Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the pathogenic virus that causes coronavirus disease 2019 (COVID-19), with major symptoms including hyper-inflammation and cytokine storm, which consequently impairs the respiratory system and multiple organs, or even cause death. SARS-CoV-2 activates inflammasomes and inflammasome-mediated inflammatory signaling pathways, which are key determinants of hyperinflammation and cytokine storm in COVID-19 patients. Additionally, SARS-CoV-2 inhibits inflammasome activation to evade the host's antiviral immunity. Therefore, regulating inflammasome initiation has received increasing attention as a preventive measure in COVID-19 patients. Ginseng and its major active constituents, ginsenosides and saponins, improve the immune system and exert anti-inflammatory effects by targeting inflammasome stimulation. Therefore, this review discussed the potential preventive and therapeutic roles of ginseng in COVID-19 based on its regulatory role in inflammasome initiation and the host's antiviral immunity.
Keywords: Ginseng, Ginsenoside, Saponin, SARS-CoV-2, COVID-19, Inflammasome
Graphical abstract
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a strain of single-stranded RNA-enveloped coronavirus that belongs to the genus Betacoronavirus of the Coronaviridae family and causes coronavirus disease 2019 (COVID-19), which is the respiratory disease responsible for the ongoing COVID-19 pandemic [1]. SARS-CoV-2 has had detrimental effects globally, with a large number of casualties and huge economic and social crises. SARS-CoV-2 encodes nucleocapsid, membrane, envelope, and spike proteins [2,3]. It primarily affects the respiratory tract, particularly lungs, and causes various symptoms such as fever, ageusia, anosmia, shortness of breath, cough, and chest pain [4]. Although COVID-19 is a respiratory disease, it increases the risk of other serious diseases and life-threatening situations [5]. Despite the recent rapid development of COVID-19 vaccines and therapeutic drugs, the number of COVID-19 patients is still sharply increasing worldwide; therefore, studies identifying and validating therapeutic targets and developing standard therapeutic agents for this deadly disease are highly needed.
COVID-19 is caused by infection-induced pathological inflammatory responses, resulting in excessive inflammatory cytokine release, known as “cytokine storm” and severe organ injuries [6]. Additionally, COVID-19 is highly associated with various comorbidities, including asthma, chronic obstructive pulmonary disease, cardiovascular diseases, hepatic diseases, diabetes, renal diseases, human immunodeficiency syndrome, and cancers, which are closely related to pathogenic inflammatory responses [5]. Therefore, SARS-CoV-2 infection can amplify pathogenic inflammatory responses by activating inflammatory signaling pathways, which worsen COVID-19 symptoms. Accordingly, the suppression of pathogenic inflammatory responses by targeting critical molecules in inflammatory responses could be a useful strategy to alleviate COVID-19 in patients.
Ginseng is a medicinal plant that has long been cultivated in East Asia and North America and is traditionally used to improve physiological conditions and ameliorate disease [7,8]. Various bioactive components have been identified in ginseng, including ginsenosides, polysaccharides, phytosterols, essential oils, glycosides, ginsenosides, and ginseng saponins, are the major active physiological and pharmacological ingredients [[9], [10], [11]]. Over 150 natural ginsenosides and saponins have been identified and reported to play multiple pharmacological roles in diseases, including inflammatory, autoimmune, cardiovascular, metabolic diseases and cancer [11]. Interestingly, recent studies have demonstrated that ginseng, particularly ginsenosides and saponins, plays an anti-inflammatory role by inhibiting the activation of inflammasomes [11,12], intracellular multiprotein complexes that provide molecular platforms to activate inflammatory responses [13,14]. Therefore, this review discussed recent studies that highlight the functional involvement of inflammasomes in SARS-CoV-2 infection as well as the inhibitory role of ginseng in inflammasome activation. This review also provided insights into the potential role of ginseng as a promising traditional medicine for the treatment and prevention of COVID-19 by inhibiting inflammasome stimulation.
2. Inflammasomes
2.1. Classification and structures of inflammasomes
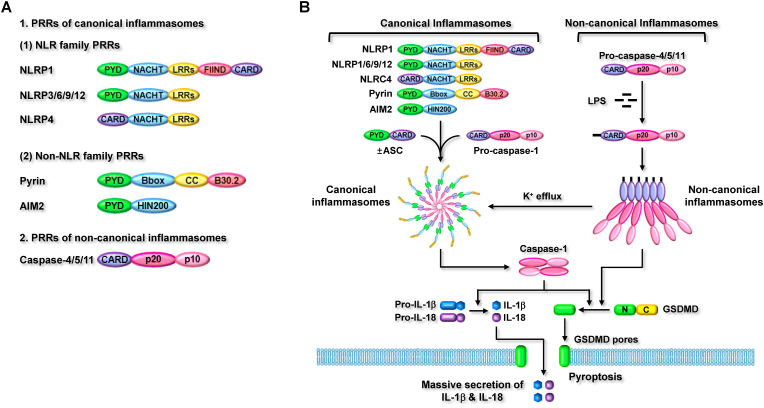
Inflammasomes are intracellular protein complexes composing PRRs and inflammatory molecules and provide platforms for inflammatory responses [13,14]. Inflammasomes are mainly classified into canonical and non-canonical inflammasomes. The canonical inflammasomes that were first identified include nucleotide-binding and oligomerization domain (NOD)-like receptor (NLR) family inflammasomes, such as NLRP1, NLRP3, NLPC4, NLPR6, NLRP9, and NLRP12 inflammasomes, and non-NLR family inflammasomes, such as absent in melanoma 2 (AIM2) and pyrin inflammasomes [13,14]. Recent studies have discovered new types of inflammasomes that are distinguishable from canonical inflammasomes and named them non-canonical inflammasomes [15]. Non-canonical inflammasomes include mouse caspase-11 and human caspase-4 and -5 inflammasomes [[16], [17], [18]].
The NLR family of PRRs has similar structures. NLRP1, which was first identified in the NLR family PRRS, has the most complex structure, composed of an N-terminal PYD, a nucleotide-binding and oligomerization domain (NACHT), leucine-rich repeats (LRRs), a functional-to-find domain, and a C-terminal caspase recruitment domain (CARD) (Fig. 1A). Interestingly, mouse NLRP1 isoforms do not contain PYD in their human homologs. NLRP3, 6, 9, and 12 have similar structures, with the same domains comprising an N-terminal PYD, a NACHT, and C-terminal LRRs (Fig. 1A). NLRC4 consists of an N-terminal CARD, NACHT, and C-terminal LRRs (Fig. 1A). Non-NLR family PRRs have different structures than those of NLR family PRRs. Pyrin consists of an N-terminal PYD, a B-box-type zinc finger, a coiled-coil, and C-terminal B30.2 (Fig. 1A). AIM2 is the simplest PRR, comprising an N-terminal PYD and C-terminal hematopoietic interferon-inducible nuclear protein 200 (HIN200) (Fig. 1A). Despite different structures of canonical inflammasome PRRs, noncanonical inflammasome PRRs have the same molecular architecture. Mouse caspase-11 and human caspase-4/5 consist of an N-terminal CARD and two catalytic domains: a p20 large domain and a p10 small domain at the C-terminus (Fig. 1A).
Fig. 1.
Inflammasome-activated signaling pathways. Canonical inflammasomes are activated by interaction with pro-caspase-1 with or without the help of ASC, but non-canonical inflammasomes are activated by direct interaction with intracellular LPS. The activated inflammasomes subsequently induce GSDMD proteolysis and GSDMD pore generation, resulting in pyroptosis. The activated inflammasomes also induce proteolytic activation of caspase-1 and caspase-1-mediated maturation of pro-inflammatory cytokines, resulting in the secretion of the pro-inflammatory cytokines through GSDMD pores.
Canonical inflammasomes are assembled through the interaction of PRRs and pro-caspase-1, with or without the help of a bipartite adaptor, ASC. PRRs with PYD (NLRP1, 3, 6, 9, 12, pyrin, and AIM2) form inflammasomes by interacting with pro-caspase-1 with the help of ASC, contrary to PRRs with an absent PYD (NLRC4) that directly interacts with pro-caspase-1 to form an inflammasome. Non-canonical inflammasomes are assembled differently than canonical inflammasomes. Mouse caspase-11 and human caspase-4/5 form non-canonical inflammasomes via oligomerization through direct CARD-CARD interaction.
