Abstract
COVID-19-associated pulmonary mucormycosis (CAPM) remains an underdiagnosed entity. Using a modified Delphi method, we have formulated a consensus statement for the diagnosis and management of CAPM. We selected 26 experts from various disciplines who are involved in managing CAPM. Three rounds of the Delphi process were held to reach consensus (≥70% agreement or disagreement) or dissensus. A consensus was achieved for 84 of the 89 statements. Pulmonary mucormycosis occurring within 3 months of COVID-19 diagnosis was labelled CAPM and classified further as proven, probable, and possible. We recommend flexible bronchoscopy to enable early diagnosis. The experts proposed definitions to categorise dual infections with aspergillosis and mucormycosis in patients with COVID-19. We recommend liposomal amphotericin B (5 mg/kg per day) and early surgery as central to the management of mucormycosis in patients with COVID-19. We recommend response assessment at 4–6 weeks using clinical and imaging parameters. Posaconazole or isavuconazole was recommended as maintenance therapy following initial response, but no consensus was reached for the duration of treatment. In patients with stable or progressive disease, the experts recommended salvage therapy with posaconazole or isavuconazole. CAPM is a rare but under-reported complication of COVID-19. Although we have proposed recommendations for defining, diagnosing, and managing CAPM, more extensive research is required.
Introduction
The COVID-19 pandemic precipitated an epidemic of mucormycosis worldwide, especially in India.1 Traditionally, the site of involvement of mucormycosis is related to the underlying predisposing factors. Rhino-orbital mucormycosis occurs in uncontrolled diabetes, whereas pulmonary mucormycosis is seen in patients with haematological malignancy and transplant recipients.2 During the COVID-19-associated mucormycosis (CAM) outbreak, rhino-orbital mucormycosis was the most common manifestation, followed by pulmonary mucormycosis.3, 4, 5 Among the various risk factors for rhino-orbital mucormycosis and pulmonary mucormycosis, uncontrolled diabetes overshadowed all others.3 In two large multicentre cohort studies from India, pulmonary mucormycosis accounted for 13·3% of the total patients with mucormycosis before the COVID-19 pandemic, and 8·6% of the total patients with mucormycosis during the COVID-19 pandemic.3, 6 The lower proportion of patients with pulmonary mucormycosis during the CAM outbreak could be due to the difficulty in diagnosis of and little awareness of pulmonary mucormycosis.7 Often, pulmonary mucormycosis is either not suspected or remains undiagnosed (due to inadequate infrastructure), despite clinical suspicion. The disruption of diagnostic and clinical services during the COVID-19 pandemic further compounded the difficulties in diagnosing COVID-19-associated pulmonary mucormycosis (CAPM).8, 9 Although there are global guidelines for the management of mucormycosis,10, 11 there is no clear guidance on the diagnosis and treatment of pulmonary mucormycosis, including CAPM. We framed the current consensus statement to address the diagnosis and management of CAPM and to identify the knowledge gaps in this area.
Methods
We formed a CAPM clinical practice guideline group (CAPM-GG), including experts from the Fungal Infection Study Forum and the Academy of Pulmonary Sciences in India. We selected experts, with specific interest in mucormycosis who were actively involved in managing CAPM and pulmonary mucormycosis, from various disciplines, including pulmonary medicine, infectious diseases, clinical mycology, pathology, radiodiagnosis, and thoracic surgery to be part of CAPM-GG. At the outset, the experts were briefed on the objectives of the CAPM-GG and the Delphi process (appendix p 5). For the systematic review, two authors (RA and VM) searched PubMed and Embase databases (from inception to Sept 25, 2021) using the search terms: (“COVID” OR “SARS-CoV” OR “coronavirus”) AND (mucor* OR “zygomycosis”). The references obtained from the search were imported into a reference manager software. Our search retrieved 306 articles. We excluded abstracts, articles in a language other than English, and animal studies. After excluding duplicate citations, we reviewed 236 articles in detail (appendix pp 9–25). We reviewed the articles reporting cases of CAPM, relevant review articles, large series of CAM, and our personal files to identify the questions to be addressed (appendix pp 26–30). On the basis of the literature review, three authors (VM, RA, and AC) formulated the initial questions. The questions were circulated by e-mail, and additional questions were invited from the CAPM-GG.
Subsequently, we followed a modified Delphi method (appendix p 5). We used the commercially available, web-based Delphi platform for circulating the questions and receiving anonymous responses from the participants. The Delphi process was continued until the predefined criteria of consensus (≥70% agreement or disagreement on a statement) was achieved, or for a maximum of three rounds. After each round of Delphi, we held virtual meetings to discuss the unresolved issues. The comments received during the two rounds and the virtual discussions were incorporated into the final round of Delphi. We recorded the responses to statements using a five-point Likert scale: strongly agree, somewhat agree, neutral, somewhat disagree, and strongly disagree. For the statements for which a response was recorded using the Likert sale, the categories strongly agree and somewhat agree, or strongly disagree and somewhat disagree, were considered together. We recommend a course of action for statements for which consensus of 70% or more was reached and suggest a course of actions for those with a consensus of less than 70%, and provided the consensus level for important summary statements. Statements failing to achieve the predefined consensus criteria even after the final meeting were recorded as dissensus.
Results
The online surveys and meetings were conducted between Oct 1 and Nov 1, 2021. 26 of the 28 invited experts participated in the survey. The CAPM-GG comprised experts from pulmonary medicine (13 [50%]), infectious diseases (six [23%]), clinical mycology (three [12%]), radiodiagnosis (two [8%]), pathology (one [4%]), and thoracic surgery (one [4%]), belonging to either public sector (14 [54%]) or private sector (12 [46%]) institutes across the country. The results of the Delphi process are presented in table 1 . We achieved a consensus for 84 of the 89 statements, based on which we provide various clinical practice statements for different questions on CAPM.
Table 1.
Results of the Delphi process for the various statements on different questions concerning CAPM
| Survey response | Consensus level (%) | |
|---|---|---|
| Definition of CAPM | ||
| Proven CAPM | Yes | 100% |
| Probable CAPM | Yes | 100% |
| Possible CAPM | Yes | 75% |
| Risk factors | ||
| Uncontrolled diabetes | Yes | 100% |
| Inappropriate steroid therapy | Yes | 100% |
| Severe COVID-19 | Yes | 78% |
| Immunosuppression | Yes | 95% |
| Immunomodulators for COVID-19 (eg, tocilizumab) | Yes | 28% |
| Altered iron metabolism | Yes | 78% |
| ICU admission for COVID-19 | No | 85% |
| Use of industrial oxygen, contaminated humidifier water, or reused masks | No | 65% |
| No or irregular use of a mask during COVID-19 or post-COVID-19 period | No | 79% |
| Zinc supplement for COVID-19 | No | 75% |
| Clinical features | ||
| Fever | Suggestive | 83% |
| Worsening or productive cough | Suggestive | 87% |
| Brownish or black sputum | Highly suggestive | 74% |
| Chest pain | Suggestive | 71% |
| Haemoptysis | Highly suggestive | 70% |
| Worsening respiratory symptoms patients with COVID-19 | Suggestive | 83% |
| Worsening chest imaging | Suggestive | 70% |
| Evaluation of CAPM | ||
| Characteristic imaging on CT with intravenous contrast | Yes | 100% |
| Routine imaging of paranasal sinuses or brain | No | 89% |
| Respiratory sample positive for Mucorales by conventional diagnostic techniques | Yes | 100% |
| Bronchoalveolar lavage sample positive for Mucorales by molecular diagnostic techniques | Yes | 74% |
| Serology | No | 83% |
| Molecular test of blood, urine, or body fluid | No | 58% |
| Imaging findings | ||
| Reversed halo sign | Highly suggestive | 100% |
| Thick-walled cavity | Highly suggestive | 94% |
| Large consolidation or necrotising pneumonia | Highly suggestive | 81% |
| Mycotic aneurysm | Highly suggestive | 100% |
| Bird's nest sign | Highly suggestive | 95% |
| Multiple large nodules | Highly suggestive | 72% |
| Serial imaging showing air-fluid levels | Suggestive | 80% |
| Pleural effusion associated with other findings | Suggestive | 74% |
| Pneumothorax | Non-specific | 100% |
| Mediastinal lymphadenopathy | Not suggestive | 89% |
| Centrilobular nodules or tree in bud appearance | Not suggestive | 100% |
| Differential diagnosis | ||
| Severe COVID-19 | Yes | 82% |
| COVID-19-associated pulmonary aspergillosis | Yes | 100% |
| Tuberculosis | Yes | 96% |
| Other cavitary pneumonias | Yes | 75% |
| Bacterial pneumonia (community and hospital acquired) | Yes | 86% |
| Diagnostic procedures | ||
| Open-lung biopsy for diagnosis | No | 73% |
| Diagnostic bronchoscopy should be performed as early as possible for the evaluation of suspected CAPM | Yes | 95% |
| Flexible bronchoscopy can be safely performed in all patients with COVID-19 (intubated and non-intubated), following standard precautions | Yes | 78% |
| CT-guided trucut biopsy (or fine-needle aspiration with on-site evaluation) | Yes | 91% |
| Laboratory processing of samples | ||
| Use of high-volume samples | Yes | 85% |
| Rapid transport to the laboratory | Yes | 90% |
| Use of Calcofluor microscopical examination | Yes | 72% |
| Semiquantitative estimation of fungus | Not recommended | 85% |
| Mincing (instead of grinding) the tissue sample | Yes | 87% |
| PCR from surgical or biopsy specimens for bronchoalveolar lavage fluid | Yes | 74% |
| The histopathology of CAPM is not different from non-CAPM | Yes | 90% |
| Immunohistochemistry is useful in differentiating mucormycosis from aspergillosis in tissues | Yes | 61% |
| Species identification and antifungal susceptibility | ||
| Does species identification help in the management? | Yes | 74% |
| Is an antifungal susceptibility test essential for optimal therapy? | Yes | 71% |
| Choice of drug and dose | ||
| Liposomal amphotericin B is the treatment of choice for CAPM | Yes | 100% |
| If liposomal formulation is unavailable, any lipid formulation can be used | Yes | 100% |
