodes
Traditional pacemakers use noble metals as the stimulating electrodes, such as gold (Au), platinum (Pt), and iridium (Ir).51 They all have high electric conductivity, high resistance to corrosion, and excellent biocompatibility.50 Particularly, Pt and Pt-based alloys (typically with a small amount of Ir) are widely used in CIEDs due to their large charge storage capability (300–350 μC/cm2). For the electrode design, a small electrode contact area (Figure 4b–i) is favorable as it provides a large current density, lowers the stimulation thresholds, decreases the battery drain, and thereby extends the battery lifetime. Some early pacemakers even confined the exposed electrode area to the tip of pacing leads. A trade-off of this configuration is its small surface area that decreases the double layer capacitance and the charge storage capacity, while a large reversible charge storage capacity is desired to avoid the irreversible Faradaic reactions. A solution is to introduce porosity to the electrodes (Figure 4b–ii). Nano- or microscale porosity in electrodes could remarkably increase the surface area in contact with biofluids, while keeping the same effective stimulation area in contact with tissue. Thus, an improved charge storage capacity and a high stimulating current density are achieved simultaneously.
Although noble metal electrodes bring excellent stability and high efficiency to cardiac stimulations, the mechanical modulus of the electrodes is drastically different from the surrounding tissues. While the cardiac tissues are soft with a Young’s modulus of ~100 kPa, the moduli of metal electrodes are usually beyond 10 GPa (Figure 4c).47,52 This huge mismatch risks the destruction of local tissue environment, inflammation, and formation of scar tissue. Moreover, the rigidity nature limits the conformality of electrode–tissue contacts, which may significantly raise the impedance at the interface or create detachment. This can lead to increased stimulation threshold, more power consumption, and potentially lower stimulation efficacy.
Conductive Hydrogel Electrodes
As discussed previously, hydrogels are soft, wet, flexible, and stretchable, closely mimicking the tissue behavior. A hydrogel can be made conductive, which may serve well as a new electrode candidate for CIEDs. Two types of conductive hydrogels could be applied. The ionic conductive hydrogel is the most common one. Its high water content and mesoporous network structure allow the dissolved ionic species to transport freely, enabling a high conductivity. Stimulation electrodes have been successfully built on ionic conductive hydrogel.53 A circuit based on a sodium salt/PEG hydrogel electrode was used for rat muscle stimulation (Figure 4d–i). Once the hydrogel electrodes were conformed to the rat tissue, they generated the same level of tetanic force (1.38 N) at a lower voltage (2.5 V) compared to bipolar gold electrodes (1.33 N at 4 V) (Figure 4d–ii). Meanwhile, ionic hydrogel electrodes could reduce adverse effects, particularly the local heating damage due to high-current injection (Figure 4d–iii). A main concern of this kind of conductive hydrogel is the diffusion of ions to tissue, which will cause biocompatibility issues as well as unstable electrode performances in the long run. Another more promising conductive hydrogel is the electroconductive hydrogel. A hydrogel based on a conducting polymer, poly(3,4-ethylenedioxythiophene):polystyrenesulfonate (PEDOT:PSS), has been used as electrodes for cellular stimulation.54 The conductive hydrogel was prepared by combing peptide solution with PEDOT:PSS to enhance the conductivity (Figure 4e). It was able to deliver electrical stimulations to the Mesenchymal stromal cells and enhance their differentiation with minimal cytotoxicity. Typical hydrogel nano-composites often have impaired mechanical properties and inhomogeneous electrical properties. Compared to other conductive polymers (such as PPy and PANi), the PEDOT:PSS possesses more tunable and improved conductivity by secondary doping (1.5 × 10−3 to 4000 S/m), together with excellent chemical stability and good biocompatibility.55
So far, there are no experimental demonstrations of conductive hydrogel for CIED applications, while it is foreseeable that the conductive hydrogels should be able to provide desired conformal attachment to cardiac tissue with enhanced interfacial compatibility. Compared to metal electrodes, hydrogel electrodes are far less implemented. Its conductivity (10−5 to 103 S/m) is still orders of magnitude lower than metals (107 S/m), which imposes high power consumption.55 It may be a more substantial challenge for i-NGs as the energy delivered by a NG is rather limited in general and a high delivery efficiency is always a major concern. Here, we have not discussed any surface-area- and charge-density-related design issues as those mentioned for metal electrodes, mostly because this factor has not been investigated in these very early stage developments. Nevertheless, it is still an essential aspect to consider in more practical device developments, and how conductive hydrogen can satisfy those charge density and capacity requirements is a very interesting question for materials and device engineering.
