Abstract
Background:
Elevated blood pressure variability (BPV) is predictive of dementia, independent of average blood pressure levels, but neuropathological mechanisms remain unclear. We examined whether BPV in older adults is related to tau accumulation in brain regions vulnerable to Alzheimer’s disease, and whether relationships are modified by apolipoprotein ϵ4 carrier status.
Methods:
286 Alzheimer’s Disease Neuroimaging Initiative participants without history of dementia underwent 3–4 blood pressure measurements over 12 months and ≥ 1 tau positron emission tomography thereafter. BPV was calculated as variability independent of mean. Each scan determined tau burden (standardized uptake value ratio) for a temporal meta-region of interest, including burden from entorhinal cortex, amygdala, parahippocampus, fusiform, inferior temporal, and middle temporal. Bayesian linear growth modelling examined the role of BPV, apolipoprotein ϵ4 carrier status, and time on regional tau accumulation after controlling for several variables, including baseline hypertension.
Results:
Elevated BPV was related to tau accumulation at follow-up in a temporal meta-region, independent of average blood pressure levels (ß: .89 [95% CI .86, .92]), and especially in entorhinal cortex (ß: 2.57 [95% 2.15, 2.99]). Apolipoprotein ϵ4 carriers with elevated BPV had the fastest tau accumulation at follow-up (ß: 1.73 [95% CI .47, 3.03]).
Conclusions:
BPV is related to tau accumulation in brain regions vulnerable to Alzheimer’s disease, independent of average blood pressure. APOE ϵ4 modified this relationship. Bidirectionality of findings is possible. BPV may represent a marker of vascular dysfunction related to early-stage tau pathology contributing to Alzheimer’s disease.
Keywords: Blood Pressure, Tau Protein, Alzheimer Disease, Aging, Dementia
INTRODUCTION
Blood pressure (BP) and its modulation by antihypertensive treatment has been extensively investigated in relation to cognitive decline and dementia.1–7 The role of BP elevation in dementia risk has garnered substantial attention in part because of the clinical availability of BP measures, the high rates of hypertension in older adults, and the wide availability of antihypertensive medications.8–10 Recent randomized controlled trial results from the SPRINT study indicate aggressive BP lowering prevents cognitive impairment, relative to standard BP control,11 underscoring the potential importance of BP and its modulation in the prevention of cognitive decline. However, the mechanisms linking BP to cognitive dysfunction have not been fully elucidated.
The role of BP in cerebrovascular disease is well established,12–15 but fewer studies have examined whether BP is related to Alzheimer’s disease (AD) pathophysiology. Findings to date have suggested that elevated BP may be associated with cerebral spinal fluid (CSF) biomarkers and positron emission tomography (PET) markers of both cerebral amyloidosis and tau-mediated neurodegeneration,16–20 as well as AD pathology at autopsy.13 More recent studies have particularly emphasized specific links between BP elevation and pathophysiological markers of hyperphosphorylated tau,13,16,17,21 as links between BP and amyloid-beta markers have been less consistent.15,17 At least one study has suggested tau specific changes may mediate relationships between BP and cognitive impairment, independent of amyloid-beta.20
Beyond average BP and the associated treatment strategies of aggressive BP lowering, other aspects of BP may be important for cognitive decline with potential implications for specific therapeutic approaches. Recent interest in blood pressure variability (BPV) over months to years (e.g., “visit-to-visit” BPV) has emerged as a vascular risk factor for cerebrovascular disease and cognitive decline, independent of average BP levels.22–30 Consistent evidence suggests elevated BPV is related both to cerebrovascular disease25–27,31,32 and cerebral perfusion decline,33 particularly within brain regions vulnerable to early-stage AD. However, it remains unclear whether increased BPV may convey risk for dementia purely through its association with cerebrovascular disease, or whether BPV is also linked to AD biomarker abnormality. The only study to examine BPV in relation to AD biomarkers showed no relationship with CSF amyloid-beta or tau levels.20 To our knowledge, no studies have evaluated the relationship between BPV and regional tau accumulation by tau-PET scans. The present study examined the longitudinal relationship between BPV and later tau accumulation in older adults, focusing on temporal regions affected during early-stage AD.34,35 The potential modifying role of the apolipoprotein ϵ (APOE)-4 allele was also investigated. While there is the possibility of a bidirectional relationship between BPV and tauopathy, the present investigation focused on tau changes after BPV was determined.
METHODS
Participants
Data were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database. The ADNI is a multisite natural history study that has collected clinical, biomarker, and neuropsychological data since 2003 to measure the progression of typical aging, mild cognitive impairment (MCI), and AD. Volunteer adults (age 55–91) were enrolled if they met the following criteria: few depressive symptoms (Geriatric Depression Scale < 6), free of history of neurological disease (other than suspected AD), no greater than mild dementia symptoms (Clinical Dementia Rating scale ≤ 1), and low vascular risk (Hachinski Ischemic Score ≤ 4). Ethical approval was obtained for each institution involved and all participants provided written informed consent. Further study details can be found online (https://adni.loni.usc.edu).
The present study included participants who underwent clinical evaluation at study baseline and BP measurement at study screening, baseline, and 6- and 12- months follow-up. Participants also underwent ≥ 1 tau-PET scan after the final BP measurement at 12-months follow-up.
Measures
Clinical assessment
Baseline clinical evaluation identified participants to be cognitively normal (CN) or MCI, as described elsewhere,33,36 and all participants were confirmed to be without history of dementia or stroke. Briefly, a clinical diagnosis of MCI was given if the following criteria were met:37 subjective memory complaint; Mini Mental State Exam (MMSE) scores between 24 and 30 (inclusive); global Clinical Dementia Rating scale score of 0.5; scores on delayed recall of Story A of the Wechsler Memory Scale Revised Logical Memory II subtest that are below expected performance based on years of education; general presentation that would disqualify for a diagnosis of AD. Participants were categorized as CN if diagnostic criteria for MCI were not met. For the present analysis, CN and MCI participants were combined into one category of older adults without history of dementia or stoke.
