Abstract
Objective
To evaluate the agreement in brain injury findings between early and late magnetic resonance imaging (MRI) in newborn infants with hypoxic ischemic encephalopathy (HIE) treated with therapeutic hypothermia and to compare the ability of early versus late MRI to predict early neurodevelopmental outcomes.
Study design
This was a prospective longitudinal study of 49 patients with HIE who underwent therapeutic hypothermia and had MRI performed at both <7 and ≥7 days of age. MRIs were reviewed by an experienced neuroradiologist and assigned brain injury severity scores according to established systems. Scores for early and late MRIs were assessed for agreement using the kappa statistic. The ability of early and late MRI scores to predict death or developmental delay at 15-30 months of age was assessed by logistic regression analyses.
Results
Agreement between the early and late MRI was substantial to near perfect (k>0.75, p<0.001) across MRI scoring systems. In cases of discrepant scoring, early MRI was more likely to identify severe injury when compared with late MRI. Early MRI scores were more consistently predictive of adverse outcomes compared with late MRI.
Conclusion
The results of this study suggest that a single MRI performed in the first week after birth is adequate to assess brain injury and offer prognostic information in this high-risk population.
Keywords: neonatal encephalopathy, magnetic resonance imaging, brain injury, neonatal intensive care
Though the introduction of therapeutic hypothermia following neonatal hypoxic-ischemic encephalopathy (HIE) has greatly reduced the risk of death and disability, nearly half of all newborn infants suffering from moderate to severe HIE still suffer from death and neurodevelopmental delay.1-4 Methods for assessing the extent and severity of brain injury in the subacute period following therapeutic hypothermia are necessary to serve as early endpoints to assess therapeutic effectiveness of current and future early neuroprotective interventions, and perhaps to direct the need for additional adjuvant therapies. Additionally, early and accurate predictors of later neurodevelopmental outcomes are needed to determine prognosis and counsel families appropriately after HIE.
Brain magnetic resonance imaging (MRI) following therapeutic hypothermia is the mainstay of assessing subacute brain injury in clinical care.5 Severity of brain injury on MRI shows good prognostic value for early childhood neurodevelopmental outcomes.6-12 Additionally, the location and extent of MRI lesions can specify outcome phenotypes. Specifically, basal ganglia and thalamic lesions have been associated with long-term motor outcomes9, 11, 12 whereas watershed lesions have been associated with verbal and intellectual outcomes.10 The timing of brain MRI, however, remains controversial.13
Current guidelines proposed by the American College of Obstetrics and Gynecology suggest performing two brain MRIs in the neonatal period following therapeutic hypothermia.14 These guidelines are based on the notion that early MRI (at 1-4 days) indicates the timing of injury, but later MRI (between 7-21 days) more fully defines the extent of the injury. However, disagreement exists regarding whether both are necessary and which is more valuable in determining injury and prognosis. Although some studies suggest there is no difference between early and late MRI scans,15 others propose that a late MRI is necessary to appropriately predict prognosis.16 Conversely, other studies report that the early MRI shows a higher specificity for brain injury than the late MRI and is sufficient to determine prognosis.6
Given the absence of clear evidence on the optimal timing of brain MRI after neonatal HIE, the primary objective of this study was to determine the agreement between early and late brain MRI after therapeutic hypothermia in newborn infants with HIE. A secondary purpose was to determine whether either MRI reliably predicted outcomes at 15-30 months.
Methods
This was a prospective longitudinal cohort study performed in a level 4 neonatal intensive care unit (NICU) at Children’s National Hospital. Enrollment occurred from 4/2012-6/2016. Infants included in the study were eligible for and underwent therapeutic hypothermia for moderate-severe HIE according to institutional protocol. All included infants had gestational ages of at least 35 weeks, had birth weights >1800g, and evidence of perinatal depression according to the National Institute of Child Health and Human Development (NICHD) Neonatal Research Network (NRN) protocol (including Apgar score <=5 at 10 minutes, prolonged resuscitation (chest compressions, mechanical ventilation, intubation) at 10 minutes, or metabolic acidosis including umbilical cord or infant blood gas within the first hour after birth.1 Moderate-severe encephalopathy was defined using the worst examination before 6 hours of life according to modified Sarnat clinical staging.1, 17 All included patients received 72 hours of hypothermia, initiated within 6 hours of birth, with a target temperature of 33.5±0.5C. Infants who underwent therapeutic hypothermia but did not have two brain MRI scans after rewarming and prior to hospital discharge (according to institutional protocol – see below) were excluded.