| If no lipid formulation is available, amphotericin B deoxycholate should be used as the primary therapy over posaconazole or isavuconazole | Yes | 94% |
| Initial dose of intravenous liposomal amphotericin B | 5 mg/kg | 80% |
| Should the amphotericin B dose be escalated in bilateral or non-operable disease? | No | 85% |
| Should the amphotericin B dose be escalated in the presence of uncontrolled risk factors for CAPM? | No | 90% |
| Should the amphotericin B dose be escalated in the presence of extrapulmonary mucormycosis (disseminated or ROCM)? | No | 52% |
| After complete or partial response is achieved, maintenance treatment with isavuconazole or posaconazole should be given | Yes | 100% |
| Preferred formulation of posaconazole is a tablet | Yes | 80% |
| Therapeutic drug monitoring of posaconazole | Yes | 74% |
| Combination of antifungals | ||
| The combination of antifungals (posaconazole or isavuconazole with amphotericin) is not evidence based and should not be recommended | Yes | 89% |
| Echinocandins in combination with amphotericin B can be given in CAPM | No | 83% |
| Salvage therapy with posaconazole or isavuconazole might be considered in refractory patients | Yes | 100% |
| Nebulised amphotericin B for CAPM | No | 95% |
| Response monitoring and duration of therapy | ||
| Duration of therapy should be based on response assessment (instead of a fixed duration) | Yes | 81% |
| Monitoring with a weekly chest radiography (along with antifungals as and when required) | Yes | 95% |
| Preferred timing of CT scan for response assessment | 4–6 weeks | 70% |
| Surgery For CAPM | ||
| All patients with potentially resectable disease of the lung (unilateral) should undergo surgery | Yes | 95% |
| Preoperative multidisciplinary team evaluation | Yes | 100% |
| Timing of surgery after diagnosis* | As early as possible (< 1 week); <2 weeks | 34%; 40% |
| Spirometry desirable in all patients preoperatively, especially before pneumonectomy or in those with pre-existing lung disease | Yes | 100% |
| Surrogate tests such as 6-MWT or other methods are sufficient to assess exercise capacity (if spirometry not possible) | Yes | 90% |
| Preoperative assessment of frailty | Yes | 82% |
| Delay surgery or continue medical management and reassess in frail patients | Yes | 89% |
| Surgery for CAPM in the presence of COVID-19-related lung disease† | After stabilistion | 80% |
| Extensive invasion of mediastinal structures and hilar vessels seen on thoracic imaging is associated with technical difficulties during surgery and poor outcome; hence, initial medical management followed by reassessment is suggested | Agreed | 81% |
| Prevention of CAPM | ||
| Prophylactic antifungals or nebulised amphotericin B to prevent CAPM in patients with COVID-19 admitted to hospital or the ICU | No | 91% |
| Universal masking | Yes | 95% |
| Avoidance of construction site | Yes | 90% |
| Control of blood sugars in diabetes | Yes | 100% |
| Immunosuppression for COVID-19, optimal dose, and duration | Yes | 95% |
| Severe COVID-19 and development of CAPM before 10 days of therapy with glucocorticoids | Stop therapy | 71% |
| No glucocorticoid use for non-severe (non-hypoxaemic) COVID-19 | Yes | 100% |
| Judicious use of corticosteroids for post-COVID-19 lung disease (at the lowest possible dose for the shortest possible duration) | Yes | 78% |
CAPM=COVID-19-associated pulmonary mucormycosis. 6-MWT=6 min walk test. CAPA=COVID-19-associated pulmonary aspergillosis. ICU=intensive care unit. ROCM=rhinoorbitocerebral mucormycosis.
After stabilising the metabolic derangements.
Except in patients with emergent indications such as massive haemoptysis.
Definitions
Pulmonary mucormycosis diagnosed either at the same time as, or within 3 months of, confirmed COVID-19 was agreed upon as the entry criterion for diagnosing CAPM.6 We further classified CAPM as proven, probable, and possible (panel 1 ). The consensus for the proven and probable CAPM categories was obtained following the first round of surveys. For the possible CAPM category, we could only reach a consensus in the third round. The experts were divided in their opinions on possible CAPM, given the potential for over-diagnosis and unnecessary empirical treatment, which is both long lasting and expensive. However, we retained the possible CAPM category, recognising the need for an epidemiological definition for facilitating research and adopting a judicious treatment approach. Furthermore, the group emphasised that an extensive evaluation of possible CAPM should be undertaken by performing bronchoscopy or other suitable diagnostic procedures to confirm or exclude the diagnosis.
Panel 1. Definitions of COVID-19 associated pulmonary mucormycosis.
COVID-19-associated pulmonary mucormycosis (CAPM) is diagnosed either simultaneously with or within 3 months of virologically confirmed COVID-19.
Proven CAPM
Histopathology or cytology showing aseptate hyphae or culture obtained by a sterile procedure from a usually sterile site (pleural fluid or lung) showing growth of Mucorales.
Probable CAPM
Presence of all the following: compatible clinical features, risk factors, and suggestive imaging (thick-walled cavity, large consolidation, reversed halo sign, or multiple large nodules) and demonstration of aseptate hyphae (with or without growth of Mucorales) in a sample representative of the lower respiratory tract (including bronchoalveolar lavage, non-bronchoscopic bronchial lavage, bronchial washings, bronchial brushing, endotracheal aspirates, and sputum).
Possible CAPM
Presence of all the following: compatible clinical features; uncontrolled diabetes, prolonged or inappropriate glucocorticoid therapy (dose, duration, or indication deviating from the current evidence-based practice for glucocorticoids in COVID-19); and highly suggestive radiology (reversed halo sign, mycotic aneurysm, or thick-walled cavity), in the absence of a definite alternative diagnosis.
Burden of CAPM
The prevalence of CAPM was reported to be 0·01% in patients with COVID-19 from the community (data from one centre in Mexico), 0·15% in patients admitted to hospital with COVID-19 (data from five tertiary-care centres in India), and 1·00% in patients with COVID-19 on mechanical ventilation (data from 59 micology laboratories in France).6, 12, 13 The burden of CAPM following the second wave of the COVID-19 pandemic in India and other countries remains largely unknown.14, 15, 16, 17
In a systematic review, CAPM accounted for 26 (9·5%) of 275 patients with CAM from across the world.1 17 (7·3%) of 233 reported CAM cases from India and nine (21·4%) of 42 reported CAM cases from the rest of the world were due to CAPM.1 On the basis of the scarce data available from India,6 France,18 and Chile,19 the pooled prevalence of CAPM was estimated to be 5 (95% CI <1 to 29) per 10 000 patients admitted to hospital with COVID-19 (appendix p 6). The prevalence of CAPM was higher in India and Chile than in France. After the second wave of COVID-19, India reported more than 40 000 patients with CAM. The experts estimated that the number of patients with CAPM in the recent CAM epidemic should have been around 4000. However, no more than 40 incidents of CAPM have been published globally.1, 20, 21, 22, 23, 24 Thus, there is a possibility of gross under-reporting of the number of patients with CAPM.
Risk factors
All participants considered uncontrolled diabetes (hyperglycaemia) and inappropriate (or excessive) glucocorticoid therapy as major risk factors for CAPM.25, 26 COVID-19 per se, and the associated dysregulation of iron metabolism, were also considered to be contributing factors.27 Although patients with COVID-19 requiring intensive care have been noted to develop CAPM,13, 16 most experts believed that intensive care was not an independent risk factor. The CAPM-GG did not consider zinc supplementation, contaminated humidifiers, industrial oxygen, or reused masks as risk factors for CAPM.1, 28 The experts also found insufficient evidence to indicate tocilizumab or other immunomodulators as risk factors for CAPM.6
Clinical features
None of the clinical features were found to be specific to CAPM, and the presentation of CAPM is often indistinguishable from COVID-19 or any pneumonic illness.29 The presence of brownish or black sputum and haemoptysis in a patient with COVID-19, particularly in the presence of risk factors (as mentioned earlier), should trigger investigations for CAPM.30 Other suggestive features include chest pain; fever despite antibiotic therapy for at least 48 h; worsening or productive cough; cavity; or worsening alveolar shadows on a chest x-ray in the appropriate setting (eg, a patient with uncontrolled diabetes).
Evaluation of suspected CAPM
Early detection of mucormycosis determines patient outcomes. In this context, CT of the chest is superior to chest x-ray. Mucorales, unlike Aspergillus, rarely colonise the respiratory tract.31, 32, 33 Thus, the isolation of Mucorales from sputum or endotracheal aspirate signifies probable mucormycosis in the presence of compatible clinicoradiological features. Sputum examination is a non-invasive procedure and can be considered the initial investigation. The diagnostic yield might be higher with respiratory samples obtained using bronchoscopy because they are more representative of the disease site. In a study of 24 patients with confirmed pulmonary mucormycosis, three were diagnosed with sputum examination, whereas nine were diagnosed with bronchoscopy.34 No specific serological markers are available for mucormycosis. Serum galactomannan and β-D-glucan are useful in diagnosing COVID-19-associated pulmonary aspergillosis (CAPA), a close mimic of CAPM. Importantly, dual infections of CAPM and CAPA might also be encountered.35, 36
We recommend CT with intravenous contrast and conventional microbiological testing from the lower respiratory tract samples as the initial steps in evaluating CAPM (consensus level: 100%).
Imaging of CAPM
A chest x-ray is often the initial imaging available, and non-specific signs such as consolidation, cavities, and pleural effusion might be encountered. A CT of the thorax is thus required to delineate the abnormalities and guide diagnostic procedures. The imaging of CAPM has a wide differential diagnosis, including CAPA, tuberculosis, other bacterial pneumonia, and even severe COVID-19.37 The presence of a halo sign, a reversed halo sign (RHS), an air crescent sign, a hypodense sign, and cavitating nodules help to differentiate invasive mould infections from other pneumonias.38 Although a cavity on imaging might be seen in vasculitis or malignancy, the setting of COVID-19, serial imaging, and the doubling time of the lesions are important differentiating features of CAPM.