BP assessment
Participants underwent seated BP measurement (taken from the dominant forearm arranged at the horizontal level of the fourth intercostal space at the sternum) 3–4 times between study screening and 12-months follow-up using a calibrated mercury sphygmomanometer, as described elsewhere.33,36 Intraindividual BPV was calculated for each participant using the 3–4 BP measurements collected over the 12-month period as variation independent of mean (VIM), a commonly used index of visit-to-visit BPV that is uncorrelated with average BP levels across visits27,29,30,33,36,38 and was recently shown to predict all-cause mortality better than other indices of BPV.39 VIM was calculated as: VIM = SD/meanx, where the power x was derived from non-linear curve fitting of BP SD against average BP using the nls package in R,40 as described elsewhere.38,41 Baseline hypertension was determined from the total sample average systolic BP taken at study baseline.
Tau-PET assessment
Participants underwent ≥ 1 tau-PET (F-AV-1451 tracer) after the final BP collection at 12-months follow-up. Image acquisition and processing details can be found online http://adni.loni.usc.edu/methods/pet-analysis-method/pet-analysis/. Briefly, tau burden (standardized uptake value ratio [SUVR]) was determined from each tau-PET. SUVR values were then intensity-normalized and partial-volume corrected, consistent with other tau-PET imaging studies,34 including those involving ADNI data.42 SUVR values were determined for a temporal meta-region of interest (ROI), which has been used in previous cross-sectional43 and longitudinal34 tau-PET studies to separate participants along the AD continuum based on cognitive impairment and abnormal AD biomarkers. The temporal meta-ROI included SUVR values in the entorhinal cortex, amygdala, parahippocampus, fusiform, inferior temporal, and middle temporal ROIs. Finally, ROI and meta-ROI SUVR values were normalized to cerebellum and used in all analyses, consistent with other cross-sectional42,43 and longitudinal34 tau-PET studies.
Amyloid-PET assessment
Nearly all (n = 256 / 286) participants also underwent amyloid-PET (Florbetapir tracer). A composite amyloid burden score was calculated and normalized to cerebellum, as described in detail on the ADNI site (http://adni.loni.usc.edu/methods/pet-analysis-method/pet-analysis/). An SUVR threshold of 1.11 was used to determine amyloid-PET status; values above this threshold were considered to be “amyloid positive” and values below this threshold were considered to be “amyloid negative” using established criteria (http://adni.loni.usc.edu/methods/pet-analysis-method/pet-analysis/).
Other measurements
Baseline clinical evaluation determined several demographic and clinical variables, including: years of education, history of smoking, history of dyslipidemia, global cognition (i.e., MMSE score), use of antihypertensive medication, use of antidementia agents. Participants were categorized at those taking antihypertensive medication (all classes) versus those who were not, and those taking antidementia agents versus those who were not. Vascular risk was also determined from baseline clinical evaluation, as described elsewhere,17,36,44 and participants were categorized as having low (≤ 1 vascular risk factor) or high (≥ 2 vascular risk factors) vascular risk.44 APOE ϵ4 carrier status was determined from baseline venipuncture as previously described45 and participants were categorized as those with at least one APOE ϵ4 allele versus those without.
Data availability statement
All data are available on the ADNI site (https://adni.loni.usc.edu).
STATISTICAL ANALYSIS
Bayesian linear growth modeling (brms46 package in R40 using default prior distributions: fixed effects = improper flat prior over the reals; random effects = non-negative, half Student-t prior with 3 degrees of freedom, sigma = 2.5 or median absolute deviation of dependent variable if greater than 2.5) was used to investigate relationships between BPV, APOE ϵ4, and tau accumulation over time. All models specified random intercepts for participant, to account for individual variation in tau accumulation, and fixed effects for BPV and APOE ϵ4 carrier status to test for differences in tau accumulation due to BPV and APOE ϵ4 carrier status, respectively. BPV was handled as a time-invariant variable, such that a participant’s single BPV value determined over study screening to 12-months follow-up was associated with SUVR values at each follow-up tau-PET scan. To help determine the temporal order of any associations, only tau-PET scans acquired after the final BP measurement at 12-months follow-up were used in analyses. Passage of time for tau-PET scans was calculated as months elapsed since BPV determination (range: 12–156 months) and grand centered at 0. Based on emerging evidence that markers of vascular aging may be especially related to tau burden,13,17,47 we first ran models investigating a BPV by time interaction on temporal meta-ROI SUVR levels. We then ran models testing the 3-way interaction of BPV by APOE ϵ4 carrier status by time on temporal meta-ROI tau burden given the established links between APOE ϵ4, tau, and AD.48 For both models, we also explored contributions of individual ROIs included in the temporal meta-ROI (entorhinal cortex, amygdala, parahippocampus, fusiform, inferior temporal, and middle temporal). All models controlled for age at tau-PET, sex, APOE ϵ4 carrier status (for main effect models), baseline MMSE score, average BP, baseline hypertension, vascular risk, and antihypertensive medication use. Systolic BPV and diastolic BPV were examined separately in all models. The current investigation conducted prespecified analyses with BPV and considered analyses with other more well-studied BP indices (e.g., average BP, pulse pressure, mean arterial pressure) to be beyond the scope of the present study. To test the robustness of primary findings in temporal meta-ROI, sensitivity analyses also covaried for 1) years of education, 2) history of smoking, 3) history of dyslipidemia, 4) use of antidementia agents, and individual contribution of vascular risk factors 5) diabetes mellitus type 2 and 6) atrial fibrillation (versus controlling for overall vascular risk that includes these two factors as defined above). Finally, additional analyses of temporal meta-ROI tau burden were stratified by amyloid-PET status. Non-linear models were attempted but did not result in better fit based on leave-one-out cross validation. All analyses were 2-tailed and effect estimates with credible intervals (CI) excluding 0 were considered significant.
RESULTS
286 participants contributed to 472 tau-PET scans (median 2 scans). The median time interval between BPV measurement and tau-PET scan was 72 months (IQR: 48 months). Table 1 summarizes baseline demographic and clinical information.
Table 1.