Magnetic Resonance Imaging
Brain MRIs were performed according to institutional protocol developed for newborn infants with suspected hypoxic-ischemic injury. All infants treated with therapeutic hypothermia were assessed by MRI as soon as possible after rewarming (target 4-6 days of age) and again at a target age of 10-12 days of age or discharge if earlier. Infants did not undergo MRI if deemed medically unstable by the clinical team. Infants were not scanned twice if discharged at age < 7 days. All scans were performed on a 3 Tesla scanner (Discovery MR750, GE Healthcare, Milwaukee, WI) using a 32-channel receive-only head coil (MR Instruments, Inc., Minneapolis, MN). Standard anatomical sequences included 3D T1-weighted Spoiled Gradient Recalled, double acquisition axial FSE T2 proton density, axial T2 propeller (in cases of patient motion), axial T2-Star Weighted Susceptibility Imaging, coronal T1 FLAIR propeller, axial pseudocontinuous arterial spin labeling, and axial 30-direction diffusion tensor imaging with generation of apparent diffusion coefficient (ADC) maps off-line.
Brain MRIs were reviewed by an experienced neuroradiologist (G.V.), who assigned an individual basal ganglia, watershed, and basal ganglia/watershed score according to Barkovich.9 Additionally, images were scored according to the National Institute of Child Health and Human Development (NICHD) scoring system.8 The neuroradiologist was blinded to the patient outcomes when reviewing MRI scans. For each scan, T1, T2, and ADC were reviewed for overall scoring of injury and both scoring systems were applied based on the pattern/extent of injury described for each system (i.e. irrespective of sequence where signal abnormality was observed).
Neurodevelopmental Follow-Up
Surviving infants underwent clinical neurodevelopmental follow-up at 15, 21 and 30 months of age per institutional protocol. Infants were assessed with the Bayley Scales of Infant-Toddler Development −3rd Edition (BSITD-III) by a certified developmental psychologist who was blinded to neonatal brain MRI scores.18 The BSITD-III is a scale commonly used to assess developmental progress in young children which measures cognitive, language (receptive and expressive), and motor (gross and fine motor) domains. A composite score of 100 is the normative mean, with a SD of 15. Given reports of overestimation of developmental performance with the BSITD-III, significant neurodevelopmental delay was defined as a BSITD-III cognitive composite score <85 or a motor composite score <85.19-21 The latest available developmental assessment was used for analysis.
Statistical Analyses
Descriptive statistics included means (standard deviations) and medians (ranges) for continuous variables, as well as counts (percentages) for categorical data. Agreement between early and late MRI scores was assessed with the weighted kappa statistic. The ability of MRI scores to predict significant neurodevelopmental delay was assessed with logistic regression analyses. Secondary exploratory models were developed controlling for clinical confounders including encephalopathy grade (moderate versus severe), Apgar score at 5 minutes and socioeconomic status (public versus private insurance). Models were compared using the C-statistic (area under the curve-AUC; where values close to 1 represent perfect model prediction) and Akaike information criterion (AIC; where lower values represent higher quality of the model).22 Additionally, the relationship between MRI scores and individual BSITD-III cognitive, language and motor scores were assessed with Spearman correlation analyses. A P value < .05 was considered statistically significant. Statistical analysis was performed using SAS 9.4 (SAS Institute).
Results
Of the 110 infants admitted for therapeutic hypothermia during the study period, 92 were enrolled. Of those enrolled in the study, 15 patients died prior to MRI, 26 had only one MRI (n=13 early and n=13 late), and 2 had both MRIs in the early window, leaving 49 infants who met the eligibility criteria of two brain MRIs at <7 days and >=7 days. Table I (available at www.jpeds.com) shows the baseline characteristics and clinical presentation of the studied infants which was typical of infants with moderate (n=43) and severe (n=6) encephalopathy. These baseline and clinical characteristics, as well as the distribution of MRI severity scores, did not significantly differ from the excluded population of babies with HIE who did not undergo MRI in both windows of interest (p>0.05). The median age for the early MRI was 4 days (range 2-6) and the median age for the late MRI was 10 days (range 7-25).
Table 1.