2.2. Inflammasome-activated inflammatory responses
Inflammasomes are activated by sensing their specific ligands by PRRs, inducing inflammatory responses via successive proteolytic stimulation cascades of several inflammatory molecules. Activation of inflammasomes induces two main inflammatory responses: pyroptosis and secretion of pro-inflammatory cytokines. Initiation of canonical inflammasomes directly promotes the proteolytic activation of caspase-1, and the activated caspase-1 subsequently induces 1) the proteolytic cleavage of GSDMD, leading to the formation of GSDMD pores in membranes and GSDMD pore-mediated pyroptosis, as well as 2) the proteolytic maturation of IL-1β and IL-18, leading to secretion through GSDMD pores [13,14]. Non-canonical inflammasome-activated inflammatory responses are similar, but not the same as those mediated by canonical inflammasomes. Stimulation of non-canonical inflammasomes directly induces proteolysis, pore formation, and pore-mediated pyroptosis of GSDMD, but indirectly activates caspase-1 through canonical inflammasome activation [12,[19], [20], [21]]. The stimulation of non-canonical inflammasomes induces canonical inflammasome activation, resulting in caspase-1 activation and caspase-1-mediated maturation and secretion of IL-1β and IL-18 through GSDMD pores [12,[19], [20], [21]].
As discussed above, the stimulation of caspase-1 by non-canonical inflammasomes is promoted by functional cooperation between canonical and non-canonical inflammasomes. Potassium ion (K+) efflux by membrane damage and membrane gate proteins, such as GSDMD pores, P2X7 channels, bacterial pore-forming toxins, and pannexin 1 channel, is the main cause of NLRP3 canonical inflammasome activation [13,14]. Recent studies have demonstrated that caspase-11 non-canonical inflammasome activation induces K+ efflux, resulting in NLRP3 canonical inflammasome activation [22,23]. This strongly suggests that canonical and non-canonical inflammasomes have functional crosstalk rather than independent roles in inducing inflammatory responses. However, the functional relationship between canonical and non-canonical inflammasomes is still poorly understood and needs to be further elucidated. Inflammasome-activated inflammatory responses are summarized in Fig. 1B.
3. Regulatory roles of SARS-CoV-2 infection in inflammasome initiation
3.1. NLRP3 inflammasome
SARS-CoV-2 infection induces hyper-inflammation and a consequent cytokine storm, and many studies have reported that SARS-CoV-2-infected hyper-inflammation and cytokine storm are mediated by stimulation of the NLRP3 inflammasome. In vitro studies have clearly demonstrated that SARS-CoV-2 infection activates the NLRP3 inflammasome in various cell types. SARS-CoV-2 S protein activates the NLRP3 inflammasome in Cacp-2 cells [24], and SARS-CoV-2 viroporin encoded by ORF3a triggers the NLRP3 inflammasome pathway in A549 cells [25]. In vitro studies of SARS-CoV-2-induced NLRP3 inflammasome activation have also been conducted in macrophages, human peripheral blood mononuclear cells (PBMCs), hematopoietic stem/progenitor cells (HSPCs), and endothelial progenitor cells (EPCs). Single-stranded RNA genome sequences of SARS-CoV-2 activate the NLRP3 inflammasome in human macrophages differentiated from PBMCs [26], and SARS-CoV-2 envelope protein also activates the NLRP3 inflammasome in mouse bone marrow-derived macrophages (BMDMs) [27]. SARS-CoV-2 nucleocapsid protein also induces hyper-inflammation by stimulating the NLRP3 inflammasome in mouse BMDMs, human monocytes, and THP-1 cells [28]. Interestingly, SARS-CoV-2 nonstructural proteins NSP1 and NSP13, however, suppress NLRP3 inflammasome activation in monocytes [29]. This indicates that different proteins in the same SARS-CoV-2 can play different roles in NLRP3 inflammasome stimulation during inflammatory response. SARS-CoV-2 replicates via the mechanism that allows for its escape from inflammasome-activated antiviral immunity. The SARS-CoV-2 spike protein induces hyper-inflammation and exaggerated production of pro-inflammatory cytokines by activating the NLRP3 inflammasome in human PBMCs [30]. The SARS-CoV-2 spike protein also damages HSPCs and EPCs by inducing NLRP3 inflammasome activation and subsequent pyroptosis [31]. The SARS-CoV-2 spike protein has been demonstrated in vitro to interact with its molecular receptor, angiotensin-converting enzyme 2 (ACE2) [32,33], leading to the stimulation of the NLRP3 inflammasome in HSPCs and EPCs [34].
The regulatory role of SARS-CoV-2 in NLRP3 inflammasome activation has also been evaluated in various types of cells in COVID-19 patients. The primary site of SARS-CoV-2 infection is the respiratory system; therefore, the modulatory role of SARS-CoV-2 in NLRP3 inflammasome initiation has been reported in lungs and airway of COVID-19 patients. SARS-CoV-2 NSP6 triggers pyroptotic death of lung epithelial cells in COVID-19 patients by activating the NLRP3 inflammasome [35]. In contrast to these results, NLRP3 inflammasome activation was inhibited and NLRP3-activated inflammatory responses were attenuated in the upper airway tissues of COVID-19 patients with further reduced recruitment of inflammatory cells, macrophages, and neutrophils [36], which may suggest that the role of SARS-CoV-2 depends on the location of respiratory tissues, either to induce hyper-inflammation by activating the NLRP3 inflammasome or evading host antiviral immune response by inhibiting NLRP3 inflammasome activation.
The effect of SARS-CoV-2 infection has also been demonstrated in blood cells and circulating monocytes of COVID-19 patients. The NLRP3 inflammasome is triggered in blood cells of COVID-19 patients, leading to elevated levels of proinflammatory cytokines in serum [37]. SARS-CoV-2 also initiates the NLRP3 inflammasome in circulating monocytes of COVID-19 patients, resulting in pyroptotic death of circulating monocytes and secretion of pro-inflammatory cytokines from the cells [38,39]. Interestingly, NLRP3 inflammasome activation in response to SARS-CoV-2 infection is associated with COVID-19 severity [39].
Although the respiratory system is the primary target of SARS-CoV-2 infection, many COVID-19 patients exert mind and severe neurological manifestations [40,41], which indicates the potential for neurotropism and neuropathogenesis by SARS-CoV-2 through inducing neuroinflammation, and some studies reported the regulatory role of SARS-CoV-2 in NLRP3 inflammasome activated neuroinflammation. An in vitro study demonstrated that SARS-CoV-2 spike protein induces neuroinflammation by activating the NLRP3 inflammasome and NLRP3 inflammasome-activated inflammatory signaling pathways in BV2 microglial cells [42]. A case study of deceased COVID-19 patients also reported that SARS-CoV-2 nucleocapsid protein was co-localized with ACE2 and NLRP3 inflammasome in the cerebral cortical tissue-resident macrophages of deceased COVID-19 patients [43], which strongly suggests the involvement of NLRP3 inflammasome in SARS-CoV-2 cerebral pathogenicity.
Age is one of the most critical factors associated with mortality risk due to SARS-CoV-2 infection. This risk rapidly increases for people in their 60s and over 80s with serious illnesses and death [44,45]. A recent study demonstrated that age is an important factor in increasing lethality in COVID-19 patients. The NLRP3 inflammasome is over-activated and the production of pro-inflammatory cytokines is highly increased in aged COVID-19 patients [46]. Studies explained the mechanistic reason why the NLRP3 inflammasome is more activated in elderly people. Aging induces deterioration of mitochondrial performance and is highly associated with the increased production of mitochondrial ROS (mtROS), leading to the accumulation of damaged mitochondria [47]. The mtROS-damaged mitochondria release mtDNA which acts as damage-associated molecular patterns (DAMPs), resulting in the induction of the formation and activation of NRLP3 inflammasome and NLRP3 inflammasome-activated inflammatory responses [48,49]. These studies suggest that NLRP3 inflammasome activation and subsequent inflammatory responses play a pivotal role in the increased mortality of elderly COVID-19 patients infected with SARS-CoV-2.
Taken together, SARS-CoV-2 infection induces hyper-inflammation and consequent cytokine storm via activation of the NLRP3 inflammasome and NLRP3 inflammasome-activated inflammatory responses in COVID-19 patients, and also plays an inhibitory role in NLRP3 inflammasome stimulation to escape from the NLRP3 inflammasome-activated host antiviral immunity.
