Abstract
Background
We reported that chemokine C-X-C motif receptor 2 (CXCR2) signaling appears to play an important role in the pathogenic signaling of gastric cancer (GC), and although CXCR2 may have a role in other solid cancers, the significance of CXCR2 in cholangiocarcinoma (CCA) has not been evaluated. Herein, we determined the clinicopathologic significance of CXCL1-CXCR2 signaling in CCA.
Materials and methods
Two human CCA cell lines, OCUG-1 and HuCCT1, were used. CXCR2 expression was examined by western blotting. We investigated the effects of CXCL1 on the proliferation (by MTT assay) and migration activity (by a wound-healing assay) of each cell line. Our immunohistochemical study of the cases of 178 CCA patients examined the expression levels of CXCR2 and CXCL1, and we analyzed the relationship between these expression levels and the patients’ clinicopathologic features.
Results
CXCR2 was expressed on both CCA cell lines. CXCL1 significantly inhibited both the proliferative activity and migratory activity of both cell lines. CXCL1 and CXCR2 were immunohistochemically expressed in 73% and 18% of the CCA cases, respectively. The CXCL1-positive group was significantly associated with negative lymph node metastasis (p = 0.043). The CXCR2-positive group showed significantly better survival (p = 0.042, Kaplan-Meier). A multivariate logistic regression analysis revealed that CXCR2 expression (p = 0.031) and lymph node metastasis (p = 0.004) were significantly correlated with the CCA patients’ overall survival.
Conclusion
CXCR2 signaling might exert a tumor-suppressive effect on CCA cells. CXCR2 might be a useful independent prognostic marker for CCA patients after surgical resection.
Introduction
Cholangiocarcinoma (CCA) is a biliary cancer that may originate from epithelial cells in the biliary tract, and patients with CCA have had an extremely poor prognosis [1]. Most CCAs are diagnosed at an advanced stage, and there are few treatment options; the development of effective treatments is thus desired [2]. Several factors that can serve as biomarkers in CCA were recently reported [3–6], and some chemokines have been shown to play an important role in the development of CCA [1, 7]. Indeed, chemokines are known to have roles in the development of various types of cancer [8–10]. Chemokine C-X-C motif receptor 2 (CXCR2) has many ligands, such as chemokine (C-X-C motif) ligand 1 (CXCL1), CXCL2, CXCL3, CXCL5, CXCL6, CXCL7, and CXCL8 [11], and the clinical significance of CXCL1 and CXCR2 and their signaling has not been clarified.
It was reported that CXCL7 and CXCL5 are involved in the development of CCA [1, 12, 13]. CXCL5/ENA-78 (epithelial cell-derived neutrophil-activating peptide-78) was identified as a factor in the interaction between CCA cells and cancer-associated fibroblasts [13]. In addition, various chemokines have been reported to act as tumor suppressors and indicators of better prognosis in gastric cancer [14], colorectal cancer [15–17], lung adenocarcinoma [18, 19], and triple-negative breast cancer [20]. These findings might indicate that CXCR2 signaling is a target in various cancers, but the effects of this signaling on cancer cells might differ among cancer types [11]. We observed that among the members of the family of CXCR2 ligands, CXCL1, one of the ligands for CXCR2, produced by cancer cells might play an important role among CXCR2 ligands family in gastric cancer [21]. These findings suggest that CXCL1-CXCR2 signaling may exert one or more actions in the development of CCA. We conducted the present study to examine the significance of CXCL1-CXCR2 signaling in CCA.
Materials and methods
Cell lines
Two human CCA cell lines, OCUG-1 and HuCCT1(RCB1960), were used. The OCUG-1 cell line was established at our laboratory [22]. The HuCCT1 line was provided by RIKEN BRC (BioResource Research Center) (Tsukuba, Japan). All cell lines in this study were authenticated by short tandem repeat (STR) profiling before distribution.
Cell culture
The culture medium consisted of Dulbecco’s modified Eagle medium (DMEM; Wako, Osaka, Japan) with high glucose, penicillin-streptomycin solution (5 mL/500 mL D-MEM, Wako), 100 mM sodium pyruvate solution (5 mL/500 mL D-MEM, Sigma-Aldrich, St. Louis, MO), and 10% fetal bovine serum (FBS, Sigma). Cells were cultured at 37°C in 20% O2.
