Abstract
BACKGROUND:
Retinal venous occlusive diseases have been recognized as a major cause of ocular morbidity. Hyperhomocysteinemia could be a potentially modifiable risk factor.
OBJECTIVE:
To determine the association of hyperhomocysteinemia with central and hemi-central retinal vein occlusion (CRVO and HCRVO), the correlation of serum levels of homocysteine with Vitamin B12 and folate levels and the association of Vitamin B12 deficiency with hyperhomocysteinemia.
METHODS:
In this case–control study, patients with CRVO and HCRVO, and age- and gender-matched controls without CRVO and HCRVO, who met the eligibility criteria, were enrolled after obtaining informed consent. Data obtained from participants using a questionnaire, complete ophthalmological examination and relevant investigations, including estimation of serum homocysteine, Vitamin B12 and folate levels, were collated and analyzed.
RESULTS:
Thirty-nine cases with CRVO and HCRVO and 39 age- and gender-matched controls were studied. We found a significant association of hypertension (P < 0.01), hyperlipidemia (P = 0.01), and abnormal blood profile (P < 0.01) with retinal vein occlusion. There was no statistically significant association of hyperhomocysteinemia with CRVO and HCRVO (P = 0.81). However, we found a high prevalence of both hyperhomocysteinemia (43.58% of cases and 53.84% of controls; P = 0.81) and Vitamin B12 deficiency (23.08% of cases and 38.46% of controls; P = 0.14) in cases and controls, without a statistically significant difference between the two groups with respect to both parameters. Our study also found a negative correlation of serum levels of homocysteine with Vitamin B12 (Pearson correlation co-efficient − 0.3874, P = 0.0005), and folate (Pearson correlation coefficient − 0.3886, P = 0.0004) of the study participants. Among the study participants (n = 78), the odds of patients with Vitamin B12 deficiency having hyperhomocysteinemia were 7.0 (2.26–21.72) times those of patients without Vitamin B12 deficiency (P = 0.001). Similarly, among the cases (CRVO, n = 39), the odds of patients with Vitamin B12 deficiency having hyperhomocysteinemia were 7.0 (1.22–40.09) times those of patients without Vitamin B12 deficiency (P = 0.029). In the control group also (non-CRVO, n = 39), the odds of patients with Vitamin B12 deficiency having hyperhomocysteinemia were 6.67 (1.47–30.21) times those of patients without Vitamin B12 deficiency (P = 0.014).
CONCLUSION:
Hyperhomocysteinemia was not found to be an independent risk factor for retinal vein occlusion in our study. However, we found a high prevalence of hyperhomocysteinemia and Vitamin B12 deficiency in both cases and controls, without a statistically significant difference between the two groups with respect to both parameters. We also found a negative correlation of serum homocysteine levels with Vitamin B12 and folate levels. The odds of patients with Vitamin B12 deficiency having hyperhomocysteinemia were seven times those of patients without Vitamin B12 deficiency. Hypertension, hyperlipidemia, and abnormal blood profile had a significant association with CRVO and HCRVO. Many of the systemic risk factors for retinal vein occlusions are found to be associated with elevation of serum homocysteine levels, which may be part of a final common pathway in bringing about a state of accelerated atherosclerosis, leading to CRVO or HCRVO. Therefore, lowering serum levels of homocysteine by Vitamin B12 and folate supplementation could have a role in the prevention of these diseases.
Keywords: Case–control study, central retinal vein occlusion, folate, hemi-central retinal vein occlusion, homocysteine, Vitamin B12
Introduction
Venous occlusive diseases of the retina have been recognized as a major cause of ocular morbidity.[1] Central retinal vein occlusion (CRVO) and hemi-central retinal vein occlusion (HCRVO) are sight-threatening conditions with a common etiopathogenesis. They arise due to formation of an intravascular thrombus at the level of the lamina cribrosa. Apart from the earlier known risk factors such as advancing age, systemic hypertension and atherosclerosis, elevated homocysteine level in blood has emerged as a possible risk factor of recent interest and research.
Homocysteine is a sulfur-containing, nonprotein amino acid, synthesized in the body from methionine. Elevation in the serum homocysteine concentration can occur due to a variety of causes, including genetic defects in the enzymes required for homocysteine metabolism, nutritional deficiencies, such as deficiencies of vitamin cofactors, systemic conditions such as chronic renal failure, drugs such as nicotinic acid and fibrates, pregnancy and smoking. Elevated homocysteine levels have been recognized to have prothrombotic and atherogenic properties.[2] Vascular changes such as thickening of the intima, disruption of the elastic lamina, hypertrophy of the smooth muscles, and occlusive thrombus formation have been seen in homocysteine-related vascular injury.