3.2. Other types of inflammasomes – NLRP1, NLRP12, and AIM2
Although most studies have focused on the NLRP3 inflammasome, other inflammasomes have also been reported to be regulated by SARS-CoV-2. A study analyzing gene expression profiles revealed that NLRP1 expression decreased in SARS-CoV-2-infected human lung epithelial cells [50]. This result is similar to those of other studies investigating the inhibitory role of SARS-CoV-2 in NLRP3 inflammasome activation [29,36], suggesting that SARS-CoV-2 also inhibits NLRP1 inflammasome initiation to escape host antiviral immunity.
Unlike ordinary inflammasomes, the NLRP12 inflammasome negatively regulates the secretion of pro-inflammatory cytokines from pyroptotic cells through GSDMD pores [51], which has been identified as the main cause of the cytokine storm observed in COVID-19 patients [52]. In addition, loss-of-function mutations in NLRP12 are associated with inflammatory responses and autoimmune diseases [53], indicating that NLRP12 inflammasome plays an anti-inflammatory role in inflammatory responses and diseases. Interestingly, an in vitro protease assay identified NLRP12 as a direct substrate of SARS-CoV-2 NSP5, resulting in its proteolysis of NLRP12 and hence indicating its role in hyperinflammation [54]. Cell-based experiments also revealed that NLRP12 levels were reduced in SARS-CoV-2-infected HEK293T-ACE2 cells expressing ACE2 [54], suggesting that SARS-CoV-2 induces hyperinflammation by proteolytic inhibition of the NLRP12 inflammasome, which is a negative regulator of inflammatory responses.
The role of SARS-CoV-2 in AIM2 inflammasome activation has also been previously reported. SARS-CoV-2 activates the AIM2 inflammasome in circulating monocytes of COVID-19 patients, hence inducing pyroptotic death and secreting pro-inflammatory cytokines from monocytes [38]. SARS-CoV-2 infection instigated hyper-inflammation by activating not only NLR family inflammasomes, but also non-NLR family inflammasomes, such as the AIM2 inflammasome, in COVID-19 patients.
Most studies have evaluated the effect of SARS-CoV-2 on NLRP3 inflammasome activation; however, those demonstrating SARS-CoV-2-regulated functions of other types of inflammasomes, particularly non-canonical inflammasomes, are still limited. Conclusively, SARS-CoV-2 modulates the function of all inflammasomes apart from that of NLRP3, resulting in either hyperinflammation via pyroptosis and pro-inflammatory cytokine secretion or evasion of host immune surveillance for viral replication and survival. The regulatory roles of SARS-CoV-2 infection in inflammasome stimulation are summarized in Table 1.
Table 1.
Regulatory roles of SARS-CoV-2 infection in inflammasome activation.
| Types | Roles | Activators/inhibitors | Models | Ref. |
|---|---|---|---|---|
| NLRP3 | Activation | SARS-CoV-2 spike protein | Caco-2 | [24] |
| SARS-CoV-2 viroporin | HEK293 & A549 | [25] | ||
| SARS-CoV-2 single-stranded RNA | Macrophages | [26] | ||
| SARS-CoV-2 envelope protein | BMDMs | [27] | ||
| SARS-CoV-2 nucleocapsid protein | BMDMs & THP-1 | [28] | ||
| SARS-CoV-2 spike protein | PBMCs | [30] | ||
| SARS-CoV-2 spike protein | HSPCs & EPCs | [31] | ||
| SARS-CoV-2 spike protein | HSPCs & EPCs | [34] | ||
| SARS-CoV-2 NSP6 | Lung epithelial cells of COVID-19 patients | [35] | ||
| SARS-CoV-2 | Blood cells of COVID-19 patients | [37] | ||
| SARS-CoV-2 | Circulating monocytes of COVID-19 patients | [38] | ||
| SARS-CoV-2 | Circulating monocytes of COVID-19 patients | [39] | ||
| SARS-CoV-2 spike protein | BV-2 | [42] | ||
| SARS-CoV-2 | Cerebral cortical tissues of COVID-19 patients | [43] | ||
| SARS-CoV-2 | Aged COVID-19 patients | [46] | ||
| Inhibition | SARS-CoV-2 NSP1 and NSP13 | HEK293 & THP-1 | [29] | |
| SARS-CoV-2 | Upper airway of COVID-19 patients | [36] | ||
| NLRP1 | Inhibition | SARS-CoV-2 | Lung epithelial cells | [50] |
| NLRP12 | Activation | SARS-CoV-2 NSP5 | HEK293T-ACE2 | [54] |
| AIM2 | Activation | SARS-CoV-2 | Circulating monocytes of COVID-19 patients | [38] |
4. Inhibitory role of ginseng in inflammasome activation
4.1. NLRP3 inflammasome
The NLRP3 inflammasome is the most studied canonical inflammasome that induces inflammatory responses and diseases. Numerous ginsenosides and ginseng saponins have been demonstrated to inhibit NLRP3 inflammasome activation, resulting in the suppression of inflammatory responses and amelioration of disease conditions. Rb1, Rg1, Rg2, Rg3, Rg5, Rd, Re, Rh1, 25-OCH3-PPD, and compound K (CK) in Panax ginseng inhibit NLRP3 inflammasome stimulation, leading to suppressed inflammatory responses and multiple disease conditions, such as obesity, gouty arthritis, atherosclerosis, non-alcoholic fatty liver disease, liver injury, hyperlipidemia, type I diabetes, myocardial hypertrophy and dysfunction, cerebral ischemia and reperfusion injury, colitis, hepatic fibrosis, sepsis, neuronal damage, kidney injury, and cognitive deficits [[55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73]]. Korean Red Ginseng (KRG) extract and KRG saponin fraction in Panax ginseng also inhibited inflammatory responses by inhibiting NLRP3 inflammasome activation in macrophages, monocytes, sepsis mice, and aging mice [65,74]. Chikusetsu saponin IVa, a major active triterpenoid saponin in Panax japonicus, inhibited NLRP3 inflammasome activation, hence ameliorating obesity and neuroinflammation in macrophages, adipocytes, primary neurons, and postoperative cognitive dysfunction rats [75,76]. PF11, pseudoginsenoside, in Panax quinquefolius and saponins in Panax notoginseng inhibited NLRP3 inflammasome initiation, attenuating age-related neuroinflammation in macrophages and neurons [77,78].
4.2. NLRP1 inflammasome
Although most studies investigating the inhibitory role of ginseng in inflammasome activation have focused on NLRP3 inflammasome, while several others have also demonstrated the inhibitory role of ginseng in NLRP1 inflammasome stimulation. The NLRP1 inflammasome is another canonical inflammasome in the NLR family, and some studies have reported the inhibitory role of ginsenoside Rg1 in NLRP1 inflammasome activation and diseases. Rg1 in Panax ginseng showed neuroprotective effects against neuronal injury and degeneration by hindering NLRP1 inflammasome-activated neuroinflammation in neurons [79]. Rg1 in Panax ginseng protected against age-related neuronal damage and senescence by suppressing NLRP1 inflammasome-activated oxidative stress and neuroinflammation in neurons [80].
4.3. AIM2 inflammasome
The inhibitory effect of ginseng on the activation and inflammatory responses of AIM2 inflammasome, a non-NLR family inflammasome, was examined. KRG extract inhibits AIM2 inflammasome initiation and AIM2 inflammasome-activated inflammatory responses in macrophages and acute septic shock, increasing the survival rate of sepsis mice [65]. Rh1 and Rg3, as the key ginsenosides in KRG extract, play an inhibitory role in AIM2 inflammasome activation and inflammatory responses in macrophages and acute septic shock [65]. Fructose-arginine, a non-ginsenoside amino-sugar in the KRG extract, also inhibited AIM2 inflammasome stimulation. Fructose-arginine in the KRG non-saponin fraction attenuated AIM2 inflammasome activation and AIM2 inflammasome-activated inflammatory responses in macrophages [81]. Interestingly, Rh1 and Rg3 in the KRG extract inhibited the stimulation of both AIM2 and NLRP3 inflammasomes [65]; however, fructose-arginine in the KRG non-saponin fraction inhibited only AIM2, but not NLRP3 [81], suggesting that fructose-arginine is a more specific inhibitor of AIM2 inflammasome activation.