Compounds
CXCL1 recombinant (BioLegend, San Diego, CA) was used.
Proliferation assay
The effect of CXCL1 on the proliferation of CCA cells was determined by an MTT assay (Dojindo, Kumamoto, Japan) as follows. A total of 2.5×103 cells were seeded into 96-well plates with CXCL1 at 20 ng/mL. After incubation for 72 h, MTT was added to each well. After incubation for 2 h at 37°C, the plate was measured with absorbance at 570 nm using a microtiter plate reader.
Wound-healing assay
Both cell lines were cultured in 96-well plates (Essen ImageLock; Essen Instruments, Birmingham, UK). After the cells reached semi confluence, a wound was generated in the cell monolayer with the 96-well WoundMaker (Essen BioScience Inc., Ann Arbor, MI). Cell lines were cultured in DMEM with 3% FBS and CXCL1 at 20 ng/mL or control. Scratched fields were imaged every 12 h and monitored for 48 h with the Incucyte ZOOM Live-Cell Imaging System and software ver. 2018A (Essen Instruments).
Western blot analysis
Cell lysates of OCUG-1 and HuCCT1 were made by standard methods. The protein concentration of each sample was measured using a Coomassie Plus Assay Kit (Thermo Fisher Scientific, Waltham, MA). Each sample was subjected to electrophoresis and transferred to a polyvinylidene difluoride membrane. The membrane was placed in phosphate-buffered saline with Tween 20 (PBS-T) solution containing each primary antibody: CXCR2 (1:2000, R&D Systems, Minneapolis, MN) or β-actin (1:5000; Sigma-Aldrich) at 4°C overnight. The blots were incubated with secondary antibody for 1 h and were detected by enhanced chemiluminescence using ECL prime (GE Health Care, Buckinghamshire, UK).
Clinical materials
We also retrospectively analyzed the cases of 178 patients with CCA who underwent surgery at Osaka City University Hospital. CCA tissues were obtained from each patient. The pathological diagnosis and classifications were made according to the Japanese classification of CCA (6th edition). This study was approved by the Osaka City University Ethics Committee (approval no. 924). Written informed consent for this research was obtained from all of the patients.
Immunohistochemical determination of CXCL1 and CXCR2
We conducted the present experiment based on the methods reported in our previous papers [21]. The immunohistochemical determination of the expressions of CXCL1 and CXCR2 in the CCA tissues from the patients was performed as follows. The tissue samples were deparaffinized, and slides were heated. After endogenous peroxidase activity was blocked, the samples were incubated with anti-CXCL1 antibody (1:200; Proteintech, Rosemont, IL) or anti-CXCR2 antibody (1:100; R&D Systems) for 1 h at room temperature. The samples were then incubated with biotinylated secondary antibody, followed by treatment with streptavidin-peroxidase reagent and counterstaining with Mayer’s hematoxylin.
The expression levels of CXCL1 and CXCR2 were analyzed by both the intensity of staining and the percentage of stained cancer cells at the invading tumor front. The CXCL1 and CXCR2 expression levels were evaluated as follows: the intensity scores were 0–3 (0 = no, 1 = weak, 2 = moderate, 3 = intense), and the percentage of immune-positive cells was given the scores 0–3 (CXCL1: 0 = 0%–20%, 1 = 21%–50%, 2 = 51%–80%, 3 = 81%–100%, and CXCR2: 0 = 0%, 1 = 1%–30%, 2 = 31%–70%, 3 = 71%–100%). The two scores were added to gain the final result of 0–6 points. Expression was considered positive when the CXCL1 score was ≥4 and when the CXCR2 score was ≥5. The evaluations were conducted by two double-blinded independent observers who were unaware of the patients’ clinical data and outcomes. When the observers reached different conclusions about an evaluation, it was rechecked and resolved.
Statistical analyses
The χ2 test or Fisher’s exact was used to determine the significance of differences between covariates. Survival durations were calculated with the Kaplan–Meier method and analyzed by the log-rank test to compare cumulative survival durations among patient groups. The Cox proportional hazards model was used to compute univariate hazards ratios for the study parameters. In all of the tests, p-values <0.05 were defined as significant. The SPSS software program (SPSS Japan, Tokyo) was used for the analyses. MTT assay and wound-healing assay data are expressed as the mean ± standard deviation (SD), and differences in these data were analyzed using the unpaired Student’s t-test.