OUTLOOKS
Six decades of evolution of CIEDs have brought them from a simple asynchronous device to a highly integrated, intelligent, and complex system. The next generation of CIEDs is moving toward a miniaturized, flexible, conformal, and less invasive system. While many materials and device innovations are pushing CIEDs closer to this goal, the battery technology still stands as a major roadblock. As discussed in this Account, the emerging cardiac i-NGs are a promising solution for replacing the batteries and realizing completely flexible and self-sustainable CIEDs. It is exciting to see that μW-level electricity output has been achieved by both TENG and PENG technologies, reaching the minimal level of power requirement of most CIEDs.
Nevertheless, current i-NGs are still far from practical clinical applications. The main perspectives of materials developments toward potential clinical translations of self-powered CIEDs are discussed in this Account. The core component of NGs demands high-performance electromechanical coupling materials with long-term dynamic stability. Soft materials with biocompatibility, antifouling, hemocompatibility, and bioadhesion are desired for packaging or encapsulation. Electrode materials directly interfacing tissues should provide capacitive charge injection and mimic the soft and wet intrinsic tissues for the sake of stable biointerfaces. While state-of-the-art technologies and associated challenges and opportunities are discussed in each category, here we further consider the key research and development directions of self-powered CIEDs toward their eventual clinical applications.
From Invasive to Minimally Invasive
Most current self-powered CIEDs are in the form of a large-sized patch. Its implantation still requires an invasive thoracotomy procedure that is associated with many potential complications. The large size also often needs suturing to affix it at the implantation site over a long operation period. Device miniaturization is the first essential step that the implantation procedure may be improved to be less invasive. The key is the NG materials, as the electrical output is directly related to the surface area of the electromechanical function material. Using materials with stronger electromechanical coupling is a typical way to improve the output power densities of NGs. Due to many other material property restrictions for an implantable device, the room for seeking for new material replacement is already small. Creating more complex 3D heterogeneous structures may possess a better possibility to improve the power density. For example, multilayered structures with a large number of microscale interfaces may substantially improve the charge output per specific area. Moreover, suture-free is another preferred feature for less invasive implantation. Integrating conductivity, adhesion, and electromechanical coupling together may provide new materials solutions for the next generation of miniaturized self-powered CIEDs.
From Epicardiac to Intracardiac
Intracardiac/endocardial application is an alternative and promising strategy to minimize the invasivity of the implantation surgery, which can be achieved by transcatheter procedures. In terms of mechanical energy harvesting, the energy availability is at least equally abundant compared to the epicardia conditions. In addition to the heart muscle strain energy from regular heartbeats, blood pressure fluctuation and blood flow provide additional routes for energy harvesting, bringing in more design possibilities for i-NGs. As discussed in this Account, the biggest challenge for intracardiac CIEDs is the dynamic and pressurized blood environment. This places much higher requirements for the packaging materials of intracardiac NGs and CIEDs. The functional materials of NGs and the package materials all need to maintain a stable mechanical elasticity and electric or dielectric properties over the entire operational lifetime (up to ten years for clinical applications). This long-term dynamic stability needs to be reevaluated in a blood environment at the pressure that at least matches the highest human blood pressure (50 kPa or higher).56 So far, understanding the dynamic stability of either packaging materials or electromechanical coupling materials in pressurized blood is still very limited. We do not know if current packaging or functional materials can survive or how long they may survive under this harsh operation conditions. As more intracardiac NGs or other electronic devices are developed, we may encounter more materials challenges regarding this critical requirement. It will not be surprising to see that the materials’ dynamic stability arises as the main hurdle for transmitting CIEDs endocardially for clinical applications.