The presence of COVID-19-related lung abnormalities on imaging poses additional challenges in the diagnosis of CAPM (appendix p 7). COVID-19 has been shown to cause both the halo sign and RHS; the prevalence of both signs varies from 0 to 18% in different series.37, 39 These signs occur early in COVID-19 pneumonia. Serial imaging studies in haematological malignancies suggest halo sign to be an early feature of pulmonary mucormycosis.40, 41 By contrast, CAPM most often occurs in patients with diabetes following COVID-19 and thus resembles pulmonary mucormycosis occurring in patients with diabetes rather than haematological malignancies.30, 42, 43, 44 The presentation in CAPM could be indolent,30, 45 and not as aggressive as in haematological malignancies. Additionally, most patients with CAPM present relatively late (usually >7 days after the onset of COVID-19), and the halo sign is not commonly seen at the time of diagnosis.42 Furthermore, serial imaging can help in differentiating between COVID-19 and CAPM. The halo sign and RHS due to COVID-19 tend to improve over time,46 whereas in pulmonary mucormycosis, cavitation is the usual course.40, 41 Thus, the timing, clinical setting (uncontrolled diabetes, persistent or new-onset fever, haemoptysis, or productive cough), and the course of disease help to differentiate acute COVID-19 from CAPM.
We classified the different imaging features of CAPM as highly suggestive, suggestive, non-specific, or not suggestive (panel 2 ), on the basis of the existing evidence and responses received from the CAPM-GG. The presence of a thick-walled cavity, bird's nest sign, RHS, and other features were considered as highly suggestive of CAPM (appendix p 7), compared with the presence of multiple nodules (described in patients with pulmonary mucormycosis with haematological malignancies).47 Digital subtraction angiography might be required in patients with a mycotic aneurysm (appendix p 7).
Panel 2. CT findings of COVID-19-associated pulmonary mucormycosis.
Highly suggestive
-
•
Thick-walled cavity
-
•
Reversed halo sign
-
•
Large consolidation or necrotising pneumonia
-
•
Mycotic aneurysm
-
•
Bird's nest sign
-
•
Multiple large nodules (nodules >1 cm)
-
•
Serial imaging showing cavity with an air-fluid level
Suggestive
-
•
Pleural effusion
Non-specific
-
•
Pneumothorax
Not suggestive
-
•
Enlarged mediastinal lymph nodes
-
•
Centrilobular nodules or tree-in-bud appearance (could be seen in patients with haemoptysis or in patients with coexisting COVID-19-associated pulmonary aspergillosis)
The portal of entry and risk factors are the same for both pulmonary mucormycosis and rhino-orbital mucormycosis, yet disseminated disease was reported in only three of the 20 patients with CAPM.29 Notably, all the patients were symptomatic for rhino-orbital mucormycosis. Thus, the presence of clinical features should guide evaluation of mucormycosis at other sites (paranasal sinuses) in patients with CAPM rather than routine imaging.
The presence of RHS, thick-walled cavity, bird's nest sign, mycotic aneurysm, large consolidation, or necrotising pneumonia, and multiple large nodules (nodules >1 cm) and serial imaging showing cavity with an air-fluid level, were considered highly suggestive imaging features of CAPM in the appropriate clinical setting (consensus level: 72–100%). We do not recommend routine imaging of the paranasal sinuses or brain in patients with CAPM (consensus level: 89%).
Bronchoscopy for CAPM in patients with COVID-19
Flexible bronchoscopy can be performed in patients with COVID-19 at any time, adhering to standard precautions.48, 49, 50, 51, 52 Bronchoscopy and bronchoalveolar lavage with 60 mL of saline has been safely done even in patients with severe acute respiratory distress syndrome due to COVID-19 (median fractional concentration of oxygen of inspired air is 0·8 and positive end-expiratory pressure of 10 cm of water).48 The median time of occurrence of CAPM following COVID-19 ranges from 2 to 3 weeks6, 30 and the majority of patients are unlikely to transmit SARS-CoV-2 during this period. Preliminary data suggest that the use of bronchoscopy is safe in patients with CAPM.53
Mycotic aneurysms encountered in CAPM have the potential to cause fatal or severe haemoptysis.22, 24, 30 Hence, a cautious approach is required when carrying out flexible bronchoscopy, bronchoalveolar lavage, or biopsies in patients with massive haemoptysis or mycotic aneurysms. Notably, the bronchoscopy unit should be equipped to handle emergencies, such as severe bleeding following diagnostic bronchoscopies.54 The choice of additional procedures, such as transbronchial lung biopsy (TBLB), endobronchial biopsy, brush cytology, and others, needs to be individualised, depending on the imaging abnormalities, bronchoscopical findings, and the patient's status.54 In general, performing more than one procedure increases the diagnostic yield of bronchoscopy in CAPM, as with other diseases. Flexible bronchoscopy can be used for peripheral and non-peripheral lung lesions.54 Radial endobronchial ultrasound-guided bronchoalveolar lavage or TBLB could improve the chances of obtaining a representative specimen.54, 55 Importantly, endobronchial abnormalities occur in up to 79% of patients with suspected pulmonary mucormycosis undergoing diagnostic bronchoscopy.56 In patients with suspected invasive mould disease based on clinical and radiological features, identification of an adherent mucus plug during bronchoscopy was noted exclusively in patients with pulmonary mucormycosis and not those with invasive pulmonary aspergillosis.54 Furthermore, invasive tracheobronchitis is a feature of fungal disease in intubated, critically ill patients, and can be diagnosed only with bronchoscopy.57
We recommend early flexible bronchoscopy in most patients with CAPM (consensus level: 95%) due to the following advantages: visualisation of airway abnormalities, performing endobronchial biopsies, and provision of samples representing the lower respiratory tract (bronchoalveolar lavage or bronchial washings).
Transthoracic biopsies and needle aspiration
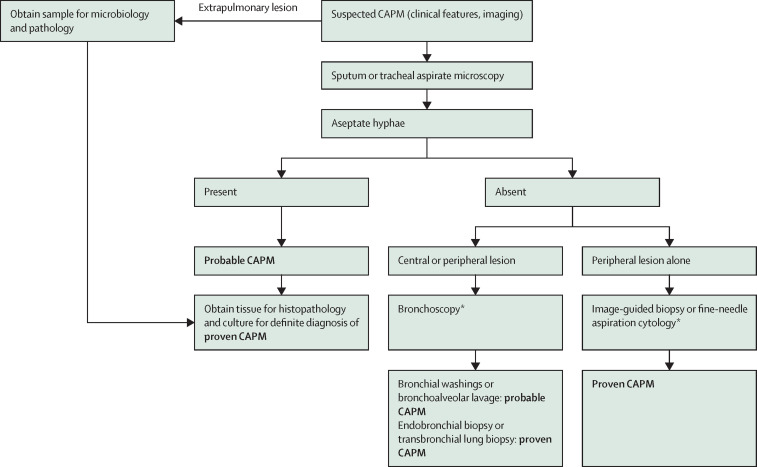
Lung biopsies obtained by video-assisted thoracoscopic surgery or thoracotomy might yield the diagnosis in suspected CAPM. However, due to the high morbidity and mortality of this test and the availability of less invasive diagnostic tests, surgical lung biopsy should rarely be used for the diagnosis of CAPM. Although both fine-needle aspiration biopsies and core-needle biopsies can be used to diagnose mould infections, a consensus was achieved for transthoracic core-needle biopsies owing to their higher diagnostic yield (figure 1 ).58, 59, 60, 61 The complication rates of transthoracic core-needle biopsies and fine-needle aspirates have been similar in the studies published before the COVID-19 pandemic.58, 59, 60, 61, 62 However, considering the possibility of severe bleeding in patients with suspected CAPM, an alternative approach of performing a fine-needle aspiration cytology with on-site cytopathological examination might be considered before proceeding with core-needle biopsies.
Figure 1.
Diagnostic algorithm for evaluating suspected CAPM
CAPM=COVID-19-associated pulmonary mucormycosis. *Direct microscopy or histopathology showing broad aseptate hyphae.
We recommend transthoracic trucut core-needle biopsy for diagnosing CAPM in patients with peripheral chest lesions (consensus level: 91%).
Quality of the clinical sample and technical details of sample processing
Obtaining a high-volume sample, avoiding contamination, and ensuring rapid transport to the laboratory increases the diagnostic yield during mycological analysis. The processing of tracheal, bronchial, and bronchoalveolar lavage fluid samples in the mycology laboratory is similar. The respiratory sample is first centrifuged, and the pellet obtained is used for microscopy and culture. The use of Calcofluor-white staining improves the detection of hyphae during microscopy.33 Mincing the tissue samples for culture should be performed instead of grinding, because grinding compromises fungal viability. Environmental contamination of a sample obtained in a non-sterile manner could result in a positive culture for Mucorales.63 Hence, the experts considered direct microscopy to be more reliable than culture when performed from a non-sterile sample. In the presence of strong clinicoradiological suspicion, isolated growth of Mucorales (despite negative direct smear microscopy) could represent probable CAPM. However, in the absence of a strong clinical or radiological suspicion, an isolated positive culture should be interpreted with caution, and repeating the biopsy or bronchoalveolar lavage could be warranted.
Role of nucleic acid amplification assays in CAPM
Molecular diagnostic tests from respiratory and other samples could help to diagnose CAPM. The diagnostic role of nucleic acid amplification (NAA) tests on blood or body fluids is still unclear, and the experts did not recommend routine use of these tests for diagnosing CAPM. However, the experts acknowledged the potential usefulness of molecular tests from blood or body fluids once standardised testing methods and more evidence are available. Molecular assays from a non-sterile site should also be cautiously interpreted. NAA assays for Mucorales in a sample obtained from a sterile site provide supportive evidence for CAPM in a compatible clinical setting.64, 65, 66 One published study in CAPM18 and a few studies67, 68, 69 in non-CAPM suggest the potential usefulness of NAA tests using bronchoalveolar lavage fluid. The group felt that a commercially available NAA test could be more reproducible than an in-house assay and should be preferred.
The expert group recommended using a standardised NAA method from sterile sites or bronchoalveolar lavage fluid (consensus level: 74%). No consensus was reached for NAA tests on samples other than bronchoalveolar lavage (consensus level: 58%).
