Abstract
Holder pasteurization (HoP) is the current recommended treatment for donor human milk. Although this method inactivates microbial contaminants, it also negatively affects various milk components. High-pressure processing (HPP, 400, 500, and 600 MPa), ultraviolet-C irradiation (UV-C, 2,430, 3,645, and 4,863 J/L) and thermoultrasonication (TUS, 1,080 and 1,620 kJ/L) were investigated as alternatives to thermal pasteurization (HoP). We assessed the effects of these methods on microbiological safety, and on concentration and functionality of immunoglobulin A, lactoferrin, lysozyme and bile salt-stimulated lipase, with LC-MS/MS-based proteomics and activity assays. HoP, HPP, TUS, and UV-C at 4863 J/L, achieved >5-log10 microbial reduction. Native protein levels and functionality showed the highest reduction following HoP, while no significant reduction was found after less intense HPP and all UV-C treatments. Immunoglobulin A, lactoferrin, and lysozyme contents were also preserved after low intensity TUS, but bile salt-stimulated lipase activity was significantly reduced. This study demonstrated that HPP and UV-C may be considered as suitable alternatives to HoP, since they were able to ensure sufficient microbial inactivation while at the same time better preserving the bioactive components of donor human milk. In summary, our results provide valuable insights regarding the evaluation and selection of suitable processing methods for donor human milk treatment, which may replace HoP in the future.
Keywords: donor human milk, non-thermal processing, bacteria inactivation, bacteriostatic properties, proteomics, antimicrobial proteins
Introduction
Human milk (HM) is universally identified as the normative standard for infant nutrition, due to its unique nutritional composition and bioactive components such as immunoactive proteins, hormones, and growth factors, that facilitate proper infant growth and development (1). An essential component of HM known for its bioactive function is the HM proteome, which is comprised of a wide array of proteins, glycoproteins, enzymes, and endogenous peptides (2). For example, HM exerts bacteriostatic activity, a function largely ascribed to the presence of bioactive proteins, such as immunoglobulin A (IgA), lactoferrin (LTF), and lysozyme (LYZ), due to their high abundance in HM [(3, 4)]. More specifically, IgA protects the infant from invasive pathogens, LTF inhibits the growth of iron-dependent pathogens and LYZ lyses the proteoglycan matrix of the cell walls in Gram-positive bacteria. In addition, a synergistic effect of LYZ and LTF is suggested against Gram-negative bacteria (4–6)].
In case mother’s own milk is unavailable, donor human milk (DHM) is the best alternative and it should be provided by established human milk banks that enforce all necessary actions to guarantee its safety (7, 8). Human milk banking guidelines recommend holder pasteurization (HoP) for the elimination of possible life-threatening pathogens in DHM (9). This method is performed by heating DHM for 30 min at a temperature of 62.5°C, followed by a rapid cooling down to <10°C (10).
Even though HoP achieves the 5-log10 reduction of vegetative bacterial cells required by all human milk banking guidelines, it also leads to the degradation of key DHM bioactive components (9). After HoP, a significant reduction has been reported in the concentration and activity of IgA, LTF, and LYZ, as well as in several enzymes, hormones, cytokines, and growth factors (10, 11). In addition, HoP completely inactivates bile salt-stimulated lipase (BSSL), a heat-labile enzyme that facilitates fat absorption and enhances lipid metabolism (11). It is thus possible that BSSL inactivation through HoP may be the cause of the reported lower growth rates of preterm infants fed with HoP-treated DHM, compared with the ones fed mother’s own milk (12). To overcome the disadvantages of this treatment, novel methods such as high-pressure processing (HPP), ultraviolet-C irradiation (UV-C), and thermoultrasonication (TUS) have been proposed as promising non-thermal alternatives to HoP (9, 13).
High-pressure processing is a mild food preservation method commonly applied in the food industry to guarantee the food safety of a product by microbial inactivation due to the high hydrostatic pressure (14, 15). UV irradiation is a non-thermal disinfection method, especially at wavelengths between 200 and 280 nm (UV-C) (16). This method effectively inactivates microbial contaminants by disrupting DNA transcription and replication, ultimately leading to cell death (16, 17). Ultrasonication (20–100 kHz) is a food preservation method that involves microbubble formation and their rapid collapse though inertial cavitation. The shock waves that are produced from this process, as well as the chemical changes induced by it, ultimately lead to bacterial cell death (9, 18). TUS, the process where ultrasonication is combined with mild heating, is considered more effective in microbial inactivation than ultrasonication alone (19). In addition to bacterial inactivation, all the aforementioned methods are able to batch process human milk, as would be required when applying these methodologies in a human milk bank.
When applied to DHM, these methods have shown promising results with regards to microbial inactivation and retention of DHM bioactive components (9, 20, 21). However, the number of studies evaluating UV-C or TUS as possible alternatives to HoP is still quite limited, while a large number of different pressure, time and temperature combinations have been applied for HPP to DHM, making direct comparison of those studies complicated (9).
The aim of this study was first to assess whether HPP, UV-C and TUS can achieve a 5-log10 bacterial reduction as found following HoP, which is a primary requirement for use of DHM. Secondly, we aimed to evaluate the effects of these methods on the DHM proteome in order to get a full overview of the changes caused, as well as on the concentration and bioactivity of IgA, LTF, LYZ, and BSSL, and compare them with HoP.
Materials and Methods
Milk Samples
The HM samples used in this study were provided by the Dutch Human Milk Bank (located at Amsterdam UMC, Amsterdam, Netherlands). Donor screening and milk collection was performed according to standardized procedures that comply to international guidelines (10). All donors signed informed consent before recruitment. Milk expression, collection, and transportation to the Dutch Human Milk Bank was performed as previously described (22). The samples were transported frozen (−20°C) to the human milk bank and were stored frozen at the same temperature, for a maximum of 3 months, until further processed. Before analysis, each donated sample was thawed overnight in a refrigerator at 4°C. Once thawed, the native microflora of the samples was assessed by pour or surface-plating of undiluted DHM (1 or 0.1 mL, respectively) in duplicate onto selective media (VRBGA, violet red bile glucose agar, CM0107B and MSA, mannitol salt agar, CM0085B, Thermo Fisher Scientific, Massachusetts, USA) and non-selective media (PCA, Plate Count Agar, CM0325, Thermo Fisher Scientific, MA, United States) followed by an incubation at 37°C for 24–48 h, while the remaining amount of each sample was again placed in the freezer (−20°C). Bacterial numbers were determined by colony counting (CFU/mL), and the samples with 1 log10 CFU/mL or less were selected. Next, the samples were again thawed overnight at 4°C, and milk from four different donors was pooled to ensure sufficient amounts of DHM for all treatments. One pool (four donors, 1,000 mL) was used to evaluate the inactivation of the selected bacterial strains and another pool (four donors, 1,000 mL) was used to evaluate the DHM proteome, the total protein content, the BSSL activity and the bacteriostatic properties. Aliquots for each treatment (HPP, UV-C, TUS, and HoP) and for the untreated milk (UN) that served as control were then created. After all treatments, the samples were cooled in an ice-water bath and all analyses were performed immediately. The analyses were performed as two independent experiments (biological replicates) in duplicate (technical replicates). The experimental approach used in this study is presented in Figure 1.
FIGURE 1.
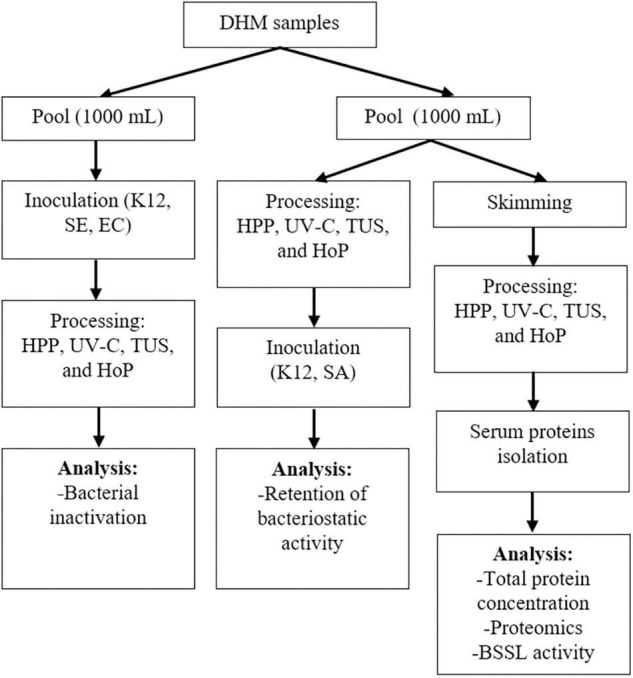
Schematic representation of the experimental approach used. K12, SE, and EC stand for E. coli K12, S. epidermidis and E. cloacae, respectively. Two independent experiments (biological replicates) were performed while all analyses were performed in technical duplicate for each sample.