4.4. Caspase-11 and caspase-4 inflammasomes
Mouse caspase-11 and human caspase-4/5 are non-canonical inflammasomes that are distinct from canonical inflammasomes. These non-canonical inflammasomes were recently discovered, and many studies have successfully demonstrated their roles in inflammatory responses and diseases, particularly in infectious diseases caused by gram-negative bacterial infection [[15], [16], [17], [18], [82]]. However, non-canonical inflammasomes have not received much attention in studies investigating the inhibitory role of ginseng in inflammasome activation and inflammasome-activated inflammatory diseases. Several natural compounds, such as flavonoids and plant extracts, have been reported to attenuate inflammatory responses and diseases by inhibiting non-canonical inflammasome [[83], [84], [85], [86], [87], [88], [89]]; however, the inhibitory role of ginseng in non-canonical inflammasome activation has been rarely demonstrated. KRG extract hindered caspase-11 non-canonical inflammasome initiation and downstream inflammatory responses in macrophages [90]. KRG extract also increased the survival rate of mice with sepsis induced by lethal doses of lipopolysaccharides by inhibiting caspase-11 non-canonical inflammasome activation [90]. Interestingly, the anti-cancer effect of Rh2 through the inhibition of caspase-4 non-canonical inflammasome stimulation has been demonstrated in lung cancer. Rh2 suppresses the proliferation of human lung cancer cells and reduces tumor growth by promoting the apoptotic death of lung cancer cells. The anti-cancer effect of Rh2 was accomplished by the restriction of caspase-4 non-canonical inflammasome activation in lung cancer cells [91].
Taken together, ginsenosides and saponins play an anti-inflammatory role by inhibiting the initiation of canonical and non-canonical inflammasomes, including NLRP1, NLRP3, AIM2, and caspase-11, in inflammatory responses and disease (Table 2).
Table 2.
Inhibitory role of ginseng in inflammasome activation.
| Target | Ginseng | Components | Models | Ref. |
|---|---|---|---|---|
| NLRP3 | Panax ginseng | Rb1 | 3T3-L1, adipose tissue | [55] |
| Gouty arthritic rats | [56] | |||
| Rg1 | NAFLD mice | [57] | ||
| Liver injury mice | [58,59] | |||
| BNCC337685, diabetic nephropathy rats | [60] | |||
| Diabetic mice | [61] | |||
| Cardiomyocytes, myocardial injury mice | [62] | |||
| Rg2 | KC, NAFLD mice | [63] | ||
| Rg3 | RAW264.7, sepsis mice | [64] | ||
| BMDMs, THP-1, sepsis mice | [65] | |||
| HK-2, kidney injury mice | [66] | |||
| AC16, HCM, cardiomyocytes, myocardial hypertrophy rats | [67] | |||
| Rg5 | Diabetic nephropathy mice | [68] | ||
| Rd | THP-1, colitis mice | [69] | ||
| Cerebral IRI mice | [70] | |||
| Re | Memory impairment mice | [71] | ||
| Rh1 | BMDMs, THP-1, sepsis mice | [65] | ||
| KC, NAFLD mice | [63] | |||
| 25-OCH3-PPD | HSC-T6, hepatic fibrosis mice | [72] | ||
| CK | Atherosclerotic mice | [73] | ||
| 3T3-L1, adipose tissue | [55] | |||
| KRG extract | BMDMs, THP-1, sepsis mice | [65] | ||
| KRG saponins | Aging mice | [74] | ||
| Panax japonicus | CS IVa | BMDMs, adipocytes, obese mice | [75] | |
| Neurons, POCD rats | [76] | |||
| Panax quinquefolius | PF11 | Cognition impaired mice | [77] | |
| Panax notoginseng | Total saponins | Aging rats | [78] | |
| NLRP1 | Panax ginseng | Rg1 | Neuronal injury mice | [79] |
| Rg1 | Hippocampal neurons, neuronal injury mice | [80] | ||
| AIM2 | Panax ginseng | Rg3 | BMDMs, THP-1, sepsis mice | [65] |
| Fructose-arginine | BMDMs | [81] | ||
| Caspase-11 | Panax ginseng | KRG extract | J774A.1, sepsis mice | In press |
| Caspase-4 | Rh2 | Human lung cancer cells | [91] |
5. Conclusions and perspectives: Therapeutic potential of ginseng in SARS-CoV-2 pathogenesis and COVID-19
After identifying SARS-CoV-2 as a pathogenic virus causing the ongoing global pandemic of COVID-19, a huge effort has been made to understand the SARS-CoV-2-infected COVID-19 pathogenesis and underlying mechanisms in COVID-19 patients. This effort has successfully demonstrated that despite cases of mild asymptomatic COVID-19 caused by SARS-CoV-2, SARS-CoV-2 also strongly activates the immune system of host, leading to hyper-inflammation and cytokine storm that cause severe COVID-19, organ injuries, and even death [6]. One of the most critical hallmarks of inflammatory responses and disease pathogenesis is inflammasome activation, which leads to GSDMD pore-mediated pyroptosis and the secretion of pro-inflammatory cytokines through the GSDMD pores [13,14]. SARS-CoV-2 activates various inflammasomes, resulting in severe COVID-19 in many patients with multiple organ injuries due to pyroptotic cell death and cytokine storms due to the massive secretion of pro-inflammatory cytokines. In contrast to these observations, several studies have documented the hindering role of SARS-CoV-2 in inflammasome activation to escape host antiviral immunity. Restricting inflammasome initiation as well as the improvement of host immunity in COVID-19 patients can provide therapeutic benefits.
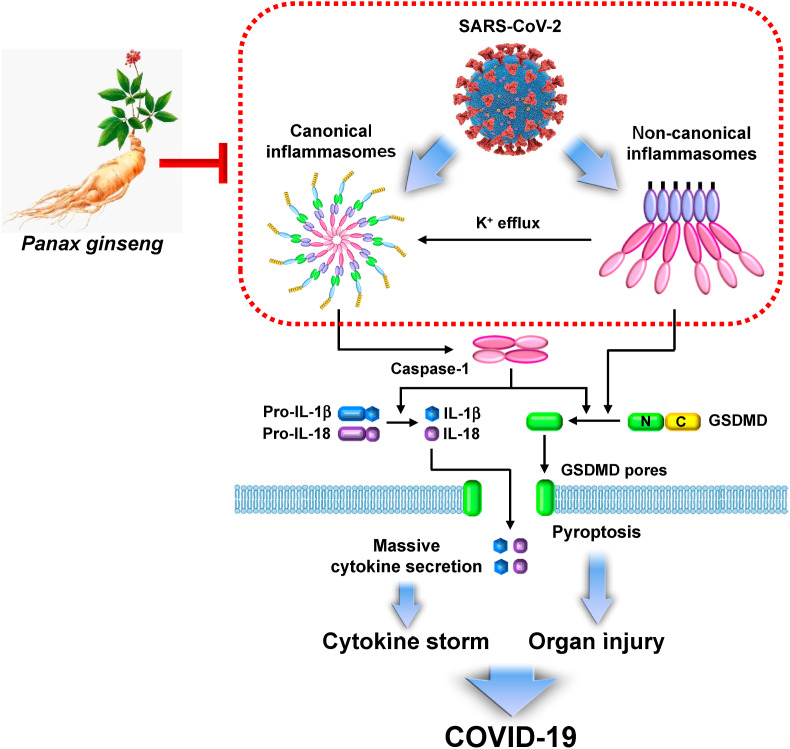
Ginseng and its main active physiological and pharmacological constituents, ginsenosides and saponins, have been successfully demonstrated as nutraceuticals that ameliorate multiple inflammatory diseases by attenuating the priming step of inflammatory responses [92,93]. Interestingly, ginsenosides and saponins also inhibit the triggering step via attenuating inflammasome activation [11,12]. This provides strong evidence that regular consumption of ginseng as a supplement may reduce the risk of COVID-19 progression, and ginseng and its constituents, ginsenosides, and saponins can be potential herbal medicines to alleviate COVID-19 by inhibiting inflammasome stimulation. Notably, ginseng can ameliorate COVID-19 through a distinct mechanism by inhibiting inflammasome activation and enhancing the host's antiviral immunity [94,95]. The preventive and therapeutic potential of ginseng against COVID-19 via modulation of inflammasome activation is shown in Fig. 2.
