Abstract
A series of 1,6-naphthyridine (L. Chan, H. Jin, T. Stefanac, J. F. Lavallee, G. Falardeau, W. Wang, J. Bedard, S. May, and L. Yuen, J. Med. Chem. 42:3023–3025, 1999) and isoquinoline (L. Chan, H. Jin, T. Stefanac, W. Wang, J. F. Lavallee, J. Bedard, and S. May, Bioorg. Med. Chem. Lett. 9:2583–2586, 1999) analogues exhibiting a high level of anti-human cytomegalovirus (HCMV) activity were investigated in a series of studies aimed at better understanding the mechanism of action of some representatives of this class of compounds. In vitro antiviral profiling revealed that these compounds were active against a narrow spectrum of viruses, essentially the human herpesviruses and type 2 rhinovirus. In HCMV assays, a 39- to 223-fold lower 50% inhibitory concentration was obtained for compound A1 than for ganciclovir against strains AD 169 and Towne. In addition, ganciclovir, foscarnet, cidofovir, and BDCRB (2-bromo-5,6-dichloro-1-β-d-ribofuranosylbenzimidazole)-resistant HCMV strains remained susceptible to 1,6-naphthyridines and 7,8-dihydroisoquinolines tested in this study, supporting the view that a novel mechanism of action could be involved. Drug combination studies showed a small but significant synergistic antiviral effect between compound B2 and ganciclovir. Cytotoxicity profiling of representative compounds under various cell growth conditions indicated a generally similar cytotoxic effect, relative to ganciclovir, in log-phase growing cells. However, in stationary cells, a relatively higher level of toxicity was observed than that for control compound. Effect of time of drug addition showed that the anti-HCMV activity of compound A1, ganciclovir, and cidofovir was lost at approximately the same time (72 h postinfection), indicating that the compound was affecting events at the early and late stage of virus replication. This interpretation is also supported by reduction of de novo synthesis of pp65 tegument protein and lack of any effect of the compound on viral adsorption. A reduction of the HCMV enhancer-promoter-directed luciferase expression was also observed in a stably transfected cell line when compound A1 was present at relatively high concentrations.
Human cytomegalovirus (HCMV) is a serious, life-threatening, opportunistic pathogen in immunocompromised individuals such as AIDS patients (20, 36) or organ transplant recipients (21). Over the past decade, there has been a tremendous effort dedicated to improving the available treatments for HCMV. At the present time, ganciclovir (GCV; DHPG; 9-[2′-hydroxy-1(hydroxymethyl)ethoxymethyl] guanine), foscarnet (PFA; Foscovir), cidofovir [CDV; HPMPC; (S)-1-(3-hydroxy-2-phosphonyl-methoxypropyl)cytosine], and fomivirsen (Vitravene; ISIS 2922) are the only drugs currently approved for the treatment of AIDS-related CMV retinitis. Unfortunately, there can be serious limitations associated with the use of these compounds (13). The emergence of drug-resistant HCMV clinical isolates through mutations within the DNA polymerase gene (UL54) has been reported for both GCV- and PFA-treated virus, and mutations affecting the protein kinase gene (UL97) activity have been reported for GCV-treated patients (1, 8, 9, 12–14, 29, 34, 35). The latter seems to be the predominant genotype found at present among clinical isolates recovered from patients after long-term GCV therapy (8, 34). Numerous examples of CDV- and GCV-cross-resistant clinical isolates due to UL54 mutations have been reported in the scientific literature (13, 32). In HCMV retinitis patients, whose isolates are GCV resistant in vitro, PFA has proven valuable in reducing the progression of retinitis (18). In some situations, CDV may have advantages over GCV and PFA since it is characterized by a long-term antiviral response and remains effective against clinical isolates defective in GCV phosphorylation. In addition to concerns over viral resistance, there are numerous reports of adverse effects associated with the use of the three approved drugs. Development of nephrotoxicity is the principal risk factor encountered with patients receiving CDV or PFA (11, 16, 24), whereas bone marrow depression resulting in granulocytopenia and thrombocytopenia is the most common dose-limiting toxic effect seen with GCV (5, 17, 22). Thus, there is a need for identifying and developing new anti-HCMV agents.
Two novel classes of relatively potent anti-HCMV molecules, namely, 1,6-naphthyridines and isoquinoline-6-carboxamides, have been identified using a plaque reduction assay (6, 7; L. Chan, T. Stefanac, N. Turcotte, Z. Hu, Y. Chen, J. Bedard, and H. Jin, Unpublished data). In the present study, we report on the antiviral profile for some derivatives of the two classes of compounds and their in vitro cytotoxicity profile. Included in the analysis are experiments which monitor the effect of time of drug addition on compound efficacy and the effect that these compounds have on the de novo synthesis of pp65 tegument protein using an indirect immunofluorescence assay. Data on compound effects on HCMV major immediate-early promoter activity are also provided. To further illustrate the mechanism of action, cross-resistance evaluations, assays of spectrum of antiviral activity, and drug combination studies with GCV were conducted.
MATERIALS AND METHODS
Antiviral assays. (i) HCMV plaque reduction assay.
Antiviral efficacy was evaluated using the laboratory-derived HCMV strain AD 169; low-passage clinical isolate P8; and GCV-resistant clinical isolates C8704, C8805-37, and D16 using MRC-5 cells (2, 30). The HFF cell line was used for testing compound activity against HCMV strains AD 169 and Towne and against PFA-, CDV-, and BDCRB (2-bromo-5,6-dichloro-1-β-d-ribofuranosylbenzimidazole)-resistant isolates as described previously (27, 37). For the evaluation of antiviral efficacy using AD 169 with Hs68 cells, human fibroblasts were plated in 12-well tissue culture dishes (no. 25815; Corning Costar Corp, Oneonta, N.Y.), at a density of 1.5 × 105 cells/well in 2 ml of Dulbecco's modified Eagle medium (DMEM)–10% fetal bovine serum (FBS) and incubated overnight in 5% CO2 at 37°C. Medium was removed, and cells were infected with 0.5 ml of DMEM–2% FBS containing approximately 125 PFU of HCMV per ml (multiplicity of infection [MOI] of 0.001). After an adsorption at 37°C for 2 h, the inoculum was removed and the monolayer was overlaid with 1 ml of DMEM–2% FBS containing the test compounds at specific concentrations. After 7 days of incubation, cells were fixed with 1 volume of 8% formaldehyde in water for 30 min, and then the solution was removed and cell monolayers were stained with 2% crystal violet in 20% ethanol for few seconds. Cells were rinsed with tap water and dried, and the monolayer was examined for the presence of plaques under an inverted microscope using an ×40 magnification.
(ii) HCMV yield reduction assay.
Compounds were further tested for anti-HCMV activity using a virus yield reduction assay. Briefly, HFF cells were seeded in a 24-well microtiter plate, infected with HCMV strain Towne at an MOI of 0.5 PFU/cell, and incubated for 7 days in the presence of compounds serially diluted to give a concentration range of 0.025 to 715 μM. Eluates obtained after one cycle of freezing and thawing were assayed for virus titer by serial dilution onto a monolayer of HFF cells in a second 96-well microtiter plate as reported previously (37).
(iii) Time-of-drug addition studies.