Out of the many studies that have been done on this subject, some have shown an association between hyperhomocysteinemia and retinal vein occlusion,[3,4,5] while others have failed to do so.[6,7] The role of Vitamin B12 and folate deficiencies as effect modifiers in this association is also still unclear.[8,9] However, the studies by Lahiri et al. and Narayanasamy et al. found a significant negative correlation of serum Vitamin B12 and folate levels with serum homocysteine levels.[5,10] Hyperhomocysteinemia could, therefore, be a potentially modifiable risk factor for retinal venous occlusive diseases, and a simple intervention such as dietary supplementation of Vitamin B12 and folate could possibly address this risk factor.
The aim of our study was to determine the association of hyperhomocysteinemia with CRVO and HCRVO, the correlation of serum levels of homocysteine with Vitamin B12 and folate levels of the study subjects, and the association of Vitamin B12 deficiency with hyperhomocysteinemia.
Methods
This was a hospital-based, case–control study, conducted from April 2014 to August 2015, at a tertiary eye care center in South India. The study was initiated after obtaining the approval of the Institutional Review Board (IRB Min No. 8731, dated March 6, 2014). Patients with CRVO or HCRVO, diagnosed as per standard diagnostic criteria, and meeting the eligibility criteria, were included in the study. Age- and gender-matched controls without CRVO or HCRVO were randomly selected from the patients in the outpatient clinics of the department. The age of each control was matched to within 2.5 years above or below that of the corresponding case. All patients were of the age group from 18 to 70 years, and those with dense media opacities, retinal vasculitis, kidney dysfunction, history of systemic vasculitis, liver disease, or previous systemic vascular events were excluded. An informed consent was obtained from all the study participants.
A questionnaire was administered to all the participants, followed by a complete ophthalmological examination and relevant investigations. Measurements of blood pressure, height and weight were done, and body mass index (BMI) was calculated. A fasting venous blood sample was collected from all the patients in the study, to estimate serum creatinine, complete blood count, lipid profile, blood sugar, serum homocysteine, Vitamin B12 and folate levels. A 2-h postprandial venous blood sample was also collected to estimate postprandial blood sugar levels. Serum homocysteine levels were analyzed by competitive immunoassay using direct chemiluminescent technology. Serum B12 and folate levels were measured by competitive immunoassay using electrochemiluminescence. The biochemistry laboratory personnel who processed and analyzed the blood samples were masked toward the group to which the participant belonged (case or control). Relevant ophthalmological investigations, including optical coherence tomography and fundus fluorescein angiography, were done for the cases.
Diabetes mellitus was diagnosed if there was a history of treatment for diabetes, or if fasting plasma glucose was ≥126 mg/dL (7.0 mmol/L) or 2-h postprandial glucose was ≥200 mg/dL (11.1 mmol/L).[11] Hypertension was diagnosed if there was a history of treatment for hypertension, or if systolic blood pressure was ≥140 mmHg or diastolic blood pressure was ≥90 mmHg. The diagnosis was based on the average of two or more properly measured readings at each of two or more visits after an initial screen.[12] Patients with a history of treatment for hyperlipidemia, or with total cholesterol ≥240 mg/dL, or low-density lipoprotein cholesterol ≥60 mg/dL were diagnosed to have hyperlipidemia.[13] Anemia was diagnosed if the hemoglobin was <13 and <12 g/dL in men and women, respectively. Polycythemia was defined as hemoglobin >18.5 g/dL and >16.5 g/dL in men and women, respectively. Leukopenia was diagnosed if the white blood cell count was <3500/μL. Leukocytosis was defined as white blood cell count >11000/μL. Thrombocytopenia was defined as platelet count <150,000/μL. Thrombocytosis was defined as platelet count >450,000/μL.[14]
Based on the BMI, the patients were categorized as underweight (BMI <18.5 kg/m2), normal weight (BMI ≥18.5–24.9 kg/m2), overweight (BMI ≥25.0–29.9 kg/m2), and obese (BMI ≥30 kg/m2).[15] Renal dysfunction was defined as estimated glomerular filtration rate <60 mL/min/1.73 m2 irrespective of the cause.[16]
Smoking was assessed as per the following classification:[17]
The patients were divided into four groups:
Group 1 (nil smoking) – participants who did not smoke at all
Group 2 (regular smoking) – participants who smoked more than once a week
Group 3 (occasional smoking) – participants who smoked less than once a week
Group 4 (quit smoking) – participants who stopped smoking at least 6 months earlier.
