Abstract
In vivo pharmacodynamic parameters have been characterized for a variety of antibacterial agents. These parameters have been studied in correlation with in vivo outcomes in order to determine (i) which dosing parameter is predictive of outcome and (ii) the magnitude of that parameter associated with efficacy. Very little is known of the pharmacodynamics of antifungal agents. We used a neutropenic murine model of disseminated candidiasis to correlate the pharmacodynamic parameters (percentage of time above the MIC, area under the concentration-time curve [AUC]/MIC and peak level/MIC) for flucytosine (5-FC) in vivo with efficacy as measured by organism number in homogenized kidney cultures after 24 h of therapy. The pharmacokinetics of 5-FC in infected mice were linear. Serum half-lives ranged from 0.36 to 0.43 h. Infection was achieved by intravenous inoculation of 106 CFU of yeast cells per ml via the lateral tail vein of neutropenic mice. Groups of mice were treated with fourfold escalating total doses of 5-FC ranging from 1.56 to 400 mg/kg of body weight/day divided into one, two, four, or eight doses over 24 h. Increasing doses produced minimal concentration-dependent killing ranging from 0 to 0.9 log10 CFU/kidneys. 5-FC did, however, produce a dose-dependent suppression of growth after levels in serum had fallen below the MIC. The fungistatic dose increased from 6 to 8 mg/kg with dosing every 3 and 6 h to 70 mg/kg at with dosing every 24 h. Nonlinear regression analysis was used to determine which pharmacodynamic parameter best correlated with efficacy. Time above the MIC was the parameter best predictive of outcome, while AUC/MIC was only slightly less predictive (time above MIC, R2 = 85%; AUC/MIC, R2 = 77%; peak level/MIC, R2 = 53%). Maximal efficacy was observed when levels exceeded the MIC for only 20 to 25% of the dosing interval. If one considers drug kinetics in humans, these results suggest reevaluation of current dosing regimens.
The incidence of nosocomial candida infections has risen sharply, and candida is now the fourth most common cause of hospital-acquired bloodstream infection (4). The currently available therapies result in unacceptably high failure rates (10, 30). In addition, the available antifungal therapies often produce significant toxicities (14, 18). Although the new antifungal agents appear to be promising, approaches that optimize the efficacies and limit the toxicities of currently available agents through rational pharmacodynamic dosing may offer more immediate impact (15).
Flucytosine is an oral administered pyrimidine analog that has been available for clinical use for more than 30 years. Studies with both experimental infection models and clinical trials have demonstrated the potency of flucytosine against a variety of yeasts (3, 5, 16, 25). While flucytosine is primarily used for the treatment of cryptococcal meningitis, several studies have shown its utility in the therapy of various infections caused by Candida, including meningitis, endophthalmitis, endocarditis, peritonitis, and candidemia associated with neutropenia (5, 14, 24). In a recent consensus publication on the therapy of candidemia, 50% of the participants would include flucytosine in combination with amphotericin B for the treatment of candidemia in those with concomitant neutropenia (13). Current use of this agent has, however, become quite limited. Clinicians have grown reluctant to use flucytosine because of (i) the common development of resistance during therapy when it is used as a single agent and (ii) the relatively narrow therapeutic window.
Pharmacodynamic characterization of flucytosine should enable the maximization of dosing efficacy and perhaps limit toxicity and the development of resistance. In the experiments described here we have characterized the pharmacodynamic parameter predictive of efficacy of flucytosine monotherapy in a neutropenic murine model of disseminated Candida albicans infection.
(Part of this work was presented at the 98th General Meeting of the American Society for Microbiology, Atlanta, Ga., 17 to 21 May 1998, and the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego, Calif., 24 to 27 September 1998.)
MATERIALS AND METHODS
Organism.
A clinical isolate of C. albicans designated K-1 was used for all experiments. The organism was recovered from a patient with endophthalmitis. The organism was maintained, grown, subcultured, and quantified on Sabouraud dextrose agar (SDA; Difco Laboratories, Detroit, Mich.). The organisms were maintained on SDA slants at 4°C. Twenty-four hours prior to study, the organisms were subcultured at 35°C.