Comparison of study population with and without follow-up data
| Overall Study Population (n=49) |
Subjects with Outcome Data (n=30) |
Lost to Follow-up (n=19) |
*P value | |
|---|---|---|---|---|
| Gestational Age (mean±SD wks) | 38.6±1.5 | 38.4±1.6 | 39±1.1 | 0.148 |
| Birth weight (mean±SD kg) | 3.24±0.75 | 3.06±0.58 | 3.51±0.92 | 0.039 |
| Sex (n, % male) | 25 (51) | 14 (47) | 11 (58) | 0.561 |
| Apgar | ||||
| 1 minute | 1 (0-5) | 1 (0-5) | 1 (0-5) | 0.602 |
| 5 minute | 4 (0-8) | 4 (0-8) | 4 (0-7) | 0.442 |
| 10 minute | 5 (0-9)a | 5 (1-9)b | 6 (0-8)c | 0.290 |
| Presenting pH | 6.96 (6.6-7.4)a | 6.9 (6.6-7.3)d | 7.1 (6.6-7.4)e | 0.015 |
| Presenting base deficit | 18.1 (3-30) | 18.6 (6-30) | 16 (3-25) | 0.141 |
| Encephalopathy grade (n, % severe) | 6 (12) | 4 (13) | 2 (11) | 1.000 |
| Public Insurance (n, %) | 24 (49) | 12 (40) | 12 (63) | 0.148 |
| Distribution of Early MRI Scores | ||||
| Basal Ganglia | ||||
| 0 | 32 (65.3) | 18 (60) | 14 (74) | 0.471 |
| 1 | 4 (8.2) | 3 (10) | 1 (5) | |
| 2 | 4 (8.2) | 4 (13) | 0 (0) | |
| 3 | 6 (12.2) | 3 (10) | 3 (16) | |
| 4 | 3 (6.1) | 2 (7) | 1 (5) | |
| Watershed | ||||
| 0 | 32 (65.3) | 18 (60) | 14 (74) | 0.405 |
| 1 | 4 (8.2) | 3 (10) | 1 (5) | |
| 2 | 5 (10.2) | 2 (7) | 3 (16) | |
| 3 | 0 (0) | 0 (0) | 0 (0) | |
| 4 | 7 (14.3) | 6 (20) | 1 (5) | |
| 5 | 1 (2) | 1 (3) | 0 (0) | |
| Basal Ganglia/Watershed | ||||
| 0 | 28 (57.2) | 16 (53) | 12 (63) | 0.701 |
| 1 | 6 (12.2) | 5 (17) | 1 (5) | |
| 2 | 5 (10.2) | 3 (10) | 2 (11) | |
| 3 | 9 (18.4) | 5 (17) | 4 (21) | |
| 4 | 1 (2) | 1 (3) | 0 (0) | |
| NICHD | ||||
| 0 | 20 (41) | 11 (37) | 9 (47) | 0.949 |
| 1A | 6 (12) | 4 (13) | 2 (11) | |
| 1B | 5 (10) | 3 (10) | 2 (11) | |
| 2A | 8 (16) | 6 (20) | 2 (11) | |
| 2B | 8 (16) | 5 (17) | 3 (16) | |
| 3 | 2 (4) | 1 (3) | 1 (5) | |
| Distribution of Late MRI Scores | ||||
| Basal Ganglia | ||||
| 0 | 35 (71) | 21 (70) | 14 (74) | 0.369 |
| 1 | 3 (6) | 2 (7) | 1 (5) | |
| 2 | 5 (10) | 4 (13) | 1 (5) | |
| 3 | 4 (8) | 1 (3) | 3 (16) | |
| 4 | 2 (4) | 2 (7) | 0 (0) | |
| Watershed | ||||
| 0 | 34 (70) | 19 (63) | 15 (79) | 0.675 |
| 1 | 3 (6) | 2 (7) | 1 (5) | |
| 2 | 4 (8) | 2 (7) | 2 (11) | |
| 3 | 1 (2) | 1 (3) | 0 (0) | |
| 4 | 6 (12) | 5 (17) | 1 (5) | |
| 5 | 1 (2) | 1 (3) | 0 (0) | |
| Basal Ganglia/Watershed | ||||
| 0 | 29 (59) | 16 (54) | 13 (69) | 0.480 |
| 1 | 5 (10) | 4 (13) | 1 (5) | |
| 2 | 6 (12) | 5 (17) | 1 (5) | |
| 3 | 8 (16) | 4 (13) | 4 (21) | |
| 4 | 1 (2) | 1 (3) | 0 (0) | |
| NICHD | ||||
| 0 | 22 (45) | 13 (44) | 9 (47) | 0.831 |
| 1A | 7 (14) | 4 (13) | 3 (16) | |
| 1B | 3 (6) | 1 (3) | 2 (10.5) | |
| 2A | 7 (14) | 5 (17) | 2 (10.5) | |
| 2B | 9 (18) | 6 (20) | 3 (16) | |
| 3 | 2 (2) | 1 (3) | 1 (5) | |
| Bayley Scales of Infant Toddler Development – III Composite Scores | ||||
| Motor | n/a | 93±17 | n/a | |
| Cognitive | n/a | 91±20 | n/a | |
| Language | n/a | 90±19 | n/a | |
Data presented as median (range) unless otherwise specified.
Comparison between patients with and without developmental follow-up
Data available for
46
29
17
27
19
Neurodevelopmental outcomes were available for 30 patients (61%) assessed at a median age of 28 months (range 13-36). Significant neurodevelopmental delay was observed in 6 (20%) patients. The patients lost to follow-up had higher birth weights and presenting pH compared with the study population with known outcomes, but were otherwise similar with regards to baseline and clinical characteristics, as well as distribution of MRI severity scores (p>0.05; Table 1).
Agreement between Early and Late MRI
Early and late basal ganglia scores demonstrated substantial agreement with k=0.772 (p<0.001), whereas early and late watershed and basal ganglia/watershed scores both demonstrated almost perfect agreement with k=0.883 (p<0.001) and k=0.8063 (p<0.001) respectively. There was also substantial agreement between the early and late NICHD scores with k=0.766 (p<0.001).