Mucorales identification and drug susceptibility testing
The identification of the species of Mucorales and antifungal susceptibility testing are necessary for epidemiological purposes and in patients who do not respond adequately to treatment.70 For example, infections caused by Mucorales, such as Cunninghamella bertholletiae, have been shown to respond poorly to amphotericin B.71, 72 By contrast, the minimum inhibitory concentration (MIC) of posaconazole is higher for Mucor circinelloides (4 μg/mL) than for other species, and mice infected with M circinelloides show poor response to posaconazole treatment.73, 74, 75, 76 Recognising the organism also becomes relevant in cases in which differentiating septate and aseptate hyphae on morphology is difficult.70 Antifungal susceptibility testing is also valuable for dual infections (CAPA and CAPM) as azole resistance is reported in Aspergillus fumigatus, and Aspergillus terreus is intrinsically resistant to amphotericin B.77, 78
The expert group recommended species identification and performing antifungal susceptibility testing for epidemiological purposes to guide the choice of antifungals, and in patients with disease progression (consensus level: 71–74%).
Histopathology in CAPM
It is not known whether the histopathology of CAPM is different from that of non-CAPM. Limited experience points towards little difference in the tissue reaction to the fungi.79 Dual infections with Aspergillus and Mucorales have also been noted in pathological specimens of patients undergoing surgery for CAPM (appendix p 8). A swollen small hyphal segment of Aspergillus might occasionally be mistaken for Mucorales. Immunohistochemistry using antibodies against Rhizopus (and other Mucorales) could help to differentiate Aspergillus from Mucorales.80 Furthermore, identification of Mucoralean DNA by PCR (and DNA sequencing) in fresh or formalin-fixed paraffin-embedded tissues might also be attempted in complex cases.81 A diagnostic algorithm for CAPM is provided in figure 1.
CAPA and CAPM dual infections
CAPA is the closest differential diagnosis of CAPM because of the shared risk factors and similar clinicoradiological features.82 Dual infections of CAPA and CAPM further add to the diagnostic conundrum.36 Although glucocorticoids are a risk factor for both CAPA and CAPM,83 poorly controlled diabetes is more often associated with CAPM than with CAPA.4, 5, 26
The imaging findings of CAPM overlap with CAPA. In contrast-enhanced CT chest scans, vessel occlusion sign (due to angioinvasion) is seen in patients with invasive pulmonary aspergillosis,84 but might also be seen in patients with pulmonary mucormycosis.41 The bird's nest sign is seen in up to a third of patients with pulmonary mucormycosis, in contrast to 3% of patients with invasive pulmonary aspergillosis,80 and might help to differentiate the two invasive mould infections.38 On the basis of clinical experience and the published literature, we have summarised the likelihood of diagnosing CAPA versus CAPM on encountering various radiological findings (appendix p 3). However, in patients with radiological features highly suggestive of CAPM (panel 2), the evaluation of CAPM might have to continue despite diagnostic evidence of CAPA (microbiological or serological), because therapy will be dictated against dual infections (use of antifungal agent active against both Aspergillus and Mucorales and the need for surgery). For example, in a patient with uncontrolled diabetes whose CT thorax shows RHS, the evaluation for mucormycosis should continue despite microbiological and serological evidence of aspergillosis.85
Biomarkers such as serum galactomannan have imperfect diagnostic performance for CAPA, especially in non-neutropenic individuals.86 The accuracy of bronchoalveolar lavage fluid β-D-glucan is poor (sensitivity is 52% and specificity is 58%) for the diagnosis of invasive fungal disease.87 The sensitivity (87%) and specificity (81%) of bronchoalveolar lavage fluid galactomannan is acceptable in diagnosing invasive pulmonary aspergillosis.88 Thus, even when performing these two tests in the bronchoalveolar lavage fluid, we cannot conclusively exclude invasive pulmonary aspergillosis. However, the sensitivity improves to 94% when combining the two tests.87, 88 Thus, the likelihood of diagnosing CAPM is higher in a patient with negative bronchoalveolar lavage fluid β-D-glucan and galactomannan.
It is possible to encounter multiple scenarios suggestive of dual infection, which can be categorised further for research and epidemiological purposes (appendix p 4).
Prevention of CAPM
The most important step in the prevention of CAPM is the judicious use of glucocorticoids and other immunosuppressants for COVID-19.89 Glucocorticoids should be used only in hypoxaemic individuals with COVID-19, with the dose and duration of glucocorticoids conforming to the current guidelines.90 The expert panel advised against using antifungal prophylaxis for preventing CAM or CAPM in patients with COVID-19. Instead, the emphasis was placed on optimal glycaemic control.91 In patients with prolonged hypoxaemia due to COVID-19 or post-COVID-19 lung abnormalities, when a longer duration of glucocorticoids might be needed, the lowest possible dose of glucocorticoids should be used (along with strict glycaemic control).92
Control of underlying risk factors
The control of underlying risk factors is essential to improve outcomes in CAPM. For example, strict glycaemic control (140–180 mg/dL [7.8–10·0 mmol/L]) is suggested in patients with CAPM, like in other critically ill patients.91 Before initiating or withholding other immunosuppressive drugs in the transplant setting, the risk–benefit ratio must be weighed. In general, in transplant recipients receiving immunosuppression who develop CAPM, low-dose glucocorticoids could be continued to avoid graft rejection.93
Management of CAPM
Surgery
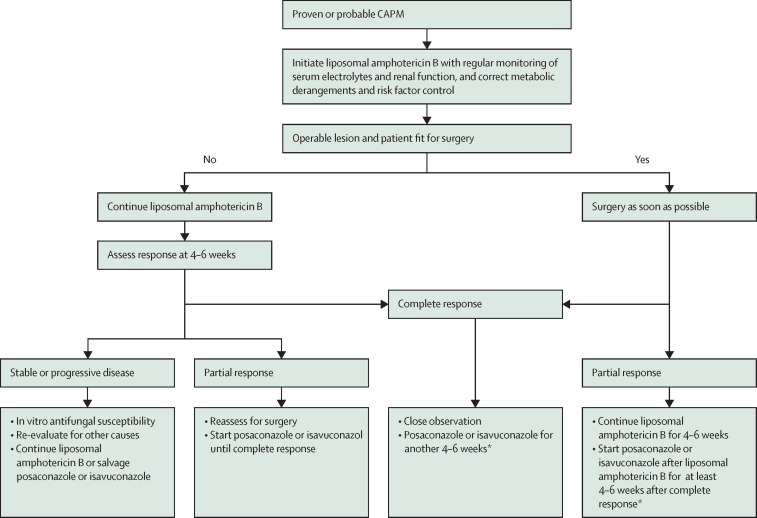
The CAPM-GG recommended surgery for all patients with potentially resectable disease (figure 2 ). Extensive invasion of mediastinal structures and hilar vessels is associated with technical difficulties during surgery and poor outcome.94 A few patients with potentially resectable disease might not be operable due to multiple comorbidities or frailty.95, 96 The treatment decision for these patients needs to be individualised, ideally after a discussion involving a multidisciplinary team, consisting of thoracic surgeons, clinicians, and radiologists.
Figure 2.
Proposed algorithm for treating CAPM
CAPM=COVID-19-associated pulmonary mucormycosis. *Treatment duration should be individualised and could be extended up to 12 weeks after complete response.
Preoperative evaluation should include spirometry (especially in patients due to receive a pneumonectomy or patients with chronic respiratory disease) and assessment of frailty and exercise capacity (eg, 6 min walk test [6MWT]).97 Spirometry might not be possible for patients with COVID-19 or patients with massive haemoptysis,98 and the decision to operate must be made on the basis of surrogate measures (ie, 6MWT and frailty assessment) and multidisciplinary team evaluation.
The optimal timing for surgery in CAPM is unknown, and there is a wide variation in practice.99 Although surgery should be performed as early as possible, the experts felt that the metabolic abnormalities (eg, glycaemic control and electrolyte imbalance) should be corrected, which generally takes 1–2 weeks. Emergent surgery is warranted in patients with massive haemoptysis.3, 97, 99, 100, 101 In patients with CAPM, surgery could be delayed due to the poor health status of patients. However, it was suggested that surgery be performed soon after stabilising COVID-19 or post-COVID-19 hypoxaemia. Uncommonly, patients might show a good response following medical therapy and, consequently, might not require surgery for CAPM. These patients should be closely observed, and the underlying risk factors such as diabetes should be controlled. However, re-evaluation and surgery should be considered at the earliest sign of deterioration.
In patients with bilateral disease, surgery could be considered if the lesion in one of the lungs shows total or near-total resolution, or when there is a complication such as massive haemoptysis. Some patients might tolerate partial resection, provided the lung reserve permits surgery of both the lungs.
All patients with potentially resectable lung disease should undergo surgery (consensus level: 95%). We recommend that surgery should be performed as soon as the metabolic derangements are corrected, generally within 1–2 weeks (consensus level: 74%). Furthermore, in patients who show invasion of the mediastinal structures during imaging, we recommend initial medical management followed by reassessment for surgery (consensus level: 81%). We also recommend that a multidisciplinary team evaluate all such patients before surgery (consensus level: 100%).
Medical management
All patients with proven or probable CAPM should be treated with antifungal agents that are effective against Mucorales. The expert group recommended against routinely treating patients with possible CAPM. However, delayed initiation of therapy is associated with high mortality in patients with mucormycosis.11 Hence, the treatment decision must be individualised, ideally after discussion by a multidisciplinary team (ie, clinicians, radiologists, microbiologists). For example, patients with highly suggestive imaging (eg, RHS, mycotic aneurysm and bird's nest sign), risk factors (eg, post-COVID-19 in a patient with diabetes, with diabetic ketoacidosis, or on glucocorticoid therapy), and clinical features (massive haemoptysis) would benefit from early therapy. The experts recommended classifying treatment in CAPM as primary and maintenance therapy.