Treatments
High-Pressure Processing
High pressure treatment was carried out in a pilot-scale equipment, custom made by Resato (1.6 L, Resato, Roden, Netherlands). The computer-controlled pressure build up was ∼30 MPa/s. The samples were subjected to three different pressures for various holding times; 400 MPa for 5, 10, and 30 min, 500 MPa for 1.5, 2 × 1.5, 3, and 5 min, 600 MPa for 1.5, 2 × 1.5, 3, and 5 min. For the treatments “2 × 1.5 min,” the process of pressure build up, holding time of 1.5 min and pressure release was carried out twice. This was done based on previous findings which demonstrated that two pressure treatments with 1.5 min holding time were more effective in microbial inactivation than one pressure treatment of 3 or 4 min at the same pressure (23). DHM samples (10 mL, 4°C) were packed into sterile pouches made of polyethylene. Two to four small sample pouches were packed in a larger pouch which was subsequently vacuumized at 95% vacuum. The larger pouches were then taped in a cylindrical holder that was placed in a sample holder as described previously (14). Samples were not preheated and tap water of 10°C was used at the start of the treatment as medium. The temperature increase during HPP treatment was described as follows (14): Tincrease/100MPa = 0.026×Tinitial+2.26, leading to a maximum sample temperature of around 18°C (after 600 MPa).
Ultraviolet-C Irradiation Treatment
The UV-C system was based on published literature (16), where a UV-C lamp (TUV PL-S 5W, UV-C radiation 1.1 W, Philips, Netherlands) was placed diagonally in a sterile beaker glass filled with 140 mL DHM. During treatment, the milk was stirred with a sterile 4 × 20 mm stirring rod at 500 rpm (IKA RH 2, Staufen, Germany). Samples (20 mL, 4°C) were taken at three different time points and were aliquoted for further analysis. The samples were exposed to three different UV-C dosages; 2,430, 3,645, and 4,863 J/L. The time needed to reach these dosages (5.15, 6.63, and 7.36 min, respectively) was calculated according to: (16). The temperature was controlled during the whole process with a temperature data logger (RS PRO 1384, RS Components B.V., Netherlands) and a maximum increase of 3°C was documented after a treatment of 4,863 J/L. This set-up was used in order to overcome the limited penetration of UV-C in milk (absorption coefficient of 300 cm–1 at 254 nm), by applying a turbulent flow (16).
Thermoultrasonication
A sonifier (Branson 450 Digital Sonifier®, Branson Ultrasonics Corporation, CT, United States) with a horn frequency of 20 kHz was outfitted with a sound enclosure (Branson Emerson Technologies, GmbH & Co, Germany), a microtip probe (length: 60 mm, diameter: 10 mm), and a circulating water bath. Samples (20 mL, 4°C) were placed into a sterile 80 mL glass beaker that was surrounded by circulating water of 40°C. The sonifier was operated in pulse mode, with a continuous pulse of 59.9 s followed by a short pause of 30 s. The samples were treated for 9 min (excluding pause time) at 40 W (1,080 kJ/L, 38% amplitude), for 6 min at 60W (1,080 kJ/L, 58% amplitude) or for 9 min at 60 W (1,620 kJ/L, 58% amplitude). The energy density (KJ/L) was calculated as power(W) x treatmenttime(sec)/volume(mL) (24). The temperature of the samples was recorded by a temperature data logger (RS PRO 1384, RS Components B.V., Netherlands). The maximum temperature increase was 20°C (maximum sample temperature, 59°C), after 9 min at 60 W.
Holder Pasteurization
Donor human milk (30 mL) was placed into a Greiner tube (50 mL) and was heated at 62.5°C for 30 min, in a shaking water bath (150 rpm). The sample was cooled in an ice-water bath immediately after treatment, until a temperature 4°C was reached. The time required for the temperature of the sample to reach the pasteurization temperature (62.5°C) was 25 min, while the cooling down time to 4°C was 15 min. A temperature data logger RS PRO 1384 (RS Components B.V., Netherlands) was used to monitor the temperatures during the whole process.
Bacterial Inactivation
Bacterial species were selected based on their clinical relevance for DHM. Fresh cultures of Enterobacter cloacae (ATCC 13047, American Type Culture Collection, Manassas, VA, United States), Escherichia coli K12 (DSM 498, Deutsche Sammlung von Mikroorganismen und Zellkulturen, Braunschweig, Germany) and Staphylococcus epidermidis (ATCC 14990, American Type Culture Collection, Manassas, VA, United States) were prepared from frozen stocks in brain heart infusion broth (CM1135, Thermo Fisher Scientific, MA, United States) after an overnight incubation at 37°C. The bacterial pellets that were obtained after centrifugation at 4,000 × g for 10 min (Microcentrifuge 5890R, Eppendorf, Hamburg, Germany), were subsequently inoculated into DHM samples at a final concentration of 108 CFU/mL and were then subjected to HPP, UV-C, TUS, or HoP treatment. Treated and untreated samples were next plated in duplicate onto VRBGA (E. coli, E. cloacae) and MSA (S. epidermidis) and were incubated overnight at optimal growth conditions. Untreated but inoculated samples with the three strains served as reference to verify the starting microbial concentration and to calculate bacterial reduction. The reduction in bacterial numbers was determined by colony counting (CFU/mL), with a detection limit of 0 log10 CFU/mL (E. coli and E. cloacae counts) and 1 log10 CFU/mL (S. aureus counts). Since the HPP unit used in this study is located in a food safe environment, inoculation with pathogenic strains was prohibited. Therefore, all the strains used in this study were biosafety level 1 strains. In addition, bacteria inactivation after HPP was tested only with the E. cloacae and S. epidermidis strains.
Milk Serum Preparation and Total Protein Content
To obtain the native milk serum proteins, after all treatments, caseins and denatured proteins were removed. To do so, untreated samples (520 mL) were first centrifuged at 6,500 × g for 30 min at 4°C (with rotor 16.250, Avanti Centrifuge J-26 XP, Beckman Coulter, United States) to remove the fat. The skimmed samples were then treated with all methods as described above (sections 2.2.1–2.2.4), apart from one sample that remained untreated (control). Next, the pH of the skimmed samples was adjusted to 4.6 by the addition of 1 mol/L HCl under stirring, to precipitate the caseins and the denatured serum proteins (25). The samples were left for 30 min at 4°C to equilibrate and were subsequently ultracentrifuged at 100,000 × g for 90 min at 30°C (Optima L-80, Beckman Coulter, United States). Finally, the casein pellet was discarded, and the supernatant containing the native serum proteins was collected. The total native protein content was then assessed using the bicinchoninic acid (BCA) assay kit (Thermo Fisher Scientific, United States), according to the manufacturer’s instructions.
Protein Quantification and Identification by Liquid Chromatography With Tandem Mass Spectrometry
Filter Aided Sample Preparation
The Filter Aided Sample Preparation (FASP)method was carried out as previously reported (26, 27). Briefly, milk serum samples were diluted with 100 mM Tris (pH 8.0) to a protein concentration of 1.0 μg/μL. The next steps included; DDT reduction (10 μL, 0.15 M), alkylation with 136 μL urea (8 M) in 100 mM Tris/HCl (0.1 M, pH 8.0) and 20 μL of acrylamide (0.2 M), placing the samples into ethanol washed Pall 3K omega filters (10–20 kDa cut off, OD003C34, Pall corporation, Port Washington, NY, United States) and centrifuging them at 14,000 × g for 30 min, adding 110 μL 50 mmol/L NH4HCO3 to the filters and centrifuging them again (14,000 × g for 30 min). The samples were then digested with 1 μL trypsin (0.5 μg/μL sequencing grade) in 100 μL of NH4HCO3 (0.05 M) and after an overnight incubation, they were centrifuged for 30 min at 14,000 × g. After the addition of 100 μL 1 mL/L HCOOH in water on the filters, another centrifugation followed (14,000 × g for 30 min). Finally, 3 μL of TFA (10% v/v) was added to the filtrate to adjust the pH of the samples to 3. All samples were stored at −20 °C prior to liquid chromatography with tandem mass spectrometry (LC-MS/MS) analysis.