Table 2 summarizes the changes that were observed between the early and late MRI scores. Although the majority demonstrated no change between serial scans, MRI scores more often decreased between the early and late scans as opposed to increasing over time, suggesting fading or pseudonormalization of injury by the second time point (Figure).
Table 2.
MRI Score Changes between Early and Late Scan (n=49)
| Measurement | Paired Difference Late – Early (Mean ±SD) |
Increase | No change |
Decrease | P value (paired comparison) |
|---|---|---|---|---|---|
| Basal ganglia score | −0.18 ± 0.73 | 2.0% | 85.7% | 12.3% | 0.083 |
| Watershed score | −0.08 ± 0.48 | 4.1% | 87.8% | 8.1% | 0.252 |
| Basal ganglia/Watershed score | −0.04 ± 0.71 | 4.1% | 85.7% | 10.2% | 0.688 |
| NICHD score | −0.14 ± 0.94 | 10.2% | 75.5% | 14.3% | 0.290 |
Figure 1.
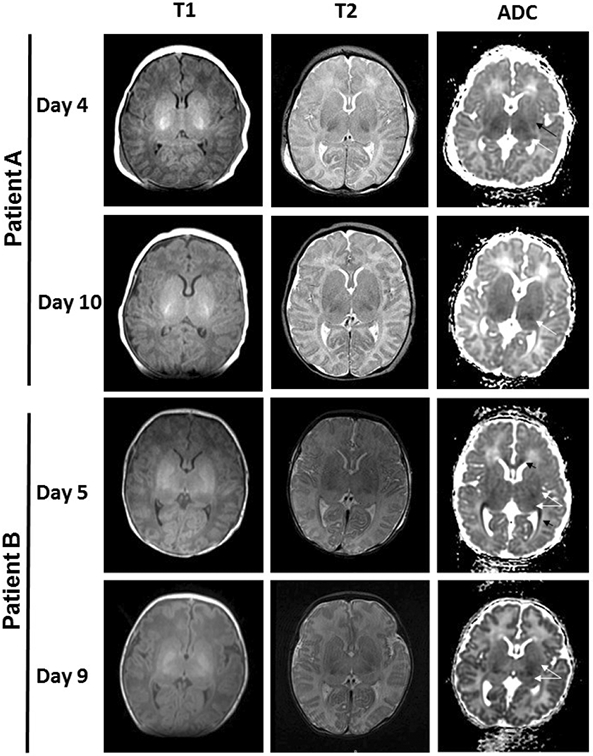
Exemplar cases with discordant scores between early and late scans. T1, T2 and diffusion weighted images are shown. In patient A, basal ganglia score was higher on day 4 (basal ganglia score=3, signifying signal abnormality in the thalamus [white arrow] and basal ganglia [black arrow]) compared with day 10 (basal ganglia score=1, signifying signal abnormality in the thalamus only [white arrow]). Similarly in patient B, basal ganglia injury was more apparent on day 5 (basal ganglia score=4, signifying extensive involvement with signal abnormality in the thalamus and basal ganglia [white arrows] and white matter tracts [black arrows]) compared with day 9 (basal ganglia score=2, signifying less extensive signal abnormality in the thalamus and basal ganglia [white arrows]).
Association between MRI Score and Neurodevelopmental Outcome
Logistic regression model results are summarized in Table 3. Early and late basal ganglia and basal ganglia/watershed scores, and early NICHD MRI score were associated with significant neurodevelopmental delay at 15-30 months of age (p<0.05). Neither the early nor the late watershed score was significantly predictive of outcome. Evaluation of model statistics suggested that early basal ganglia, basal ganglia /watershed, and NICHD scores had AUCs >0.8. Based on AIC, other than late basal ganglia score, which performed slightly better than early basal ganglia score, early MRI scores were better predictors of neurodevelopmental delay than late scores. These results were similar after adjusting for clinical covariates.
Table 3.