Antifungal agent.
Flucytosine was obtained as a powder from Sigma (St. Louis, Mo.). The powder was stored at −70°C. Drug solutions were prepared on the day of study by dissolving the powder in sterile distilled H2O.
In vitro susceptibility testing.
The MIC for the organism was determined by a broth microdilution modification of the M27-A method of the National Committee for Clinical Laboratory Standards (21). Determinations were performed in duplicate on at least two separate occasions (21). Final results are expressed as the geometric mean of those results.
Animals.
Six-week-old specific-pathogen free female ICR/Swiss mice (weight, 23 to 27 g; (Harlan Sprague-Dawley, Madison, Wis.) were used for all studies.
Infection model.
Mice were rendered neutropenic (polymorphonuclear leukocyte count, <100/mm3) by injecting cyclophosphamide (Mead Johnson Pharmaceuticals, Evansville, Ind.) intraperitoneally 4 days (150 mg/kg of body weight) and 1 day (100 mg/kg) before infection.
Organisms were subcultured on SDA 24 h prior to infection. The inoculum was prepared by placing six colonies into 5 ml of sterile pyrogen-free 0.9% saline that had been warmed at 35°C. The fungal counts of the inoculum as determined from viable counts on SDA were 106 CFU/ml.
Disseminated infection with C. albicans organisms was achieved by injection of 0.1 ml of inoculum via the lateral tail vein 2 h prior to the start of drug therapy. At the end of the study period the animals were killed by CO2 asphyxiation. After the mice were killed, the kidneys of each mouse were immediately removed and placed in sterile 0.9% saline at 4°C. The homogenate was then serially diluted 1:10 and aliquots were plated onto SDA for determination of viable fungal colony counts after incubation for 24 h at 35°C. Results were expressed as the mean number of CFU per kidneys for two mice (four kidneys).
Pharmacokinetics.
The single-dose pharmacokinetics of flucytosine were determined for individual neutropenic, infected ICR/Swiss mice following the administration of subcutaneous doses of 6.25, 25, and 100 mg/kg in 0.2-ml volumes. For each dose examined, groups of three mice were sampled three or four times by retro-orbital puncture, and the samples were collected in heparinized capillary tubes (Fisher Scientific, Pittsburgh, Pa.) at 30- to 60-min intervals. The tubes were centrifuged (model MB centrifuge; International Equipment Co.) at 10,000 × g for 5 min. The serum was subsequently removed and drug levels were determined by a standard drug diffusion bioassay with Saccharomyces cerevisiae as the assay organism in Noble agar and yeast nitrogen base (23). Assays of serum samples and preparation of standard curves prepared with mouse serum were performed on the same day. Intraday variation ranged from 0 to 2.7 mg/liter. The lower level of detection for this assay was 2 mg/liter. Pharmacokinetic constants including elimination half-life and concentration at time zero were calculated by using a one-compartment model with first-order absorption via nonlinear least-squares techniques (MINSQ; Micromath Inc., Salt Lake City, Utah). The area under the concentration-time curve (AUC) was calculated by the trapezoidal rule. For doses for which no kinetics were determined, pharmacokinetic parameter values were extrapolated from the values obtained in the actual studies. Total levels in serum were used for all calculations because of the negligible amount of protein binding to flucytosine.
In vivo PAE.
Infection in neutropenic mice was produced as described above. Two hours after infection with C. albicans K-1, the mice were treated with single subcutaneous doses of flucytosine (6.25, 25, and 100 mg/kg). Groups of two treated mice and two control mice were killed at each sampling time interval ranging from 1 to 12 h. Control growth was determined over 24 h at five sampling times. The treated groups were sampled nine times over 30 h. The kidneys were removed at each time point and were immediately processed for CFU determination as outlined above. The time that serum flucytosine levels remained above the MIC for the organism following administration of the three doses was calculated from the pharmacokinetic data. The postantibiotic effect (PAE) was calculated by determining the amount of time that it took for the organism numbers in the controls to increase 1 log10 CFU/kidneys (C) and subtracting this from the amount of time that it took organisms from the treated animals to grow 1 log10 CFU/kidneys (T) after levels in serum fell below the MIC for the organism, i.e., PAE = T − C (7, 28).