Results
CXCR2 expression on CCA cells
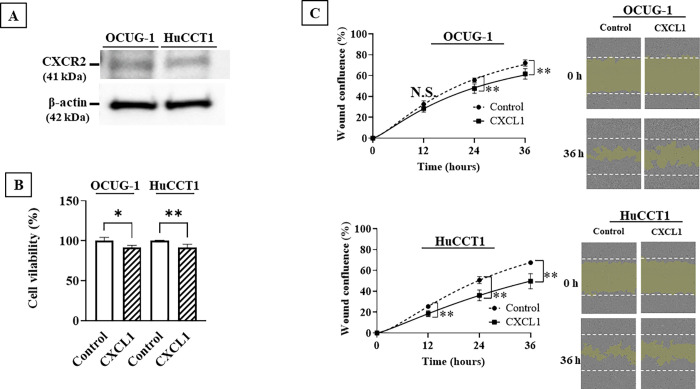
Fig 1A provides an example of the CXCR2 expression level on both OCUG-1 cells and HuCCT1 cells are revealed by the western blotting analysis.
Fig 1. Effect of CXCL1 on the proliferation and migration of CCA cells.
A, CXCR2 expression on OCUG-1 cells and HuCCT1 cells. B, CXCL1 significantly inhibited the proliferative potential of OCUG-1 cells and HuCCT1 cells at 72 h. **, p<0.01 vs control. C, CXCL1 significantly inhibited the migration potential of OCUG-1 cells after 24 h and HuCCT1 cells after 12 h. **, p<0.01 vs control.
Effects of CXCL1 on CCA cell proliferation and migration
CXCL1 decreased the proliferation of both OCUG-1 cells (p = 0.013) and HuCCT1 cells (p = 0.002) (Fig 1B) and significantly decreased the migration activities of OCUG-1 cells (p<0.001) and HuCCT1 cells (p = 0.01) (Fig 1C).
Immunostaining of CXCL1 and CXCR2
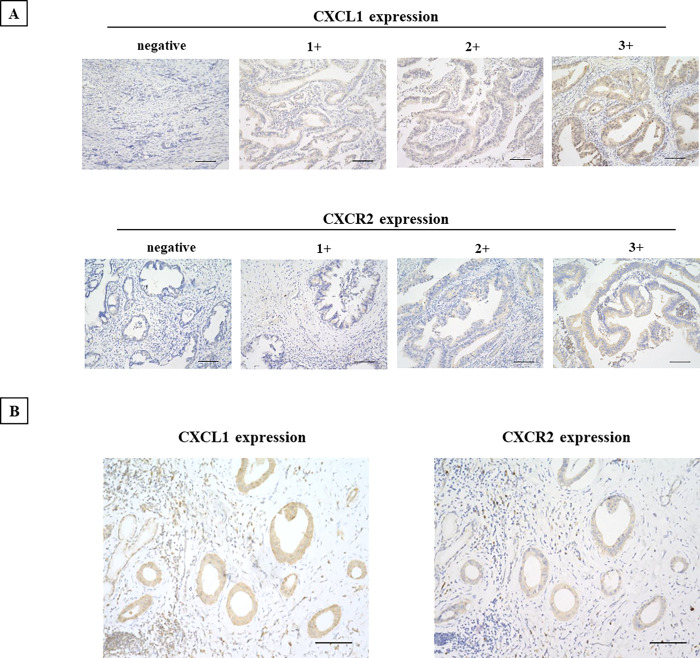
The results of the immunostaining study demonstrated that CXCL1 was expressed in the cytoplasm of CCA cells, and CXCR2 was expressed mainly at the cell membrane and the cytoplasm of the CCA cells (Fig 2). CXCL1 was positive in 130 of the 177 CCA cases (73.0%), and CXCR2 was positive in 32 cases (18.5%). Thirty-one cases (17.9%) were positive for both CXCL1 and CXCR2.
Fig 2. Representative immunostaining images of CXCL1 and CXCR2 expression.
A, CXCL1 was expressed in CCA cells. CXCR2 was observed at both the cell membrane and the cytoplasm. Intensity score: 0, negative; 1+, weakly positive; 2+, positive; 3+. Bar: 100 μm. B, CXCL1 and CXCR2 expression in a case. Bar: 100 μm.