Baseline clinical and demographic information.
| Total sample (N = 286) | |
|---|---|
| Age (years) | 78.3 (7.3) |
| Sex (n, % female) | 125 (43.7%) |
| Education (years) | 16.4 (2.6) |
| APOE ϵ4 carriers (n, %) | 100 (35.0%) |
| ADNI MCI diagnosis (n, %) | 130 (45.5%) |
| MMSE score | 28.7 (1.4) |
| BMI (kg/m2) | 27.7 (5.2) |
| Vascular risk* (n, % low) | 233 (81.5%) |
| Vascular risk factors (n, %) | |
| Cardiovascular disease | 28 (9.8%) |
| Diabetes mellitus type 2 | 22 (7.7%) |
| Atrial fibrillation | 4 (1.4%) |
| Carotid artery disease | 0 (0.0%) |
| TIA/minor stroke | 8 (2.8%) |
| Medication use (n, %) | |
| Antihypertensive agents | 105 (36.7%) |
| ACE inhibitors | 46 (16.1%) |
| ARBs | 15 (5.3%) |
| Alpha blockers | 10 (3.5%) |
| Calcium channel blockers | 17 (5.9%) |
| Diuretics | 13 (4.6%) |
| Antidementia agents | 14 (4.9%) |
| Systolic BP (mmHg) | |
| Baseline | 133.9 (15.2) |
| Average | 131.4 (12.5) |
| VIM | 4.9 (3.1) |
| Diastolic BP (mmHg) | |
| Baseline | 75.7 (9.7) |
| Average | 73.8 (7.8) |
| VIM | 5.9 (1.0) |
Means and SDs shown unless otherwise indicated.
Baseline vascular risk level determined from presence/absence of individual risk factors (history of cardiovascular disease, history of diabetes mellitus type 2, history of atrial fibrillation, history of carotid artery disease, history of TIA/minor stroke). Risk level is low (≤ 1 individual vascular risk factor) or high (≥ 2 individual vascular risk factors), as described elsewhere.17,36,44
Abbreviations: MMSE = Mini Mental State Exam; BP = blood pressure; BMI = body mass index: VIM = variability independent of mean; APOE ϵ4 = apolipoprotein ϵ4; MCI = mild cognitive impairment; CDR-sb = Clinical Dementia Rating Scale sum of box score; Aβ = amyloid-beta; Ptau = phosphorylated tau; ACE inhibitors = angiotensin-converting enzyme inhibitors; ARBs = angiotensin II receptor blockers; ADNI = Alzheimer’s Disease Neuroimaging Initiative; TIA = transient ischemic attack
Temporal meta-ROI
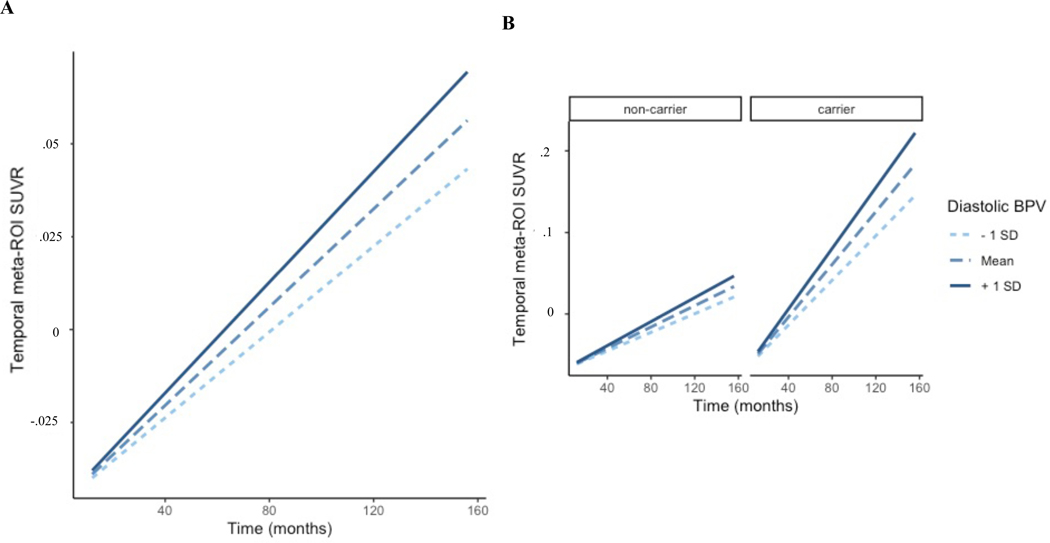
Increased diastolic BPV was associated with greater tau accumulation in the temporal meta-ROI at follow-up (ß: .89 [95% CI .86, .92]) (Figure 1A). Additionally, temporal meta-ROI tau burden increased the fastest for APOE ϵ4 carriers with elevated diastolic BPV (ß: 1.73 [95% CI .47, 3.03]) (Figure 1B).
Figure 1. BPV and temporal meta-ROI tau accumulation in older adults.
Conditional effects of the interaction of A) diastolic BPV by time and B) diastolic BPV by APOE ϵ4 carrier status by time on tau accumulation in temporal meta-ROI in older adults without history of dementia or stroke.
Abbreviations: BPV = blood pressure variability; SUVR = standardized uptake value ratio; ROI = region of interest
Systolic BPV was not significantly related to tau accumulation in the temporal meta-ROI (ß: .04 [95% CI −.27, .36]), and this did not differ by APOE ϵ4 carrier status (ß: −.39 [95% CI −1.24, .44]).
Individual ROIs
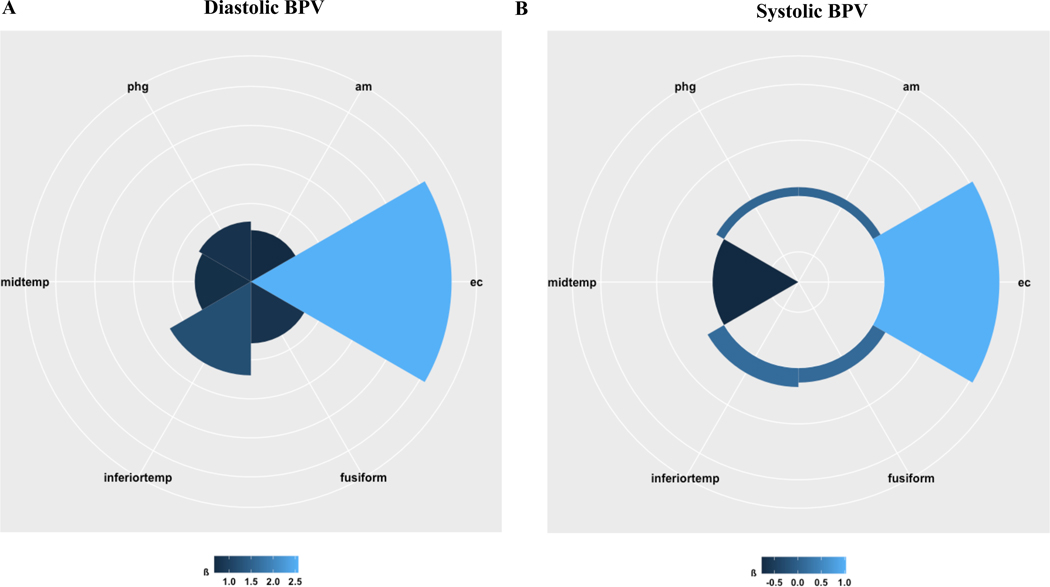
As shown in Table 2 and Figure 2, BPV predicted tau accumulation in all six individual ROIs included in the temporal meta-ROI. Specifically, elevated diastolic BPV was associated with greater tau accumulation in entorhinal cortex (ß: 2.57 [95% 2.15, 2.99]), amygdala (ß: .66 [95% .56, .76]), parahippocampus (ß: .77 [95% .72, .80]), inferior temporal (ß: 1.20 [95% .88, 1.52]), fusiform (ß: .79 [95% .75, .82]), and middle temporal (ß: .72 [95% .70, .75]). Systolic BPV was significantly related to increased tau burden in entorhinal cortex (ß: 1.03 [95% .81, 1.25]) and decreased tau burden in middle temporal (ß: −.77 [95% −.80, −.74]), but did not significantly predict tau accumulation in other individual ROIs (see Table 2).