Pharmacodynamic parameter determination.
Neutropenic mice were infected with C. albicans K-1 2 h prior to the start of therapy. Twenty dosing regimens were chosen to minimize the interdependence between the three pharmacodynamic parameters studied and also to describe the complete dose-response relationship. Groups of two mice each were treated for 24 h with different flucytosine dosing regimens by using fourfold increasing total doses administered at 3-, 6-, 12-, and 24-h intervals. Total doses ranged from 1.56 to 400 mg/kg/day. Drug was administered in 0.2-ml volumes. The mice were killed after 24 h of therapy, and the kidneys were removed for CFU determination as described above. Untreated control mice were killed just before treatment and after 24 h. Efficacy was defined as the change in log10 the number of CFU per kidneys over the 24-h treatment period and was calculated by subtracting the mean log10 number of CFU per kidneys in untreated control mice after 24 h from the mean number of CFU in the kidneys of two mice at the end of therapy.
Data analysis.
A sigmoid dose-effect model was used to measure the in vivo potency of flucytosine. The model is derived from the Hill equation: E = (Emax × DN)/(ED50N + DN), where E is the observed effect (change in log10 number of CFU per kidney compared with that in untreated controls at 24 h), D is the cumulative 24-h dose, Emax is the maximum effect, ED50 is the dose required to achieve 50% of Emax, and N is the slope of the dose-effect relationship. The correlation between efficacy and each of the three parameters studied was determined by nonlinear least-squares multivariate regression analysis (Sigma Stat; Jandel Scientific Software, San Rafael, Calif.). The coefficient of determination (R2) was used to estimate the percent variance in the change in the log10 number of CFU per kidneys over the treatment period for the different dosing regimens that could be attributed to each of the pharmacodynamic parameters.
To allow a more meaningful comparison of potency among the dosing regimens studied, we calculated the dose required to produce a fungistatic effect or no net growth over 24 h. The static dose was estimated from the following equation: log10 static dose = {log10 [E/(Emax − E)]}/N + log10 ED50. If the static doses for the different dosing intervals were similar, this would suggest that AUC/MIC is the parameter most important in the prediction of outcome. If the static dose increased significantly as the dosing interval was lengthened from every 3 h through every 24 h, the duration of time that the levels in serum remained above the MIC was the parameter predictive of efficacy.
RESULTS
In vitro susceptibility testing.
The MIC for flucytosine for C. albicans K-1 was 1.0 mg/liter.
Pharmacokinetics.
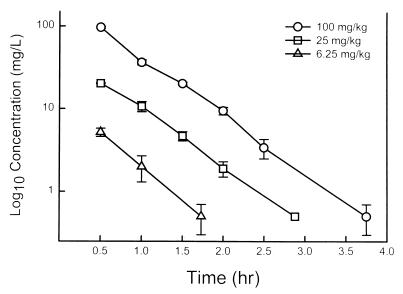
The time course of the levels of flucytosine in the sera of infected neutropenic mice following the administration of subcutaneous doses of 6.25, 25, and 100 mg/kg are shown in Fig. 1. The pharmacokinetics were linear and were well described by a one-compartment model. Peak levels were achieved within 30 min for each of the doses and ranged from 5.2 ± 0.6 to 97 ± 2.5 mg/liter. The elimination half-life did not change significantly with higher doses and ranged from 0.36 to 0.43 h. The AUC, as determined by the trapezoidal rule, ranged from 3.2 to 60 mg · h/liter with the lowest and highest doses, respectively.