From Single Function to Multifunction
Current pacemakers are highly integrated, intelligent, and complex systems with multiple functionalities. The device not only senses the real-time movement of the heart but also provides adaptive pacing depending on their activities. Discussion of i-NGs in this Account are focused on the power source for pacemakers. Indeed, the unique correlation between the electric output from a NG with the heartbeat or blood pressure offer more functionality capabilities, such as sensing, monitoring, and even direct electrostimulation. Recent research has demonstrated such possibilities. For example, an implantable triboelectric active sensor (iTEAS) was developed and applied on pig hearts, where the generated electricity exhibited a 99% accuracy to ECG waves.16 It is thus feasible for such a device to directly serve as a CIED for real-time monitoring the cardiac physiological information. Furthermore, the high-voltage and low-current electrical outputs from NGs make them a good candidate for direct electrostimulations. Some high-performance NGs, such as those built on ferroelectric relaxors, may be able to produce electric peaks exceeding the stimulation threshold of regulate heartbeat without involving any additional electronics and power sources. An in vitro demonstration showed that instantaneous electrical energy of 2.7 μJ per pulse from a NG was able to trigger heart contraction.23 This is a closed-loop stimulation in terms of regulation and energy flow. It may be very effective to quickly adjust heartbeats to the optimal rhythms. So far, as the in vivo output from a NG is still far from optimal, how to design a fully implantable NG-based pacemaker to achieve effective in vivo stimulation is still challenging.
In general, we envision that the self-powering capability enabled by the NG technology for biomechanical energy harvesting from heartbeats may become one prevailing strategy in future CIEDs. New materials or composites with tissue-like flexibility, high electromechanical coupling, and long-term stability and biosafety are exciting opportunities for materials innovations in this field. Success along this emerging research direction will establish a materials framework necessary to move self-powered CIEDs to clinical applications.
ACKNOWLEDGMENTS
This publication was supported by the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health under Award Numbers R01EB021336 and R01HL157077. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Biographies

Jun Li received his B.E. degree in Materials Science and Engineering at Zhejiang University, China, in 2016. He is now a Ph.D. student of Materials Science and Engineering at University of Wisconsin—Madison, under the supervision of Prof. Xudong Wang since September, 2016. His current research interests focus on the nanogenerator design and innovation for implantable medical system, 3D printing ferroelectri materials, and piezoelectric biomaterials.

Xudong Wang is now a Professor and Associate Chair of Materials Science and Engineering at University of Wisconsin—Madison. He received his Ph.D. degree in Materials Science and Engineering from Georgia Tech in 2005. His current research interests include understanding the coupling effect between piezoelectric polarization and semiconductor functionalities; developing advanced nanomaterials and nanodevices for mechanical energy harvesting from human activities and ambient environment; and studying the growth mechanisms and developing assembly techniques of oxide nanostructures. He is the author of more than 150 publications and holds more than 10 patents on oxide and piezoelectric materials processing and applications.
Footnotes
Complete contact information is available at: https://pubs.acs.org/10.1021/accountsmr.1c00078
The authors declare no competing financial interest.