LC-MS/MS Analysis
The LC-MS/MS analysis was performed as previously described (28). Briefly, the samples (5 μL) were injected onto a 0.10 × 250 mm ReproSil-Pur 120 C18-AQ 1.9 μm beads analytical column that was prepared in house, using pressure of 800 bar. The peptides were then eluted at a flow of 0.5 μL/min with an acetonitrile gradient. The gradient elution increased from 9 to 34% acetonitrile in water with 1 mL/L formic acid in 50 min. Next, an electrospray potential of 3.5 kV was applied straight to the eluent, through a needle that was equipped to the P777 Upchurch micro cross waste line. Using a Q Exactive HF-X quadrupole-Orbitrap mass spectrometer (Thermo Electron, San Jose, CA, United States), full scan Fourier Transform MS in positive mode between m/z 380 and 1400 were measured. MS/MS scans of the most abundant multiply-charged peaks were recorded in data-dependent mode. The obtained MS/MS data was analyzed using the Andromeda search engine of the MaxQuant software (v1.6.3.4). The Uniprot human protein database was used, together with a database containing the sequences of common contaminants (29). Protein identification and quantification was performed as previously described (30, 31). To calculate the false discovery rate (FDR), MaxQuant created a decoy database of reversed sequences. The FDR cut off used was 0.01. The required peptide length was set to at least seven amino acids, with a maximum of 2 missed cleavages allowed. Protein modifications were set for propionamide (C) (fixed) and oxidation (M) (variable). Contaminants (e.g., keratins, trypsin) were removed from the set of identified proteins, as well as the proteins that were detected in less than half of our samples.
BSSL Activity
Bile salt-stimulated lipase activity was determined according to Krewinkel et al. (32), with minor modifications. This fluorometric assay allows the determination of lipase activity in DHM by utilizing the synthetic substrates 4-methylumbelliferyl butyrate (4-MUB) and 4-methylumbelliferyl laurate (4-MUL). Milk samples were first skimmed as described in section 2.4 and were then preincubated at 40°C for 3 min, under shaking (800 rpm) in a ThermoMixer (SmartBlock 1.5 mL, Eppendorf, Hamburg, Germany). The conversion of the added substrate was stopped by the addition of a stop solution containing GuHCl (8 M) and HCl (1 M) in water. Next, a neutralizing solution with Bis-tris (1 M), NaOH (0.85 M), and EDTA (0.25 M) in water was added to clarify the samples. The fluorescence was then measured with a fluorimeter (excitation 355 nm, emission 460 nm).
DHM Bacteriostatic Capacity
To evaluate the effect of processing on the bacteriostatic capacity of DHM, the growth rate of E. coli and S. aureus, which are known to be sensitive to these proteins was characterized (5, 33, 34). Bacterial pellets of E. coli K12 (DSM 498) and S. aureus (ATCC6538, American Type Culture Collection, Manassas, VA, United States) were prepared as described in section 2.3 and were dissolved in peptone physiological salt solutions (PFZ; Tritium Microbiology, Netherlands). After determining the optical density (OD) with a spectrophotometer (Cary 50 UV–Visible Spectrophotometer, Agilent Technologies, United States) for bacterial culture standardization (35, 36), E. coli and S. aureus cultures were diluted and inoculated into untreated samples and samples that were previously treated with HPP, UV-C, TUS, and HoP, to a final concentration of around 103 CFU/mL. This inoculation level was selected because higher levels may overcome the ability of the milk to inhibit the growth of E. coli and S. aureus (37). Next, the samples inoculated with E. coli and S. aureus were incubated at 37°C for 2 and 4 h, respectively. All samples were then plated in duplicate onto VRBGA (selective for E. coli) and MSA (selective for S. aureus) and the plates were subsequently incubated overnight at 37°C. The amount of inoculated DHM sample plated was 1 and 0.1 mL for VRBGA and MSA, respectively. The bacterial concentrations were then determined by colony counting (CFU/mL). The growth rate per hour was calculated as , were Nt = bacterial counts after 2 or 4 h incubation, N0 = bacterial counts immediately after inoculation and t = incubation time.
Data Analysis
Data analysis and visualization were performed using GraphPad Prism software 8.0 (GraphPad Inc., La Jolla, CA, United States). For multiple comparisons of means and to determine significant differences among the treatments, ANOVA and Tukey’s HSD for post-hoc testing were performed. Protein retentions (% compared to untreated) were calculated as the ratio of the concentration after each treatment to the concentration of untreated samples, multiplied by 100. Perseus software v.1.6.2.1 was used to analyze the intensity based absolute quantitation (iBAQ) values that were determined by MaxQuant. These values refer to the sum of all peptide peak intensities divided by the number of theoretically generated tryptic peptides and are considered as a good indicator for the absolute protein concentration (30). To indicate significant differences in the DHM proteome after the different treatments, student’s t-tests were performed in Perseus after imputation of missing values, using permutation-based false discovery rate (FDR) correction. The cluster analysis was performed and visualized with the circos.heatmap package in R version 4.1.2 (38) on the imputed log10 scaled IBAQ values. Pearson correlations were also calculated to determine the relationship between the bacterial growth rate and the retention of IgA, LTF, and LYZ. A correlation matrix was created using R version 3.4.0 (38). Significant differences in all analyses were indicated by a p-value < 0.05. Two independent experiments (biological replicates) were conducted and all analyses were performed in technical duplicates. Data are presented as mean ± standard deviation of the two independent experiments.
Results
Bacterial Inactivation
The bacterial count reductions after HPP, UV-C, TUS and HoP are presented in Table 1. A >7.8–log10 inactivation of E. cloacae and S. epidermidis was obtained after all the different HPP conditions tested. The same inactivation was obtained after HoP for all tested bacterial strains. A UV-C dosage of 4,863 J/L was the only UV-C dosage effective in causing a >5-log10 reduction of E. cloacae, E. coli K12 and S. epidermidis counts. All TUS conditions tested were able to achieve a >5-log10 reduction for all tested bacterial strains.
TABLE 1.
Reduction in bacterial counts in HoP, HPP, UV-C, and TUS treated DHM samples.
| Methods | Parameters | Log10 reduction (CFU/mL), mean ± SD |
||
| Enterobacter cloacae | Staphylococcus epidermidis | E. coli K12 | ||
| HoP | >7.8 (below the detection limit) | |||
| HPP | 400 MPa, 5–30 min | >7.8 (below the detection limit) | ND* | |
| 500 MPa, 1.5–5 min | >7.8 (below the detection limit) | |||
| 600 MPa, 1.5–5 min | >7.8 (below the detection limit) | |||
| UV-C | 2,430 J/L | 4.25 ± 0.1 | 5.00 ± 0.3 | 4. 36 ± 0.1 |
| 3,645 J/L | 4.64 ± 0.1 | 5.95 ± 0.1 | 5.30 ± 0.3 | |
| 4,863 J/L | 5.78 ± 0.2 | 6.95 ± 0.4 | 6.92 ± 0.1 | |
| TUS | 40 W for 9 min | 6.31 ± 0.4 | 6.07 ± 0.3 | 6.40 ± 0.1 |
| 60 W for 6 min | 6.63 ± 0.6 | 6.50 ± 0.5 | 6.73 ± 0.3 | |
| 60 W for 9 min | 6.52 ± 0.5 | 6.21 ± 0.2 | 6.79 ± 0.4 | |
The results are presented as mean ± standard deviation of two independent experiments and all analyses were performed in technical duplicate. *ND, Not determined. The detection limit was 0 log10 CFU/mL for E. coli and E. cloacae and 1 log10 CFU/mL for S. aureus.
Protein Damage
Native Milk Serum Protein Concentration
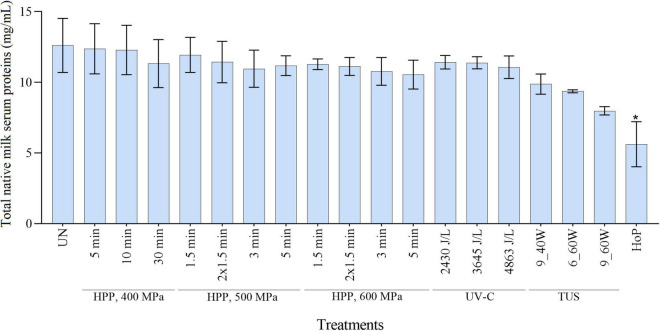
The total native milk serum protein concentration after HPP, UV-C, TUS, and HoP is shown in Figure 2. When compared to the untreated samples, a significant decrease in protein concentration was observed only after HoP (p < 0.05).
FIGURE 2.
Native milk serum protein concentration as determined with a BCA assay. The results are presented as mean ± standard deviation of two independent experiments and all analyses were performed in technical duplicate. UN represents the untreated values. *Indicates significant differences to untreated samples (p < 0.05).