In order to determine the phase within the viral replication cycle targeted by the 1,6-naphthyridines, a time course experiment was performed with compound A1 in parallel with GCV, CDV, and 2,5,6-trichloro-1-β-d-ribofuranosylbenzimidazole (TCRB). Briefly, 96-well plates were seeded with 12,500 HFF cells/well 18 h prior to infection. On the day of the infection, medium was removed and replaced with 0.180 ml of virus inoculum. The inoculum was prepared by diluting previously titered stock of HCMV strain Towne to obtain an MOI of 0.5 PFU/cell. At each time point, 0.020 ml of a 10× solution of compound was added to a subset of wells on the plates. Each drug was tested in duplicate at each time point. In addition, each time point contained duplicate virus control samples which received medium without drug. At 96 h postinfection, the plates were frozen at −80°C. The titer of virus produced in each well was determined by end-point dilution and plaque enumeration as detailed previously (37).
(iv) HIV-inhibitory assays.
The anti-human immunodeficiency virus (HIV) activity of some 1,6-naphthyridine analogues was evaluated in CEM-SS cells acutely infected with HIV strains RF and IIIB in a 96-well microtiter plate (23). Quantification of inhibition of HIV-induced cell killing in this assay was performed by using the tetrazolium dye 2,3-bis(2-methyl-4-nitro-5-sulfophenyl)-2H-tetrazolium-S-carboxamide salt (XTT), which is metabolized by viable cells to a colored formazan product. Anti-HIV activity was also measured by the reduction of reverse transcriptase activity in culture supernatants of peripheral blood mononuclear cells (PBMC) infected with HIV ROJO (syncytium-inducing) and TEKI (non-syncytium-inducing) clinical isolates as described previously (23).
(v) Additional antiviral assays.
The increase in neutral dye uptake was the method used to measure potential inhibitory activity of 1,6-naphthyridine representatives on cytopathic effects due to selected viruses (15, 30, 31). Included in these studies were the herpes simplex viruses type 1 (HSV-1 strain McKrae) and type 2 (HSV-2 strain E194), the respiratory viruses influenza virus A (strain H3N2) and virus B, respiratory syncytial virus (RSV strain A2), type 2 rhinovirus (RV/2 strain GPH), type 1 adenovirus (AV/1 strain 65089), and type 3 parainfluenza virus (PIV/3 strain 14702).
Drug combination studies.
Compound B2 interaction with GCV was studied using an HCMV enzyme-linked immunosorbent assay (ELISA) by the modification of a procedure previously used to assay HSV-1 (26, 28). MRC-5 cells were incubated at 37°C overnight, and the cells were infected with HCMV strain Towne (MOI, 0.001 PFU/cell). Following a 1-h adsorption, drug was added to triplicate plates. Use of a 10-by-7 grid on a 96-well cluster plate allowed evaluation of compound B2 at concentrations of 0, 0.005, 0.014, 0.041, 0.123, 0.37, 1.11, 3.33, 10, and 30 μM in combination with GCV at 0, 0.41, 1.23, 3.7, 11, 33, and 100 μM. After a 7-day incubation, cells were fixed with 95% ethanol. The ELISA was performed in the wells containing the infected cell sheets. Wells were blocked with 10% calf serum in HEPES-buffered saline with 0.05% Tween 20 and then treated with a 1:400 dilution of mouse monoclonal antibody to HCMV (the epitope has been mapped to the UL44 reading frame of the HCMV genome and reacts with the delayed-early DNA-binding protein p52). After 1 h, a 1:1,000 dilution of peroxidase-conjugated rabbit anti-mouse antibody was added to each well and incubated for 2 h, and plates were developed and read at 450 or 570 nm in a microplate kinetics reader. Data were plotted as a three-dimensional dose-response surface and analyzed using MacSynergy II (26; M. N. Prichard, K. R. Aseltine, and C. Shipman, 1993). Data derived from quintuplicate plates as described in the preceding paragraph were used to construct dose-response surfaces. Theoretical additive interactions were calculated from the dose-response curves for each drug used individually. This calculated surface, which represents additive interactions, was subtracted from the experimentally determined dose-response surface to reveal regions of nonadditive activity. The resulting surface would appear as a horizontal plane at 0% inhibition if the interactions were merely additive. Depressions in this plane were indicative of antagonism; similarly, peaks above the plane indicate synergy. Confidence intervals (95%) around each of the points which defined the dose-response surface were calculated from the quintuplicate data. This provided limits for the experimental dose-response surface. If the lower confidence limit of the experimental data was greater than the calculated additive surface, the synergy was considered significant at that confidence level. Conversely, if the upper confidence limit of the experimental data was less than the calculated additive surface, then the antagonism was significant.
Cytotoxicity determination.
Cell lines investigated in the cytotoxicity studies included human embryonic lung cells (MRC-5), human foreskin fibroblasts (Hs68 and HFF), human lung carcinoma cells (A549), African green monkey embryonic kidney cells (MA-104), African green monkey kidney cells (Vero), Madin-Darby canine kidney cells (MDCK), human CEM-SS T lymphocytes, and PBMC.
In vitro toxicity profiling was performed using several different methods. Drug-induced cytotoxicity, produced in exponentially growing Hs68 and Vero cells, was measured by [3H]thymidine incorporation and by the use of WST-1 cell proliferation reagent (Boehringer Mannheim GmbH, Laval, Quebec, Canada). Cytotoxicity was also determined on stationary cells by visual examination (31, 37), by monitoring neutral red uptake (15, 30), or by the use of XTT reagent as described previously (4, 23).
For experiments using exponentially growing cells, a total of 1,000 cells/well were seeded in 96-well cluster dishes in a volume of 150 μl of DMEM (Life Technologies, Inc., Gaithersburg, Md.) supplemented with 10% FBS (HyClone Laboratories, Inc., Logan, Utah) and 2 mM glutamine (Life Technologies, Inc.). Penicillin and streptomycin (Life Technologies, Inc.) were added to 500 U/ml and 50-μg/ml final concentrations, respectively. After an incubation of 18 h at 37°C and 5% CO2, the medium was removed and replaced with compounds diluted in culture medium. Six serial twofold dilutions of drugs were tested in triplicate. After a further 72-h incubation, a volume of 50 μl of a 10-μCi/ml solution of [3H]methyl thymidine (Amersham Life Science, Inc., Arlington Heights, Ill.; 2 Ci/mmol) was added in culture medium, and accumulation was allowed to proceed for 18 h at 37°C. Cells were then washed with phosphate-buffered saline (PBS), trypsinized for 2 min, and then collected onto a fiberglass filter using a Tomtec cell harvester (Tomtec, Orange, Conn.). Filters were dried at 37°C for 1 h and placed into a bag with 4.5 ml of liquid scintillation cocktail (Wallac Oy, Turku, Finland). Radioactivity was measured using a liquid scintillation counter (1450-Microbeta; Wallac Oy). When the cell proliferation reagent WST-1 was used, the indication of toxicity was measured after the 72-h incubation by the addition of 100 μl of prewarmed DMEM–2% FBS containing WST-1 reagent diluted 1/40 to each well. After a 2-h incubation at 37°C, the absorbance was measured in a microtiter plate Dynatech MR5000 micro-ELISA autoreader (Dynatech, Alexandria, Va.) set at a wavelength of 410 nm.
Transfection and luciferase assays.