The relationship between clinicopathological features and the expression of the CXCL1 and/or CXCR2
Table 1 summarizes the correlations between the patients’ clinicopathologic features and the expression of CXCL1 or CXCR2 in their CCAs. The CXCL1 expression was significantly correlated with no distant metastasis (p = 0.043). The CXCR2 expression was not correlated with any clinicopathological features.
Table 1. The correlation between the clinicopathologic features and CXCL1 and/or CXCR2 in CCAs.
| CXCL1 | CXCR2 | CXCL1 and CXCR2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Clinicopathological features | Positive (n = 130) | Negative (n = 48) | p-value | Positive (n = 32) | Negative (n = 141) | p-value | Positive (n = 31) | Negative (n = 142) | p-value | |
| Age | ≤70 | 63 (72.4%) | 24 (27.6%) | 0.865 | 16 (19.0%) | 68 (81.0%) | 0.692 | 16 (19.0%) | 68 (81.0%) | 0.843 |
| >70 | 67 (74.4%) | 23 (25.6%) | 16 (18.2%) | 72 (81.8%) | 15 (17.0%) | 73 (83.0%) | ||||
| Gender | female | 57 (75.0%) | 19 (25.0%) | 0.611 | 15 (20.8%) | 57 (79.2%) | 0.692 | 15 (20.8%) | 57 (79.2%) | 0.548 |
| male | 70 (71.4%) | 28 (28.6%) | 17 (17.5%) | 80 (82.5%) | 16 (16.5%) | 81 (83.5%) | ||||
| Histological type | well/mode | 103 (75.2%) | 34 (24.8%) | 0.408 | 25 (18.9%) | 107 (81.1%) | 1 | 24 (18.2%) | 108 (81.8%) | 1 |
| por | 12 (66.7%) | 6 (33.3%) | 3 (16.7%) | 15 (83.3%) | 3 (16.7%) | 15 (83.3%) | ||||
| Stroma in tumors | med/int | 75 (74.3%) | 26 (25.7%) | 0.212 | 19 (19.2%) | 80 (80.8%) | 0.347 | 19 (19.2%) | 80 (80.8%) | 0.347 |
| sci | 4 (50.0%) | 4 (50.0%) | 0 (0.0%) | 7 (100.0%) | 0 (0.0%) | 7 (100%) | ||||
| T invasion | T1 | 23 (79.3%) | 6 (20.7%) | 0.344 | 7 (25.0%) | 21 (75.0%) | 0.423 | 6 (21.4%) | 22 (78.6%) | 0.781 |
| T2-4 | 57 (67.9%) | 27 (32.1%) | 15 (18.1%) | 68 (81.9%) | 15 (18.1%) | 68 (81.9%) | ||||
| Lymph node metastasis | Negative | 83 (73.5%) | 30 (26.5%) | 1 | 21 (18.9%) | 90 (81.8%) | 1 | 20 (18.0%) | 91 (82.0%) | 1 |
| Positive | 42 (72.4%) | 16 (27.6%) | 10 (17.9%) | 46 (82.1%) | 10 (17.9%) | 46 (82.1%) | ||||
| Distant metastasis | Negative | 112 (75.2%) | 37 (24.8%) | 0.043 | 28 (19.3%) | 117 (80.7%) | 1 | 27 (18.6%) | 118 (81.4%) | 1 |
| Positive | 2 (33.3%) | 4 (66.7%) | 1 (16.7%) | 5 (83.3%) | 1 (16.7%) | 5 (83.3%) | ||||
| Infiltration | Type A/B | 99 (74.4%) | 34 (25.6%) | 0.514 | 26 (20.2%) | 103 (79.8%) | 0.217 | 25 (19.4%) | 104 (80.6%) | 0.213 |
| Type C | 8 (66.7%) | 4 (33.3%) | 0 (0%) | 11 (100.0%) | 0 (0%) | 11 (100%) | ||||