REFERENCES
- (1).Mulpuru SK; Madhavan M; McLeod CJ; Cha YM; Friedman PA Cardiac Pacemakers: Function, Troubleshooting, and Management Part 1 of a 2-Part Series. J. Am. Coll. Cardiol 2017, 69, 189–210. [DOI] [PubMed] [Google Scholar]
- (2).Steffen MM; Osborn JS; Cutler MJ Cardiac Implantable Electronic Device Therapy Permanent Pacemakers, Implantable Cardioverter Defibrillators, and Cardiac Resynchronization Devices. Med. Clin. North Am 2019, 103, 931–943. [DOI] [PubMed] [Google Scholar]
- (3).Dean J; Sulke N Pacemaker battery scandal Much can and should be done to maximise the longevity of existing devices. BMJ-BRIT MED J. . 2016, 352, i228. [DOI] [PubMed] [Google Scholar]
- (4).Richter S; Doring M; Ebert M; Bode K; Mussigbrodt A; Sommer P; Husser D; Hindricks G Battery Malfunction of a Leadless Cardiac Pacemaker Worrisome Single-Center Experience. Circulation 2018, 137, 2408–2410. [DOI] [PubMed] [Google Scholar]
- (5).Basaeri H; Christensen DB; Roundy S A review of acoustic power transfer for bio-medical implants. Smart Mater. Struct 2016, 25, 123001. [Google Scholar]
- (6).Khan SR; Pavuluri SK; Cummins G; Desmulliez MPY Wireless Power Transfer Techniques for Implantable Medical Devices: A Review. Sensors 2020, 20, 3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Jawad AM; Nordin R; Gharghan SK; Jawad HM; Ismail M Opportunities and Challenges for Near-Field Wireless Power Transfer: A Review. Energies 2017, 10, 1022. [Google Scholar]
- (8).Starner T Human-powered wearable computing. IBM Syst. J 1996, 35, 618–629. [Google Scholar]
- (9).Li J; Wang XD Research Update: Materials design of implantable nanogenerators for biomechanical energy harvesting. APL Mater. 2017, 5, No. 073801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Yoon HJ; Kim SW Nanogenerators to Power Implantable Medical Systems. Joule 2020, 4, 1398–1407. [Google Scholar]
- (11).Jiang DJ; Shi BJ; Ouyang H; Fan YB; Wang ZL; Li Z Emerging Implantable Energy Harvesters and Self-Powered Implantable Medical Electronics. ACS Nano 2020, 14, 6436–6448. [DOI] [PubMed] [Google Scholar]
- (12).Long Y; Li J; Yang F; Wang JY; Wang XD Wearable and Implantable Electroceuticals for Therapeutic Electrostimulations. Adv. Sci 2021, 8, 2004023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Li J; Long Y; Yang F; Wang X Respiration-driven triboelectric nanogenerators for biomedical applications. EcoMat 2020, 2, No. e12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Li J; Kang L; Long Y; Wei H; Yu YH; Wang YH; Ferreira CA; Yao G; Zhang ZY; Carlos C; German L; Lan XL; Cai WB; Wang XD Implanted Battery-Free Direct-Current Micro-Power Supply from in Vivo Breath Energy Harvesting. ACS Appl. Mater. Interfaces 2018, 10, 42030–42038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Zheng Q; Zhang H; Shi BJ; Xue X; Liu Z; Jin YM; Ma Y; Zou Y; Wang XX; An Z; Tang W; Zhang W; Yang F; Liu Y; Lang XL; Xu ZY; Li Z; Wang ZL In Vivo Self-Powered Wireless Cardiac Monitoring via Implantable Triboelectric Nanogenerator. ACS Nano 2016, 10, 6510–6518. [DOI] [PubMed] [Google Scholar]