For inhibition studies of the HCMV ie1/ie2 enhancer-promoter activity, a hepatocyte cell line, HCF, containing the HCMV ie1/ie2 enhancer-promoter upstream of a luciferase reporter gene, stably transfected and expressed, was kindly provided by OSI Pharmaceuticals, Inc. (Uniondale, N.Y.). Cells were seeded in a 96-well microtiter plate (Nunc, Roskilde, Denmark) at a density of 10,000 cells/well in 100 μl of DMEM–10% FBS. An aliquot of 100 μl of culture medium containing the test compounds at concentrations ranging from 1.8 to 39.0 μM was added in appropriate wells. Cells were incubated for 18 h in 5% CO2 at 37°C, at which time the cells were lysed and light production was measured by cleavage of luciferin according to the specifications of OSI Pharmaceuticals, Inc. All experiments were performed in triplicate.
Immunofluorescence studies.
MRC-5 cells were seeded onto glass coverslips sitting in 24-well culture plates, at a density of 2 × 105 cells/well. After 18 h, confluent monolayers were infected with HCMV strain AD 169 using an MOI of 0.1, and virus was allowed to adsorb at room temperature for 2 h. After removal of the inoculum, the cells were maintained at 37°C in medium containing 0.62 μM compound A1, 10.2 μM GCV, or no compound. Three days postinfection, the coverslips were fixed for 10 min with ice-cold acetone. The fixed monolayers were blocked with rabbit serum and reacted with the monoclonal antibody 0841 or 0831 (diluted 1:20) (Virostat, Portland, Maine), which recognizes the 70-kDa immediate-early nuclear antigen or the 65-kDa late major matrix protein (pp65, tegument protein), respectively. After incubation at 37°C for 1 h, the coverslips were washed thoroughly with PBS and incubated with anti-mouse immunoglobulin G conjugated to fluorescein isothiocyanate (Sigma F3008) at a final concentration of 10 μg/ml for 30 min at 37°C. The coverslips were briefly counterstained with Evans blue (Sigma) prior to being washed with PBS, mounted on slides using 70% glycerol in PBS, and photographed using a Zeiss Axiphot UV microscope. Uninfected monolayers were stained in parallel to detect nonspecific reaction.
Data analysis.
The 50% inhibitory concentrations (IC50s) for antiviral activity and 50% cytotoxic concentrations (CC50s) for cell toxicity were determined from dose-response curves using six to eight concentrations per drug in triplicate. Curves were fitted to data points using nonlinear regression analysis, and IC50s were interpolated from the resulting curves using GraphPad Prism software, version 2.0 (GraphPad Software, Inc., San Diego, Calif.).
RESULTS
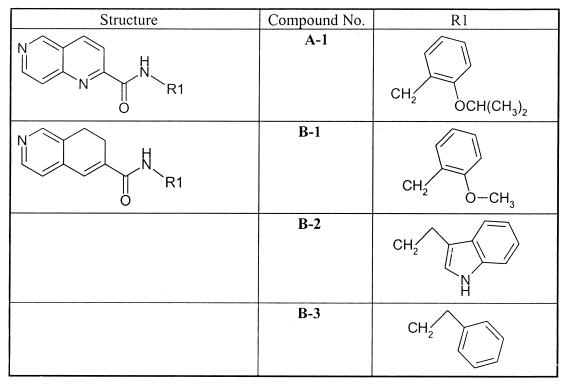
Structure-activity relationship studies of a novel series of 1,6-naphthyridine isoquinoline derivatives have demonstrated that excellent activity against HCMV is obtained with these classes of compounds (6, 7; L. Chan et al., unpublished data). In this study, we investigated the antiviral properties of some representative members of these classes of compounds (Fig. 1). The selected compounds are characterized by the presence of a 1,6-naphthyridine (compound A1) or a dihydroisoquinoline (compounds B1, B2, and B3) scaffold linked through an amide group to an aromatic moiety such as phenyl or indole with one or two methylenes as a spacer.
FIG. 1.
Chemical structures of a 1,6-naphthyridine derivative (A) and 7,8-dihydroisoquinoline derivatives (B).
Antiviral activity against HCMV, HSV-1, and HSV-2.
The antiviral activity of the compounds listed in Fig. 1 against HCMV was determined using two different HCMV laboratory-derived strains (AD 169 and Towne) in Hs68, MRC-5, or HFF cells. Compounds B1 and B3 with a dihydroisoquinoline scaffold were relatively equipotent to or less potent than GCV against strains AD 169 and Towne in Hs68, MRC-5, and HFF cells, respectively, whereas compound B2 was three- to ninefold more active than GCV (Table 1). The naphthyridine derivative (A1) was found to have the highest anti-HCMV activity with an IC50 39- to 223-fold lower than that of GCV (Table 1). Anti-HCMV activity was also determined using the viral yield reduction assay in HFF cells infected with HCMV Towne at an MOI of 0.5. It is interesting to note that all 1,6-naphthyridine and 7,8-dihydroisoquinoline derivatives were less potent in the yield reduction assay than in the plaque reduction assay (IC90s) using the HCMV Towne strain in the HFF cell line (Table 1). The IC90s obtained in the yield reduction assay were 0.28, 2.0, 143, 3.4, >28.2, 7.0, and >34 μM for BDCRB, GCV, PFA, and compounds A1, B1, B2, and B3, respectively.
TABLE 1.
Antiviral activities against human herpesviruses of 1,6-naphthyridine and 7,8-dihydroisoquinoline representatives
| Virus | Cell line | IC50 (μM)a
|
||||
|---|---|---|---|---|---|---|
| Controlb | A1 | B1 | B2 | B3 | ||
| HCMV isolates | ||||||
| AD 169 | Hs68 | 0.78 ± 0.16 | 0.02 ± 0.01 | 1.8 ± 1.3 | 0.09 ± 0.01 | 2.4 ± 1.0 |
| AD 169 | MRC-5 | 6.7 ± 2.0 | 0.03 ± 0.003 | 15.5 ± 1.1 | 1.5 ± 0.1 | 8.8 ± 1.4 |
| P8c | MRC-5 | 3.0 ± 0.9 | 0.3 ± 0.03 | 11.8 ± 2.8 | 0.3 ± 0.2 | 18.0 ± 4.4 |
| C8704c | MRC-5 | 36.9 ± 6.3 | 0.03 ± 0.01 | ND | 0.09 ± 0.01 | 5.8 ± 0.7 |
| C8805-37c | MRC-5 | 59.1 ± 11.3 | 0.02 ± 0.003 | 27.1 ± 2.8 | 0.3 ± 0.06 | 6.5 ± 0.7 |
| D16c | MRC-5 | 30.7 ± 5.5 | 0.2 ± 0.06 | 23.7 ± 8.5 | 1.6 ± 0.8 | 4.1 ± 1.7 |
| AD 169 | HFF | 3.0 ± 1.0 | 0.05 | 13.8 | 1.0 | 13.6 |
| Towne | HFF | 2.94 ± 0.98 | 0.06 ± 0.01 | 9.04 ± 0.42 | 0.41 ± 0.1 | 12.9 ± 1.7 |
| Towned | HFF | 0.9 ± 0.3 | 1.8 ± 0.01 | >28.2 | 8.9 | >34.0 |
| 4760recPolA1-1-1e | HFF | 595 | 0.04 | 5.9 | 0.3 | 6.8 |
| 1117r73-1-2e | HFF | 7.2 | 0.04 | 5.6 | 6.4 | 0.3 |
| D-10 C4e | HFF | >25.3 | 0.04 | 6.2 | 0.6 | 11.2 |
| HSV isolates | ||||||
| HSV-1 (McKrae) | MA-104 | 3.2 ± 0.6 | 1.1 ± 0.3 | 15.5 ± 7.3 | 2.3 ± 1.2 | 54.4 ± 19.4 |
| HSV-2 (E194) | MA-104 | 12.9 ± 3.6 | 0.6 ± 0.1 | 42.4 ± 9.0 | 3.1 ± 0.4 | 292 ± 65 |
The IC50 was defined as the concentration of compound that resulted in a 50% reduction in plaque number compared to the number observed in control samples without drug. For the experiments performed with the Hs68 fibroblasts, the data are presented as the averages of three or more experiments with the standard deviation. For the experiments performed with the MRC-5, HFF, and MA-104 cells, all dose-response curves for all HCMV and HSV strains were constructed by using seven and eight drug concentrations, respectively, in duplicate. ND, not determined.