Effects of Processing on the DHM Proteome
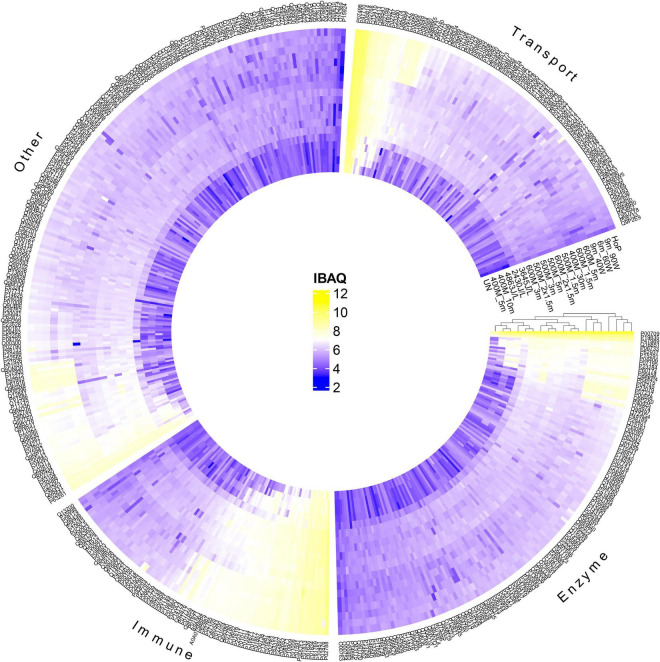
To further evaluate the effect of the different treatments on the native milk serum proteins, a detailed characterization of the DHM proteome was obtained, by means of LC-MS/MS. Next, a clustered heat map based on the obtained iBAQ values was created, for visualization of the protein profile of the different treated DHM samples (Figure 3). Samples with similar protein patterns are clustered together. The samples formed two main clusters; one that consists of the untreated, the HPP and the UV-C samples and one that includes the TUS and HoP samples. This clustering pattern indicates that HoP and TUS affect the DHM proteome the most, and similarly. In addition, the separation of the HPP treatments with the highest intensities (600MPa for 3 and 5 min) in the cluster with the other samples suggests that these most intense HPP treatments have a larger effect on the proteome compare to the less intense HPP and UV/C treatments.
FIGURE 3.
Hierarchical cluster analysis and heatmap showing the changes in the protein profile after HPP, UV-C, TUS, and HoP, based on iBAQ values (log10 scale from 2 to 12 according to color bar). Proteins are labeled by their UniProt ID. Functional categories (enzyme, immune, transport, and other) were based on GO annotation of biological function. Two independent experiments (biological replicates) were performed while all analyses were performed in technical duplicate for each sample. UN represents the untreated values. HPP; 400 MPa for 5, 10, and 30 min, 500 MPa for 1.5, 2 × 1.5, 3, and 5 min, 600 MPa for 1.5, 2 × 1.5, 3, and 5 min, UV-C; 2430J/L, 3645J/L, and 4863L/L,TUS; 9_40W, 6_60W, and 9_60W.
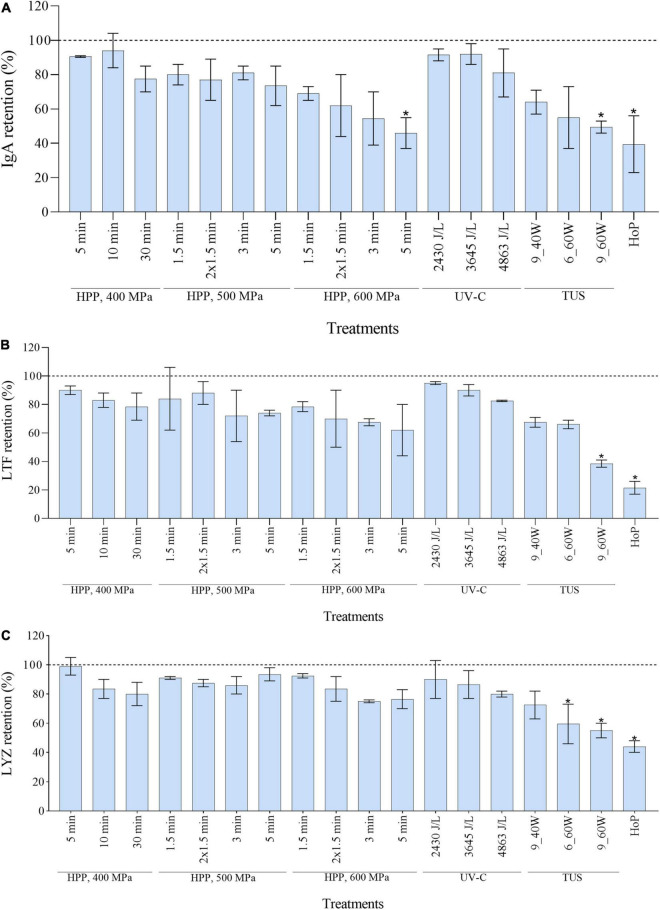
Retention of IgA, LTF and LYZ After Processing
Of all the proteins analyzed by LC-MS/MS as shown in Figure 3, IgA, LTF, and LYZ are of importance due to their bacteriostatic activity. The retention values of the three proteins, as calculated from the LC-MS/MS data, were significantly reduced after HoP (p < 0.05), with only 40, 22, and 44% of IgA, LTF, and LYZ levels being retained after HoP, respectively (Figure 4). At the same time, none of the HPP treatments tested caused a significant reduction in LTF and LYZ levels. Furthermore, no IgA losses occurred after HPP at 400 and 500 MPa, regardless of the treatment time. When the pressure intensity increased (600 MPa), a treatment of 3 min caused a major decrease (55% IgA retention), although statistically non-significant, while a treatment of 5 min caused a statistically significant decrease (47% IgA retention). None of the applied UV-C dosages caused a significant reduction on the levels of the three proteins. The retentions of the three studied proteins showed a decreasing tendency with increasing TUS intensity and exposure time. After 6 min at 60 W, LYZ levels were significantly reduced, while after 9 min at 60 W, all three proteins were significantly reduced (p < 0.05).
FIGURE 4.
Effect of HPP, UV-C, TUS and HoP on the IgA (A), LTF (B), and LYZ (C) content. The retention values were calculated based on the iBAQ intensities obtained by LC-MS/MS analysis. Untreated values were set at 100% (dotted line). The results are presented as mean ± standard deviation of two independent experiments and all analyses were performed in technical duplicate.*Indicates significant differences to untreated samples (p < 0.05).
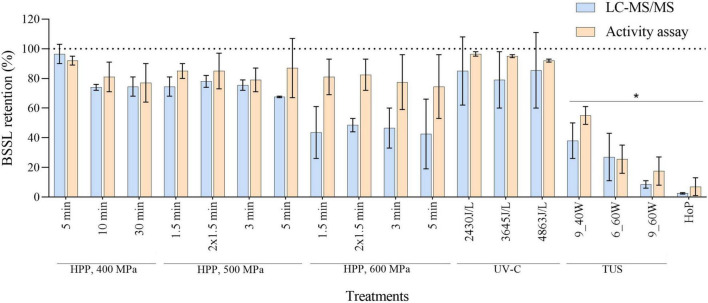
BSSL Retention After Processing
To evaluate whether the different methods affected the BSSL levels and activity, we first determined the BSSL retention, based on the LC-MS/MS results. Then, a specific lipase activity assay was used (as described in section 2.6), and the percentage of BSSL activity retained after the different treatments was compared to the LC-MS/MS values (Figure 5). Although the majority of the values obtained by the activity assay were higher than the LC-MS/MS values, no significant differences between the retention values from both analytical methods were observed (p > 0.05). After HoP, BSSL was almost completely diminished (LC-MS/MS, 3% and activity assay, 7%). On the contrary, the different HPP and UV-C treatments applied in this study did not lead to a significant decrease. However, BSSL retention decreased significantly after TUS, regardless of the intensity and the exposure time applied.
FIGURE 5.
BSSL retention after HPP, UV-C, TUS, and HoP, based on iBAQ intensities and a lipase activity assay. Untreated values were set at 100% (dotted line). The results are presented as mean ± standard deviation of two independent experiments and all analyses were performed in technical duplicate.*Indicates significant differences to untreated samples (p < 0.05).