Fig. 2.
Graphical summary demonstrating the potential roles of ginseng in SARS-CoV-2-infected COVID-19. SARS-CoV-2 infection induces the activation of canonical and non-canonical inflammasomes, leading to the cytokine storm by massive cytokine secretion and organ injuries by pyroptosis. Ginseng regulates the activation of inflammasomes, which could show potential benefits in COVID-19.
Despite these successful studies, direct evidence of ginseng-mediated hindrance of SARS-CoV-2 and alleviation of COVID-19 symptoms have not yet been reported. In addition, the identification and validation of ginsenosides and saponins that can ameliorate COVID-19 by inhibiting inflammasome initiation are in high demand. Moreover, toxicological issues of ginsenosides and saponins need to be evaluated for their effective and safe use in COVID-19 patients.
In conclusion, accumulating evidence strongly suggests that ginseng and its main active ingredients, ginsenosides and saponins, provide preventive and therapeutic benefits by not only inhibiting inflammasome activation and consequent hyper-inflammation, but also promoting the host's antiviral immunity in COVID-19 patients.
Declaration of competing interest
The author declares no conflict of interest.
Acknowledgments
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (NRF-2020R1F1A1074415).
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W., China Novel Coronavirus I., Research T. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coronaviridae Study Group of the International Committee on Taxonomy of V The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tan Y.J., Lim S.G., Hong W. Characterization of viral proteins encoded by the SARS-coronavirus genome. Antivir Res. 2005;65:69–78. doi: 10.1016/j.antiviral.2004.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dixon B.E., Wools-Kaloustian K.K., Fadel W.F., Duszynski T.J., Yiannoutsos C., Halverson P.K., Menachemi N. Symptoms and symptom clusters associated with SARS-CoV-2 infection in community-based populations: results from a statewide epidemiological study. PLoS One. 2021;16 doi: 10.1371/journal.pone.0241875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ejaz H., Alsrhani A., Zafar A., Javed H., Junaid K., Abdalla A.E., Abosalif K.O.A., Ahmed Z., Younas S. COVID-19 and comorbidities: deleterious impact on infected patients. J Infect Publ Health. 2020;13:1833–1839. doi: 10.1016/j.jiph.2020.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merad M., Martin J.C. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020;20:355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu J.M., Yao Q., Chen C. Ginseng compounds: an update on their molecular mechanisms and medical applications. Curr Vasc Pharmacol. 2009;7:293–302. doi: 10.2174/157016109788340767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao B., Lv C., Lu J. Natural occurring polysaccharides from Panax ginseng C. A. Meyer: a review of isolation, structures, and bioactivities. Int J Biol Macromol. 2019;133:324–336. doi: 10.1016/j.ijbiomac.2019.03.229. [DOI] [PubMed] [Google Scholar]
- 9.Attele A.S., Wu J.A., Yuan C.S. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58:1685–1693. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 10.Sun Y.J., Chen H., Hao Z.Y., Wang J.M., Zhang Y.L., Zhao X., Zheng Y.N. [Chemical constituents from fruit of Panax ginseng] Zhong Yao Cai. 2014;37:1387–1390. [PubMed] [Google Scholar]
- 11.Yi Y.S. New mechanisms of ginseng saponin-mediated anti-inflammatory action via targeting canonical inflammasome signaling pathways. J Ethnopharmacol. 2021;278:114292. doi: 10.1016/j.jep.2021.114292. [DOI] [PubMed] [Google Scholar]
- 12.Yi Y.S. Roles of ginsenosides in inflammasome activation. J Ginseng Res. 2019;43:172–178. doi: 10.1016/j.jgr.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng D., Liwinski T., Elinav E. Inflammasome activation and regulation: toward a better understanding of complex mechanisms. Cell Discov. 2020;6:36. doi: 10.1038/s41421-020-0167-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xue Y., Enosi Tuipulotu D., Tan W.H., Kay C., Man S.M. Emerging activators and regulators of inflammasomes and pyroptosis. Trends Immunol. 2019;40:1035–1052. doi: 10.1016/j.it.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 15.Kayagaki N., Warming S., Lamkanfi M., Vande Walle L., Louie S., Dong J., Newton K., Qu Y., Liu J., Heldens S., Zhang J., Lee W.P., Roose-Girma M., Dixit V.M. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 16.Hagar J.A., Powell D.A., Aachoui Y., Ernst R.K., Miao E.A. Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science. 2013;341:1250–1253. doi: 10.1126/science.1240988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kayagaki N., Wong M.T., Stowe I.B., Ramani S.R., Gonzalez L.C., Akashi-Takamura S., Miyake K., Zhang J., Lee W.P., Muszynski A., Forsberg L.S., Carlson R.W., Dixit V.M. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science. 2013;341:1246–1249. doi: 10.1126/science.1240248. [DOI] [PubMed] [Google Scholar]
- 18.Shi J., Zhao Y., Wang Y., Gao W., Ding J., Li P., Hu L., Shao F. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014;514:187–192. doi: 10.1038/nature13683. [DOI] [PubMed] [Google Scholar]
- 19.Yi Y.S. Caspase-11 non-canonical inflammasome: a critical sensor of intracellular lipopolysaccharide in macrophage-mediated inflammatory responses. Immunology. 2017;152:207–217. doi: 10.1111/imm.12787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yi Y.S. Regulatory roles of the caspase-11 non-canonical inflammasome in inflammatory diseases. Immune Netw. 2018;18:e41. doi: 10.4110/in.2018.18.e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matikainen S., Nyman T.A., Cypryk W. Function and regulation of noncanonical caspase-4/5/11 inflammasome. J Immunol. 2020;204:3063–3069. doi: 10.4049/jimmunol.2000373. [DOI] [PubMed] [Google Scholar]
- 22.Ruhl S., Broz P. Caspase-11 activates a canonical NLRP3 inflammasome by promoting K(+) efflux. Eur J Immunol. 2015;45:2927–2936. doi: 10.1002/eji.201545772. [DOI] [PubMed] [Google Scholar]
- 23.Yi Y.S. Functional crosstalk between non-canonical caspase-11 and canonical NLRP3 inflammasomes during infection-mediated inflammation. Immunology. 2020;159:142–155. doi: 10.1111/imm.13134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corpetti C., Del Re A., Seguella L., Palenca I., Rurgo S., De Conno B., Pesce M., Sarnelli G., Esposito G. Cannabidiol inhibits SARS-Cov-2 spike (S) protein-induced cytotoxicity and inflammation through a PPARgamma-dependent TLR4/NLRP3/Caspase-1 signaling suppression in Caco-2 cell line. Phytother Res. 2021;35:6893–6903. doi: 10.1002/ptr.7302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu H., Akinyemi I.A., Chitre S.A., Loeb J.C., Lednicky J.A., McIntosh M.T., Bhaduri-McIntosh S. SARS-CoV-2 viroporin encoded by ORF3a triggers the NLRP3 inflammatory pathway. Virology. 2022;568:13–22. doi: 10.1016/j.virol.2022.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campbell G.R., To R.K., Hanna J., Spector S.A. SARS-CoV-2, SARS-CoV-1, and HIV-1 derived ssRNA sequences activate the NLRP3 inflammasome in human macrophages through a non-classical pathway. iScience. 