Table 2.
Model estimates of BPV, APOE predicting regional tau accumulation
| ß (95% credible interval) | ||
|---|---|---|
| ROI/meta-ROI | Systolic BPV | Diastolic BPV |
| Meta-ROI | ||
| Temporal | ||
| BPV x time | .04 (−.27, .36) | .89 (.86, .92) |
| BPV x time x APOE ϵ4 | −.39 (−1.24, .44) | 1.73 (.47, 3.03) |
|
| ||
| Individual ROI | ||
| Entorhinal cortex | ||
| BPV x time | 1.03 (.81, 1.25) | 2.57 (2.15, 2.99) |
| BPV x time x APOE ϵ4 | 2.13 (.45, 3.86) | 4.24 (2.15, 6.18) |
| Amygdala | ||
| BPV x time | .08 (−.26, .42) | .66 (.56, .76) |
| BPV x time x APOE ϵ4 | −.27 (−1.14, .52) | 5.07 (4.73, 5.42) |
| Parahippocampus | ||
| BPV x time | .08 (−.25, .41) | .77 (.72, .80) |
| BPV x time x APOE ϵ4 | −.38 (−1.22, .45) | 1.99 (.47, 3.55) |
| Inferior temporal | ||
| BPV x time | .17 (−.29, .65) | 1.20 (.88, 1.52) |
| BPV x time x APOE ϵ4 | −.41 (−1.26, .43) | −.39 (−1.23, .44) |
| Fusiform | ||
| BPV x time | .13 (−.23, .49) | .79 (.75, .82) |
| BPV x time x APOE ϵ4 | −.38 (−1.21, .45) | 1.84 (.48, 3.21) |
| Middle temporal | ||
| BPV x time | −.77 (−.80, −.74) | .72 (.70, .75) |
| BPV x time x APOE ϵ4 | .54 (.42, .68) | .19 (−1.23, 1.60) |
Models adjusted for age at tau-PET scan, sex, APOE ϵ4 carrier status, baseline MMSE score, average BP, baseline hypertension, antihypertensive medication use and vascular risk.
Abbreviations: BPV = blood pressure variability; APOE ϵ4 = apolipoprotein ϵ4; ROI = region of interest
Figure 2. BPV and regional tau accumulation in older adults.
Radial plot of the effect estimates (ß) of A) diastolic BPV and B) systolic BPV predicting tau accumulation in individual ROIs included in the temporal meta-ROI.
Abbreviations: BPV = blood pressure variability; phg = parahippocampus; am = amygdala; ec = entorhinal cortex; inferiortemp = inferior temporal; midtemp = middle temporal
Similar patterns emerged at this level for APOE ϵ4 carriers; regional tau burden at follow-up was related to elevated BPV specifically in APOE ϵ4 carriers. As summarized in Table 2, APOE ϵ4 carriers with elevated diastolic BPV were observed to have significantly greater tau accumulation in entorhinal cortex (ß: 4.24 [95% 2.15, 6.18]), amygdala (ß: 5.07 [95% 4.73, 5.42]), parahippocampus (ß: 1.99 [95% .47, 3.55]), and fusiform (ß: 1.84 [95% .48, 3.21]), and not significantly greater tau accumulation in inferior temporal (ß: −.39 [95% −1.23, .44]) or middle temporal (ß: .19 [95% −1.23, 1.60]). APOE ϵ4 carriers with elevated systolic BPV had significantly greater tau accumulation in entorhinal cortex (ß: 2.13 [95% .45, 3.86]) and middle temporal (ß: .54 [95% .42, .68]), but tau accumulation did not significantly differ by APOE ϵ4 carrier status in other ROIs (see Table 2).
Primary findings remained statistically significant (e.g., CI excluded 0) in sensitivity analyses controlling for years of education, history of smoking, history of dyslipidemia, use of antidementia agents, history of diabetes mellitus type 2, and history of atrial fibrillation (see Supplementary Table S1).
Sensitivity analyses stratified by amyloid-PET status revealed that temporal meta-ROI tau burden increased the fastest for individuals with amyloid-PET positivity and elevated diastolic BPV (ß: 4.93 [95% CI 4.62, 5.20]) (Supplementary Figure S1).