Retention of DHM Bacteriostatic Properties After Processing
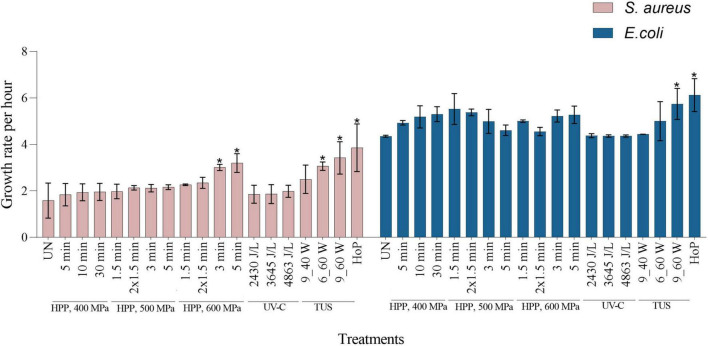
To evaluate whether the bacteriostatic capacity of DHM was retained after the different treatments, the growth rate of S. aureus and E. coli was characterized, in both untreated and treated samples (Figure 6). Untreated samples showed the lowest bacterial growth rate, thus the highest inhibition rate for both strains (1.6 ± 0.75 and 4.4 ± 0.04-fold per hour, for S. aureus and E. coli, respectively). In contrast, the highest bacterial growth rate was observed after HoP (3.9 ± 1.02 and 6.1 ± 0.70-fold per hour, for S. aureus and E. coli, respectively, p < 0.05), which indicates a significant decrease in the DHM bacteriostatic capacity. When compared to the untreated samples, E. coli growth rate after HPP was not significantly different, while a significant increase in S. aureus growth rate was only observed at the highest intensities (3.0 ± 0.13 and 3.2 ± 0.40-fold per hour, after 600 MPa for 3 min and 600 MPa for 5min, respectively, p < 0.05). After UV-C, as well as after TUS for 9 min at 40 W, bacterial growth rates were not significantly different to those of untreated samples. The growth rate of S. aureus was significantly increased after 6 min at 60 W (3.0 ± 0.18-fold per hour), while after 9 min at the same intensity, the growth rates of both strains were significantly increased (S. aureus, 3.4 ± 0.69 and E. coli, 5.7 ± 0.66-fold per hour, p < 0.05).
FIGURE 6.
Growth rate per hour of S. aureus and E. coli in untreated, HPP, UV-C, TUS, and HoP DHM samples. The results are presented as mean ± standard deviation of two independent experiments and all analyses were performed in technical duplicate. UN represents the untreated values. *Indicates significant differences to untreated samples (p < 0.05).
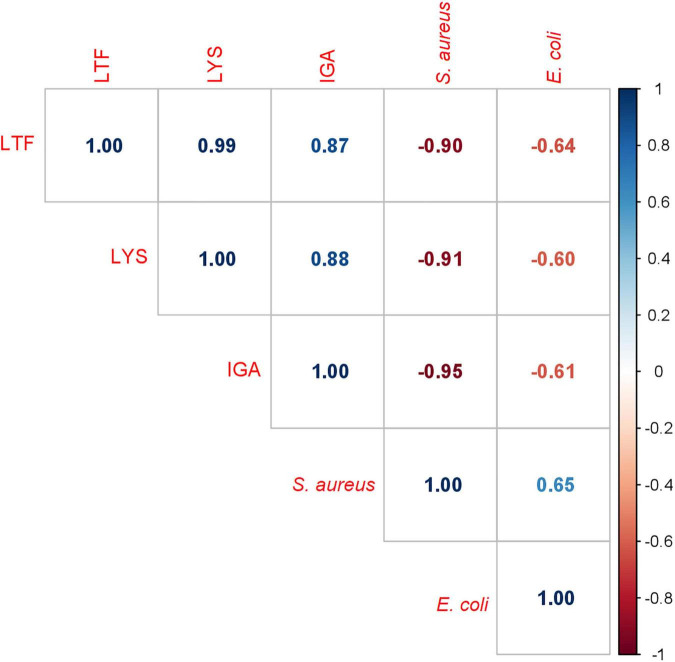
When the bacterial growth increased while the IgA, LTF, and/or LYZ levels decreased, a negative correlation could be expected between the S. aureus and E. coli growth rate and the retentions of these three antimicrobial proteins. To confirm this bacteriostatic activity, a correlation matrix was created (Figure 7). Figure 7 shows that S. aureus growth was strongly negatively correlated with the levels of these three antimicrobial proteins (IgA, r = −0.95, LTF, r = −0.90, and LYZ, r = −0.91, p < 0.05). Although the correlation between the inhibition of E. coli growth and the concentrations of these proteins was weaker, it was still significant (IgA, r = −0.61, LTF, r = −0.64, and LYZ, r = −0.64, p < 0.05).
FIGURE 7.
Correlation matrix of S. aureus and E. coli growth rates and IgA, LTF and LYZ iBAQ values. Each box contains an r value (Pearson correlation coefficient).
Discussion
The present study demonstrates that the tested HPP and UV-C conditions preserved the levels and functionality of key DHM bioactive components better than HoP, while at the same time ensured sufficient microbial inactivation. Although the tested TUS conditions resulted in similar bacterial inactivation, this method was generally less efficient in retaining the DHM bioactive components.
Effects of Processing on Bacteria Inactivation
All different HPP intensity-time combinations were able to achieve a reduction >7.8-log10 CFU/mL of E. cloacae and S. epidermidis counts (Table 1), even at the lowest condition of 400 MPa for 5 min. Similarly, coliform and Enterobacteriaceae counts were reduced to undetectable levels after 5 min at pressures of 400–600 MPa (39, 40). Viazis et al. (41) also found a ≥6-log10 reduction of E. coli and S. aureus counts after 400 MPa for 30 min, while an 8-log10 reduction of Listeria monocytogenes and Streptococcus agalactiae counts was already achieved after ≤4 min at the same intensity. Although some S. aureus strains were found to be more pressure resistant, at higher pressures intensities significant reductions were achieved (500–600 MPa, or 400 MPa for 30 min for >5-log10 reduction) (41–43).
Only the highest UV-C dosage of 4,863 J/L was capable of achieving a >5-log10 CFU/mL reduction of E. cloacae, S. epidermidis, and E. coli K12 counts (Table 1). Christen et al. (16) showed similar reductions of S. epidermidis, E. cloacae, Bacillus cereus, and E. coli counts at 4,863 J/L, and according to Li et al. (44), the same dosage reduced DHM bacterial counts as effectively as HoP. Martysiak-Żurowska et al. (17) reported a 5-log10 reduction of S. aureus and E. coli K12 counts already at much lower dosages (400 and 700 J/L, respectively). The differences in the experimental set up (e.g., actual UV-C output power, milk flow around lamp, milk compositional differences) used in these studies may account for the observed variation.
All TUS treatments tested in this study achieved a >6-log10 reduction of E. cloacae, S. epidermidis, and E. coli K12 counts (Table 1). Similar results were observed by Czank et al. (45), who found that the decimal reduction time of S. epidermidis and E. coli K12 was 1.74 and 2.08 min, respectively after TUS at 60 W and 45°C.
In the current study, all HPP treatments, the highest UV-C dosage (4,863 J/L), and all three TUS treatments were capable of reducing bacterial counts in DHM samples >5-log10, thus meeting the requirements of the human milk banking guidelines.
Effects of Processing on the DHM Proteome, IgA, LTF, LYZ, and BSSL Levels and Activity
In order to get a full overview of the impact of the different processing methods on the DHM proteome, the native milk serum protein levels were assessed by means of the BCA assay and LC-MS/MS. With regard to HoP, our results (Figures 2, 3) confirm the major decrease in native protein abundance (46). Moreover, the proteomic analysis of the differently treated DHM samples showed that HoP affected the native serum protein levels the most (Figure 3), an outcome that supported the results of the BCA assay (Figure 2). Of all the treatments tested in this study, HoP caused the highest reduction in IgA, LTF and LYZ levels (Figure 4), which is consistent with the losses previously reported (11, 47). As expected, the highest reduction in bacteriostatic capacity was also documented after HoP (Figure 6), which is in line with previous studies (5). These results can be attributed to the thermally induced denaturation and aggregation during HoP, which caused a loss in the functionality of these bioactive components (45). As BSSL is a heat-labile enzyme that inactivates at temperatures around 45°C (48), the complete loss of BSSL that was observed in this study was to be expected (11, 16, 21, 49).