FIG. 1.
Serum flucytosine concentrations after the administration of subcutaneous doses of 100, 25, and 6.25 mg/kg in neutropenic infected mice. Each symbol represents the geometric mean ± standard deviation levels in serum for three mice.
In vivo PAE.
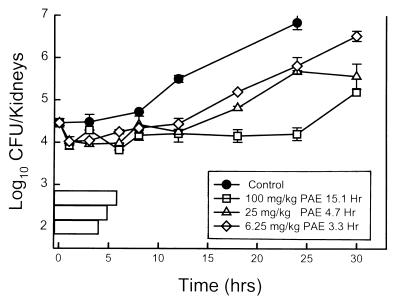
Following inoculation of 106 CFU/ml into the tail vein, the growth of candida organisms in the kidneys of untreated mice increased 2.4 ± 0.12 log10 CFU/kidneys over 24 h. Growth of 1 log10 CFU/kidneys in untreated control mice was achieved in 14.3 h. On the basis of the pharmacokinetics described above, the three doses of flucytosine studied (6.25, 25, and 100 mg/kg) would produce levels in serum above the MIC for the candida organism (1.0 mg/l) for 1.5, 2.4, and 3.3 h, respectively. Treatment with each of the doses produced modest reductions in colony counts compared with the numbers at the start of therapy, ranging from 0.44 ± 0.13 log10 CFU/kidneys at the lowest dose to 0.64 ± 0.08 log10 CFU/thigh at the highest dose. Growth curves for both the control group and treated mice after the administration of single doses of flucytosine are shown in Fig. 2. At each of the three doses studied, flucytosine suppressed regrowth of the organisms in a dose-dependent fashion. PAEs increased from 3.3 to 15.1 h with escalating flucytosine doses. These in vivo studies are unable to determine what degree of growth suppression could be due to the antimicrobial effects of sub-MICs. In addition, although serum drug concentrations have been shown to be a relatively good surrogate of the concentrations in tissue, the magnitude of the PAE in these experiments may have been different if we were able to accurately measure the flucytosine concentrations at the site of infection.
FIG. 2.
In vivo PAE of flucytosine against C. albicans in neutropenic mice after administration of doses of 100, 25, and 6.25 mg/kg. Each symbol represents the mean ± standard deviation for two mice (four kidneys).
Pharmacodynamic parameter determination.
At the start of therapy the kidneys had 4.14 ± 0.09 log10 CFU/kidneys. After 24 h the organisms grew by 2.58 ± 0.01 log10 CFU/kidneys in untreated mice and resulted in the death of each of the control mice. Escalating doses of flucytosine produced little net killing of organisms in treated animals compared to the inoculum in control animals at the start of therapy. The highest total doses for the different regimens resulted in a reduction of 0.40 ± 0.06 log10 CFU/kidneys with dosing every 24 h and a reduction of 0.73 ± 0.09 log10 CFU/kidneys with the shortest dosing interval.
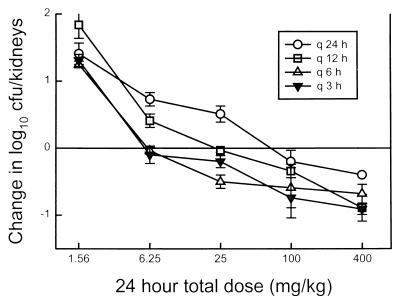
As shown in Fig. 3, there was a significant increase in the dose required to produce a net static effect over the 24-h treatment period as the dosing interval was lengthened. The fungistatic dose ranged from 6 to 8 mg/kg/day for the 3- and 6-h dosing regimens but increased to 70 mg/kg/day with the 24-h dosing regimen.
FIG. 3.
Relationship between the 24-h total dose for four lengthening dosing intervals and log10 number of CFU per kidneys in a neutropenic murine model of disseminated candidiasis. Symbols above the reference line represent growth, while those below the reference line represent net killing compared to control growth at the beginning of therapy. Each symbol represents data for two mice (four kidneys).