Smoking was considered as positive in all participants who “smoked regularly” (Group 2).
With regard to alcoholism, the patients were divided into the following four groups:
Group 1 (nil alcohol consumption) – participants who did not consume alcohol at all
Group 2 (regular alcohol consumption) – participants who consumed alcohol more than once a week
Group 3 (occasional alcohol consumption) – participants who consumed alcohol less than once a week
Group 4 (quit alcohol consumption) – participants who stopped alcohol consumption at least 6 months earlier.
For the purpose of this study, alcoholism was considered as positive in all those falling under Group 2.
Vitamin B12 deficiency was defined as serum Vitamin B12 level < 200 pg/mL.[18] Folate deficiency was defined as serum folate level < 3 ng/mL.[19]
As hyperhomocysteinemia is a relatively recently postulated risk factor for vascular occlusive disease, the cutoff values for hyperhomocysteinemia have differed between studies. The prevalence of hyperhomocysteinemia and odds ratios reported in various studies also show wide variation (range: 1.9–13).[3,5,20] For the purpose of this study, the median serum homocysteine value of controls was taken as the cutoff for hyperhomocysteinemia.
Descriptive statistics were reported as mean with standard deviation for the continuous variables and frequency with percentage for the categorical variables. The comparison of demographic, clinical, and biochemical characteristics between cases and controls was performed using the Chi-square test. The variables, namely homocysteine, Vitamin B12, and folate levels, were log transformed to achieve normality, and Pearson correlation coefficient was used to assess the strength of correlation between them. Statistical analysis was performed using SPSS 21.0, IBM, Bangalore, India.
Results
Over the study period, from April 2014 to August 2015, 39 cases who were diagnosed with CRVO and HCRVO, and fulfilled the eligibility criteria, were recruited into the study. Age- and gender-matched controls were chosen for each of the cases. Data obtained from 78 participants, including 39 cases and 39 controls, were analyzed.
The study participants ranged in age from 20 to 69 years, with men constituting 69.23% of the cases. There was no statistically significant difference in the dietary pattern between the cases and controls (P = 0.46) [Table 1]. Out of the 39 cases, 29 (74.36%) had CRVO and 10 (25.64%) had HCRVO. Sixteen patients (41%) had ischemic venous occlusion, and 23 (59%) had nonischemic venous occlusion, as confirmed by fundus fluorescein angiography.
Table 1.
Demographic pattern of study participants
| Cases, n (%) | Controls, n (%) | Total | P | |
|---|---|---|---|---|
| Age (years), mean±SD | 52.69±11.87 | 52.59±11.89 | 0.96 | |
| Male | 27 (69.23) | 27 (69.23) | 54 (69.23) | 1 |
| Female | 12 (30.77) | 12 (30.77) | 24 (30.77) | |
| Vegetarian | 3 (7.69) | 5 (12.82) | 8 (10.26) | 0.455 |
| Nonvegetarian | 36 (92.31) | 34 (87.18) | 70 (89.74) |
SD: Standard deviation
Out of the risk factors analyzed, the prevalence of hypertension, hyperlipidemia, and abnormal blood profile was found to be significantly higher in the cases than in the controls [Table 2].
Table 2.