Control: GCV, HCMV isolates, except for 4760recPolA1-1-1, 1117r73-1-2, and D-10 C4, where PFA, CDV, and BDCRB, respectively, were used; acyclovir, HSV isolates.
HCMV strains used to infect MRC-5 cells: P8, a low-passage clinical isolate which is GCV sensitive; C8704 and C8805-37, which are GCV resistant due to UL97 phosphotransferase gene mutation (3, 34); and D16, which is GCV resistant through a mutation within the UL54 DNA polymerase gene (35).
Virus yield reduction assay.
HCMV strains used to infect HFF cells: 4760recPolA1-1-1 (genotype unknown) is PFA resistant (2.5-fold resistance) and is derived from a clinical isolate (1), 1117r73-1-2 (genotype unknown) is CDV resistant (10-fold resistance) and is derived from the AD 169 strain, D-10 C4 (D344E and Q204R mutations within the UL89 and UL56 genes, respectively) is TCRB and BDCRB resistant (20-fold resistance) and is derived from the Towne strain (19).
To further investigate the utility of the test compounds as anti-HCMV agents and the mechanism of action of the naphthyridine-dihydroisoquinoline scaffold, we tested these compounds against HCMV isolates harboring resistance to GCV, PFA, CDV, or BDCRB. The IC50s obtained against GCV-resistant viruses remained relatively unchanged for compounds A1, B1, B2, and B3 (Table 1), whereas HCMV strains C8704, C8805-37, and D16 displayed 12.3-, 19.7-, and 10.2-fold resistance, respectively, to GCV compared to the low-passage clinical isolate P8 (Table 1). Viral strains 4760recPolA1-1-1, 1117r73-1-2, and D-10 C4 displayed 2.5-, 10-, and 20-fold resistance to PFA, CDV, and BDCRB, respectively, compared to the wild-type strains (data not shown), whereas, again, the susceptibility to compounds A1, B1, B2, and B3 remained unchanged (Table 1).
To further elucidate the activity of the naphthyridine-dihydroisoquinoline scaffolds toward herpesviruses, we determined the antiviral efficacy of this series of compounds against HSV-1 and HSV-2. In these experiments, compounds A1 and B2 were found to be relatively equipotent to acyclovir against HSV-1, and compound A1 was 21.5-fold more potent than acyclovir against HSV-2 (Table 1). However, in all cases the therapeutic index of acyclovir was clearly superior (Table 1, see also Table 3).
TABLE 3.
Cytotoxicity profile for 1,6-naphthyridine and 7,8-dihydroisoquinoline representatives
| Cell line | CC50 (μM)
|
||||
|---|---|---|---|---|---|
| Controla | A1 | B1 | B2 | B3 | |
| MRC-5b | >3,921 | 8 | 90 | 26 | 255 |
| HFFb | >102 | 31 | >23 | 26 | >28 |
| MA-104c | >444 | 6.2 ± 0.3 | 93.1 ± 8.5 | 63.6 ± 1.3 | 91.8 ± 0.2 |
| MDCKc | >410 | 17.4 ± 2.5 | >282 | 35 ± 5.1 | >344 |
| A549c | 125.4 ± 16.5 | 15.6 ± 3.1 | 225.6 ± 2.3 | 95.4 ± 3.1 | 116 ± 10.2 |
| Hs68d | 54.2 ± 4.9 | 5.9 ± 2.1 | 175 | 15 ± 1.6 | 85 |
| Verod | 31.2 ± 2.5 | 1 | >282 | 20 | 170 |
| Hs68e | >392 | >312 | >282 | >318 | >344 |
| Veroe | >100 | NDg | >282 | 80 | 344 |
| CEM-SSf | >2.0 | 3 | 87 | 24 | 71 |
| PBMCf | >1.1 | 19 | >180 | 23 | 255 |
Controls: GCV for MRC-5, HFF, and Hs68 cells; ribavirin for MDCK cells; HPMPA for A549 cells; acyclovir for MA-104 and Vero cells; dideoxycytosine for CEM-SS cells; and zidovudine for PBMC.
Visual assessment on MRC-5 and HFF stationary cells (31, 37) was performed in six separate experiments. On a one to four-plus basis of scoring the cytopathology, no variation around the reported values was noticed.
Neutral red uptake on stationary cells (15, 30). MA-104, MDCK, and A549 cells were observed microscopically for drug-induced morphological changes by using seven concentrations of test compound in duplicate.
Thymidine uptake on exponentially growing Hs68 and Vero cells as described in Materials and Methods.
WST-1 cell proliferating agent on exponentially growing Hs68 and Vero cells as described in Materials and Methods.
XTT cell proliferating agent method (4). Experiments were performed in triplicate. Standard deviation was less than 15%.
ND, not determined.
Antiviral activity spectrum.
No significant activity against influenza virus, RSV, adenovirus, or parainfluenza viruses was detected for any of the test compounds (Table 2). Despite the fact that IC50s obtained with the test compounds were similar, in some cases, to those of the control substances used, a relatively large selectivity window for these viruses was nonexistent. However, there was a strong activity observed for compound A1 against RV/2 with a selectivity index greater than 400. In addition, no significant activity was observed against HIV strains RF and IIIB in CEM-SS cells. A marginal activity was observed against HIV clinical isolates ROJO and TEKI tested in PBMC for compounds A1 and B3 (Table 2).
TABLE 2.
Spectrum of antiviral activities of 1,6-naphthyridine and 7,8-dihydroisoquinoline representatives
| Virus (strain)a | Antiviral activity (IC50 [μM]) and selectivity index (SI)b
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Controlc
|
A1
|
B1
|
B2
|
B3
|
||||||
| IC50 | SI | IC50 | SI | IC50 | SI | IC50 | SI | IC50 | SI | |
| Influenza virus A | 25 ± 2 | >16 | >10 | <1 | 169 ± 31 | 1 | >32 | <1 | >344 | >1 |
| Influenza virus B | 23 ± 3 | >36 | 10 ± 1.6 | 2 | 158 ± 10 | >2 | 23 ± 8 | 2 | >344 | >1 |
| RSV (A2) | 8.2 ± 4.1 | 17 | 19 ± 13 | <1 | 118 ± 25 | <1 | 178 ± 13 | <1 | >344 | <1 |
| RV/2 (GPH) | 4.4 ± 1.2 | >7 | <0.03 | >400 | 31 ± 1.4 | 3 | 11 ± 2.5 | 3 | 74 ± 7 | 2 |
| AV/1 (65098) | 9.9 ± 1.7 | 13 | 13 ± 0.6 | 1 | >282 | <1 | 64 ± 12 | 1.5 | 136 ± 14 | <1 |
| PIV/3 (14702) | 12.3 ± 0.4 | 10 | 28.1 ± 3 | <1 | 282 ± 141 | <1 | 127 ± 32 | <1 | >344 | <1 |
| HIV RFd | 0.018 | >404 | >31 | <1 | >181 | <1 | >51 | <1 | >34 | <1 |
| HIV IIIBd | 0.028 | >388 | >31 | <1 | ND | ND | ND | ND | >34 | <1 |
| HIV ROJOc | 0.004 | >769 | 12.4 | 1.5 | 127 | >1.4 | 9.2 | 2 | 30 | 9 |
| HIV TEKIe | 0.004 | >1,250 | 0.93 | 22 | ND | ND | ND | ND | 139 | 2 |
Cell lines used for antiviral activity evaluation were MDCK for influenza viruses A and B, MA-104 for RSV and PIV/3, and A549 for RV/2 and AV/1.