DISCUSSION
The present study findings indicate elevated BPV is related to increased tau accumulation over time, specifically within temporal regions known to show tau deposition on tau-PET scans during the early stages of AD. Both systolic and diastolic BPV were examined since prior studies have observed either or both of these to be related to neurocognitive outcomes. Interestingly, diastolic BPV was more consistently related to tau accumulation throughout all temporal regions, but both systolic and diastolic BPV were related to tau within the entorhinal cortex and middle temporal cortex. APOE ϵ4 carriers with elevated BPV were observed to have the fastest regional tau accumulation, suggesting BPV may be an important vascular risk factor particularly in those at increased genetic risk for AD due to the presence of the APOE ϵ4 allele. Together these data add to mounting evidence that potentially modifiable hemodynamic factors are related to cerebral tau abnormalities in older adults.13,16,17,20,21
Increased BPV was especially related to tau accumulation in entorhinal cortex, amygdala, and parahippocampus, consistent with other cohort and postmortem studies of tau deposition and spread in AD.35,42,49 Classic models of Braak and Braak staging of AD indicate early tau deposition in entorhinal cortex.35 Newer models suggest that other regions may also be vulnerable to tau accumulation earlier in the disease process, which has led to growing investigation of tau burden in meta-ROIs.34 Interestingly, the amygdala is also critical for cortical control of autonomic nervous system activity,50 especially sympathetic function,51,52 and neurodegeneration in nearby insula has been linked with increases in BPV.53 Regional tau accumulation in the present study was robustly associated with diastolic BPV, which has been hypothesized to reflect endothelial dysfunction and sympathetic nervous system over-activation - compared with systolic BPV reflecting arterial stiffness – although more research is needed.30,54,55 Despite the longitudinal design and temporal order of tau-PET scans following BPV measurement, it is not possible to determine causality or directionality of the present findings. Nevertheless, it could be hypothesized that the hemodynamic effects of BPV on the cerebral microvasculature may potentiate tau-mediated neurodegeneration, which has a predilection for autonomic centers such as the amygdala.53 The resulting neurodegenerative effects on autonomic centers could in turn diminish BP regulation and drive further BPV fluctuations.53 This hypothesized scenario of bidirectional causality suggests a potential vicious cycle contributing to AD pathological progression. Future studies should investigate this possibility in order to explore potential treatment implications. Additionally, findings were predominantly observed in individuals with amyloid-PET positivity, consistent with studies suggesting that tauopathy most often occurs in the context of elevated amyloid.56
The association between BPV elevation and tau accumulation was particularly apparent in APOE ϵ4 carriers. There is an increasing appreciation of the vascular contributions of APOE ϵ4 to cognitive dysfunction in AD. A recent study found APOE ϵ4 carriers exhibit blood-brain barrier breakdown in the medial temporal lobe,57,58 a region where blood-brain barrier permeability59 and tau accumulation have been independently linked to cognitive dysfunction.34,35 It has been hypothesized that large fluctuations in BP may stretch and distress tight junctions and other intercellular connections23,24,60–62 that are necessary to maintain the blood-brain barrier,63 a theory that has found support in rodent calcium chloride models of arterial stiffening.64 In APOE ϵ4 carriers, a blood-brain barrier already prone to “leaking”65 may be particularly vulnerable to these hemodynamic forces, which have been coined a “tsunami effect”,62 potentially setting the stage for microvascular damage and possibly exacerbating tau vulnerability. Alternatively, tau pathophysiology may exacerbate microvascular damage as seen in tau-overexpressing mice,47 adding to the vulnerability of medial temporal regions to vascular insult.59 More studies are needed to determine the causal pathways linking BPV to regional tau accumulation in APOE ϵ4 carriers.
The present findings suggest BPV is related to tau accumulation, a marker of disease progression with growing clinical utility. Importantly, F-AV-1451 tau-PET tracer was recently approved by the FDA for clinical applicability of AD.66 BPV may also be a useful marker of disease progression, and one that is both highly modifiable and easily accessible.22,54 Some classes of antihypertensive medication may have differential effects on BPV in risk for stroke, independent of average BP levels.67,68 While the current study was not able to address potential class effects on tau accumulation, understanding these differences could inform treatment targets beyond BP lowering.
To our knowledge, this is the first study to examine BPV and tau-PET imaging. One prior study failed to detect any relationship between BPV and CSF tau levels.20 It is unclear why the present study found a different pattern of results, but it could be that CSF tau levels are less sensitive to regional tau deposition within specific brain areas affected in the earliest stages of AD (e.g., the entorhinal cortex) where robust relationships between BPV and tau were observed. This particular study examined day-to-day BPV over an average of 9.1 days,20 suggesting mechanisms underlying BPV may differ by timespan of BP measurement.22 The findings of the present study could suggest vascular dysfunction represented by increased BPV contributes to pathologic tau accumulation; however, replication of the present study finding is needed to further support a role for increased BPV in cerebral tau changes. The longitudinal design and assessment of tau-PET changes after the measurement of BPV represent strengths of the present study. Additionally, BPV was determined from BP measurements obtained using methods standard in routine clinical examination, suggesting BPV is a readily obtained clinic measure. Study limitations include the fact that some aspects of BP collection were not explicitly standardized across sites and may have been collected by individuals who did not have expertise in diagnosing hypertension. BPV was determined from 3–4 BP measurements, consistent with other studies of visit-to-visit BPV in ADNI,33,36 but additional BP measurements may improve measurement of BPV.69 Antihypertensive treatment initiation, discontinuation, or dosage change over the course of BP measurements were not addressed. The majority of participants in the present study were non-Hispanic White, limiting findings to other racial and ethnic groups. ADNI excludes participants with extensive cerebrovascular burden (Hachinski Ischemic Score ≤ 4), precluding investigation of older adults with greater cerebrovascular disease burden.
Supplementary Material
PATHOPHYSIOLOGIC NOVELTY AND RELEVANCE.
What is New?
Beyond average blood pressure, high blood pressure variability is linked to dementia risk, although potential mechanisms remain understudied.
What is Relevant?
Elevated blood pressure variability over one year was associated with tau accumulation in brain regions vulnerable to Alzheimer’s disease, especially in individuals at increased genetic risk for Alzheimer’s disease.
What are the Pathophysiological Implications?
Our results support growing evidence linking vascular dysfunction to tau accumulation in older adults at risk for dementia.
PERSPECTIVES.
Older adults with greater BPV exhibit increased tau accumulation in temporal regions affected during early-stage AD. The relationship between higher BPV and greater tau accumulation is greatest in APOE ϵ4 carriers. Findings add to evidence of a link between hemodynamic factors and tau accumulation in older adults at risk for dementia.
ACKNOWLEDGEMENTS
We would like to thank the participants and their families, investigators, and researchers from the ADNI study.