Risk factors among study participants
| Cases, n (%) | Controls, n (%) | Total | P | |
|---|---|---|---|---|
| Diabetes | 16 (41.03) | 11 (28.21) | 27 (34.62) | 0.23 |
| No diabetes | 23 (58.97) | 28 (71.79) | 51 (65.38) | |
| Hypertension | 27 (69.23) | 13 (33.33) | 40 (51.28) | <0.01 |
| No hypertension | 12 (30.77) | 26 (66.67) | 38 (48.72) | |
| Hyperlipidemia | 20 (51.28) | 9 (23.08) | 29 (37.18) | 0.01 |
| No hyperlipidemia | 19 (48.72) | 30 (76.92) | 49 (62.82) | |
| Abnormal blood profile | 19 (48.72) | 7 (17.95) | 26 (33.33) | <0.01 |
| Normal blood profile | 20 (51.28) | 32 (82.05) | 52 (66.67) | |
| Overweight and obese | 20 (51.28) | 25 (64.10) | 45 (57.69) | 0.25 |
| Underweight and normal | 19 (48.72) | 14 (35.90) | 33 (42.31) | |
| No vitamin supplementation | 36 (92.30) | 39 (100) | 75 (96.15) | 0.07 |
| Vitamin supplementation | 3 (7.70) | 0 (0) | 3 (3.85) | |
| Smoking | 10 (25.64) | 4 (10.26) | 14 (17.95) | 0.07 |
| No smoking | 29 (74.36) | 35 (89.74) | 64 (82.05) | |
| Alcoholism | 5 (12.82) | 7 (17.95) | 12 (15.38) | 0.53 |
| No alcoholism | 34 (87.18) | 32 (82.05) | 66 (84.62) |
The mean homocysteine level was 19.4 ± 7.72 μmol/L among cases and 21.94 ± 11.35 μmol/L among controls. This difference was not statistically significant (P = 0.13). The median value of serum homocysteine among controls was 19.15 μmol/L. This was taken as the cutoff to define hyperhomocysteinemia. Based on this cutoff, 17 (43.58%) cases and 21 (53.84%) controls had hyperhomocysteinemia, and this difference too, was not statistically significant (P = 0.81) [Table 3].
Table 3.
Homocysteine, Vitamin B12, and folate levels among study participants
| Cases, n (%) | Controls, n (%) | Total | P | |
|---|---|---|---|---|
| Homocysteine levels (µmol/L), mean±SD | 19.40±7.72 | 21.94±11.35 | 0.13 | |
| Hyperhomocysteinemia | 17 (43.58) | 21 (53.84) | 38 (48.72) | 0.81 |
| No hyperhomocysteinemia | 22 (56.42) | 18 (46.16) | 40 (51.28) | |
| Vitamin B12 levels (pg/mL), mean±SD | 419.56±292.87 | 382.78±392.73 | 0.22 | |
| Vitamin B12 deficiency | 9 (23.08) | 15 (38.46) | 24 (30.77) | 0.14 |
| No Vitamin B12 deficiency | 30 (76.92) | 24 (61.54) | 54 (69.23) | |
| Folate levels (ng/mL), mean±SD | 8.44±4.17 | 8.55±4.59 | 0.88 | |
| Folate deficiency | 0 (0) | 0 (0) | 0 (0) | 1 |
| No folate deficiency | 39 (100) | 39 (100) | 78 (100) |
SD: Standard deviation
The mean serum folate levels in cases and controls were 8.44 ± 4.17 and 8.55 ± 4.59 ng/mL, respectively, and this difference too was not statistically significant (P = 0.88). None of the study participants had folate deficiency. The mean Vitamin B12 levels in cases and controls were 419.56 ± 292.87 and 382.78 ± 392.73 pg/mL, respectively, and the difference was not statistically significant (P = 0.22). Nine (23.08%) cases and 15 (38.46%) controls had Vitamin B12 deficiency, and there was no statistically significant difference between the prevalence of Vitamin B12 deficiency between the case and control groups (P = 0.14).
However, there was a significant negative correlation between serum levels of homocysteine and Vitamin B12 (Pearson correlation coefficient − 0.3874, P = 0.0005) and between serum levels of homocysteine and folate (Pearson correlation coefficient − 0.3886, P = 0.0004) of the study participants [Figures 1 and 2].
Figure 1.
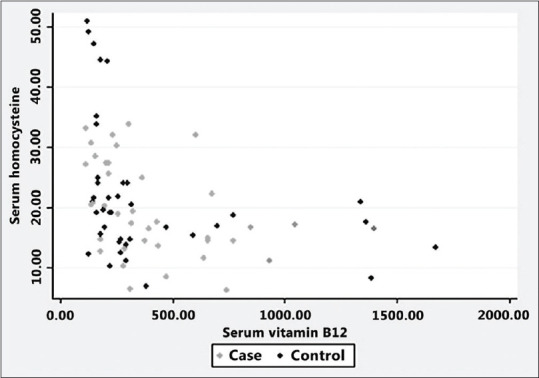
Serum homocysteine versus Vitamin B12
Figure 2.