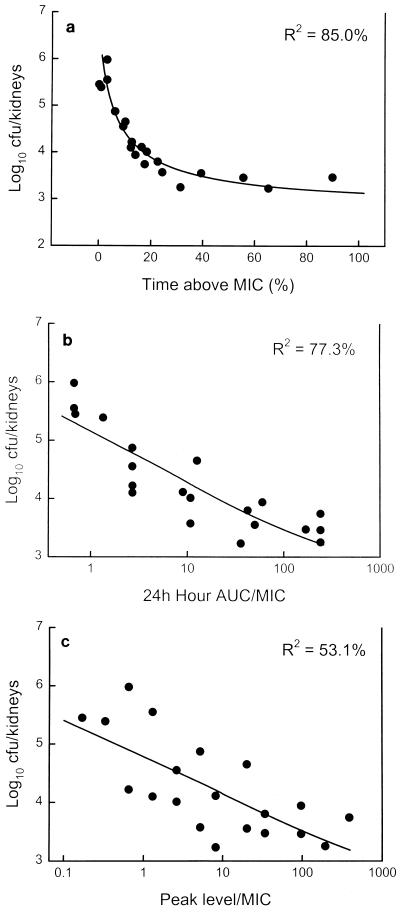
The relationship between microbiologic effect and each of the pharmacodynamic parameters, percent time above the MIC, AUC/MIC, and peak level/MIC, are shown in Fig. 4a to c. Minimal in vivo killing made it difficult to correlate parameters with efficacy. Both the time that the levels in serum remained above the MIC for the organism and the AUC/MIC appeared to be important in predicting efficacy; however, time above the MIC had a relatively stronger regression coefficient (R2 = 85% [P < 0.001] versus 77% [P < 0.001]). The peak level/MIC was the least important pharmacodynamic parameter in determining efficacy (R2 = 53% [P = 0.002]). Maximal efficacy was observed when levels in serum exceeded the MIC for this organism for only 20 to 25% of the 24-h dosing interval.
FIG. 4.
(a) Relationship between the percentage of the dosing interval that levels in serum remained above the MIC for the organism and log10 number of CFU per kidneys after 24 h of therapy. Each symbol represents data for two mice (four kidneys). (b) Relationship between the 24-h AUC/MIC and log10 number of CFU per kidneys after 24 h of therapy. Each symbol represents data for two mice (four kidneys). (c) Relationship between the peak level in serum/MIC and log10 number of CFU per kidneys after 24 h of therapy. Each symbol represents data for two mice (four kidneys).
DISCUSSION
The time course of antimicrobial activity can be determined by two characteristics: (i) the effect of increasing drug concentrations on the extent of organism killing and (ii) the presence or absence of antimicrobial effects which persist after the levels in serum have fallen below the MIC (9). For example, demonstration of the concentration-dependent killing and prolonged PAE with the aminoglycoside class has provided the basis for once-daily dosing of these drugs (8). This regimen optimizes the concentration-dependent parameters peak level/MIC and AUC/MIC, which have been shown to predict efficacy, limit toxicity, and reduce the development of organism resistance (6, 19, 29).
Previous animal infection models have demonstrated the potency of flucytosine both alone and in combination with other agents against several Candida species (3, 16, 22, 26). In addition, several studies have demonstrated the concordance between in vitro susceptibility and in vivo endpoints (1, 25). These studies have, however, used only a single dosing interval, limiting one's ability to determine which pharmacodynamic parameter best predicts efficacy. With only a single dosing interval, escalating doses increase the levels of all three parameters. The interdependence between the parameters with single-dosing-interval studies is too great to determine if one is more important than another. We were able to locate only a single study, by Karytotakis and Anaissie (17), in which more than one dosing length was used. Continuous infusion of flucytosine via a subcutaneous pump was compared to once-daily administration in the therapy of disseminated Candida lusitaniae infection in an immunocompromised mouse model. They found significantly greater reductions in yeast numbers in the kidneys of mice treated by continuous infusion. These results were thought to be due simply to the short elimination half-life of flucytosine in this animal model. Several pharmacodynamic studies, however, have shown that these parameters can be independent of differences in animal and human pharmacokinetics. For example, the durations of time that the levels in serum need to exceed the MIC for efficacy are similar in animal infection models and for bacteriologic cure in acute otitis media (10; W. A. Craig, S. Ebert, and Y. Watanabe, Program Abstr. 33rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. 86, p. 135, 1993).