Primary therapy
Liposomal amphotericin B is the therapy of choice for patients with CAPM. The experts agreed on an initial dose of 5 mg/kg per day of intravenous liposomal amphotericin B as recommended by the global guidelines for mucormycosis.11 A higher dose (10 mg/kg per day) has been suggested to treat intracranial disease.11, 102, 103 The experts advocated against dose escalation of liposomal amphotericin B for patients with bilateral pulmonary disease or inoperable CAPM, although no consensus was reached for critically ill patients with CAPM. When liposomal amphotericin B is not available, other lipid formulations of amphotericin B could be used.10, 11 Posaconazole or isavuconazole should not be routinely used as primary therapy because there is no supporting randomised controlled trial, except in situations in which the organism is known to have a high minimum inhibitory concentration for amphotericin B (eg, C bertholletiae). The experts agreed that triazoles might also be the primary therapy when none of the amphotericin B formulations are available.
When administering amphotericin B, serum electrolytes, renal and liver functions, and complete blood count should be closely monitored. Hypokalaemia induced by amphotericin B could be aggravated by glucocorticoids used for COVID-19. Daily electrolyte monitoring with electrolyte supplementation was proposed for hypokalaemia and hypomagnesaemia, and amphotericin B can be continued with electrolyte supplementation. The renal dysfunction associated with amphotericin B is generally reversible after discontinuing therapy. All formulations of amphotericin B have been safely used in a standard dosage, even in patients on renal replacement therapy.104, 105 The experts advised temporarily discontinuing amphotericin B if the serum creatinine values double from baseline and advised restarting amphotericin B once the values normalise. Another approach is to reduce the dose of amphotericin B (from 5 mg/kg per day to 1–3 mg/kg per day) and increase the dose once the serum creatinine values return to baseline. Some patients with progressive renal dysfunction, anaphylaxis, drug non-availability, or drug intolerance might need to switch over to salvage therapy with posaconazole or isavuconazonium.
The optimal duration of primary therapy for CAPM is unclear. The experts recommended that the duration of therapy be based on response assessment rather than fixed duration (table 2 ). However, most experts agreed that complete or partial response is generally achieved by 4–6 weeks of primary therapy.
Table 2.
Response assessment criteria in COVID-19 associated pulmonary mucormycosis
| Definition | |
|---|---|
| Success | |
| Complete response | Survival and resolution of all attributable clinical features (symptoms and signs) of disease, and resolution of the radiological lesion (or lesions) or persistence of only a scar or postoperative changes that can be equated with a complete radiological response |
| Partial response | Survival and resolution of all attributable clinical features (symptoms and signs) of disease, and a 25% or higher reduction in the diameter of radiological lesion (or lesions); or radiological stabilisation (<25% reduction in the diameter of the lesion), and resolution of all attributable symptoms and signs of fungal disease |
| Failure | |
| Stable disease | Survival and minor or no improvement in all attributable clinical features (symptoms and signs) of disease and radiological stabilisation (<25% reduction in the diameter of the lesion) |
| Progressive disease | Worsening clinical symptoms or signs of disease, and new sites of disease or radiological worsening of pre-existing lesions or persistent isolation of Mucorales |
| Death | Death due to any cause during the period of assessment |
The criteria have been adapted from the Mycoses Study Group and European Organisation for Research and Treatment of Cancer consensus criteria for response assessment in invasive mould disease.106
We recommend liposomal amphotericin B (5 mg/kg per day) as the treatment of choice for CAPM (consensus level: 100%). This dose could be escalated (10 mg/kg per day) in patients with intracranial involvement (consensus level: 48%). If the liposomal formulation is unavailable, any amphotericin formulation can be used for primary therapy rather than posaconazole or isavuconazole (consensus level: 94–100%). We do not recommend escalating the dose of amphotericin B in patients with bilateral or non-operable disease or uncontrolled risk factors (consensus level: 85–90%).
The ideal time for response assessment using a CT scan remains unclear. Chest radiography might be performed weekly, or as clinically indicated, to detect radiological deterioration rather than improvement. Unlike in invasive pulmonary aspergillosis, improvement in pulmonary mucormycosis takes longer, particularly in patients with diabetes.103 The expert group recommended performing a CT scan of the thorax at 4–6 weeks to assess treatment response.106 The response should be categorised as complete response, partial response, stable disease, or progressive disease (table 2). A complete or partial response is classified as a successful outcome, whereas stable or progressive disease is considered a treatment failure.106
Maintenance therapy
Once a complete or partial response is achieved, maintenance treatment with isavuconazole or posaconazole should be initiated (except when the organism isolated is resistant to azoles, eg, M circinelloides).107 A clear consensus was not achieved even after three rounds of Delphi regarding the duration of treatment after attaining a complete or partial response. Although most (two-thirds) participants in the expert group suggested at least 4–6 weeks of maintenance therapy after complete response, a few experts felt the need for a longer duration (up to 3 months). Importantly, all of the experts agreed that the duration of maintenance treatment needs to be personalised. The factors to be considered are the predisposing conditions (COVID-19 only vs COVID-19 with coexisting diabetes vs COVID-19 in organ transplant recipients, for whom the reversal of immune status differs), the extent of lung involvement, complete or partial surgical resection, response to initial therapy, the type of Mucorales, and coexisting pulmonary illnesses for which a delayed resolution is expected.
A delayed-release tablet of posaconazole is preferred to the suspension form.25 The suspension form has variable absorption and less dependable pharmacokinetics than the posaconazole tablet.108 The expert group favoured posaconazole over isavuconazole due to its wider availability, reduced cost of therapy, more experience of its use by health-care professionals, and published evidence. However, therapeutic drug monitoring is recommended for patients on posaconazole therapy, and the target concentration should be more than 1 mg/L. By contrast, isavuconazole does not need therapeutic drug monitoring, might have lesser toxicity and better bioavailability than posaconazole, but has a higher cost than posaconazole.10, 109, 110
In patients with stable disease or partial response, surgery should be considered along with maintenance therapy with posaconazole or isavuconazole. The experts suggested the following measures in patients with progressive disease: excluding secondary infections, species identification, and antifungal susceptibility testing (if not performed earlier). Additionally, the underlying risk factors for CAPM should be addressed. The experts reached a consensus on the following therapeutic approach in patients with progressive disease: continuing liposomal amphotericin B, or using posaconazole or isavuconazole as salvage therapy for a longer duration until a complete or partial response is achieved. The experts suggested against using a combination of antifungal agents in patients with treatment failure.111, 112 Despite a theoretical advantage of drug synergy, 113 there is currently no clear evidence supporting the use of a combination of antifungal drugs for the treatment of pulmonary mucormycosis.11, 112 A management algorithm is provided in figure 2.
We recommend maintenance treatment with isavuconazole or posaconazole after the patient achieves a complete or partial response (consensus: 100%). No consensus could be reached regarding the duration of treatment after the patient attains a complete or partial response. We do not recommend a combination of antifungal drugs (posaconazole or isavuconazole with amphotericin) in patients with treatment failure (consensus level: 89%). We recommend continuing liposomal amphotericin B or using posaconazole or isavuconazole for a longer duration until a complete or partial response is achieved, as salvage therapy in patients with treatment failure (consensus level: 89–100%).
In the absence of high-quality data for CAPM or non-CAPM, nebulised amphotericin B was not recommended for treatment. Colony-stimulating factors (G-CSF, GM-CSF) or neutrophil transfusions should not be routinely used in patients with CAPM.
Future directions
The expert group identified the following broad areas for conducting research: incidence and epidemiology of CAPM at different geographical locations; factors contributing to the development of CAPM in patients with COVID-19; imaging findings of CAPM versus CAPA; the role of molecular diagnostics (ie, blood, endotracheal aspirate, or sputum) in CAPM; and duration of medical therapy for CAPM and timing of surgery.
Limitations and strengths
Data on CAPM are scarce. This Review is primarily based on the opinions of a small group of experts, although this group does have considerable experience and were actively managing CAPM cases during the pandemic. The opinions of this group might have inherent biases, as all the experts are from a single country. Furthermore, the experiences of the experts are derived from a population comprising patients from a single country, with possible common geographical, racial, genetic, and environmental factors. CAPM could behave differently in other parts of the world. The strengths of the decision process include obtaining anonymous responses from the experts and performing three rounds of Delphi to clarify ambiguous statements and questions. Although CAPA has been recognised as an important complication of COVID-19 in many countries, CAPM remains under-diagnosed. The absence of a biomarker for CAPM is a substantial drawback, and the true incidence of CAPM might be underestimated. The current consensus opinion therefore provides a framework to improve awareness of, identify, and manage CAPM until well conducted studies are available. The various possible categories of dual infections of CAPA and CAPM are also categorised.
Conclusions
In conclusion, this Review provides current knowledge on the epidemiology, risk factors, and expert guidance on defining CAPM for patient care and research. Furthermore, this Review summarises the available data on imaging, diagnostic challenges, and management issues unique to CAPM, which are likely to evolve with further research. The widespread dissemination of these guidelines could improve awareness about CAPM, and possibly research into CAPM, to meet the unmet needs in this field.
This online publication has been corrected. The corrected version first appeared at thelancet.com/infection on April 14, 2022
Declaration of interests
AP received honoraria and lecture fees from Gilead Sciences, Pfizer (India), Intas Pharmaceuticals, Mylan (India), Bharat Serum Vaccine, and Cipla India. TS received lecture fees from Mylan (India), Cipla India, Merck Sharp & Dohme, Intas Pharmaceuticals, Pfizer (India), and Glenmark Pharmaceuticals. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
The Fungal Infection Study Forum and Academy of Pulmonary Sciences supported the Delphi process and organised the virtual discussion.
Contributors
AC and RA contributed to the conceptualisation and funding acquisition. VM, AP, AK, UM, SMR, and ASB contributed to data curation. VM, RA, ANA, and SK contributed to formal analysis. RA, VM, and AC contributed to investigation and resources. AC, SK, RA, VM, AP, GMV, and RS contributed to methodology. AC, RA, and SMR contributed to project administration. SMR and VM contributed to software. ANA, AC, RG, DC, AM, and RS contributed to validation. RA, SK, and AC contributed to visualistion. VM, RA, and SK contributed to writing the original draft. All authors contributed to writing, reviewing, and editing the submitted work.