- (16).Ma Y; Zheng Q; Liu Y; Shi BJ; Xue X; Ji WP; Liu Z; Jin YM; Zou Y; An Z; Zhang W; Wang XX; Jiang W; Xu ZY; Wang ZL; Li Z; Zhang H Self-Powered, One-Stop, and Multifunctional Implantable Triboelectric Active Sensor for Real-Time Biomedical Monitoring. Nano Lett. 2016, 16, 6042–6051. [DOI] [PubMed] [Google Scholar]
- (17).Liu Z; Ma Y; Ouyang H; Shi BJ; Li N; Jiang DJ; Xie F; Qu D; Zou Y; Huang Y; Li H; Zhao CC; Tan PC; Yu M; Fan YB; Zhang H; Wang ZL; Li Z Transcatheter Self-Powered Ultrasensitive Endocardial Pressure Sensor. Adv. Funct. Mater 2019, 29, 1807560. [Google Scholar]
- (18).Kim DH; Shin HJ; Lee H; Jeong CK; Park H; Hwang GT; Lee HY; Joe DJ; Han JH; Lee SH; Kim J; Joung B; Lee KJ In Vivo Self-Powered Wireless Transmission Using Biocompatible Flexible Energy Harvesters. Adv. Funct. Mater 2017, 27, 1700341. [Google Scholar]
- (19).Ouyang H; Liu Z; Li N; Shi BJ; Zou Y; Xie F; Ma Y; Li Z; Li H; Zheng Q; Qu XC; Fan YB; Wang ZL; Zhang H; Li Z Symbiotic cardiac pacemaker. Nat. Commun 2019, 10, 1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Yi ZR; Xie F; Tian YW; Li N; Dong XX; Ma Y; Huang Y; Hu YL; Xu XB; Qu D; Lang XL; Xu ZY; Liu JQ; Zhang H; Yang B A Battery- and Leadless Heart-Worn Pacemaker Strategy. Adv. Funct. Mater 2020, 30, 2000477. [Google Scholar]
- (21).Li N; Yi ZR; Ma Y; Xie F; Huang Y; Tian YW; Dong XX; Liu Y; Shao X; Li Y; Jin L; Liu JQ; Xu ZY; Yang B; Zhang H Direct Powering a Real Cardiac Pacemaker by Natural Energy of a Heartbeat. ACS Nano 2019, 13, 2822–2830. [DOI] [PubMed] [Google Scholar]
- (22).Azimi S; Golabchi A; Nekookar A; Rabbani S; Amiri MH; Asadi K; Abolhasani MMJNE Self-powered cardiac pacemaker by piezoelectric polymer nanogenerator implant. Nano Energy 2021, 83, 105781. [Google Scholar]
- (23).Hwang GT; Park H; Lee JH; Oh S; Park KI; Byun M; Park H; Ahn G; Jeong CK; No K; Kwon H; Lee SG; Joung B; Lee KJ Self-Powered Cardiac Pacemaker Enabled by Flexible Single Crystalline PMN-PT Piezoelectric Energy Harvester. Adv. Mater 2014, 26, 4880–4887. [DOI] [PubMed] [Google Scholar]
- (24).Zheng Q; Tang QZ; Wang ZL; Li Z Self-powered cardiovascular electronic devices and systems. Nat. Rev. Cardiol 2021, 18, 7–21. [DOI] [PubMed] [Google Scholar]
- (25).Morris DA; Takeuchi M; Krisper M; Kohncke C; Bekfani T; Carstensen T; Hassfeld S; Dorenkamp M; Otani K; Takigiku K; Izumi C; Yuda S; Sakata K; Ohte N; Tanabe K; Osmanoglou E; Kuhnle Y; Dungen HD; Nakatani S; Otsuji Y; Haverkamp W; Boldt LH Normal values and clinical relevance of left atrial myocardial function analysed by speckle-tracking echocardiography: multicentre study. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 364–372. [DOI] [PubMed] [Google Scholar]
- (26).Mizuguchi Y; Oishi Y; Miyoshi H; Iuchi A; Nagase N; Oki T The functional role of longitudinal, circumferential, and radial myocardial deformation for regulating the early impairment of left ventricular contraction and relaxation in patients with cardiovascular risk factors: A study with two-dimensional strain Imaging. J. Am. Soc. Echocardiogr 2008, 21, 1138–1144. [DOI] [PubMed] [Google Scholar]
- (27).Starling MR LEFT VENTRICULAR-ARTERIAL COUPLING RELATIONS IN THE NORMAL HUMAN HEART. Am. Heart J 1993, 125, 1659–1666. [DOI] [PubMed] [Google Scholar]