| Lymphatic invasion | Negative | 51 (77.3%) | 15 (22.7%) | 0.344 | 13 (20.0%) | 52 (80.0%) | 1 | 12 (18.5%) | 53 (81.5%) | 1 |
| Positive | 51 (69.9%) | 22 (30.1%) | 14 (19.7%) | 57 (80.3%) | 14 (19.7%) | 57 (80.3%) | ||||
| Venous invasion | Negative | 87 (76.3%) | 27 (23.7%) | 0.132 | 24 (21.2%) | 89 (78.8%) | 0.567 | 23 (20.4%) | 90 (79.6%) | 0.566 |
| Positive | 15 (60.0%) | 10 (40.0%) | 3 (13.0%) | 20 (87.0%) | 3 (13.0%) | 20 (87.0%) | ||||
| Stage | Stage 1 | 34 (73.9%) | 12 (26.1%) | 1 | 6 (13.0%) | 40 (87.0%) | 1 | 6 (13.0%) | 40 (87.0%) | 1 |
| Stage 2–4 | 60 (72.3%) | 23 (27.7%) | 12 (14.8%) | 69 (85.2%) | 12 (14.8%) | 69 (85.2%) | ||||
CXCL1 and/or CXCR2 expression and the CCA patients’ survival
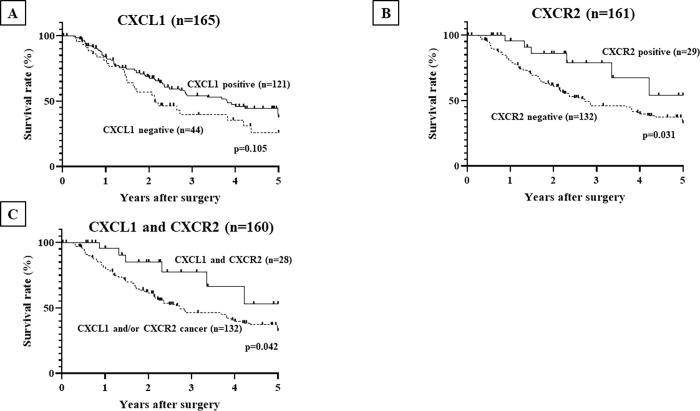
Fig 3 provides the Kaplan-Meier survival curves for the CCA patients in terms of CXCL1 and/or CXCR2 expression. The overall survival of the CCA patients was not significantly different between the CXCL1-positive patients (n = 121) and the CXCL1-negative patients (n = 44) (Fig 3A). The CXCR2-positive patients (n = 29) had significantly better prognoses than the CXCR2-negative patients (n = 132) (p = 0.031) (Fig 3B). Patients with both CXCL1- and CXCR2-positive cancer (n = 28) had significantly better prognoses than those with CXCL1- and/or CXCR2-negative cancer (n = 132) (p = 0.042) (Fig 3C).
Fig 3. The overall survival rate of CCA patients according to CXCR2 expression.
The patients with a positive expression of CXCR2 (n = 29) had a significantly better survival rate than those without CXCR2 expression (n = 132). The patients with positive expressions of both CXCL1 and CXCR2 (n = 28) had a significantly better survival rate than those with no CXCR2 expression (n = 132), but the patients with positive CXCL1 expression (n = 121) did not have a significantly better survival rate than those without CXCL1 expression (n = 44).
Univariate and multivariate analyses of overall survival
The univariate analysis revealed that the overall survival of the patients was significantly correlated with CXCR2 expression in cancer cells, high age (≥70 years), T invasion (T2–T4), lymph node metastasis, and distant metastasis. The multivariate logistic regression analysis revealed that CXCR2 expression (p = 0.031) and lymph node metastasis (p = 0.004) were significantly correlated with the CCA patients’ overall survival (Table 2).
Table 2. Univariate and multivariate analysis with respect to overall survival.