Supplementary Material
References
- 1.Muthu V, Rudramurthy SM, Chakrabarti A, Agarwal R. Epidemiology and pathophysiology of COVID-19-associated mucormycosis: India versus the rest of the world. Mycopathologia. 2021;186:739–754. doi: 10.1007/s11046-021-00584-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prakash H, Chakrabarti A. Global epidemiology of mucormycosis. J Fungi (Basel) 2019;5:26. doi: 10.3390/jof5010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel A, Kaur H, Xess I, Michael JS, Savio J, Rudramurthy S, et al. A multicentre observational study on the epidemiology, risk factors, management and outcomes of mucormycosis in India. Clin Microbiol Infect. 2020;26:e9–15. doi: 10.1016/j.cmi.2019.11.021. [DOI] [PubMed] [Google Scholar]
- 4.Hussain S, Riad A, Singh A, et al. Global prevalence of COVID-19-associated mucormycosis (CAM): living systematic review and meta-analysis. J Fungi (Basel) 2021;7:985. doi: 10.3390/jof7110985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hussain S, Baxi H, Riad A, et al. COVID-19-associated mucormycosis (CAM): an updated evidence mapping. Int J Environ Res Public Health. 2021;18 doi: 10.3390/ijerph181910340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel A, Agarwal R, Rudramurthy SM, et al. Multicenter epidemiologic study of coronavirus disease-associated mucormycosis, India. Emerg Infect Dis. 2021;27:2349–2359. doi: 10.3201/eid2709.210934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muthu V, Agarwal R, Dhooria S, et al. Has the mortality from pulmonary mucormycosis changed over time? A systematic review and meta-analysis. Clin Microbiol Infect. 2021;27:538–549. doi: 10.1016/j.cmi.2020.12.035. [DOI] [PubMed] [Google Scholar]
- 8.Garg D, Muthu V, Sehgal IS, et al. Coronavirus disease (Covid-19) associated mucormycosis (CAM): case report and systematic review of literature. Mycopathologia. 2021;186:289–298. doi: 10.1007/s11046-021-00528-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pandey N, Kaushal V, Puri GD, et al. Transforming a general hospital to an infectious disease hospital for COVID-19 over 2 weeks. Front Public Health. 2020;8:382. doi: 10.3389/fpubh.2020.00382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rudramurthy SM, Hoenigl M, Meis JF, et al. ECMM/ISHAM recommendations for clinical management of COVID-19 associated mucormycosis in low- and middle-income countries. Mycoses. 2021;64:1028–1037. doi: 10.1111/myc.13335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cornely OA, Alastruey-Izquierdo A, Arenz D, et al. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis. 2019;19:e405–e421. doi: 10.1016/S1473-3099(19)30312-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guzman-Castro S, Chora-Hernandez LD, Trujillo-Alonso G, et al. COVID-19-associated mucormycosis, diabetes and steroid therapy: experience in a single centre in western Mexico. Mycoses. 2021;65:65–70. doi: 10.1111/myc.13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gangneux JP, Dannaoui E, Fekkar A, et al. Fungal infections in mechanically ventilated patients with COVID-19 during the first wave: the French multicentre MYCOVID study. Lancet Respir Med. 2021;10:180–190. doi: 10.1016/S2213-2600(21)00442-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramaswami A, Sahu AK, Kumar A, et al. COVID-19-associated mucormycosis presenting to the emergency department-an observational study of 70 patients. QJM. 2021;114:464–470. doi: 10.1093/qjmed/hcab190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Selarka L, Sharma S, Saini D, et al. Mucormycosis and COVID-19: an epidemic within a pandemic in India. Mycoses. 2021;64:1253–1260. doi: 10.1111/myc.13353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seidel D, Simon M, Sprute R, et al. Results from a national survey on COVID-19-associated mucormycosis in Germany: 13 patients from six tertiary hospitals. Mycoses. 2021;65:103–109. doi: 10.1111/myc.13379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bayram N, Ozsaygılı C, Sav H, et al. Susceptibility of severe COVID-19 patients to rhino-orbital mucormycosis fungal infection in different clinical manifestations. Jpn J Ophthalmol. 2021;65:515–525. doi: 10.1007/s10384-021-00845-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Danion F, Letscher-Bru V, Guitard J, et al. COVID-19 associated mucormycosis in France: a rare but deadly complication. Open Forum Infect Dis. 2021;9 doi: 10.1093/ofid/ofab566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rabagliati R, Rodríguez N, Núñez C, Huete A, Bravo S, Garcia P. COVID-19-associated mold infection in critically ill patients, Chile. Emerg Infect Dis. 2021;27:1454–1456. doi: 10.3201/eid2705.204412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alfishawy M, Elbendary A, Younes A, et al. Diabetes mellitus and coronavirus disease (Covid-19) associated mucormycosis (CAM): a wake-up call from Egypt. Diabetes Metab Syndr. 2021;15 doi: 10.1016/j.dsx.2021.102195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chennamchetty VK, Adimulapu S, Kola BP, et al. Post-COVID pulmonary mucormycosis—a case report. IP Indian J Immunol Respir Med. 2021;6:62–66. [Google Scholar]
- 22.Dantis K, Rathore V, Kashyap NK, Gupta N, De S, Singha SK. SARS-CoV-2 sequel: pulmonary mucormycosis with a mycotic aneurysm in a transplant recipient. Tuberc Respir Dis (Seoul) 2021;84:335–337. doi: 10.4046/trd.2021.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rabagliati R, Rodríguez N, Núñez C, Huete A, Bravo S, Garcia P. Covid-19-associated mold infection in critically ill patients, chile. Emerg Infect Dis. 2021;27:1454–1456. doi: 10.3201/eid2705.204412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rana G, Gautam S, Mawari G, Daga MK, Kumar N, Raghu RV. Massive hemoptysis causing mortality in a post COVID-19 infected Asian male patient: presenting as pulmonary mucormycosis, pulmonary tuberculosis and later sino-nasal mucormycosis. Respir Med Case Rep. 2021;34 doi: 10.1016/j.rmcr.2021.101511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cornely OA, Duarte RF, Haider S, et al. Phase 3 pharmacokinetics and safety study of a posaconazole tablet formulation in patients at risk for invasive fungal disease. J Antimicrob Chemother. 2016;71:718–726. doi: 10.1093/jac/dkv380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riad A, Shabaan AA, Issa J, et al. COVID-19-associated mucormycosis (CAM): case-series and global analysis of mortality risk factors. J Fungi. 2021;7:837. doi: 10.3390/jof7100837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar HM, Sharma P, Rudramurthy SM, et al. Serum iron indices in COVID-19-associated mucormycosis: a case-control study. Mycoses. 2021;65:120–127. doi: 10.1111/myc.13391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muthu V, Kumar M, Paul RA, et al. Is there an association between zinc and COVID-19-associated mucormycosis? Results of an experimental and clinical study. Mycoses. 2021;64:1291–1297. doi: 10.1111/myc.13365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoenigl M, Seidel D, Carvalho A, et al. The emergence of COVID-19 associated mucormycosis: a review of cases from 18 countries. Lancet Microbe. 2022 doi: 10.1016/S2666-5247(21)00237-8. published online Jan 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pruthi H, Muthu V, Bhujade H, et al. Pulmonary artery pseudoaneurysm in COVID-19-associated pulmonary mucormycosis: case series and systematic review of the literature. Mycopathologia. 2021;187:31–37. doi: 10.1007/s11046-021-00610-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lass-Flörl C, Salzer GM, Schmid T, Rabl W, Ulmer H, Dierichi MP. Pulmonary Aspergillus colonization in humans and its impact on management of critically ill patients. Br J Haematol. 1999;104:745–747. doi: 10.1046/j.1365-2141.1999.01260.x. [DOI] [PubMed] [Google Scholar]
- 32.Ribes JA, Vanover-Sams CL, Baker DJ. Zygomycetes in human disease. Clin Microbiol Rev. 2000;13:236–301. doi: 10.1128/cmr.13.2.236-301.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lass-Flörl C. Zygomycosis: conventional laboratory diagnosis. Clin Microbiol Infect. 2009;15(suppl 5):60–65. doi: 10.1111/j.1469-0691.2009.02999.x. [DOI] [PubMed] [Google Scholar]
- 34.Peng M, Meng H, Sun Y, et al. Clinical features of pulmonary mucormycosis in patients with different immune status. J Thorac Dis. 2019;11:5042–5052. doi: 10.21037/jtd.2019.12.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bellanger AP, Navellou JC, Lepiller Q, et al. Mixed mold infection with Aspergillus fumigatus and Rhizopus microsporus in a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) patient. Infect Dis Now. 2021;51:633–635. doi: 10.1016/j.idnow.2021.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson AK, Ghazarian Z, Cendrowski KD, Persichino JG. Pulmonary aspergillosis and mucormycosis in a patient with COVID-19. Med Mycol Case Rep. 2021;32:64–67. doi: 10.1016/j.mmcr.2021.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yılmaz Demirci N, Uğraş Dikmen A, Taşçı C, et al. Relationship between chest computed tomography findings and clinical conditions of coronavirus disease (COVID-19): a multicentre experience. Int J Clin Pract. 2021;75 doi: 10.1111/ijcp.14459. [DOI] [PubMed] [Google Scholar]
- 38.Alexander BD, Lamoth F, Heussel CP, et al. Guidance on imaging for invasive pulmonary aspergillosis and mucormycosis: from the imaging working group for the revision and update of the consensus definitions of fungal disease from the EORTC/MSGERC. Clin Infect Dis. 2021;72(suppl 2):S79–S88. doi: 10.1093/cid/ciaa1855. [DOI] [PubMed] [Google Scholar]