Prediction of Developmental Delay by MRI Score (n=30)
| Variable Name | Odds Ratio (95% CI) |
β | Standard Error |
P Value | AUC (C- Statistic) (95% CI) |
R-Square | % Concordant | AIC | Adjusted Odds Ratio* |
P Value |
|---|---|---|---|---|---|---|---|---|---|---|
| Early Basal Ganglia | 2.63 (1.22-5.69) | 0.968 | 0.393 | 0.014 | 0.819 (1.22-5.96) | 0.36 | 73.6 | 26.24 | 3.31 (1.26-8.66) | 0.015 |
| Late Basal Ganglia | 3.20 (1.31-7.80) | 1.163 | 0.455 | 0.011 | 0.788 (1.31-7.80) | 0.43 | 64.6 | 24.50 | 3.14 (1.31-7.51) | 0.010 |
| Early Watershed | 1.44 (0.89-2.35) | 0.367 | 0.249 | 0.141 | 0.628 (0.89-2.35) | 0.11 | 44.4 | 31.85 | ||
| Late Watershed | 1.50 (0.91-2.47) | 0.404 | 0.254 | 0.112 | 0.646 (0.91-2.47) | 0.13 | 45.8 | 31.47 | ||
| Early Basal Ganglia/Watershed | 2.84 (1.24-6.51) | 1.043 | 0.424 | 0.014 | 0.816 (1.24-6.51) | 0.37 | 72.9 | 26.14 | 2.98 (1.26-7.06) | 0.013 |
| Late Basal Ganglia / Watershed | 2.56 (1.13-5.79) | 0.940 | 0.416 | 0.024 | 0.792 (1.13-5.79) | 0.30 | 70.1 | 27.72 | 2.43 (1.07-5.51) | 0.033 |
| Early NICHD | 2.41 (1.10-5.30) | 0.881 | 0.401 | 0.028 | 0.816 (1.10-5.30) | 0.34 | 74.3 | 26.74 | 2.68 (1.11-6.47) | 0.029 |
| Late NICHD | 1.79 (0.99-3.24) | 0.582 | 0.303 | 0.055 | 0.771 (0.99-3.24) | 0.22 | 68.8 | 29.59 |
β = regression coefficient, AUC= area under the receiver operating curve, AIC= Akaike information criterion
Adjusted for encephalopathy grade, encephalopathy grade (moderate versus severe), Apgar score at 5 minutes and socioeconomic status (public versus private insurance)
The relationships between MRI scores and BSITD-III cognitive, language, and motor composite score, are summarized in Table 4. Early MRI basal ganglia, and basal ganglia /watershed scores as well as early and late NICHD scores were associated with cognitive scores (p<0.05). Early MRI NICHD as well as late MRI basal ganglia and NICHD were associated with language scores (p<0.05). None of the MRI scores were significantly associated with the continuous motor composite score.
Table 4.
Spearman Correlation Between Bayley Scales of Infant-Toddler Development-III and MRI scores (n=30)
| Variable Name | Cognitive composite score |
P value |
Language composite score |
P value |
Motor composite score |
P value |
|---|---|---|---|---|---|---|
| Early Basal Ganglia | −0.400 | 0.039 | −0.357 | 0.067 | −0.225 | 0.258 |
| Late Basal Ganglia | −0.278 | 0.160 | −0.506 | 0.007 | −0.180 | 0.368 |
| Early Watershed | −0.359 | 0.066 | −0.162 | 0.419 | −0.197 | 0.326 |
| Late Watershed | −0.256 | 0.198 | −0.130 | 0.519 | −0.228 | 0.252 |
| Early Basal Ganglia / Watershed | −0.478 | 0.012 | −0.301 | 0.127 | −0.157 | 0.433 |
| Late Basal Ganglia / Watershed | −0.309 | 0.117 | −0.358 | 0.067 | −0.122 | 0.544 |
| Early NICHD | −0.530 | 0.004 | −0.485 | 0.010 | −0.254 | 0.201 |
| Late NICHD | −0.474 | 0.013 | −0.395 | 0.041 | −0.213 | 0.286 |
Discussion
Lifelong injury following neonatal HIE remains a significant problem associated with high physical, psychological and financial burden of disease. Ongoing and future studies require outcomes that can be assessed in the neonatal period to provide early assessment of treatment effect. Additionally, detailed early prognostication can help guide care following discharge and enable appropriate counseling of families. Although MRI serves as the putative subacute biomarker of brain injury in neonatal HIE, optimal timing of MRI after therapeutic hypothermia remains controversial. In this cohort, we found substantial agreement between early and late MRI across several established scoring systems. Furthermore, although both early and late MRI scans were associated with prediction of later neurodevelopmental outcomes, these data suggest that the information provided by the early MRI is sufficient, and possibly more reliable, for establishing prognosis and counseling families. Given the substantial agreement between serial MRI scans and limited evidence for additive prognostic value, the practice of serial MRI in babies with HIE may add unnecessary cost in a condition where cost of care is known to be high.23
Our study evaluated agreement between early and late MRI using well-established scoring systems in 49 newborn infants with HIE treated with therapeutic hypothermia. Previously, Wintermark et al reported preliminary findings on serial MRI in 12 newborn infants with HIE treated with therapeutic hypothermia. These authors used the Barkovich scoring system in infants who underwent 2-4 MRI scans in the first month after birth and concluded that early MRI at day 2-3 of life demonstrated injuries seen in later scans24. Similarly, Agut et al compared sequential MRI in a small cohort of 15 infants with HIE and found no significant differences between the early and late scans.25 In a study of 43 infants with HIE, Boudes et al compared early MRI performed during therapeutic hypothermia, with late MRI performed after the completion of therapeutic hypothermia.26 Although the investigators found that MRI done during therapeutic hypothermia was sufficient to assess the extent of brain injury, performing MRI during therapeutic hypothermia has practical challenges. Skranes et al studied a cohort of 41 newborn infants with HIE treated with therapeutic hypothermia and compared early MRI (4 days) with late MRI (11 days) using different scoring methods than were used in this study.27 Another large cohort of 89 infants with neonatal encephalopathy (including 43 who were cooled) was reported by Chakkarapani et al and likewise observed substantial agreement between early (3-6 days) and late (10-14 days) MRI16. One other study compared predictive abilities of early versus late MRI for developmental outcomes in infants with HIE. Using a visual analysis system to classify MRIs as normal versus abnormal in 33 infants with HIE, Charon et al reported that both early (<7 days) and late (≥7 days) MRIs yielded 100% sensitivity for adverse outcome at median age 24 months, but that early MRI had a higher specificity than late MRI (96.3% versus 89.3%)6. Our findings are consistent with these prior reports suggesting that findings on early and late MRI substantially agree and that early MRI provides a fuller picture of the extent of injury that can be used to most accurately predict early cognitive and motor outcomes. It should be noted, however, that although one early MRI is likely sufficient for clinicians in most circumstances, a second image may be warranted in a setting in which the MRI quality is suboptimal for confident interpretation or if findings are incongruent with the clinical picture.