We showed that HPP treatments at intensities of 400, 500 MPa and of short duration (<3 min) at 600 MPa, preserve the levels of the three main antimicrobial proteins in DHM (Figure 4). Furthermore, our proteomic analysis showed that HPP treatments at these intensities had only minimal effects on the levels of the native milk serum protein levels, while more intense conditions showed a larger change in these levels. The enhanced denaturation observed after HPP at 600 MPa at longer treatment times might be explained by the fact that HPP can cause native conformation unfolding and formation of inter/intra protein complexes, where these changes may only be reversible at lower treatment intensities (40, 50, 51). Moreover, increased protein denaturation has been observed at higher pressures and holding times, suggesting an effect of both pressure and holding time (52, 53). Other studies have also reported significant reductions in IgA levels after HPP at 600 MPa for >2.5 min (40, 54–57), while after treatment at 400–500 MPa, only 0–15% of IgA losses were documented (15, 39, 40). Our data showed that treatments of 400, 500, and 600 MPa retained LTF levels within a range of 62–90%, as previously described (52, 54, 58). In addition, none of the HPP treatments tested had an effect on LYZ levels, as well as on BSSL levels and activity, in line with previous studies (13, 15, 49, 56, 57, 59, 60). Since pressure and temperature have a synergistic effect on protein denaturation, the low initial temperature (4°C) and the limited temperature increase during HPP treatment (around 14°C at the most intense pressure of 600 MPa), may have additionally contributed to the improved protein retentions observed (61–63).
As a non-thermal method, UV-C does not inactivate pathogens by thermally-induced protein denaturation and aggregation, but by DNA disruption, that often results from pyrimidine dimerization (44). Hence, this method may effectively reduce bacterial counts in HM without causing detrimental losses of bioactive components (5, 44). All UV-C treatments in our study preserved both the levels and the bioactivity of IgA, LTF, and LYZ. In fact, the three antimicrobial proteins were retained within a range of 80–95% after UV-C treatment, while the bacteriostatic activity was similar to that observed in untreated HM (Figures 4, 6). The clustering pattern observed for the three dosages additionally suggests that the changes occurring in the DHM proteome after UV-C are minimal (Figure 3). Christen et al. (5) also reported retention of IgA, LTF, and LYZ within a range of 75–95% and no loss of bacteriostatic activity, after treatments of the same intensity. As it is possible that UV-C induces protein photo-oxidation (direct or indirect) the authors speculated that the reductions (∼25%) in LYZ levels at the highest dosage could be attributed to the fact that LYZ contains several amino acid residues that may absorb photons at this wavelength (5). With respect to the BSSL levels and activity, none of the dosages in this study showed a significant reduction compared to untreated milk (Figure 5), supporting previous findings (16, 44, 60).
After TUS at the highest ultrasound power (60 W) for the longest exposure time (9 min), the IgA, LTF, LYZ and BSSL levels and bioactivity retained were comparable to those after HoP (Figure 4). Similar reductions have been previously reported after 10 min at 60 W and 45°C (64). Treatments at 60 W for a shorter time (6 min) caused significant reductions in LYZ and BSSL levels and bioactivity, whereas at 40 W for 9 min, only BSSL was significantly reduced. Our findings suggest that at constant exposure times, higher ultrasound power will result in more protein damage. In addition, the differences observed when ultrasound energy was held constant (1,080 kJ/L after 9 min at 40 W or 6 min at 60 W), suggest that higher ultrasound power rather than the longer exposure time may lead to more protein damage. The impact of those treatments on the DHM proteome was confirmed by the hierarchical clustering analysis, that showed a similar pattern of protein damage to HoP-treated DHM (Fig. 3). These results could be further attributed to the temperature increase documented during such treatments, and to the denaturation that might be caused due to the shear effects generated during ultrasound cavitation (64, 65).
Specifically for BSSL, published reports have used both activity assays and quantification techniques (e.g., ELISA) to evaluate its retention (16, 66). Since the loss of protein as measured through LC-MS/MS approaches or ELISA assays is not necessarily correlated to loss of function (67), we compared the retention of the BSSL levels (LC-MS/MS) and the BSSL activity (activity assay) after the different treatments. Our findings suggest that although the activity assay produced higher values than LC-MS/MS values, no significant differences were observed between the results of the two methods.
Lastly, the correlations in this study between native IgA, LTF, and LYZ levels and the growth rate of bacteria sensitive to these proteins, suggest that these proteins may significantly contribute in limiting their growth (Figure 7). However, as DHM contains large numbers of antimicrobial components, the exact proportion of bacteriostatic activity attributed to those proteins is difficult to determine. These findings underline the importance of using complementary assays to determine protein levels and functionality, to accurately assess the effects of different processing methods. In this regard, many analytical techniques are available that can be used for future, more detailed characterization of such HM components and their functionality (28, 46, 68). In addition, the outcomes of our study suggest that certain proteins may be more sensitive to specific non-thermal treatments than others, due to the different underlying mechanisms of these treatments. Our proteomic analysis, for example, showed that even though TUS and high HPP intensities both cause protein damage, they cluster separately (Figure 3), indicating that different underlying mechanisms lead to a different profile of resulting protein damage. In summary, HPP at 400, 500, and 600 MPa for <3 min, as well as UV-C at 4,863 J/L, may be promising alternatives to HoP, when considering the sufficient microbial inactivation achieved and the improved outcomes on the preservation of important HM bioactive components.
Conclusion
Although HoP is the method currently recommended for DHM processing, the results of the current study indicate that non-thermal methods such as HPP and UV-C may offer improved retention of key DHM bioactive components, while at the same time effectively reduce bacterial contaminants. These findings are of particular importance in the context of providing DHM to high-risk infants. However, before full-scale implementation of these technologies in a human milk bank setting, additional studies are needed to investigate both viral inactivation and the clinical significance of this study’s observations, especially with regards to growth rates and health status of infants fed DHM treated with HPP or UV-C.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: ProteomeXchange Consortium via the PRIDE [Vizcaíno 2016] partner repository and PXD031216.
Author Contributions
EK: conceptualization, methodology, investigation, and writing—original draft preparation. BS, RV, and JG: conceptualization, supervision, investigation, resources, and writing—reviewing and editing. SB: methodology, validation, and writing—reviewing and editing. RT and HB: methodology and writing—reviewing and editing. KH: conceptualization, supervision, investigation, resources, validation, and writing—reviewing and editing. All authors contributed to the article and approved the submitted version.
Conflict of Interest
JG is the founder and director of the Dutch National Human Milk Bank and member of the Dutch National Health Council. BS is as Science Director of Human Milk Research & Analytical Sciences an employee of Danone Nutricia Research, Utrecht, Netherlands. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank the Dutch Human Milk Bank for providing DHM samples. Additionally, we gratefully acknowledge the support of Jiska Oostveen, Mirte Doomen, Roosje de Jong, Jaap Hulstein, Louise Nederhoff, and Mariette Helmond in the experimental execution.