In vitro selectivity index value, which is the ratio of CC50 to IC50. ND, not determined.
Control: influenza viruses A and B, RSV, and PIV/3, ribavirin; AV/1, HPMPA; RV/2, pirodavir; HIV RF and IIIB strains, dideoxycytosine; HIV ROJO and TEKI strains, zidovudine.
Anti-HIV activity was evaluated in CEM-SS cells. Experiments were performed in triplicate. Standard deviation was less than 10%.
Anti-HIV activity was evaluated in PBMC. Experiments were performed in triplicate. Standard deviation was less than 10%.
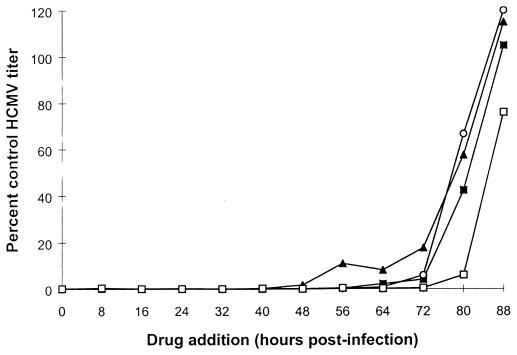
Time-of-drug addition studies.
To determine the events in the virus replication cycle affected by the 1,6-naphthyridine compound, various drugs known to act at early (GCV and CDV) and late (TCRB) phases within the HCMV replication cycle were used in a parallel experiment with the test compounds. GCV, CDV, and compound A1 were all found to be effective without loss of activity when added up to 72 h postinfection (Fig. 2), whereas TCRB was found to remain active when added between 72 and 80 h postinfection (Fig. 2), consistent with its inhibitory effect on viral DNA processing. These data indicate that compound A1 affects events at an early phase of the HCMV life cycle up to and including viral DNA synthesis.
FIG. 2.
Effect of time of drug addition on anti-HCMV activity of compound A1. HFF cells were infected with HCMV strain Towne at an MOI of 0.5 PFU/cell and exposed to GCV (○), CDV (▴), TCRB (□), and compound A1 (■) at 100, 10, 100, and 10 μM, respectively. Drugs were added at 8, 16, 24, 32, 40, 48, 56, 64, 72, 80, and 88 h postinfection and were allowed to remain on the infected cultured cells for the 96-h duration of the experiment. Thereafter, infected cells were subjected to one cycle of freezing and thawing, and the titer of virus produced was determined as described previously (37). The 0-h time point represents the end of the viral adsorption step. The antiviral activity of the drugs is depicted as a percentage of the virus titer obtained with the infected untreated cells.
Drug combination studies.
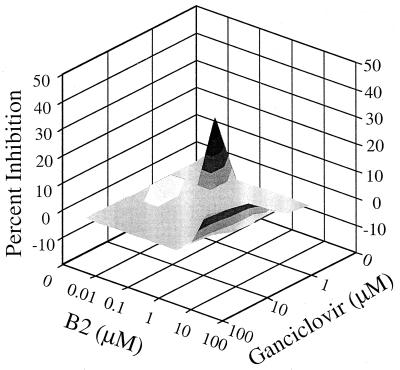
In vitro studies of antiviral activity using different drug combinations have suggested a synergistic inhibition of HCMV replication when GCV was combined with CDV (33). To determine if the activity of a currently employed anti-HCMV drug would be affected by the concomitant use of the new compounds, the effect of compound B2 on the activity of GCV against HCMV was determined. In these experiments, noncytotoxic combinations of B2 (0.005 to 30 μM) and GCV (0.4 to 100 μM) were employed. Following coadministration of test compounds, HCMV replication was determined by ELISA. The data show that there were drug combination concentrations in which the interaction rose above additivity, volume being 92.7 μM2% at 95% confidence level (Fig. 3). There also was a small area of slight antagonism at one combination of lower drug concentrations (Fig. 3), which gave a volume of antagonism of 9.4 μM2%, 95% confidence level. Nonetheless, the amount of synergy is similar to that observed for other interactions. For example, Prichard et al. observed synergy volume of 112 μM2% at 95% confidence level between acyclovir and a ribonucleotide reductase inhibitor (25), leading them and us to conclude that such interactions of two drugs are synergistic.
FIG. 3.
Interaction of compound B2 and GCV. MRC-5 cells were infected with HCMV at an MOI of 0.001 and treated with combinations of the two compounds. The amount of HCMV replication was determined by an ELISA. Data analysis with MacSynergy II revealed a volume of synergy of 92.7 μM2% at 95% confidence level.
Cytotoxicity determination.
Various methods, using different cell lines and growth conditions, were used to investigate the cytotoxic effects of the indicated compounds. These assays involved the measurement of the incorporation of [3H]thymidine, the use of WST-1 and XTT cell proliferation reagents, neutral red uptake, and visual assessment for the determination of cell viability. In these assays, the naphthyridine and dihydroisoquinoline compounds were found to be more toxic than GCV when assessed using stationary cell cultures (Table 3). It is interesting to note, however, that when log-phase growing Hs68 cells were used, the CC50s were generally equivalent to those of GCV, at least up to 282 μM. Of the four compounds evaluated in this study, B1 and B3 were better tolerated than were A1 and B2 in all of the different toxicity assays.
Effect of 1,6-naphthyridine derivatives on the HCMV major immediate-early promoter activity.
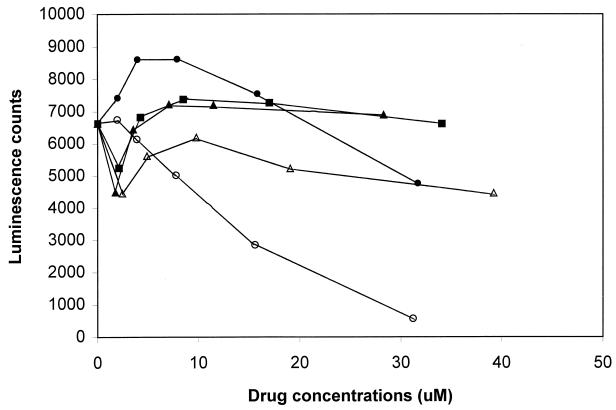
Since the time-of-drug addition studies suggested that the compounds were interfering with viral functions up to or including the initiation of DNA replication, we used the HCF cell line, in which a luciferase gene is stably expressed under the ie1/ie2 enhancer-promoter, to examine the potential effects on HCMV immediate-early promoter functions. In these studies, no inhibition of HCMV major immediate-early promoter-dependent luciferase activity was observed in the HCF hepatocyte stable cell line when cells were treated with GCV, B1, B2, or B3 at concentrations up to 39 μM (Fig. 4). However, a reduction was noted with compound A1 used at concentrations above 3.9 μM in a dose-dependent fashion with 90% inhibition at 31 μM. A similar observation was made for other 1,6-naphthyridine derivatives associated with IC50s comparable to or lower than that of compound A1 (data not shown). In parallel experiments, no toxicity was observed using the WST-1 cell proliferation reagent for any of the test compounds with the concentration range used in this study (data not shown). The marked inhibition of HCMV major immediate-early promoter activity by 1,6-naphthyridine representatives at nontoxic concentrations was also confirmed in an independent laboratory (data not shown).