Although the agreement between early and late MRI in our study was substantial, the data also provide insights into the evolution of specific changes that occurred from early to late scores to better understand how visualization of injury by MRI progresses over the first weeks of life. Late MRI scores were more likely to decrease compared with early scores. That early scores were overall slightly more predictive of later outcomes, suggests that imaging in the second week of life may be capturing potential pseudonormalization of injury on diffusion-weighted images, whereas signal abnormalities on T1 and T2-weighted images may be less apparent or not fully evolved, affecting the sensitivity of assessment of tissue injury by MRI at this timepoint. Prior reports have suggested that the time course of pseudonormalization is delayed in the setting of therapeutic hypothermia, with pseudonormalization occurring after 10 days in cooled infants compared with day 6-8 in controls.28 Thus, it would be expected that brain injury may continue to be visible on diffusion weighted imaging throughout the first postnatal week in cooled infants. We recognize that both the Barkovich9 and NICHD systems8 did not include analysis of diffusion-weighted images and were described for application to T1 and T2-weighted images beyond the first week of life. Thus, our reported predictive abilities cannot be directly compared with the source publications. However, our goal was to extend the application of these commonly used systems to pragmatic timepoints in order to provide data to refine optimal timing of MRI in clinical practice.
Although the aim of our study was to assess the ability of MRI at alternative timepoints to predict later neurodevelopmental adverse outcomes categorically, we also evaluated individual Bayley composite scores as continuous measures to assess the association of MRI findings with individual developmental domains. Although the relationship with MRI scores and Bayley cognitive composite scores largely mirrored our primary analyses, it was of interest that we did not observe any significant correlations between MRI and Bayley motor composite scores. This may be due to a relatively narrow distribution of scores across a small sample size or may reflect the limitations of the Bayley motor composite as a reliable measure of motor function.29-31 We did not have systematic capture of concurrent neurological examination for identification and classification of cerebral palsy to augment our assessment of the association between neonatal brain injury by MRI and later motor performance.
There were some limitations to this study. There is an inherent selection bias in this cohort of patients as those who did not receive two brain MRIs were excluded. Sicker patients may have been too medically unstable for early MRI or may not have survived to the second MRI timepoint, and healthier infants may have been discharged prior to obtaining a second MRI, limiting the MRIs available for analysis and potentially selecting out extreme phenotypes in our cohort. We used a single experienced reader for MRI scoring in our study based on the high intra-observer reliability (k=0.85-1) reported for scoring system used,9 as well as prior experience using multiple readers with high reliability in our earlier studies.32, 33 Whereas our results may be dependent on the reliability of scoring, prior large studies have relied on a single central scorer for MRI in this population.8 A large portion of the study cohort was lost to follow-up, limiting the data available to analyze the correlation between MRI scores and early neurodevelopmental outcome and the ability to control for all of the possible confounders in this study. Furthermore, because clinical follow-up was used in this study, there was a large age range at neurodevelopmental assessment. We used any available developmental assessment to optimize the sample size and power for the study, leading to a relatively wide age range used in these analyses which was not ideal. Although studies of outcomes after HIE routinely assessed and reported outcomes at 18 months,1, 2, 4 additional studies are needed to assess predictive abilities for later school ages. These factors limit our ability to draw robust conclusions about the relationship between MRI and neurodevelopmental outcome. Although our study involved a relatively large cohort of infants who underwent serial MRI to assess agreement between early and late scans, a future study with more complete follow-up may be helpful to confirm our findings with regards to optimal timing for developmental prognosis.