The current studies demonstrated that time above the MIC is the pharmacokinetic or pharmacodynamic parameter that most strongly correlated with the outcome of flucytosine monotherapy. We also showed that less total drug needed to be given to achieve a similar effect when the drug was administered more frequently, maximizing this time-dependent parameter. Tenfold more drug was required to produce a net static effect when drug was administered once daily than when it was administered four to eight times daily. Thus, these data suggest that low-dose, frequently administered regimens of flucytosine can be as effective as higher-dose regimens of flucytosine, provided that the concentrations in serum are maintained above the MIC for greater than 25% of the dosing interval. However, we demonstrated that efficacy is dependent upon the time that serum flucytosine levels remain above the MIC for the C. albicans organism, not simply upon the frequency of the dosing interval. Similar outcomes were observed with the various flucytosine dosing intervals when the doses used produced similar times above the MIC.
Flucytosine would only rarely be used as monotherapy in humans, such as for urinary tract infections (27). However, studies of flucytosine in combination with several classes of antibiotics have shown that the pharmacodynamic parameter predictive of efficacy in combination therapy is the same as that observed with monotherapy (12, 20). Thus, we expect that in combination with either amphotericin B or fluconazole, dosing of flucytosine to maximize the time that levels in serum remain above the MIC would still be most efficacious.
Although the experiments described here included only a single strain of C. albicans, we observed near maximal microbiologic efficacy when levels in serum remained above the MIC for only a quarter of the dosing interval. We believe that this relatively brief time requirement is most likely due to the significant PAEs observed. If one were to consider the human kinetics of the most frequently recommended flucytosine dosing of 150 mg/kg/day divided into four doses, each dose of 37.5 mg/kg would remain above the MIC for 90% of C. albicans isolates tested for 12 to 14 h (11). Many experts have suggested the use of significantly lower doses of flucytosine (100 mg/kg/day) (13, 14). If the findings of these studies are confirmed in strains with a wide variety of susceptibilities, a reevaluation of current flucytosine dosing regimens would be suggested. Use of significantly smaller amounts of drug or dosing to achieve lower peak levels may allow flucytosine administration with much less concern about related toxicities. Several studies have shown that bone marrow toxicities are essentially eliminated when levels in serum remain between 40 and 60 mg/liter (14, 18). Even these “safe” levels would not be necessary if the pharmacodynamic predictions provided here are valid. Future studies should characterize the time above the MIC necessary for flucytosine to have efficacy against other yeast species as well as the time above the MIC for flucytosine in combination with other antifungal agents. In addition, studies should attempt to correlate these parameters with the development of toxicity and resistance.