FIG. 4.
Effect on HCMV ie1/ie2 promoter-enhancer-dependent luciferase activity. HCF cells were incubated in the presence of GCV (▵) and compounds A1 (○), B1 (▴), B2 (●), and B3 (■) at concentrations ranging from 1.8 to 39 μM for 40 h. Luciferase activity was measured as described in Materials and Methods. All points are averages of triplicate experiments. Standard deviation was less than 15%.
Effect of 1,6-naphthyridine derivatives on the expression of viral antigens in HCMV-infected cells.
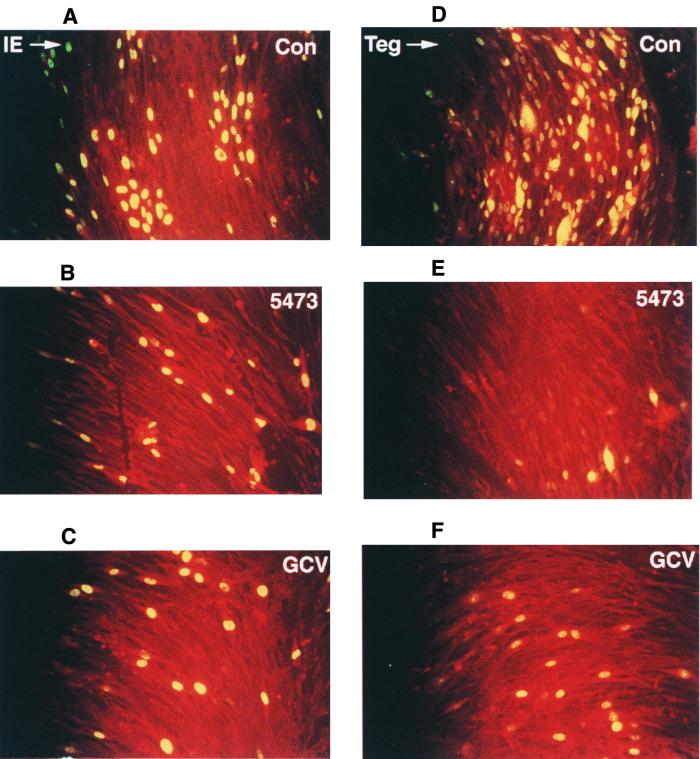
To help further define the potential mechanism of action for this series of compounds, we infected cells with HCMV and monitored temporal expression of the immediate-early and tegument proteins. In the control infected cells sampled at early time points postinfection (6 and 24 h), the nuclei were found to contain both IE 1 and tegument proteins, the latter deriving in part from the incoming virus, as determined by the lack of effect of cycloheximide treatment (data not shown). A similar pattern of staining was observed at these early times when infected cells were treated with GCV (10.2 μM) or compound A1 (0.62 μM). This indicated that virus infection was not prevented by, and IE 1 protein was synthesized in the presence of, the naphthyridine under the experimental conditions used (data not shown). Virus control samples stained for IE 1 at 72 h postinfection showed increased numbers of infected cells due to secondary infection, which was clearly restricted by either treatment (Fig. 5a to c). Similar effects were observed at this time when cells were stained for tegument protein. Secondary spread was even more in evidence in the virus controls in this case, with compound A1 significantly reducing the appearance of tegument protein in the cytoplasm of infected cells, a measure of de novo synthesis (Fig. 5d to f). Once again, it was clear that treatment with either compound A1 or GCV prevented normal progression of virus replication and secondary spread by infective progeny virus.
FIG. 5.
Effect of compound A1 (5473) on the expression of IE 1 and the tegument proteins of HCMV strain AD 169 using indirect immunofluorescence assay. MRC-5 cells were infected at an MOI of 0.1 PFU/cell in the presence of either no compound (A and D), compound A1 (B and E), or GCV (C and F) at 0.62 and 10.2 μM, respectively. Cell cultures were sampled and examined at 72 h postinfection for the expression of the IE 1 (A to C) and tegument (D to F) proteins as described in Materials and Methods.
DISCUSSION
The 1,6-naphthyridines and isoquinolines were identified as novel classes of compounds with anti-HCMV activity through screening using a plaque reduction assay (6, 7; L. Chan et al., unpublished data). A series of analogues were then synthesized and evaluated for structure-activity relationships (6, 7; L. Chan et al., unpublished data). The antiviral properties of compound A1, a representative of the 1,6-naphthyridines, as well as of three 7,8-dihydroisoquinolines, namely, compounds B1, B2, and B3, were presented in this study.
The drug-induced cytotoxicity and antiviral activity of four compounds, one 1,6-naphthyridine and three dihydroisoquinolines, were investigated using a variety of methods, viral strains, and cell lines. The 1,6-naphthyridine (A1) was clearly the most potent compound evaluated in this report. Its selectivity index was, in most cases, superior to that of the dihydroisoquinolines tested (Tables 1 and 3) despite its being more cytotoxic than B1, B2, and B3 (Table 3). Because of the large therapeutic index, it is very unlikely that the antiviral activity observed is related to the drug-induced toxicity to the host cells. In addition to the observed anti-HCMV activity, the four compounds tested were also active, albeit to a lesser extent, against HSV-1 and HSV-2 (Table 1). Compound A1 was also found to be an effective inhibitor of the rhinovirus strain RV/2 with a selective index of >400 (Table 2). A slight inhibitory effect on HIV (TEKI) with a selective index of 22 was also observed with A1. None of the compounds investigated were active against the influenza virus, RSV, adenovirus, or parainfluenza virus strains tested (Table 2). These data suggest a specific mechanism of viral inhibition which may result from either a direct effect on the susceptible virus or an effect on virus-cell interactions.
Among the set of molecules examined, compound A1 was the most potent inhibitor of laboratory-derived and clinical isolates of HCMV. The reduction in potency observed in the virus yield reduction assay may demonstrate that the antiviral activity of these compounds is MOI dependent. Amino acid changes within the UL54 and UL97 genes associated with a GCV resistance phenotype and mutations leading to PFA, CDV, and BDCRB drug resistance had no effect, for the specific viruses used in the experiments, on the activity of the compounds presented in this study. The HCMV phosphotransferase, DNA polymerase, or UL89 gene products cannot be excluded, despite lack of cross-resistance, as potential targets for these molecules since, for example, a large spectrum of mutations in the UL54 gene, which confers various levels of GCV, PFA, or CDV resistance, have been identified (13). Furthermore, it has been reported that HCMV strains derived under selective pressure of PFA were cross-resistant to phosphonylmethoxyethyl derivatives in vitro but not to HPMPA [(S)-9-(3-hydroxy-2-phosphonylmethoxypropyl)adenine] or CDV, suggesting a different mutation pattern within the same molecular target. Therefore, it is possible that a similar situation could be envisaged for the 1,6-naphthyridines and 7,8-dihydroisoquinolines. However, it should be emphasized that these compounds do not share any common structural features with GCV and CDV nucleoside analogues, which could suggest that a viral protein distinct from the HCMV DNA polymerase is targeted by these molecules.