2021;24:102295. doi: 10.1016/j.isci.2021.102295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yalcinkaya M., Liu W., Islam M.N., Kotini A.G., Gusarova G.A., Fidler T.P., Papapetrou E.P., Bhattacharya J., Wang N., Tall A.R. Modulation of the NLRP3 inflammasome by sars-CoV-2 envelope protein. Sci Rep. 2021;11:24432. doi: 10.1038/s41598-021-04133-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pan P., Shen M., Yu Z., Ge W., Chen K., Tian M., Xiao F., Wang Z., Wang J., Jia Y., Wang W., Wan P., Zhang J., Chen W., Lei Z., Chen X., Luo Z., Zhang Q., Xu M., Li G., Li Y., Wu J. SARS-CoV-2 N protein promotes NLRP3 inflammasome activation to induce hyperinflammation. Nat Commun. 2021;12:4664. doi: 10.1038/s41467-021-25015-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim N.E., Kim D.K., Song Y.J. SARS-CoV-2 nonstructural proteins 1 and 13 suppress caspase-1 and the NLRP3 inflammasome activation. Microorganisms. 2021;9 doi: 10.3390/microorganisms9030494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olajide O.A., Iwuanyanwu V.U., Lepiarz-Raba I., Al-Hindawi A.A. Induction of exaggerated cytokine production in human peripheral blood mononuclear cells by a recombinant SARS-CoV-2 spike glycoprotein S1 and its inhibition by dexamethasone. Inflammation. 2021;44:1865–1877. doi: 10.1007/s10753-021-01464-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kucia M., Ratajczak J., Bujko K., Adamiak M., Ciechanowicz A., Chumak V., Brzezniakiewicz-Janus K., Ratajczak M.Z. An evidence that SARS-Cov-2/COVID-19 spike protein (SP) damages hematopoietic stem/progenitor cells in the mechanism of pyroptosis in Nlrp3 inflammasome-dependent manner. Leukemia. 2021;35:3026–3029. doi: 10.1038/s41375-021-01332-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C., Choe H., Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., Huan Y., Yang P., Zhang Y., Deng W., Bao L., Zhang B., Liu G., Wang Z., Chappell M., Liu Y., Zheng D., Leibbrandt A., Wada T., Slutsky A.S., Liu D., Qin C., Jiang C., Penninger J.M. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ratajczak M.Z., Bujko K., Ciechanowicz A., Sielatycka K., Cymer M., Marlicz W., Kucia M. SARS-CoV-2 entry receptor ACE2 is expressed on very small CD45(-) precursors of hematopoietic and endothelial cells and in response to virus spike protein activates the Nlrp3 inflammasome. Stem Cell Rev Rep. 2021;17:266–277. doi: 10.1007/s12015-020-10010-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun X., Liu Y., Huang Z., Xu W., Hu W., Yi L., Liu Z., Chan H., Zeng J., Liu X., Chen H., Yu J., Chan F.K.L., Ng S.C., Wong S.H., Wang M.H., Gin T., Joynt G.M., Hui D.S.C., Zou X., Shu Y., Cheng C.H.K., Fang S., Luo H., Lu J., Chan M.T.V., Zhang L., Wu W.K.K. SARS-CoV-2 non-structural protein 6 triggers NLRP3-dependent pyroptosis by targeting ATP6AP1. Cell Death Differ. 2022 doi: 10.1038/s41418-021-00916-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mick E., Kamm J., Pisco A.O., Ratnasiri K., Babik J.M., Castaneda G., DeRisi J.L., Detweiler A.M., Hao S.L., Kangelaris K.N., Kumar G.R., Li L.M., Mann S.A., Neff N., Prasad P.A., Serpa P.H., Shah S.J., Spottiswoode N., Tan M., Calfee C.S., Christenson S.A., Kistler A., Langelier C. Upper airway gene expression reveals suppressed immune responses to SARS-CoV-2 compared with other respiratory viruses. Nat Commun. 2020;11:5854. doi: 10.1038/s41467-020-19587-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Esmaeili Gouvarchin Ghaleh H., Hosseini A., Aghamollaei H., Fasihi-Ramandi M., Alishiri G., Saeedi-Boroujeni A., Hassanpour K., Mahmoudian-Sani M.R., Farnoosh G. NLRP3 inflammasome activation and oxidative stress status in the mild and moderate SARS-CoV-2 infected patients: impact of melatonin as a medicinal supplement. Z Naturforsch C J Biosci. 2022;77:37–42. doi: 10.1515/znc-2021-0101. [DOI] [PubMed] [Google Scholar]
- 38.Junqueira C., Crespo A., Ranjbar S., Lewandrowski M., Ingber J., de Lacerda L.B., Parry B., Ravid S., Clark S., Ho F., Vora S.M., Leger V., Beakes C., Margolin J., Russell N., Kays K., Gehrke L., Adhikari U.D., Henderson L., Janssen E., Kwon D., Sander C., Abraham J., Filbin M., Goldberg M.B., Wu H., Mehta G., Bell S., Goldfeld A.E., Lieberman J. SARS-CoV-2 infects blood monocytes to activate NLRP3 and AIM2 inflammasomes, pyroptosis and cytokine release. Res Sq. 2021 [Google Scholar]
- 39.Rodrigues T.S., de Sa K.S.G., Ishimoto A.Y., Becerra A., Oliveira S., Almeida L., Goncalves A.V., Perucello D.B., Andrade W.A., Castro R., Veras F.P., Toller-Kawahisa J.E., Nascimento D.C., de Lima M.H.F., Silva C.M.S., Caetite D.B., Martins R.B., Castro I.A., Pontelli M.C., de Barros F.C., do Amaral N.B., Giannini M.C., Bonjorno L.P., Lopes M.I.F., Santana R.C., Vilar F.C., Auxiliadora-Martins M., Luppino-Assad R., de Almeida S.C.L., de Oliveira F.R., Batah S.S., Siyuan L., Benatti M.N., Cunha T.M., Alves-Filho J.C., Cunha F.Q., Cunha L.D., Frantz F.G., Kohlsdorf T., Fabro A.T., Arruda E., de Oliveira R.D.R., Louzada-Junior P., Zamboni D.S. Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients. J Exp Med. 2021:218. doi: 10.1084/jem.20201707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iadecola C., Anrather J., Kamel H. Effects of COVID-19 on the nervous system. Cell. 2020;183:16–27 e11. doi: 10.1016/j.cell.2020.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abboud H., Abboud F.Z., Kharbouch H., Arkha Y., El Abbadi N., El Ouahabi A. COVID-19 and SARS-cov-2 infection: pathophysiology and clinical effects on the nervous system. World Neurosurg. 2020;140:49–53. doi: 10.1016/j.wneu.2020.05.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olajide O.A., Iwuanyanwu V.U., Adegbola O.D., Al-Hindawi A.A. SARS-CoV-2 spike glycoprotein S1 induces neuroinflammation in BV-2 microglia. Mol Neurobiol. 2022;59:445–458. doi: 10.1007/s12035-021-02593-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cama V.F., Marin-Prida J., Acosta-Rivero N., Acosta E.F., Diaz L.O., Casadesus A.V., Fernandez-Marrero B., Gilva-Rodriguez N., Cremata-Garcia D., Cervantes-Llanos M., Piniella-Matamoros B., Sanchez D., Del Rosario-Cruz L., Borrajero I., Diaz A., Gonzalez Y., Penton-Arias E., Montero-Gonzalez T., Guillen-Nieto G., Penton-Rol G. The microglial NLRP3 inflammasome is involved in human SARS-CoV-2 cerebral pathogenicity: a report of three post-mortem cases. J Neuroimmunol. 2021;361:577728. doi: 10.1016/j.jneuroim.2021.577728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen Y., Klein S.L., Garibaldi B.T., Li H., Wu C., Osevala N.M., Li T., Margolick J.B., Pawelec G., Leng S.X. Aging in COVID-19: vulnerability, immunity and intervention. Ageing Res Rev. 2021;65:101205. doi: 10.1016/j.arr.2020.101205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Akbar A.N., Gilroy D.W. Aging immunity may exacerbate COVID-19. Science. 2020;369:256–257. doi: 10.1126/science.abb0762. [DOI] [PubMed] [Google Scholar]
- 46.Lara P.C., Macias-Verde D., Burgos-Burgos J. Age-induced NLRP3 inflammasome over-activation increases lethality of SARS-CoV-2 pneumonia in elderly patients. Aging Dis. 2020;11:756–762. doi: 10.14336/AD.2020.0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee H.C., Wei Y.H. Mitochondria and aging. Adv Exp Med Biol. 2012;942:311–327. doi: 10.1007/978-94-007-2869-1_14. [DOI] [PubMed] [Google Scholar]