| Clinicopathologic features | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | p-value | Hazard Ratio | 95% CI | p-value | |
| CXCL1 | 0.432–1.084 | 0.684 | 0.106 | |||
| CXCR2 | 0.177–0.933 | 0.406 | 0.034 | 0.139–0.910 | 0.356 | 0.031 |
| CXCL1 and CXCR2 | 0.185–0.980 | 0.426 | 0.045 | |||
| Age ≥ 70 | 1.040–2.486 | 1.608 | 0.033 | 1.082–4.024 | 2.093 | 0.028 |
| Gender | 0.571–1.363 | 0.882 | 0.572 | 0.724–2.391 | 1.315 | 0.367 |
| Histological type; por | 0.793–2.849 | 1.503 | 0.212 | 0.424–2.292 | 0.987 | 0.973 |
| T invasion | 1.884–11.830 | 4.721 | 0.001 | 0.966–8.552 | 2.927 | 0.058 |
| Lymph node metastasis | 1.710–4.118 | 2.654 | <0.001 | 1.337–4.596 | 2.481 | 0.004 |
| Distant metastasis | 1.279–8.147 | 3.228 | 0.013 | 0.795–19.704 | 3.927 | 0.093 |
Discussion
We evaluated the significance of CXCL1 and CXCR2 signaling in CCA patients after surgical resection. CXCL1, one of the ligands of CXCR2, significantly decreased the proliferative and migratory potential of CCA cells. The immunohistochemical study also revealed an inverse correlation between CXCL1 and distant metastasis. CXCL1 may inhibit distant metastasis by suppressing the migratory potential of CCA cells. These results suggest that CXCL1 may act as a suppressor of CCA progression. Although other members of the CXCR2 ligand family, i.e., CXCL7 and CXCL5, have frequently been reported to promote the progression of CCA [1, 12, 13], our present results indicate that CXCL1 may exert a tumor-inhibitory effect on CCA cells. We considered that the reducing 80–90% viability in a 3-days might be clinically meaningful because the clinical effect is usually evaluated in a couple of months. It has been reported that the senescence of cancer cells is one of anti-proliferative responses against tumor progression via CXCL1-CXCR2 signaling, resulting in promoting apoptosis [23]. These mechanisms might be one of reasons for the tumor suppressive effect of CXCL1. These findings suggest that CXCL1-CXCR2 axis may play a tumor-suppressive role in the progression of CCA.
Since there has been no report about the clinicopathologic significance of CXCL1 or CXCR2 signaling in CCA, we next analyzed the correlation between the CXCR2 expression level and the clinicopathologic features in patients with CCA. The Kaplan-Meier curves demonstrated that the CXCR2-positive patients achieved significantly better survival, and the multivariate logistic regression analysis revealed that CXCR2 expression was significantly correlated with the overall survival of CCA patients. These findings suggested that CXCR2 might be a useful independent prognostic marker for CCA patients after surgical resection.
It has been reported that in triple-negative breast cancer, the expression of CXCR2 is associated with higher immune infiltration and more favorable outcomes [24], whereas CXCR2 ligands have been reported to have cancer-promoting effects [25–27]. Tumor progression-enhancing effects of CXCL7 and CXCL5 on CCA cells were described [1, 12, 13], in contrast, it has also been reported that CCL14 inhibited the proliferation and invasion of colon cancer cells [28]. Moreover, CCL14 attenuated the proliferation of hepatocellular carcinoma (HCC) cells by inhibiting the cell-cycle progression and promoting apoptosis, and it suppressed the growth via the Wnt/β-catenin signaling [23]. These findings may indicate that CXCL1-CXCR2 signaling exerts an inhibitory effect on the proliferative and migratory activity of CCA cells and thus improve the survival of patients with CCA.
In conclusion, the CXCL1-CXCR2 axis may play a tumor-suppressive role in the progression of CCA. CXCR2 might be a useful independent prognostic marker for CCA patients after surgical resection.
Supporting information
(PDF)
Acknowledgments
We thank Akiko Tsuda (Molecular Oncology and Therapeutics, Osaka City University Graduate School of Medicine) for technical support.
Data Availability
The data supporting these findings during the current study are not publicly available since data contain potentially sensitive information. However, this data could be available from the corresponding author or the Ethics Committee (ethics@med.osaka-cu.ac.jp) on reasonable request.