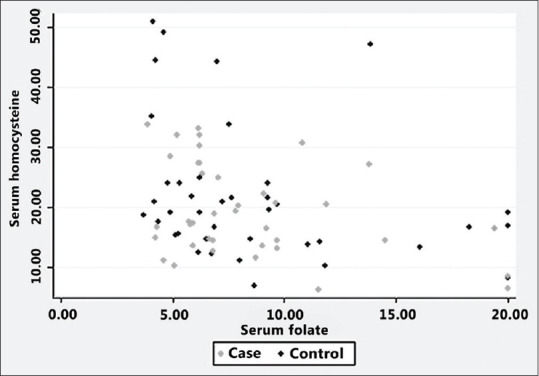
Serum homocysteine versus folate
Among the study patients (n = 78), the odds of patients with Vitamin B12 deficiency having hyperhomocysteinemia were 7.0 (2.26–21.72) times those of patients without Vitamin B12 deficiency (P = 0.001). Similarly, among the cases (CRVO, n = 39), the odds of patients with Vitamin B12 deficiency having hyperhomocysteinemia were 7.0 (1.22–40.09) times those of patients without Vitamin B12 deficiency (P = 0.029). In the control group also (non-CRVO, n = 39), the odds of patients with Vitamin B12 deficiency having hyperhomocysteinemia were 6.67 (1.47–30.21) times those of patients without Vitamin B12 deficiency (P = 0.014).
Discussion
Over the past two decades, hyperhomocysteinemia has been identified as a possible risk factor in the etiopathogenesis of diseases such as coronary artery disease and stroke.[21,22] Our study looked at the possible association of hyperhomocysteinemia with CRVO and HCRVO, the correlation of serum homocysteine levels with Vitamin B12 and folate levels in serum, and the association of Vitamin B12 deficiency with hyperhomocysteinemia. As a part of the study, most of the known risk factors for CRVO and HCRVO, such as diabetes, hypertension, hyperlipidemia, obesity, smoking, alcoholism, and blood profile abnormalities were also studied.
Hypertension has been one of the well-known risk factors associated with CRVO.[23,24] The results of our study also showed that 69% of the cases were hypertensive, while only 33% of the controls had hypertension (P < 0.01).
Hyperlipidemia was another risk factor, which was found to be significantly higher among the cases (51% cases vs. 23% controls, P = 0.01). Elevated plasma lipid levels have been shown to hasten the process of atherosclerosis, which in turn, acts as a predisposing factor for CRVO and HCRVO.[25]
Abnormal blood profiles were also found to be significantly higher among the cases (P < 0.01). The various abnormalities noted among the patients in the study were anemia (14 patients), leucocytosis (11 patients) and thrombocytosis (one patient). Increase in blood cell numbers creates a hypercoagulable state, which may result in increased risk of intravascular thrombus formation. The possible mechanism by which anemia causes a venous occlusion is by secondary thrombocytosis, which frequently accompanies the less severe anemias and may be related to platelet stimulation in a manner analogous to the erythropoietin increase seen in many of the anemic states.[26] The other risk factors that did not show a statistically significant difference between cases and controls were diabetes, BMI, oral vitamin supplementation, smoking, and alcohol consumption.
We did not find a statistically significant difference in the prevalence of hyperhomocysteinemia between the cases and controls in our study (P = 0.81). Our results are comparable to those of other studies, which have also concluded that hyperhomocysteinemia may not be an independent risk factor for retinal venous occlusions. Parodi and Di Crecchio concluded from the results of their study, that plasma homocysteine level was not a primary and independent risk factor for CRVO, but was more likely, a marker of atherosclerosis.[6] Di Crecchio et al. also concluded from their study, that elevated plasma homocysteine level was not a primary and independent risk factor for CRVO.[27]
However, we found a high prevalence of hyperhomocysteinemia among both the cases (43.58%) and controls (53.84%) in our study. Several studies have shown a similar pattern of high prevalence of hyperhomocysteinemia among the Indian population. The study by Kalita et al. among normal adult Indian population found that hyperhomocysteinemia was present in 113 (56.5%) participants.[28] The study by Kamdi and Palkar among healthy Indian doctors also showed a similar result.[29]
Decreased serum Vitamin B12 levels have been noted to be associated with elevated homocysteine levels in serum.[30] Worldwide, the prevalence of Vitamin B12 deficiency has been reported to be 2% to 12%, whereas it is 16% in the urban Indian population.[31] In our study, we found that 30.77% of the study patients had Vitamin B12 deficiency. The relatively high prevalence of Vitamin B12 deficiency noted in the Indian population may be related to the general dietary habits of the Indian population, which often include factors such as overcooking, vegetarianism, and lack of fortification of cereals. We found a negative correlation of the homocysteine levels with Vitamin B12 levels in the serum of the study subjects, with a Pearson correlation coefficient of − 0.3874 and P value of 0.0005. We also found that among the study patients (n = 78), the odds of patients with Vitamin B12 deficiency having hyperhomocysteinemia were 7.0 (2.26–21.72) times those of patients without Vitamin B12 deficiency (P = 0.001). This trend was similar in both the case and control groups. Studies by Savage et al. and Narayanasamy A et al. have also found a similar association between Vitamin B12 deficiency and hyperhomocysteinemia.[5,32]
Analysis of the serum folate levels of our study subjects showed a mean value of 8.44 (±4.17) ng/mL among cases and 8.55 (±4.59) ng/mL among controls (P = 0.88). There was no folate deficiency seen in any of the cases or controls. However, when we analyzed the correlation of serum homocysteine levels with folate levels in the serum of the study participants, we found that there was a negative correlation with a Pearson correlation coefficient of − 0.3886 and P value of 0.0004.