- (28).Li Z; Zhu GA; Yang RS; Wang AC; Wang ZL Muscle-Driven In Vivo Nanogenerator. Adv. Mater 2010, 22, 2534–2537. [DOI] [PubMed] [Google Scholar]
- (29).Yang RS; Qin Y; Dai LM; Wang ZL Power generation with laterally packaged piezoelectric fine wires. Nat. Nanotechnol 2009, 4, 34–39. [DOI] [PubMed] [Google Scholar]
- (30).Dagdeviren C; Yang BD; Su YW; Tran PL; Joe P; Anderson E; Xia J; Doraiswamy V; Dehdashti B; Feng X; Lu BW; Poston R; Khalpey Z; Ghaffari R; Huang YG; Slepian MJ; Rogers JA Conformal piezoelectric energy harvesting and storage from motions of the heart, lung, and diaphragm. Proc. Natl. Acad. Sci. U. S. A 2014, 111, 1927–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Jeong CK; Han JH; Palneedi H; Park H; Hwang GT; Joung B; Kim SG; Shin HJ; Kang IS; Ryu J; Lee KJ Comprehensive biocompatibility of nontoxic and high-output flexible energy harvester using lead-free piezoceramic thin film. APL Mater. 2017, 5, No. 074102. [Google Scholar]
- (32).Zheng Q; Shi BJ; Fan FR; Wang XX; Yan L; Yuan WW; Wang SH; Liu H; Li Z; Wang ZL In Vivo Powering of Pacemaker by Breathing-Driven Implanted Triboelectric Nanogenerator. Adv. Mater 2014, 26, 5851–5856. [DOI] [PubMed] [Google Scholar]
- (33).Cheng XL; Xue X; Ma Y; Han MD; Zhang W; Xu ZY; Zhang H; Zhang HX Implantable and self-powered blood pressure monitoring based on a piezoelectric thinfilm: Simulated, in vitro and in vivo studies. Nano Energy 2016, 22, 453–460. [Google Scholar]
- (34).Dong L; Closson AB; Oglesby M; Escobedo D; Han XM; Nie Y; Huang SC; Feldman MD; Chen Z; Zhang JXJ In vivo cardiac power generation enabled by an integrated helical piezoelectric pacemaker lead. Nano Energy 2019, 66, 104085. [Google Scholar]
- (35).Yu YH; Sun HY; Orbay H; Chen F; England CG; Cai WB; Wang XD Biocompatibility and in vivo operation of implantable mesoporous PVDF-based nanogenerators. Nano Energy 2016, 27, 275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Anderson JM; Rodriguez A; Chang DT Foreign body reaction to biomaterials. Semin. Immunol 2008, 20, 86–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Ahn SH; Jeong J; Kim SJ Emerging Encapsulation Technologies for Long-Term Reliability of Microfabricated Implantable Devices. Micromachines 2019, 10, 508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Liu YX; Liu J; Chen SC; Lei T; Kim Y; Niu SM; Wang HL; Wang X; Foudeh AM; Tok JBH; Bao ZN Soft and elastic hydrogel-based microelectronics for localized low-voltage neuromodulation. Nat. Biomed. Eng 2019, 3, 58–68. [DOI] [PubMed] [Google Scholar]
- (39).Li J; Kang L; Yu YH; Long Y; Jeffery JJ; Cai WB; Wang XD Study of long-term biocompatibility and bio-safety of implantable nanogenerators. Nano Energy 2018, 51, 728–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Zhang HB; Chiao M Anti-fouling Coatings of Poly-(dimethylsiloxane) Devices for Biological and Biomedical Applications. J. Med. Biol. Eng 2015, 35, 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Murosaki T; Ahmed N; Gong JP Antifouling properties of hydrogels. Sci. Technol. Adv. Mater 2011, 12, 064706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Zhang L; Cao ZQ; Bai T; Carr L; Ella-Menye JR; Irvin C; Ratner BD; Jiang SY Zwitterionic hydrogels implanted in mice resist the foreign-body reaction. Nat. Biotechnol 2013, 31, 553–556. [DOI] [PubMed] [Google Scholar]