Data derived from time-of-drug addition studies indicate that the compound A1 is acting at an early event within the viral replication cycle. A reduction of efficacy was observed at the same time as GCV and CDV when infected cells were exposed to the drugs at various time points during a single HCMV replication cycle. This is further supported by the reduction of de novo expression of pp65 tegument protein in the presence of compound A1 at a concentration of 0.62 μM which is well below the CC50 for the MRC-5 cells (Table 3) and suggests that the function of other cellular or viral proteins regulating the expression of the pp65 gene could be affected by compound A1. This could suggest a drug-related interference with the immediate-early- to early-phase transition of viral gene expression. The expression of other early gene products which were not monitored in these studies could be affected as well in the presence of compound A1. Experiments designed to elucidate the effects of compound A1 on RNA level and protein synthesis arising from various HCMV genes and viral polymerase activity are currently under way to further clarify the specificity of the inhibitory action. Alternatively, it is also possible that the naphthyridines and dihydroisoquinolines may act by disrupting cellular factors involved in viral cycle events, leading to an abnormal progression of the HCMV replication cycle. The immunofluorescence studies also demonstrate that the viral adsorption was not prevented since the amount of IE 1 protein staining positively was not affected in the presence of compound A1. Interestingly, an inhibition of the HCMV major immediate-early promoter-dependent luciferase activity in the HCF hepatocyte stably transfected cell line was noted in the presence of compound A1 at concentrations ranging from 3.9 to 31 μM (Fig. 4). These concentrations, however, were well above the concentrations used in the immunofluorescence studies where the expression of IE 1 protein was found to be unaffected by these concentrations of drug. The fact that different cell lines were used in the two studies could also explain the discrepancies observed between the two assays. In addition, no inhibitory and cytotoxicity effects were observed on the HIV type 1 long terminal repeat-driven chloramphenicol acetyltransferase reporter gene expression in Jurkat T cells (10) incubated with compound A1 (data not shown), suggesting a promoter or/and cell type specificity. It should be emphasized that the relevance of the marked reduction of the HCMV major immediate-early promoter activity with respect to the anti-CMV activity of these compounds remains to be determined.
In this study, we have reported on novel anti-HCMV agents, namely, 1,6-naphthyridines and 7,8-dihydroisoquinolines, having potent antiviral activity and in some cases relatively wide selectivity indices. Cross-resistance evaluation studies have revealed that a novel mechanism of action could be involved, making these compounds attractive for potential therapeutic use against HCMV infections where isolates have become resistant to current drug therapies. Identification of a slight synergistic effect between compound B2 and GCV raises the possibility for a use in combination therapy which could be beneficial to the infected individuals by preventing the emergence of drug resistance. Further exploratory work such as characterization of 1,6-naphthyridine- or 7,8-dihydroisoquinoline-resistant viruses is required to provide a better understanding of their mechanism of action.
ACKNOWLEDGMENT
The assistance of Tomislav Stefanac in carrying out the synthesis of some of the compounds is gratefully acknowledged.
REFERENCES
- 1.Baldanti F, Underwood M R, Stanat S C, Biron K K, Chou S, Sarasini A, Silini E, Gerna G. Single amino acid changes in the DNA polymerase confer foscarnet resistance and slow-growth phenotype, while mutations in the UL97-encoded phosphotransferase confer ganciclovir resistance in three double-resistant human cytomegalovirus strains recovered from patients with AIDS. J Virol. 1996;70:1390–1395. doi: 10.1128/jvi.70.3.1390-1395.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnard J A, Huffman J H, Sidwell R W, Reist E J. Selective inhibition of cytomegalovirus by 9-(3′-ethylphosphono-1′-propyloxy-methyl)guanine. Antivir Res. 1993;22:77–89. doi: 10.1016/0166-3542(93)90086-x. [DOI] [PubMed] [Google Scholar]
- 3.Biron K K. Ganciclovir-resistant human cytomegalovirus clinical isolates: resistance mechanisms and in vitro susceptibility to antiviral agents. Transplant Proc. 1991;23:162–167. [PubMed] [Google Scholar]
- 4.Buckheit R W, Kinjerski T L, Fliakas-Boltz V, Russell J D, Stup T L, Pallansch L A, Brouwer W G, Dao D C, Harrison W A, Schultz R J, Bader J P, Yang S S. Structure-activity and cross-resistance evaluations of a series of human immunodeficiency virus type 1-specific compounds related to oxathin carboxanilide. Antimicrob Agents Chemother. 1995;39:2718–2727. doi: 10.1128/aac.39.12.2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Causey D. Concomitant ganciclovir and zidovudine treatment for cytomegalovirus retinitis with HIV infection: an approach to treatment. J Acquir Immune Defic Syndr. 1991;4:S16–S21. [PubMed] [Google Scholar]
- 6.Chan L, Jin H, Stefanac T, Lavallee J F, Falardeau G, Wang W, Bedard J, May S, Yuen L. Discovery of the 1,6-naphthyridines as novel class of potent and selective human cytomegalovirus inhibitors. J Med Chem. 1999;42:3023–3025. doi: 10.1021/jm9902483. [DOI] [PubMed] [Google Scholar]
- 7.Chan L, Jin H, Stefanac T, Wang W, Lavallee J F, Bedard J, May S. Isoquinoline-6-carboxamides as potent and selective anti-human cytomegalovirus (HCMV) inhibitors. Bioorg Med Chem Lett. 1999;9:2583–2586. doi: 10.1016/s0960-894x(99)00435-7. [DOI] [PubMed] [Google Scholar]
- 8.Chou S, Erice A, Jordan M C, Vercellotti G M, Michels K R, Talarico C L, Stanat S C, Biron K K. Analysis of the UL97 phosphotransferase coding sequence in clinical cytomegalovirus isolates and identification of mutations conferring ganciclovir resistance. J Infect Dis. 1995;171:576–583. doi: 10.1093/infdis/171.3.576. [DOI] [PubMed] [Google Scholar]
- 9.Chou S, Guentzel S, Michel K R, Miner R C, Drew W L. Frequency of UL97 phosphotransferase mutations related to ganciclovir resistance in clinical cytomegalovirus isolates. J Infect Dis. 1995;172:239–242. doi: 10.1093/infdis/172.1.239. [DOI] [PubMed] [Google Scholar]
- 10.Copeland K F T, McKay P J, Rosenthal K L. Suppression of activation of the human immunodeficiency virus long terminal repeat by CD8+ T cells is not lentivirus specific. AIDS Res Hum Retroviru. 1995;11:1321–1326. doi: 10.1089/aid.1995.11.1321. [DOI] [PubMed] [Google Scholar]