The serum homocysteine levels in blood are also influenced by other factors such as advancing age and male gender. The majority of patients in our study were above 60 years of age. Men constituted 69.23% of our study population. The coexistence of hyperhomocysteinemia along with the other known risk factors for venous occlusive diseases may further increase the risk of developing CRVO and HCRVO.
Our study findings correlating Vitamin B12 and folate levels with homocysteine levels suggest that supplementation of Vitamin B12 and folate could help decrease serum homocysteine levels. Several studies have proved the association of hyperhomocysteinemia with increased incidence of diseases such as stroke and ischemic heart disease. Therefore, oral supplementation of Vitamin B12 and folate could possibly be beneficial for those who are at risk for these diseases, especially those with other systemic risk factors such as diabetes, hypertension, hyperlipidemia, and obesity.
The cases in the study included both fresh and old retinal vein occlusions. Hence, the serum homocysteine levels measured during the study may have been different from those at the time of occurrence of the vein occlusion. Moreover, several factors, including diet and stress levels, influence the short-term fluctuation of serum homocysteine levels. Since our study included only a one-time measurement of homocysteine levels, such factors could possibly have influenced the results.
Conclusion
Hyperhomocysteinemia was not found to be an independent risk factor for CRVO and HCRVO in our study. However, we found a high prevalence of hyperhomocysteinemia among both the cases and controls. Similarly, we also found a high prevalence of Vitamin B12 deficiency both among cases and controls. There was a negative correlation of serum homocysteine levels with Vitamin B12 and folate levels. We also found that patients with Vitamin B12 deficiency have significantly higher odds of developing hyperhomocysteinemia, compared to patients without Vitamin B12 deficiency.
Systemic risk factors such as hypertension, hyperlipidemia, and blood count abnormalities were found to have a strong association with CRVO and HCRVO on our study, consistent with the findings of previous studies. Several investigators have found that many of these systemic risk factors could be associated with elevated serum homocysteine levels, which may be part of a final common pathway in bringing about a state of accelerated atherosclerosis, thus promoting intravascular thrombosis, leading to vasoocclusive events.
Our study has established the negative correlation of serum homocysteine levels with Vitamin B12 and folate levels, with significantly higher odds of developing hyperhomocysteinemia among patients with Vitamin B12 deficiency. This could be particularly relevant in the Indian scenario, in which a high prevalence of both Vitamin B12 deficiency and hyperhomocysteinemia has been documented. Our study also found a similarly high prevalence of both parameters among our participants. Lowering serum levels of homocysteine by Vitamin B12 and folate supplementation could have a role in the prevention of these diseases.
There is a lot of scope for further research on this subject. Prospective studies with long-term follow-up to record the change in serum homocysteine levels with Vitamin B12 and folate supplementation, and the impact of this change on the incidence and prevalence of ocular and systemic vaso-occlusive diseases are needed to prove the effectiveness of such therapies.