Funding Statement
This study is partially funded by KAKENHI (Grant-in-Aid for Scientific Research) in the form of grants to MY [18H02883, 21H03008], the Japan Science and Technology Agency (JST)-SPRING in the form of funds to YY [JPMJSP2139], and the Osaka Community Foundation in the form of funds to YY. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Caligiuri A, Pastore M, Lori G, Raggi C, Di Maira G, Marra F, et al. Role of Chemokines in the Biology of Cholangiocarcinoma. Cancers (Basel). 2020;12(8). doi: 10.3390/cancers12082215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sarcognato S, Sacchi D, Fassan M, Fabris L, Cadamuro M, Zanus G, et al. Cholangiocarcinoma. Pathologica. 2021;113(3):158–69. doi: 10.32074/1591-951X-252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Macias RIR, Banales JM, Sangro B, Muntané J, Avila MA, Lozano E, et al. The search for novel diagnostic and prognostic biomarkers in cholangiocarcinoma. Biochim Biophys Acta Mol Basis Dis. 2018;1864(4 Pt B):1468–77. doi: 10.1016/j.bbadis.2017.08.002 [DOI] [PubMed] [Google Scholar]
- 4.Macias RIR, Kornek M, Rodrigues PM, Paiva NA, Castro RE, Urban S, et al. Diagnostic and prognostic biomarkers in cholangiocarcinoma. Liver Int. 2019;39 Suppl 1:108–22. doi: 10.1111/liv.14090 [DOI] [PubMed] [Google Scholar]
- 5.Rodrigues PM, Vogel A, Arrese M, Balderramo DC, Valle JW, Banales JM. Next-Generation Biomarkers for Cholangiocarcinoma. Cancers (Basel). 2021;13(13). doi: 10.3390/cancers13133222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haga H, Yan IK, Takahashi K, Wood J, Zubair A, Patel T. Tumour cell-derived extracellular vesicles interact with mesenchymal stem cells to modulate the microenvironment and enhance cholangiocarcinoma growth. J Extracell Vesicles. 2015;4:24900. doi: 10.3402/jev.v4.24900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ehling J, Tacke F. Role of chemokine pathways in hepatobiliary cancer. Cancer Lett. 2016;379(2):173–83. doi: 10.1016/j.canlet.2015.06.017 [DOI] [PubMed] [Google Scholar]
- 8.Tanaka T, Bai Z, Srinoulprasert Y, Yang BG, Hayasaka H, Miyasaka M. Chemokines in tumor progression and metastasis. Cancer Sci. 2005;96(6):317–22. doi: 10.1111/j.1349-7006.2005.00059.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chow MT, Luster AD. Chemokines in cancer. Cancer Immunol Res. 2014;2(12):1125–31. doi: 10.1158/2326-6066.CIR-14-0160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vandercappellen J, Van Damme J, Struyf S. The role of CXC chemokines and their receptors in cancer. Cancer Lett. 2008;267(2):226–44. doi: 10.1016/j.canlet.2008.04.050 [DOI] [PubMed] [Google Scholar]
- 11.Bizzarri C, Beccari AR, Bertini R, Cavicchia MR, Giorgini S, Allegretti M. ELR+ CXC chemokines and their receptors (CXC chemokine receptor 1 and CXC chemokine receptor 2) as new therapeutic targets. Pharmacol Ther. 2006;112(1):139–49. doi: 10.1016/j.pharmthera.2006.04.002 [DOI] [PubMed] [Google Scholar]
- 12.Guo Q, Jian Z, Jia B, Chang L. CXCL7 promotes proliferation and invasion of cholangiocarcinoma cells. Oncol Rep. 2017;37(2):1114–22. doi: 10.3892/or.2016.5312 [DOI] [PubMed] [Google Scholar]
- 13.Okabe H, Beppu T, Ueda M, Hayashi H, Ishiko T, Masuda T, et al. Identification of CXCL5/ENA-78 as a factor involved in the interaction between cholangiocarcinoma cells and cancer-associated fibroblasts. Int J Cancer. 2012;131(10):2234–41. doi: 10.1002/ijc.27496 [DOI] [PubMed] [Google Scholar]
- 14.Fang H, Li R, Gu Y, Fei Y, Jin K, Chen Y, et al. Intratumoral interleukin-9 delineates a distinct immunogenic class of gastric cancer patients with better prognosis and adjuvant chemotherapeutic response. Oncoimmunology. 2020;9(1):1856468. doi: 10.1080/2162402X.2020.1856468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohta M, Tanaka F, Yamaguchi H, Sadanaga N, Inoue H, Mori M. The high expression of Fractalkine results in a better prognosis for colorectal cancer patients. Int J Oncol. 2005;26(1):41–7. [PubMed] [Google Scholar]