References
- 1.Ballard O, Morrow AL. Human milk composition. nutrients and bioactive factors. Pediatr Clin North Am. (2013) 60:49–74. 10.1016/j.pcl.2012.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu J, Dingess KA. The functional power of the human milk proteome. Nutrients. (2019) 11:1834. 10.3390/nu11081834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ellison RT, Giehl TJ. Killing of gram-negative bacteria by lactofernn and lysozyme. J Clin Invest. (1991) 88:1080–91. 10.1172/jci115407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lönnerdal B. Nutritional and physiologic significance of human milk proteins. Am J Clin Nutr. (2003) 77:1537S–43S. 10.1093/ajcn/77.6.1537s [DOI] [PubMed] [Google Scholar]
- 5.Christen L, Lai CT, Hartmann B, Hartmann PE, Geddes DT. The effect of UV-C pasteurization on bacteriostatic properties and immunological proteins of donor human milk. PLoS One. (2013) 8:e85867. 10.1371/journal.pone.0085867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monaco MH, Kim J, Donovan SM. Human milk: composition and nutritional value. value. 1st ed. In: Caballero B, Finglas PM, Toldrá F. editors. Encyclopedia of Food and Health. Amsterdam: Elsevier Ltd; (2015). 10.1016/B978-0-12-384947-2.00413-X. [DOI] [Google Scholar]
- 7.Arslanoglu S, Corpeleijn W, Moro G, Braegger C, Campoy C, Colomb V, et al. Donor human milk for preterm infants: current evidence and research directions. J Pediatr Gastroenterol Nutr. (2013) 57:535–42. 10.1097/MPG.0b013e3182a3af0a [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. Guidelines on Optimal Feeding of Low Birth-Weight Infants in Low-and Middle-Income Countries. Geneva: WHO; (2011). p. 16–45. [PubMed] [Google Scholar]
- 9.Peila C, Emmerik NE, Giribaldi M, Stahl B, Ruitenberg JE, Van Elburg RM, et al. Human milk processing: a systematic review of innovative techniques to ensure the safety and quality of donor Milk. J Pediatr Gastroenterol Nutr. (2017) 64:353–61. 10.1097/MPG.0000000000001435 [DOI] [PubMed] [Google Scholar]
- 10.Arslanoglu S, Bertino E, Tonetto P, Nisi GDE, Ambruzzi M, Biasini A, et al. Guidelines for the establishment and operation of a donor human milk bank. J Matern Fetal Neonatal Med. (2010) 23(Suppl. 2):1–20. 10.3109/14767058.2010.512414 [DOI] [PubMed] [Google Scholar]
- 11.Peila C, Moro GE, Bertino E, Cavallarin L, Giribaldi M, Giuliani F, et al. The effect of holder pasteurization on nutrients and biologically-active components in donor human milk: a review. Nutrients. (2016) 8:1–19. 10.3390/nu8080477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andersson Y, Sävman K, Bläckberg L, Hernell O. Pasteurization of mother’s own milk reduces fat absorption and growth in preterm infants. Acta Paediatr Int J Paediatr. (2007) 96:1445–9. 10.1111/j.1651-2227.2007.00450.x [DOI] [PubMed] [Google Scholar]
- 13.Wesolowska A, Sinkiewicz-Darol E, Barbarska O, Bernatowicz-Lojko U, Borszewska-Kornacka MK, van Goudoever JB. Innovative techniques of processing human milk to preserve key components. Nutrients. (2019) 11:1–17. 10.3390/nu11051169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Timmermans R, Hayrapetyan H, Vollebregt M, Dijksterhuis J. Comparing thermal inactivation to a combined process of moderate heat and high pressure: effect on ascospores in strawberry puree. Int J Food Microbiol. (2020) 325:108629. 10.1016/j.ijfoodmicro.2020.108629 [DOI] [PubMed] [Google Scholar]
- 15.Viazis S, Farkas BE, Allen JC. Effects of high-pressure processing on immunoglobulin a and lysozyme activity in human milk. J Hum Lactat. (2007) 23:253–61. 10.1177/0890334407303945 [DOI] [Google Scholar]
- 16.Christen L, Lai CT, Hartmann B, Hartmann PE, Geddes DT. Ultraviolet-C irradiation: a novel pasteurization method for donor human milk. PLoS One. (2013) 8:e68120. 10.1371/journal.pone.0068120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martysiak-Żurowska D, Puta M, Kotarska J, Cybula K, Malinowska-Pańczyk E, Kołodziejska I. The effect of UV-C irradiation on lipids and selected biologically active compounds in human milk. Int Dairy J. (2017) 66:42–8. 10.1016/j.idairyj.2016.10.009 [DOI] [Google Scholar]
- 18.Christen L, Lai CT, Hartmann PE. Ultrasonication and the quality of human milk: variation of power and time of exposure. J Dairy Res. (2012) 79:361–6. 10.1017/S0022029912000246 [DOI] [PubMed] [Google Scholar]
- 19.Anaya-Esparza LM, Velázquez-Estrada RM, Roig AX, García-Galindo HS, Sayago-Ayerdi SG, Montalvo-González E. Thermosonication: an alternative processing for fruit and vegetable juices. Trends Food Sci Technol. (2017) 61:26–37. 10.1016/j.tifs.2016.11.020 [DOI] [Google Scholar]
- 20.Moro GE, Billeaud C, Rachel B, Calvo J, Cavallarin L, Christen L, et al. Processing of donor human milk: update and recommendations from the European milk bank association (EMBA). Front Pediatr. (2019) 7:49. 10.3389/fped.2019.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wesolowska A, Brys J, Barbarska O, Strom K, Szymanska-Majchrzak J, Karzel K, et al. Lipid profile, lipase bioactivity, and lipophilic antioxidant content in high pressure processed donor human milk. Nutrients. (2019) 11:1–15. 10.3390/nu11091972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Waard M, Mank E, van Dijk K, Schoonderwoerd A, van Goudoever JB. Holder-pasteurized human donor milk: how long can it be preserved? J Pediatr Gastroenterol Nutr. (2018) 66:10–2. 10.1097/MPG.0000000000001782 [DOI] [PubMed] [Google Scholar]
- 23.Cornell A. HPP Process for Dairy Foods (p. WO2017054052A1). Geneva: World Intellectual Property Organization; (2015). [Google Scholar]
- 24.Zhang W, Liu Y, Li Z, Xu S, Zhang J, Hettinga K, et al. Effects of microfiltration combined with ultrasonication on shelf life and bioactive protein of skim milk. Ultrason Sonochem. (2021) 77:105668. 10.1016/j.ultsonch.2021.105668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Law AJR, Leaver J. Effect of pH on the thermal denaturation of whey proteins in milk. J Agric Food Chem. (2000) 48:672–9. 10.1021/jf981302b [DOI] [PubMed] [Google Scholar]
- 26.Hettinga K, van Valenberg H, de Vries S, Boeren S, van Hooijdonk T, van Arendonk J, et al. The host defense proteome of human and bovine milk. PLoS One. (2011) 6:e19433. 10.1371/journal.pone.0019433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kontopodi E, Boeren S, Stahl B, van Goudoever JB. High-temperature short-time preserves human milk’s bioactive proteins and their function better than pasteurization techniques with long processing times. Front Pediatr. (2022) 9:798609. 10.3389/fped.2021.798609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y, Xiong L, Kontopodi E, Boeren S, Zhang L, Zhou P, et al. Changes in the milk serum proteome after thermal and non-thermal treatment. Innov Food Sci Emerg Technol. (2020) 66:102544. 10.1016/j.ifset.2020.102544 [DOI] [Google Scholar]
- 29.Cox J, Mann M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat Biotechnol. (2008) 26:1367–72. 10.1038/nbt.1511 [DOI] [PubMed] [Google Scholar]
- 30.Hettinga K, Reina FM, Boeren S, Zhang L, Koppelman GH, Postma DS, et al. Difference in the breast milk proteome between allergic and non-allergic mothers. PLoS One. (2015) 10:e0122234. 10.1371/journal.pone.0122234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang L, van Dijk ADJ, Hettinga K. An interactomics overview of the human and bovine milk proteome over lactation. Proteome Sci. (2017) 15:1–15. 10.1186/s12953-016-0110-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krewinkel M, Baur C, Kranz B, von Neubeck M, Wenning M, Scherer S, et al. A sensitive and robust method for direct determination of lipolytic activity in natural milk environment. Food Anal Methods. (2016) 9:646–55. 10.1007/s12161-015-0233-4 [DOI] [Google Scholar]
- 33.Leitch EC, Willcox MDP. Elucidation of the antistaphylococcal action of lactoferrin and lysozyme. J Med Microbiol. (1999) 48:867–71. 10.1099/00222615-48-9-867 [DOI] [PubMed] [Google Scholar]
- 34.Lönnerdal B. Bioactive proteins in human milk: health, nutrition, and implications for infant formulas. J Pediatr. (2016) 173:S4–9. 10.1016/j.jpeds.2016.02.070 [DOI] [PubMed] [Google Scholar]
- 35.Campbell J. High-throughput assessment of bacterial growth inhibition by optical density measurements. Curr Protoc Chem Biol. (2010) 2:195–208. 10.1002/9780470559277.ch100115 [DOI] [PubMed] [Google Scholar]
- 36.Monod J. The growth of bacterial cultures. Ann Rev Microbiol. (1949) 3:371–94. [Google Scholar]