Funding
The study data analysis was supported by NIH/NIA grants (R01AG064228, R01AG060049, P50AG016573, P01AG052350) and Alzheimer’s Association grant AARG-17–532905. Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12–2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.1
APPENDIX: AUTHOR GROUPS
ADNI executive committee: Michael Weiner, MD; Paul Aisen, MD; Ronald Petersen, MD, PhD; Clifford R. Jack, Jr., MD; William Jagust, MD; John Q. Trojanowki, MD, PhD; Arthur W. Toga, PhD; Laura Beckett, PhD; Robert C. Green, MD, MPH; Andrew J. Saykin, PsyD; John Morris, MD; Leslie M. Shaw, PhD
Footnotes
Disclosures: none
REFERENCES
- 1.Levi Marpillat N, Macquin-Mavier I, Tropeano A-I, Levi-Bachoud A-C, Maison P. Antihypertensive classes, cognitive decline and incidence of dementia: a network meta-analysis. J. Hypertens 2013;31:1073–1082. [DOI] [PubMed] [Google Scholar]
- 2.Amenta F, Mignini F, Rabbia F, Tomassoni D, Veglio F. Protective effect of antihypertensive treatment on cognitive function in essential hypertension: Analysis of published clinical data. J. Neurol. Sci 2002;203–204:147–151. [DOI] [PubMed]
- 3.Xu W, Tan L, Wang HF, Jiang T, Tan MS, Tan L, Zhao QF, Li JQ, Wang J, Yu JT. Meta-analysis of modifiable risk factors for Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 2015;86:1299–1306. [DOI] [PubMed] [Google Scholar]
- 4.Iadecola C, Yaffe K, Biller J, Bratzke LC, Faraci FM, Gorelick PB, Gulati M, Kamel H, Knopman DS, Launer LJ, et al. Impact of hypertension on cognitive function: A scientific statement from the American Heart Association. Hypertension. 2016;68:e67–e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGrath ER, Beiser AS, DeCarli C, Plourde KL, Vasan RS, Greenberg SM, Seshadri S. Blood pressure from mid- to late life and risk of incident dementia. Neurology. 2017;89:2447–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ho JK, Moriarty F, Manly JJ, Larson EB, Evans DA, Rajan KB, Hudak EM, Hassan L, Liu E, Sato N, et al. Blood-Brain Barrier Crossing Renin-Angiotensin Drugs and Cognition in the Elderly: A Meta-Analysis. Hypertens. (Dallas, Tex. 1979) [Internet] 2021;78:629–643. Available from: http://www.ncbi.nlm.nih.gov/pubmed/34148364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol. 2005;4:487–499. [DOI] [PubMed] [Google Scholar]
- 8.Yaffe K. Prevention of cognitive impairment with intensive systolic blood pressure control. JAMA. 2019;321:548–549. [DOI] [PubMed] [Google Scholar]
- 9.Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011;10:819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, Ballard C, Banerjee S, Burns A, Cohen-Mansfield J, et al. Dementia prevention, intervention, and care. Lancet. 2017;390:2673–2734. [DOI] [PubMed] [Google Scholar]
- 11.Wright JT, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV., Reboussin DM, Rahman M, Oparil S, Lewis CE, et al. A randomized trial of intensive versus standard blood-pressure control. N. Engl. J. Med 2015;373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat. Rev. Neurosci 2004;5:347–360. [DOI] [PubMed] [Google Scholar]
- 13.Arvanitakis Z, Capuano AW, Lamar M, Shah RC, Barnes LL, Bennett DA, Schneider JA. Late-life blood pressure association with cerebrovascular and Alzheimer disease pathology. Neurology. 2018;91:e517–e525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iadecola C. Hypertension in dementia. Hypertension. 2014;64:3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lane CA, Barnes J, Nicholas JM, Sudre CH, Cash DM, Parker TD, Malone IB, Lu K, James SN, Keshavan A, et al. Associations between blood pressure across adulthood and late-life brain structure and pathology in the neuroscience substudy of the 1946 British birth cohort (Insight 46): an epidemiological study. Lancet Neurol. [Internet] 2019;18:942–952. Available from: 10.1016/S1474-4422(19)30228-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nation DA, Edland SD, Bondi MW, Salmon DP, Delano-Wood L, Peskind ER, Quinn JF, Galasko DR. Pulse pressure is associated with Alzheimer biomarkers in cognitively normal older adults. Neurology. 2013;81:2024–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nation DA, Edmonds EC, Bangen KJ, Delano-Wood L, Scanlon BK, Han SD, Edland SD, Salmon DP, Galasko DR, Bondi MW. Pulse pressure in relation to tau-mediated neurodegeneration, cerebral amyloidosis, and progression to dementia in very old adults. JAMA Neurol. 2015;72:546–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Langbaum JBS, Chen K, Launer LJ, Fleisher AS, Lee W, Liu X, Protas HD, Reeder SA, Bandy D, Yu M, et al. Blood pressure is associated with higher brain amyloid burden and lower glucose metabolism in healthy late middle-age persons. Neurobiol. Aging 2012;33:827.e11–827.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodrigue KM, Rieck JR, Kennedy KM, Devous MD, Diaz-Arrastia R, Park DC. Risk factors for β-amyloid deposition in healthy aging: Vascular and genetic effects. JAMA Neurol. 2013;70:600–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu H, Meng L, Bi Y-L, Zhang W, Xu W, Shen X-N, Ou Y-N, Ma Y-H, Dong Q, Tan L, et al. Tau pathologies mediate the association of blood pressure with cognitive impairment in adults without dementia: The CABLE study. Alzheimer’s Dement. [Internet] 2021;alz.12377. Available from: 10.1002/alz.12377 [DOI] [PubMed]
- 21.Glodzik L, Rusinek H, Pirraglia E, McHugh P, Tsui W, Williams S, Cummings M, Li Y, Rich K, Randall C, et al. Blood pressure decrease correlates with tau pathology and memory decline in hypertensive elderly. Neurobiol. Aging 2014;35:64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parati G, Ochoa JE, Lombardi C, Bilo G. Assessment and management of blood-pressure variability. Nat. Rev. Cardiol 2013;10:143–155. [DOI] [PubMed] [Google Scholar]
- 23.Ma Y, Tully PJ, Hofman A, Tzourio C. Blood pressure variability and dementia: A state-of-the art review. Am. J. Hypertens 2020;33:1059–1066. [DOI] [PubMed] [Google Scholar]