- 16.Hojo S, Koizumi K, Tsuneyama K, Arita Y, Cui Z, Shinohara K, et al. High-level expression of chemokine CXCL16 by tumor cells correlates with a good prognosis and increased tumor-infiltrating lymphocytes in colorectal cancer. Cancer Res. 2007;67(10):4725–31. doi: 10.1158/0008-5472.CAN-06-3424 [DOI] [PubMed] [Google Scholar]
- 17.Wu Z, Huang X, Han X, Li Z, Zhu Q, Yan J, et al. The chemokine CXCL9 expression is associated with better prognosis for colorectal carcinoma patients. Biomed Pharmacother. 2016;78:8–13. doi: 10.1016/j.biopha.2015.12.021 [DOI] [PubMed] [Google Scholar]
- 18.Minamiya Y, Saito H, Takahashi N, Ito M, Imai K, Ono T, et al. Expression of the chemokine receptor CXCR4 correlates with a favorable prognosis in patients with adenocarcinoma of the lung. Lung Cancer. 2010;68(3):466–71. doi: 10.1016/j.lungcan.2009.07.015 [DOI] [PubMed] [Google Scholar]
- 19.Minamiya Y, Saito H, Takahashi N, Ito M, Toda H, Ono T, et al. Expression of the chemokine receptor CCR6 correlates with a favorable prognosis in patients with adenocarcinoma of the lung. Tumour Biol. 2011;32(1):197–202. doi: 10.1007/s13277-010-0113-x [DOI] [PubMed] [Google Scholar]
- 20.Li X, Sun S, Li N, Gao J, Yu J, Zhao J, et al. High Expression of CCR7 Predicts Lymph Node Metastasis and Good Prognosis in Triple Negative Breast Cancer. Cell Physiol Biochem. 2017;43(2):531–9. doi: 10.1159/000480526 [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto Y, Kuroda K, Sera T, Sugimoto A, Kushiyama S, Nishimura S, et al. The Clinicopathological Significance of the CXCR2 Ligands, CXCL1, CXCL2, CXCL3, CXCL5, CXCL6, CXCL7, and CXCL8 in Gastric Cancer. Anticancer Res. 2019;39(12):6645–52. doi: 10.21873/anticanres.13879 [DOI] [PubMed] [Google Scholar]
- 22.Yamada N, Chung Y, Ohtani H, Ikeda T, Onoda N, Sawada T, et al. Establishment and characterization of a new human gallbladder carcinoma cell line (OCUG-1) producing TA-4. Int J Oncol. 1997;10(6):1251–5. doi: 10.3892/ijo.10.6.1251 [DOI] [PubMed] [Google Scholar]
- 23.Do HTT, Lee CH, Cho J. Chemokines and their Receptors: Multifaceted Roles in Cancer Progression and Potential Value as Cancer Prognostic Markers. Cancers (Basel). 2020;12(2). doi: 10.3390/cancers12020287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boissière-Michot F, Jacot W, Massol O, Mollevi C, Lazennec G. CXCR2 Levels Correlate with Immune Infiltration and a Better Prognosis of Triple-Negative Breast Cancers. Cancers (Basel). 2021;13(10). doi: 10.3390/cancers13102328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luan J, Furuta Y, Du J, Richmond A. Developmental expression of two CXC chemokines, MIP-2 and KC, and their receptors. Cytokine. 2001;14(5):253–63. doi: 10.1006/cyto.2001.0882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Erin N, Tavşan E, Akdeniz Ö, Isca VMS, Rijo P. Rebound increases in chemokines by CXCR2 antagonist in breast cancer can be prevented by PKCδ and PKCε activators. Cytokine. 2021;142:155498. [DOI] [PubMed] [Google Scholar]
- 27.Li C, Deng H, Zhou Y, Ye Y, Zhao S, Liang S, et al. Expression and clinical significance of CXC chemokines in the glioblastoma microenvironment. Life Sci. 2020;261:118486. doi: 10.1016/j.lfs.2020.118486 [DOI] [PubMed] [Google Scholar]
- 28.Li N, Liang X, Li J, Zhang D, Li T, Guo Z. C-C motif chemokine ligand 14 inhibited colon cancer cell proliferation and invasion through suppressing M2 polarization of tumor-associated macrophages. Histol Histopathol. 2021:18348. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
The data supporting these findings during the current study are not publicly available since data contain potentially sensitive information. However, this data could be available from the corresponding author or the Ethics Committee (ethics@med.osaka-cu.ac.jp) on reasonable request.