We found substantial agreement across multiple MRI scoring systems between early (<7 days) and late (≥7) MRI in newborn infants with HIE following therapeutic hypothermia. Although both early and late MRI showed predictive value for later significant developmental delay, early MRI scores generally demonstrated better predictive ability compared with late MRI scores. These data support that a single MRI performed in the first week after birth is sufficient to assess the degree and extent of subacute brain injury after neonatal HIE.
Acknowledgments
Supported by the Clinical and Translational Science Institute at Children’s National (UL1TR000075, 1KL2RR031987-01) and the National Institutes of Health Intellectual and Developmental Disabilities Research Consortium (U54 HD090257). The sponsors had no role in study design, conduct or reporting of results. A.M. serves on the Editorial Board of The Journal of Pediatrics. The authors declare no conflicts of interest.
Abbreviations:
- HIE
hypoxic ischemic encephalopathy
- MRI
magnetic resonance imaging
- BSITD-III
Bayley Scales of Infant Toddler Development −3rd Edition
- NICHD
National Institute of Child Health and Human Development
- AUC
area under the curve
- AIC
Akaike information criterion
- k
kappa statistic
Footnotes
Portions of this study were presented at the Pediatric Academic Society annual meeting, << >>, 2018, << >>; and at the Eastern Society for Pediatric Research meeting, << >>, 2018, << >>.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. The New England journal of medicine. 2005;353:1574–84. [DOI] [PubMed] [Google Scholar]
- [2].Azzopardi DV, Strohm B, Edwards AD, Dyet L, Halliday HL, Juszczak E, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. The New England journal of medicine. 2009;361:1349–58. [DOI] [PubMed] [Google Scholar]
- [3].Jacobs SE, Morley CJ, Inder TE, Stewart MJ, Smith KR, McNamara PJ, et al. Whole-body hypothermia for term and near-term newborns with hypoxic-ischemic encephalopathy: a randomized controlled trial. Archives of pediatrics & adolescent medicine. 2011;165:692–700. [DOI] [PubMed] [Google Scholar]
- [4].Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365:663–70. [DOI] [PubMed] [Google Scholar]
- [5].Ment LR, Bada HS, Barnes P, Grant PE, Hirtz D, Papile LA, et al. Practice parameter: neuroimaging of the neonate: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2002;58:1726–38. [DOI] [PubMed] [Google Scholar]
- [6].Charon V, Proisy M, Bretaudeau G, Bruneau B, Pladys P, Beuchee A, et al. Early MRI in neonatal hypoxic-ischaemic encephalopathy treated with hypothermia: Prognostic role at 2-year follow-up. European journal of radiology. 2016;85:1366–74. [DOI] [PubMed] [Google Scholar]
- [7].Rutherford M, Ramenghi LA, Edwards AD, Brocklehurst P, Halliday H, Levene M, et al. Assessment of brain tissue injury after moderate hypothermia in neonates with hypoxic-ischaemic encephalopathy: a nested substudy of a randomised controlled trial. The Lancet Neurology. 2010;9:39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Shankaran S, Barnes PD, Hintz SR, Laptook AR, Zaterka-Baxter KM, McDonald SA, et al. Brain injury following trial of hypothermia for neonatal hypoxic-ischaemic encephalopathy. Archives of disease in childhood Fetal and neonatal edition. 2012;97:F398–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Barkovich AJ, Hajnal BL, Vigneron D, Sola A, Partridge JC, Allen F, et al. Prediction of neuromotor outcome in perinatal asphyxia: evaluation of MR scoring systems. AJNR American journal of neuroradiology. 1998;19:143–9. [PMC free article] [PubMed] [Google Scholar]
- [10].Steinman KJ, Gorno-Tempini ML, Glidden DV, Kramer JH, Miller SP, Barkovich AJ, et al. Neonatal watershed brain injury on magnetic resonance imaging correlates with verbal IQ at 4 years. Pediatrics. 2009;123:1025–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ferrari F, Todeschini A, Guidotti I, Martinez-Biarge M, Roversi MF, Berardi A, et al. General movements in full-term infants with perinatal asphyxia are related to Basal Ganglia and thalamic lesions. The Journal of pediatrics. 2011;158:904–11. [DOI] [PubMed] [Google Scholar]
- [12].Miller SP, Ramaswamy V, Michelson D, Barkovich AJ, Holshouser B, Wycliffe N, et al. Patterns of brain injury in term neonatal encephalopathy. The Journal of pediatrics. 2005;146:453–60. [DOI] [PubMed] [Google Scholar]
- [13].Higgins RD, Raju T, Edwards AD, Azzopardi DV, Bose CL, Clark RH, et al. Hypothermia and other treatment options for neonatal encephalopathy: an executive summary of the Eunice Kennedy Shriver NICHD workshop. The Journal of pediatrics. 2011;159:851–8 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Executive summary: Neonatal encephalopathy and neurologic outcome, second edition. Report of the American College of Obstetricians and Gynecologists' Task Force on Neonatal Encephalopathy. Obstetrics and gynecology. 2014;123:896–901. [DOI] [PubMed] [Google Scholar]