REFERENCES
- 1.Anaissie E J, Karyotakis N C, Hachem R, Dignani M C, Rex J H, Paetznick V. Correlation between in vitro and in vivo activity of antifungal agents against Candida species. J Infect Dis. 1994;170:384–389. doi: 10.1093/infdis/170.2.384. [DOI] [PubMed] [Google Scholar]
- 2.Andes D, van Ogtrop M. Characterization and quantitation of the pharmacodynamics of fluconazole in a neutropenic murine disseminated candidiasis infection model. Antimicrob Agents Chemother. 1999;43:2116–2120. doi: 10.1128/aac.43.9.2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atkinson B A, Bouthet C, Bocanegra R, Correa A, Luther M F, Graybill J R. Comparison of fluconazole, amphotericin B and flucytosine in treatment of a murine model of disseminated infection with Candida glabrata in immunocompromised mice. J Antimicrob Chemother. 1995;35:631–640. doi: 10.1093/jac/35.5.631. [DOI] [PubMed] [Google Scholar]
- 4.Banerjee S N, Emori T G, Culver D H, Gaynes R P, Jarvis W R, Horan T, Edwards J R, Tolson J S, Henderson T, Martone W J. Secular trends in nosocomial primary blood stream infections in the United States, 1980–1989. Am J Med. 1991;91(Suppl. 3B):86–89. doi: 10.1016/0002-9343(91)90349-3. [DOI] [PubMed] [Google Scholar]
- 5.Bennett J E, Dismukes W E, Duma R J, Medoff G, Sande M A, Gallis H, Leonard J, Fields B T, Bradshaw M, Haywood H, McGee Z A, Cate T R, Cobs C G, Warner J F, Alling D W. A comparison of amphotericin B alone and combined with flucytosine in the treatment of cryptococcal meningitis. N Engl J Med. 1979;301:126–131. doi: 10.1056/NEJM197907193010303. [DOI] [PubMed] [Google Scholar]
- 6.Blaser J, Stone B B, Groner M C, Zinner S H. Comparative study with enoxacin and netilmicin, a pharmacodynamic model to determine importance of ratio of antibiotic peak concentration to MIC for bactericidal activity and emergence of resistance. Antimicrob Agents Chemother. 1987;31:1054–1060. doi: 10.1128/aac.31.7.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Craig W A, Gudmundsson S. Postantibiotic effect. In: Lorian V, editor. Antibiotics in laboratory medicine. 4th ed. Baltimore, Md: Baltimore: The Williams & Wilkins Co.; 1996. pp. 296–329. [Google Scholar]
- 8.Craig W A, Redington J, Ebert S C. Pharmacodynamics of amikacin in vitro and in mouse thigh and lung infections. J Antimicrob Chemother. 1991;27(Suppl. C):29–40. doi: 10.1093/jac/27.suppl_c.29. [DOI] [PubMed] [Google Scholar]
- 9.Craig W A. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis. 1998;26:1–12. doi: 10.1086/516284. [DOI] [PubMed] [Google Scholar]
- 10.Craig W A, Andes D. Pharmacokinetics and pharmacodynamics of antibiotics in otitis media. Pediatr Infect Dis J. 1996;15:955–962. doi: 10.1097/00006454-199603000-00015. [DOI] [PubMed] [Google Scholar]
- 11.Cutler R E, Blair A D, Kelly M R. Flucytosine kinetics in subjects with normal and impaired renal function. Clin Pharmacol Ther. 1978;24:333–342. doi: 10.1002/cpt1978243333. [DOI] [PubMed] [Google Scholar]
- 12.den Hollander J G, Mouton J W, Verbrugh H A. Use of pharmacodynamic parameters to predict efficacy of combination therapy by using fractional inhibitory concentration kinetics. Antimicrob Agents Chemother. 1998;42:744–748. doi: 10.1128/aac.42.4.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edwards J E Conference Participants. International Conference for the Development of a Consensus on the Management and Prevention of Severe Candida Infections. Clin Infect Dis. 1997;25:43–59. doi: 10.1086/514504. [DOI] [PubMed] [Google Scholar]
- 14.Francis P, Walsh T J. Evolving role of flucytosine in immunocompromised patients: new insights into safety, pharmacokinetics, and antifungal therapy. Clin Infect Dis. 1992;15:1003–1018. doi: 10.1093/clind/15.6.1003. [DOI] [PubMed] [Google Scholar]