- (43).Fu M; Liang Y; Lv X; Li C; Yang YY; Yuan P; Ding X Recent advances in hydrogel-based anti-infective coatings. J. Mater. Sci. Technol 2021, 85, 169–183. [Google Scholar]
- (44).Long Y; Yu YH; Yin X; Li J; Carlos C; Du XS; Jiang YD; Wang XD Effective anti-biofouling enabled by surface electric disturbance from water wave-driven nanogenerator. Nano Energy 2019, 57, 558–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Weber M; Steinle H; Golombek S; Hann L; Schlensak C; Wendel HP; Avci-Adali M Blood-Contacting Biomaterials: In Vitro Evaluation of the Hemocompatibility. Front. Bioeng. Biotechnol 2018, 6, 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Movafaghi S; Wang W; Bark DL; Dasi LP; Popat KC; Kota AK Hemocompatibility of super-repellent surfaces: current and future. Mater. Horiz 2019, 6, 1596–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Yuk H; Lu BY; Zhao XH Hydrogel bioelectronics. Chem. Soc. Rev 2019, 48, 1642–1667. [DOI] [PubMed] [Google Scholar]
- (48).Yuk H; Zhang T; Lin ST; Parada GA; Zhao XH Tough bonding of hydrogels to diverse non-porous surfaces. Nat. Mater 2016, 15, 190–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Yuk H; Varela CE; Nabzdyk CS; Mao XY; Padera RF; Roche ET; Zhao XH Dry double-sided tape for adhesion of wet tissues and devices. Nature 2019, 575, 169–174. [DOI] [PubMed] [Google Scholar]
- (50).Merrill DR; Bikson M; Jefferys JGR Electrical stimulation of excitable tissue: design of efficacious and safe protocols. J. Neurosci. Methods 2005, 141, 171–198. [DOI] [PubMed] [Google Scholar]
- (51).Mond HG; Helland JR; Stokes K; Bornzin GA; McVenes R The Electrode-Tissue Interface: The Revolutionary Role of Steroid-Elution. Pace-Pacing and Clinical Electrophysiology 2014, 37, 1232–1249. [DOI] [PubMed] [Google Scholar]
- (52).Lacour SP; Courtine G; Guck J Materials and technologies for soft implantable neuroprostheses. Nat. Rev. Mater 2016, 1, 16063. [Google Scholar]
- (53).Zhao SW; Tseng P; Grasman J; Wang Y; Li WY; Napier B; Yavuz B; Chen Y; Howell L; Rincon J; Omenetto FG; Kaplan DL Programmable Hydrogel Ionic Circuits for Biologically Matched Electronic Interfaces. Adv. Mater 2018, 30, 1800598. [DOI] [PubMed] [Google Scholar]
- (54).Xu Y; Yang XG; Thomas AK; Patsis PA; Kurth T; Krater M; Eckert K; Bornhauser M; Zhang YX Noncovalently Assembled Electroconductive Hydrogel. ACS Appl. Mater. Interfaces 2018, 10, 14418–14425. [DOI] [PubMed] [Google Scholar]
- (55).Peng QY; Chen JS; Wang T; Peng XW; Liu JF; Wang XG; Wang JM; Zeng HB Recent advances in designing conductive hydrogels for flexible electronics. Infomat 2020, 2, 843–865. [Google Scholar]
- (56).Narloch JA; Brandstater ME Influence of breating technique on arterial blood pressure during heavy weight lifting. Arch. Phys. Med. Rehabil 1995, 76, 457–462. [DOI] [PubMed] [Google Scholar]