- 24.Nagai M, Dote K, Kato M, Sasaki S, Oda N, Kagawa E, Nakano Y, Yamane A, Higashihara T, Miyauchi S, et al. Visit-to-visit blood pressure variability and Alzheimer’s disease: Links and risks. J. Alzheimer’s Dis 2017;59:515–526. [DOI] [PubMed] [Google Scholar]
- 25.Tully PJ, Yano Y, Launer LJ, Kario K, Nagai M, Mooijaart SP, Claassen JAHR, Lattanzi S, Vincent AD, Tzourio C, et al. Association Between Blood Pressure Variability and Cerebral Small-Vessel Disease: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma Y, Song A, Viswanathan A, Blacker D, Vernooij MW, Hofman A, Papatheodorou S. Blood Pressure Variability and Cerebral Small Vessel Disease: A Systematic Review and Meta-Analysis of Population-Based Cohorts. Stroke. 2020;51:82–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sible IJ, Bangen KJ, Blanken AE, Ho JK, Nation DA. Antemortem Visit-To-Visit Blood Pressure Variability Predicts Cerebrovascular Lesion Burden in Autopsy-Confirmed Alzheimer’s Disease. J. Alzheimers. Dis. [Internet] 2021;83:65–75. Available from: 10.3233/JAD-210435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alpérovitch A, Blachier M, Soumaré A, Ritchie K, Dartigues JF, Richard-Harston S, Tzourio C. Blood pressure variability and risk of dementia in an elderly cohort, the Three-City Study. Alzheimer’s Dement. 2014;10:S330–S337. [DOI] [PubMed] [Google Scholar]
- 29.Rouch L, Cestac P, Sallerin B, Piccoli M, Benattar-Zibi L, Bertin P, Berrut G, Corruble E, Derumeaux G, Falissard B, et al. Visit-to-visit blood pressure variability is associated with cognitive decline and incident dementia: The S.AGES cohort. Hypertension. 2020;76:1280–1288. [DOI] [PubMed] [Google Scholar]
- 30.de Heus RAA, Olde Rikkert MGM, Tully PJ, Lawlor BA, Claassen JAHR. Blood pressure variability and progression of clinical Alzheimer disease. Hypertension. 2019;74:1172–1180. [DOI] [PubMed] [Google Scholar]
- 31.Brickman AM, Reitz C, Luchsinger JA, Manly JJ, Schupf N, Muraskin J, DeCarli C, Brown TR, Mayeux R. Long-term blood pressure fluctuation and cerebrovascular disease in an elderly cohort. Arch. Neurol 2010;67:564–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma Y, Blacker D, Viswanathan A, van Veluw SJ, Bos D, Vernooij MW, Hyman BT, Tzourio C, Das S, Hofman A. Visit-to-visit blood pressure variability, neuropathology, and cognitive decline. Neurology [Internet]. 2021;96:e2812 LP-e2823. Available from: http://n.neurology.org/content/96/23/e2812.abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sible IJ, Yew B, Dutt S, Bangen KJ, Li Y, Nation DA. Visit-to-visit blood pressure variability and regional cerebral perfusion decline in older adults. Neurobiol. Aging [Internet] 2021;105:57–63. Available from: 10.1016/j.neurobiolaging.2021.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jack CR Jr, Wiste HJ, Schwarz CG, Lowe VJ, Senjem ML, Vemuri P, Weigand SD, Therneau TM, Knopman DS, Gunter JL, et al. Longitudinal tau PET in ageing and Alzheimer’s disease. Brain [Internet]. 2018;141:1517–1528. Available from: https://pubmed.ncbi.nlm.nih.gov/29538647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. [Internet] 1991;82:239–259. Available from: 10.1007/BF00308809 [DOI] [PubMed] [Google Scholar]
- 36.Sible IJ, Nation DA, Alzheimer’s Disease Neuroimaging Initiative. Long-term blood pressure variability across the clinical and biomarker spectrum of Alzheimer’s disease. J. Alzheimer’s Dis. [Internet] 2020;77:1655–1669. Available from: http://www.ncbi.nlm.nih.gov/pubmed/32925032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petersen RC, Aisen PS, Beckett LA, Donohue MC, Gamst AC, Harvey DJ, Jack CR, Jagust WJ, Shaw LM, Toga AW, et al. Alzheimer’s Disease Neuroimaging Initiative (ADNI): Clinical characterization. Neurology. 2010;74:201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rothwell PM, Howard SC, Dolan E, O’Brien E, Dobson JE, Dahlöf B, Sever PS, Poulter NR. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet [Internet]. 2010;375:895–905. Available from: 10.1016/S0140-6736(10)60308-X [DOI] [PubMed] [Google Scholar]
- 39.Cheng Y, Li J, Ren X, Wang D, Yang Y, Miao Y, Sheng C-S, Tian J. Visit-to-visit office blood pressure variability combined with Framingham risk score to predict all-cause mortality: A post hoc analysis of the systolic blood pressure intervention trial. J. Clin. Hypertens. [Internet] 2021;23:1516–1525. Available from: 10.1111/jch.14314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.R Core Team. R: A language and environment for statistical computing. 2018;
- 41.Yano Y. Visit-to-visit blood pressure variability - What is the current challenge? Am. J. Hypertens 2017;30:112–114. [DOI] [PubMed] [Google Scholar]
- 42.Zhao Q, Liu M, Ha L, Zhou Y, Alzheimer’s Disease Neuroimaging Initiative. Quantitative 18F-AV1451 brain Tau PET imaging in cognitively normal older adults, mild cognitive impairment, and Alzheimer’s disease patients [Internet]. Front. Neurol 2019;10:486. Available from: 10.3389/fneur.2019.00486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jack CR, Wiste HJ, Weigand SD, Therneau TM, Lowe VJ, Knopman DS, Gunter JL, Senjem ML, Jones DT, Kantarci K, et al. Defining imaging biomarker cut points for brain aging and Alzheimer’s disease. Alzheimer’s Dement. [Internet] 2017;13:205–216. Available from: 10.1016/j.jalz.2016.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.D’Agostino RB, Wolf PA, Belanger AJ, Kannel WB. Stroke risk profile: Adjustment for antihypertensive medication: The Framingham Study. Stroke. 1994;25:40–43. [DOI] [PubMed] [Google Scholar]
- 45.Saykin AJ, Shen L, Foroud TM, Potkin SG, Swaminathan S, Kim S, Risacher SL, Nho K, Huentelman MJ, Craig DW, et al. Alzheimer’s Disease Neuroimaging Initiative biomarkers as quantitative phenotypes: Genetics core aims, progress, and plans. Alzheimer’s Dement. 2010;6:265–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bürkner PC. brms: An R package for Bayesian multilevel models using Stan. J. Stat. Softw 2017;80. [Google Scholar]