- 39.Sales AR, Casagrande EM, Hochhegger B, Zanetti G, Marchiori E. The reversed halo sign and COVID-19: possible histopathological mechanisms related to the appearance of this imaging finding. Arch Bronconeumol. 2021;57:73–75. doi: 10.1016/j.arbres.2020.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nam BD, Kim TJ, Lee KS, Kim TS, Han J, Chung MJ. Pulmonary mucormycosis: serial morphologic changes on computed tomography correlate with clinical and pathologic findings. Eur Radiol. 2018;28:788–795. doi: 10.1007/s00330-017-5007-5. [DOI] [PubMed] [Google Scholar]
- 41.Hammer MM, Madan R, Hatabu H. Pulmonary mucormycosis: radiologic features at presentation and over time. AJR Am J Roentgenol. 2018;210:742–747. doi: 10.2214/AJR.17.18792. [DOI] [PubMed] [Google Scholar]
- 42.Garg M, Prabhakar N, Muthu V, et al. CT Findings of COVID-19-associated pulmonary mucormycosis: a case series and literature review. Radiology. 2022;302:214–217. doi: 10.1148/radiol.2021211583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muthu V, Agarwal R. Cavity in pulmonary mucormycosis: is it rare? Trop Doct. 2021;51:673. doi: 10.1177/00494755211010007. [DOI] [PubMed] [Google Scholar]
- 44.Agarwal R, Kumar V, Gupta D. Pulmonary mucormycosis: two of a kind. Eur J Intern Med. 2006;17:63–65. doi: 10.1016/j.ejim.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 45.Iqbal N, Irfan M, Jabeen K, Kazmi MM, Tariq MU. Chronic pulmonary mucormycosis: an emerging fungal infection in diabetes mellitus. J Thorac Dis. 2017;9:e121–e125. doi: 10.21037/jtd.2017.02.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu J, Tang J, Zhang T, Chen YC, Du C. Follow-up CT of “reversed halo sign” in SARS-CoV-2 delta VOC pneumonia: a report of two cases. J Med Virol. 2021;94:1289–1291. doi: 10.1002/jmv.27533. [DOI] [PubMed] [Google Scholar]
- 47.Chamilos G, Marom EM, Lewis RE, Lionakis MS, Kontoyiannis DP. Predictors of pulmonary zygomycosis versus invasive pulmonary aspergillosis in patients with cancer. Clin Infect Dis. 2005;41:60–66. doi: 10.1086/430710. [DOI] [PubMed] [Google Scholar]
- 48.Torrego A, Pajares V, Fernández-Arias C, Vera P, Mancebo J. Bronchoscopy in patients with COVID-19 with invasive mechanical ventilation: a single-center experience. Am J Respir Crit Care Med. 2020;202:284–287. doi: 10.1164/rccm.202004-0945LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gao CA, Bailey JI, Walter JM, et al. Bronchoscopy on intubated patients with COVID-19 is associated with low infectious risk to operators. Ann Am Thorac Soc. 2021;18:1243–1246. doi: 10.1513/AnnalsATS.202009-1225RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chang SH, Jiang J, Kon ZN, et al. Safety and efficacy of bronchoscopy in critically ill patients with coronavirus disease 2019. Chest. 2021;159:870–872. doi: 10.1016/j.chest.2020.09.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chhajed PN, Nene A, Abhyankar N, et al. Conventional flexible bronchoscopy during the COVID pandemic: a consensus statement from the Indian Association for Bronchology. Lung India. 2021;38(suppl):S105–S115. doi: 10.4103/lungindia.lungindia_953_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koehler P, Cornely OA, Kochanek M. Bronchoscopy safety precautions for diagnosing COVID-19 associated pulmonary aspergillosis—a simulation study. Mycoses. 2021;64:55–59. doi: 10.1111/myc.13183. [DOI] [PubMed] [Google Scholar]
- 53.Mehta R, Bansal S, Kalpakkam H. Critical COVID-19 associated pulmonary mucormycosis (CAPM): the underreported life-threatening spectrum of the mucormycosis epidemic. Lung India (in press). [DOI] [PMC free article] [PubMed]
- 54.Muthu V, Gandra RR, Dhooria S, et al. Role of flexible bronchoscopy in the diagnosis of invasive fungal infections. Mycoses. 2021;64:668–677. doi: 10.1111/myc.13263. [DOI] [PubMed] [Google Scholar]
- 55.Chen CH, Cheng WC, Wu BR, et al. Improved diagnostic yield of bronchoscopy in peripheral pulmonary lesions: combination of radial probe endobronchial ultrasound and rapid on-site evaluation. J Thorac Dis. 2015;7(suppl 4):S418–S425. doi: 10.3978/j.issn.2072-1439.2015.12.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin CY, Wang IT, Chang CC, et al. Comparison of clinical manifestation, diagnosis, and outcomes of invasive pulmonary aspergillosis and pulmonary mucormycosis. Microorganisms. 2019;7:e531. doi: 10.3390/microorganisms7110531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yazıcıoğlu Moçin O, Karakurt Z, Aksoy F, et al. Bronchoscopy as an indicator of tracheobronchial fungal infection in non-neutropenic intensive-care unit patients. Clin Microbiol Infect. 2013;19:e136–e141. doi: 10.1111/1469-0691.12112. [DOI] [PubMed] [Google Scholar]
- 58.Watane GV, Hammer MM, Barile MF. CT-guided core-needle biopsy of the lung Is safe and more effective than fine-needle aspiration biopsy in patients with hematologic malignancies. Radiol Cardiothorac Imaging. 2019;1 doi: 10.1148/ryct.2019180030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Haas BM, Clayton JD, Elicker BM, Ordovas KG, Naeger DM. CT-guided percutaneous lung biopsies in patients with suspicion for infection may yield clinically useful information. AJR Am J Roentgenol. 2017;208:459–463. doi: 10.2214/AJR.16.16255. [DOI] [PubMed] [Google Scholar]
- 60.Sharma SK, Kumar S, Singh AK, et al. Feasibility and outcome of CT-guided lung biopsy in patients with hematological diseases and suspected fungal pneumonia. J Infect Dev Ctries. 2013;7:748–752. doi: 10.3855/jidc.2823. [DOI] [PubMed] [Google Scholar]
- 61.Carrafiello G, Laganà D, Nosari AM, et al. Utility of computed tomography (CT) and of fine needle aspiration biopsy (FNAB) in early diagnosis of fungal pulmonary infections. Study of infections from filamentous fungi in haematologically immunodeficient patients. Radiol Med (Torino) 2006;111:33–41. doi: 10.1007/s11547-006-0004-9. [DOI] [PubMed] [Google Scholar]
- 62.Lass-Flörl C, Aigner M, Nachbaur D, et al. Diagnosing filamentous fungal infections in immunocompromised patients applying computed tomography-guided percutaneous lung biopsies: a 12-year experience. Infection. 2017;45:867–875. doi: 10.1007/s15010-017-1072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Prakash H, Singh S, Rudramurthy SM, et al. An aero mycological analysis of mucormycetes in indoor and outdoor environments of northern India. Med Mycol. 2020;58:118–123. doi: 10.1093/mmy/myz031. [DOI] [PubMed] [Google Scholar]
- 64.Millon L, Herbrecht R, Grenouillet F, Morio F, Alanio A, Letscher-Bru V, et al. Early diagnosis and monitoring of mucormycosis by detection of circulating DNA in serum: retrospective analysis of 44 cases collected through the French Surveillance Network of Invasive Fungal Infections (RESSIF) Clin Microbiol Infect. 2016;22:e1–e8. doi: 10.1016/j.cmi.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 65.Millon L, Scherer E, Rocchi S, Bellanger AP. Molecular strategies to diagnose mucormycosis. J Fungi (Basel) 2019;5:e24. doi: 10.3390/jof5010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Caillot D, Legouge C, Lafon I, et al. Retrospective study of 25 cases of pulmonary mucormycosis in acute leukaemia. Rev Mal Respir. 2018;35:452–464. doi: 10.1016/j.rmr.2017.11.009. (in French). [DOI] [PubMed] [Google Scholar]
- 67.Scherer E, Iriart X, Bellanger AP, et al. Quantitative PCR (qPCR) detection of Mucorales DNA in bronchoalveolar lavage fluid to diagnose pulmonary mucormycosis. J Clin Microbiol. 2018;56:e00289–e00318. doi: 10.1128/JCM.00289-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guegan H, Iriart X, Bougnoux ME, Berry A, Robert-Gangneux F, Gangneux JP. Evaluation of MucorGenius mucorales PCR assay for the diagnosis of pulmonary mucormycosis. J Infect. 2020;81:311–317. doi: 10.1016/j.jinf.2020.05.051. [DOI] [PubMed] [Google Scholar]
- 69.Wehrle-Wieland E, Affolter K, Goldenberger D, et al. Diagnosis of invasive mold diseases in patients with hematological malignancies using Aspergillus, Mucorales, and panfungal PCR in BAL. Transpl Infect Dis. 2018;20 doi: 10.1111/tid.12953. [DOI] [PubMed] [Google Scholar]
- 70.Skiada A, Lass-Floerl C, Klimko N, Ibrahim A, Roilides E, Petrikkos G. Challenges in the diagnosis and treatment of mucormycosis. Med Mycol. 2018;56(suppl 1):93–101. doi: 10.1093/mmy/myx101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Garey KW, Pendland SL, Huynh VT, Bunch TH, Jensen GM, Pursell KZ. splant patient: amphotericin lung penetration, MIC determinations, and review of the literature. Pharmacotherapy. 2001;21:855–860. doi: 10.1592/phco.21.9.855.34560. [DOI] [PubMed] [Google Scholar]
- 72.Badali H, Cañete-Gibas C, McCarthy D, et al. Epidemiology and antifungal susceptibilities of Mucoralean fungi in clinical samples from the United States. J Clin Microbiol. 2021;59 doi: 10.1128/JCM.01230-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Salas V, Pastor FJ, Calvo E, et al. In vitro and in vivo activities of posaconazole and amphotericin B in a murine invasive infection by Mucor circinelloides: poor efficacy of posaconazole. Antimicrob Agents Chemother. 2012;56:2246–2250. doi: 10.1128/AAC.05956-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Espinel-Ingroff A, Chakrabarti A, Chowdhary A, et al. Multicenter evaluation of MIC distributions for epidemiologic cutoff value definition to detect amphotericin B, posaconazole, and itraconazole resistance among the most clinically relevant species of Mucorales. Antimicrob Agents Chemother. 2015;59:1745–1750. doi: 10.1128/AAC.04435-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dannaoui E. Antifungal resistance in mucorales. Int J Antimicrob Agents. 2017;50:617–621. doi: 10.1016/j.ijantimicag.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 76.Vitale RG, de Hoog GS, Schwarz P, et al. Antifungal susceptibility and phylogeny of opportunistic members of the order mucorales. J Clin Microbiol. 2012;50:66–75. doi: 10.1128/JCM.06133-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Meijer EFJ, Dofferhoff ASM, Hoiting O, Meis JF. COVID-19-associated pulmonary aspergillosis: a prospective single-center dual case series. Mycoses. 2021;64:457–464. doi: 10.1111/myc.13254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lass-Flörl C, Dietl AM, Kontoyiannis DP, Brock M. Aspergillus terreus species complex. Clin Microbiol Rev. 2021;34 doi: 10.1128/CMR.00311-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zurl C, Hoenigl M, Schulz E, et al. Autopsy proven pulmonary mucormycosis due to Rhizopus microsporus in a critically ill COVID-19 patient with underlying hematological malignancy. J Fungi (Basel) 2021;7:88. doi: 10.3390/jof7020088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jung J, Kim MY, Lee HJ, et al. Comparison of computed tomographic findings in pulmonary mucormycosis and invasive pulmonary aspergillosis. Clin Microbiol Infect. 2015;21:e11–e18. doi: 10.1016/j.cmi.2015.03.019. [DOI] [PubMed] [Google Scholar]