- 48.Oka T., Hikoso S., Yamaguchi O., Taneike M., Takeda T., Tamai T., Oyabu J., Murakawa T., Nakayama H., Nishida K., Akira S., Yamamoto A., Komuro I., Otsu K. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature. 2012;485:251–255. doi: 10.1038/nature10992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakahira K., Haspel J.A., Rathinam V.A., Lee S.J., Dolinay T., Lam H.C., Englert J.A., Rabinovitch M., Cernadas M., Kim H.P., Fitzgerald K.A., Ryter S.W., Choi A.M. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011;12:222–230. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jha P.K., Vijay A., Halu A., Uchida S., Aikawa M. Gene expression profiling reveals the shared and distinct transcriptional signatures in human lung epithelial cells infected with SARS-CoV-2, MERS-CoV, or SARS-CoV: potential implications in cardiovascular complications of COVID-19. Front Cardiovasc Med. 2020;7:623012. doi: 10.3389/fcvm.2020.623012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tuncer S., Fiorillo M.T., Sorrentino R. The multifaceted nature of NLRP12. J Leukoc Biol. 2014;96:991–1000. doi: 10.1189/jlb.3RU0514-265RR. [DOI] [PubMed] [Google Scholar]
- 52.Tay M.Z., Poh C.M., Renia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20:363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gurung P., Kanneganti T.D. NLRP12 in autoimmune diseases. Oncotarget. 2015;6:19950–19951. doi: 10.18632/oncotarget.4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moustaqil M., Ollivier E., Chiu H.P., Van Tol S., Rudolffi-Soto P., Stevens C., Bhumkar A., Hunter D.J.B., Freiberg A.N., Jacques D., Lee B., Sierecki E., Gambin Y. SARS-CoV-2 proteases PLpro and 3CLpro cleave IRF3 and critical modulators of inflammatory pathways (NLRP12 and TAB1): implications for disease presentation across species. Emerg Microb Infect. 2021;10:178–195. doi: 10.1080/22221751.2020.1870414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen W., Wang J., Luo Y., Wang T., Li X., Li A., Li J., Liu K., Liu B. Ginsenoside Rb1 and compound K improve insulin signaling and inhibit ER stress-associated NLRP3 inflammasome activation in adipose tissue. J Ginseng Res. 2016;40:351–358. doi: 10.1016/j.jgr.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu Y., Zhu H., Zhou W., Ye Q. Anti-inflammatory and anti-gouty-arthritic effect of free Ginsenoside Rb1 and nano Ginsenoside Rb1 against MSU induced gouty arthritis in experimental animals. Chem Biol Interact. 2020;332:109285. doi: 10.1016/j.cbi.2020.109285. [DOI] [PubMed] [Google Scholar]
- 57.Xu Y., Yang C., Zhang S., Li J., Xiao Q., Huang W. Ginsenoside Rg1 protects against non-alcoholic fatty liver disease by ameliorating lipid peroxidation, endoplasmic reticulum stress, and inflammasome activation. Biol Pharm Bull. 2018;41:1638–1644. doi: 10.1248/bpb.b18-00132. [DOI] [PubMed] [Google Scholar]
- 58.Li Y., Zhang D., Li L., Han Y., Dong X., Yang L., Li X., Li W., Li W. Ginsenoside Rg1 ameliorates aginginduced liver fibrosis by inhibiting the NOX4/NLRP3 inflammasome in SAMP8 mice. Mol Med Rep. 2021;24 doi: 10.3892/mmr.2021.12441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao J., He B., Zhang S., Huang W., Li X. Ginsenoside Rg1 alleviates acute liver injury through the induction of autophagy and suppressing NF-kappaB/NLRP3 inflammasome signaling pathway. Int J Med Sci. 2021;18:1382–1389. doi: 10.7150/ijms.50919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang T., Gao Y., Yue R., Wang X., Shi Y., Xu J., Wu B., Li Y. Ginsenoside Rg1 alleviates podocyte injury induced by hyperlipidemia via targeting the mTOR/NF-kappaB/NLRP3 Axis. Evid Based Complement Alternat Med. 2020;2020:2735714. doi: 10.1155/2020/2735714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gao Y., Li J., Chu S., Zhang Z., Chen N., Li L., Zhang L. Ginsenoside Rg1 protects mice against streptozotocin-induced type 1 diabetic by modulating the NLRP3 and Keap1/Nrf2/HO-1 pathways. Eur J Pharmacol. 2020;866:172801. doi: 10.1016/j.ejphar.2019.172801. [DOI] [PubMed] [Google Scholar]
- 62.Luo M., Yan D., Sun Q., Tao J., Xu L., Sun H., Zhao H. Ginsenoside Rg1 attenuates cardiomyocyte apoptosis and inflammation via the TLR4/NF-kB/NLRP3 pathway. J Cell Biochem. 2020;121:2994–3004. doi: 10.1002/jcb.29556. [DOI] [PubMed] [Google Scholar]
- 63.Wang F., Park J.S., Ma Y., Ma H., Lee Y.J., Lee G.R., Yoo H.S., Hong J.T., Roh Y.S. Ginseng saponin enriched in Rh1 and Rg2 ameliorates nonalcoholic fatty liver disease by inhibiting inflammasome activation. Nutrients. 2021;13 doi: 10.3390/nu13030856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yoon S.J., Park J.Y., Choi S., Lee J.B., Jung H., Kim T.D., Yoon S.R., Choi I., Shim S., Park Y.J. Ginsenoside Rg3 regulates S-nitrosylation of the NLRP3 inflammasome via suppression of iNOS. Biochem Biophys Res Commun. 2015;463:1184–1189. doi: 10.1016/j.bbrc.2015.06.080. [DOI] [PubMed] [Google Scholar]
- 65.Kim J., Ahn H., Han B.C., Lee S.H., Cho Y.W., Kim C.H., Hong E.J., An B.S., Jeung E.B., Lee G.S. Korean red ginseng extracts inhibit NLRP3 and AIM2 inflammasome activation. Immunol Lett. 2014;158:143–150. doi: 10.1016/j.imlet.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 66.Zhai J., Gao H., Wang S., Zhang S., Qu X., Zhang Y., Tao L., Sun J., Song Y., Fu L. Ginsenoside Rg3 attenuates cisplatin-induced kidney injury through inhibition of apoptosis and autophagy-inhibited NLRP3. J Biochem Mol Toxicol. 2021;35 doi: 10.1002/jbt.22896. [DOI] [PubMed] [Google Scholar]
- 67.Ren B., Feng J., Yang N., Guo Y., Chen C., Qin Q. Ginsenoside Rg3 attenuates angiotensin II-induced myocardial hypertrophy through repressing NLRP3 inflammasome and oxidative stress via modulating SIRT1/NF-kappaB pathway. Int Immunopharm. 2021;98:107841. doi: 10.1016/j.intimp.2021.107841. [DOI] [PubMed] [Google Scholar]
- 68.Zhu Y., Zhu C., Yang H., Deng J., Fan D. Protective effect of ginsenoside Rg5 against kidney injury via inhibition of NLRP3 inflammasome activation and the MAPK signaling pathway in high-fat diet/streptozotocin-induced diabetic mice. Pharmacol Res. 2020;155:104746. doi: 10.1016/j.phrs.2020.104746. [DOI] [PubMed] [Google Scholar]
- 69.Liu C., Wang J., Yang Y., Liu X., Zhu Y., Zou J., Peng S., Le T.H., Chen Y., Zhao S., He B., Mi Q., Zhang X., Du Q. Ginsenoside Rd ameliorates colitis by inducing p62-driven mitophagy-mediated NLRP3 inflammasome inactivation in mice. Biochem Pharmacol. 2018;155:366–379. doi: 10.1016/j.bcp.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 70.Yao Y., Hu S., Zhang C., Zhou Q., Wang H., Yang Y., Liu C., Ding H. Ginsenoside Rd attenuates cerebral ischemia/reperfusion injury by exerting an anti-pyroptotic effect via the miR-139-5p/FoxO1/Keap1/Nrf2 axis. Int Immunopharm. 2022;105:108582. doi: 10.1016/j.intimp.2022.108582. [DOI] [PubMed] [Google Scholar]
- 71.Wang H., Lv J., Jiang N., Huang H., Wang Q., Liu X. Ginsenoside Re protects against chronic restraint stress-induced cognitive deficits through regulation of NLRP3 and Nrf2 pathways in mice. Phytother Res. 2021 doi: 10.1002/ptr.6947. [DOI] [PubMed] [Google Scholar]