Financial support and sponsorship
This study was supported by the Institutional Fluid Research Grant, Christian Medical College, Vellore, India.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Cugati S, Wang JJ, Rochtchina E, Mitchell P. Ten-year incidence of retinal vein occlusion in an older population: The Blue Mountains Eye Study. Arch Ophthalmol. 2006;124:726–32. doi: 10.1001/archopht.124.5.726. [DOI] [PubMed] [Google Scholar]
- 2.Cattaneo M. Hyperhomocysteinemia, atherosclerosis and thrombosis. Thromb Haemost. 1999;81:165–76. [PubMed] [Google Scholar]
- 3.Moghimi S, Najmi Z, Faghihi H, Karkhaneh R, Farahvash MS, Maghsoudipour M. Hyperhomocysteinemia and central retinal vein occlusion in Iranian population. Int Ophthalmol. 2008;28:23–8. doi: 10.1007/s10792-007-9103-4. [DOI] [PubMed] [Google Scholar]
- 4.Gao W, Wang YS, Zhang P, Wang HY. Hyperhomocysteinemia and low plasma folate as risk factors for central retinal vein occlusion: A case-control study in a Chinese population. Graefes Arch Clin Exp Ophthalmol. 2006;244:1246–9. doi: 10.1007/s00417-005-0191-4. [DOI] [PubMed] [Google Scholar]
- 5.Narayanasamy A, Subramaniam B, Karunakaran C, Ranganathan P, Sivaramakrishnan R, Sharma T, et al. Hyperhomocysteinemia and low methionine stress are risk factors for central retinal venous occlusion in an Indian population. Invest Ophthalmol Vis Sci. 2007 Apr;48(4):1441–6. doi: 10.1167/iovs.06-0905. [DOI] [PubMed] [Google Scholar]
- 6.Parodi MB, Di Crecchio L. Hyperhomocysteinemia in central retinal vein occlusion in young adults. Semin Ophthalmol. 2003;18:154–9. doi: 10.1076/soph.18.3.154.29809. [DOI] [PubMed] [Google Scholar]
- 7.McGimpsey SJ, Woodside JV, Cardwell C, Cahill M, Chakravarthy U. Homocysteine, methylenetetrahydrofolate reductase C677T polymorphism, and risk of retinal vein occlusion: A meta-analysis. Ophthalmology. 2009;116:1778–87.e1. doi: 10.1016/j.ophtha.2009.02.033. [DOI] [PubMed] [Google Scholar]
- 8.Malinow MR, Nieto FJ, Kruger WD, Duell PB, Hess DL, Gluckman RA, et al. The effects of folic acid supplementation on plasma total homocysteine are modulated by multivitamin use and methylenetetrahydrofolate reductase genotypes. Arterioscler Thromb Vasc Biol. 1997;17:1157–62. doi: 10.1161/01.atv.17.6.1157. [DOI] [PubMed] [Google Scholar]
- 9.Neal B, MacMahon S, Ohkubo T, Tonkin A, Wilcken D PACIFIC Study Group. Dose-dependent effects of folic acid on plasma homocysteine in a randomized trial conducted among 723 individuals with coronary heart disease. Eur Heart J. 2002;23:1509–15. doi: 10.1053/euhj.2002.3161. [DOI] [PubMed] [Google Scholar]
- 10.Lahiri KD, Dutta J, Datta H, Das HN. Hyperhomocysteinemia, as an independent risk factor for retinal venous occlusion in an Indian population. Indian J Clin Biochem. 2013;28:61–4. doi: 10.1007/s12291-012-0238-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Diabetes Association, Standards of medical care in diabetes – 2011. Diabetes Care. 2011;34(Suppl 1):S11–61. doi: 10.2337/dc11-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311:507–20. doi: 10.1001/jama.2013.284427. [doi: 10.1001/jama. 2013.284427] [DOI] [PubMed] [Google Scholar]
- 13.Fedder DO, Koro CE, L’Italien GJ. New National Cholesterol Education Program III guidelines for primary prevention lipid-lowering drug therapy: Projected impact on the size, sex, and age distribution of the treatment-eligible population. Circulation. 2002;105:152–6. doi: 10.1161/hc0202.101971. [DOI] [PubMed] [Google Scholar]
- 14.Beutler E, Waalen J. The definition of anemia: What is the lower limit of normal of the blood hemoglobin concentration? Blood. 2006;107:1747–50. doi: 10.1182/blood-2005-07-3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhaskaran K, Douglas I, Forbes H, dos-Santos-Silva I, Leon DA, Smeeth L. Body-mass index and risk of 22 specific cancers: A population-based cohort study of 5·24 million UK adults. Lancet. 2014;384:755–65. doi: 10.1016/S0140-6736(14)60892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levin A, Stevens PE, Bilous RW, Coresh J, de Francisco ALM, de Jong PE, et al. Kidney disease: Improving global outcomes (KDIGO) CKD work group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. [doi: 10.1038/kisup. 2012.73] [Google Scholar]
- 17.Gupta P, John D, Rebekah G, John SS. Role of hyperhomocysteinemia in proliferative diabetic retinopathy: A case–control study. Indian J Ophthalmol. 2018;66:1435. doi: 10.4103/ijo.IJO_350_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matchar DB, McCrory DC, Millington DS, Feussner JR. Performance of the serum cobalamin assay for diagnosis of cobalamin deficiency. Am J Med Sci. 1994;308:276–83. doi: 10.1097/00000441-199411000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Organization WH. Serum and Red Blood Cell Folate Concentrations for Assessing Folate Status in Populations. Published Online. 2015. [Last accessed on 2018 Aug 27]. Available from: http://apps.who.int/iris/handle/10665/162114 .