- 81.Jillwin J, Rudramurthy SM, Singh S, et al. Molecular identification of pathogenic fungi in formalin-fixed and paraffin-embedded tissues. J Med Microbiol. 2021;70:1–8. doi: 10.1099/jmm.0.001282. [DOI] [PubMed] [Google Scholar]
- 82.Verweij PE, Brüggemann RJM, Azoulay E, et al. Taskforce report on the diagnosis and clinical management of COVID-19 associated pulmonary aspergillosis. Intensive Care Med. 2021;47:819–834. doi: 10.1007/s00134-021-06449-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Koehler P, Bassetti M, Chakrabarti A, et al. Defining and managing COVID-19-associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect Dis. 2021;21:e149–e162. doi: 10.1016/S1473-3099(20)30847-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Henzler C, Henzler T, Buchheidt D, et al. Diagnostic performance of contrast enhanced pulmonary computed tomography angiography for the detection of angioinvasive pulmonary aspergillosis in immunocompromised patients. Sci Rep. 2017;7 doi: 10.1038/s41598-017-04470-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Crone CG, Helweg-Larsen J, Steensen M, Arendrup MC, Helleberg M. Pulmonary mucormycosis in the aftermath of critical COVID-19 in an immunocompromised patient: mind the diagnostic gap. J Mycol Med. 2021;32 doi: 10.1016/j.mycmed.2021.101228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhou W, Li H, Zhang Y, et al. Diagnostic value of galactomannan antigen test in serum and bronchoalveolar lavage fluid samples from patients with nonneutropenic invasive pulmonary aspergillosis. J Clin Microbiol. 2017;55:2153–2161. doi: 10.1128/JCM.00345-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shi XY, Liu Y, Gu XM, et al. Diagnostic value of (1 → 3)-β-D-glucan in bronchoalveolar lavage fluid for invasive fungal disease: a meta-analysis. Respir Med. 2016;117:48–53. doi: 10.1016/j.rmed.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 88.Cao XJ, Li YP, Xie LM, Zhang HL, Qin YS, Guo XG. Diagnostic accuracy of bronchoalveolar lavage fluid galactomannan for invasive aspergillosis. BioMed Res Int. 2020;2020 doi: 10.1155/2020/5434589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Muthu V, Sehgal IS, Prasad KT, Agarwal R. Is high-dose glucocorticoid beneficial in COVID-19? Eur Respir J. 2021;57 doi: 10.1183/13993003.00065-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Agarwal A, Rochwerg B, Lamontagne F, et al. A living WHO guideline on drugs for covid-19. BMJ. 2020;370 doi: 10.1136/bmj.m3379. [DOI] [PubMed] [Google Scholar]
- 91.Mulakavalupil B, Vaity C, Joshi S, Misra A, Pandit RA. Absence of case of mucormycosis (March 2020–May 2021) under strict protocol driven management care in a COVID-19 specific tertiary care intensive care unit. Diabetes Metab Syndr. 2021;15 doi: 10.1016/j.dsx.2021.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dhooria S, Chaudhary S, Sehgal IS, et al. High-dose versus low-dose prednisolone in symptomatic patients with post-COVID-19 diffuse parenchymal lung abnormalities: an open-label, randomised trial (Acronym: COLDSTER) Eur Respir J. 2022;59 doi: 10.1183/13993003.02930-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Meshram HS, Kute VB, Chauhan S, et al. Mucormycosis as SARS-CoV2 sequelae in kidney transplant recipients: a single-center experience from India. Int Urol Nephrol. 2021;18:1–11. doi: 10.1007/s11255-021-03057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Saxena P, Shen SH, Morrissey O, Gooi JH. Challenges in the management of invasive pulmonary zygomycosis: the Alfred experience. ANZ J Surg. 2015;85:700–701. doi: 10.1111/ans.13264. [DOI] [PubMed] [Google Scholar]
- 95.Benjamin SR, Narayanan D, Chandy ST, Gnanamuthu BR, Michael JS, Kodiatte TA. Pulmonary mucormycosis—a case series. Indian J Thorac Cardiovasc Surg. 2021 doi: 10.1007/s12055-021-01272-4. published online Oct 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hong HL, Lee YM, Kim T, et al. Risk factors for mortality in patients with invasive mucormycosis. Infect Chemother. 2013;45:292–298. doi: 10.3947/ic.2013.45.3.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Multani A, Reveron-Thornton R, Garvert DW, Gomez CA, Montoya JG, Lui NS. Cut it out! Thoracic surgeon's approach to pulmonary mucormycosis and the role of surgical resection in survival. Mycoses. 2019;62:893–907. doi: 10.1111/myc.12954. [DOI] [PubMed] [Google Scholar]
- 98.Muthu V, Singh H, Gorsi U, Agarwal R. Large pulmonary artery pseudoaneurysm in mucormycosis: successfully managed with surgery and amphotericin. BMJ Case Rep. 2021;14 doi: 10.1136/bcr-2020-240813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pulle MV, Puri HV, Asaf BB, Bishnoi S, Sharma S, Kumar A. Outcomes of early anti-fungal therapy with aggressive surgical resection in pulmonary mucormycosis. Lung India. 2021;38:314–320. doi: 10.4103/lungindia.lungindia_758_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Choi H, Lee H, Jeon K, et al. Factors affecting surgical resection and treatment outcomes in patients with pulmonary mucormycosis. J Thorac Dis. 2019;11:892–900. doi: 10.21037/jtd.2019.01.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mills SEA, Yeldandi AV, Odell DD. Surgical treatment of multifocal pulmonary mucormycosis. Ann Thorac Surg. 2018;106:e93–e95. doi: 10.1016/j.athoracsur.2017.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Soni K, Das A, Sharma V, et al. Surgical & medical management of ROCM (rhino-orbito-cerebral mucormycosis) epidemic in COVID-19 era and its outcomes—a tertiary care center experience. J Mycol Med. 2021;32 doi: 10.1016/j.mycmed.2021.101238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lanternier F, Poiree S, Elie C, et al. Prospective pilot study of high-dose (10 mg/kg/day) liposomal amphotericin B (L-AMB) for the initial treatment of mucormycosis. J Antimicrob Chemother. 2015;70:3116–3123. doi: 10.1093/jac/dkv236. [DOI] [PubMed] [Google Scholar]
- 104.Anaissie EJ, Mattiuzzi GN, Miller CB, et al. Treatment of invasive fungal infections in renally impaired patients with amphotericin B colloidal dispersion. Antimicrob Agents Chemother. 1998;42:606–611. doi: 10.1128/aac.42.3.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wood JE, Mahnensmith MP, Mahnensmith RL, Perazella MA. Intradialytic administration of amphotericin B: clinical observations on efficacy and safety. Am J Med Sci. 2004;327:5–8. doi: 10.1097/00000441-200401000-00002. [DOI] [PubMed] [Google Scholar]
- 106.Segal BH, Herbrecht R, Stevens DA, et al. Defining responses to therapy and study outcomes in clinical trials of invasive fungal diseases: Mycoses Study Group and European Organization for Research and Treatment of Cancer consensus criteria. Clin Infect Dis. 2008;47:674–683. doi: 10.1086/590566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Borman AM, Fraser M, Patterson Z, Palmer MD, Johnson EM. In vitro antifungal drug resistance profiles of clinically relevant members of the Mucorales (Mucoromycota) especially with the newer triazoles. J Fungi (Basel) 2021;7:271. doi: 10.3390/jof7040271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen L, Krekels EHJ, Verweij PE, Buil JB, Knibbe CAJ, Brüggemann RJM. Pharmacokinetics and pharmacodynamics of posaconazole. Drugs. 2020;80:671–695. doi: 10.1007/s40265-020-01306-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.McCarthy MW, Moriyama B, Petraitiene R, Walsh TJ, Petraitis V. Clinical pharmacokinetics and pharmacodynamics of isavuconazole. Clin Pharmacokinet. 2018;57:1483–1491. doi: 10.1007/s40262-018-0673-2. [DOI] [PubMed] [Google Scholar]
- 110.Zurl C, Waller M, Schwameis F, et al. Isavuconazole treatment in a mixed patient cohort with invasive fungal infections: outcome, tolerability and clinical implications of isavuconazole plasma concentrations. J Fungi (Basel) 2020;6:e90. doi: 10.3390/jof6020090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fortun J, Gioia F, Cardozo C, et al. Posaconazole salvage therapy: the Posifi study. Mycoses. 2019;62:526–533. doi: 10.1111/myc.12911. [DOI] [PubMed] [Google Scholar]
- 112.van Burik JA, Hare RS, Solomon HF, Corrado ML, Kontoyiannis DP. Posaconazole is effective as salvage therapy in zygomycosis: a retrospective summary of 91 cases. Clin Infect Dis. 2006;42:e61–e65. doi: 10.1086/500212. [DOI] [PubMed] [Google Scholar]
- 113.Schwarz P, Cornely OA, Dannaoui E. Antifungal combinations in Mucorales: a microbiological perspective. Mycoses. 2019;62:746–760. doi: 10.1111/myc.12909. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.