- 47.Bennett RE, Robbins AB, Hu M, Cao X, Betensky RA, Clark T, Das S, Hyman BT. Tau induces blood vessel abnormalities and angiogenesis-related gene expression in P301L transgenic mice and human Alzheimer’s disease. Proc. Natl. Acad. Sci. U. S. A 2018;115:E1289–E1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shi Y, Yamada K, Liddelow SA, Smith ST, Zhao L, Luo W, Tsai RM, Spina S, Grinberg LT, Rojas JC, et al. ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy. Nature [Internet]. 2017;549:523–527. Available from: 10.1038/nature24016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maass A, Landau S, Baker SL, Horng A, Lockhart SN, La Joie R, Rabinovici GD, Jagust WJ. Comparison of multiple tau-PET measures as biomarkers in aging and Alzheimer’s disease. Neuroimage [Internet]. 2017;157:448–463. Available from: https://www.sciencedirect.com/science/article/pii/S1053811917304585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nagai M, Hoshide S, Kario K. The insular cortex and cardiovascular system: a new insight into the brain-heart axis. J. Am. Soc. Hypertens 2010;4:174–182. [DOI] [PubMed] [Google Scholar]
- 51.Sturm VE, Brown JA, Hua AY, Lwi SJ, Zhou J, Kurth F, Eickhoff SB, Rosen HJ, Kramer JH, Miller BL, et al. Network architecture underlying basal autonomic outflow: Evidence from frontotemporal dementia. J. Neurosci 2018;38:8943–8955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guo CC, Sturm VE, Zhou J, Gennatas ED, Trujillo AJ, Hua AY, Crawford R, Stables L, Kramer JH, Rankin K, et al. Dominant hemisphere lateralization of cortical parasympathetic control as revealed by frontotemporal dementia. Proc. Natl. Acad. Sci. U. S. A 2016;113:E2430–E2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kitamura J, Nagai M, Ueno H, Ohshita T, Kikumoto M, Toko M, Kato M, Dote K, Yamashita H, Kario K. The Insular Cortex, Alzheimer Disease Pathology, and Their Effects on Blood Pressure Variability. Alzheimer Dis. Assoc. Disord. [Internet] 2020;34:282–291. Available from: 10.1097/WAD.0000000000000340 [DOI] [PubMed] [Google Scholar]
- 54.Parati G, Stergiou GS, Dolan E, Bilo G. Blood pressure variability: clinical relevance and application. J. Clin. Hypertens 2018;20:1133–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bilo G, Parati G. Blood pressure variability and kidney disease: Another vicious circle? J. Hypertens 2018;36:1019–1021. [DOI] [PubMed] [Google Scholar]
- 56.Hanseeuw BJ, Betensky RA, Jacobs HIL, Schultz AP, Sepulcre J, Becker JA, Cosio DMO, Farrell M, Quiroz YT, Mormino EC, et al. Association of Amyloid and Tau with Cognition in Preclinical Alzheimer Disease: A Longitudinal Study. JAMA Neurol. 2019;76:915–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Montagne A, Nation DA, Sagare AP, Barisano G, Sweeney MD, Chakhoyan A, Pachicano M, Joe E, Nelson R, Orazio LMD, et al. APOE4 leads to early blood-brain barrier dysfunction predicting human cognitive decline. Nature. 2020;581:71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nation DA, Sweeney MD, Montagne A, Sagare AP, D’Orazio LM, Pachicano M, Sepehrband F, Nelson AR, Buennagel DP, Harrington MG, et al. Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat. Med 2019;25:270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zlokovic B V. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat. Rev. Neurosci 2011;12:723–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lattanzi S, Vernieri F, Silvestrini M. Blood pressure variability and neurocognitive functioning. J. Clin. Hypertens 2018;20:645–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yoo JE, Shin DW, Han K, Kim D, Lee SP, Jeong SM, Lee J, Kim SY. Blood pressure variability and the risk of dementia: a nationwide cohort study. Hypertension. 2020;75:982–990. [DOI] [PubMed] [Google Scholar]
- 62.Saji N, Toba K, Sakurai T. Cerebral Small Vessel Disease and Arterial Stiffness: Tsunami Effect in the Brain? Pulse. 2016;3:182–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Winder NR, Reeve EH, Walker AE. Large artery stiffness and brain health: Insights from animal models. Am. J. Physiol. - Hear. Circ. Physiol 2021;320:H424–H431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Muhire G, Iulita MF, Vallerand D, Youwakim J, Gratuze M, Petry FR, Planel E, Ferland G, Girouard H. Arterial stiffness due to carotid calcification disrupts cerebral blood flow regulation and leads to cognitive deficits. J. Am. Heart Assoc 2019;8:e011630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Salloway S, Gur T, Berzin T, Tavares R, Zipser B, Correia S, Hovanesian V, Fallon J, Kuo-Leblanc V, Glass D, et al. Effect of APOE genotype on microvascular basement membrane in Alzheimer’s disease. J. Neurol. Sci 2002;203–204:183–187. [DOI] [PubMed]
- 66.Beyer L, Brendel M. Imaging of Tau Pathology in Neurodegenerative Diseases: An Update. Semin. Nucl. Med. [Internet] 2021;51:253–263. Available from: https://www.sciencedirect.com/science/article/pii/S0001299820301276 [DOI] [PubMed] [Google Scholar]
- 67.Webb AJ, Fischer U, Mehta Z, Rothwell PM. Effects of antihypertensive-drug class on interindividual variation in blood pressure and risk of stroke: A systematic review and meta-analysis. Lancet [Internet]. 2010;375:906–915. Available from: 10.1016/S0140-6736(10)60235-8 [DOI] [PubMed] [Google Scholar]
- 68.Rothwell PM, Howard SC, Dolan E, Brien EO, Dobson JE, Dahlöf B, Poulter NR, Sever PS. Effects of β blockers and calcium-channel blockers on within-individual variability in blood pressure and risk of stroke. Lancet Neurol. [Internet] 2010;9:469–480. Available from: 10.1016/S1474-4422(10)70066-1 [DOI] [PubMed] [Google Scholar]
- 69.Lim HM, Chia YC, Ching SM, Chinna K. Number of blood pressure measurements needed to estimate long-term visit-to-visit systolic blood pressure variability for predicting cardiovascular risk: A 10-year retrospective cohort study in a primary care clinic in Malaysia. BMJ Open. 2019;9:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available on the ADNI site (https://adni.loni.usc.edu).