- 20.Al Wadani F, Khandekar R, Salim G, Al Ali M, Ramzi S. Hyperhomocysteinia is a risk factor for retinal venous occlusion: A case control study. Indian J Ophthalmol. 2014;62:291–4. doi: 10.4103/0301-4738.111213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collaboration HS. Homocysteine and Risk of Ischemic Heart Disease and Stroke: A Meta-Analysis. [Last accessed on 2018 Aug 24]. Available from: https://www.ncbi.nlm.nih.gov/pubmed/12387654 .
- 22.Klerk M, Verhoef P, Clarke R, Blom HJ, Kok FJ, Schouten EG, et al. MTHFR 677C-->T polymorphism and risk of coronary heart disease: A meta-analysis. JAMA. 2002;288:2023–31. doi: 10.1001/jama.288.16.2023. [DOI] [PubMed] [Google Scholar]
- 23.Glacet-Bernard A, Coscas G, Chabanel A, Zourdani A, Lelong F, Samama MM. Prognostic factors for retinal vein occlusion: Prospective study of 175 cases. Ophthalmology. 1996;103:551–60. doi: 10.1016/s0161-6420(96)30653-2. [DOI] [PubMed] [Google Scholar]
- 24.Risk factors for central retinal vein occlusion. The Eye Disease Case-Control Study Group. Arch Ophthalmol. 1996;114:545–54. [PubMed] [Google Scholar]
- 25.Sodi A, Giambene B, Marcucci R, Sofi F, Bolli P, Abbate R, et al. Atherosclerotic and thrombophilic risk factors in patients with recurrent central retinal vein occlusion. Eur J Ophthalmol. 2008;18:233–8. doi: 10.1177/112067210801800211. [DOI] [PubMed] [Google Scholar]
- 26.Hayreh SS, Zimmerman MB, Podhajsky P. Hematologic abnormalities associated with various types of retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol. 2002;240:180–96. doi: 10.1007/s00417-001-0421-3. [DOI] [PubMed] [Google Scholar]
- 27.Di Crecchio L, Parodi MB, Sanguinetti G, Iacono P, Ravalico G. Hyperhomocysteinemia and the methylenetetrahydrofolate reductase 677C-T mutation in patients under 50 years of age affected by central retinal vein occlusion. Ophthalmology. 2004;111:940–5. doi: 10.1016/j.ophtha.2003.08.028. [DOI] [PubMed] [Google Scholar]
- 28.Kalita J, Misra UK, Srivastava AK, Bindu IS. A study of homocysteine level in North Indian subjects with special reference to their dietary habit. Eur Ejournal Clin Nutr Metab. 2007;2:e116–9. [Google Scholar]
- 29.Kamdi SP, Palkar P. Prevalence of hyperhomocysteinemia in healthy Indian doctors. Bioinformation. 2013;9:193–6. doi: 10.6026/97320630009193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yajnik CS, Deshpande SS, Lubree HG, Naik SS, Bhat DS, Uradey BS, et al. Vitamin B12 deficiency and hyperhomocysteinemia in rural and urban Indians. J Assoc Physicians India. 2006;54:775–82. [PubMed] [Google Scholar]
- 31.Shobha V, Tarey SD, Singh RG, Shetty P, Unni US, Srinivasan K, et al. Vitamin B12 deficiency and levels of metabolites in an apparently normal urban south Indian elderly population. Indian J Med Res. 2011;134:432–9. [PMC free article] [PubMed] [Google Scholar]
- 32.Savage DG, Lindenbaum J, Stabler SP, Allen RH. Sensitivity of serum methylmalonic acid and total homocysteine determinations for diagnosing cobalamin and folate deficiencies. Am J Med. 1994;96:239–46. doi: 10.1016/0002-9343(94)90149-x. [DOI] [PubMed] [Google Scholar]