- 15.Georgeopapadakou N H, Walsh T J. Antifungal agents: chemotherapeutic targets and immunologic strategies. Antimicrob Agents Chemother. 1996;40:279–291. doi: 10.1128/aac.40.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graybill J R, Najvar L K, Holmberg J D, Luther M F. Fluconazole, D0870, and flucytosine treatment of disseminated Candida tropicalis infections in mice. Antimicrob Agents Chemother. 1995;39:924–929. doi: 10.1128/aac.39.4.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karytotakis N C, Anaissie E J. Efficacy of continuous flucytosine infusion against Candida lusitaniae in experimental hematogenous murine candidiasis. Antimicrob Agents Chemother. 1996;40:2907–2908. doi: 10.1128/aac.40.12.2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kauffman C A, Frame P T. Bone marrow toxicity associated with 5-fluorocytosine therapy. Antimicrob Agents Chemother. 1977;11:244–247. doi: 10.1128/aac.11.2.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore R D, Lietman P S, Smith C R. Clinical response to aminoglycoside therapy: importance of the ratio of peak concentration to minimal inhibitory concentration. J Infect Dis. 1987;155:93–99. doi: 10.1093/infdis/155.1.93. [DOI] [PubMed] [Google Scholar]
- 20.Mouton J W, van Ogtrop M L, Andes D, Craig W A. Use of pharmacodynamic indices to predict efficacy of combination therapy in vivo. Antimicrob Agents Chemother. 1999;43:2473–2478. doi: 10.1128/aac.43.10.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing for yeast; approved standard. M27-A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 22.Polak A. Combination therapy for systemic mycosis. Infection. 1989;17:203–208. doi: 10.1007/BF01639520. [DOI] [PubMed] [Google Scholar]
- 23.Shadomy S, Espinel-Ingroff A, Cartwright Y. Laboratory studies with antifungal agents: susceptibility tests and bioassays. In: Lennette E H, Balows A, Hausler W J Jr, Shadomy H J, editors. Manual of clinical microbiology. 4th ed. Washington, D.C.: American Society for Microbiology; 1985. pp. 991–999. [Google Scholar]
- 24.Smego R A, Perfect J R, Durack D T. Combined therapy with amphotericin B and 5-fluorocytosine for candida meningitis. Rev Infect Dis. 1984;6:791–800. doi: 10.1093/clinids/6.6.791. [DOI] [PubMed] [Google Scholar]
- 25.Stiller R L, Bennett J E, Scholer H J, Wall M, Polak A, Stevens D A. Correlation of in vitro susceptibility test results with in vivo response: flucytosine therapy in a systemic candidiasis model. J Infect Dis. 1983;147:1070–1077. doi: 10.1093/infdis/147.6.1070. [DOI] [PubMed] [Google Scholar]
- 26.Thaler M, Bacher J, O'Leary T, Pizzo P A. Evaluation of single-drug and combination antifungal therapy in an experimental model of candidiasis in rabbits with prolonged neutropenia. J Infect Dis. 1988;158:80–88. doi: 10.1093/infdis/158.1.80. [DOI] [PubMed] [Google Scholar]
- 27.Viviani M A. Flucytosine—what is its future? J Antimicrob Chemother. 1995;35:241–244. doi: 10.1093/jac/35.2.241. [DOI] [PubMed] [Google Scholar]
- 28.Vogelman B, Gudmundsson S, Turnidge J, Leggett J, Craig W A. In vivo postantibiotic effect in a thigh infection in neutropenic mice. J Infect Dis. 1988;157:287–298. doi: 10.1093/infdis/157.2.287. [DOI] [PubMed] [Google Scholar]
- 29.Vogelman B, Gudmundsson S, Leggett J, Turnidge J, Ebert S, Craig W A. Correlation of antimicrobial pharmacokinetic parameters with therapeutic efficacy in an animal model. J Infect Dis. 1988;158:831–847. doi: 10.1093/infdis/158.4.831. [DOI] [PubMed] [Google Scholar]
- 30.Wey S B, Mori M, Pfaller M A, Woolson R F, Wenzel R P. Hospital acquired candidemia: the attributable mortality and excess length of stay. Arch Intern Med. 1988;148:2642–2645. doi: 10.1001/archinte.148.12.2642. [DOI] [PubMed] [Google Scholar]