- [15].Rollins N, Booth T, Morriss MC, Sanchez P, Heyne R, Chalak L. Predictive value of neonatal MRI showing no or minor degrees of brain injury after hypothermia. Pediatric neurology. 2014;50:447–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chakkarapani E, Poskitt KJ, Miller SP, Zwicker JG, Xu Q, Wong DS, et al. Reliability of Early Magnetic Resonance Imaging (MRI) and Necessity of Repeating MRI in Noncooled and Cooled Infants With Neonatal Encephalopathy. Journal of child neurology. 2016;31:553–9. [DOI] [PubMed] [Google Scholar]
- [17].Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Archives of neurology. 1976;33:696–705. [DOI] [PubMed] [Google Scholar]
- [18].Bayley N Bayley Scales of Infant Development - Third Edition San Antonio, TX: Harcourt Assessment; 2006. [Google Scholar]
- [19].Chalak LF, DuPont TL, Sanchez PJ, Lucke A, Heyne RJ, Morriss MC, et al. Neurodevelopmental outcomes after hypothermia therapy in the era of Bayley-III. Journal of perinatology : official journal of the California Perinatal Association. 2014;34:629–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Anderson PJ, De Luca CR, Hutchinson E, Roberts G, Doyle LW. Underestimation of developmental delay by the new Bayley-III Scale. Archives of pediatrics & adolescent medicine. 2010;164:352–6. [DOI] [PubMed] [Google Scholar]
- [21].Yu YT, Hsieh WS, Hsu CH, Chen LC, Lee WT, Chiu NC, et al. A psychometric study of the Bayley Scales of Infant and Toddler Development - 3rd Edition for term and preterm Taiwanese infants. Research in developmental disabilities. 2013;34:3875–83. [DOI] [PubMed] [Google Scholar]
- [22].Akaike H [Data analysis by statistical models]. No to hattatsu = Brain and development. 1992;24:127–33. [PubMed] [Google Scholar]
- [23].Massaro AN, Murthy K, Zaniletti I, Cook N, DiGeronimo R, Dizon ML, et al. Intercenter Cost Variation for Perinatal Hypoxic-Ischemic Encephalopathy in the Era of Therapeutic Hypothermia. The Journal of pediatrics. 2016;173:76–83 e1. [DOI] [PubMed] [Google Scholar]
- [24].Wintermark P, Hansen A, Soul J, Labrecque M, Robertson RL, Warfield SK. Early versus late MRI in asphyxiated newborns treated with hypothermia. Archives of disease in childhood Fetal and neonatal edition. 2011;96:F36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Agut T, Leon M, Rebollo M, Muchart J, Arca G, Garcia-Alix A. Early identification of brain injury in infants with hypoxic ischemic encephalopathy at high risk for severe impairments: accuracy of MRI performed in the first days of life. BMC pediatrics. 2014;14:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Boudes E, Tan X, Saint-Martin C, Shevell M, Wintermark P. MRI obtained during versus after hypothermia in asphyxiated newborns. Archives of disease in childhood Fetal and neonatal edition. 2015;100:F238–42. [DOI] [PubMed] [Google Scholar]
- [27].Skranes JH, Cowan FM, Stiris T, Fugelseth D, Thoresen M, Server A. Brain imaging in cooled encephalopathic neonates does not differ between four and 11 days after birth. Acta Paediatr. 2015;104:752–8. [DOI] [PubMed] [Google Scholar]
- [28].Bednarek N, Mathur A, Inder T, Wilkinson J, Neil J, Shimony J. Impact of therapeutic hypothermia on MRI diffusion changes in neonatal encephalopathy. Neurology. 2012;78:1420–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Spittle AJ, Spencer-Smith MM, Eeles AL, Lee KJ, Lorefice LE, Anderson PJ, et al. Does the Bayley-III Motor Scale at 2 years predict motor outcome at 4 years in very preterm children? Developmental medicine and child neurology. 2013;55:448–52. [DOI] [PubMed] [Google Scholar]
- [30].Burakevych N, McKinlay CJ, Alsweiler JM, Wouldes TA, Harding JE. Bayley-III motor scale and neurological examination at 2 years do not predict motor skills at 4.5 years. Developmental medicine and child neurology. 2017;59:216–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Griffiths A, Toovey R, Morgan PE, Spittle AJ. Psychometric properties of gross motor assessment tools for children: a systematic review. BMJ open. 2018;8:e021734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Massaro AN, Chang T, Kadom N, Tsuchida T, Scafidi J, Glass P, et al. Biomarkers of brain injury in neonatal encephalopathy treated with hypothermia. The Journal of pediatrics. 2012;161:434–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Massaro AN, Jeromin A, Kadom N, Vezina G, Hayes RL, Wang KK, et al. Serum biomarkers of MRI brain injury in neonatal hypoxic ischemic encephalopathy treated with whole-body hypothermia: a pilot study. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2013;14:310–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