- 37.Spanu C, Scarano C, Ibba M, Pala C, Spanu V, De Santis EPL. Microbiological challenge testing for Listeria monocytogenes in ready-to-eat food: a practical approach. Ital J Food Saf. (2014) 3:231–7. 10.4081/ijfs.2014.4518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.R Core Team. A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; (2018). 10.1111/j.1365-2699.2008.01969.x [DOI] [Google Scholar]
- 39.Irazusta A, Rodríguez-Camejo C, Jorcin S, Puyol A, Fazio L, Arias F, et al. High-pressure homogenization and high hydrostatic pressure processing of human milk: preservation of immunological components for human milk banks. J Dairy Sci. (2020) 103:5978–91. 10.3168/jds.2019-17569 [DOI] [PubMed] [Google Scholar]
- 40.Permanyer M, Castellote C, Ramírez-Santana C, Audí C, Pérez-Cano FJ, Castell M, et al. Maintenance of breast milk immunoglobulin A after high-pressure processing. J Dairy Sci. (2010) 93:877–83. 10.3168/jds.2009-2643 [DOI] [PubMed] [Google Scholar]
- 41.Viazis S, Farkas BE, Jaykus LA. Inactivation of bacterial pathogens in human milk by high-pressure processing. J Food Protect. (2008) 71:109–18. 10.4315/0362-028X-71.1.109 [DOI] [PubMed] [Google Scholar]
- 42.Rocha-Pimienta J, Martillanes S, Ramírez R, Garcia-Parra J, Delgado-Adamez J. Bacillus cereus spores and Staphylococcus aureus sub. aureus vegetative cells inactivation in human milk by high-pressure processing. Food Control. (2020) 113:107212. 10.1016/j.foodcont.2020.107212 [DOI] [Google Scholar]
- 43.Windyga B, Rutkowska M, Sokołowska B, Skąpska S, Wesołowska A, Wilińska M, et al. Inactivation of Staphylococcus aureus and native microflora in human milk by high pressure processing. High Press Res. (2015) 35:181–8. 10.1080/08957959.2015.1007972 [DOI] [Google Scholar]
- 44.Li Y, Nguyen DN, de Waard M, Christensen L, Zhou P, Jiang P, et al. Pasteurization procedures for donor human milk affect body growth, intestinal structure, and resistance against bacterial infections in preterm pigs. J Nutr. (2017) 147:1121–30. 10.3945/jn.116.244822 [DOI] [PubMed] [Google Scholar]
- 45.Czank C, Prime DK, Hartmann BEN, Simmer K, Hartmann PE, Biochemistry D. Retention of the immunological proteins of pasteurized human milk in relation to pasteurizer design and practice. Pediatr Res. (2009) 66:374–9. 10.1203/PDR.0b013e3181b4554a [DOI] [PubMed] [Google Scholar]
- 46.Baro C, Giribaldi M, Arslanoglu S, Giuffrida MG, Dellavalle G, Conti A, et al. Effect of two pasteurization methods on the protein content of human milk. Front Biosci. (2011) 3E:818–29. 10.2741/e289 [DOI] [PubMed] [Google Scholar]
- 47.Heiman H, Schanler RJ. Enteral nutrition for premature infants: the role of human milk. Semin Fetal Neonat Med. (2007) 12:26–34. 10.1016/j.siny.2006.10.004 [DOI] [PubMed] [Google Scholar]
- 48.Wardell JM, Wright AJ, Bardsley WG, D’Souza SW. Bile salt–stimulated lipase and esterase activity in human milk after collection, storage, and heating: nutritional implications. Pediatr Res. (1984) 18:382–6. 10.1203/00006450-198404000-00017 [DOI] [PubMed] [Google Scholar]
- 49.Pitino MA, Unger S, Doyen A, Pouliot Y, Aufreiter S, Stone D, et al. High hydrostatic pressure processing better preserves the nutrient and bioactive compound composition of human donor milk. J Nutr. (2019) 149:497–504. 10.1093/jn/nxy302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bogahawaththa D, Buckow R, Chandrapala J, Vasiljevic T. Comparison between thermal pasteurization and high pressure processing of bovine skim milk in relation to denaturation and immunogenicity of native milk proteins. Innov Food Sci Emerg Technol. (2018) 47:301–8. 10.1016/j.ifset.2018.03.016 [DOI] [Google Scholar]
- 51.Bolumar T, Middendorf D, Toepfl S, Heinz V. Structural changes in foods caused by high-pressure processing. In: Balasubramaniam V, Barbosa-Cánovas G, Lelieveld H. editors. High Pressure Processing of Food. Food Engineering Series. New York, NY: Springer; (2016). 10.1007/978-1-4939-3234-4_23 [DOI] [Google Scholar]
- 52.Mayayo C, Montserrat M, Ramos SJ, Martínez-Lorenzo MJ, Calvo M, Sánchez L, et al. Kinetic parameters for high-pressure-induced denaturation of lactoferrin in human milk. Int Dairy J. (2014) 39:246–52. 10.1016/j.idairyj.2014.07.001 [DOI] [Google Scholar]
- 53.Mazri C, Sánchez L, Ramos S, Calvo M. Effect of high-pressure treatment on denaturation of bovine lactoferrin and lactoperoxidase. J Dairy Sci. (2012) 95:549–57. 10.3168/jds.2011-4665 [DOI] [PubMed] [Google Scholar]
- 54.Aceti A, Cavallarin L, Martini S, Giribaldi M, Vitali F, Ambretti S, et al. Effect of alternative pasteurization techniques on human milk’s bioactive proteins. J Pediatr Gastroenterol Nutr. (2020) 70:508–12. 10.1097/MPG.0000000000002598 [DOI] [PubMed] [Google Scholar]
- 55.Contador R, Delgado-Adámez J, Delgado FJ, Cava R, Ramírez R. Effect of thermal pasteurisation or high pressure processing on immunoglobulin and leukocyte contents of human milk. Int Dairy J. (2013) 32:1–5. 10.1016/j.idairyj.2013.03.006 [DOI] [Google Scholar]
- 56.Mayayo C, Ramos SJ, Martínez MJ, Miguel L, Lourdes C, María S. Effect of high pressure and heat treatments on IgA immunoreactivity and lysozyme activity in human milk. Eur Food Res Technol. (2016) 242:891–8. 10.1007/s00217-015-2595-7 [DOI] [Google Scholar]
- 57.Sousa SG, Santos MD, Fidalgo LG, Delgadillo I, Saraiva JA. Effect of thermal pasteurisation and high-pressure processing on immunoglobulin content and lysozyme and lactoperoxidase activity in human colostrum. Food Chem. (2014) 151:79–85. 10.1016/j.foodchem.2013.11.024 [DOI] [PubMed] [Google Scholar]
- 58.Wesolowska A, Sinkiewicz-Darol E, Barbarska O, Strom K, Rutkowska M, Karzel K, et al. New achievements in high-pressure processing to preserve human milk bioactivity. Front Pediatr. (2018) 6:323. 10.3389/fped.2018.00323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Demazeau G, Plumecocq A, Lehours P, Martin P, Couëdelo L, Billeaud C. A new high hydrostatic pressure process to assure the microbial safety of human milk while preserving the biological activity of its main components. Front Public Health. (2018) 6:306. 10.3389/fpubh.2018.00306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Koh J, Victor AF, Howell ML, Yeo JG, Qu Y, Selover B, et al. Bile Salt-stimulated lipase activity in donor breast milk influenced by pasteurization techniques. Front Nutr. (2020) 7:264. 10.3389/fnut.2020.552362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Delgado FJ, Contador R, Álvarez-Barrientos A, Cava R, Delgado-Adámez J, Ramírez R. Effect of high pressure thermal processing on some essential nutrients and immunological components present in breast milk. Innov Food Sci Emerg Technol. (2013) 19:50–6. 10.1016/j.ifset.2013.05.006 [DOI] [Google Scholar]
- 62.Huppertz T, Kelly AL, Fox PF. Effects of high pressure on constituents and properties of milk. Int Dairy J. (2002) 12:561–72. [DOI] [PubMed] [Google Scholar]
- 63.Huppertz T, Fox PF, Kelly AL. High pressure-induced denaturation of a-lactalbumin and b-lactoglobulin in bovine milk and whey: a possible mechanism. J Dairy Res. (2004) 71:489–95. 10.1017/S0022029904000500 [DOI] [PubMed] [Google Scholar]
- 64.Czank C, Simmer K, Hartmann PE. Simultaneous pasteurization and homogenization of human milk by combining heat and ultrasound: effect on milk quality. J Dairy Res. (2010) 77:183–9. 10.1017/S0022029909990483 [DOI] [PubMed] [Google Scholar]
- 65.Shanmugam A, Chandrapala J, Ashokkumar M. The effect of ultrasound on the physical and functional properties of skim milk. Innov Food Sci Emerg Technol. (2012) 16:251–8. 10.1016/j.ifset.2012.06.005 [DOI] [Google Scholar]
- 66.Dussault N, Cayer MP, Landry P, de Grandmont MJ, Cloutier M, Thibault L, et al. Comparison of the effect of holder pasteurization and high-pressure processing on human milk bacterial load and bioactive factors preservation. J Pediatr Gastroenterol Nutr. (2021) 72:756–62. 10.1097/MPG.0000000000003065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sha L, Zhou S, Xi Y, Li R, Li X. The level of bile salt-stimulated lipase in the milk of Chinese women and its association with maternal BMI. J Biomed Res. (2019) 34:122–8. 10.7555/JBR.33.20180107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dingess KA, van Dam P, Zhu J, Mank M, Knipping K, Heck AJR. Optimization of a human milk – directed quantitative sIgA ELISA method substantiated by mass spectrometry. Anal Bioanal Chem. (2021) 413:5037–49. 10.1007/s00216-021-03468-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: ProteomeXchange Consortium via the PRIDE [Vizcaíno 2016] partner repository and PXD031216.