- 11.Drew W L, Lalezari J P, Glutzer E, Flaherty J, Martin J C, Fisher J P, Jaffe H S. The safety, pharmacokinetics, and anti-CMV activity of weekly HPMPC in HIV positive patients excreting CMV. Antivir Res. 1993;20(Suppl. 1):55. [Google Scholar]
- 12.Erice A. Resistance of human cytomegalovirus to antiviral drugs. Clin Microbiol Rev. 1999;12:286–297. doi: 10.1128/cmr.12.2.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Field A K. Human cytomegalovirus: challenges, opportunities and new drug development. Antivir Chem Chemother. 1999;10:219–232. doi: 10.1177/095632029901000501. [DOI] [PubMed] [Google Scholar]
- 14.Hanson M H, Preheim L C, Chou S, Talarico C L, Biron K K, Erice A. Novel mutation in the UL97 gene of a clinical cytomegalovirus strain conferring resistance to ganciclovir. Antimicrob Agents Chemother. 1995;39:1204–1205. doi: 10.1128/aac.39.5.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huffman J H, Sidwell R W, Barnard D L, Morrison A, Otto M J, Hill C L, Schinazi R F. Influenza virus-inhibitory effects of a series of germanium and silicon centred polyoxometalates. Antivir Chem Chemother. 1997;8:75–83. [Google Scholar]
- 16.Jacobson M A. Review of the toxicities of foscarnet. J Acquir Immune Defic Syndr. 1992;5(Suppl.):S11–S17. [PubMed] [Google Scholar]
- 17.Jacobson M A. Treatment of cytomegalovirus retinitis in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1997;337:105–114. doi: 10.1056/NEJM199707103370207. [DOI] [PubMed] [Google Scholar]
- 18.Jacobson M A, Drew W L, Feinberg J, O'Donnell J J, Whitmore P V, Miner R D, Parenti D. Foscarnet therapy for ganciclovir resistant cytomegalovirus retinitis in patients with AIDS. J Infect Dis. 1991;163:1348–1351. doi: 10.1093/infdis/163.6.1348. [DOI] [PubMed] [Google Scholar]
- 19.Krosky P M, Underwood M N, Turk S R, Feng K W H, Jain R K, Ptark R G, Westerman A C, Biron K K, Townsend L B, Drach J C. Resistance of human cytomegalovirus to benzimidazole ribonucleosides maps to two open reading frames: UL89 and UL56. J Virol. 1998;72:4721–4728. doi: 10.1128/jvi.72.6.4721-4728.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Macher A M, Reichert C M, Straus S E, Longo D L, Parrillo J, Lane H C, Fauci A S, Rook A H, Manischewitz J F, Quinnan G V J. Death in the AIDS patient: role of cytomegalovirus. N Engl J Med. 1983;309:1454. doi: 10.1056/NEJM198312083092312. [DOI] [PubMed] [Google Scholar]
- 21.Meyers J D. Infection in bone marrow transplant recipients. Am J Med. 1986;81:27–38. doi: 10.1016/0002-9343(86)90511-5. [DOI] [PubMed] [Google Scholar]
- 22.Meyers J D. Prevention and treatment of cytomegalovirus infection. Annu Rev Med. 1991;42:179–187. doi: 10.1146/annurev.me.42.020191.001143. [DOI] [PubMed] [Google Scholar]
- 23.Ojwang J O, Buckheit R W, Pommier Y, Mazumder A, De Vreese K, Este J A, Reymen D, Pallansch L, Lackman-Smith C, Wallace T L, DeClercq E, McGrath M S, Rando R F. T30177, an oligonucleotide stabilized by an intramolecular guanosine octet, is a potent inhibitor of laboratory strains and clinical isolates of human immunodeficiency virus type 1. Antimicrob Agents Chemother. 1995;39:2426–2435. doi: 10.1128/aac.39.11.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palestine A G, Polis M A, DeSmet M D, Baird B F, Falloon J, Kovacs J A, Davey R T, Zurlo J J, Zunich K M, Davis M. A randomized, controlled trial of foscarnet in the treatment of cytomegalovirus retinitis in patients with AIDS. Appl Microbiol. 1991;22:797–801. doi: 10.7326/0003-4819-115-9-665. [DOI] [PubMed] [Google Scholar]
- 25.Prichard M N, Prichard L E, Shipman C. Strategic design and three-dimensional analysis of antiviral drug combinations. Antimicrob Agents Chemother. 1993;37:540–545. doi: 10.1128/aac.37.3.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prichard M N, Shipman C., Jr A three-dimensional model to analyze drug-drug interactions. Antivir Res. 1990;14:181–206. doi: 10.1016/0166-3542(90)90001-n. [DOI] [PubMed] [Google Scholar]
- 27.Prichard M N, Turk S R, Coleman L A, Engelhardt S L, Shipman C, Jr, Drach J C. A microtiter virus yield reduction assay for the evaluation of antiviral compounds against human cytomegalovirus and herpes simplex virus. J Virol Methods. 1990;29:101–106. doi: 10.1016/0166-0934(90)90091-s. [DOI] [PubMed] [Google Scholar]
- 28.Renau T E, Wotring J C, Drach J C, Townsend L B. Synthesis of non-nucleoside analogs of toyocamycin and thiosangivamycin: influence of various 7-substituents on antiviral activity. J Med Chem. 1996;39:873–880. doi: 10.1021/jm950444j. [DOI] [PubMed] [Google Scholar]
- 29.Sarasini A, Baldanti F, Furione M, Percevalle E, Brerra R, Barbi M, Gerna G. Double resistance to ganciclovir and foscarnet of four human cytomegalovirus strains recovered from AIDS patients. J Med Virol. 1995;47:237–244. doi: 10.1002/jmv.1890470309. [DOI] [PubMed] [Google Scholar]
- 30.Sidwell R W, Huffman J H. Use of disposable micro tissue culture plates for antiviral and interferon induction studies. Appl Microbiol. 1971;22:797–801. doi: 10.1128/am.22.5.797-801.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sidwell R W, Huffman J H, Khare G P, Allen L B, Witkowski T J, Robins R K. Broad-spectrum antiviral activity of virazole: 1-D-ribofuranosyl-1,2,4-triazole-3-carboxamide. Science. 1972;177:705–706. doi: 10.1126/science.177.4050.705. [DOI] [PubMed] [Google Scholar]
- 32.Smith I, Cherrington J M, Jiles R E, Fuller M D, Freeman W R, Spector S A. High-level resistance of cytomegalovirus to ganciclovir is associated with alterations in both the UL97 and DNA polymerase genes. J Infect Dis. 1997;176:69–77. doi: 10.1086/514041. [DOI] [PubMed] [Google Scholar]
- 33.Snoeck R, Andrei G, Schols D, Balzarini J, De Clercq E. Activity of different drug combinations against human cytomegalovirus replication in vitro. Eur J Clin Microbiol Infect Dis. 1998;11:1144–1155. doi: 10.1007/BF01961133. [DOI] [PubMed] [Google Scholar]
- 34.Stanat S C, Reardon J E, Erice A, Jordan M C, Drew W L, Biron K K. Ganciclovir-resistant cytomegalovirus clinical isolates: mode of resistance to ganciclovir. Antimicrob Agents Chemother. 1991;35:2191–2197. doi: 10.1128/aac.35.11.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tatarowicz W A, Lurain N S, Thompson K D. A ganciclovir-resistant clinical isolate of human cytomegalovirus exhibiting cross-resistance to other DNA polymerase inhibitors. J Infect Dis. 1992;166:904–907. doi: 10.1093/infdis/166.4.904. [DOI] [PubMed] [Google Scholar]
- 36.Tyms A S, Taylor D L, Parkin J M. Cytomegalovirus and the acquired immunodeficiency syndrome. J Antimicrob Chemother. 1989;23:89–105. doi: 10.1093/jac/23.suppl_a.89. [DOI] [PubMed] [Google Scholar]
- 37.Zou R, Drach J C, Townsend L B. Design, synthesis, and antiviral evaluation of 2-substituted 4,5-dichloro- and 4,6-dichloro-1-D-ribofuranosylbenzimidazoles as potential agents for human cytomegalovirus infections. J Med Chem. 1997;40:802–810. doi: 10.1021/jm960533b. [DOI] [PubMed] [Google Scholar]