- 72.Kim M., Yi Y.S., Kim J., Han S.Y., Kim S.H., Seo D.B., Cho J.Y., Shin S.S. Effect of polysaccharides from a Korean ginseng berry on the immunosenescence of aged mice. J Ginseng Res. 2018;42:447–454. doi: 10.1016/j.jgr.2017.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhou L., Zheng Y., Li Z., Bao L., Dou Y., Tang Y., Zhang J., Zhou J., Liu Y., Jia Y., Li X. Compound K attenuates the development of atherosclerosis in ApoE(-/-) mice via LXRalpha activation. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17071054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chei S., Oh H.J., Jang H., Lee K., Jin H., Choi Y., Lee B.Y. Korean red ginseng suppresses the expression of oxidative stress response and NLRP3 inflammasome genes in aged C57BL/6 mouse ovaries. Foods. 2020;9 doi: 10.3390/foods9040526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yuan C., Liu C., Wang T., He Y., Zhou Z., Dun Y., Zhao H., Ren D., Wang J., Zhang C., Yuan D. Chikusetsu saponin IVa ameliorates high fat diet-induced inflammation in adipose tissue of mice through inhibition of NLRP3 inflammasome activation and NF-kappaB signaling. Oncotarget. 2017;8:31023–31040. doi: 10.18632/oncotarget.16052. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 76.Shao A., Fei J., Feng S., Weng J. Chikusetsu saponin IVa alleviated sevoflurane-induced neuroinflammation and cognitive impairment by blocking NLRP3/caspase-1 pathway. Pharmacol Rep. 2020;72:833–845. doi: 10.1007/s43440-020-00078-2. [DOI] [PubMed] [Google Scholar]
- 77.Zhang Z., Yang H., Yang J., Xie J., Xu J., Liu C., Wu C. Pseudoginsenoside-F11 attenuates cognitive impairment by ameliorating oxidative stress and neuroinflammation in dgalactose-treated mice. Int Immunopharm. 2019;67:78–86. doi: 10.1016/j.intimp.2018.11.026. [DOI] [PubMed] [Google Scholar]
- 78.Zhou Z., He M., Zhao Q., Wang D., Zhang C., Liu C., Zhao H., Dun Y., He Y., Yuan C., Yuan D., Wang T. Panax notoginseng saponins attenuate neuroinflammation through TXNIP-mediated NLRP3 inflammasome activation in aging rats. Curr Pharmaceut Biotechnol. 2021;22:1369–1379. doi: 10.2174/1389201021999201110204735. [DOI] [PubMed] [Google Scholar]
- 79.Zhang Y., Hu W., Zhang B., Yin Y., Zhang J., Huang D., Huang R., Li W., Li W. Ginsenoside Rg1 protects against neuronal degeneration induced by chronic dexamethasone treatment by inhibiting NLRP-1 inflammasomes in mice. Int J Mol Med. 2017;40:1134–1142. doi: 10.3892/ijmm.2017.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xu T.Z., Shen X.Y., Sun L.L., Chen Y.L., Zhang B.Q., Huang D.K., Li W.Z. Ginsenoside Rg1 protects against H2O2induced neuronal damage due to inhibition of the NLRP1 inflammasome signalling pathway in hippocampal neurons in vitro. Int J Mol Med. 2019;43:717–726. doi: 10.3892/ijmm.2018.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ahn H., Han B.C., Lee S.H., Lee G.S. Fructose-arginine, a non-saponin molecule of Korean Red Ginseng, attenuates AIM2 inflammasome activation. J Ginseng Res. 2020;44:808–814. doi: 10.1016/j.jgr.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Young-Su Yi. Dual roles of the caspase-11 non-canonical inflammasome in inflammatory bowel disease. Int Immunopharmacol. 2022;108 doi: 10.1016/j.intimp.2022.108739. [DOI] [PubMed] [Google Scholar]
- 83.Marquez-Flores Y.K., Villegas I., Cardeno A., Rosillo M.A., Alarcon-de-la-Lastra C. Apigenin supplementation protects the development of dextran sulfate sodium-induced murine experimental colitis by inhibiting canonical and non-canonical inflammasome signaling pathways. J Nutr Biochem. 2016;30:143–152. doi: 10.1016/j.jnutbio.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 84.Zhong X., Liu M., Yao W., Du K., He M., Jin X., Jiao L., Ma G., Wei B., Wei M. Epigallocatechin-3-Gallate attenuates microglial inflammation and neurotoxicity by suppressing the activation of canonical and noncanonical inflammasome via TLR4/NF-kappaB pathway. Mol Nutr Food Res. 2019;63 doi: 10.1002/mnfr.201801230. [DOI] [PubMed] [Google Scholar]
- 85.Ye J., Zeng B., Zhong M., Li H., Xu L., Shu J., Wang Y., Yang F., Zhong C., Ye X., He X., Ouyang D. Scutellarin inhibits caspase-11 activation and pyroptosis in macrophages via regulating PKA signaling. Acta Pharm Sin B. 2021;11:112–126. doi: 10.1016/j.apsb.2020.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang Z.T., Zhang D.Y., Xie K., Wang C.J., Xu F. Luteolin activates Tregs to promote IL-10 expression and alleviating caspase-11-dependent pyroptosis in sepsis-induced lung injury. Int Immunopharm. 2021;99:107914. doi: 10.1016/j.intimp.2021.107914. [DOI] [PubMed] [Google Scholar]
- 87.Xu J., Li S., Jiang L., Gao X., Liu W., Zhu X., Huang W., Zhao H., Wei Z., Wang K., Yang Z. Baicalin protects against zearalenone-induced chicks liver and kidney injury by inhibiting expression of oxidative stress, inflammatory cytokines and caspase signaling pathway. Int Immunopharm. 2021;100:108097. doi: 10.1016/j.intimp.2021.108097. [DOI] [PubMed] [Google Scholar]
- 88.Gao X., Xu J., Jiang L., Liu W., Hong H., Qian Y., Li S., Huang W., Zhao H., Yang Z., Liu Q., Wei Z. Morin alleviates aflatoxin B1-induced liver and kidney injury by inhibiting heterophil extracellular traps release, oxidative stress and inflammatory responses in chicks. Poultry Sci. 2021;100:101513. doi: 10.1016/j.psj.2021.101513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hwang S.H., Lorz L.R., Yi D.K., Noh J.K., Yi Y.S., Cho J.Y. Viburnum pichinchense methanol extract exerts anti-inflammatory effects via targeting the NF-kappaB and caspase-11 non-canonical inflammasome pathways in macrophages. J Ethnopharmacol. 2019;245:112161. doi: 10.1016/j.jep.2019.112161. [DOI] [PubMed] [Google Scholar]
- 90.Min Ji-Hyun, Cho Hui-Jin, Young-Su Yi. A novel mechanism of Korean red ginseng-mediated anti-inflammatory action via targeting caspase-11 non-canonical inflammasome in macrophages. J Ginseng Res. 2021:In Press. doi: 10.1016/j.jgr.2021.12.009. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ge G., Yan Y., Cai H. Ginsenoside Rh2 inhibited proliferation by inducing ROS mediated ER stress dependent apoptosis in lung cancer cells. Biol Pharm Bull. 2017;40:2117–2124. doi: 10.1248/bpb.b17-00463. [DOI] [PubMed] [Google Scholar]
- 92.Kim J.H., Yi Y.S., Kim M.Y., Cho J.Y. Role of ginsenosides, the main active components of Panax ginseng, in inflammatory responses and diseases. J Ginseng Res. 2017;41:435–443. doi: 10.1016/j.jgr.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee Y.Y., Kim S.D., Park S.C., Rhee M.H. Panax ginseng: inflammation, platelet aggregation, thrombus formation, and atherosclerosis crosstalk. J Ginseng Res. 2022;46:54–61. doi: 10.1016/j.jgr.2021.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hyun S.H., Ahn H.Y., Kim H.J., Kim S.W., So S.H., In G., Park C.K., Han C.K. Immuno-enhancement effects of Korean Red Ginseng in healthy adults: a randomized, double-blind, placebo-controlled trial. J Ginseng Res. 2021;45:191–198. doi: 10.1016/j.jgr.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shin M.S., Song J.H., Choi P., Lee J.H., Kim S.Y., Shin K.S., Ham J., Kang K.S. Stimulation of innate immune function by Panax ginseng after heat processing. J Agric Food Chem. 2018;66:4652–4659. doi: 10.1021/acs.jafc.8b00152. [DOI] [PubMed] [Google Scholar]